Monoclonal Antibody Functionalized, and L-lysine α-Oxidase Loaded PEGylated-Chitosan Nanoparticle for HER2/Neu Targeted Breast Cancer Therapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Cell Line, and Animals
2.2. Preparation of CS-LO NPs/CS-LO-PEG NPs
2.3. Functionalization of Antibody Anti-HER2 (Trastuzumab) in CS-LO-PEG NPs
2.4. Enzyme Assay
2.5. Enzyme Loading and Entrapment Assay
2.6. Characterization of Nanoparticles
2.7. In Vitro Enzyme Release Assay
2.8. Cell Culture and Blood Compatibility Assay
2.8.1. Cellular Internalization
2.8.2. Hemolysis Assay
2.8.3. Cytotoxicity
2.8.4. Fluorescent Microscopic Assay
2.8.5. FITC, Annexin V Apoptosis Assay
2.8.6. Cell Cycle Assay
2.9. In Vivo Animal Model
2.10. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterization
3.1.1. DLS, Enzyme Entrapment, Loading Efficiency
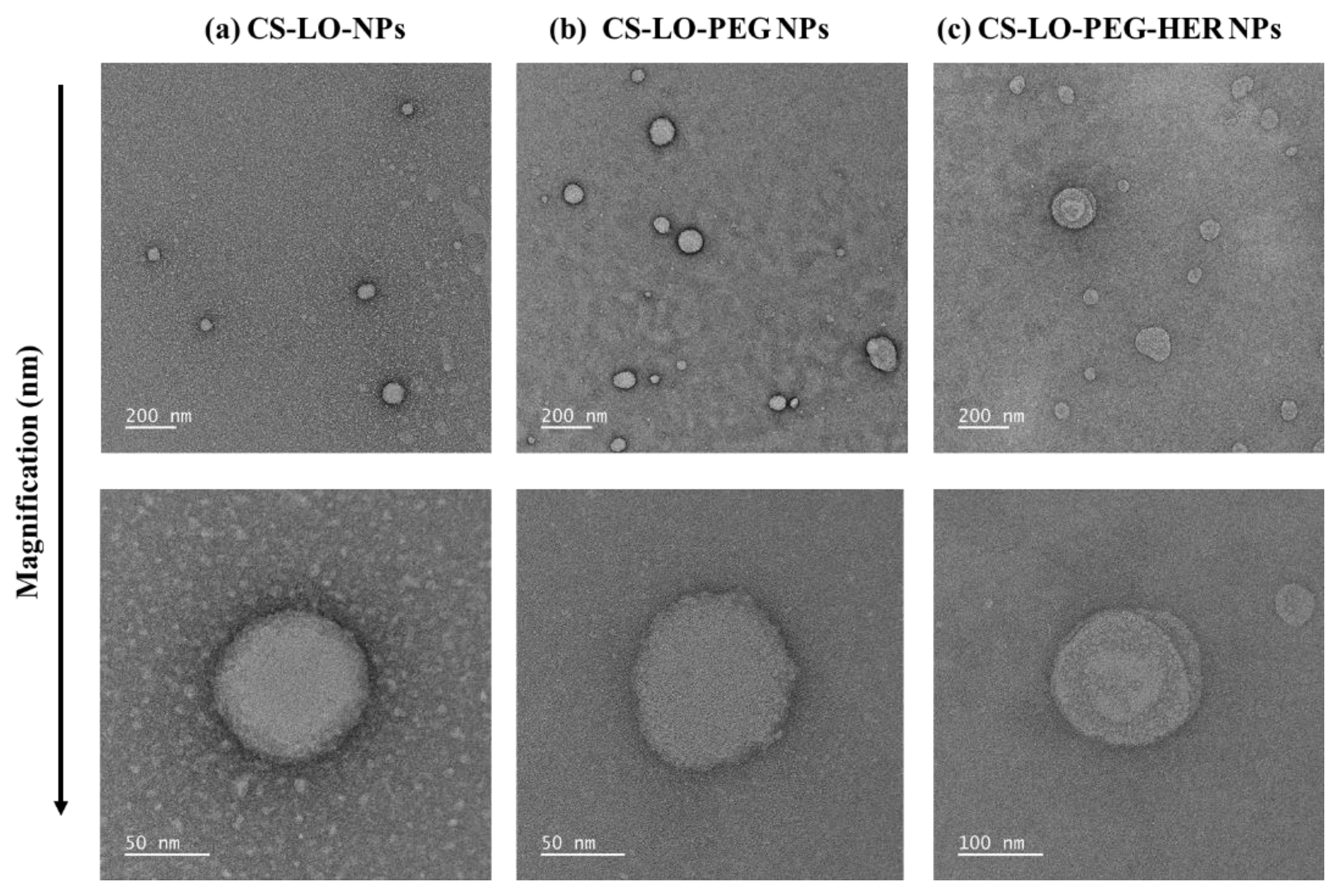
3.1.2. Morphological Characterization by TEM
3.1.3. Surface Chemistry Analysis
3.2. In-Vitro Experiments
3.2.1. Drug Release and Intracellular Distribution
3.2.2. Blood Compatibility
3.2.3. Cytotoxicity
3.2.4. Fluorescent Staining Assay
3.2.5. Measurement of Apoptosis and Cell Cycle Arrest by Flow Cytometer
3.3. In Vivo Experiments
3.3.1. Survival, Tumor Size, and Body Weight
3.3.2. Histopathology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kusakabe, H.; Kodama, K.; Kuninaka, A.; Yoshino, H.; Misono, H.; Soda, K. A new antitumor enzyme, L-lysine alpha-oxidase from Trichoderma viride. Purification and enzymological properties. J. Biol. Chem. 1980, 255, 976–981. [Google Scholar] [CrossRef]
- Lukasheva, E.V.; Berezov, T.T. L-Lysine α-Oxidase: Physicochemical and Biological Properties. Biochemistry 2002, 67, 1152–1158. [Google Scholar] [CrossRef]
- Lukasheva, E.V.; Babayeva, G.; Karshieva, S.S.; Zhdanov, D.D.; Pokrovsky, V.S. L-Lysine α-Oxidase: Enzyme with Anticancer Properties. Pharmaceuticals 2021, 14, 1070. [Google Scholar] [CrossRef]
- Amano, M.; Mizuguchi, H.; Sano, T.; Kondo, H.; Shinyashiki, K.; Inagaki, J.; Tamura, T.; Kawaguchi, T.; Kusakabe, H.; Imada, K.; et al. Recombinant expression, molecular characterization and crystal structure of antitumor enzyme, L-lysine α-oxidase from Trichoderma viride. J. Biochem. 2015, 157, 549–559. [Google Scholar] [CrossRef]
- Pokrovsky, V.S.; Treshalina, H.M.; Lukasheva, E.V.; Sedakova, L.A.; Medentzev, A.G.; Arinbasarova, A.Y.; Berezov, T.T. Enzymatic properties and anticancer activity of L-lysine α-oxidase from Trichoderma cf. aureoviride Rifai BKMF-4268D. Anti.-Cancer Drugs 2013, 24, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Kusakabe, H.; Kodama, K.; Kuninaka, A.; Yoshino, H.; Soda, K. Effect ofl-Lysine α-Oxidase on Growth of Mouse Leukemic Cells. Agric. Biol. Chem. 1980, 44, 387–392. [Google Scholar] [CrossRef]
- Griffith, R.S.; Norins, A.L.; Kagan, C. A Multicentered Study of Lysine Therapy in Herpes simplex Infection. Dermatology 1978, 156, 257–267. [Google Scholar] [CrossRef]
- Lukasheva, E.V.; Makletsova, M.G.; Lukashev, A.N.; Babayeva, G.; Arinbasarova, A.Y.; Medentsev, A.G. Fungal Enzyme l-Lysine α-Oxidase Affects the Amino Acid Metabolism in the Brain and Decreases the Polyamine Level. Pharmaceuticals 2020, 13, 398. [Google Scholar] [CrossRef]
- Molina, M.A.; Codony-Servat, J.; Albanell, J.; Rojo, F.; Arribas, J.; Baselga, J. Trastuzumab (herceptin), a humanized anti-Her2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cells. Cancer Res. 2001, 61, 4744–4749. [Google Scholar]
- Saravanakumar, K.; Sathiyaseelan, A.; Mariadoss, A.V.A.; Jeevithan, E.; Hu, X.; Shin, S.; Wang, M.-H. Dual stimuli-responsive release of aptamer AS1411 decorated erlotinib loaded chitosan nanoparticles for non-small-cell lung carcinoma therapy. Carbohydr. Polym. 2020, 245, 116407. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Min, H.S.; Ku, S.H.; Son, S.; Kwon, I.C.; Kim, S.H.; Kim, K. Tumor-targeting glycol chitosan nanoparticles as a platform delivery carrier in cancer diagnosis and therapy. Nanomedicine 2014, 9, 1697–1713. [Google Scholar] [CrossRef]
- Nascimento, A.V.; Singh, A.; Bousbaa, H.; Ferreira, D.; Sarmento, B.; Amiji, M.M. Overcoming cisplatin resistance in non-small cell lung cancer with Mad2 silencing siRNA delivered systemically using EGFR-targeted chitosan nanoparticles. Acta Biomater. 2016, 47, 71–80. [Google Scholar] [CrossRef]
- Schellmann, N.; Deckert, P.M.; Bachran, D.; Fuchs, H.; Bachran, C. Targeted enzyme prodrug therapies. Mini-Rev. Med. Chem. 2010, 10, 887–904. [Google Scholar] [CrossRef]
- Appel, E.; Rabinkov, A.; Neeman, M.; Kohen, F.; Mirelman, D. Conjugates of daidzein-alliinase as a targeted pro-drug enzyme system against ovarian carcinoma. J. Drug Target. 2010, 19, 326–335. [Google Scholar] [CrossRef]
- Majd, M.H.; Asgari, D.; Barar, J.; Valizadeh, H.; Kafil, V.; Coukos, G.; Omidi, Y. Specific targeting of cancer cells by multifunctional mitoxantrone-conjugated magnetic nanoparticles. J. Drug Target. 2013, 21, 328–340. [Google Scholar] [CrossRef]
- Kumar, A.; Zhang, X.; Liang, X.-J. Gold nanoparticles: Emerging paradigm for targeted drug delivery system. Biotechnol. Adv. 2013, 31, 593–606. [Google Scholar] [CrossRef]
- Singh, R.; Lillard, J.W., Jr. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef]
- Baharifar, H.; Khoobi, M.; Bidgoli, S.A.; Amani, A. Preparation of PEG-grafted chitosan/streptokinase nanoparticles to improve biological half-life and reduce immunogenicity of the enzyme. Int. J. Biol. Macromol. 2019, 143, 181–189. [Google Scholar] [CrossRef]
- Singh, A.P.; Biswas, A.; Shukla, A.; Maiti, P. Targeted therapy in chronic diseases using nanomaterial-based drug delivery vehicles. Signal Transduct. Target. Ther. 2019, 4, 33. [Google Scholar] [CrossRef]
- Yuan, S.; Hua, J.; Zhou, Y.; Ding, Y.; Hu, Y. Doxorubicin Loaded Chitosan-W18 O49 Hybrid Nanoparticles for Combined Photothermal-Chemotherapy. Macromol. Biosci. 2017, 17, 1700033. [Google Scholar] [CrossRef]
- Kumar, S.; Jana, A.K.; Dhamija, I.; Maiti, M. Chitosan-assisted immobilization of serratiopeptidase on magnetic nanoparticles, characterization and its target delivery. J. Drug Target. 2013, 22, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Sathiyaseelan, A.; Saravanakumar, K.; Mariadoss, A.V.A.; Wang, M.-H. pH-controlled nucleolin targeted release of dual drug from chitosan-gold based aptamer functionalized nano drug delivery system for improved glioblastoma treatment. Carbohydr. Polym. 2021, 262, 117907. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.; Remuñán-López, C.; Vila-Jato, J.L.; Alonso, M.J. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J. Appl. Polym. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Scheeren, L.E.; Nogueira, D.R.; Macedo, L.B.; Vinardell, M.P.; Mitjans, M.; Infante, M.R.; Rolim, C.M.B. PEGylated and poloxamer-modified chitosan nanoparticles incorporating a lysine-based surfactant for pH-triggered doxorubicin release. Colloids Surfaces B Biointerfaces 2016, 138, 117–127. [Google Scholar] [CrossRef]
- Dziawer, Ł.; Majkowska-Pilip, A.; Gaweł, D.; Godlewska, M.; Pruszyński, M.; Jastrzębski, J.; Wąs, B.; Bilewicz, A. Trastuzumab-Modified Gold Nanoparticles Labeled with 211At as a Prospective Tool for Local Treatment of HER2-Positive Breast Cancer. Nanomaterials 2019, 9, 632. [Google Scholar] [CrossRef]
- Arinbasarova, A.Y.; Ashin, V.V.; Makrushin, K.V.; Medentsev, A.G.; Lukasheva, E.V.; Berezov, T.T. Isolation and properties of L-lysine-α-oxidase from the fungus Trichoderma cf. aureoviride RIFAI VKM F-4268D. Microbiology 2012, 81, 549–554. [Google Scholar] [CrossRef]
- Yoon, H.Y.; Son, S.; Lee, S.J.; Gil You, D.; Yhee, J.Y.; Park, J.H.; Swierczewska, M.; Lee, S.; Kwon, I.C.; Kim, S.H.; et al. Glycol chitosan nanoparticles as specialized cancer therapeutic vehicles: Sequential delivery of doxorubicin and Bcl-2 siRNA. Sci. Rep. 2014, 4, 6878. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Mariadoss, A.V.A.; Sathiyaseelan, A.; Wang, M.-H. Synthesis and characterization of nano-chitosan capped gold nanoparticles with multifunctional bioactive properties. Int. J. Biol. Macromol. 2020, 165, 747–757. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Sriram, B.; Sathiyaseelan, A.; Hu, X.; Mariadoss, A.V.A.; MubarakAli, D.; Wang, M.-H. Molecular identification, volatile metabolites profiling, and bioactivities of an indigenous endophytic fungus (Diaporthe sp.). Process Biochem. 2020, 102, 72–81. [Google Scholar] [CrossRef]
- Jaiswal, Y.; Tatke, P.; Gabhe, S.; Vaidya, A. Antidiabetic activity of extracts of Anacardium occidentale Linn. leaves on n -streptozotocin diabetic rats. J. Tradit. Complement. Med. 2016, 7, 421–427. [Google Scholar] [CrossRef]
- Sakhtianchi, R.; Atyabi, F.; Yousefpour, P.; Vasheghani-Farahani, E.; Movahedi, A.-A.M.; Dinarvand, R. Targeted delivery of doxorubicin-utilizing chitosan nanoparticles surface-functionalized with anti-Her2 trastuzumab. Int. J. Nanomed. 2011, 6, 1977–1990. [Google Scholar] [CrossRef][Green Version]
- Niu, S.; Williams, G.R.; Wu, J.; Wu, J.; Zhang, X.; Chen, X.; Li, S.; Jiao, J.; Zhu, L.-M. A chitosan-based cascade-responsive drug delivery system for triple-negative breast cancer therapy. J. Nanobiotechnology 2019, 17, 95. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.; Mittal, N.K.; Balabathula, P.; Thoma, L.A.; Wood, G.C. Development and in vitro evaluation of core–shell type lipid–polymer hybrid nanoparticles for the delivery of erlotinib in non-small cell lung cancer. Eur. J. Pharm. Sci. 2015, 81, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Forest, V.; Pourchez, J. Preferential binding of positive nanoparticles on cell membranes is due to electrostatic interactions: A too simplistic explanation that does not take into account the nanoparticle protein corona. Mater. Sci. Eng. C 2017, 70, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Pan, M.; Yang, Y.; Sun, A.; Chen, Y.; Yuan, L.; Huang, K.; Qu, Y.; He, C.; Wei, Q.; et al. Trimodal Sono/Photoinduced Focal Therapy for Localized Prostate Cancer: Single-Drug-Based Nanosensitizer under Dual-Activation. Adv. Funct. Mater. 2021, 31, 2104473. [Google Scholar] [CrossRef]
- Ito, T.; Sun, L.; Bevan, M.A.; Crooks, R.M. Comparison of Nanoparticle Size and Electrophoretic Mobility Measurements Using a Carbon-Nanotube-Based Coulter Counter, Dynamic Light Scattering, Transmission Electron Microscopy, and Phase Analysis Light Scattering. Langmuir 2004, 20, 6940–6945. [Google Scholar] [CrossRef] [PubMed]
- Sathiyaseelan, A.; Saravanakumar, K.; Mariadoss, A.V.A.; Wang, M.-H. Biocompatible fungal chitosan encapsulated phytogenic silver nanoparticles enhanced antidiabetic, antioxidant and antibacterial activity. Int. J. Biol. Macromol. 2020, 153, 63–71. [Google Scholar] [CrossRef]
- Melo, M.N.; Pereira, F.M.; Rocha, M.A.; Ribeiro, J.G.; Diz, F.M.; Monteiro, W.F.; Ligabue, R.A.; Severino, P.; Fricks, A.T. Immobilization and characterization of horseradish peroxidase into chitosan and chitosan/PEG nanoparticles: A comparative study. Process Biochem. 2020, 98, 160–171. [Google Scholar] [CrossRef]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef]
- Cipolatti, E.P.; Valério, A.; Nicoletti, G.; Theilacker, E.; Araújo, P.H.H.; Sayer, C.; Ninow, J.L.; de Oliveira, D. Immobilization of Candida antarctica lipase B on PEGylated poly(urea-urethane) nanoparticles by step miniemulsion polymerization. J. Mol. Catal. B Enzym. 2014, 109, 116–121. [Google Scholar] [CrossRef]
- Garg, N.K.; Dwivedi, P.; Campbell, C.; Tyagi, R.K. Site specific/targeted delivery of gemcitabine through anisamide anchored chitosan/poly ethylene glycol nanoparticles: An improved understanding of lung cancer therapeutic intervention. Eur. J. Pharm. Sci. 2012, 47, 1006–1014. [Google Scholar] [CrossRef]
- Antoniou, J.; Liu, F.; Majeed, H.; Qi, J.; Yokoyama, W.; Zhong, F. Physicochemical and morphological properties of size-controlled chitosan–tripolyphosphate nanoparticles. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 465, 137–146. [Google Scholar] [CrossRef]
- Niu, S.-J.C.C.-C.; Kuo, C.-F.H.S.-M. Evaluation of chitosan-g-PEG copolymer for cell anti-adhesion application. J. Med. Biol. Eng. 2007, 27, 41–46. [Google Scholar]
- Helmi, O.; Elshishiny, F.; Mamdouh, W. Targeted doxorubicin delivery and release within breast cancer environment using PEGylated chitosan nanoparticles labeled with monoclonal antibodies. Int. J. Biol. Macromol. 2021, 184, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Ye, Y.; Gao, F.; Yuan, H.; Lan, M.; Lou, K.; Wang, W. Chitosan-graft-β-cyclodextrin nanoparticles as a carrier for controlled drug release. Int. J. Pharm. 2013, 446, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Gatenby, R.A.; Gillies, R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 2004, 4, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Kesavan, S.; Meena, K.; Sharmili, S.A.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Alobaidi, A.S.; Alanzi, K.F.; Vaseeharan, B. Ulvan loaded graphene oxide nanoparticle fabricated with chitosan and d-mannose for targeted anticancer drug delivery. J. Drug Deliv. Sci. Technol. 2021, 65, 102760. [Google Scholar] [CrossRef]
- Wu, S.; Xu, C.; Zhu, Y.; Zheng, L.; Zhang, L.; Hu, Y.; Yu, B.; Wang, Y.; Xu, F.-J. Biofilm-Sensitive Photodynamic Nanoparticles for Enhanced Penetration and Antibacterial Efficiency. Adv. Funct. Mater. 2021, 31, 2103591. [Google Scholar] [CrossRef]
- Wang, F.; Li, J.; Tang, X.; Huang, K.; Chen, L. Polyelectrolyte three layer nanoparticles of chitosan/dextran sulfate/chitosan for dual drug delivery. Colloids Surf. B Biointerfaces 2020, 190, 110925. [Google Scholar] [CrossRef] [PubMed]
- Tai, W.; Mahato, R.; Cheng, K. The role of HER2 in cancer therapy and targeted drug delivery. J. Control. Release 2010, 146, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Accardo, A.; Aloj, L.; Aurilio, M.; Morelli, G.; Tesauro, D. Receptor binding peptides for target-selective delivery of nanoparticles encapsulated drugs. Int. J. Nanomed. 2014, 9, 1537–1557. [Google Scholar] [CrossRef][Green Version]
- Master, A.M.; Gupta, A.S. EGF receptor-targeted nanocarriers for enhanced cancer treatment. Nanomedicine 2012, 7, 1895–1906. [Google Scholar] [CrossRef]
- Ando, T.; Nagumo, M.; Ninomiya, M.; Tanaka, K.; Linhardt, R.J.; Koketsu, M. Synthesis of coumarin derivatives and their cytoprotective effects on t -BHP-induced oxidative damage in HepG2 cells. Bioorganic Med. Chem. Lett. 2018, 28, 2422–2425. [Google Scholar] [CrossRef] [PubMed]
- Pajaniradje, S.; Mohankumar, K.; Pamidimukkala, R.; Subramanian, S.; Rajagopalan, R. Antiproliferative and Apoptotic Effects ofSesbania grandifloraLeaves in Human Cancer Cells. BioMed Res. Int. 2014, 2014, 474953. [Google Scholar] [CrossRef]
- Murad, H.; Hawat, M.; Ekhtiar, A.; Aljapawe, A.; Abbas, A.; Darwish, H.; Sbenati, O.; Ghannam, A. Induction of G1-phase cell cycle arrest and apoptosis pathway in MDA-MB-231 human breast cancer cells by sulfated polysaccharide extracted from Laurencia papillosa. Cancer Cell Int. 2016, 16, 39. [Google Scholar] [CrossRef] [PubMed]
- Kuang, W.; Hu, W.; Ren, H.; Shao, Y.; Liu, B. Plant derived coumestrol phytochemical targets human skin carcinoma cells by inducing mitochondrial-mediated apoptosis, cell cycle arrest, inhibition of cell migration and invasion and modulation of m-TOR/PI3K/AKT signalling pathway. Saudi J. Biol. Sci. 2021, 28, 2739–2746. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, C.; Zheng, Y.; Zheng, Y.; Liu, Z.; Xu, K.; Zhong, W. Triple Enzyme-Regulated Molecular Hydrogels for Carrier-Free Delivery of Lonidamine. Adv. Funct. Mater. 2021, 31, 2104418. [Google Scholar] [CrossRef]
- Liao, T.; Liu, C.; Ren, J.; Chen, H.; Kuang, Y.; Jiang, B.; Chen, J.; Sun, Z.; Li, C. A chitosan/mesoporous silica nanoparticle-based anticancer drug delivery system with a “tumor-triggered targeting” property. Int. J. Biol. Macromol. 2021, 183, 2017–2029. [Google Scholar] [CrossRef]






| Initial Enzyme Input (mg) | Size (nm) | Zeta Potential (mV) | PDI | Enzyme Entrapment (%) | Enzyme Loading (%) |
|---|---|---|---|---|---|
| 0.1 | 138.53 ± 4.68 a | 26.73 ± 1.02 a | 0.35 ± 0.04 c | 75.77 ± 0.76 d | 5.26 ± 0.31 a |
| 0.2 | 143.76 ± 2.43 b | 31.10 ± 0.92 b | 0.44 ± 0.01 d | 62.82 ± 0.82 c | 10.59 ± 0.64 b |
| 0.5 | 144.1 ± 2.96 b | 35.86 ± 1.33 c | 0.28 ± 0.03 b | 59.15 ± 0.44 b | 14.26 ± 0.85 c |
| 1 | 182.60 ± 2.34 c | 36.60 ± 0.30 d | 0.12 ± 0.05 a | 53.38 ± 0.57 a | 17.49 ± 0.81 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saravanakumar, K.; Sathiyaseelan, A.; Park, S.; Kim, S.-R.; Priya, V.V.; Wang, M.-H. Monoclonal Antibody Functionalized, and L-lysine α-Oxidase Loaded PEGylated-Chitosan Nanoparticle for HER2/Neu Targeted Breast Cancer Therapy. Pharmaceutics 2022, 14, 927. https://doi.org/10.3390/pharmaceutics14050927
Saravanakumar K, Sathiyaseelan A, Park S, Kim S-R, Priya VV, Wang M-H. Monoclonal Antibody Functionalized, and L-lysine α-Oxidase Loaded PEGylated-Chitosan Nanoparticle for HER2/Neu Targeted Breast Cancer Therapy. Pharmaceutics. 2022; 14(5):927. https://doi.org/10.3390/pharmaceutics14050927
Chicago/Turabian StyleSaravanakumar, Kandasamy, Anbazhagan Sathiyaseelan, Soyoung Park, Song-Rae Kim, Veeraraghavan Vishnu Priya, and Myeong-Hyeon Wang. 2022. "Monoclonal Antibody Functionalized, and L-lysine α-Oxidase Loaded PEGylated-Chitosan Nanoparticle for HER2/Neu Targeted Breast Cancer Therapy" Pharmaceutics 14, no. 5: 927. https://doi.org/10.3390/pharmaceutics14050927
APA StyleSaravanakumar, K., Sathiyaseelan, A., Park, S., Kim, S.-R., Priya, V. V., & Wang, M.-H. (2022). Monoclonal Antibody Functionalized, and L-lysine α-Oxidase Loaded PEGylated-Chitosan Nanoparticle for HER2/Neu Targeted Breast Cancer Therapy. Pharmaceutics, 14(5), 927. https://doi.org/10.3390/pharmaceutics14050927








