Growth of MIN-6 Cells on Salmon Fibrinogen Scaffold Improves Insulin Secretion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electrospinning of Chitosan and Fibrinogen/Chitosan Scaffolds
2.2. Scaffold Neutralization and Sterilization
2.3. Scanning Electron Microscopy and Image Analysis
2.4. MIN-6 Cell Culture
2.5. Phase Contrast and Stereomicroscopy
2.6. Cell Proliferation Assay
2.7. Insulin Secretion Assay
2.8. RNA Extraction
2.9. cDNA Synthesis and Gene Expression Analysis
2.10. Statistical Analysis
3. Results and Discussion
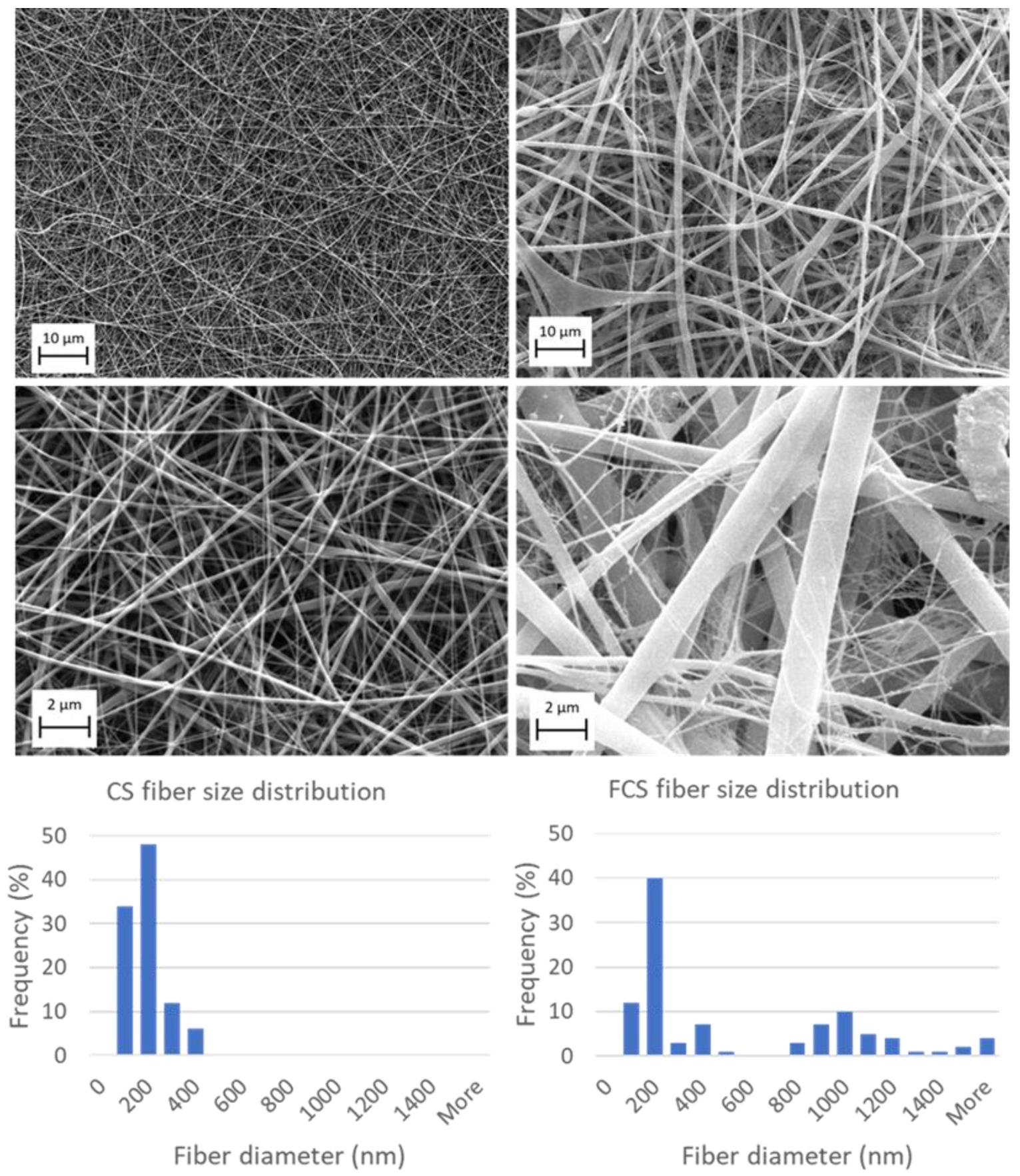
3.1. Electrospinning of Chitosan and Fibrinogen/Chitosan Scaffolds
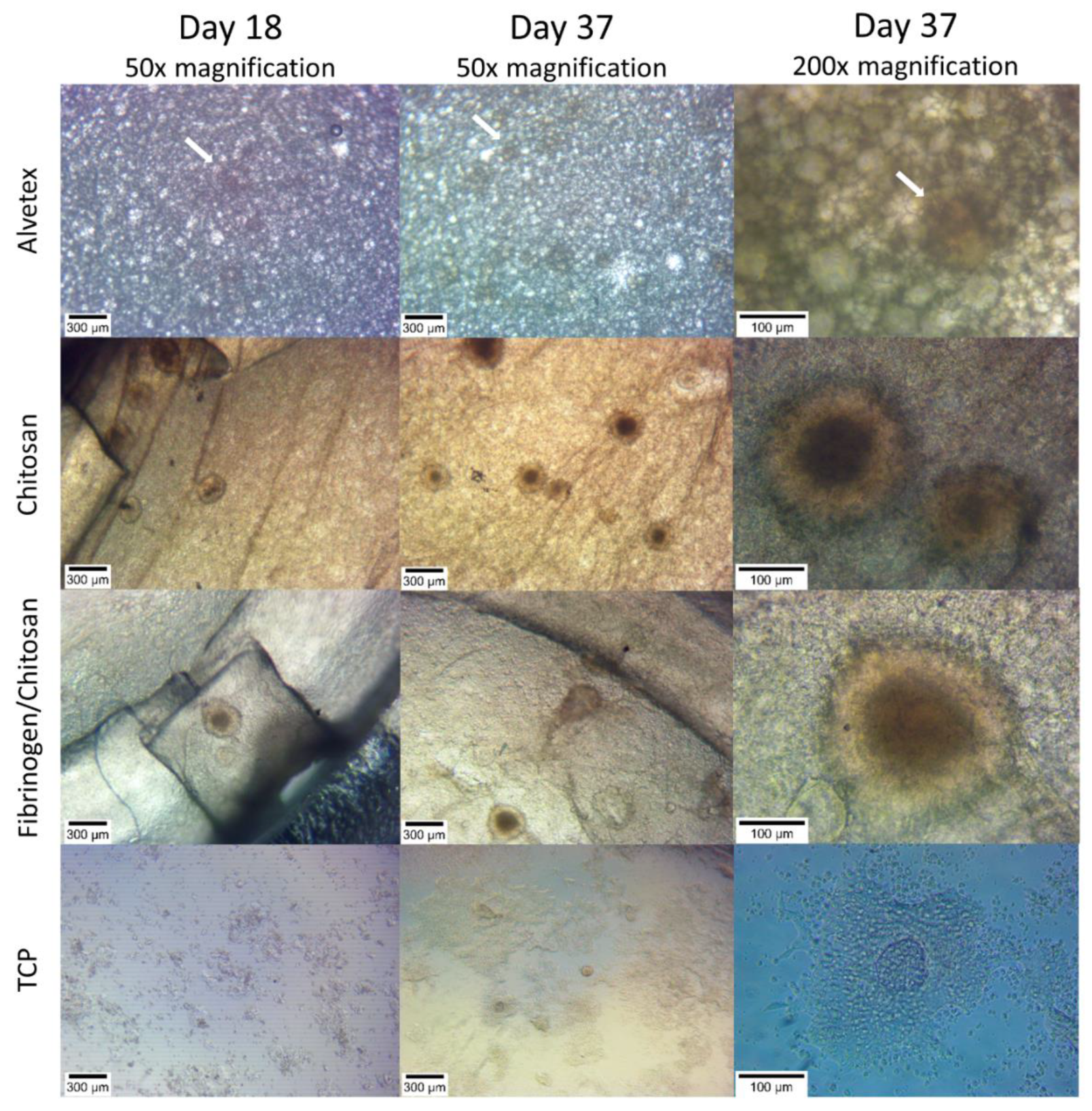
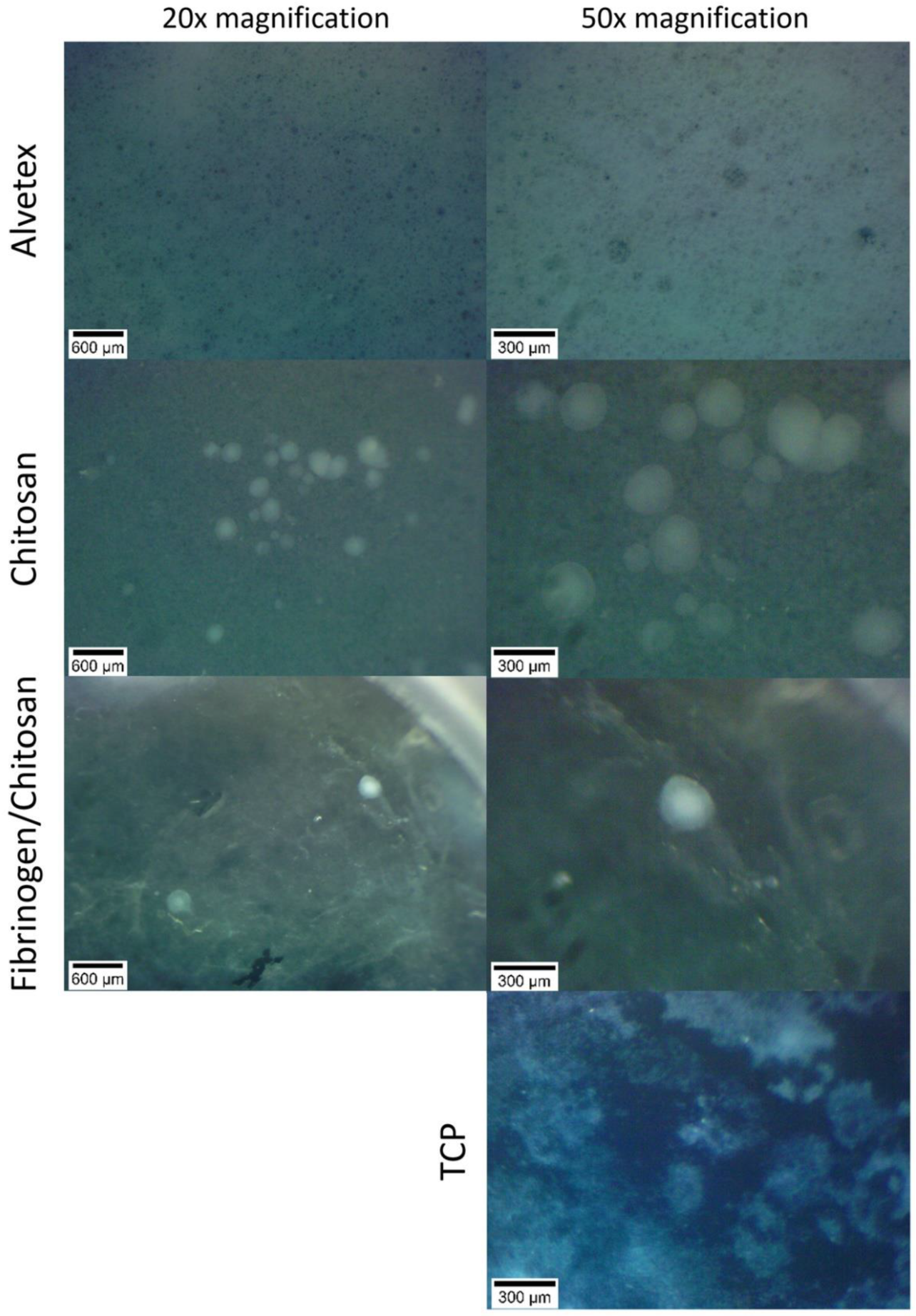
3.2. Cell Cluster Morphology
3.3. Insulin Secretion
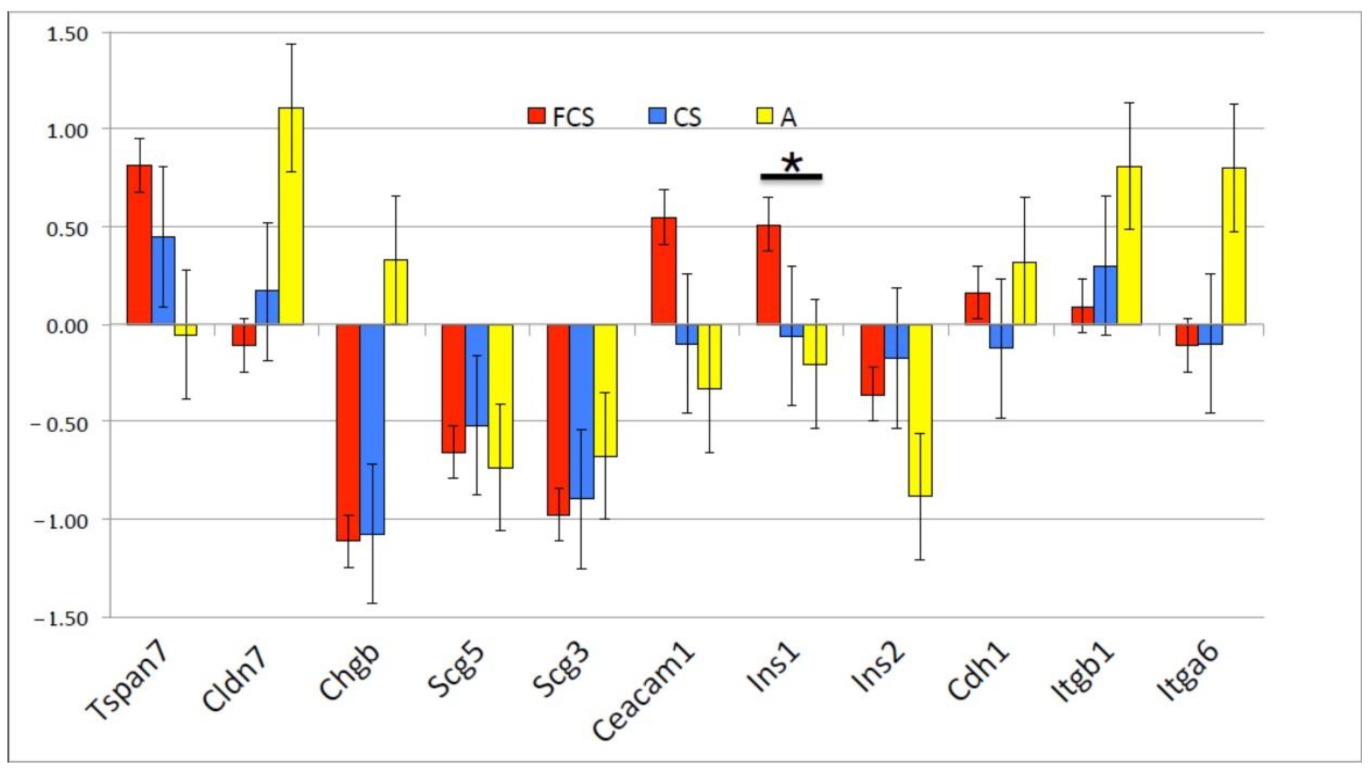
3.4. Gene Expression
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Reports on Diabetes 2016. Available online: http://www.who.int/diabetes/global-report/en/ (accessed on 8 December 2021).
- Borchers, A.T.; Uibo, R.; Gershwin, M.E. The geoepidemiology of type 1 diabetes. Autoimmun. Rev. 2010, 9, A355–A365. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; Desai, C.S. Islet Transplantation in Children. Curr. Gastroenterol. Rep. 2019, 21, 26. [Google Scholar] [CrossRef] [PubMed]
- Rickels, M.R.; Robertson, R.P. Pancreatic Islet Transplantation in Humans: Recent Progress and Future Directions. Endocr. Rev. 2019, 40, 631–668. [Google Scholar] [CrossRef] [Green Version]
- Vlahos, A.E.; Kinney, S.M.; Kingston, B.R.; Keshavjee, S.; Won, S.-Y.; Martyts, A.; Chan, W.C.W.; Sefton, M.V. Endothelialized collagen based pseudo-islets enables tuneable subcutaneous diabetes therapy. Biomaterials 2020, 232, 119710. [Google Scholar] [CrossRef] [PubMed]
- Aloysious, N.; Nair, P.D. Enhanced survival and function of islet-like clusters differentiated from adipose stem cells on a three-dimensional natural polymeric scaffold: An in vitro study. Tissue Eng. Part A 2014, 20, 1508–1522. [Google Scholar] [CrossRef]
- Green, A.D.; Vasu, S.; Flatt, P.R. Cellular models for beta-cell function and diabetes gene therapy. Acta Physiol. 2018, 222, e13012. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, H.; Asano, T.; Tsukuda, K.; Katagiri, H.; Inukai, K.; Anai, M.; Kikuchi, M.; Yazaki, Y.; Miyazaki, J.I.; Oka, Y. Pancreatic beta cell line MIN6 exhibits characteristics of glucose metabolism and glucose-stimulated insulin secretion similar to those of normal islets. Diabetologia 1993, 36, 1139–1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spelios, M.G.; Olsen, J.A.; Kenna, L.A.; Akirav, E.M. Islet Endothelial Cells Induce Glycosylation and Increase Cell-surface Expression of Integrin β1 in β Cells. J. Biol. Chem. 2015, 290, 15250–15259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velasco-Mallorquí, F.; Rodríguez-Comas, J.; Ramón-Azcón, J. Cellulose-based scaffolds enhance pseudoislets formation and functionality. Biofabrication 2021, 13, 35044. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yan, S.; Xu, X.; Yu, T.; Guo, Z.; Ma, M.; Zhang, Y.; Gu, Z.; Feng, Y.; Du, C.; et al. Three-dimensional cell-culture platform based on hydrogel with tunable microenvironmental properties to improve insulin-secreting function of MIN6 cells. Biomaterials 2021, 270, 120687. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.S.; Tian, Y.; Zhang, C.; Ye, Z.; Roy, K.; Chinnappan, A.; Ramakrishna, S.; Liu, W.; Ghosh, R. A Review on Electrospun Nanofibers Based Advanced Applications: From Health Care to Energy Devices. Polymers 2021, 13, 3746. [Google Scholar] [CrossRef] [PubMed]
- Suh, T.C.; Amanah, A.Y.; Gluck, J.M. Electrospun Scaffolds and Induced Pluripotent Stem Cell-Derived Cardiomyocytes for Cardiac Tissue Engineering Applications. Bioengineering 2020, 7, 105. [Google Scholar] [CrossRef]
- Yao, Q.; Cosme, J.G.L.; Xu, T.; Miszuk, J.M.; Picciani, P.H.S.; Fong, H.; Sun, H. Three dimensional electrospun PCL/PLA blend nanofibrous scaffolds with significantly improved stem cells osteogenic differentiation and cranial bone formation. Biomaterials 2017, 115, 115–127. [Google Scholar] [CrossRef] [Green Version]
- El-Ghazali, S.; Khatri, M.; Hussain, N.; Khatri, Z.; Yamamoto, T.; Kim, S.H.; Kobayashi, S.; Kim, I.S. Characterization and biocompatibility evaluation of artificial blood vessels prepared from pristine poly (Ethylene-glycol-co-1,4-cyclohexane dimethylene-co-isosorbide terephthalate), poly (1, 4 cyclohexane di-methylene-co-isosorbide terephthalate) nanofibers and their blended composition. Mater. Today Commun. 2021, 26, 102113. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Q.; Luk, B.T.; Fang, R.H.; Liu, Y.; Gao, W.; Zhang, L. Coating nanofiber scaffolds with beta cell membrane to promote cell proliferation and function. Nanoscale 2016, 8, 10364–10370. [Google Scholar] [CrossRef] [PubMed]
- Mridha, A.R.; Dargaville, T.R.; Dalton, P.D.; Carroll, L.; Morris, M.B.; Vaithilingam, V.; Tuch, B.E. Prevascularized Retrievable Hybrid Implant to Enhance Function of Subcutaneous Encapsulated Islets. Tissue Eng. Part A 2022, 28, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Hoveizi, E.; Tavakol, S.; Shirian, S.; Sanamiri, K. Electrospun Nanofibers for Diabetes: Tissue Engineering and Cell-Based Therapies. Curr. Stem Cell Res. Ther. 2019, 14, 152–168. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Shi, G.; Tang, J.; Cheng, R.; Shen, X.; Gu, Y.; Wu, L.; Xi, K.; Zhao, Y.; Cui, W.; et al. ECM-inspired micro/nanofibers for modulating cell function and tissue generation. Sci. Adv. 2020, 6, eabc2036. [Google Scholar] [CrossRef]
- Kim, J.I.; Hwang, T.I.; Aguilar, L.E.; Park, C.H.; Kim, C.S. A Controlled Design of Aligned and Random Nanofibers for 3D Bi-functionalized Nerve Conduits Fabricated via a Novel Electrospinning Set-up. Sci. Rep. 2016, 6, 23761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieminen, H.J.; Laidmäe, I.; Salmi, A.; Rauhala, T.; Paulin, T.; Heinämäki, J.; Hæggström, E. Ultrasound-enhanced electrospinning. Sci. Rep. 2018, 8, 4437. [Google Scholar] [CrossRef] [Green Version]
- Janmey, P.A.; Winer, J.P.; Murray, M.E.; Wen, Q. The hard life of soft cells. Cell Motil. Cytoskelet. 2009, 66, 597–605. [Google Scholar] [CrossRef]
- Bhattarai, D.P.; Aguilar, L.E.; Park, C.H.; Kim, C.S. A Review on Properties of Natural and Synthetic Based Electrospun Fibrous Materials for Bone Tissue Engineering. Membranes 2018, 8, 62. [Google Scholar] [CrossRef] [Green Version]
- Laidmäe, I.; Ērglis, K.; Cēbers, A.; Janmey, P.A.; Uibo, R. Salmon fibrinogen and chitosan scaffold for tissue engineering: In vitro and in vivo evaluation. J. Mater. Sci. Mater. Med. 2018, 29, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uibo, R.; Laidmäe, I.; Sawyer, E.S.; Flanagan, L.A.; Georges, P.C.; Winer, J.P.; Janmey, P.A. Soft materials to treat central nervous system injuries: Evaluation of the suitability of non-mammalian fibrin gels. Biochim. Biophys. Acta 2009, 1793, 924–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laurens, N.; Koolwijk, P.; de Maat, M.P.M. Fibrin structure and wound healing. J. Thromb. Haemost. 2006, 4, 932–939. [Google Scholar] [CrossRef]
- Sieminski, A.L.; Gooch, K.J. Salmon fibrin supports an increased number of sprouts and decreased degradation while maintaining sprout length relative to human fibrin in an in vitro angiogenesis model. J. Biomater. Sci. Polym. Ed. 2004, 15, 237–242. [Google Scholar] [CrossRef]
- Ju, Y.-E.; Janmey, P.A.; McCormick, M.E.; Sawyer, E.S.; Flanagan, L.A. Enhanced neurite growth from mammalian neurons in three-dimensional salmon fibrin gels. Biomaterials 2007, 28, 2097–2108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharp, K.G.; Dickson, A.R.; Marchenko, S.A.; Yee, K.M.; Emery, P.N.; Laidmåe, I.; Uibo, R.; Sawyer, E.S.; Steward, O.; Flanagan, L.A. Salmon fibrin treatment of spinal cord injury promotes functional recovery and density of serotonergic innervation. Exp. Neurol. 2012, 235, 345–356. [Google Scholar] [CrossRef] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.-C.; Wu, C.-C.; Yang, S.-H.; Chiu, C.-C.; Sumi, S.; Lee, H.-S. Investigating the suspension culture on aggregation and function of mouse pancreatic β-cells. J. Biomed. Mater. Res. A 2013, 101, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- Zhi, Z.-L.; Liu, B.; Jones, P.M.; Pickup, J.C. Polysaccharide multilayer nanoencapsulation of insulin-producing beta-cells grown as pseudoislets for potential cellular delivery of insulin. Biomacromolecules 2010, 11, 610–616. [Google Scholar] [CrossRef] [Green Version]
- Kelly, C.; Guo, H.; McCluskey, J.T.; Flatt, P.R.; McClenaghan, N.H. Comparison of insulin release from MIN6 pseudoislets and pancreatic islets of Langerhans reveals importance of homotypic cell interactions. Pancreas 2010, 39, 1016–1023. [Google Scholar] [CrossRef]
- Nyitray, C.E.; Chavez, M.G.; Desai, T.A. Compliant 3D microenvironment improves β-cell cluster insulin expression through mechanosensing and β-catenin signaling. Tissue Eng. Part A 2014, 20, 1888–1895. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.K.; Park, S.J.; Kim, M.S.; Kang, C.M.; Kim, J.-I.; Kim, C.-H. Fabrication of sonicated chitosan nanofiber mat with enlarged porosity for use as hemostatic materials. Carbohydr. Polym. 2013, 97, 65–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuzlakoglu, K.; Alves, C.M.; Mano, J.F.; Reis, R.L. Production and characterization of chitosan fibers and 3-D fiber mesh scaffolds for tissue engineering applications. Macromol. Biosci. 2004, 4, 811–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asthana, A.; Kisaalita, W.S. Biophysical microenvironment and 3D culture physiological relevance. Drug Discov. Today 2013, 18, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Tibbitt, M.W.; Basta, L.; Anseth, K.S. Mechanical memory and dosing influence stem cell fate. Nat. Mater. 2014, 13, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Laidmäe, I.; McCormick, M.E.; Herod, J.L.; Pastore, J.J.; Salum, T.; Sawyer, E.S.; Janmey, P.A.; Uibo, R. Stability, sterility, coagulation, and immunologic studies of salmon coagulation proteins with potential use for mammalian wound healing and cell engineering. Biomaterials 2006, 27, 5771–5779. [Google Scholar] [CrossRef] [PubMed]

| Genes | Sequences |
|---|---|
| tspan7_Mm_5′974 | TCTGCCTTTCAGCCCACGTC |
| tspan7_Mm_3′1167 | CTGAAGCCTCCCCTACTACATGC |
| cldn7_Mm_5′612 | GCGGGCGACAACATCATCACA |
| cldn7_Mm_3′784 | ATCGTGGCGACAAACATGGCTA |
| chgb_Mm_5′223 | CCCTATCCAAGTCCAGTGTTCCAA |
| chgb_Mm_3′434 | CACTTCTCATTGCCTACCTTCGTC |
| scg5_Mm_5′260 | CTCACCAGGCCATGAATCTTGTTG |
| scg5_Mm_3′489 | ACTGGAATTCTCGGCTGAACTCT |
| scg3_Mm_5′202 | CTCTCCCTTCCCGCACCCAG |
| scg3_Mm_3′344 | CAGTATCCAAGAGCCGGTCCA |
| ceacam1_Mm_5′529 | GCCCTTCCTCCAAGTCACCAAC |
| ceacam1_Mm_3′730 | CGCTGACTGGATTCGAGATTTCACAC |
| ins1_Mm_5′268 | AACCCACCCAGGCTTTTGTCA |
| ins1_Mm_3′464 | ACTGATCCACAATGCCACGCTTC |
| ins2_Mm_5′139 | CCCCACCCAGGCTTTTGTCA |
| ins2_Mm_3′340 | ACTGATCTACAATGCCACGCTTC |
| actb_Mm_5′367 | GCACCACACCTTCTACAATGAGC |
| actb_Mm_3′558 | CTCCGGAGTCCATCACAATGC |
| cdh1_Mm_5′930 | CAGAGTTTACCCAGCCGGTCT |
| cdh1_Mm_3′1149 | ATGTAGGGTAACTCTCTCGGTCCA |
| itgb1_Mm_5′532 | CAGCCAAGTGACATAGAGAATCCCA |
| itgb1_Mm_3′840 | GCCAAAGCCAATGCGGAAGTCT |
| itga6_Mm_5′1080 | AGAGACATGAAGTCCGCGCATC |
| itga6_Mm_3′1282 | ACGAATCGGCTTCACATTACTCC |
| CS Scaffold | FCS Scaffold | |
|---|---|---|
| Mean diameter (nm ± SD) | 144.1 ± 70.6 | 525.9 ± 576.1 |
| Median diameter (nm) | 115.0 | 192.8 |
| Minimum diameter (nm) | 67.4 | 76.9 |
| Maximum diameter (nm) | 391.7 | 3743.4 |
| Mid-range value (nm ± SD) | 229.6 ± 229.3 | 1910.2 ± 2592.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laidmäe, I.; Aints, A.; Uibo, R. Growth of MIN-6 Cells on Salmon Fibrinogen Scaffold Improves Insulin Secretion. Pharmaceutics 2022, 14, 941. https://doi.org/10.3390/pharmaceutics14050941
Laidmäe I, Aints A, Uibo R. Growth of MIN-6 Cells on Salmon Fibrinogen Scaffold Improves Insulin Secretion. Pharmaceutics. 2022; 14(5):941. https://doi.org/10.3390/pharmaceutics14050941
Chicago/Turabian StyleLaidmäe, Ivo, Alar Aints, and Raivo Uibo. 2022. "Growth of MIN-6 Cells on Salmon Fibrinogen Scaffold Improves Insulin Secretion" Pharmaceutics 14, no. 5: 941. https://doi.org/10.3390/pharmaceutics14050941
APA StyleLaidmäe, I., Aints, A., & Uibo, R. (2022). Growth of MIN-6 Cells on Salmon Fibrinogen Scaffold Improves Insulin Secretion. Pharmaceutics, 14(5), 941. https://doi.org/10.3390/pharmaceutics14050941







