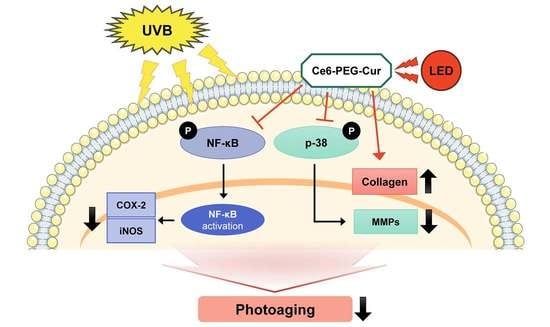
The Potential Anti-Photoaging Effect of Photodynamic Therapy Using Chlorin e6-Curcumin Conjugate in UVB-Irradiated Fibroblasts and Hairless Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ce6-PEG-Cur Synthesis
2.2. In Vitro Assay
2.2.1. Determination of the Antioxidant Capacity of Ce6-PEG-Cur
2.2.2. Cell Culture
2.2.3. UVB Irradiation and Ce6-PEG-Cur-Mediated PDT
2.2.4. MTT Cell Viability Assay
2.2.5. Sircol Collagen Assay
2.2.6. Western Blot Analysis
2.3. In Vivo Assay
2.3.1. Experimental Animals
2.3.2. Measurement of Wrinkles Induced by UVB Irradiation
2.3.3. Histological Analysis
2.4. Statistical Analysis
3. Results
3.1. Absorption Spectrum and Antioxidant Capacity of Ce6-PEG-Cur
3.2. The Effects of Ce6-PEG-Cur-Mediated PDT on the Viability of Fibroblasts
3.3. Inhibitory Effect of Ce6-PEG-Cur-Mediated PDT on UVB-Induced MMP and Procollagen Expression
3.4. Inhibitory Effects of Ce6-PEG-Cur-Mediated PDT on the Expressions of NF-kB-Dependent Proteins
3.5. Effects of Ce6-PEG-Cur-Mediated PDT on UVB-Induced Activation of p38 MAP Kinase
3.6. Effect of Ce6-PEG-Cur-Mediated PDT on In Vitro Collagen Production
3.7. In Vivo Expression of Type Ⅰ Procollagen and MMP Proteins in the Skin of Mice Treated with UVB-Irradiated Mice
3.8. Suppressive Effect of Ce6-PEG-Cur-Mediated PDT on Wrinkle Formation in UVB-Irradiated Hairless Mice
3.9. Effects of Ce6-PEG-Cur-Mediated PDT on the Skin Thickness and Collagen in UVB-Irradiated Hairless Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yaar, M.; Gilchrest, B.A. Aging versus photoaging: Postulated mechanisms and effectors. J. Investig. Dermatol. Symp. Proc. 1998, 3, 47–51. [Google Scholar]
- Zhang, S.B.; Duan, E.K. Fighting against skin aging: The way from bench to bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.; Yano, K.; Lee, M.K.; Youn, C.S.; Seo, J.Y.; Kim, K.H.; Cho, K.H.; Eun, H.C.; Detmar, M. Differential effects of photoaging vs. intrinsic aging on the vascularization of human skin. Arch. Dermatol. 2002, 138, 1437–1442. [Google Scholar] [CrossRef] [PubMed]
- Bosset, S.; Bonnet-Duquennoy, M.; Barre, P.; Chalon, A.; Kurfurst, R.; Bonte, F.; Schnebert, S.; Le Varlet, B.; Nicolas, J.F. Photoageing shows histological features of chronic skin inflammation without clinical and molecular abnormalities. Br. J. Dermatol. 2003, 149, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Lephart, E.D. Skin aging and oxidative stress: Equol’s anti-aging effects via biochemical and molecular mechanisms. Ageing Res. Rev. 2016, 31, 36–54. [Google Scholar] [CrossRef]
- Lam, P.Y.; Yan, C.W.; Chiu, P.Y.; Leung, H.Y.; Ko, K.M. Schisandrin B protects against solar irradiation-induced oxidative stress in rat skin tissue. Fitoterapia 2011, 82, 393–400. [Google Scholar] [CrossRef]
- Rittie, L.; Fisher, G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, S.; Rho, H.S.; Kim, D.H.; Chang, I.S.; Chung, J.H. The effects of a novel synthetic retinoid, seletinoid G, on the expression of extracellular matrix proteins in aged human skin in vivo. Clin. Chim. Acta 2005, 362, 161–169. [Google Scholar] [CrossRef]
- Bell, S.; Degitz, K.; Quirling, M.; Jilg, N.; Page, S.; Brand, K. Involvement of NF-kappaB signalling in skin physiology and disease. Cell. Signal. 2003, 15, 1–7. [Google Scholar] [CrossRef]
- Cooper, S.J.; Bowden, G.T. Ultraviolet B regulation of transcription factor families: Roles of nuclear factor-kappa B (NF-kappaB) and activator protein-1 (AP-1) in UVB-induced skin carcinogenesis. Curr. Cancer Drug Targets 2007, 7, 325–334. [Google Scholar] [CrossRef]
- Brenneisen, P.; Sies, H.; Scharffetter-Kochanek, K. Ultraviolet-B irradiation and matrix metalloproteinases—From induction via signaling to initial events. Ann. N. Y. Acad. Sci. 2002, 973, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Sardy, M. Role of matrix metalloproteinases in skin ageing. Connect. Tissue Res. 2009, 50, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Castranova, V.; Shi, X.; Demers, L.M. New insights into the role of nuclear factor-kappaB, a ubiquitous transcription factor in the initiation of diseases. Clin. Chem. 1999, 45, 7–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debacq-Chainiaux, F.; Leduc, C.; Verbeke, A.; Toussaint, O. UV, stress and aging. Dermato-Endocrinology 2012, 4, 236–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, K.; Asamitsu, K.; Uranishi, H.; Iddamalgoda, A.; Ito, K.; Kojima, H.; Okamoto, T. Protecting skin photoaging by NF-kappaB inhibitor. Curr. Drug Metab. 2010, 11, 431–435. [Google Scholar] [CrossRef]
- Birkedalhansen, H. Proteolytic remodeling of extracellular-matrix. Curr. Opin. Cell Biol. 1995, 7, 728–735. [Google Scholar] [CrossRef]
- O’Grady, A.; Dunne, C.; O’Kelly, P.; Murphy, G.M.; Leader, M.; Kay, E. Differential expression of matrix metalloproteinase (MMP)-2, MMP-9 and tissue inhibitor of metalloproteinase (TIMP)-1 and TIMP-2 in non-melanoma skin cancer: Implications for tumour progression. Histopathology 2007, 51, 793–804. [Google Scholar] [CrossRef]
- Inomata, S.; Matsunaga, Y.; Amano, S.; Takada, K.; Kobayashi, K.; Tsunenaga, M.; Nishiyama, T.; Kohno, Y.; Fukuda, M. Possible involvement of gelatinases in basement membrane damage and wrinkle formation in chronically ultraviolet B-exposed hairless mouse. J. Invest. Dermatol. 2003, 120, 128–134. [Google Scholar] [CrossRef] [Green Version]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [Green Version]
- Pedrosa, T.D.; Barros, A.O.; Nogueira, J.R.; Fruet, A.C.; Rodrigues, I.C.; Calcagno, D.Q.; Smith, M.D.C.; de Souza, T.P.; Barros, S.B.D.; de Vasconcellos, M.C.; et al. Anti-wrinkle and anti-whitening effects of juca (Libidibia ferrea Mart.) extracts. Arch. Dermatol. Res. 2016, 308, 643–654. [Google Scholar] [CrossRef]
- Chaves, Y.N.; Torezan, L.A.; Niwa, A.B.M.; Sanches, J.A.; Neto, C.F. Pain in photodynamic therapy: Mechanism of action and management strategies. An. Bras. Dermatol. 2012, 87, 521–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morton, C.A.; Szeimies, R.M.; Sidoroff, A.; Braathen, L.R. European guidelines for topical photodynamic therapy part 1: Treatment delivery and current indications actinic keratoses, Bowen’s disease, basal cell carcinoma. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Jung, H.Y.; Park, H.J. Topical PDT in the treatment of benign skin diseases: Principles and new applications. Int. J. Mol. Sci. 2015, 16, 23259–23278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baez, F.; Reilly, L.R. The use of light-emitting diode therapy in the treatment of photoaged skin. J. Cosmet. Dermatol. 2007, 6, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Tong, R.; Kohane, D.S. Shedding light on nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012, 4, 638–662. [Google Scholar] [CrossRef] [PubMed]
- MacCormack, M.A. Photodynamic therapy in dermatology: An update on applications and outcomes. Semin. Cutan. Med. Surg. 2008, 27, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Han, C.S.; Chun, S.N.; Lee, M.Y. Photodynamic inactivation of chlorin e6 with halogen light against dermatophytes. Toxicol. Environ. Health Sci. 2014, 6, 170–175. [Google Scholar] [CrossRef]
- Jeon, Y.M.; Lee, H.S.; Jeong, D.; Oh, H.K.; Ra, K.H.; Lee, M.Y. Antimicrobial photodynamic therapy using chlorin e6 with halogen light for acne bacteria-induced inflammation. Life Sci. 2015, 124, 56–63. [Google Scholar] [CrossRef]
- Ryu, A.R.; Lee, M.Y. Chlorin e6-mediated photodynamic therapy promotes collagen production and suppresses MMPs expression via modulating AP-1 signaling in P. acnes-stimulated HaCaT cells. Photodiagnosis Photodyn. Ther. 2017, 20, 71–77. [Google Scholar] [CrossRef]
- Ryu, A.R.; Kim, Y.W.; Lee, M.Y. Chlorin e6 and halogen light as a sebostatic photomedicine modulates linoleic acid-induced lipogenesis. Mol. Cell. Toxicol. 2019, 15, 49–56. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Ryu, A.R.; Jin, S.; Jeon, Y.M.; Lee, M.Y. Chlorin e6-mediated photodynamic therapy suppresses P. acnes-induced inflammatory response via NF kappa B and MAPKs signaling pathway. PLoS ONE 2017, 12, e0170599. [Google Scholar] [CrossRef]
- Ryu, A.R.; Kim, Y.W.; Lee, M.Y. Chlorin e6-mediated photodynamic therapy modulates adipocyte differentiation and lipogenesis in 3T3-L1 cells. Photodiagnosis Photodyn. Ther. 2020, 31, 101917. [Google Scholar] [CrossRef] [PubMed]
- Jalde, S.S.; Chauhan, A.K.; Lee, J.H.; Chaturvedi, P.K.; Park, J.S.; Kim, Y.W. Synthesis of novel Chlorin e6-curcumin conjugates as photosensitizers for photodynamic therapy against pancreatic carcinoma. Eur. J. Med. Chem. 2018, 147, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ou, B.X.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Kawada, S.; Ohtani, M.; Ishii, N. Increased oxygen tension attenuates acute ultraviolet-B-induced skin angiogenesis and wrinkle formation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R694–R701. [Google Scholar] [CrossRef]
- Hwang, B.M.; Noh, E.M.; Kim, J.S.; Kim, J.M.; Hwang, J.K.; Kim, H.K.; Kang, J.S.; Kim, D.S.; Chae, H.J.; You, Y.O.; et al. Decursin inhibits UVB-induced MMP expression in human dermal fibroblasts via regulation of nuclear factor-kappa B. Int. J. Mol. Med. 2013, 31, 477–483. [Google Scholar] [CrossRef] [Green Version]
- Kwon, K.R.; Alam, M.B.; Park, J.H.; Kim, T.H.; Lee, S.H. Attenuation of UVB-induced photo-aging by polyphenolic-rich Spatholobus suberectus stem extract via modulation of MAPK/AP-1/MMPs signaling in human keratinocytes. Nutrients 2019, 11, 1341. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kim, M.B.; Yun, J.G.; Hwang, J.K. Protective effects of standardized Siegesbeckia glabrescens extract and Its active compound Kirenol against UVB-induced photoaging through inhibition of MAPK/NF-kappa B pathways. J. Microbiol. Biotechnol. 2017, 27, 242–250. [Google Scholar] [CrossRef] [Green Version]
- Pillai, S.; Oresajo, C.; Hayward, J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation—A review. Int. J. Cosmet. Sci. 2005, 27, 17–34. [Google Scholar] [CrossRef]
- Kim, J.M.; Kim, S.Y.; Noh, E.M.; Song, H.K.; Lee, G.S.; Kwon, K.B.; Lee, Y.R. Reversine inhibits MMP-1 and MMP-3 expressions by suppressing of ROS/MAPK/AP-1 activation in UV-stimulated human keratinocytes and dermal fibroblasts. Exp. Dermatol. 2018, 27, 298–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quan, T.; Fisher, G.J. Role of age-associated alterations of the dermal extracellular matrix microenvironment in human skin aging: A mini-review. Gerontology 2015, 61, 427–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Matrix-degrading metalloproteinases in photoaging. J. Invest. Dermatol. Symp. Proc. 2009, 14, 20–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, T.; Park, J.; Moon, Y.; Kang, W.; Park, T. alpha-Ionone protects against UVB-induced photoaging in human dermal fibroblasts. Molecules 2019, 24, 1804. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Photosensitizer | μM | ABTS | ORAC (μM TE) |
|---|---|---|---|
| EC50 (μM) | |||
| Ce6-PEG-Curcumin/Light (−) | 1 | 18.77 ± 4.55 | 9.25 |
| 10 | - | 34.48 | |
| Ce6-PEG-Curcumin/Light (+) | 1 | 23.31 ± 9.82 | 1.40 |
| 10 | - | 27.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hur, G.-H.; Ryu, A.-R.; Kim, Y.-W.; Lee, M.-Y. The Potential Anti-Photoaging Effect of Photodynamic Therapy Using Chlorin e6-Curcumin Conjugate in UVB-Irradiated Fibroblasts and Hairless Mice. Pharmaceutics 2022, 14, 968. https://doi.org/10.3390/pharmaceutics14050968
Hur G-H, Ryu A-R, Kim Y-W, Lee M-Y. The Potential Anti-Photoaging Effect of Photodynamic Therapy Using Chlorin e6-Curcumin Conjugate in UVB-Irradiated Fibroblasts and Hairless Mice. Pharmaceutics. 2022; 14(5):968. https://doi.org/10.3390/pharmaceutics14050968
Chicago/Turabian StyleHur, Ga-Hee, A-Reum Ryu, Yong-Wan Kim, and Mi-Young Lee. 2022. "The Potential Anti-Photoaging Effect of Photodynamic Therapy Using Chlorin e6-Curcumin Conjugate in UVB-Irradiated Fibroblasts and Hairless Mice" Pharmaceutics 14, no. 5: 968. https://doi.org/10.3390/pharmaceutics14050968