A Comparative Study on Inhibition of Breast Cancer Cells and Tumors in Mice by Carotenoid Extract and Nanoemulsion Prepared from Sweet Potato (Ipomoea batatas L.) Peel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Chemicals and Reagents
2.1.2. Instrumentation
2.1.3. Cell Culture and Animal Study
2.2. Methods
2.2.1. HPLC (High-Performance Liquid Chromatography) Analysis of Carotenoids in Sweet Potato Peel
2.2.2. Method Validation
2.2.3. Quantitation of Carotenoids in Sweet Potato Peel
2.2.4. Preparation of Carotenoid Nanoemulsion
2.2.5. Determination of Carotenoid Nanoemulsion Characteristics
2.2.6. Stability Test
2.2.7. In Vitro Release Test
2.2.8. Cell Culture Experiment
2.2.9. MTT (3-[4,5-Dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) Assay
2.2.10. Cell Cycle Study
2.2.11. FITC-Annexin V/PI Study
2.2.12. Determination of Caspase-3, Caspase-8 and Caspase-9
2.2.13. Animal Study
2.2.14. Statistical Analysis
3. Results and Discussion
3.1. Analysis of Carotenoids in Sweet Potato Peel
3.2. Preparation of Carotenoid Nanoemulsion
3.3. Stability of Carotenoid Nanoemulsion
3.4. In Vitro Release Study
3.5. Cell Culture Study
3.6. Growth of MCF-7 and H184B5F5/M10 Cells as Affected by Carotenoid Nanoemulsion and Extract
3.7. Cell Cycle Study
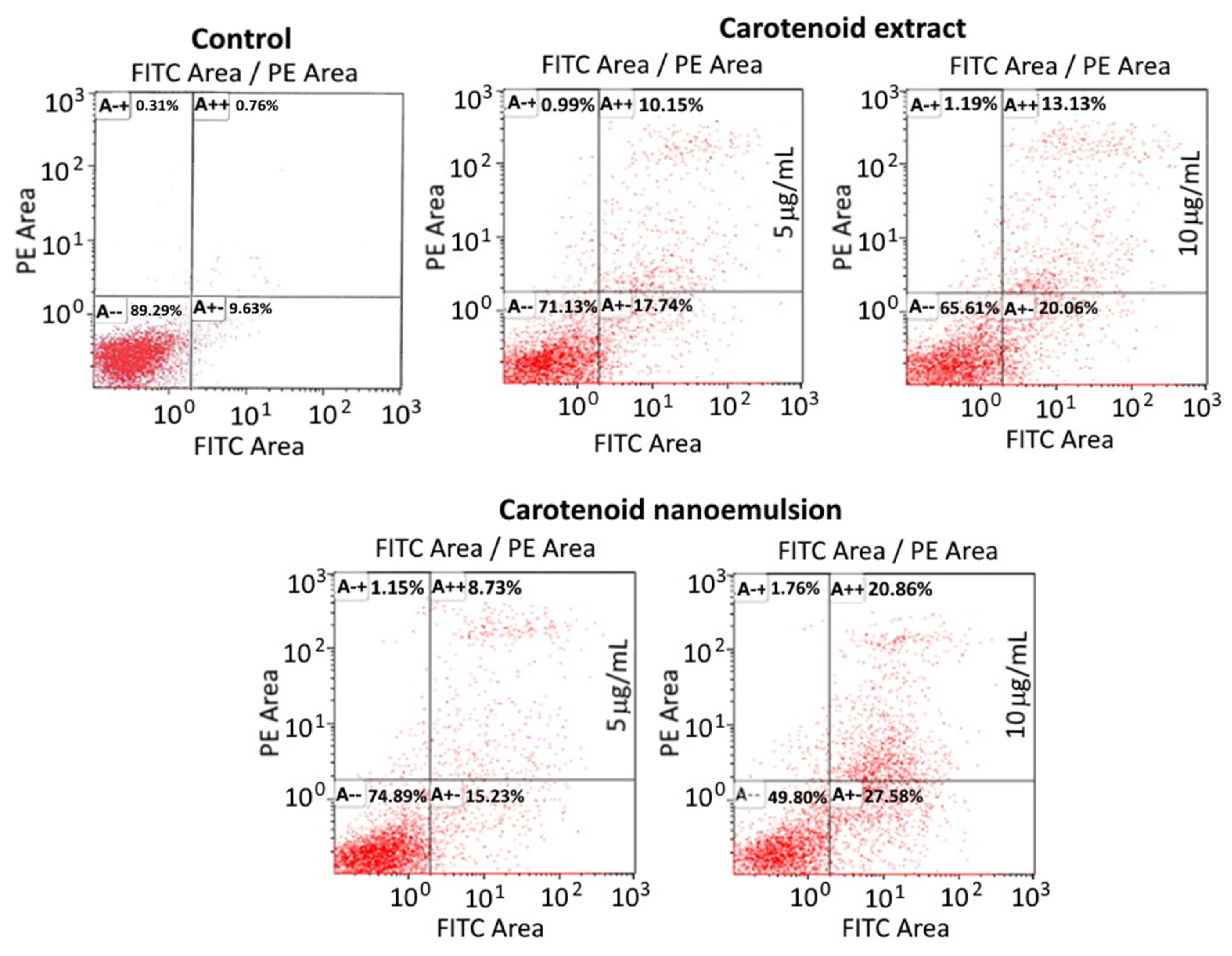
3.8. Analysis of Cell Apoptosis
3.9. Activities of Caspase-3, Caspase-8 and Caspase-9
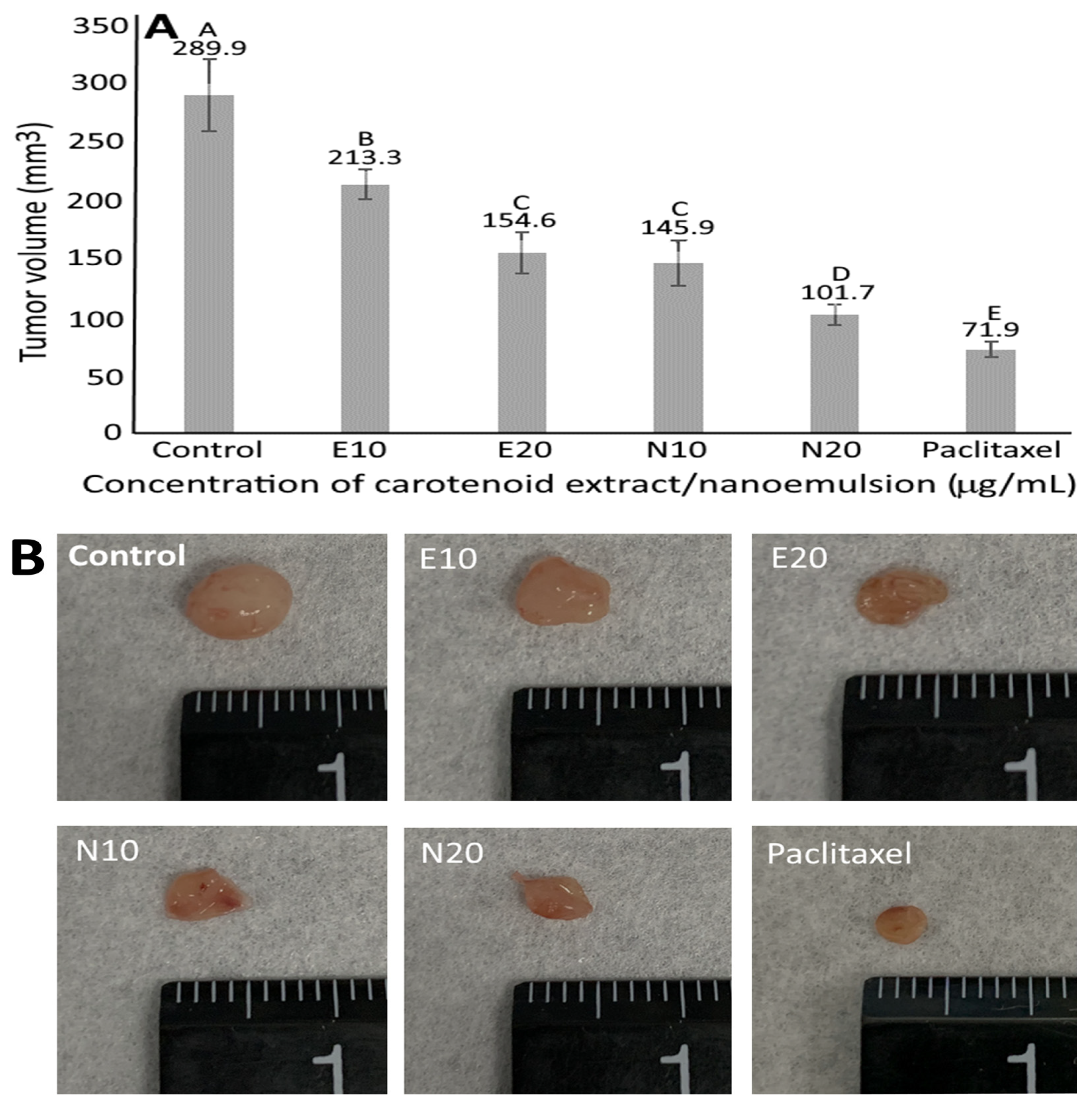
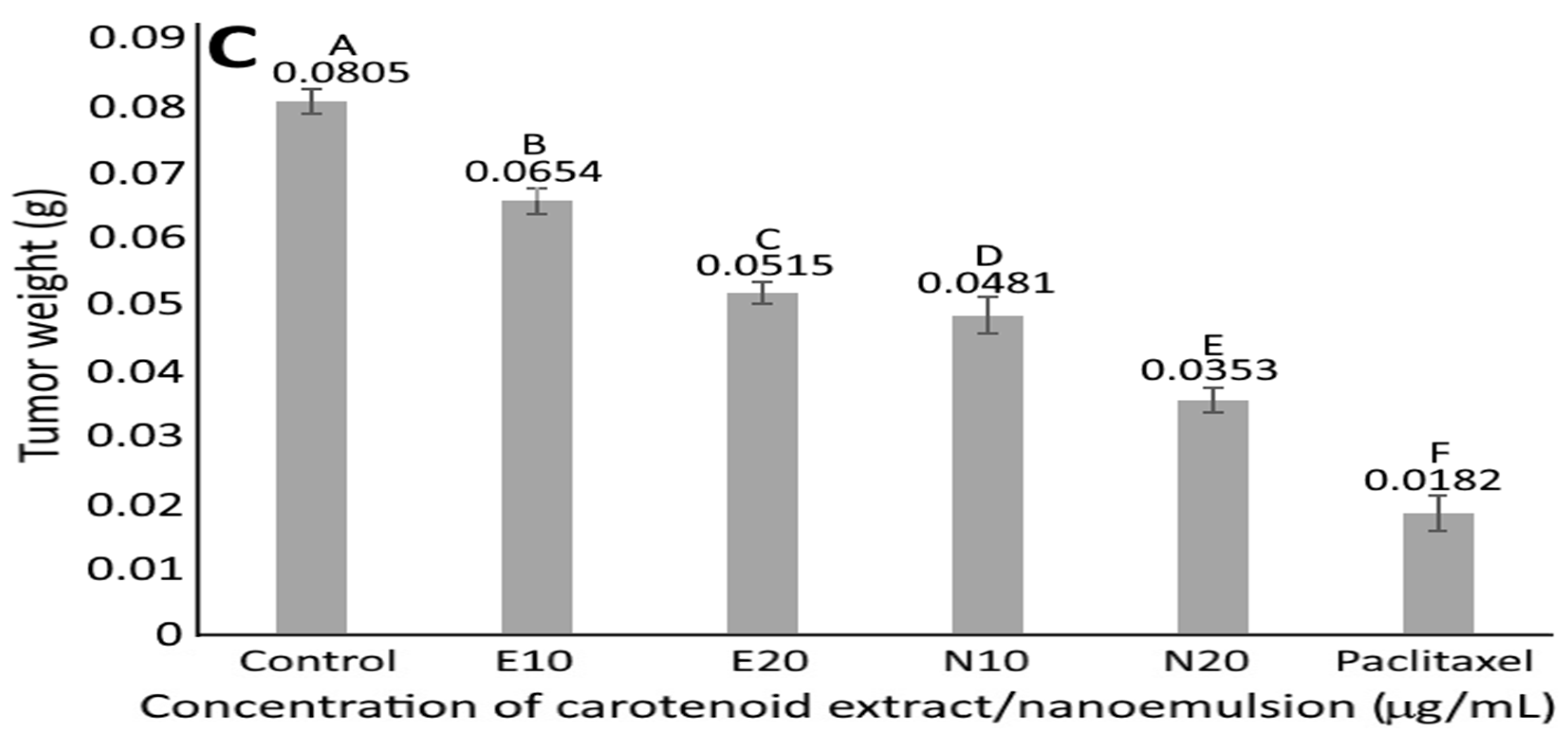
3.10. Animal Study
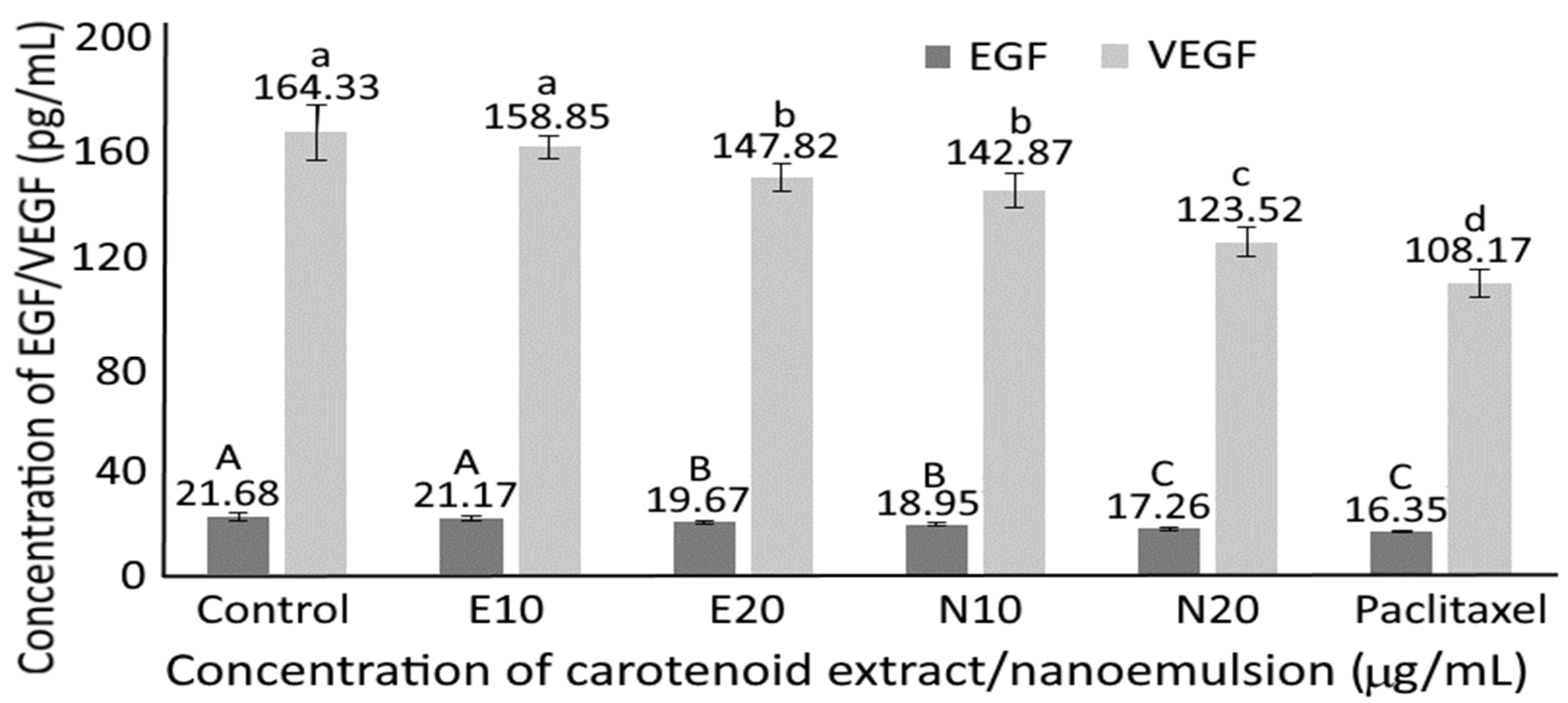
3.11. Serum Growth Factor in Nude Mice
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, G.W. Phytochemical Analysis of Different Cultivars of Sweet Potato Root Tubers Cultivated in Taiwan. Master’s Thesis, National Chung-Hsin University, Taichung, Taiwan, 2019. [Google Scholar]
- Vimala, B.; Nambisan, B.; Hariprakash, B. Retention of carotenoids in orange-fleshed sweet potato during processing. J. Food Sci. Technol. 2011, 48, 520–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salawu, S.O.; Udi, E.; Akindahunsi, A.A.; Boligon, A.A.; Athayde, M.L. Antioxidant potential, phenolic profile and nutrient composition of flesh and peels from Nigerian white and purple skinned sweet potato (Ipomea batatas L.). Asian J. Plant Sci. Res. 2015, 5, 14–23. [Google Scholar]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sridhar, K.; Inbaraj, B.S.; Chen, B.H. Recent advances on nanoparticle based strategies for improving carotenoid stability and biological activity. Antioxidants 2021, 10, 713. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Choi, C.H.; Zuckerman, J.E.; Webster, P.; Davis, M.E. Targeting kidney mesangium by nanoparticles of defined size. Proc. Natl. Acad. Sci. USA 2011, 108, 6656–6661. [Google Scholar] [CrossRef] [Green Version]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [Green Version]
- Peretz, V.; Motiei, M.; Sukenik, C.N.; Popovtzer, R. The effect of nanoparticle size on cellular binding probability. J. At. Mol. Opt. Phys. 2012, 2012, 404536. [Google Scholar] [CrossRef] [Green Version]
- MOHW. Ministry of Health and Welfare. 2019. Available online: https://dep.mohw.gov.tw/DOS/Ip-4927-113.html (accessed on 23 March 2021).
- Kao, T.H.; Loh, C.H.; Inbaraj, B.S.; Chen, B.H. Determination of carotenoids in Taraxacum formosanum by HPLC-DAD-APCI-MS and preparation by column chromatography. J. Pharm. Biomed. Anal. 2012, 66, 144–153. [Google Scholar] [CrossRef]
- Inbaraj, B.S.; Chien, J.T.; Chen, B.H. Improved high performance liquid chromatographic method for determination of carotenoids in the microalga Chlorella pyrenoidosa. J. Chromatogr. A 2006, 1102, 193–199. [Google Scholar] [CrossRef]
- Inbaraj, B.S.; Lu, H.; Hung, C.F.; Wu, W.B.; Lin, C.L.; Chen, B.H. Determination of carotenoids and their esters in fruits of Lycium barbarum Linnaeus by HPLC-DAD-APCI-MS. J. Pharm. Biomed. Anal. 2008, 47, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Hung, C.F.; Chen, B.H. Preparation of coffee oil-algae oil-based nanoemulsions and the study of their inhibition effect on UVA-induced skin damage in mice and melanoma cell growth. Int. J. Nanomed. 2017, 12, 6559–6580. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.H.; Li, Y.F.; Chen, B.H. Inhibition of melanoma cells A375 by carotenoid extract and nanoemulsion prepared from pomelo leaves. Plants 2021, 10, 2129. [Google Scholar] [CrossRef] [PubMed]
- SAS. Statistical Analysis System, Version 6, SAS Procedures and SAS/Graph User’s Guide; SAS: Cary, NC, USA, 2014. [Google Scholar]
- Straiti, I.F.; Sinanoglou, V.J.; Kora, L.; Miniadis-Meimaroglou, S.; Oreopoulou, V. Carotenoids from foods of plant, animal and marine origin: An efficient HPLC-DAD separation method. Foods 2012, 1, 52–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kao, T.H.; Chen, C.J.; Chen, B.H. Carotenoid composition in Rhinacanthus nasutus (L.) Kurz as determined by HPLC-MS and affected by freeze-drying and hot-air-drying. Anal. 2011, 136, 3194–3202. [Google Scholar] [CrossRef]
- Schex, R.; Lieb, V.M.; Jiménez, V.M.; Esquivel, P.; Schweiggert, R.M.; Carle, R.; Steingass, C.B. HPLC-DAD-APCI/ESI-MSn analysis of carotenoid and α-tocopherol in Costa Rican Acrocomia aculeata fruits of varying maturity stages. Food Res. Int. 2018, 105, 645–653. [Google Scholar] [CrossRef]
- Liu, H.L.; Chen, B.H.; Kao, T.H.; Shiau, C.Y. Carotenoids composition in Scutellaria barbata D. Don as detected by high performance liquid chromatography-diode array detection-mass spectrometry-atmospheric pressure chemical ionization. J. Funct. Foods 2014, 8, 605–610. [Google Scholar] [CrossRef]
- Gupta, P.; Sreelakshmi, T.; Sharma, R. A rapid and sensitive method for determination of carotenoids in plant tissues by high performance liquid chromatography. Plant Methods 2015, 11, 5. [Google Scholar] [CrossRef] [Green Version]
- TFDA. Method Validation Specification of Food Analytical Methods; Taiwan Food and Drug Administration: Taipei, Taiwan, 2013.
- Islam, S.N.; Nusrat, T.; Begum, P.; Ahsan, M. Carotenoids and β-carotene in orange fleshed sweet potato: A possible solution to vitamin A deficiency. Food Chem. 2016, 199, 628–631. [Google Scholar] [CrossRef]
- Drapal, M.; Fraser, P.D. Determination of carotenoids in sweet potato (Ipomoea batatas L., Lam) tubers: Implications for accurate provitamin A determination in staple sturdy tuber crops. Phytochemistry 2019, 167, 112102. [Google Scholar] [CrossRef]
- Jing, X.; Deng, L.; Gao, B.; Xiao, L.; Zhang, Y.; Ke, X.; Lian, J.; Zhao, Q.; Ma, L.; Yao, J.; et al. A novel polyethylene glycol mediated lipid nanoemulsion as drug delivery carrier for paclitaxel. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 371–380. [Google Scholar] [CrossRef]
- Vinarov, Z.; Petkova, Y.; Tcholakova, S.; Denkov, N.; Stoyanov, S.; Pelan, E.; Lips, A. Effects of emulsifier charge and concentration on pancreatic lipolysis. 1. In the absence of bile salts. Langmuir 2012, 28, 8127–8139. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Jafari, S.M. Improving emulsion formation, stability and performance using mixed emulsifiers: A review. Adv. Coll. Interf. Sci. 2018, 251, 55–79. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Costa, A.L.R.; Cardoso, D.D.; Náthia-Neves, G.; Meireles, M.A.A.; Cunha, R.L. Interactions of β-carotene with WPI/Tween 80 mixture and oil phase: Effect on the behavior of O/W emulsions during in vitro digestion. Food Chem. 2021, 341, 128155. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, P.; Kumar, G.A. Nanosuspension technology: A review. Int. J. Pharm. Pharm. Sci. 2010, 2, 35–40. [Google Scholar]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Decker, E.A.; Xiao, H.; McClements, D.J. Nanoemulsion delivery systems: Influence of carrier oil on β-carotene bioaccessibility. Food Chem. 2012, 135, 1440–1447. [Google Scholar] [CrossRef]
- Jo, Y.-J.; Kwon, Y.-J. Characterization of β-carotene nanoemulsions prepared by microfluidization technique. Food Sci. Biotechnol. 2014, 23, 107–113. [Google Scholar] [CrossRef]
- Tan, C.; Xue, J.; Lou, X.; Abbas, S.; Guan, Y.; Feng, B.; Zhang, X.; Xia, S. Liposomes as delivery systems for carotenoids: Comparative studies of loading ability, storage stability and in vitro release. Food Funct. 2014, 5, 1232–1240. [Google Scholar] [CrossRef]
- Yi, J.; Lam, T.I.; Yokoyama, W.; Cheng, L.W.; Zhong, F. Controlled release of β-carotene in β-lactoglobulin-dextran-conjugated nanoparticles’ in vitro digestion and transport with Caco-2 monolayers. J. Agric. Food Chem. 2014, 62, 8900–8907. [Google Scholar] [CrossRef]
- Cui, Y.; Lu, Z.; Bai, L.; Shi, Z.; Zhao, W.; Zhao, B. β-Carotene induces apoptosis and up-regulates peroxisome proliferator-activated receptor γ expression and reactive oxygen species production in MCF-7 cancer cells. Eur. J. Cancer 2007, 43, 2590–2601. [Google Scholar] [CrossRef] [PubMed]
- Shree, G.S.; Prasad, K.Y.; Arpitha, H.S.; Deepika, U.R.; Kumar, K.N.; Mondal, P.; Ganesan, P. β-carotene at physiologically attainable concentration induces apoptosis and down-regulates cell survival and antioxidant markers in human breast cancer (MCF-7) cells. Mol. Cell. Biochem. 2017, 436, 1–12. [Google Scholar] [CrossRef]
- Gloria, N.F.; Soares, N.; Brand, C.; Oliveira, F.L.; Borojevic, R.; Teodoro, A.J. Lycopene and β-carotene induce cell-cycle arrest and apoptosis in human breast cancer cell lines. Anticancer. Res. 2014, 34, 1377–1386. [Google Scholar] [PubMed]
- Nahum, A.; Hirsch, K.; Danilenko, M.; Watts, C.K.W.; Prall, O.W.J.; Levy, J.; Sharoni, Y. Lycopene inhibition of cell cycle progression in breast and endometrial cancer cells is associated with reduction in cyclin D levels and retention of p27Kip1 in the cyclin E–cdk2 complexes. Oncogene 2001, 20, 3428–3436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijay, K.; Sowmya, P.R.R.; Arathi, B.P.; Shilpa, S.; Shwetha, H.J.; Raju, M.; Baskaran, V.; Lakshminarayana, R. Low-dose doxorubicin with carotenoids selectively alters redox status and upregulates oxidative stress-mediated apoptosis in breast cancer cells. Food Chem. Toxicol. 2018, 118, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Nesaretnam, K.; Radhakrishnan, A.; Selvaduray, K.R.; Reimann, K.; Pailoor, J.; Razak, G.; Mahmood, M.M.; Dahliwal, J.S. Effect of palm oil carotene on breast cancer tumorigenicity in nude mice. Lipids 2002, 37, 557–560. [Google Scholar] [CrossRef]
- Park, J.S.; Chew, B.P.; Wong, T.S. Dietary lutein from marigold extract inhibits mammary tumor development in BALB/c mice. J. Nutr. 1998, 128, 1650–1656. [Google Scholar] [CrossRef] [PubMed]
- Sahin, K.; Tuzcu, M.; Sahin, N.; Akdemir, F.; Ozercan, I.; Bayraktar, S.; Kucuk, O. Inhibitory effects of combination of lycopene and genistein on 7,12-dimethyl benz(a)anthracene-induced breast cancer in rats. Nutr. Can. 2011, 63, 1279–1286. [Google Scholar] [CrossRef]
- Srinivasan, R.; Chaitanyakumar, A.; Mageswari, A.; Gomathi, A.; Kumar, J.G.S.P.; Jayasindu, M.; Bharath, G.; Shravan, J.S.; Gothandam, K.M. Oral administration of lyophilized Dunaliella salina, a carotenoid-rich marine alga, reduces tumor progression in mammary cancer induced rats. Food Funct. 2017, 8, 4517–4527. [Google Scholar] [CrossRef]
- Kubatka, P.; Kapinová, A.; Kružliak, P.; Kello, M.; Výbohová, D.; Kajo, K.; Novák, M.; Chripková, M.; Adamkov, M.; Péč, M.; et al. Antineoplastic effects of Chlorella pyrenoidosa in the breast cancer model. Nutrition 2015, 31, 560–569. [Google Scholar] [CrossRef]
- Arzi, L.; Riazi, G.; Sadeghizadeh, M.; Hoshyar, R.; Jafarzadeh, N. A comparative study on anti-Invasion, antimigration, and antiadhesion effects of the bioactive carotenoids of saffron on 4T1 breast cancer cells through their effects on Wnt/β-catenin pathway genes. DNA Cell Biol. 2018, 37, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Pelicanom, H.; Zhang, W.; Liu, J.; Hammoudi, N.; Dia, J.; Xu, R.H.; Pusztai, L.; Huang, P. Mitochondrial dysfunction in some triple-negative breast cancer cell lines: Role of mTOR pathway and therapeutic potential. Breast Can. Res. 2014, 16, 434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulaiman, A.; Wang, L. Bridging the divide: Preclinical research discrepancies between triple-negative breast cancer cell lines and patient tumors. Oncotarget 2017, 8, 113269–113281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waks, A.G.; Winer, E.P. Breast cancer treatment: A review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Huang, R.F.S.; Wei, Y.J.; Inbaraj, B.S.; Chen, B.H. Inhibition of colon cancer cell growth by nanoemulsion carrying gold nanoparticles and lycopene. Int. J. Nanomed. 2015, 10, 2823–2846. [Google Scholar]
- Farokhzad, O.C.; Langer, R. Impact of nanotechnology on drug delivery. ACS Nano 2009, 3, 16–20. [Google Scholar] [CrossRef]
- Kulkarni, S.A.; Feng, S.S. Effects of particle size and surface modification on cellular uptake and biodistribution of polymeric nanoparticles for drug delivery. Pharmaceut. Res. 2013, 30, 2512–2522. [Google Scholar] [CrossRef]






| Peak No. | Compound | Retention Time (min) | Retention Factor (k) a | Separation Factor (α) b | Peak Purity (%) | Contents (μg/g) |
|---|---|---|---|---|---|---|
| 1 | All-trans-violaxanthin | 8.69 | 2.03 | 1.46 (1, 2) c | 92.1 | 5.48 |
| 2 | cis-lutein | 11.17 | 2.38 | 1.07 (2, 3) | 96.7 | 18.35 |
| 3 | All-trans-lutein | 11.70 | 2.54 | 1.31 (3, 4) | 96.8 | 42.93 |
| 4 | All-trans-zeaxanthin | 14.32 | 3.34 | 1.54 (4, 5) | 90.7 | 25.57 |
| IS d | All-trans-canthaxanthin | 14.82 | 3.63 | 1.04 (5, IS) | 99.7 | - |
| 5 | 15- or 15′-cis-cryptoxanthin | 20.30 | 5.15 | 1.47 (IS, 6) | 93.0 | 72.37 |
| 6 | All-trans-β-cryptoxanthin | 20.78 | 5.30 | 1.22 (6, 7) | 94.6 | 155.9 |
| 7 | 15- or 15′-cis-β-carotene | 24.56 | 6.44 | 1.03 (7, 8) | 98.7 | 65.67 |
| 8 | 13- or 13′-cis-β-carotene | 25.12 | 6.62 | 1.10 (8, 9) | 97.7 | 255.8 |
| 9 | All-trans-β-carotene | 27.23 | 7.25 | 1.05 (9, 10) | 95.6 | 663.8 |
| 10 | All-trans-α-carotene | 28.36 | 7.60 | 1.05 (9, 10) | 92.3 | 75.59 |
| Total | 1381.46 |
| Peak No. | Carotenoids | Repeatability | Intermediate Precision | Accuracy | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Contents (μg/g) a | RSD (%) b | Contents (μg/g) a | RSD (%) b | Original (μg) | Spiked (μg) | Found (μg) | Recovery (%) c | Mean ± SD (%) | RSD (%) b | ||
| 1 | All-trans-violanxanthin | 5.17 ± 0.2 | 0.9 | 5.73 ± 1.9 | 1.6 | - | - | - | - | - | - |
| 2 | cis-lutein | 18.42 ± 0.4 | 2.1 | 18.75 ± 0.8 | 4.2 | - | - | - | - | - | - |
| 3 | All-trans-lutein | 43.21 ± 0.5 | 1.1 | 42.95 ± 1.2 | 2.7 | 45.2 | 5 | 50 | 96.0 | 94.6 ± 2.0 | 2.1 |
| 45.2 | 50 | 91.8 | 93.2 | ||||||||
| 4 | All-trans-zeaxanthin | 26.13 ± 0.1 | 0.3 | 26.70 ± 0.4 | 1.4 | 27.4 | 5 | 32.1 | 94.0 | 92.1 ± 2.7 | 2.9 |
| 27.4 | 50 | 72.5 | 90.2 | ||||||||
| 5 | 15- or15′-cis-β-cryptoxanthin | 75.07 ± 1.1 | 1.4 | 75.51 ± 0.7 | 0.9 | - | - | - | - | - | - |
| 6 | All-trans-β-cryptoxanthin | 162.45 ± 2.5 | 1.5 | 163.02 ± 1.8 | 1.1 | 161.2 | 5 | 165.9 | 94.0 | 91.9 ± 3.0 | 3.2 |
| 161.2 | 50 | 206.1 | 89.8 | ||||||||
| 7 | 15- or 15′-cis-β-carotene | 71.08 ± 0.3 | 0.4 | 71.44 ± 0.4 | 0.5 | - | - | - | - | - | - |
| 8 | 13- or 13′-cis-β-carotene | 258.70 ± 2.1 | 0.8 | 258.53 ± 1.8 | 0.6 | - | - | - | - | - | - |
| 9 | All-trans-β-carotene | 688.15 ± 3.2 | 0.4 | 670.28 ± 2.4 | 0.3 | 673.9 | 50 | 719.1 | 90.4 | 94.7 ± 6.0 | 6.4 |
| 673.9 | 500 | 1168.5 | 98.9 | ||||||||
| 10 | All-trans-α-carotene | 77.95 ± 1.4 | 1.7 | 76.87 ± 2.0 | 2.6 | 77.3 | 5 | 81.4 | 82.0 | 86.1 ± 5.8 | 6.7 |
| 77.3 | 50 | 122.4 | 90.2 | ||||||||
| Storage Time (Day) | Particle Size (nm) a | Polydispersity Index a | Zeta-Potential (mV) a | |||||
| 0 | 13.3 ± 0.4 B | 0.238 ± 0.01 B | −69.8 ± 0.5 D | |||||
| 15 | 13.3 ± 0.3 B | 0.249 ± 0.01 AB | −69.0 ± 0.2 D | |||||
| 30 | 13.6 ± 0.1 AB | 0.265 ± 0.02 AB | −67.4 ± 1.0 C | |||||
| 45 | 13.9 ± 0.3 A | 0.267 ± 0.01 A | −65.3 ± 0.8 B | |||||
| 60 | 13.5 ± 0.4 AB | 0.267 ± 0.02 A | −66.6 ± 0.6 C | |||||
| 75 | 14.0 ± 0.2 A | 0.270 ± 0.03 A | −64.5 ± 0.7 B | |||||
| 90 | 13.8 ± 0.4 AB | 0.263 ± 0.02 AB | −63.0 ± 0.9 A | |||||
| Heating Time | Particle Size (nm) a | Zeta Potential (mV) a | ||||||
| 0.5 h | 1 h | 1.5 h | 2 h | 0.5 h | 1 h | 1.5 h | 2 h | |
| Control (unheated) | 13.3 | - | - | - | −69.0 | - | - | - |
| 40 ℃ | 13.4 | 13.9 | 13.6 | 14.0 | −66.0 | −62.3 | −62.1 | −60.1 |
| 70 ℃ | 14.2 | 14.5 | 15.1 | 15.0 | −57.4 | −60.1 | −42.4 | −41.6 |
| 100 ℃ | 16.9 | 17.5 | 18.0 | 18.2 | −41.2 | −40.9 | −34.3 | −31.2 |
| Concentration (μg/mL) | Sub-G1 (%) | G0/G1 (%) | S (%) | G2/M (%) |
|---|---|---|---|---|
| Control | 5.68 ± 0.2 A | 47.12 ± 0.4 A | 18.28 ± 0.3 A | 28.38 ± 0.1 A |
| Carotenoid extract | ||||
| 5 | 7.77 ± 0.5 B | 49.68 ± 0.3 B | 15.75 ± 0.4 B | 26.36 ± 0.7 B |
| 10 | 8.09 ± 0.2 B | 51.75 ± 1.2 C | 15.85 ± 0.4 B | 24.35 ± 0.5 C |
| Carotenoid nanoemulsion | ||||
| 5 | 8.48 ± 0.8 B | 53.43 ± 0.4 D | 15.23 ± 0.6 B | 22.17 ± 0.3 D |
| 10 | 8.09 ± 0.7 B | 54.65 ± 0.3 E | 14.68 ± 1.2 B | 21.97 ± 0.7 D |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, H.-Y.; Chen, B.-H. A Comparative Study on Inhibition of Breast Cancer Cells and Tumors in Mice by Carotenoid Extract and Nanoemulsion Prepared from Sweet Potato (Ipomoea batatas L.) Peel. Pharmaceutics 2022, 14, 980. https://doi.org/10.3390/pharmaceutics14050980
Hsu H-Y, Chen B-H. A Comparative Study on Inhibition of Breast Cancer Cells and Tumors in Mice by Carotenoid Extract and Nanoemulsion Prepared from Sweet Potato (Ipomoea batatas L.) Peel. Pharmaceutics. 2022; 14(5):980. https://doi.org/10.3390/pharmaceutics14050980
Chicago/Turabian StyleHsu, Hsin-Yen, and Bing-Huei Chen. 2022. "A Comparative Study on Inhibition of Breast Cancer Cells and Tumors in Mice by Carotenoid Extract and Nanoemulsion Prepared from Sweet Potato (Ipomoea batatas L.) Peel" Pharmaceutics 14, no. 5: 980. https://doi.org/10.3390/pharmaceutics14050980
APA StyleHsu, H.-Y., & Chen, B.-H. (2022). A Comparative Study on Inhibition of Breast Cancer Cells and Tumors in Mice by Carotenoid Extract and Nanoemulsion Prepared from Sweet Potato (Ipomoea batatas L.) Peel. Pharmaceutics, 14(5), 980. https://doi.org/10.3390/pharmaceutics14050980







