Vesicular Nanocarriers for Phytocompounds in Wound Care: Preparation and Characterization
Abstract
:1. Introduction
2. Presentation of the Cellular and Molecular Mechanisms of Wound Healing
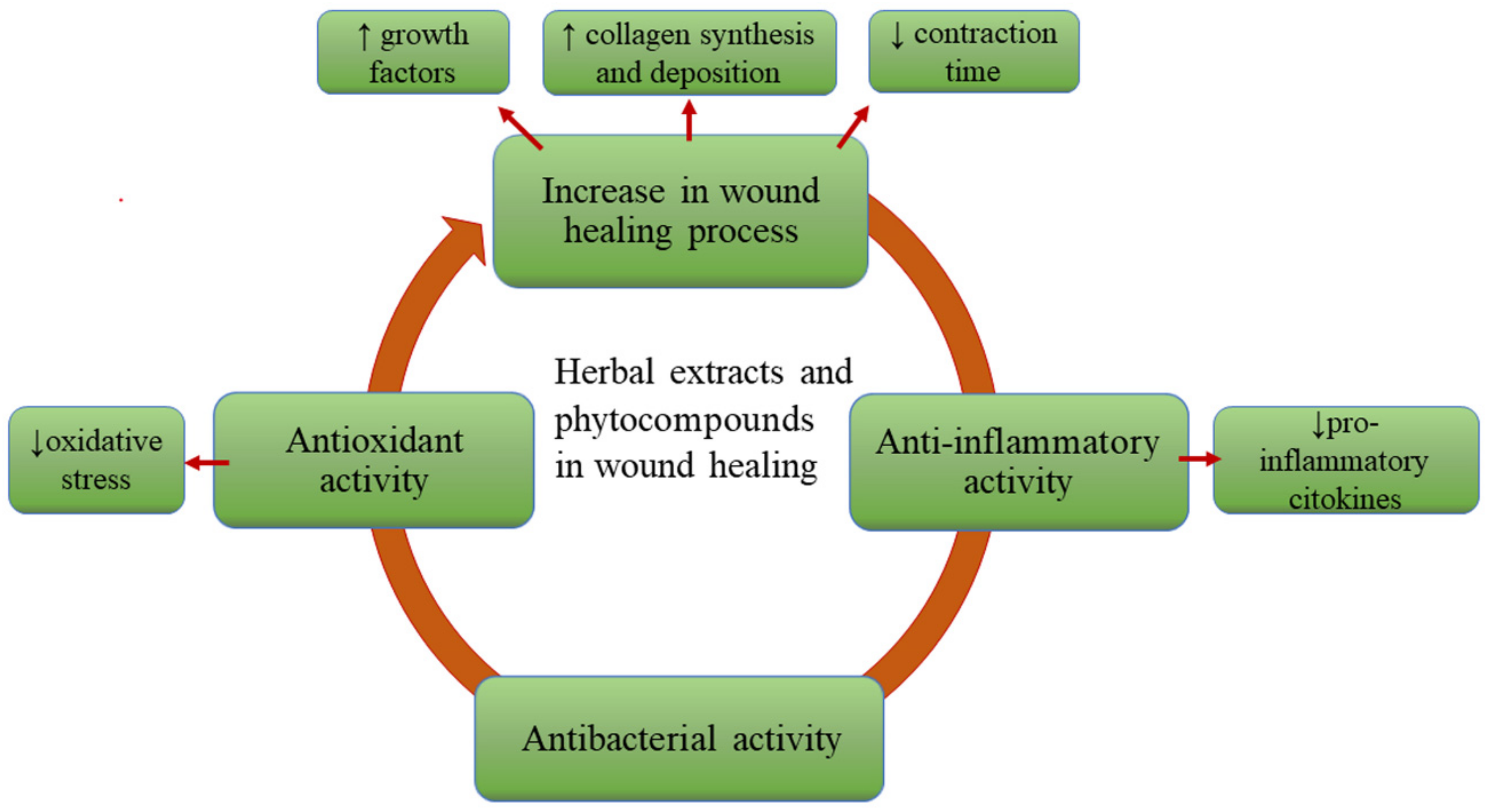
3. The Role of the Phytocompounds in Wound Care
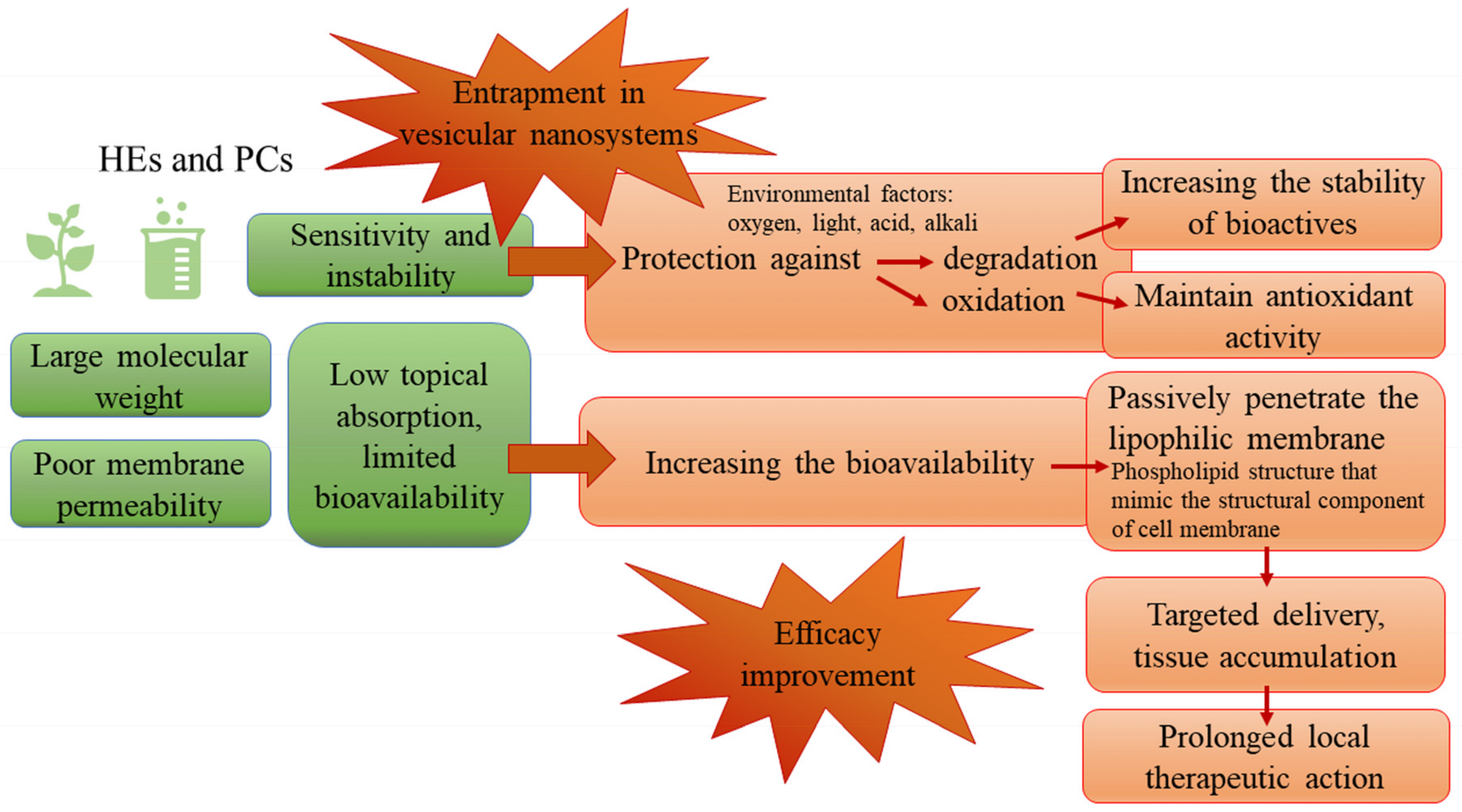
4. The Entrapment of Herbal Extracts into Vesicular Nanosystems—Challenges in the Formulation
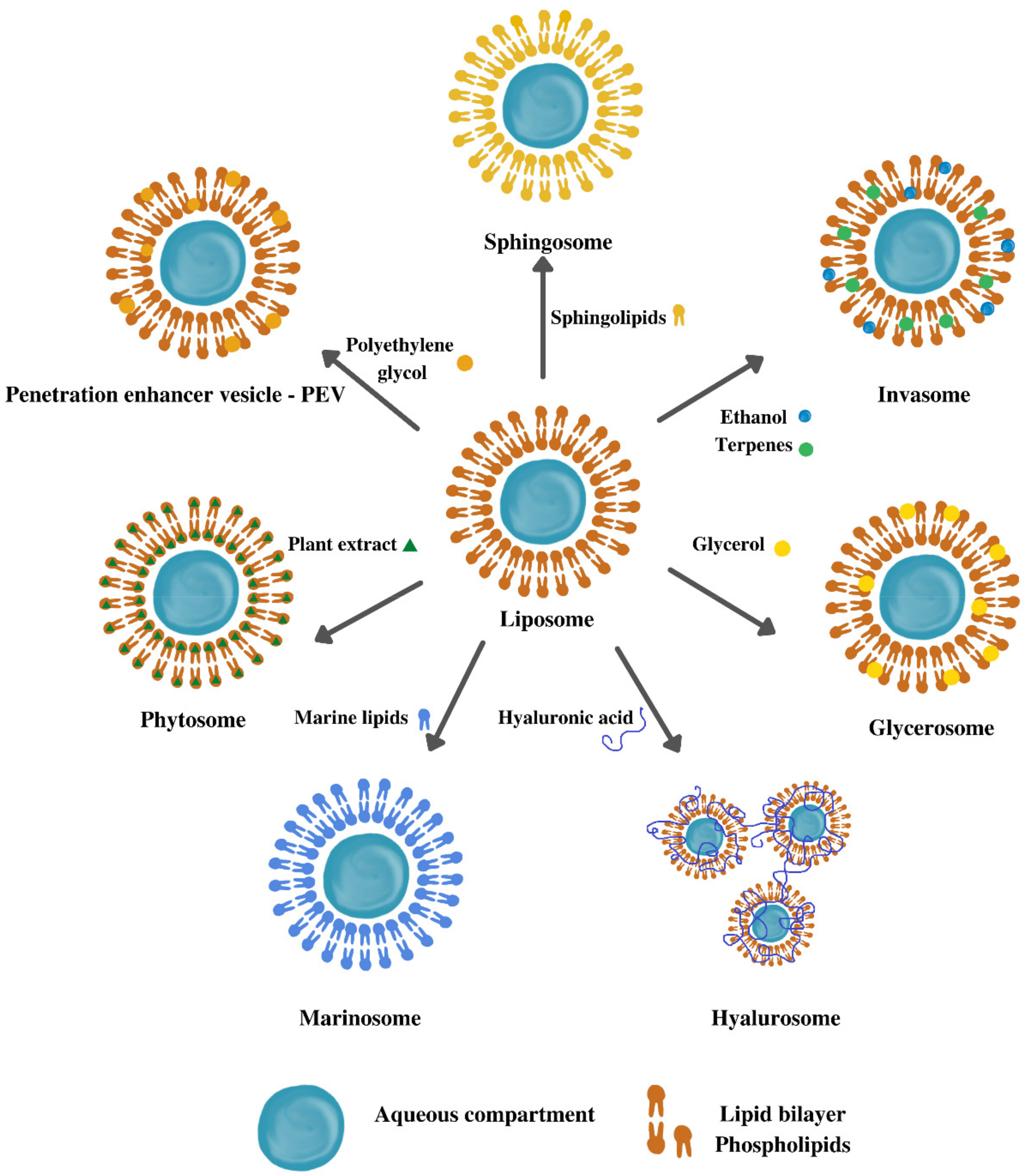
5. Types of Vesicular Nanosystems
5.1. Liposomes
5.2. Ethosomes
5.3. Niosomes
5.4. Transferosomes
5.5. Cubosomes
6. Preparation Methods of Vesicular Nanosystems
6.1. Hydration of Dry Thin Lipid Film: Bangham Method, Film Dispersion, or Thin-Layer Evaporation Method
6.2. Emulsion Evaporation
6.3. Direct Sonication
6.4. Reverse Phase Evaporation Technique
6.5. Antisolvent Precipitation Technique
6.6. Single-Step Injection Technique
7. Characterization Methods of Vesicular Nanosystems
7.1. Particle Size
7.2. Polydispersity Index
7.3. Particle Shape
7.4. Zeta ζ-Potential (Surface Charge)
7.5. The Lipid Content of the Vesicle Dispersions
7.6. Phase Behavior
7.7. Entrapment Efficiency
7.8. In Vitro Drug Release of the Active Substance
7.9. Physical Stability
7.10. Leakage Rate
7.11. Chemical Stability
7.12. Other Characterization Methods
8. Methods of Evaluation of Vesicular Nanosystems for Wound Healing
8.1. In Vitro Skin Delivery of the Active Compounds
8.2. Cellular Uptake by Vesicles
8.3. Cell Viability Studies
8.4. Scratch Assay—In Vitro Wound Healing Effect, Cell Migration Assay
8.5. In Vivo Wound Healing Effect on Animal Model
9. Topical Delivery Systems Containing Herbal Extracts
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Falanga, V. Mechanisms of Cutaneous Wound Repair. In Fitzpatrick’s Dermatology in General Medicine; McGraw Hill Medical: New York, NY, USA, 2003; pp. 278–291. [Google Scholar]
- Sen, C.K. Human Wound and Its Burden: Updated 2020 Compendium of Estimates. In Advances in Wound Care; Mary Ann Liebert Inc.: New York, NY, USA, 2021; Volume 10, pp. 281–292. [Google Scholar]
- Suliman, Y.A.; Bruni, C.; Johnson, S.R.; Praino, E.; Alemam, M.; Borazan, N.; Cometi, L.; Myers, B.; Khanna, D.; Allanore, Y.; et al. Defining Skin Ulcers in Systemic Sclerosis: Systematic Literature Review and Proposed World Scleroderma Foundation (WSF) Definition. J. Scleroderma Relat. Disord. 2017, 2, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Giuggioli, D.; Manfredi, A.; Lumetti, F.; Colaci, M.; Ferri, C. Scleroderma Skin Ulcers Definition, Classification and Treatment Strategies Our Experience and Review of the Literature. Autoimmun. Rev. 2018, 17, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lu, K.J.; Yu, C.H.; Huang, Q.L.; Du, Y.Z. Nano-Drug Delivery Systems in Wound Treatment and Skin Regeneration. J. Nanobiotechnol. 2019, 17, 1–15. [Google Scholar] [CrossRef]
- Bhatia, S. Nanoparticles Types, Classification, Characterization, Fabrication Methods and Drug Delivery Applications. In Natural Polymer Drug Delivery Systems; Springer International Publishing: New York, NY, USA, 2016; pp. 33–93. [Google Scholar]
- Allaw, M.; Manca, M.L.; Gómez-Fernández, J.C.; Pedraz, J.L.; Terencio, M.C.; Sales, O.D.; Nacher, A.; Manconi, M. Oleuropein Multicompartment Nanovesicles Enriched with Collagen as a Natural Strategy for the Treatment of Skin Wounds Connected with Oxidative Stress. Nanomedicine 2021, 16, 2363–2376. [Google Scholar] [CrossRef]
- Caddeo, C.; Manca, M.L.; Peris, J.E.; Usach, I.; Diez-Sales, O.; Matos, M.; Fernàndez-Busquets, X.; Fadda, A.M.; Manconi, M. Tocopherol-Loaded Transfersomes: In Vitro Antioxidant Activity and Efficacy in Skin Regeneration. Int. J. Pharm. 2018, 551, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Ghiulai, R.; Roşca, O.J.; Antal, D.S.; Mioc, M.; Mioc, A.; Racoviceanu, R.; Macaşoi, I.; Olariu, T.; Dehelean, C.; Creţu, O.M.; et al. Tetracyclic and Pentacyclic Triterpenes with High Therapeutic Efficiency in Wound Healing Approaches. Molecules 2020, 25, 5557. [Google Scholar] [CrossRef] [PubMed]
- Barakat, H.; Aljabeili, H.S.; Rahman, H.A.A. Synergistic Effect of Thymus Vulgaris Essential Oil Oral Administration on Topically Treated Wound with Chitosan, Thyme Essential Oil and Their Combination in Rats. J. Nutr. Food Sci. 2018, 8, 1–6. [Google Scholar] [CrossRef]
- Javed, N.; Ijaz, S.; Akhtar, N.; Khan, H.M.S. Nanostructured Ethosomal Gel Loaded with Arctostaphylosuva-Ursi Extract; In-Vitro/In-Vivo Evaluation as a Cosmeceutical Product for Skin Rejuvenation. Curr. Drug Deliv. 2022, 19, 720–734. [Google Scholar] [CrossRef]
- Sasindran, S.; Easwaran, M.; Shyamala, G.; Karuppaiah, A.; Siram, K.; Veintramuthu, S. Phytochemical Screening and Cytotoxicity Evaluation of Crude Extracts: Toxicity Comparison of Crude Extracts and Its Ethosomal Formulations. J. Cosmet. Dermatol. 2020, 19, 1794–1803. [Google Scholar] [CrossRef]
- Gupta, B.S.; Edwards, J.V. Textile Materials and Structures for Topical Management of Wounds. In Advanced Textiles for Wound Care; Elsevier Ltd.: London, UK, 2019; pp. 55–104. [Google Scholar]
- Matter, M.T.; Probst, S.; Läuchli, S.; Herrmann, I.K. Uniting Drug and Delivery: Metal Oxide Hybrid Nanotherapeutics for Skin Wound Care. Pharmaceutics 2020, 12, 780. [Google Scholar] [CrossRef]
- Falanga, V.; Iwamoto, S. Chapter 248: Mechanisms of Wound Repair, Wound Healing, and Wound Dressing. In Fitzpatrick’s Dermatology in General Medicine; McGraw Hill: New York, NY, USA, 2022; pp. 1–28. [Google Scholar]
- Matei, A.M.; Caruntu, C.; Tampa, M.; Georgescu, S.R.; Matei, C.; Constantin, M.M.; Constantin, T.V.; Calina, D.; Ciubotaru, D.A.; Badarau, I.A.; et al. Applications of Nanosized-Lipid-Based Drug Delivery Systems in Wound Care. Appl. Sci. 2021, 11, 4915. [Google Scholar] [CrossRef]
- Schultz, G.S.; Chin, G.A.; Moldawer, L.; Diegelmann, R.F. Principles of Wound Healing. In Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists; Fitridge, R., Thompson, M., Eds.; University of Adelaide Press: Adelaide, Australia, 2011; pp. 423–435. [Google Scholar]
- Ilomuanya, M.O.; Cardoso-Daodu, I.M.; Ubani-Ukoma, U.N.; Adebona, A.C. Polymeric Biomaterials for Wound Healing Incorporating Plant Extracts and Extracellular Matrix Components. In Recent Advances in Wound Healing; Aghaei, S., Ed.; IntechOpen: London, UK, 2021; pp. 1–15. [Google Scholar]
- Darvishi, B.; Dinarvand, R.; Mohammadpour, H.; Kamarul, T.; Sharifi, A.M. Dual l-Carnosine/Aloe Vera Nanophytosomes with Synergistically Enhanced Protective Effects against Methylglyoxal-Induced Angiogenesis Impairment. Mol. Pharm. 2021, 18, 3302–3325. [Google Scholar] [CrossRef] [PubMed]
- Koga, A.Y.; Pereira, A.V.; Lipinski, L.C.; Oliveira, M.R.P. Evaluation of Wound Healing Effect of Alginate Films Containing Aloe Vera (Aloe barbadensis Miller) Gel. J. Biomater. Appl. 2018, 32, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Motaal, A.A.; Ahmed, M.A.; Alsayari, A.; El-Gazayerly, O.N. An in Vivo Study of Hypericum Perforatum in a Niosomal Topical Drug Delivery System. Drug Deliv. 2018, 25, 417–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Güneş, S.; Tıhmınlıoğlu, F. Hypericum Perforatum Incorporated Chitosan Films as Potential Bioactive Wound Dressing Material. Int. J. Biol. Macromol. 2017, 102, 933–943. [Google Scholar] [CrossRef]
- Nasab, M.E.; Takzaree, N.; Saffaria, P.M. In Vitro Antioxidant Activity and in Vivo Wound-Healing Effect of Lecithin Liposomes: A Comparative Study. J. Comp. Eff. Res. 2019, 8, 633–643. [Google Scholar] [CrossRef]
- Pandey, V.K.; Ajmal, G.; Upadhyay, S.N.; Mishra, P.K. Nano-Fibrous Scaffold with Curcumin for Anti-Scar Wound Healing. Int. J. Pharm. 2020, 589, 1–10. [Google Scholar] [CrossRef]
- Castangia, I.; Nácher, A.; Caddeo, C.; Valenti, D.; Fadda, A.M.; Díez-Sales, O.; Ruiz-Saurí, A.; Manconi, M. Fabrication of Quercetin and Curcumin Bionanovesicles for the Prevention and Rapid Regeneration of Full-Thickness Skin Defects on Mice. Acta Biomater. 2014, 10, 1292–1300. [Google Scholar] [CrossRef]
- Manconi, M.; Manca, M.L.; Marongiu, F.; Caddeo, C.; Castangia, I.; Petretto, G.L.; Pintore, G.; Sarais, G.; D’Hallewin, G.; Zaru, M.; et al. Chemical Characterization of Citrus Limon Var. Pompia and Incorporation in Phospholipid Vesicles for Skin Delivery. Int. J. Pharm. 2016, 506, 449–457. [Google Scholar] [CrossRef]
- Moulaoui, K.; Caddeo, C.; Manca, M.L.; Castangia, I.; Valenti, D.; Escribano, E.; Atmani, D.; Fadda, A.M.; Manconi, M. Identification and Nanoentrapment of Polyphenolic Phytocomplex from Fraxinus Angustifolia: In Vitro and in Vivo Wound Healing Potential. Eur. J. Med. Chem. 2015, 89, 179–188. [Google Scholar] [CrossRef]
- Kaltschmidt, B.P.; Ennen, I.; Greiner, J.F.W.; Dietsch, R.; Patel, A.; Kaltschmidt, B.; Kaltschmidt, C.; Hütten, A. Preparation of Terpenoid-Invasomes with Selective Activity against S. aureus and Characterization by Cryo Transmission Electron Microscopy. Biomedicines 2020, 8, 105. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.W.; Ng, P.Y.; Chieng, N.; Ng, S.F. Moringa Oleifera Leaf Extract–Loaded Phytophospholipid Complex for Potential Application as Wound Dressing. J. Drug Deliv. Sci. Technol. 2019, 54, 101329. [Google Scholar] [CrossRef]
- Castangia, I.; Manca, M.L.; Allaw, M.; Hellström, J.; Granato, D.; Manconi, M. Jabuticaba (Myrciaria jaboticaba) Peel as a Sustainable Source of Anthocyanins and Ellagitannins Delivered by Phospholipid Vesicles for Alleviating Oxidative Stress in Human Keratinocytes. Molecules 2021, 26, 6697. [Google Scholar] [CrossRef] [PubMed]
- Arajo, A.A.S.; Nunes, P.S.; Albuquerque-Jnior, R.L.C.; Cavalcante, D.R.R.; Dantas, M.D.M.; Cardoso, J.C.; Bezerra, M.S.; Souza, J.C.C.; Serafini, M.R.; Quitans, L.J., Jr.; et al. Collagen-Based Films Containing Liposome-Loaded Usnic Acid as Dressing for Dermal Burn Healing. J. Biomed. Biotechnol. 2011, 2011, 1–9. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, Y.; Li, Y.; Bai, H.; Ma, T.; Song, X.; Zhao, J.; Gao, L. The Effects of Sodium Usnic Acid by Topical Application on Skin Wound Healing in Rats. Biomed. Pharmacother. 2018, 97, 587–593. [Google Scholar] [CrossRef]
- Kassam, N.A.; Damian, D.J.; Kajeguka, D.; Nyombi, B.; Kibiki, G.S. Spectrum and Antibiogram of Bacteria Isolated from Patients Presenting with Infected Wounds in a Tertiary Hospital, Northern Tanzania. BMC Res. Notes 2017, 10, 19–21. [Google Scholar] [CrossRef] [Green Version]
- Capoor, M.R.; Sarabahi, S.; Tiwari, V.K.; Narayanan, R.P. Fungal Infections in Burns: Diagnosis and Management. Indian J. Plast. Surg. 2010, 43, S37–S42. [Google Scholar] [CrossRef]
- Soliman, S.; Alnajdy, D.; El-Keblawy, A.A.; Mosa, K.A.; Khoder, G.; Noreddin, A.M. Plants’ Natural Products as Alternative Promising Anti-Candida Drugs. Pharmacogn. Rev. 2017, 11, 104–122. [Google Scholar] [CrossRef] [Green Version]
- Usach, I.; Margarucci, E.; Manca, M.L.; Caddeo, C.; Aroffu, M.; Petretto, G.L.; Manconi, M.; Peris, J.E. Comparison between Citral and Pompia Essential Oil Loaded in Phospholipid Vesicles for the Treatment of Skin and Mucosal Infections. Nanomaterials 2020, 10, 286. [Google Scholar] [CrossRef] [Green Version]
- Manconi, M.; Petretto, G.; D’hallewin, G.; Escribano, E.; Milia, E.; Pinna, R.; Palmieri, A.; Firoznezhad, M.; Peris, J.E.; Usach, I.; et al. Thymus Essential Oil Extraction, Characterization and Incorporation in Phospholipid Vesicles for the Antioxidant/Antibacterial Treatment of Oral Cavity Diseases. Colloids Surfaces B Biointerfaces 2018, 171, 115–122. [Google Scholar] [CrossRef]
- Rahman, H.S.; Othman, H.H.; Hammadi, N.I.; Yeap, S.K.; Amin, K.M.; Samad, N.A.; Alitheen, N.B. Novel Drug Delivery Systems for Loading of Natural Plant Extracts and Their Biomedical Applications. Int. J. Nanomed. 2020, 15, 2439–2483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behyari, M.; Imani, R.; Keshvari, H. Evaluation of Silk Fibroin Nanofibrous Dressing Incorporating Niosomal Propolis, for Potential Use in Wound Healing. Fibers Polym. 2021, 22, 2090–2101. [Google Scholar] [CrossRef]
- Allaw, M.; Manconi, M.; Caboni, P.; Bacchetta, G.; Escribano-Ferrer, E.; Peris, J.E.; Nacher, A.; Diez-Sales, O.; Manca, M.L. Formulation of Liposomes Loading Lentisk Oil to Ameliorate Topical Delivery, Attenuate Oxidative Stress Damage and Improve Cell Migration in Scratch Assay. Biomed. Pharmacother. 2021, 144, 112351. [Google Scholar] [CrossRef]
- Allaw, M.; Pleguezuelos-Villa, M.; Manca, M.L.; Caddeo, C.; Aroffu, M.; Nacher, A.; Diez-Sales, O.; Saurí, A.R.; Ferrer, E.E.; Fadda, A.M.; et al. Innovative Strategies to Treat Skin Wounds with Mangiferin: Fabrication of Transfersomes Modified with Glycols and Mucin. Nanomedicine 2020, 15, 1671–1685. [Google Scholar] [CrossRef]
- Mota, A.H.; Prazeres, I.; Mestre, H.; Bento-Silva, A.; Rodrigues, M.J.; Duarte, N.; Serra, A.T.; Bronze, M.R.; Rijo, P.; Gaspar, M.M.; et al. A Newfangled Collagenase Inhibitor Topical Formulation Based on Ethosomes with Sambucus Nigra l. Extract. Pharmaceuticals 2021, 14, 467. [Google Scholar] [CrossRef]
- Manconi, M.; Marongiu, F.; Castangia, I.; Manca, M.L.; Caddeo, C.; Tuberoso, C.I.G.; D’hallewin, G.; Bacchetta, G.; Fadda, A.M. Polymer-Associated Liposomes for the Oral Delivery of Grape Pomace Extract. Colloids Surfaces B Biointerfaces 2016, 146, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, M.; Du, S. Optimization of Madecassoside Liposomes Using Response Surface Methodology and Evaluation of Its Stability. Int. J. Pharm. 2014, 473, 280–285. [Google Scholar] [CrossRef]
- Allaw, M.; Manconi, M.; Aroffu, M.; Marongiu, F.; Porceddu, M.; Bacchetta, G.; Usach, I.; Rached, R.A.; Rajha, H.N.; Maroun, R.G.; et al. Extraction, Characterization and Incorporation of Hypericum Scruglii Extract in Ad Hoc Formulated Phospholipid Vesicles Designed for the Treatment of Skin Diseases Connected with Oxidative Stress. Pharmaceutics 2020, 12, 1010. [Google Scholar] [CrossRef]
- Pleguezuelos-Villa, M.; Diez-Sales, O.; Manca, M.L.; Manconi, M.; Sauri, A.R.; Escribano-Ferrer, E.; Nácher, A. Mangiferin Glycethosomes as a New Potential Adjuvant for the Treatment of Psoriasis. Int. J. Pharm. 2020, 573, 118844. [Google Scholar] [CrossRef]
- Nafisi, S.; Maibach, H.I. Nanotechnology in Cosmetics. In Cosmetic Science and Technology: Theoretical Principles and Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 337–365. [Google Scholar]
- Patzelt, A.; Lademann, J. Recent Advances in Follicular Drug Delivery of Nanoparticles. Expert Opin. Drug Deliv. 2020, 17, 49–60. [Google Scholar] [CrossRef]
- Gattu, S.; Maibach, H.I. Enhanced Absorption through Damaged Skin: An Overview of the in Vitro Human Model. Skin Pharmacol. Physiol. 2010, 23, 171–176. [Google Scholar] [CrossRef]
- Un, R.N.; Barlas, F.B.; Yavuz, M.; Ag Seleci, D.; Seleci, M.; Gumus, Z.P.; Guler, E.; Demir, B.; Can, M.; Coskunol, H.; et al. Phyto-Niosomes: In Vitro Assessment of the Novel Nanovesicles Containing Marigold Extract. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 927–937. [Google Scholar] [CrossRef]
- Jahanfar, S.; Gahavami, M.; Khosravi-Darani, K.; Jahadi, M.; Mozafari, M.R. Entrapment of Rosemary Extract by Liposomes Formulated by Mozafari Method: Physicochemical Characterization and Optimization. Heliyon 2021, 7, e08632. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Li, W.; Li, C.; Vittayapadung, S.; Lin, L. Liposome Containing Cinnamon Oil with Antibacterial Activity against Methicillin-Resistant Staphylococcus aureus Biofilm. Biofouling 2016, 32, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Risaliti, L.; Pini, G.; Ascrizzi, R.; Donato, R.; Sacco, C.; Bergonzi, M.C.; Salvatici, M.C.; Bilia, A.R. Artemisia annua Essential Oil Extraction, Characterization, and Incorporation in Nanoliposomes, Smart Drug Delivery Systems against Candida Species. J. Drug Deliv. Sci. Technol. 2020, 59, 101849. [Google Scholar] [CrossRef]
- Khogta, S.; Patel, J.; Barve, K.; Londhe, V. Herbal Nano-Formulations for Topical Delivery. J. Herb. Med. 2020, 20, 100300. [Google Scholar] [CrossRef]
- Ferraris, C.; Rimicci, C.; Garelli, S.; Ugazio, E.; Battaglia, L. Nanosystems in Cosmetic Products: A Brief Overview of Functional, Market, Regulatory and Safety Concerns. Pharmaceutics 2021, 13, 1408. [Google Scholar] [CrossRef]
- Kashyap, N.; Kumari, A.; Raina, N.; Zakir, F.; Gupta, M. Prospects of Essential Oil Loaded Nanosystems for Skincare. Phytomed. Plus 2022, 2, 100198. [Google Scholar] [CrossRef]
- Sebaaly, C.; Charcosset, C.; Stainmesse, S.; Fessi, H.; Greige-Gerges, H. Clove Essential Oil-in-Cyclodextrin-in-Liposomes in the Aqueous and Lyophilized States: From Laboratory to Large Scale Using a Membrane Contactor. Carbohydr. Polym. 2016, 138, 75–85. [Google Scholar] [CrossRef]
- Karatoprak, G.Ş.; Yücel, Ç.; Göger, F.; Sobarzo-Sánchez, E.; Küpeli Akkol, E. Potential Antioxidant and Enzyme Inhibitory Effects of Nanoliposomal Formulation Prepared from Salvia aramiensis Rech. f. Extract. Antioxidants 2020, 9, 293. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, M.H.; Vu, N.B.D.; Nguyen, T.H.N.; Le, H.S.; Le, H.T.; Tran, T.T.; Le, X.-C.; Le, V.-T.; Nguyen, T.-T.; Bui, C.-B.; et al. In Vivo Comparison of Wound Healing and Scar Treatment Effect between Curcumin-Oligochitosan Nanoparticle Complex and Oligochitosan-Coated Curcumin-Loaded-Liposome. J. Microencapsul. 2019, 36, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Castangia, I.; Manca, M.L.; Caddeo, C.; Bacchetta, G.; Pons, R.; Demurtas, D.; Diez-Sales, O.; Fadda, A.M.; Manconi, M. Santosomes as Natural and Efficient Carriers for the Improvement of Phycocyanin Reepithelising Ability in Vitro and in Vivo. Eur. J. Pharm. Biopharm. 2016, 103, 149–158. [Google Scholar] [CrossRef]
- Fu, X.; Shi, Y.; Wang, H.; Zhao, X.; Sun, Q.; Huang, Y.; Qi, T.; Lin, G. Ethosomal Gel for Improving Transdermal Delivery of Thymosin β-4. Int. J. Nanomed. 2019, 14, 9275–9284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cristiano, M.C.; Mancuso, A.; Fresta, M.; Torella, D.; De Gaetano, F.; Ventura, C.A.; Paolino, D. Topical Unsaturated Fatty Acid Vesicles Improve Antioxidant Activity of Ammonium Glycyrrhizinate. Pharmaceutics 2021, 13, 548. [Google Scholar] [CrossRef] [PubMed]
- Magnani, C.; Montis, C.; Mangiapia, G.; Mingotaud, A.F.; Mingotaud, C.; Roux, C.; Joseph, P.; Berti, D.; Lonetti, B. Hybrid Vesicles from Lipids and Block Copolymers: Phase Behavior from the Micro-to the Nano-Scale. Colloids Surfaces B Biointerfaces 2018, 168, 18–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, Y.; Wang, Y.; Li, X.; Zhang, G.; Zhang, G.; Hu, J. Light-Triggered Nitric Oxide (NO) Release from Photoresponsive Polymersomes for Corneal Wound Healing. Chem. Sci. 2020, 11, 186. [Google Scholar] [CrossRef] [Green Version]
- Moquin, A.; Ji, J.; Neibert, K.; Winnik, F.M.; Maysinger, D. Encapsulation and Delivery of Neutrophic Proteins and Hydrophobic Agents Using PMOXA-PDMS-PMOXA Triblock Polymersomes. ACS Omega 2018, 3, 13882–13893. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Liao, Y.; Cornel, E.J.; Lv, M.; Wu, T.; Zhang, X.; Fan, L.; Sun, M.; Zhu, Y.; Fan, Z.; et al. Polymersome Wound Dressing Spray Capable of Bacterial Inhibition and H2S Generation for Complete Diabetic Wound Healing. Chem. Mater. 2021, 33, 7972–7985. [Google Scholar] [CrossRef]
- El-Nabarawi, M.A.; Shamma, R.N.; Farouk, F.; Nasralla, S.M. Dapsone-Loaded Invasomes as a Potential Treatment of Acne: Preparation, Characterization, and In Vivo Skin Deposition Assay. AAPS PharmSciTech 2018, 19, 2174–2184. [Google Scholar] [CrossRef]
- Hoque, M.; Agarwal, S.; Gupta, S.; Garg, S.; Syed, I.; Rupesh, A.; Mohapatra, N.; Bose, S.; Sarkar, P. Lipid Nanostructures in Food Applications. In Innovative Food Processing Technologies: A Comprehensive Review; Knoerzer, K., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 565–579. [Google Scholar]
- Barani, M.; Sangiovanni, E.; Angarano, M.; Rajizadeh, M.A.; Mehrabani, M.; Piazza, S.; Gangadharappa, H.V.; Pardakhty, A.; Mehrbani, M.; Dell’agli, M.; et al. Phytosomes as Innovative Delivery Systems for Phytochemicals: A Comprehensive Review of Literature. Int. J. Nanomed. 2021, 16, 6983–7022. [Google Scholar] [CrossRef]
- Demir, B.; Barlas, F.B.; Guler, E.; Gumus, P.Z.; Can, M.; Yavuz, M.; Coskunol, H.; Timur, S. Gold Nanoparticle Loaded Phytosomal Systems: Synthesis, Characterization and in Vitro Investigations. RSC Adv. 2014, 4, 34687–34695. [Google Scholar] [CrossRef]
- Mazumder, A.; Dwivedi, A.; Du Preez, J.L.; Du Plessis, J. In Vitro Wound Healing and Cytotoxic Effects of Sinigrin-Phytosome Complex. Int. J. Pharm. 2016, 498, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Kianvash, N.; Bahador, A.; Pourhajibagher, M.; Ghafari, H.; Nikoui, V.; Rezayat, S.M.; Dehpour, A.R.; Partoazar, A. Evaluation of Propylene Glycol Nanoliposomes Containing Curcumin on Burn Wound Model in Rat: Biocompatibility, Wound Healing, and Anti-Bacterial Effects. Drug Deliv. Transl. Res. 2017, 7, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Castangia, I.; Caddeo, C.; Manca, M.L.; Casu, L.; Latorre, A.C.; Díez-Sales, O.; Ruiz-Saurí, A.; Bacchetta, G.; Fadda, A.M.; Manconi, M. Delivery of Liquorice Extract by Liposomes and Hyalurosomes to Protect the Skin against Oxidative Stress Injuries. Carbohydr. Polym. 2015, 134, 657–663. [Google Scholar] [CrossRef] [PubMed]
- El-Refaie, W.M.; Elnaggar, Y.S.R.; El-Massik, M.A.; Abdallah, O.Y. Novel Curcumin-Loaded Gel-Core Hyaluosomes with Promising Burn-Wound Healing Potential: Development, in-Vitro Appraisal and in-Vivo Studies. Int. J. Pharm. 2015, 486, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; Tagami, T.; Kishi, T.; Ozeki, T. Curcumin Marinosomes as Promising Nano-Drug Delivery System for Lung Cancer. Int. J. Pharm. 2018, 540, 40–49. [Google Scholar] [CrossRef]
- Thomas, D.A.; Sarris, A.H.; Cortes, J.; Faderl, S.; O’Brien, S.; Giles, F.J.; Garcia-Manero, G.; Rodriguez, M.A.; Cabanillas, F.; Kantarjian, H. Phase II Study of Sphingosomal Vincristine in Patients with Recurrent or Refractory Adult Acute Lymphocytic Leukemia. Cancer 2006, 106, 120–127. [Google Scholar] [CrossRef]
- Lopez, C.; Mériadec, C.; David-Briand, E.; Dupont, A.; Bizien, T.; Artzner, F.; Riaublanc, A.; Anton, M. Loading of Lutein in Egg-Sphingomyelin Vesicles as Lipid Carriers: Thermotropic Phase Behaviour, Structure of Sphingosome Membranes and Lutein Crystals. Food Res. Int. 2020, 138, 109770. [Google Scholar] [CrossRef]
- Tokudome, Y.; Uchida, R.; Yokote, T.; Todo, H.; Hada, N.; Kon, T.; Yasuda, J.; Hayashi, H.; Hashimoto, F.; Sugibayashi, K. Effect of Topically Applied Sphingomyelin-Based Liposomes on the Ceramide Level in the Three Dimensional Cultured Human Skin Model. J. Liposome Res. 2010, 20, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Kazemi, M.; Mombeiny, R.; Tavakol, S.; Keyhanvar, P.; Mousavizadeh, K. A Combination Therapy of Nanoethosomal Piroxicam Formulation along with Iontophoresis as an Anti-Inflammatory Transdermal Delivery System for Wound Healing. Int. Wound J. 2019, 16, 1144–1152. [Google Scholar] [CrossRef]
- Partoazar, A.; Kianvash, N.; Darvishi, M.H.; Nasoohi, S.; Rezayat, S.M.; Bahador, A. Ethosomal Curcumin Promoted Wound Healing and Reduced Bacterial Flora in Second Degree Burn in Rat. Drug Res. 2016, 66, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Lin, L.; Choi, Y.; Michniak-Kohn, B. Development and In-Vitro Evaluation of Co-Loaded Berberine Chloride and Evodiamine Ethosomes for Treatment of Melanoma. Int. J. Pharm. 2020, 581, 119278. [Google Scholar] [CrossRef] [PubMed]
- Sguizzato, M.; Ferrara, F.; Mariani, P.; Pepe, A.; Cortesi, R.; Huang, N.; Simelière, F.; Boldrini, P.; Baldisserotto, A.; Valacchi, G.; et al. “Plurethosome” as Vesicular System for Cutaneous Administration of Mangiferin: Formulative Study and 3D Skin Tissue Evaluation. Pharmaceutics 2021, 13, 1124. [Google Scholar] [CrossRef] [PubMed]
- Touitou, E.; Natsheh, H. Topical Administration of Drugs Incorporated in Carriers Containing Phospholipid Soft Vesicles for the Treatment of Skin Medical Conditions. Pharmaceutics 2021, 13, 2129. [Google Scholar] [CrossRef]
- Rameshk, M.; Sharififar, F.; Mehrabani, M.; Pardakhty, A.; Farsinejad, A.; Mehrabani, M. Proliferation and In Vitro Wound Healing Effects of the Microniosomes Containing Narcissus tazetta L. Bulb Extract on Primary Human Fibroblasts (HDFs). DARU J. Pharm. Sci. 2018, 26, 31–42. [Google Scholar] [CrossRef]
- Priprem, A.; Damrongrungruang, T.; Limsitthichaikoon, S.; Khampaenjiraroch, B.; Nukulkit, C.; Thapphasaraphong, S.; Limphirat, W. Topical Niosome Gel Containing an Anthocyanin Complex: A Potential Oral Wound Healing in Rats. AAPS PharmSciTech 2018, 19, 1681–1692. [Google Scholar] [CrossRef] [PubMed]
- Ou, N.; Sun, Y.; Zhou, S.; Gu, P.; Liu, Z.; Bo, R.; Hu, Y.; Liu, J.; Wang, D. Evaluation of Optimum Conditions for Achyranthes Bidentata Polysaccharides Encapsulated in Cubosomes and Immunological Activity in Vitro. Int. J. Biol. Macromol. 2018, 109, 748–760. [Google Scholar] [CrossRef]
- Morsi, N.M.; Abdelbary, G.A.; Ahmed, M.A. Silver Sulfadiazine Based Cubosome Hydrogels for Topical Treatment of Burns: Development and in Vitro/in Vivo Characterization. Eur. J. Pharm. Biopharm. 2014, 86, 178–189. [Google Scholar] [CrossRef]
- Thakkar, V.; Korat, V.; Baldaniya, L.; Gohel, M.; Gandhi, T.; Patel, N. Development and Characterization of Novel Hydrogel Containing Antimicrobial Drug for Treatment of Burns. Int. J. Pharm. Investig. 2016, 6, 158. [Google Scholar] [CrossRef] [Green Version]
- Farag, D.B.E.; Yousry, C.; Al-Mahallawi, A.M.; El-Askary, H.I.; Meselhy, M.R.; AbuBakr, N. The Efficacy of Origanum Majorana Nanocubosomal Systems in Ameliorating Submandibular Salivary Gland Alterations in Streptozotocin-Induced Diabetic Rats. Drug Deliv. 2022, 29, 62–74. [Google Scholar] [CrossRef]
- Gunal, M.; Ayla, S.; Caglayan, B.; Beker, M.; Bedri, N.; Aslan, I.; Ozdemir, E.; Kilic, E.Y. Can Carpobrotus Edulis, Heal Incisional and Excisional Wounds on the Skin? Bratisl. Med. J. 2021, 112, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Kitamoto, D.; Asikin, Y.; Takara, K.; Wada, K. Liposomes Encapsulating Aloe Vera Leaf Gel Extract Significantly Enhance Proliferation and Collagen Synthesis in Human Skin Cell Lines. J. Oleo Sci. 2009, 58, 643–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Cui, M.; Pan, Z.H.; Pan, L.Q. Danggui Buxue Extract-Loaded Liposomes in Thermosensitive Gel Enhance in Vivo Dermal Wound Healing via Activation of the VEGF/PI3K/Akt and TGF-β/Smads Signaling Pathway. Evid.-Based Complement. Altern. Med. 2017, 2017, 8407249. [Google Scholar] [CrossRef] [Green Version]
- Manca, M.L.; Manconi, M.; Meloni, M.C.; Marongiu, F.; Allaw, M.; Usach, I.; Peris, J.E.; Escribano-Ferrer, E.; Tuberoso, C.I.G.; Gutierrez, G.; et al. Nanotechnology for Natural Medicine: Formulation of Neem Oil Loaded Phospholipid Vesicles Modified with Argan Oil as a Strategy to Protect the Skin from Oxidative Stress and Promote Wound Healing. Antioxidants 2021, 10, 670. [Google Scholar] [CrossRef]
- Miranda, Í.K.S.P.B.; Santana, F.R.; Camilloto, G.P.; Detoni, C.B.; Souza, F.V.D.; de Cabral-Albuquerque, E.C.M.; Alves, S.L.; Neco, G.L.; de Lima, F.O.; de Assis, S.A. Development of Membranes Based on Carboxymethyl Cellulose/Acetylated Arrowroot Starch Containing Bromelain Extract Carried on Nanoparticles and Liposomes. J. Pharm. Sci. 2021, 110, 2372–2378. [Google Scholar] [CrossRef]
- Jangde, R.; Singh, D. Preparation and Optimization of Quercetin-Loaded Liposomes for Wound Healing, Using Response Surface Methodology. Artif. Cells Nanomed. Biotechnol. 2016, 44, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Lee, J.S.; Sung, D.; Lim, J.M.; Choi, W. Il Potential Antioxidant and Wound Healing Effect of Nano-Liposol with High Loading Amount of Astaxanthin. Int. J. Nanomed. 2020, 15, 9231–9240. [Google Scholar] [CrossRef]
- Kontogiannopoulos, K.N.; Assimopoulou, A.N.; Hatziantoniou, S.; Karatasos, K.; Demetzos, C.; Papageorgiou, V.P. Chimeric Advanced Drug Delivery Nano Systems (Chi-ADDnSs) for Shikonin Combining Dendritic and Liposomal Technology. Int. J. Pharm. 2012, 422, 381–389. [Google Scholar] [CrossRef]
- Caddeo, C.; Díez-Sales, O.; Pons, R.; Fernàndez-Busquets, X.; Fadda, A.M.; Manconi, M. Topical Anti-Inflammatory Potential of Quercetin in Lipid-Based Nanosystems: In Vivo and in Vitro Evaluation. Pharm. Res. 2014, 31, 959–968. [Google Scholar] [CrossRef] [Green Version]
- Aldalaen, S.; Nasr, M.; El-Gogary, R.I. Angiogenesis and Collagen Promoting Nutraceutical-Loaded Nanovesicles for Wound Healing. J. Drug Deliv. Sci. Technol. 2020, 56, 101548. [Google Scholar] [CrossRef]
- Manca, M.L.; Castangia, I.; Zaru, M.; Nácher, A.; Valenti, D.; Fernàndez-Busquets, X.; Fadda, A.M.; Manconi, M. Development of Curcumin Loaded Sodium Hyaluronate Immobilized Vesicles (Hyalurosomes) and Their Potential on Skin Inflammation and Wound Restoring. Biomaterials 2015, 71, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Paolino, D.; Cosco, D.; Cilurzo, F.; Trapasso, E.; Morittu, V.M.; Celia, C.; Fresta, M. Improved in Vitro and in Vivo Collagen Biosynthesis by Asiaticoside-Loaded Ultradeformable Vesicles. J. Control. Release 2012, 162, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Risaliti, L.; Kehagia, A.; Daoultzi, E.; Lazari, D.; Bergonzi, M.C.; Vergkizi-Nikolakaki, S.; Hadjipavlou-Litina, D.; Bilia, A.R. Liposomes Loaded with Salvia triloba and Rosmarinus officinalis Essential Oils: In Vitro Assessment of Antioxidant, Antiinflammatory and Antibacterial Activities. J. Drug Deliv. Sci. Technol. 2019, 51, 493–498. [Google Scholar] [CrossRef]
- Andleeb, M.; Shoaib Khan, H.M.; Daniyal, M. Development, Characterization and Stability Evaluation of Topical Gel Loaded With Ethosomes Containing Achillea millefolium L. Extract. Front. Pharmacol. 2021, 12, 603227. [Google Scholar] [CrossRef]
- Pando, D.; Caddeo, C.; Manconi, M.; Fadda, A.M.; Pazos, C. Nanodesign of Olein Vesicles for the Topical Delivery of the Antioxidant Resveratrol. J. Pharm. Pharmacol. 2013, 65, 1158–1167. [Google Scholar] [CrossRef] [PubMed]
- Barone, A.; Cristiano, M.C.; Cilurzo, F.; Locatelli, M.; Iannotta, D.; Di Marzio, L.; Celia, C.; Paolino, D. Ammonium Glycyrrhizate Skin Delivery from Ultradeformable Liposomes: A Novel Use as an Anti-Inflammatory Agent in Topical Drug Delivery. Colloids Surfaces B Biointerfaces 2020, 193, 111152. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of Liposomes as Drug Delivery System for Therapeutic Applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef]
- Tefas, L.R.; Sylvester, B.; Tomuta, I.; Sesarman, A.; Licarete, E.; Banciu, M.; Porfire, A. Development of Antiproliferative Long-Circulating Liposomes Co-Encapsulating Doxorubicin and Curcumin, through the Use of a Quality-by-Design Approach. Drug Des. Devel. Ther. 2017, 11, 1605–1621. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.J.; Kazemzadeh-Narbat, M. Innovation in Wound Care Products: A FDA Regulatory Perspective. J. Wound Care 2021, 30, S3–S4. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound Healing Dressings and Drug Delivery Systems: A Review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef] [PubMed]
- Han, S.K. Innovations and Advances in Wound Healing; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–287. [Google Scholar]
- Avachat, A.M.; Takudage, P.J. Design and Characterization of Multifaceted Lyophilized Liposomal Wafers with Promising Wound Healing Potential. J. Liposome Res. 2018, 28, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, S. Advanced Textiles for Wound Care, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; pp. 1–591. [Google Scholar]
- Dunne, C.P.; Gouveia, I.C.; Isabel, C. Gouveia Designing New Antibacterial Wound Dressings: Development of a Dual Layer Cotton Material Coated with Polyvinyl Alcohol and Chitosan Nanofibers Incorporating Agrimonia eupatoria L. Extract. Molecules 2021, 26, 83. [Google Scholar]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Preparation and Optimization of Chitosan-Gelatin Films for Sustained Delivery of Lupeol for Wound Healing. Int. J. Biol. Macromol. 2017, 107, 1888–1897. [Google Scholar] [CrossRef]
- Colobatiu, L.; Gavan, A.; Mocan, A.; Bogdan, C.; Mirel, S.; Tomuta, I. Development of Bioactive Compounds-Loaded Chitosan Films by Using a QbD Approach—A Novel and Potential Wound Dressing Material. React. Funct. Polym. 2019, 138, 46–54. [Google Scholar] [CrossRef]
- Sağıroğlu, A.A.; Çelik, B.; Güler, E.M.; Koçyiğit, A.; Özer, Ö. Evaluation of Wound Healing Potential of New Composite Liposomal Films Containing Coenzyme Q10 and D-Panthenyl Triacetate as Combinational Treatment. Pharm. Dev. Technol. 2021, 26, 444–454. [Google Scholar] [CrossRef]
- Umar, A.K.; Sriwidodo, S.; Maksum, I.P.; Wathoni, N. Film-Forming Spray of Water-Soluble Chitosan Containing Liposome-Coated Human Epidermal Growth Factor for Wound Healing. Molecules 2021, 26, 5326. [Google Scholar] [CrossRef]
- Vedovatto, S.; Facchini, J.C.; Batista, R.K.; Paim, T.C.; Lionzo, M.I.Z.; Wink, M.R. Development of Chitosan, Gelatin and Liposome Film and Analysis of Its Biocompatibility in Vitro. Int. J. Biol. Macromol. 2020, 160, 750–757. [Google Scholar] [CrossRef]
- Eid, H.M.; Ali, A.A.; Ali, A.M.A.; Eissa, E.M.; Hassan, R.M.; El-Ela, F.I.A.; Hassan, A.H. Potential Use of Tailored Citicoline Chitosan-Coated Liposomes for Effective Wound Healing in Diabetic Rat Model. Int. J. Nanomed. 2022, 17, 555–575. [Google Scholar] [CrossRef]


| Entrapped HEs | Method of Extraction | Active Compounds | Components of VNs | VNs’ Preparation | Ref. |
|---|---|---|---|---|---|
| Liposomes | |||||
| Carpobrotus edulis powder extract | Fresh leaves homogenized in distilled water, frozen, and lyophilized | Flavonoids | Hydrogenated phosphatidylcholine, cholesterol | Dry thin-film method | [90] |
| Pistacia lentiscus oil | Marketed product | Fatty acids, phenolic compounds a | Soy lecithin | Hydration, sonication | [40] |
| Aloe vera leaf gel extract | Fresh gel homogenized, frozen, defrosted, centrifugated | Glycoproteins, aloesin | Soy lecithin | Bangham method, mechanochemical method | [91] |
| Angelica sinensis and
Astragali radix ratio 1:5 | Decoction | Ferulic acid coupled with astragaloside IV | Soy phosphatidylcholine, cholesterol | Thin-film dispersion, ultrasonic method | [92] |
| Salvia aramiensis aerial part extract | Methanol, ethanol extraction in shaking bath, water extraction-infusion | n.a. | Dipalmitoylphosphatidylcholine, cholesterol | Dry thin-film hydration | [58] |
| Liposomes and hyalurosomes | |||||
| Azadirachta indica oil (Neem oil) | Marketed product | Glycerides, fatty acids, sulfur-containing compounds, flavonoids b | Soy lecithin, argan oil, sodium hyaluronate | Direct sonication | [93] |
| Glycyrrhiza glabra root extract | Percolation in ethanol | Glycyrrhizin-triterpenoid saponin glycoside, polyphenols | Soy phosphatidylcholine, Phospholipon 90G, Sodium hyaluronate LMW | Hydration, sonication | [73] |
| Niosomes | |||||
| Calendula officinalis flowers and leaves extract | Methanol extraction | Steroids, terpenoids, triterpenoids, phenolic acids, flavonoids, carotenes | Tween 60, cholesterol | Dry film hydration, sonication | [50] |
| Hypericum perforatum flowering tops | Ethanol extract, DIG–MAZ multifunctional extraction system | Hyperforin, hypericins, flavonoids | Span 20, 60, 80, cholesterol | Modified reverse phase evaporation | [21] |
| Transferosomes | |||||
| Myrciaria jaboticaba fruits peel | Pressurized hot water extraction | Flavonoids, anthocyanins, ellagitannins | Lipoid S75, Tween 80, hydroxyethylcellulose, Sodium hyaluronate | Hydration, sonication | [30] |
| Phytosomes | |||||
| Moringa oleifera leaves extract | Maceration, ultrasound-assisted extraction | Quercetin, kaempferol, chlorogenic acid, rosmarinic acid | l-α-lecithin, cholesterol, polysorbate 80 | Thin-film hydration, sonication | [29] |
| AuNP–phytosomes and liposomes | |||||
| Calendula officinalis flowers extract | Methanol for 6 h, at room temperature | Chlorogenic acid, quercetin | Egg phosphatidylcholine, cholesterol | Thin-film hydration, extrusion | [70] |
| Ethosomes and PEVs | |||||
| Fraxinus angustifolia leaves and bark extract | Extraction in ethanol (1:4 w/v) under continuous stirring at room temperature, for 24 h. | Polyphenolic compounds c | Phospholipon50, Transcutol P, ethylene glycol triglycerides, fatty acids | Hydration, sonication | [27] |
| Glycerosomes, liposomes, gluglycerosomes, gel-gluglycerosomes, hyal-glulycerosomes | |||||
| Hypericum scruglii aerial parts extract | Marketed product | Naphthodianthrones d, phloroglucinols e, phenolic acids, flavonoids f, xanthones, terpenes | Phospholipid S75, glycerol, dextrin, gelatin, hyaluronan | Hydration, sonication | [45] |
| Entrapped PC | Source of PCs | Components of VNs | VNs’ Preparation | Ref. |
|---|---|---|---|---|
| Liposomes | ||||
|
Bromelain
extract | Ananas comosus | Egg phosphatidylcholine, cholesterol | Thin-film hydration | [94] |
| Madecassoside | Centella asiatica | Egg yolk lecithin, cholesterol | Thin-film hydration | [44] |
| Curcumin | Curcuma longa | Lecithin, cholesterol, propylene glycol | Hydration, sonication | [72] |
| Quercetin | Various species | Phosphatidylcholine, cholesterol | Thin-film hydration, sonication | [95] |
| Curcumin | Curcuma longa | Phospholipon 90G, oligochitosan (used for coating liposomes) | Thin-film hydration, sonication | [59] |
| Usnic acid | Lichens-Cladonia substellata | Phosphatidylcholine (Lipoid GMBH 75%) | Thin-film hydration | [31] |
| Nano-liposol | ||||
| Astaxanthin | Yeast, algae, and otheraquatic species | L-α-phosphatidylcholine from soybean | Modified emulsion evaporation method | [96] |
| Liposomal locked-in dendrimers | ||||
| Shikonin | Species of genera Alkanna, Lithospermum, Echium, Onosma, Anchusa | Egg phosphatidylcholine | Thin-film hydration | [97] |
| Santosomes | ||||
| Phycocianin | Blue-green algae | Santolina insularis essential oil, hydrogenated phosphatidylcholine, propylene glycol | Hydration, sonication | [60] |
| Liposomes and PEVs | ||||
| Quercetin | Fruits, vegetables a | Lipoid S75, PEG 400 | Hydration, sonication | [98] |
| Quercetin and curcumin | Various species | Lipoid S75, octyl-decyl polyglucoside, PEG 400 | Hydration, sonication | [25] |
| PEVs | ||||
| Oryzanol and alpha-bisabolol | Oryzanol-rice bran oil and alpha bisabolol-Chamomile essential oil | Phospholipid (Epikuron 200), penetration enhancers (labrasol, transcutol) | Thin-film hydration | [99] |
| Phytosomes | ||||
| Sinigrin | Brassicaceae family | l-α-phosphatidylcholine hydrogenated (soybean) | Thin-film hydration | [71] |
| Ethosomes | ||||
| Curcumin | Curcuma longa | Egg lechitin, cholesterol | Ethanol injection, sonication | [80] |
| Hyalurosomes | ||||
| Curcumin | Curcuma longa | Enriched soy phosphatidylcholine (Phospholipon 90G) | Hydration, sonication | [100] |
| Gel-core hyaluosomes | ||||
| Curcumin | Curcuma longa | Lipoid S100, Hyaluronic acid, Tween 80, Poloxamer 407 | Thin film evaporation, extrusion | [74] |
| ULs | ||||
| Asiaticoside | Centella asiatica | Fully saturated pure lecithin, saturated/unsaturated lecithins (Phospholipon 100G), sodium cholate | Thin-film hydration | [101] |
| Transferosomes, glycoltransferosomes | ||||
| Mangiferin | Various plants: mango leaves, fruits, by-products (e.g., peel, kernel seed) | Soy lecithin, glycerol, propylene glycol, Tween 80, mucin | Mangiferin dispersed in hydrating blend b sonicated, added in phospholipid and Tween 80, sonicated | [41] |
| Collagen-enriched transferosomes, glycerosomes, and glytransferosomes | ||||
| Oleuropein | Olive oil | Lipoid S75, collagen, Tween 80 | Direct sonication | [7] |
| Entrapped HEs | Active Compounds | Effect of HEs | Method of Extraction | Components of VNs | VNs’ Preparation | Ref. |
|---|---|---|---|---|---|---|
| Liposomes | ||||||
| Salvia triloba and Rosmarinus officinalis essential oils | Eucalyptol and camphor | Antioxidant, anti-inflammatory, antibacterial | Marketed product | Phospholipon 90G, cholesterol | Dry thin-film hydration | [102] |
| Citrus limon
var. pompia essential oil or raw citral | Terpenes-citral | Antibacterial activity | Citral/ essential oil-steam distillation | Lipoid S75 | Hydration, sonication | [36] |
| Cinnamon oil | Essential oil | Antimicrobial effect | Marketed product | Soy lecithin and cholesterol | Thin-film hydration | [52] |
| Glycerosomes | ||||||
| Rosmarinus officinalis leaves extract | Polyphenolic compounds a | Antioxidant, antimicrobial | 24 h stirring at room temperature with 70% ethanol | Phosphatidylcholine, glycerol | Hydration of phospholipids -Mozafari method | [51] |
| Liposomes, glycerosomes, PEVs | ||||||
| Thymus capitatus essential oil | Carvacrol | Antimicrobial effect | Extraction with circulatory Clevenger-type apparatus | Soy lecithin, water/glycerol, water/propylene glycol | Hydration, sonication, dialysis | [37] |
| Glycerosomes, hyalurosomes, gly-hyalurosomes | ||||||
| Citrus limon var. pompia fruits | Flavones b | Anti-inflammatory, antioxidant | Sonication, hydroethanolic extract | Lipoid S75, sodium hyaluronate | Hydration, sonication, dialysis | [26] |
| Ethosomes | ||||||
| Achillea millefolium antenna parts | Flavonoids, caffeic acid derivatives | Antibacterial, antioxidant, wound healing | Maceration with 70% ethanol | Phospholipid, ethanol, propylene glycol | Cold method, sonication | [103] |
| Phytosomes | ||||||
| Aloe vera dry extract c | Acemannan, β-sitosterol, glycosides (aloins), anthraquinone (aloe emodin) | Anti-inflammatory, antioxidant, cytoprotective, ↑ VEGF expression, ↑ NO synthesis | Marketed product | Soy lecithin | Antisolvent precipitation technique | [19] |
| Entrapped PC | Effect of PCs | Source of PCs | Components of VNs | VNs’ Preparation | Ref. |
|---|---|---|---|---|---|
| Invasomes | |||||
| Terpenoids a | Antibacterial, anti-inflammatory | Oil fraction of various plants | Soybean lecithin | Mixing the terpenoid with ethanol and phospholipids, extrusion | [28] |
| Liposomes and niosomes | |||||
| Resveratrol | Antioxidant | Grapes, nuts, berries | Soy phosphatidylcholine (Phospholipon 90G), glycerol monooleate, polyglyceryl-3 dioleate | Direct sonication | [104] |
| ULs | |||||
|
Ammonium glycyrrhizate | Anti-inflammatory | Glycyrrhiza glabra | Soy phosphatidylcholine (Phospholipon 90G) | Thin-film hydration | [105] |
| Entrapped HE or PC | In Vitro Release Study/In Vitro Skin Permeation Study | Cell Culture Studies | In Vivo Studies | Main Results | Ref. |
|---|---|---|---|---|---|
| Liposomes | |||||
| Carpobrotus edulis powder extract | No/No | No | Male Wistar-albino rats | Positive effects on the healing process in both incisional and excisional wound tissues | [90] |
| Pistacia lentiscus oil | No/Franz cells, pig skin | HaCaT, primary mouse embryonic fibroblasts (3T3) | No | Stability of the system in dispersion, ↑ the PCs in the skin, ↑ the ability to counteract damages induced by oxidative processes, beneficial effect on lesion regeneration and healing | [40] |
| Aloe vera leaf gel extract | No/No | NB1RGB cells, NHEK(F) cells | No | ↑ the cell proliferation and collagen synthesis, ↑ bioavailability of the HE, ↑ skin properties | [91] |
| Angelica sinensis and Astragali radix ratio 1:5 | No/No | No | Adult male Sprague-Dawley rats | ↑ therapeutic efficacy, ↑ wound closure; histological improvements, ↑ hydroxyproline levels; ↑ CD34, KI67, COL1α1, COL3α1 expression levels in wound granulation tissues compared to control groups in vivo, ↑ VEGF/PI3K/AKT and TGF-β/SMADS signaling pathways, which might contribute to the ability to ↑ full-thickness excisional wound healing in rats | [92] |
| Salvia aramiensis aerial part extract | Franz cells, dialysis membrane/No | L929 cell line (mouse fibroblast) | No | Strong antioxidant effect and potential wound-healing activity | [58] |
| Bromelain extract | No/No | No | Male Wistar rats | Absence of edema on the 14th day in animals treated with bromelain entrapped in nanocarriers | [94] |
| Curcumin | Dialysis, dialysis membranes/No | HDF | Male Wistar rats, New Zealand rabbits | Monodispersity and no vesicle aggregation even in long-term storage, considerable wound-healing properties in the early stage, antibacterial activity on burn wounds similar to SSD cream application | [72] |
| Quercetin | Diffusion cells system /No | No | No | Acceptable stability, biphasic pattern of drug release behavior | [95] |
| Curcumin | No/No | 3T3 cells (mouse fibroblasts) | Mice Mus musculus var. albino. | ↑ healing rates, ↑ scar treatment effects by incorporation in liposomes, compared to native curcumin, ↑ wound healing, ↑ scar treatment effect of curcumin liposomes compared to curcumin nanoplexes | [59] |
| Usnic acid | No/No | No | Male Rattus norvegicus albinus, Wistar lineage | ↑ burn healing, probably related to the modulation of the inflammatory response, epithelialization, and collagen formation | [31] |
| Salvia triloba and Rosmarinus officinalis E.O.s | Dialysis, permeable bag membrane/No | No | No | Preservation of antioxidant properties of E.O. constituents, ↓ anti-inflammatory activity of the pure E.O. ↑ activities for the liposome-encapsulating E.O. | [102] |
| Citrus limon var. pompia E.O. or raw citral | No/No | HaCaT | No | Citral-loaded liposomes more effective than pompia E.O. liposomes in counteracting the growth of bacteria (E. coli and S. aureus) and fungi (C. albicans) | [36] |
| Cinnamon oil | No/No | No | No | ↑ E.O. stability by liposome encapsulation ↑ antibacterial activity on MRSA and MRSA biofilms, ↑ antibiofilm activities and active time of liposome-encapsulating E.O. compared to free E.O. | [52] |
| Nano-liposol | |||||
| Astaxanthin | No/No | NIH 3T3 fibroblast cells | No | %EE ↑ of astaxanthin, good stability ↓ ROS, ↑ wound healing of fibroblasts without cytotoxicity | [96] |
| Liposomal locked-in dendrimers | |||||
| Shikonin | Dialysis, dialysis sacks/No | No | No | Adequate drug encapsulation, advantageous release profiles, satisfactory stability of liposomal formulations | [97] |
| Liposomes and hyalurosomes | |||||
| Glycyrrhiza glabra root extract | Dialysis, tubing Spectra/Por® membranes/No | Primary mouse embryonic fibroblasts (3T3) | Female CD-1 mice | ↑ effect of licorice extract, especially when delivered from hyalurosomes, ability to retain the extract components over time, ↑ in vitro and in vivo biological activity | [73] |
| Liposomes and hyalurosomes | |||||
| Neem oil (Azadirachta indica oil) | No/No | HaCaT and fibroblasts (3T3) | No | ↑ biocompatibility, effective protection of the skin cells from oxidative stress, ↑ efficacy of the oil; argan-hyalurosomes → more viscous, more suitable for skin application | [93] |
| Liposomes and PEVs | |||||
| Quercetin and curcumin | No/ Franz cells, pigskin | No | Female Hsd:ICR(CD-1) mice | ↑ Anti-inflammatory activity → inhibition the onset of skin wounds during TPA treatment; protective effect, more relevant in curcumin PEG-PEV formulation, ↑ drug bioavailability in the target tissue | [25] |
| Quercetin | No/No | 3T3 mouse fibroblasts, cellular uptake | Female cd-1 mice | In vitro studies—massive uptake and diffusion of PEVs in dermal fibroblasts; in vivo studies—amelioration of the tissue damage in TPA-inflamed skin, attenuation of edema and leukocyte infiltration, especially using 5% PEG-PEVs | [98] |
| PEVs | |||||
| Oryzanol and alpha-bisabolol | No/Franz cells, dorsal rat skin- ex vivo deposition/permeation | No | Male Wistar rats | Favorable properties in terms of size, charge, stability, skin deposition for studied PEVs; faster onset, superior wound healing for oryzanol and alpha-bisabolol-loaded PEVs compared to a marketed product; early signs of neo-angiogenesis and collagen production compared to groups treated with PEVs loaded with oryzanol only or the marketed product | [99] |
| ULs | |||||
| Asiaticoside | No/Franz cells, adult human skin | Primary human dermal fibroblasts | Rats—Male Sprague-Dawley | ↑ asiaticoside permeation through human SCE, ↑ intracellular drug delivery into primary human fibroblasts, significant ↑ collagen biosynthesis both in vitro and in vivo compared to the simple aqueous drug solution. | [101] |
| Ammonium glycyrrhizate | Franz cells, human SCE/synthetic membrane | No | Human volunteers | Biocompatible, deformable, allowed passage of ULs, delivery of A.G. in specific skin layers, pseudo-zero-order release kinetic, 50% of the entrapped drug is released in 24 h—potential depot effect of ULs in the skin; ↑ anti-inflammatory activity of drug of 15- and 30-fold compared to equivalent topical application of A.G. solution on healthy volunteers, time-dependent effect | [105] |
| Transferosomes | |||||
| Myrciariajaboticaba fruits peel | No/No | HaCaT | No | ↓ H2O2 damage induced in cells, ↑ wound healing in HaCaT | [30] |
| Transferosomes, glycoltransferosomes | |||||
| Mangiferin | No/No | Mouse embryonic fibroblasts (3T3) | Female CD-1 mice | Optimal performances of mangiferin delivery, ↑ wound-healing properties; cytocompatibility, protection of fibroblasts from oxidative stress, ↑ proliferation, migration, wound closure in vitro; protection of mouse skin from chemically induced injury in vivo, ↓ inflammatory infiltration; glycoltransferosomes and mucin-glycoltransferosomes, ↑ deposition of mangiferin in epidermis and dermis; ↑ ability to pass across the biological membranes | [41] |
| Collagen-enriched transferosomes, glycerosomes, and glytransferosomes | |||||
| Oleuropein | Dissolution tester/No | Mouse embryonic fibroblasts, keratinocytes | No | ↑ woundhealing efficacy, ↓ production of NO along with the damage induced by ROS, especially when cells were treated with collagen-enriched vesicles | [7] |
| Hyalurosomes | |||||
| Curcumin | No/ Franz cells, pigskin | HaCaT | No | ↑ physicochemical properties, ↑ biological performances by using sodium hyaluronate dispersion as a hydrating medium of phospholipids; immobilization of vesicles by hyaluronan → ↑ EE%, stability, rheological properties, local drug availability, therapeutic activity, in vivo fast healing process | [100] |
| Gel-core hyaluosome | |||||
| Curcumin | Dialysis/No | No | Female Sprague-Dawley rats | ↑ curcumin skin penetration, dermal localization, protection against degradation, ↑ healing, ↑ histological progress, ↓ scar formation | [74] |
| Glycerosomes, hyalurosomes, gly-hyalurosomes | |||||
| Citrus limon var. pompia fruits | No/No | Primary mouse embryonic fibroblasts (3T3), HaCaT | No | Prevention of oxidative damage; ↑ viability; ↑ biological activity by incorporation of the extract in vesicles, especially gly-hyalurosomes | [26] |
| Glycerosomes, liposomes, gluglycerosomes, gel-gluglycerosomes, hyal-glulycerosomes | |||||
| Hypericum scruglii aerial parts extract | Polycarbonate dialysis tubes/No | HaCaT | No | ↑ antioxidant activity, ↑cell uptake and wound-healing effects | [45] |
| Glycerosomes | |||||
| Rosmarinus officinalis leaves extract | No/No | No | No | ↑ antioxidant activity by liposomal entrapment, better stability of the extract during storage in comparison to free extract | [51] |
| Niosomes | |||||
| Hypericum perforatum flowering tops | USP dissolution test apparatus/No | No | Adult Mongrel dogs | ↓ inflammatory phase, ↑ early beginning of proliferative phase of wound healing, significant ↓ wound size compared to control and Panthenol® 2% cream | [21] |
| Calendula officinalis flowers and leaves extract | No/No | Vero cell line | No | ↑ wound-healing and protective effect against oxidative stress of Calendula officinalis methanolic extract after entrapment into Tween 60 niosomes | [50] |
| Santosomes | |||||
| Phycocianin | No/No | HaCaT, endothelial cells, cell uptake | Female CD-1 mice | Easy internalization of phycocyanin from santosomes by keratinocytes and endothelial cells, protective effect against H2O2 stress; in vivo studies—wound-healing activity | [60] |
| Phytosomes | |||||
| Moringa oleifera leaves extract | No/No | NHDF | No | Cytocompatibility; ↑ dose-dependent effect in wound closure of filtered Moringa oleifera compared to unfiltered samples and controls | [29] |
| Sinigrin | No/No | HaCaT | No | At the highest tested concentration, 0.14 mg/mL (42 h), the sinigrin–phytosome complex completely cured the wound, whereas the sinigrin alone displayed only 71% wound healing | [71] |
| Aloe vera dry extract | Dialysis/No | HUVECs | No | ↑ protective effects in suppressing MGO-induced endothelial cell cytotoxicity, anti-angiogenic effects, ↓ ROS overproduction and induction of oxidative stress; restorative effect on NO production; ↑ expression of several proangiogenic genes: VEGF-A, bFGF, KDR, Ang II, ↓ expression of anti-angiogenic such as Notch I, TGF-β | [19] |
| AuNP-phytosomes and liposomes | |||||
| Calendula officinalis flowers extract | No/No | NHDF, Vero cell line | No | ↑ Antioxidant and wound-healing activity, ↑ stability compared to free forms of each encapsulated material, plain liposome, phytosome form | [70] |
| Ethosomes | |||||
| Curcumin | No/No | No | Male Wistar rats | ↑ early stages of wound healing, antibacterial activity similar to SSD cream | [80] |
| Achillea millefolium antenna parts | No/Franz cells, rat skin | No | No | ↑ skin penetration compared to conventional gel | [103] |
| Ethosomes and PEVs | |||||
| Fraxinus angustifolia leaves and bark extract | No/No | HaCaT, cell uptake fluorescent vesicles | Male CD-1 mice | ↑ local bioavailability of the leaf phytocomplex, ↑ intracellular antioxidant activity in HaCaT, ↑ wound healing in TPA-mouse model for the simple extract ethanolic solution | [27] |
| Invasomes | |||||
| Terpenoids a | No/No | No | No | ↑ bioavailability of terpenoid-based drugs, strong selective activity against Gram-positive bacteria. | [28] |
| VN | Topical Delivery System | Entrapped HE or PC | Ref. |
|---|---|---|---|
| Niosomes | Gel-sodium carboxymethyl cellulose and hydroxyethylcellulose | Hypericum perforatum flowering tops | [21] |
| Liposomes | Thermosensitive gel | Angelica sinensis and Astragali radix ratio 1:5 | [92] |
| Liposomes | Membranes—CMC, acetylated arrowroot starch | Bromelain extract | [94] |
| Ethosomes | Carbopol gel | Curcumin | [80] |
| Liposomes | Collagen-based films | Usnic acid | [31] |
| Ethosomes | Gel-carbopol 940, hydroxyethylcellulose | Achillea millefolium antenna parts | [103] |
| Niosomes * | Sodium polyacrylate and carbomer mucoadhesive gel | Zea mays cobs and Clitoria ternatea petals | [85] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safta, D.A.; Bogdan, C.; Moldovan, M.L. Vesicular Nanocarriers for Phytocompounds in Wound Care: Preparation and Characterization. Pharmaceutics 2022, 14, 991. https://doi.org/10.3390/pharmaceutics14050991
Safta DA, Bogdan C, Moldovan ML. Vesicular Nanocarriers for Phytocompounds in Wound Care: Preparation and Characterization. Pharmaceutics. 2022; 14(5):991. https://doi.org/10.3390/pharmaceutics14050991
Chicago/Turabian StyleSafta, Diana Antonia, Cătălina Bogdan, and Mirela Liliana Moldovan. 2022. "Vesicular Nanocarriers for Phytocompounds in Wound Care: Preparation and Characterization" Pharmaceutics 14, no. 5: 991. https://doi.org/10.3390/pharmaceutics14050991
APA StyleSafta, D. A., Bogdan, C., & Moldovan, M. L. (2022). Vesicular Nanocarriers for Phytocompounds in Wound Care: Preparation and Characterization. Pharmaceutics, 14(5), 991. https://doi.org/10.3390/pharmaceutics14050991







