Evaluation of the Cytotoxic Activity and Anti-Migratory Effect of Berberine–Phytantriol Liquid Crystalline Nanoparticle Formulation on Non-Small-Cell Lung Cancer In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of BP-LCN Formulation
2.2. Optimization of Formulation and Process Parameters
2.3. Physicochemical Characterization of Formulation
2.4. Entrapment Efficiency (EE)
2.5. Morphology
2.6. In Vitro Release Study
2.7. Cell Culture
2.8. Measurement of Cell Proliferation
2.8.1. MTT Assay
2.8.2. Trypan Blue Staining
2.9. Wound Healing Assay
2.10. Colony Formation Assay
2.11. RNA Isolation and Real-Time qPCR
2.12. Protein Array
2.13. Statistical Analysis
3. Results
3.1. Preparation and Optimization of BP-LCNs
3.2. Physicochemical Characterization of Optimized Formulation
3.3. BP-LCNs Inhibited Proliferation of A549 Cells in a Dose-Dependent Manner
3.4. BP-LCNs Inhibited Migration of A549 Cells in a Dose-Dependent Manner
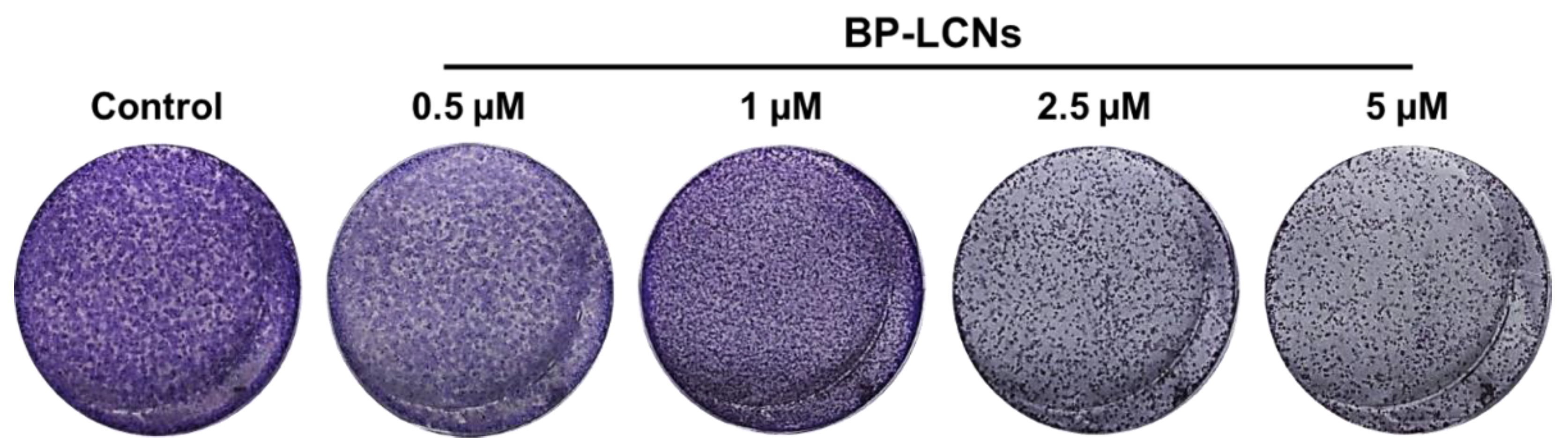
3.5. BP-LCNs Inhibited the Colony Formation of A549 Cells in a Dose-Dependent Manner
3.6. BP-LCNs Decreased the mRNA Levels of KRT18 and Increased mRNA Levels of PTEN and P53
3.7. BP-LCNs Decreased Expression of Proteins Associated with Proliferation in A549 Cells
3.8. BP-LCNs Decreased Expression of Proteins Associated with Migration or Metastasis in A549 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thai, A.A.; Solomon, B.J.; Sequist, L.V.; Gainor, J.F.; Heist, R.S. Lung cancer. Lancet 2021, 398, 535–554. [Google Scholar] [CrossRef]
- Malyla, V.; Paudel, K.R.; Shukla, S.D.; Donovan, C.; Wadhwa, R.; Pickles, S.; Chimankar, V.; Sahu, P.; Bielefeldt-Ohmann, H.; Bebawy, M.; et al. Recent advances in experimental animal models of lung cancer. Future Med. Chem. 2020, 12, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The biology and management of non-small cell lung cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Zappa, C.; Mousa, S.A. Non-small cell lung cancer: Current treatment and future advances. Transl. Lung Cancer Res. 2016, 5, 288–300. [Google Scholar] [CrossRef] [Green Version]
- Paudel, K.R.; Panth, N.; Pangeni, R.; Awasthi, R.; Chawla, V.; Mehta, M.; Tambuwala, M.M.; Hansbro, P.M. Targeting lung cancer using advanced drug delivery systems. In Targeting Chronic Inflammatory Lung Diseases Using Advanced Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2020; pp. 493–516. [Google Scholar]
- Hanna, N.; Johnson, D.; Temin, S.; Masters, G. Systemic Therapy for Stage IV Non–Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update Summary. J. Oncol. Pract. 2017, 13, 832–837. [Google Scholar] [CrossRef]
- Sun, Y.; Xun, K.; Wang, Y.; Chen, X. A systematic review of the anticancer properties of berberine, a natural product from Chinese herbs. Anti-Cancer Drugs 2009, 20, 757–769. [Google Scholar] [CrossRef] [Green Version]
- Maung, K.U.; Myo, K.; Nyunt, W.N.; Aye, K.; Tin, U. Clinical trial of berberine in acute watery diarrhoea. Br. Med. J. 1985, 291, 1601–1605. [Google Scholar] [CrossRef] [Green Version]
- Amin, A.H.; Subbaiah, T.V.; Abbasi, K.M. Berberine sulfate: Antimicrobial activity, bioassay, and mode of action. Can. J. Microbiol. 1969, 15, 1067–1076. [Google Scholar] [CrossRef]
- Rabbani, G.H.; Butler, T.; Knight, J.; Sanyal, S.C.; Alam, K. Randomized Controlled Trial of Berberine Sulfate Therapy for Diarrhea Due to Enterotoxigenic Escherichia coli and Vibrio cholerae. J. Infect. Dis. 1987, 155, 979–984. [Google Scholar] [CrossRef]
- Kowalewski, Z.; Kedzia, W.; Mirska, I. The effect of berberine sulfate on staphylococci. Arch. Immunol. Ther. Exp. 1972, 20, 353–360. [Google Scholar]
- Mirska, I.; Kedzia, H.; Kowalewski, Z.; Kedzia, W. The effect of berberine sulfate on healthy mice infected with Candida albicans. Arch. Immunol. Ther. Exp. 1972, 20, 921–929. [Google Scholar]
- Akhter, M.H.; Sabir, M.; Bhide, N.K. Anti-inflammatory effect of berberine in rats injected locally with cholera toxin. Indian J. Med. Res. 1977, 65, 133–141. [Google Scholar] [PubMed]
- Mo, C.; Wang, L.; Zhang, J.; Numazawa, S.; Tang, H.; Tang, X.; Han, X.; Li, J.; Yang, M.; Wang, Z.; et al. The Crosstalk between Nrf2 and AMPK Signal Pathways Is Important for the Anti-Inflammatory Effect of Berberine in LPS-Stimulated Macrophages and Endotoxin-Shocked Mice. Antioxid. Redox Signal. 2014, 20, 574–588. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-L.; Chi, C.-W.; Liu, T.-Y. The anti-inflammatory potential of berberine in vitro and in vivo. Cancer Lett. 2004, 203, 127–137. [Google Scholar] [CrossRef]
- El-Wahab, A.E.A.; Ghareeb, D.A.; Sarhan, E.E.; Abu-Serie, M.M.; El Demellawy, M.A. In vitro biological assessment of berberis vulgaris and its active constituent, berberine: Antioxidants, anti-acetylcholinesterase, anti-diabetic and anticancer effects. BMC Complement. Altern. Med. 2013, 13, 218. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Deng, J.; Cui, X.; Chen, Q.; Wang, W. Berberine exhibits antioxidative effects and reduces apoptosis of the vaginal epithelium in bacterial vaginosis. Exp. Ther. Med. 2019, 18, 2122–2130. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Geng, Y.-N.; Jiang, J.-D.; Kong, W.-J. Antioxidant and Anti-Inflammatory Activities of Berberine in the Treatment of Diabetes Mellitus. Evid.-Based Complement. Altern. Med. 2014, 2014, 289264. [Google Scholar] [CrossRef]
- Qi, H.-W.; Xin, L.-Y.; Xu, X.; Ji, X.-X.; Fan, L.-H. Epithelial-to-mesenchymal transition markers to predict response of Berberine in suppressing lung cancer invasion and metastasis. J. Transl. Med. 2014, 12, 22. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Jing, Z.; Lv, J.; Zhang, Z.; Lin, J.; Cao, X.; Zhao, Z.; Liu, P.; Mao, W. Berberine activates caspase-9/cytochrome c-mediated apoptosis to suppress triple-negative breast cancer cells in vitro and in vivo. Biomed. Pharmacother. 2017, 95, 18–24. [Google Scholar] [CrossRef]
- Jie, S.; Li, H.; Tian, Y.; Guo, D.; Zhu, J.; Gao, S.; Jiang, L. Berberine inhibits angiogenic potential of Hep G2 cell line through VEGF down-regulation in vitro. J. Gastroenterol. Hepatol. 2011, 26, 179–185. [Google Scholar] [CrossRef]
- Tian, Y.; Zhao, L.; Wang, Y.; Zhang, H.; Xu, D.; Zhao, X.; Li, Y.; Li, J. Berberine inhibits androgen synthesis by interaction with aldo-keto reductase 1C3 in 22Rv1 prostate cancer cells. Asian J. Androl. 2016, 18, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.-C.; Yu, C.-C.; Hsu, L.-S.; Chen, K.-S.; Su, M.-Y.; Chen, P.-N. Berberine Reverses Epithelial-to-Mesenchymal Transition and Inhibits Metastasis and Tumor-Induced Angiogenesis in Human Cervical Cancer Cells. Mol. Pharmacol. 2014, 86, 609–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, L.; Li, Z.; Ren, H.; Kong, L.; Chen, X.; Xiong, M.; Zhang, X.; Ning, B.; Li, J. Berberine inhibits non-small cell lung cancer cell growth through repressing DNA repair and replication rather than through apoptosis. Clin. Exp. Pharmacol. Physiol. 2022, 49, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.-Q.; Shi, J.-M.; Ding, Z.; Xia, Q.; Zheng, T.-S.; Ren, Y.-B.; Li, M.; Fan, L.-H. Berberine induces apoptosis in non-small-cell lung cancer cells by upregulating miR-19a targeting tissue factor. Cancer Manag. Res. 2019, 11, 9005–9015. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, F.; Jiang, S.; Liu, J.; Chen, X.; Zhang, S.; Zhao, H. Berberine hydrochloride inhibits cell proliferation and promotes apoptosis of non-small cell lung cancer via the suppression of the MMP2 and Bcl-2/Bax signaling pathways. Oncol. Lett. 2018, 15, 7409–7414. [Google Scholar] [CrossRef]
- Fu, L.; Chen, W.; Guo, W.; Wang, J.; Tian, Y.; Shi, D.; Zhang, X.; Qiu, H.; Xiao, X.; Kang, T.; et al. Berberine Targets AP-2/hTERT, NF-κB/COX-2, HIF-1α/VEGF and Cytochrome-c/Caspase Signaling to Suppress Human Cancer Cell Growth. PLoS ONE 2013, 8, e69240. [Google Scholar] [CrossRef] [Green Version]
- James, M.A.; Fu, H.; Liu, Y.; Chen, D.-R.; You, M. Dietary administration of berberine or Phellodendron amurense extract inhibits cell cycle progression and lung tumorigenesis. Mol. Carcinog. 2011, 50, 1–7. [Google Scholar] [CrossRef]
- Meng, M.; Geng, S.; Du, Z.; Yao, J.; Zheng, Y.; Li, Z.; Zhang, Z.; Li, J.; Duan, Y.; Du, G. Berberine and cinnamaldehyde together prevent lung carcinogenesis. Oncotarget 2017, 8, 76385–76397. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.-X.; Leung, E.L.-H.; Xie, Y.; Liu, Z.Q.; Zheng, Y.F.; Yao, X.J.; Lu, L.L.; Wu, J.L.; He, J.-X.; Yuan, Z.-W.; et al. Suppression of Lipogenesis via Reactive Oxygen Species–AMPK Signaling for Treating Malignant and Proliferative Diseases. Antioxid. Redox Signal. 2018, 28, 339–357. [Google Scholar] [CrossRef]
- Katiyar, S.K.; Meeran, S.M.; Katiyar, N.; Akhtar, S. p53 cooperates berberine-induced growth inhibition and apoptosis of non-small cell human lung cancer cells in vitro and tumor xenograft growth in vivo. Mol. Carcinog. 2009, 48, 24–37. [Google Scholar] [CrossRef]
- Zhang, C.; Sheng, J.; Li, G.; Zhao, L.; Wang, Y.; Yang, W.; Yao, X.; Sun, L.; Zhang, Z.; Cui, R. Effects of Berberine and Its Derivatives on Cancer: A Systems Pharmacology Review. Front. Pharmacol. 2019, 10, 1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Zhang, C.; Liang, W.; Zhang, Y.; Shen, Y.; Tian, X. Berberine regulates the Notch1/PTEN/PI3K/AKT/mTOR pathway and acts synergistically with 17-AAG and SAHA in SW480 colon cancer cells. Pharm. Biol. 2021, 59, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Qin, R.; Fang, Y.; Li, H. Berberine Sensitizes Human Ovarian Cancer Cells to Cisplatin Through miR-93/PTEN/Akt Signaling Pathway. Cell. Physiol. Biochem. 2015, 36, 956–965. [Google Scholar] [CrossRef]
- Chen, W.; Miao, Y.-Q.; Fan, D.-J.; Yang, S.-S.; Lin, X.; Meng, L.-K.; Tang, X. Bioavailability Study of Berberine and the Enhancing Effects of TPGS on Intestinal Absorption in Rats. AAPS PharmSciTech 2011, 12, 705–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baird, A.W.; Taylor, C.T.; Brayden, D.J. Non-antibiotic anti-diarrhoeal drugs: Factors affecting oral bioavailability of berberine and loperamide in intestinal tissue. Adv. Drug Deliv. Rev. 1997, 23, 111–120. [Google Scholar] [CrossRef]
- Madheswaran, T.; Kandasamy, M.; Bose, R.J.; Karuppagounder, V. Current potential and challenges in the advances of liquid crystalline nanoparticles as drug delivery systems. Drug Discov. Today 2019, 24, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Khursheed, R.; Dua, K.; Vishwas, S.; Gulati, M.; Jha, N.K.; Aldhafeeri, G.M.; Alanazi, F.G.; Goh, B.H.; Gupta, G.; Paudel, K.R.; et al. Biomedical applications of metallic nanoparticles in cancer: Current status and future perspectives. Biomed. Pharmacother. 2022, 150, 112951. [Google Scholar] [CrossRef]
- Ramanunny, A.K.; Wadhwa, S.; Gulati, M.; Vishwas, S.; Khursheed, R.; Paudel, K.R.; Gupta, S.; Porwal, O.; Alshahrani, S.M.; Jha, N.K.; et al. Journey of Alpinia galanga from kitchen spice to nutraceutical to folk medicine to nanomedicine. J. Ethnopharmacol. 2022, 291, 115144. [Google Scholar] [CrossRef]
- Imran, M.; Jha, L.A.; Hasan, N.; Shrestha, J.; Pangeni, R.; Parvez, N.; Mohammed, Y.; Jha, S.K.; Paudel, K.R. “Nanodecoys”—Future of drug delivery by encapsulating nanoparticles in natural cell membranes. Int. J. Pharm. 2022, 621, 121790. [Google Scholar] [CrossRef]
- Khursheed, R.; Paudel, K.R.; Gulati, M.; Vishwas, S.; Jha, N.K.; Hansbro, P.M.; Oliver, B.G.; Dua, K.; Singh, S.K. Expanding the arsenal against pulmonary diseases using surface-functionalized polymeric micelles: Breakthroughs and bottlenecks. Nanomedicine 2022. [Google Scholar] [CrossRef]
- Kabir, M.T.; Rahman, M.H.; Akter, R.; Behl, T.; Kaushik, D.; Mittal, V.; Pandey, P.; Akhtar, M.F.; Saleem, A.; Albadrani, G.M.; et al. Potential Role of Curcumin and Its Nanoformulations to Treat Various Types of Cancers. Biomolecules 2021, 11, 392. [Google Scholar] [CrossRef] [PubMed]
- Solanki, N.; Mehta, M.; Chellappan, D.K.; Gupta, G.; Hansbro, N.G.; Tambuwala, M.M.; Aljabali, A.A.; Paudel, K.R.; Liu, G.; Satija, S.; et al. Antiproliferative effects of boswellic acid-loaded chitosan nanoparticles on human lung cancer cell line A549. Future Med. Chem. 2020, 12, 2019–2034. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, T.; Maishu, S.P.; Akter, R.; Rahman, M.H.; Akhtar, M.F.; Saleem, A.; Bin-Jumah, M.; Kamel, M.; Abdel-Latif, M.A.; Abdel-Daim, M.M. A Review on Natural Sources Derived Protein Nanoparticles as Anticancer Agents. Curr. Top. Med. Chem. 2021, 21, 1014–1026. [Google Scholar] [CrossRef] [PubMed]
- Hardwick, J.; Taylor, J.; Mehta, M.; Satija, S.; Paudel, K.R.; Hansbro, P.M.; Chellappan, D.K.; Bebawy, M.; Dua, K. Targeting Cancer using Curcumin Encapsulated Vesicular Drug Delivery Systems. Curr. Pharm. Des. 2021, 27, 2–14. [Google Scholar] [CrossRef]
- Imran, M.; Paudel, K.R.; Jha, S.K.; Hansbro, P.M.; Dua, K.; Mohammed, Y. Dressing of multifunctional nanoparticles with natural cell-derived membranes for the superior chemotherapy. Nanomedicine 2022. [Google Scholar] [CrossRef]
- Paudel, K.R.; Panth, N.; Manandhar, B.; Singh, S.K.; Gupta, G.; Wich, P.R.; Nammi, S.; MacLoughlin, R.; Adams, J.; Warkiani, M.E. Attenuation of Cigarette-Smoke-Induced Oxidative Stress, Senescence, and Inflammation by Berberine-Loaded Liquid Crystalline Nanoparticles: In Vitro Study in 16HBE and RAW264.7 Cells. Antioxidants 2022, 11, 873. [Google Scholar] [CrossRef]
- Wadhwa, R.; Paudel, K.R.; Chin, L.H.; Hon, C.M.; Madheswaran, T.; Gupta, G.; Panneerselvam, J.; Lakshmi, T.; Singh, S.K.; Gulati, M.; et al. Anti-inflammatory and anticancer activities of Naringenin-loaded liquid crystalline nanoparticles in vitro. J. Food Biochem. 2021, 45, e13572. [Google Scholar] [CrossRef]
- Paudel, K.R.; Wadhwa, R.; Tew, X.N.; Lau, N.J.X.; Madheswaran, T.; Panneerselvam, J.; Zeeshan, F.; Kumar, P.; Gupta, G.; Anand, K.; et al. Rutin loaded liquid crystalline nanoparticles inhibit non-small cell lung cancer proliferation and migration in vitro. Life Sci. 2021, 276, 119436. [Google Scholar] [CrossRef]
- Mehta, M.; Malyla, V.; Paudel, K.R.; Chellappan, D.K.; Hansbro, P.M.; Oliver, B.G.; Dua, K. Berberine loaded liquid crystalline nanostructure inhibits cancer progression in adenocarcinomic human alveolar basal epithelial cells in vitro. J. Food Biochem. 2021, 45, e13954. [Google Scholar] [CrossRef]
- Paudel, K.R.; Mehta, M.; Yin, G.H.S.; Yen, L.L.; Malyla, V.; Patel, V.K.; Panneerselvam, J.; Madheswaran, T.; MacLoughlin, R.; Jha, N.K.; et al. Berberine-loaded liquid crystalline nanoparticles inhibit non-small cell lung cancer proliferation and migration in vitro. Environ. Sci. Pollut. Res. Int. 2022. [Google Scholar] [CrossRef]
- Tran, N.; Mulet, X.; Hawley, A.M.; Fong, C.; Zhai, J.; Le, T.C.; Ratcliffe, J.; Drummond, C.J. Manipulating the Ordered Nanostructure of Self-Assembled Monoolein and Phytantriol Nanoparticles with Unsaturated Fatty Acids. Langmuir 2018, 34, 2764–2773. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, I.R.; Immich, M.F.; Lundberg, D.; Poletto, F.; Loh, W. Physiological neutral pH drives a gradual lamellar-to-reverse cubic-to-reverse hexagonal phase transition in phytantriol-based nanoparticles. Colloids Surf. B Biointerfaces 2019, 177, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Fong, C.; Tran, N.; Drummond, C.J. Non-Lamellar Lyotropic Liquid Crystalline Lipid Nanoparticles for the Next Generation of Nanomedicine. ACS Nano 2019, 13, 6178–6206. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ma, P.; Gui, S. Cubic and Hexagonal Liquid Crystals as Drug Delivery Systems. Biomed. Res. Int. 2014, 2014, 815981. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, J.; Liu, W.; Yin, Y.; Qian, D.; Zhang, H.; Shi, B.; Li, C.; Zhu, J.; Zhang, L.; et al. Cytokeratin 18 knockdown decreases cell migration and increases chemosensitivity in non-small cell lung cancer. J. Cancer Res. Clin. Oncol. 2016, 142, 2479–2487. [Google Scholar] [CrossRef]
- Mehta, M.; Dhanjal, D.S.; Paudel, K.R.; Singh, B.; Gupta, G.; Rajeshkumar, S.; Thangavelu, L.; Tambuwala, M.M.; Bakshi, H.A.; Chellappan, D.K.; et al. Cellular signalling pathways mediating the pathogenesis of chronic inflammatory respiratory diseases: An update. Inflammopharmacology 2020, 28, 795–817. [Google Scholar] [CrossRef]
- Guo, R.; Zhang, Y.; Li, X.; Song, X.; Li, D.; Zhao, Y. Discovery of ERBB3 inhibitors for non-small cell lung cancer (NSCLC) via virtual screening. J. Mol. Model. 2016, 22, 135. [Google Scholar] [CrossRef]
- Zhu, C.; Wei, Y.; Wei, X. AXL receptor tyrosine kinase as a promising anti-cancer approach: Functions, molecular mechanisms and clinical applications. Mol. Cancer 2019, 18, 153. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Rabbani, Z.N.; Vollmer, R.T.; Schreiber, E.-G.; Oosterwijk, E.; Dewhirst, M.W.; Vujaskovic, Z.; Kelley, M.J. Carbonic Anhydrase IX in Early-Stage Non–Small Cell Lung Cancer. Clin. Cancer Res. 2004, 10, 7925–7933. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Mao, Y.; Chen, C.; Zhu, F.; Lu, W.; Ma, H. Expression patterns and clinical significances of ENO2 in lung cancer: An analysis based on Oncomine database. Ann. Transl. Med. 2020, 8, 639. [Google Scholar] [CrossRef]
- Bethune, G.; Bethune, D.; Ridgway, N.; Xu, Z. Epidermal growth factor receptor (EGFR) in lung cancer: An overview and update. J. Thorac. Dis. 2010, 2, 48–51. [Google Scholar] [PubMed]
- Arechavaleta-Velasco, F.; Perez-Juarez, C.E.; Gerton, G.L.; Diaz-Cueto, L. Progranulin and its biological effects in cancer. Med. Oncol. 2017, 34, 194. [Google Scholar] [CrossRef] [PubMed]
- Shikada, Y.; Yonemitsu, Y.; Koga, T.; Onimaru, M.; Nakano, T.; Okano, S.; Sata, S.; Nakagawa, K.; Yoshino, I.; Maehara, Y.; et al. Platelet-Derived Growth Factor-AA Is an Essential and Autocrine Regulator of Vascular Endothelial Growth Factor Expression in Non-Small Cell Lung Carcinomas. Cancer Res. 2005, 65, 7241–7248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, X.; Liu, J.; Zhong, X.; Li, X.; Zhang, Q. Role of AXL expression in non-small cell lung cancer. Oncol. Lett. 2016, 12, 5085–5091. [Google Scholar] [CrossRef] [Green Version]
- Uemura, Y.; Kobayashi, M.; Nakata, H.; Kubota, T.; Bandobashi, K.; Saito, T.; Taguchi, H. Effects of GM-CSF and M-CSF on tumor progression of lung cancer: Roles of MEK1/ERK and AKT/PKB pathways. Int. J. Mol. Med. 2006, 18, 365–373. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhang, X.; Zhao, X.; Jiang, M.; Gu, M.; Wang, Z.; Yue, W. DKK1 promotes migration and invasion of non–small cell lung cancer via β-catenin signaling pathway. Tumor Biol. 2017, 39, 1010428317703820. [Google Scholar] [CrossRef] [Green Version]
- Gong, F.; Peng, X.; Luo, C.; Shen, G.; Zhao, C.; Zou, L.; Li, L.; Sang, Y.; Zhao, Y.; Zhao, X. Cathepsin B as a potential prognostic and therapeutic marker for human lung squamous cell carcinoma. Mol. Cancer 2013, 12, 125. [Google Scholar] [CrossRef] [Green Version]
- Tan, G.-J.; Peng, Z.-K.; Lu, J.-P.; Tang, F.-Q. Cathepsins mediate tumor metastasis. World J. Biol. Chem. 2013, 4, 91–101. [Google Scholar] [CrossRef]
- Shao, F.C.; Zhang, R.F.; Dong, L.L.; Ying, K.J. Overexpression of Gelsolin-Like Actin-Capping Protein Is Associated with Progression of Lung Adenocarcinoma. Tohoku J. Exp. Med. 2011, 225, 95–101. [Google Scholar] [CrossRef] [Green Version]
- Um, H.-D. Bcl-2 family proteins as regulators of cancer cell invasion and metastasis: A review focusing on mitochondrial respiration and reactive oxygen species. Oncotarget 2016, 7, 5193–5203. [Google Scholar] [CrossRef] [Green Version]
- Hung, J.Y.; Horn, D.; Woodruff, K.; Prihoda, T.; LeSaux, C.; Peters, J.; Tio, F.; Abboud-Werner, S.L. Colony-stimulating factor 1 potentiates lung cancer bone metastasis. Lab. Investig. 2014, 94, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Paudel, K.R.; Karki, R.; Kim, D.-W. Cepharanthine inhibits in vitro VSMC proliferation and migration and vascular inflammatory responses mediated by RAW264.7. Toxicol. In Vitro 2016, 34, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Paudel, K.R.; Lee, U.-W.; Kim, D.-W. Chungtaejeon, a Korean fermented tea, prevents the risk of atherosclerosis in rats fed a high-fat atherogenic diet. J. Integr. Med. 2016, 14, 134–142. [Google Scholar] [CrossRef]
- Lee, H.-H.; Paudel, K.R.; Kim, D.-W. Terminalia chebula Fructus Inhibits Migration and Proliferation of Vascular Smooth Muscle Cells and Production of Inflammatory Mediators in RAW 264.7. Evid.-Based Complement. Altern. Med. 2015, 2015, 502182. [Google Scholar] [CrossRef] [Green Version]
- Manandhar, B.; Kim, H.J.; Rhyu, D.Y. Caulerpa okamurae extract attenuates inflammatory interaction, regulates glucose metabolism and increases insulin sensitivity in 3T3-L1 adipocytes and RAW 264.7 macrophages. J. Integr. Med. 2020, 18, 253–264. [Google Scholar] [CrossRef]
- Jun, M.Y.; Karki, R.; Paudel, K.R.; Sharma, B.R.; Adhikari, D.; Kim, D.-W. Alkaloid rich fraction from Nelumbo nucifera targets VSMC proliferation and migration to suppress restenosis in balloon-injured rat carotid artery. Atherosclerosis 2016, 248, 179–189. [Google Scholar] [CrossRef]
- Singh, M.; Bhowal, R.; Vishwakarma, R.; Chopra, D. Assessing the impact on aqueous solubility of berberine chloride via co-crystallization with different stoichiometric ratios of pyromellitic dianhydride. J. Mol. Struct. 2020, 1200, 127086. [Google Scholar] [CrossRef]
- Devkota, H.P.; Paudel, K.R.; Hassan, M.; Dirar, A.I.; Das, N.; Adhikari-Devkota, A.; Echeverría, J.; Logesh, R.; Jha, N.K.; Singh, S.K.; et al. Bioactive Compounds from Zingiber montanum and Their Pharmacological Activities with Focus on Zerumbone. Appl. Sci. 2021, 11, 10205. [Google Scholar] [CrossRef]
- Singh, N.; Sharma, B. Toxicological Effects of Berberine and Sanguinarine. Front. Mol. Biosci. 2018, 5, 21. [Google Scholar] [CrossRef] [Green Version]
- Küpeli, E.; Koşar, M.; Yeşilada, E.; Baser, K.H.C. A comparative study on the anti-inflammatory, antinociceptive and antipyretic effects of isoquinoline alkaloids from the roots of Turkish Berberis species. Life Sci. 2002, 72, 645–657. [Google Scholar] [CrossRef]
- Kwon, I.H.; Choi, H.S.; Shin, K.S.; Lee, B.K.; Lee, C.K.; Hwang, B.Y.; Lim, S.C. Effects of berberine on 6-hydroxydopamine-induced neurotoxicity in PC12 cells and a rat model of Parkinson’s disease. Neurosci. Lett. 2010, 486, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, S.; Li, Y. KRT18 is correlated with the malignant status and acts as an oncogene in colorectal cancer. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, C.Y.-C.; Cheng, C.-C.; Lai, Y.-S.; Liu, Y.-H. Cytokeratin 18-associated Histone 3 Modulation in Hepatocellular Carcinoma: A Mini Review. Cancer Genom. Proteom. 2017, 14, 219–223. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.L.; Chan, Y.; Candasamy, M.; Chellian, J.; Madheswaran, T.; Sakthivel, L.P.; Patel, V.K.; Chakraborty, A.; MacLoughlin, R.; Kumar, D.; et al. Unravelling the molecular mechanisms underlying chronic respiratory diseases for the development of novel therapeutics via in vitro experimental models. Eur. J. Pharmacol. 2022, 919, 174821. [Google Scholar] [CrossRef]












| Formulation | Conc. of Phytantriol (% w/w) | Conc. of P407 (% w/w) | Berberine Hydrochloride (% w/w) | Water | Sonication Amplitude (%) | PS (nm) | EE (%) |
|---|---|---|---|---|---|---|---|
| BP-LCN1 | 1 | 10 | 0.1 | Up to 5 mL | 20 | 288 ± 1.8 | 62 ± 0.6 |
| BP-LCN2 | 2 | 10 | 0.1 | Up to 5 mL | 20 | 460 ± 2.0 | 75 ± 0.7 |
| BP-LCN3 | 4 | 10 | 0.1 | Up to 5 mL | 20 | 566 ± 2.5 | 75 ± 0.7 |
| BP-LCN4 | 1 | 10 | 0.1 | Up to 5 mL | 40 | 185 ± 1.2 | 74 ± 0.7 |
| BP-LCN5 | 2 | 10 | 0.1 | Up to 5 mL | 40 | 228 ± 1.7 | 75 ± 0.7 |
| BP-LCN6 | 4 | 10 | 0.1 | Up to 5 mL | 40 | 324 ± 2.0 | 84 ± 0.9 |
| BP-LCN7 | 1 | 10 | 0.1 | Up to 5 mL | 80 | 247 ± 1.8 | 76 ± 0.7 |
| BP-LCN8 | 2 | 10 | 0.1 | Up to 5 mL | 80 | 209 ± 1.7 | 74 ± 0.7 |
| BP-LCN9 | 4 | 10 | 0.1 | Up to 5 mL | 80 | 369 ± 2.0 | 84 ± 0.9 |
| Source | Sum of Squares | Df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 1.420 × 105 | 5 | 28,409.95 | 18.34 | 0.0007 | Significant |
| A—Conc. of lipid | 46,542.57 | 1 | 46,542.57 | 30.04 | 0.0009 | Significant |
| B—Sonication amplitude | 40,264.63 | 1 | 40,264.63 | 25.99 | 0.0014 | Significant |
| AB | 2365.18 | 1 | 2365.18 | 1.53 | 0.2565 | Not significant |
| A² | 0.0057 | 1 | 0.0057 | 3.697 × 10−6 | 0.9985 | Not significant |
| B² | 49,193.02 | 1 | 49,193.02 | 31.75 | 0.0008 | Significant |
| Residual | 10,845.03 | 7 | 1549.29 | |||
| Lack of Fit | 10,845.03 | 3 | 3615.01 | |||
| Pure Error | 0.0000 | 4 | 0.0000 | |||
| Cor Total | 1.529 × 105 | 12 |
| Source | Sum of Squares | Df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 216.72 | 2 | 108.36 | 9.39 | 0.0051 | Significant |
| A—Conc. of lipid | 158.94 | 1 | 158.94 | 13.78 | 0.0040 | Significant |
| B—Sonication amplitude | 53.71 | 1 | 53.71 | 4.66 | 0.0563 | Significant |
| Residual | 115.37 | 10 | 11.54 | |||
| Lack of Fit | 115.37 | 6 | 19.23 | |||
| Pure Error | 0.0000 | 4 | 0.0000 | |||
| Cor Total | 332.09 | 12 |
| Parameters | BP-LCNs |
|---|---|
| Z-average (nm) | 223 ± 1.8 |
| Polydispersity Index (PdI) | 0.34 ± 0.01 |
| Zeta potential (mV) | −15.7 ± 0.1 |
| Encapsulation efficiency (%) | 75 ± 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alnuqaydan, A.M.; Almutary, A.G.; Azam, M.; Manandhar, B.; Yin, G.H.S.; Yen, L.L.; Madheswaran, T.; Paudel, K.R.; Hansbro, P.M.; Chellappan, D.K.; et al. Evaluation of the Cytotoxic Activity and Anti-Migratory Effect of Berberine–Phytantriol Liquid Crystalline Nanoparticle Formulation on Non-Small-Cell Lung Cancer In Vitro. Pharmaceutics 2022, 14, 1119. https://doi.org/10.3390/pharmaceutics14061119
Alnuqaydan AM, Almutary AG, Azam M, Manandhar B, Yin GHS, Yen LL, Madheswaran T, Paudel KR, Hansbro PM, Chellappan DK, et al. Evaluation of the Cytotoxic Activity and Anti-Migratory Effect of Berberine–Phytantriol Liquid Crystalline Nanoparticle Formulation on Non-Small-Cell Lung Cancer In Vitro. Pharmaceutics. 2022; 14(6):1119. https://doi.org/10.3390/pharmaceutics14061119
Chicago/Turabian StyleAlnuqaydan, Abdullah M., Abdulmajeed G. Almutary, Mohd Azam, Bikash Manandhar, Geena Hew Suet Yin, Lee Li Yen, Thiagarajan Madheswaran, Keshav Raj Paudel, Philip M. Hansbro, Dinesh Kumar Chellappan, and et al. 2022. "Evaluation of the Cytotoxic Activity and Anti-Migratory Effect of Berberine–Phytantriol Liquid Crystalline Nanoparticle Formulation on Non-Small-Cell Lung Cancer In Vitro" Pharmaceutics 14, no. 6: 1119. https://doi.org/10.3390/pharmaceutics14061119
APA StyleAlnuqaydan, A. M., Almutary, A. G., Azam, M., Manandhar, B., Yin, G. H. S., Yen, L. L., Madheswaran, T., Paudel, K. R., Hansbro, P. M., Chellappan, D. K., & Dua, K. (2022). Evaluation of the Cytotoxic Activity and Anti-Migratory Effect of Berberine–Phytantriol Liquid Crystalline Nanoparticle Formulation on Non-Small-Cell Lung Cancer In Vitro. Pharmaceutics, 14(6), 1119. https://doi.org/10.3390/pharmaceutics14061119








