Strategies and Mechanism in Reversing Intestinal Drug Efflux in Oral Drug Delivery
Abstract
:1. Introduction
2. Intestinal Efflux Transporters
2.1. P-gp
2.2. MRPs
2.3. BCRP
| Transporters | Substrates | Inhibitors | Refs. |
|---|---|---|---|
| P-gp | digoxin, rhodamine-123, verapamil, rapamycin, cimetidine, silybin, atenolol, citalopram, mitoxantrone, doxorubicin, fexofenadine, rhodamine 123, aliskiren, betrixaban, celiprolol, paclitaxel and vincristine. | verapamil, cyclosporine A, elacridar, tariquidar, zosuquidar, alkaloids, flavonoids, pyrimidine aminobenzene derivatives, 4-indolyl quinazoline derivatives, quercetin, ivermectin, Royleanone, HM30181A, thilphenylbenzofuran derivatives, encequidar, CBT-1®. | [14,25,26,27,28,29,30,31,32,33,34,35,36] |
| MRP2 | Valsartan, pravastatin, cisplatin, silybin, doxorubicin, sulfobromophthalein, dinitrophenyl-s-glutathione, calcein, methotrexate, ezetimibe glucuronide, resveratrol, etoposide, statins, and fexofenadine. | MK571, indomethacin, cyclosporin A, Nomegestrol acetate sulfated metabolites, indomethacin, ivermectin. | [31,37,38,39,40,41,42,43,44,45,46,47] |
| ABCG2 | 5-FU, silybin, zidovudine, cimetidine, nilotinib, bisantrene, ciprofloxacin, resveratrol, doxorubicin, mitoxantrone and topotecan. | pyrimidine aminobenzene derivatives, reserpine, Ko143, reserpine, ivermectin. | [38,41,43,44,45,48,49,50,51] |
3. Models
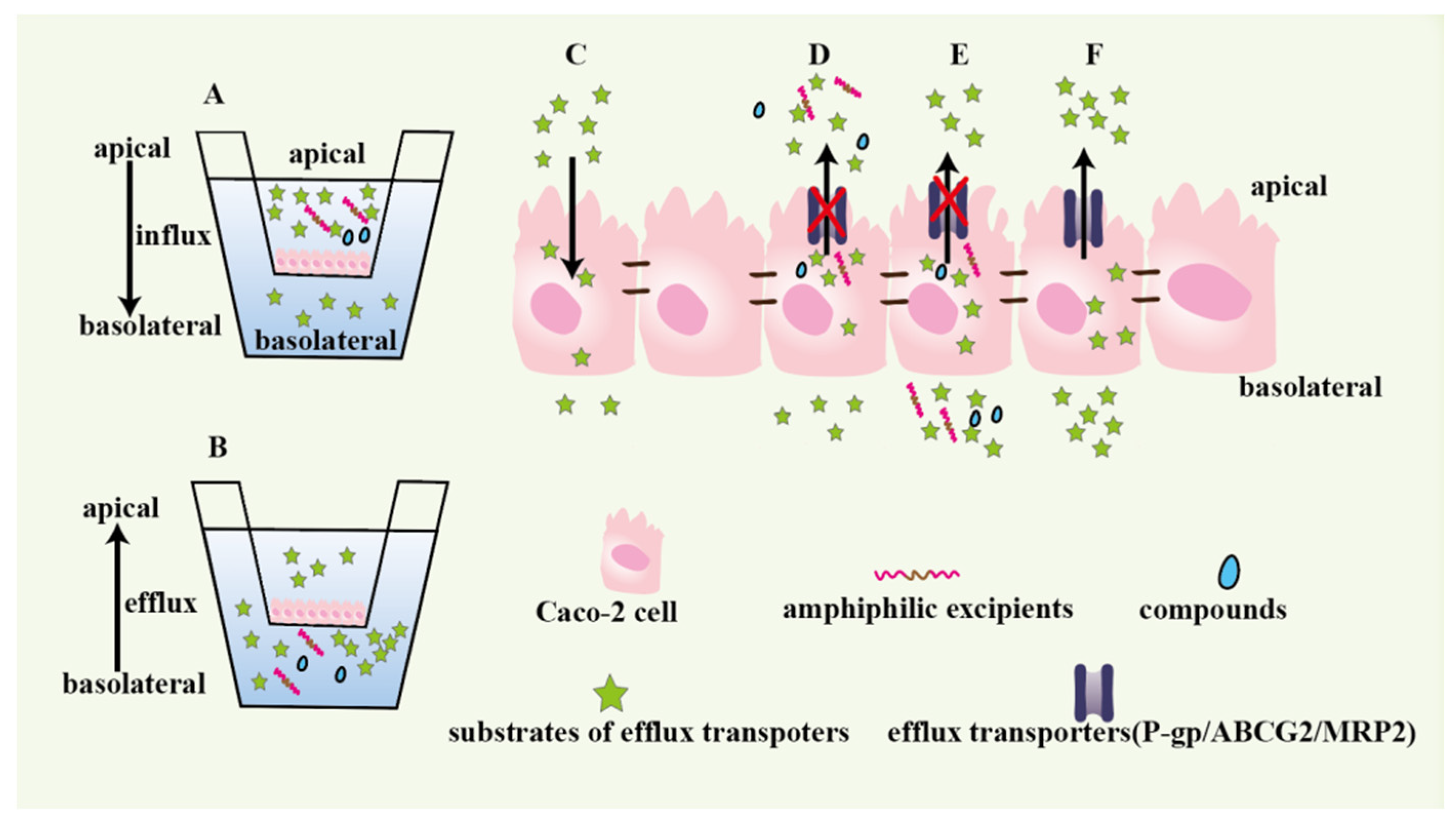
3.1. Cell Models
3.1.1. Caco-2 Monolayer
3.1.2. MDCK Monolayer
3.2. Everted Gut Sac Model
4. Excipients to Inhibit Efflux Transporters Activity
4.1. TPGS
4.2. β-Cyclodextrin
4.3. Pluronic
4.4. PEGs
4.5. Others
5. Strategies to Reverse Inhibit Intestinal Drug Efflux
5.1. Silencing Transporters
5.2. Inhibit Efflux Transporters by Excipients
5.3. Co-Delivery
- effectively inhibiting the activity of several efflux transporters in cell and animal models;
- Being harmless for healthy cells;
- Having not influence metabolic enzymes activity;
- Efficiently inhibiting efflux transporters activities in clinical trials.
6. Mechanism of Reversing Intestinal Drug Efflux
6.1. Liposomes
6.2. Solid Lipid Nanoparticles
6.3. Nanoemulsion and Self-Emulsified Drug Delivery Systems
6.4. Polymer Micelles
6.5. Nanoparticles
6.6. Nanocrystals
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sims, K.R.; Yuan, L. Enhanced design and formulation of nanoparticles for anti-biofilm drug delivery. Nanoscale 2019, 11, 219–236. [Google Scholar] [CrossRef] [PubMed]
- Okagu, O.D.; Verma, O. Utilization of insect proteins to formulate nutraceutical delivery systems: Encapsulation and release of curcumin using mealworm protein-chitosan nano-complexes. Int. J. Biol. Macromol. 2020, 151, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qiu, L.Z. Evaluation of intestinal permeation enhancement with carboxymethyl chitosan-rhein polymeric micelles for oral delivery of paclitaxel. Int. J. Pharm. 2020, 573, 118840. [Google Scholar] [CrossRef] [PubMed]
- Negi, L.M.; Tariq, M. Nano scale self-emulsifying oil based carrier system for improved oral bioavailability of camptothecin derivative by P-Glycoprotein modulation. Colloids Surf. 2013, 111, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, C.J.; Tocchetti, G.N. Acute regulation of apical ABC transporters in the gut. Potential influence on drug bioavailability. Pharmacol. Res. 2021, 163, 105251. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Yong, Y. Therapeutic Nanoparticles Based on Curcumin and Bamboo Charcoal Nanoparticles for Chemo-Photothermal Synergistic Treatment of Cancer and Radioprotection of Normal Cells. ACS Appl. Mater. Interfaces 2017, 9, 14281–14291. [Google Scholar] [CrossRef]
- Ji, H.; Tang, J.L. Curcumin-loaded solid lipid nanoparticles with Brij78 and TPGS improved in vivo oral bioavailability and in situ intestinal absorption of curcumin. Drug Deliv. 2016, 23, 459–470. [Google Scholar] [CrossRef]
- Gurjar, R.; Chan, C.Y.S. Inhibitory Effects of Commonly Used Excipients on P-Glycoprotein in Vitro. Mol. Pharm. 2018, 15, 4835–4842. [Google Scholar] [CrossRef]
- Patil, S.; Choudhary, B. Enhanced oral bioavailability and anticancer activity of novel curcumin loaded mixed micelles in human lung cancer cells. Phytomedicine 2015, 22, 1103–1111. [Google Scholar] [CrossRef]
- Mu, Y.; Fu, Y.M. Multifunctional quercetin conjugated chitosan nano-micelles with P-gp inhibition and permeation enhancement of anticancer drug. Carbohydr. Polym. 2019, 203, 10–18. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, S.W. Quercetin and doxorubicin co-delivery using mesoporous silica nanoparticles enhance the efficacy of gastric carcinoma chemotherapy. Int. J. Nanomed. 2018, 13, 5113–5126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Arranz, E. Mucus interactions with liposomes encapsulating bioactives: Interfacial tensiometry and cellular uptake on Caco-2 and cocultures of Caco-2/HT29-MTX. Food Res. Int. 2017, 92, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.; Wang, L.X. Oral Nanomedicine Based on Multicomponent Microemulsions for Drug-Resistant Breast Cancer Treatment. Biomacromolecules 2017, 18, 1268–1280. [Google Scholar] [CrossRef] [PubMed]
- Qu, G.; Hou, S.Y. Self-assembled micelles based on N-octyl-N’-phthalyl-O-phosphoryl chitosan derivative as an effective oral carrier of paclitaxel. Carbohydr. Polym. 2019, 207, 428–439. [Google Scholar] [CrossRef]
- Silva, R.; Vinia-Boas, V. Modulation of P-glycoprotein efflux pump: Induction and activation as a therapeutic strategy. Pharmacol. Ther. 2015, 149, 1–123. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, T.P. The Circadian Clock Gene Bmal1 Controls Intestinal Exporter MRP2 and Drug Disposition. Theranostics 2019, 9, 2754–2767. [Google Scholar] [CrossRef]
- Hira, D.; Terada, T. BCRP/ABCG2 and high-alert medications: Biochemical, pharmacokinetic, pharmacogenetic, and clinical implications. Biochem. Pharmacol. 2018, 147, 201–210. [Google Scholar] [CrossRef]
- Sajid, A.; Lusvarghi, S. Reversing the direction of drug transport mediated by the human multidrug transporter P-glycoprotein. Proc. Natl. Acad. Sci. USA 2020, 117, 29609–29617. [Google Scholar] [CrossRef]
- Yano, K.; Tomono, T. Ogihara, Advances in Studies of P-Glycoprotein and Its Expression Regulators. Biol. Pharm. Bull. 2018, 41, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Mai, Y.; Gavins, F.K.H. A Non-Nutritive Feeding Intervention Alters the Expression of Efflux Transporters in the Gastrointestinal Tract. Pharmaceutics 2021, 13, 1789. [Google Scholar] [CrossRef]
- Drozdzik, M.; Busch, D. Protein Abundance of Clinically Relevant Drug Transporters in the Human Liver and Intestine: A Comparative Analysis in Paired Tissue Specimens. Clin. Pharmacol. Ther. 2019, 105, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.G.; Geier, A. ABC of oral bioavailability: Transporters as gatekeepers in the gut. Gut 2003, 52, 1788–1795. [Google Scholar] [CrossRef] [PubMed]
- Zecchinati, F.; Barranco, M.M. Reversion of down-regulation of intestinal multidrug resistance-associated protein 2 in fructose-fed rats by geraniol and vitamin C: Potential role of inflammatory response and oxidative stress. J. Nutr. Biochem. 2019, 68, 7–15. [Google Scholar] [CrossRef]
- Durmus, S.; Hendrikx, J.J. Apical ABC transporters and cancer chemotherapeutic drug disposition. Adv. Cancer Res. 2015, 125, 1–41. [Google Scholar]
- Yin, T.; Zhang, Y. The efficiency and mechanism of N-octyl-O, N-carboxymethyl chitosan-based micelles to enhance the oral absorption of silybin. Int. J. Pharm. 2018, 536, 231–240. [Google Scholar] [CrossRef]
- Semeniuk, M.; Mottino, A.D. Regulation of hepatic P-gp expression and activity by genistein in rats. Arch. Toxicol. 2020, 94, 1625–1635. [Google Scholar] [CrossRef]
- Al-Saraf, A.; Holm, R. Tween 20 increases intestinal transport of doxorubicin in vitro but not in vivo. Int. J. Pharm. 2016, 498, 66–69. [Google Scholar] [CrossRef]
- Nielsen, R.B.; Kahnt, A. Montmorillonite-surfactant hybrid particles for modulating intestinal P-glycoprotein-mediated transport. Int. J. Pharm. 2019, 571, 118696. [Google Scholar] [CrossRef]
- Mai, Y.; Ashiru-Oredope, D.A.I. Boosting drug bioavailability in men but not women through the action of an excipient. Int. J. Pharm. 2020, 587, 119678. [Google Scholar] [CrossRef]
- Saaby, L.; Helms, H.C. IPEC-J2 MDR1, a Novel High-Resistance Cell Line with Functional Expression of Human P-glycoprotein (ABCB1) for Drug Screening Studies. Mol. Pharm. 2016, 13, 640–652. [Google Scholar] [CrossRef]
- Jin, S.; Wang, X.T. P-glycoprotein and multidrug resistance-associated protein 2 are oppositely altered in brain of rats with thioacetamide-induced acute liver failure. Liver Int. 2013, 33, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Wang, B. Discovery of New 4-Indolyl Quinazoline Derivatives as Highly Potent and Orally Bioavailable P-Glycoprotein Inhibitors. J. Med. Chem. 2021, 64, 14895–14911. [Google Scholar] [CrossRef]
- Joshi, P.; Vishwakarma, R.A. Natural alkaloids as P-gp inhibitors for multidrug resistance reversal in cancer. Eur. J. Med. Chem. 2017, 138, 273–292. [Google Scholar] [CrossRef]
- Sajid, A.; Raju, N. Synthesis and Characterization of Bodipy-FL-Cyclosporine A as a Substrate for Multidrug Resistance-Linked P-Glycoprotein (ABCB1). Drug Metab. Dispos. 2019, 47, 1013–1023. [Google Scholar] [CrossRef]
- Cui, J.; Liu, X. Chow, Flavonoids as P-gp Inhibitors: A Systematic Review of SARs. Curr. Med. Chem. 2019, 26, 4799–4831. [Google Scholar] [CrossRef]
- Lee, K.W.; Lee, K.H. Phase I/II Study of Weekly Oraxol for the Second-Line Treatment of Patients With Metastatic or Recurrent Gastric Cancer. Oncologist 2015, 20, 896–897. [Google Scholar] [CrossRef]
- Arnold, Y.E.; Kalia, Y.N. Using Ex Vivo Porcine Jejunum to Identify Membrane Transporter Substrates: A Screening Tool for Early-Stage Drug Development. Biomedicines 2020, 8, 340. [Google Scholar] [CrossRef]
- Yuan, Z.W.; Li, Y.Z. Role of tangeretin as a potential bioavailability enhancer for silybin: Pharmacokinetic and pharmacological studies. Pharmacol. Res. 2018, 128, 153–166. [Google Scholar] [CrossRef]
- Huang, J.; Guo, L. Interactions between Emodin and Efflux Transporters on Rat Enterocyte by a Validated Ussing Chamber Technique. Front. Pharmacol. 2018, 9, 646. [Google Scholar] [CrossRef]
- Ali, I.; Slizgi, J.R. Transporter-Mediated Alterations in Patients with NASH Increase Systemic and Hepatic Exposure to an OATP and MRP2 Substrate. Clin. Pharmacol. Ther. 2017, 104, 748–756. [Google Scholar] [CrossRef]
- Rigalli, J.P.; Reichel, M. Human papilloma virus (HPV) 18 proteins E6 and E7 up-regulate ABC transporters in oropharyngeal carcinoma. Involvement of the nonsense-mediated decay (NMD) pathway. Cancer Lett. 2018, 428, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Di Giacomo, S.; Briz, O. Chemosensitization of hepatocellular carcinoma cells to sorafenib by beta-caryophyllene oxide-induced inhibition of ABC export pumps. Arch. Toxicol. 2019, 93, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Rigalli, J.P.; Scholz, P.N. The phytoestrogens daidzein and equol inhibit the drug transporter BCRP/ABCG2 in breast cancer cells: Potential chemosensitizing effect. Eur. J. Nutr. 2019, 58, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Rendic, S.P. Metabolism and interactions of Ivermectin with human cytochrome P450 enzymes and drug transporters, possible adverse and toxic effects. Arch. Toxicol. 2021, 95, 1535–1546. [Google Scholar] [CrossRef]
- Yang, H.; Zhai, B.T. Intestinal absorption mechanisms of araloside A in situ single-pass intestinal perfusion and in vitro Caco-2 cell model. Biomed. Pharmacother. 2018, 106, 1563–1569. [Google Scholar] [CrossRef]
- Nielsen, S.; Westerhoff, A.M. MRP2-mediated transport of etoposide in MDCKII MRP2 cells is unaffected by commonly used non-ionic surfactants. Int. J. Pharm. 2019, 565, 306–315. [Google Scholar] [CrossRef]
- Tocchetti, G.N.; Zecchinati, F. Inhibition of multidrug resistance-associated protein 2 (MRP2) activity by the contraceptive nomegestrol acetate in HepG2 and Caco-2 cells. Eur. J. Pharm. Sci. 2018, 122, 205–213. [Google Scholar] [CrossRef]
- Yu, Q.; Ni, D.C. Structures of ABCG2 under turnover conditions reveal a key step in the drug transport mechanism. Nat. Commun. 2021, 12, 4376. [Google Scholar] [CrossRef]
- Ashar, Y.V.; Zhou, J.C. BMS-599626, a Highly Selective Pan-HER Kinase Inhibitor, Antagonizes ABCG2-Mediated Drug Resistance. Cancers 2020, 12, 2502. [Google Scholar] [CrossRef]
- Jiang, H.; Yu, J. Breast Cancer Resistance Protein and Multidrug Resistance Protein 2 Regulate the Disposition of Acacetin Glucuronides. Pharm. Res. 2017, 34, 1402–1415. [Google Scholar] [CrossRef]
- Hernandez-Lozano, I.; Wanek, T. Influence of ABC transporters on the excretion of ciprofloxacin assessed with PET imaging in mice. Eur. J. Pharm. Sci. 2021, 163, 105854. [Google Scholar] [CrossRef] [PubMed]
- Kono, Y.; Kawahara, L. Characterization of P-Glycoprotein Inhibitors for Evaluating the Effect of P-Glycoprotein on the Intestinal Absorption of Drugs. Pharmaceutics 2021, 13, 388. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; Rey-Rico, A. Inhibition of P-glycoprotein pumps by PEO-PPO amphiphiles: Branched versus linear derivatives. Nanomedicine 2010, 5, 1371–1383. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Qin, Y. Aristolochic acid I is a substrate of BCRP but not P-glycoprotein or MRP2. J. Ethnopharmacol. 2015, 172, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wu, Y. Inhibitory Influence of Panax notoginseng Saponins on Aspirin Hydrolysis in Human Intestinal Caco-2 Cells. Molecules 2018, 23, 455. [Google Scholar] [CrossRef] [Green Version]
- Lea, T. Caco-2 Cell Line. In The Impact of Food Bioactives on Health; Verhoeckx, K., Cotter, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 103–111. [Google Scholar]
- Qu, F.; Ai, Z. Study on mechanism of low bioavailability of black tea theaflavins by using Caco-2 cell monolayer. Drug Deliv. 2021, 28, 1737–1747. [Google Scholar] [CrossRef]
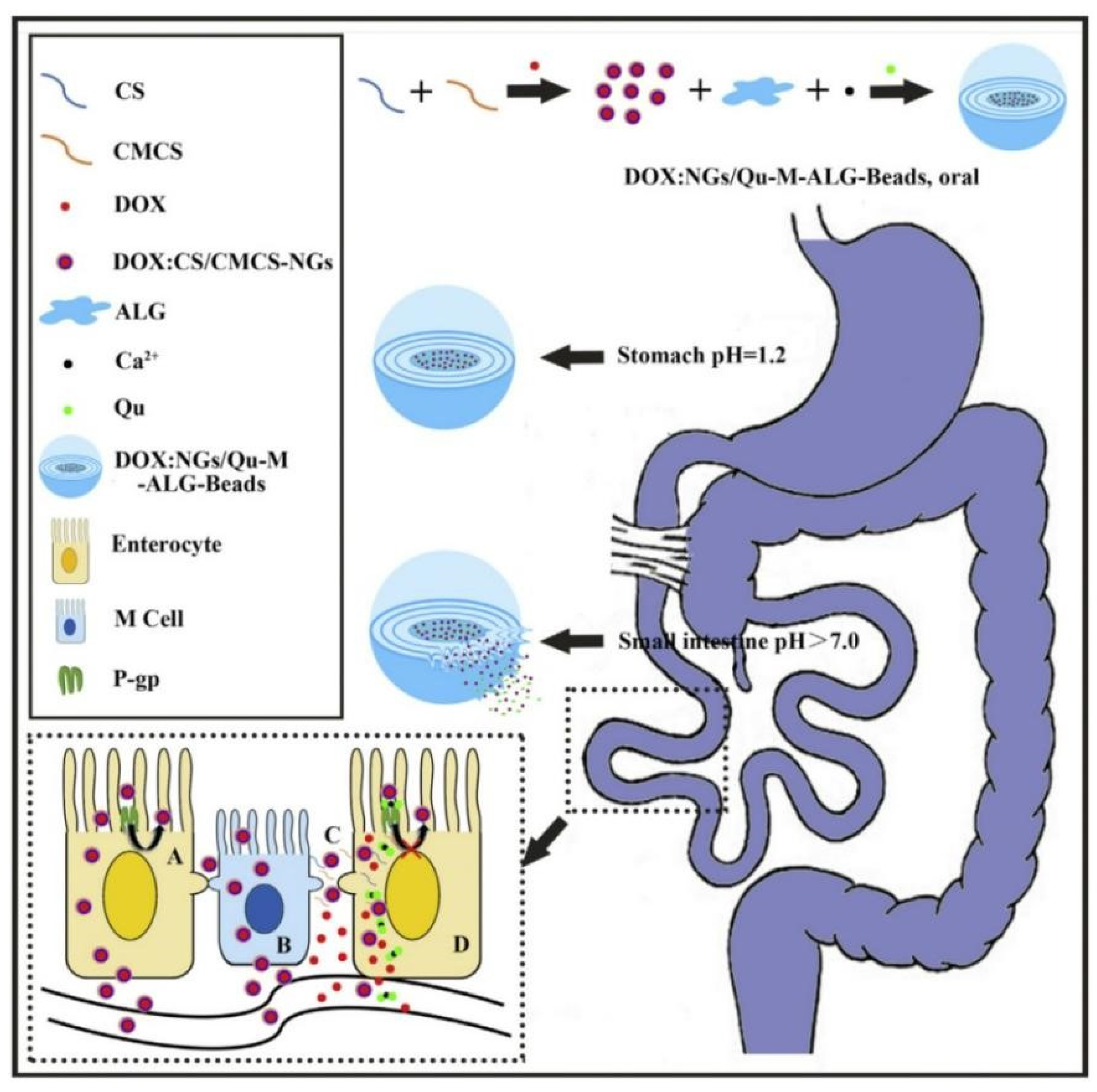
- Raza, A.; Alavi, S.E. Microfluidic assembly of pomegranate-like hierarchical microspheres for efflux regulation in oral drug delivery. Acta Biomater. 2021, 126, 277–290. [Google Scholar] [CrossRef]
- Sze, L.P.; Li, H.Y. Oral delivery of paclitaxel by polymeric micelles: A comparison of different block length on uptake, permeability and oral bioavailability. Colloids Surf. B Biointerfaces 2019, 184, 110554. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, Q. Natural Nano-Drug Delivery System in Coptidis Rhizoma Extract with Modified Berberine Hydrochloride Pharmacokinetics. Int. J. Nanomed. 2021, 16, 6297–6311. [Google Scholar] [CrossRef]
- Yamagata, T.; Morishita, M. Characterization of the inhibition of breast cancer resistance protein-mediated efflux of mitoxantrone by pharmaceutical excipients. Int. J. Pharm. 2009, 370, 216–219. [Google Scholar] [CrossRef]
- Olivas-Aguirre, F.J.; Mendoza, S. First-Pass Metabolism of Polyphenols from Selected Berries: A High-Throughput Bioanalytical Approach. Antioxidants 2020, 9, 311. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Bai, Y. Self-Assembly Nanoparticles for Overcoming Multidrug Resistance and Imaging-Guided Chemo-Photothermal Synergistic Cancer Therapy. Int. J. Nanomed. 2020, 15, 809–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, G.; Zheng, X.P. TPGS-stabilized NaYbF4: Er upconversion nanoparticles for dual-modal fluorescent/CT imaging and anticancer drug delivery to overcome multi-drug resistance. Biomaterials 2015, 40, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, X.F. Systematic evaluation of multifunctional paclitaxel-loaded polymeric mixed micelles as a potential anticancer remedy to overcome multidrug resistance. Acta Biomater. 2017, 50, 381–395. [Google Scholar] [CrossRef]
- Wempe, M.F.; Wright, C. Inhibiting efflux with novel non-ionic surfactants: Rational design based on vitamin E TPGS. Int. J. Pharm. 2009, 370, 93–102. [Google Scholar] [CrossRef]
- Chen, G.; Svirskis, D. N-trimethyl chitosan coated nano-complexes enhance the oral bioavailability and chemotherapeutic effects of gemcitabine. Carbohydr. Polym. 2021, 273, 118592. [Google Scholar] [CrossRef]
- Subongkot, T. Development and mechanistic study of a microemulsion containing vitamin E TPGS for the enhancement of oral absorption of celecoxib. Int. J. Nanomed. 2019, 14, 3087–3102. [Google Scholar] [CrossRef] [Green Version]
- Zou, T.; Gu, L. TPGS emulsified zein nanoparticles enhanced oral bioavailability of daidzin: In vitro characteristics and in vivo performance. Mol. Pharm. 2013, 10, 2062–2070. [Google Scholar] [CrossRef]
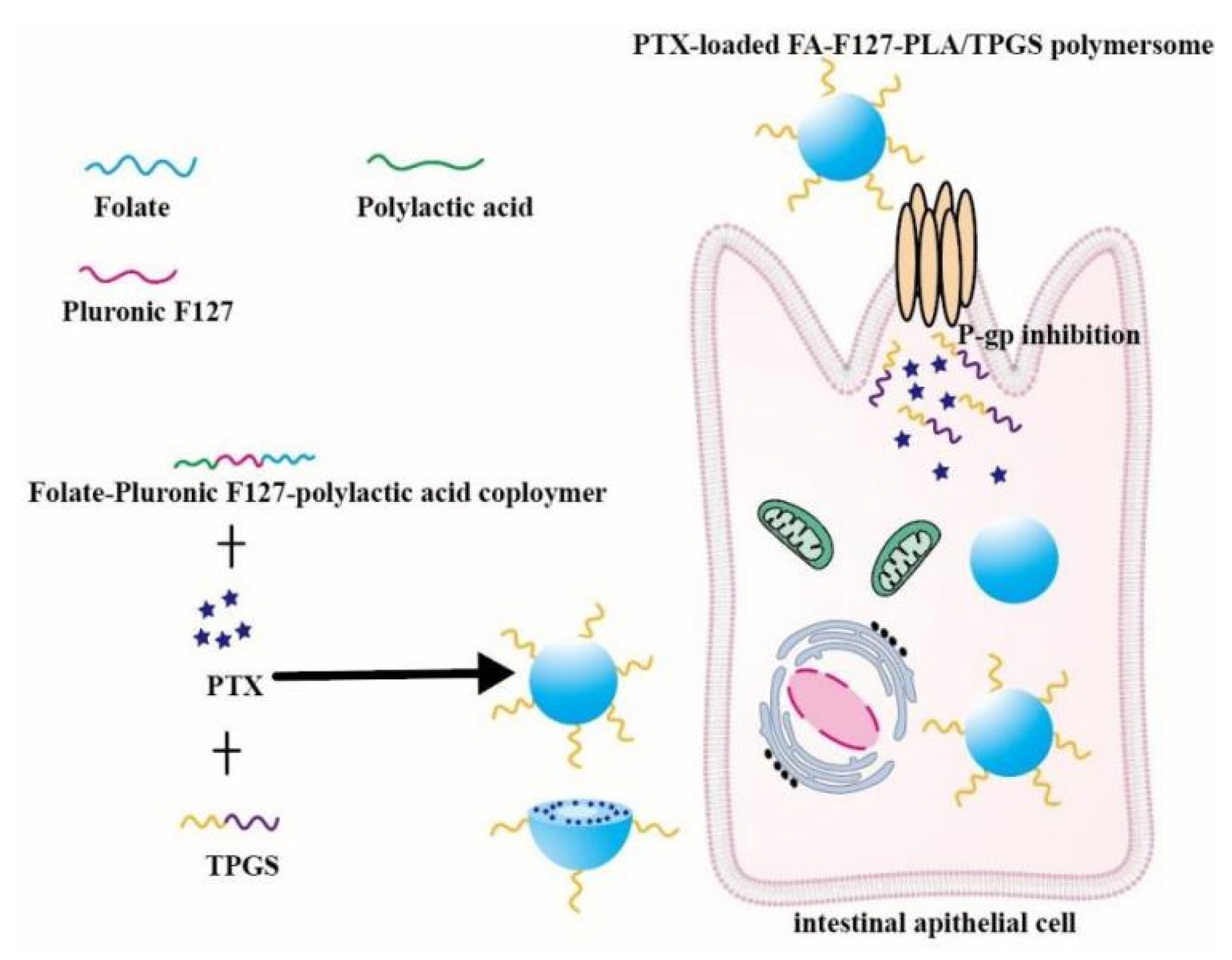
- Pan, X.Q.; Gong, Y.C. Folate-conjugated pluronic/polylactic acid polymersomes for oral delivery of paclitaxel. Int. J. Biol. Macromol. 2019, 139, 377–386. [Google Scholar] [CrossRef]
- Li, X.; Uehara, S. Improvement of intestinal absorption of curcumin by cyclodextrins and the mechanisms underlying absorption enhancement. Int. J. Pharm. 2018, 535, 340–349. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y. Carboxymethyl beta-cyclodextrin grafted carboxymethyl chitosan hydrogel-based microparticles for oral insulin delivery. Carbohydr. Polym. 2020, 246, 116617. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cui, Y.L. Effects of beta-cyclodextrin on the intestinal absorption of berberine hydrochloride, a P-glycoprotein substrate. Int. J. Biol. Macromol. 2013, 59, 363–371. [Google Scholar] [CrossRef]
- Sosnik, A. Reversal of multidrug resistance by the inhibition of ATP-binding cassette pumps employing “Generally Recognized As Safe” (GRAS) nanopharmaceuticals: A review. Adv. Drug Deliv. Rev. 2013, 65, 1828–1851. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Pan, X.L. Multifunctional Poly(methyl vinyl ether-co-maleic anhydride)-graft-hydroxypropyl-beta-cyclodextrin Amphiphilic Copolymer as an Oral High-Performance Delivery Carrier of Tacrolimus. Mol. Pharm. 2015, 12, 2337–2351. [Google Scholar] [CrossRef]
- Yang, L.; Li, M.A. A cell-penetrating peptide conjugated carboxymethyl-beta-cyclodextrin to improve intestinal absorption of insulin. Int. J. Biol. Macromol. 2018, 111, 685–695. [Google Scholar] [CrossRef]
- Vaidya, B.; Shukla, S.K. Nintedanib-cyclodextrin complex to improve bio-activity and intestinal permeability. Carbohydr. Polym. 2019, 204, 68–77. [Google Scholar] [CrossRef]
- Batrakova, E.V.; Kabanov, A.V. Pluronic block copolymers: Evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J. Control. Release 2008, 130, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Kwon, M.; Lim, D.Y. Enhanced Intestinal Absorption and Pharmacokinetic Modulation of Berberine and Its Metabolites through the Inhibition of P-Glycoprotein and Intestinal Metabolism in Rats Using a Berberine Mixed Micelle Formulation. Pharmaceutics 2020, 12, 882. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Z. The construction and characterization of hybrid paclitaxel-in-micelle-in-liposome systems for enhanced oral drug delivery. Colloids Surf. B Biointerfaces 2017, 160, 572–580. [Google Scholar] [CrossRef]
- Li, M.; Si, L.Q. Excipients enhance intestinal absorption of ganciclovir by P-gp inhibition: Assessed in vitro by everted gut sac and in situ by improved intestinal perfusion. Int. J. Pharm. 2011, 403, 37–45. [Google Scholar] [CrossRef]
- Sharma, S.; Verma, A. Investigating the role of Pluronic-g-Cationic polyelectrolyte as functional stabilizer for nanocrystals: Impact on Paclitaxel oral bioavailability and tumor growth. Acta Biomater. 2015, 26, 169–183. [Google Scholar] [CrossRef] [PubMed]
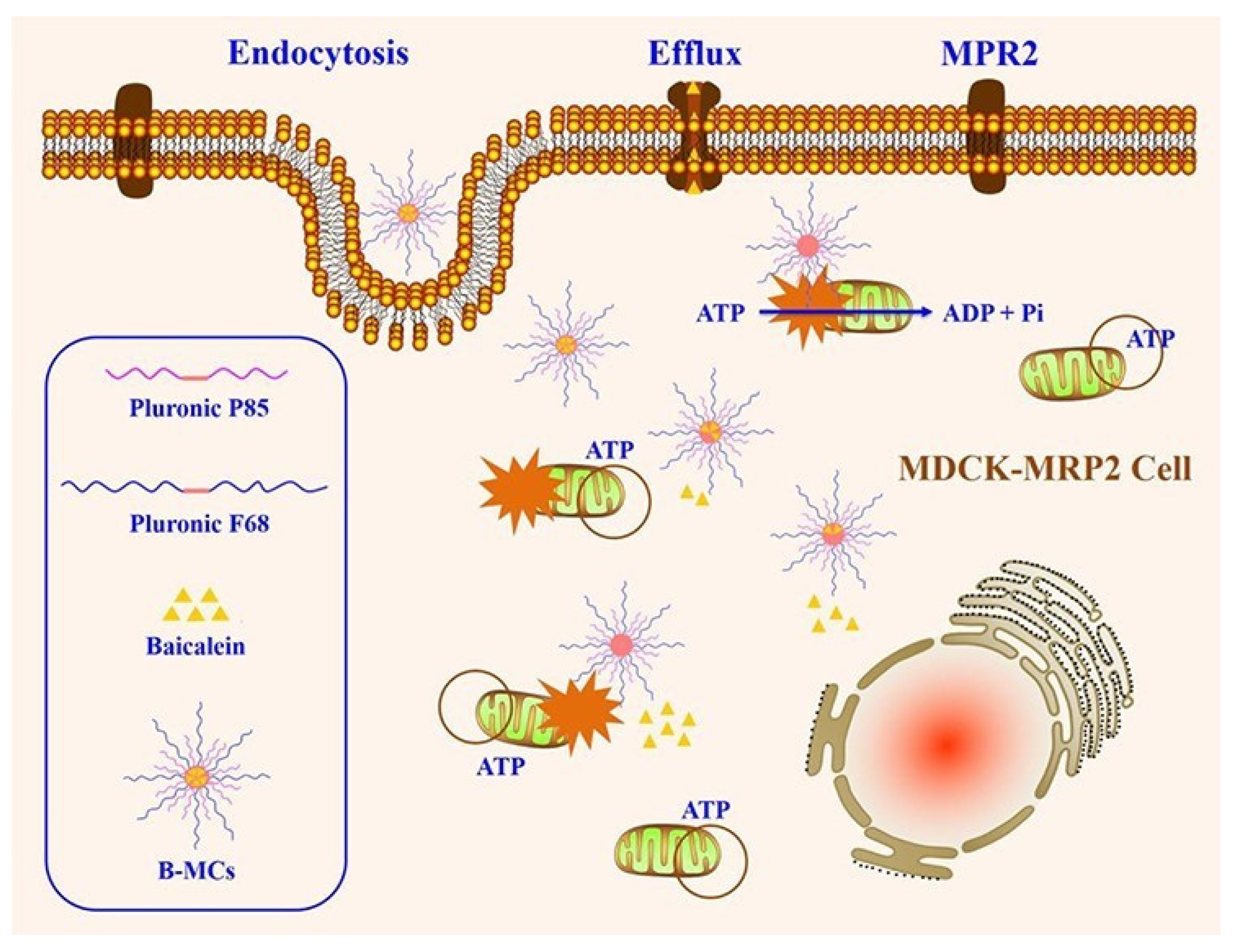
- Chen, T.; Li, Y. Pluronic P85/F68 Micelles of Baicalein Could Interfere with Mitochondria to Overcome MRP2-Mediated Efflux and Offer Improved Anti-Parkinsonian Activity. Mol. Pharm. 2017, 14, 3331–3342. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Lin, Y.L. Modulation of intestinal P-glycoprotein function by polyethylene glycols and their derivatives by in vitro transport and in situ absorption studies. Int. J. Pharm. 2006, 313, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Hombach, J.L. Design and in vitro evaluation of a novel polymeric P-glycoprotein (P-gp) inhibitor. J. Control. Release 2010, 147, 62–69. [Google Scholar] [CrossRef]
- Yamagata, T.; Kusuhara, H. Effect of excipients on breast cancer resistance protein substrate uptake activity. J. Control. Release 2007, 124, 1–5. [Google Scholar] [CrossRef]
- Zou, L.; Pottel, J. Interactions of Oral Molecular Excipients with Breast Cancer Resistance Protein, BCRP. Mol. Pharm. 2020, 17, 748–756. [Google Scholar] [CrossRef]
- Al-Ali, A.A.A.; Quach, J.R.C. Polysorbate 20 alters the oral bioavailability of etoposide in wild type and mdr1a deficient Sprague-Dawley rats. Int. J. Pharm. 2018, 543, 352–360. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, C.U.; Abdulhussein, A.A. Polysorbate 20 increases oral absorption of digoxin in wild-type Sprague Dawley rats, but not in mdr1a (-/-) Sprague Dawley rats. Int. J. Pharm. 2016, 513, 78–87. [Google Scholar] [CrossRef]
- Batrakova, E.V.; Li, S. Mechanism of sensitization of MDR cancer cells by Pluronic block copolymers: Selective energy depletion. Br. J. Cancer 2001, 85, 1987–1997. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.B. Silencing the Breast Cancer Resistance Protein Expression and Function in Caco-2 Cells Using Lentiviral Vector-Based Short Hairpin RNA. Drug Metab. Dispos. 2009, 37, 737–744. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.; Zhao, J. Development and Characterization of MDR1 (Mdr1a/b) CRISPR/Cas9 Knockout Rat Model. Drug Metab. Dispos. 2019, 47, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Jin, G.; Li, Y. SMS regulates the expression and function of P-gp and MRP2 in Caco-2 cells. Cell Biol. Toxicol. 2016, 32, 483–497. [Google Scholar] [CrossRef]
- Lian, H.; He, Z. Rational design of hybrid nanomicelles integrating mucosal penetration and P-glycoprotein inhibition for efficient oral delivery of paclitaxel. Colloids Surf. B Biointerfaces 2017, 155, 429–439. [Google Scholar] [CrossRef]
- Bugde, P.; Biswas, R. The therapeutic potential of targeting ABC transporters to combat multi-drug resistance. Expert Opin. Ther. Targets 2017, 21, 511–530. [Google Scholar] [CrossRef]
- Cui, W.; Zhao, H.Q. Co-encapsulation of docetaxel and cyclosporin A into SNEDDS to promote oral cancer chemotherapy. Drug Deliv. 2019, 26, 542–550. [Google Scholar] [CrossRef]
- Feng, C.; Li, J. Multilayer micro-dispersing system as oral carriers for co-delivery of doxorubicin hydrochloride and P-gp inhibitor. Int. J. Biol. Macromol. 2017, 94, 170–180. [Google Scholar] [CrossRef]
- Romana, B.; Hassan, M.M. A liposome-micelle-hybrid (LMH) oral delivery system for poorly water-soluble drugs: Enhancing solubilisation and intestinal transport. Eur. J. Pharm. Biopharm. 2020, 154, 338–347. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, T. Mucus adhesion- and penetration-enhanced liposomes for paclitaxel oral delivery. Int. J. Pharm. 2018, 537, 245–256. [Google Scholar] [CrossRef]
- Zhao, M.; Lee, S.H. Enhanced oral absorption of sorafenib via the layer-by-layer deposition of a pH-sensitive polymer and glycol chitosan on the liposome. Int. J. Pharm. 2018, 544, 14–20. [Google Scholar] [CrossRef]
- Sohail, M.F.; Javed, L. Folate grafted thiolated chitosan enveloped nanoliposomes with enhanced oral bioavailability and anticancer activity of docetaxel. J. Mater. Chem. B 2016, 4, 6240–6248. [Google Scholar] [CrossRef]
- Shi, L.L.; Xie, H.J. Positively Charged Surface-Modified Solid Lipid Nanoparticles Promote the Intestinal Transport of Docetaxel through Multifunctional Mechanisms in Rats. Mol. Pharm. 2016, 13, 2667–2676. [Google Scholar] [CrossRef]
- Garg, A.; Bhalala, K. In-situ single pass intestinal permeability and pharmacokinetic study of developed Lumefantrine loaded solid lipid nanoparticles. Int. J. Pharm. 2017, 516, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Ling, L. Redox sensitive lipid-camptothecin conjugate encapsulated solid lipid nanoparticles for oral delivery. Int. J. Pharm. 2018, 549, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.; Madureria, A.R. Solid lipid nanoparticles as oral delivery systems of phenolic compounds: Overcoming pharmacokinetic limitations for nutraceutical applications. Crit. Rev. Food Sci. Nutr. 2017, 57, 1863–1873. [Google Scholar] [CrossRef]
- Yu, Z.; Fan, W.F. Slowing down lipolysis significantly enhances the oral absorption of intact solid lipid nanoparticles. Biomater. Sci. 2019, 7, 4273–4282. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.M.; Hou, X.C. Co-delivery of honokiol, a constituent of Magnolia species, in a self-microemulsifying drug delivery system for improved oral transport of lipophilic sirolimus. Drug Deliv. 2016, 23, 2513–2523. [Google Scholar] [CrossRef]
- Li, Y.J.; Hu, X.B. Nanoemulsion-based delivery system for enhanced oral bioavailability and caco-2 cell monolayers permeability of berberine hydrochloride. Drug Deliv. 2017, 24, 1868–1873. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.H.; Jeong, S.H. Enhanced Lymphatic Delivery of Methotrexate Using W/O/W Nanoemulsion: In Vitro Characterization and Pharmacokinetic Study. Pharmaceutics 2020, 12, 978. [Google Scholar] [CrossRef]
- Rosso, A.; Andretto, V. Nanocomposite sponges for enhancing intestinal residence time following oral administration. J. Control. Release 2021, 333, 579–592. [Google Scholar] [CrossRef]
- Cagel, M.; Tesan, F.C. Polymeric mixed micelles as nanomedicines: Achievements and perspectives. Eur. J. Pharm. Biopharm. 2017, 113, 211–228. [Google Scholar] [CrossRef]
- Mochida, Y.; Cabral, H. Polymeric micelles for targeted tumor therapy of platinum anticancer drugs. Expert Opin. Drug Deliv. 2017, 14, 1423–1438. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhang, L. Stimuli-responsive polymeric micelles for drug delivery and cancer therapy. Int. J. Nanomed. 2018, 13, 2921–2942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Wang, L.F. Apically targeted oral micelles exhibit highly efficient intestinal uptake and oral absorption. Int. J. Nanomed. 2018, 13, 7997–8012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huo, M.; Fu, Y. N-mercapto acetyl-N′-octyl-O, N″-glycol chitosan as an efficiency oral delivery system of paclitaxel. Carbohydr. Polym. 2018, 181, 477–488. [Google Scholar] [CrossRef]
- Wang, Q.; Li, C. Poly(vinyl methyl ether/maleic anhydride)-Doped PEG-PLA Nanoparticles for Oral Paclitaxel Delivery To Improve Bioadhesive Efficiency. Mol. Pharm. 2017, 14, 3598–3608. [Google Scholar] [CrossRef]
- Xiong, W.; Xiong, S.H. Brij-functionalized chitosan nanocarrier system enhances the intestinal permeability of P-glycoprotein substrate-like drugs. Carbohydr. Polym. 2021, 266, 118112. [Google Scholar] [CrossRef]
- Wang, T.; Shen, L.A. A novel core-shell lipid nanoparticle for improving oral administration of water soluble chemotherapeutic agents: Inhibited intestinal hydrolysis and enhanced lymphatic absorption. Drug Deliv. 2017, 24, 1565–1573. [Google Scholar] [CrossRef] [Green Version]
- Xia, D.; He, Y. Transport mechanism of lipid covered saquinavir pure drug nanoparticles in intestinal epithelium. J. Control. Release 2018, 269, 159–170. [Google Scholar] [CrossRef]



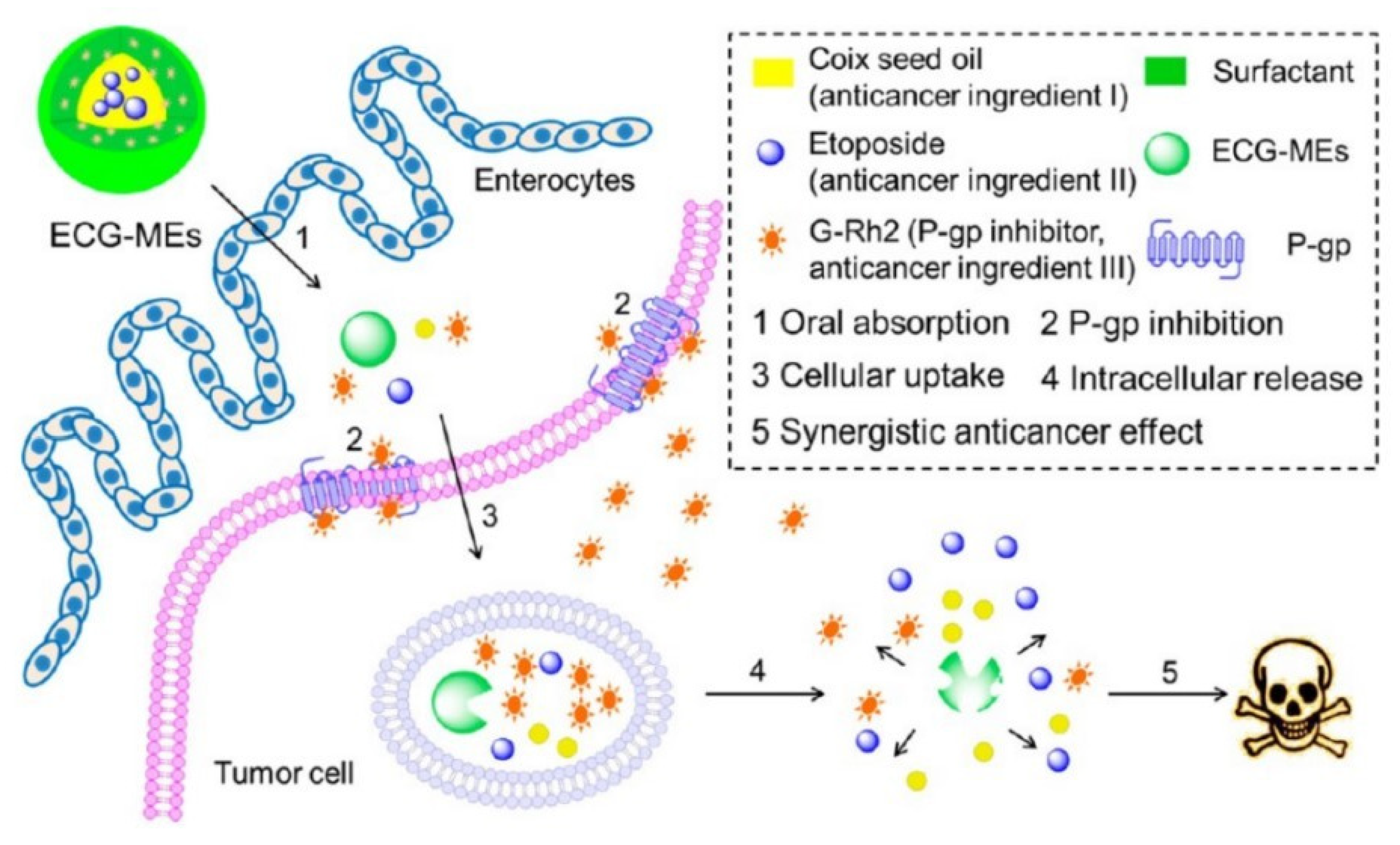

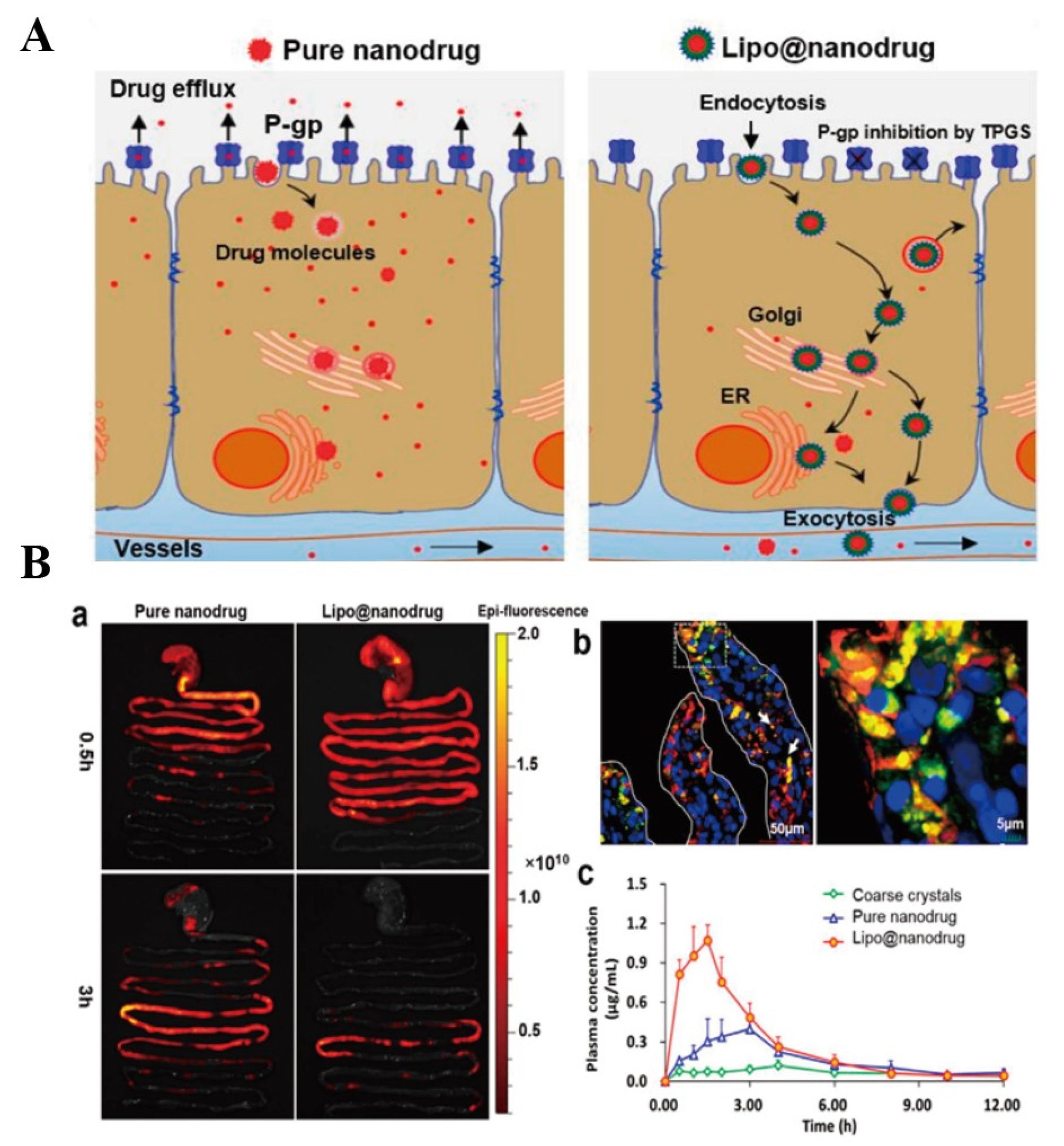
| Materials | Mechanism of Improving Oral Drug Bioavailability | Refs. |
|---|---|---|
| TPGS | Inhibiting P-gp and increasing solubility of insoluble drugs. | [63,64,65,66] |
| PEGs | Inhibiting drugs efflux mediated by P-gp. | [81] |
| β-CD | Reducing P-gp activity and improving solubility of insoluble drugs. | [71,72,74] |
| Pluronic | Inhibiting the activity of MRP2 and P-gp. | [82,83,90] |
| Polysorbate 20 | Inhibiting P-gp activity. | [88,89] |
| Tween 20 | Inhibiting drugs efflux mediated ABCG2 and P-gp. | [86] |
| Tween 80 | Inhibiting P-gp activity. | [8] |
| Docusate sodium, sodium lauryl sulfate and sucrose monolaurate | Increasing the absorption of ABCG2 substrates. | [87] |
| Cremophor EL | Inhibiting ABCG2 and P-gp activities. | [86] |
| Brij 30/58 | Reducing activities of P-gp and ABCG2. | [8,86] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, R.; Zhou, Y.; Ma, J.; Wang, Y.; Miao, X. Strategies and Mechanism in Reversing Intestinal Drug Efflux in Oral Drug Delivery. Pharmaceutics 2022, 14, 1131. https://doi.org/10.3390/pharmaceutics14061131
Lu R, Zhou Y, Ma J, Wang Y, Miao X. Strategies and Mechanism in Reversing Intestinal Drug Efflux in Oral Drug Delivery. Pharmaceutics. 2022; 14(6):1131. https://doi.org/10.3390/pharmaceutics14061131
Chicago/Turabian StyleLu, Rong, Yun Zhou, Jinqian Ma, Yuchen Wang, and Xiaoqing Miao. 2022. "Strategies and Mechanism in Reversing Intestinal Drug Efflux in Oral Drug Delivery" Pharmaceutics 14, no. 6: 1131. https://doi.org/10.3390/pharmaceutics14061131
APA StyleLu, R., Zhou, Y., Ma, J., Wang, Y., & Miao, X. (2022). Strategies and Mechanism in Reversing Intestinal Drug Efflux in Oral Drug Delivery. Pharmaceutics, 14(6), 1131. https://doi.org/10.3390/pharmaceutics14061131







