Characterization of Physical and Biological Properties of a Caries-Arresting Liquid Containing Copper Doped Bioglass Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of CuBGns
2.2. Physical Analysis of SDF@CuBGn (SDF Mixed with CuBGn)
2.3. Physicochemical Analysis of SDF@CuBGn with Hydroxyapatite (HA) Discs
2.4. In Vitro Study of Cytotoxicity on Pulp Stem Cells from Human Exfoliated Deciduous Teeth (SHED) and Antibacterial Effect on Cariogenic Organisms
2.5. Statistical Analysis
3. Results
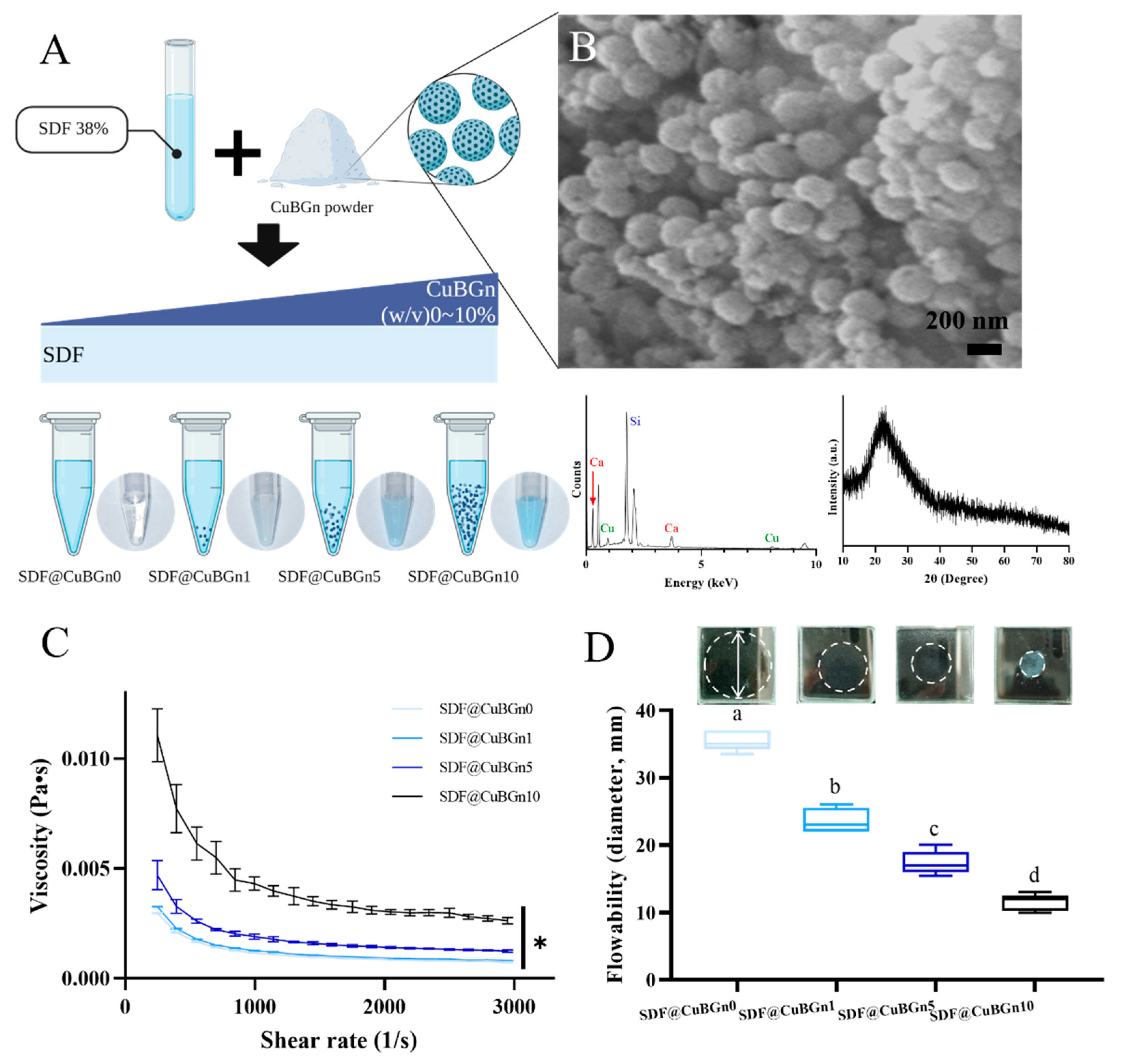
3.1. Characterization of CuBGns and Physical Analysis of SDF@CuBGns
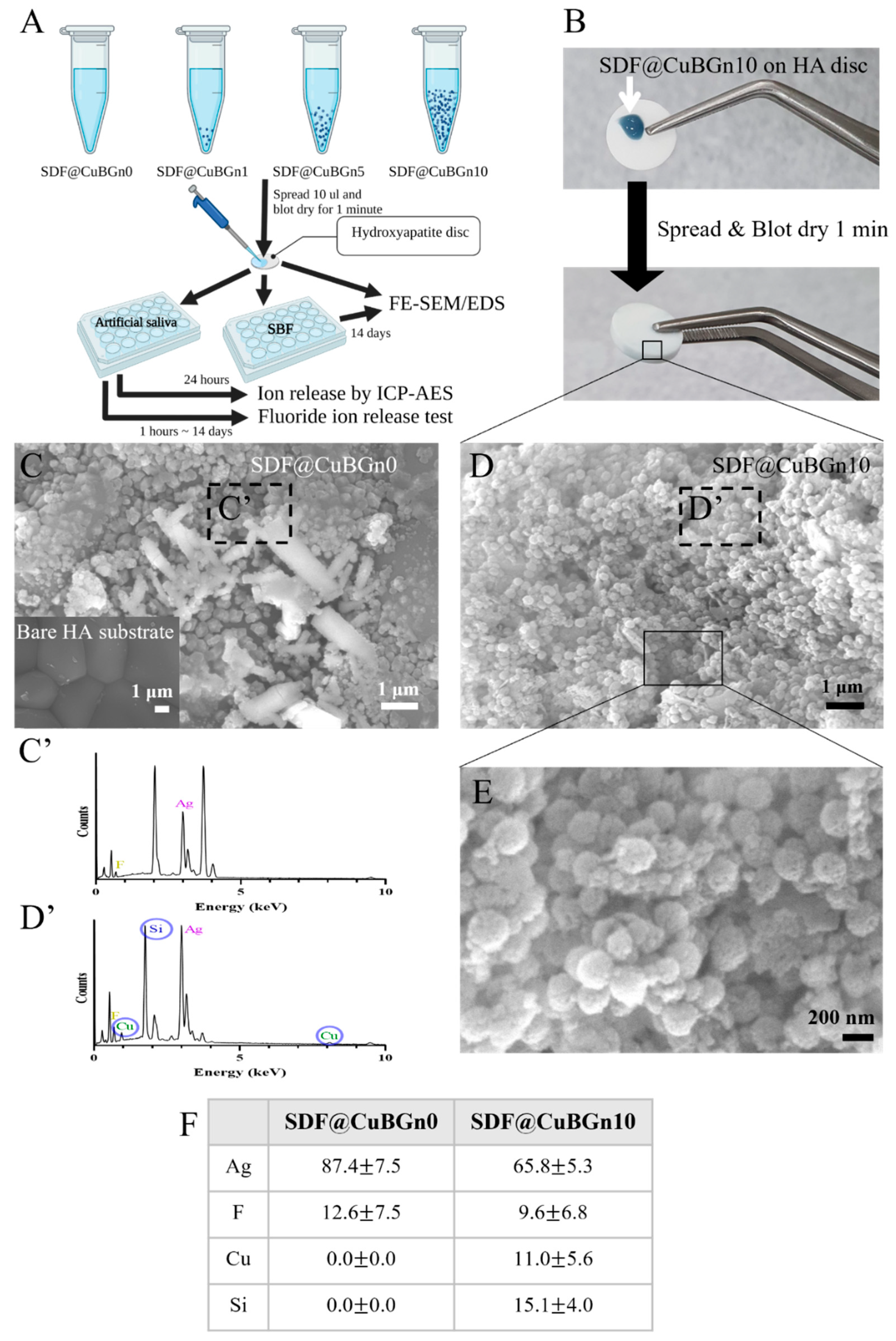
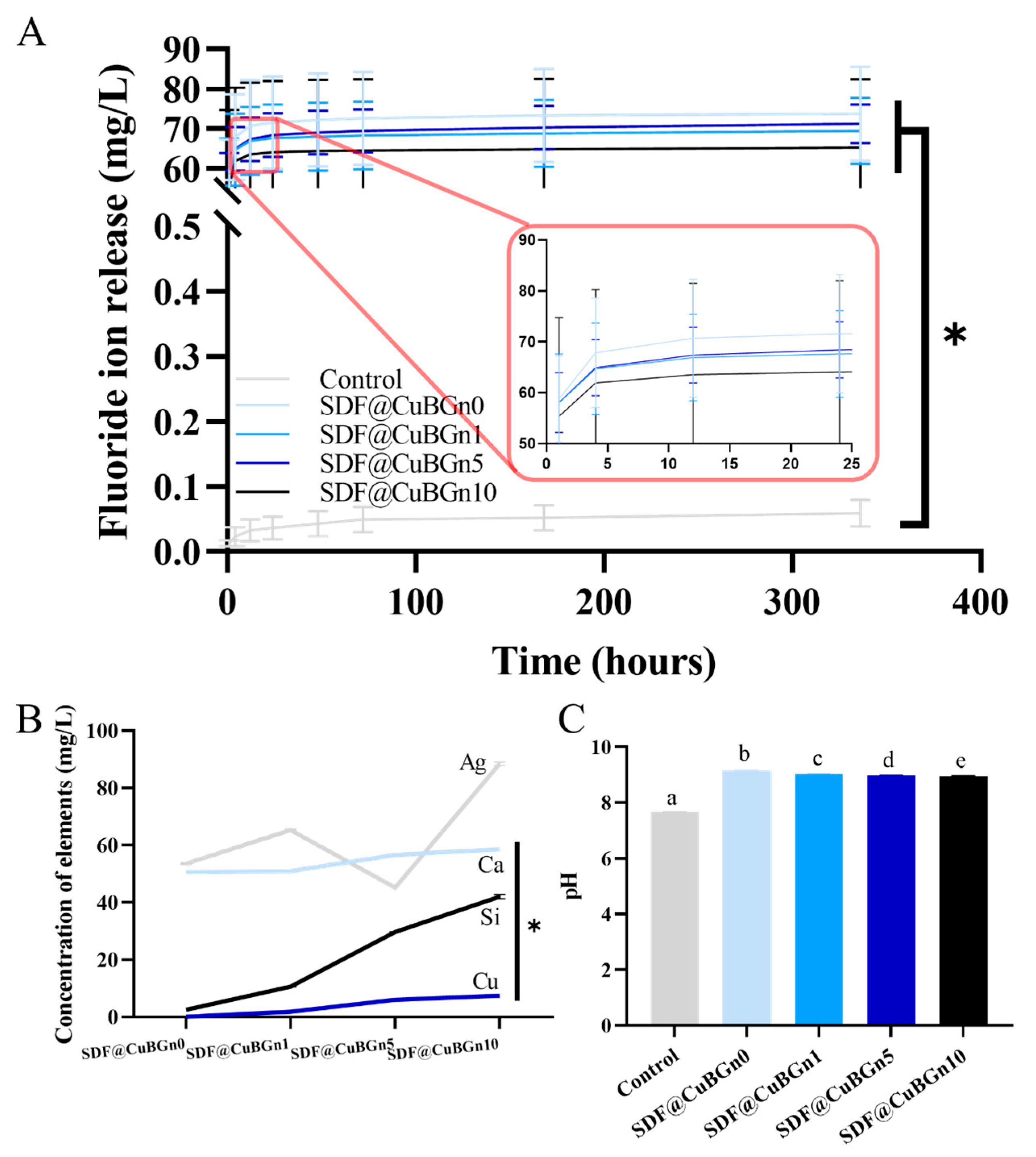
3.2. Physicochemical Analysis of SDF@CuBGn with HA Discs
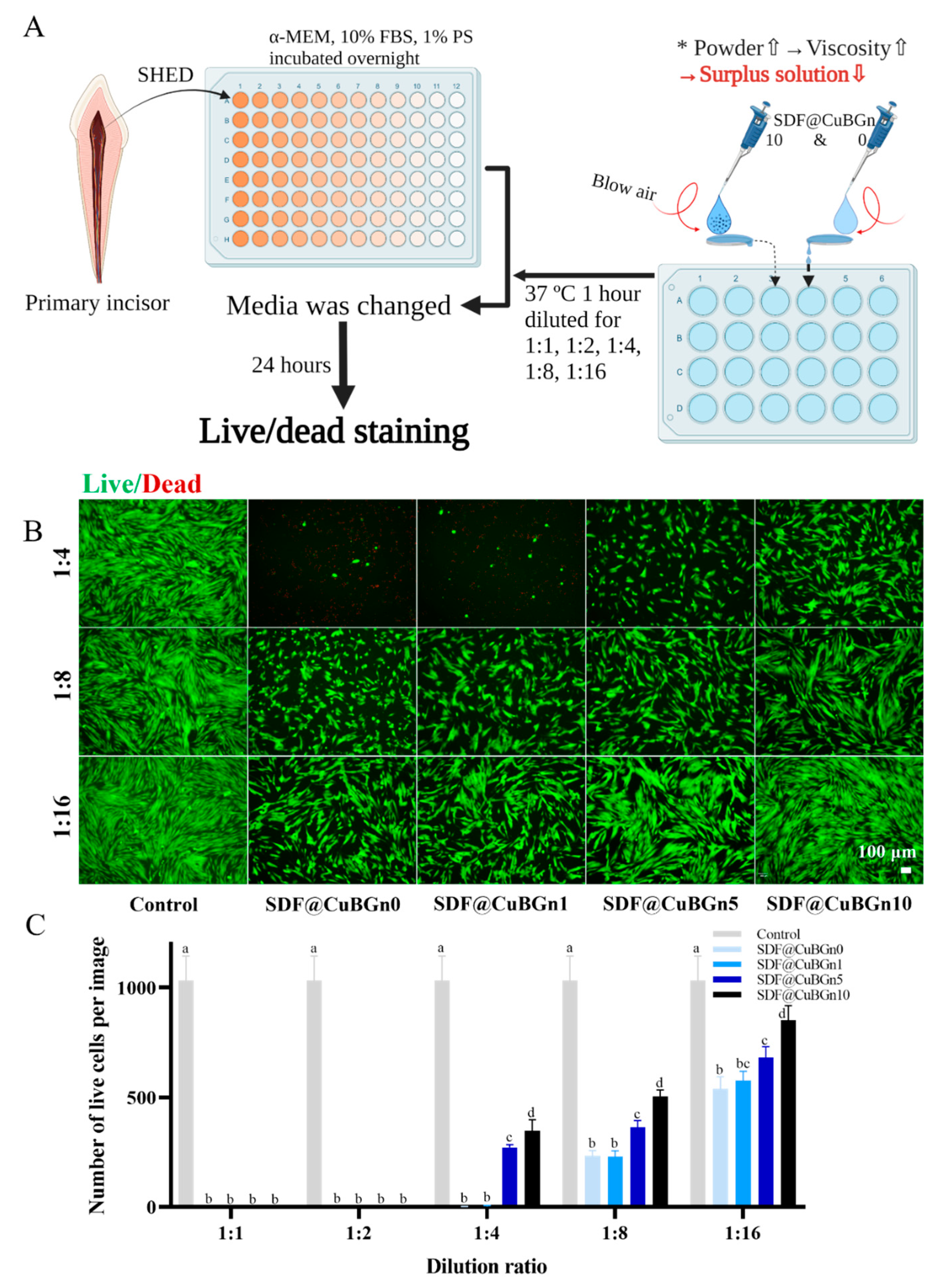
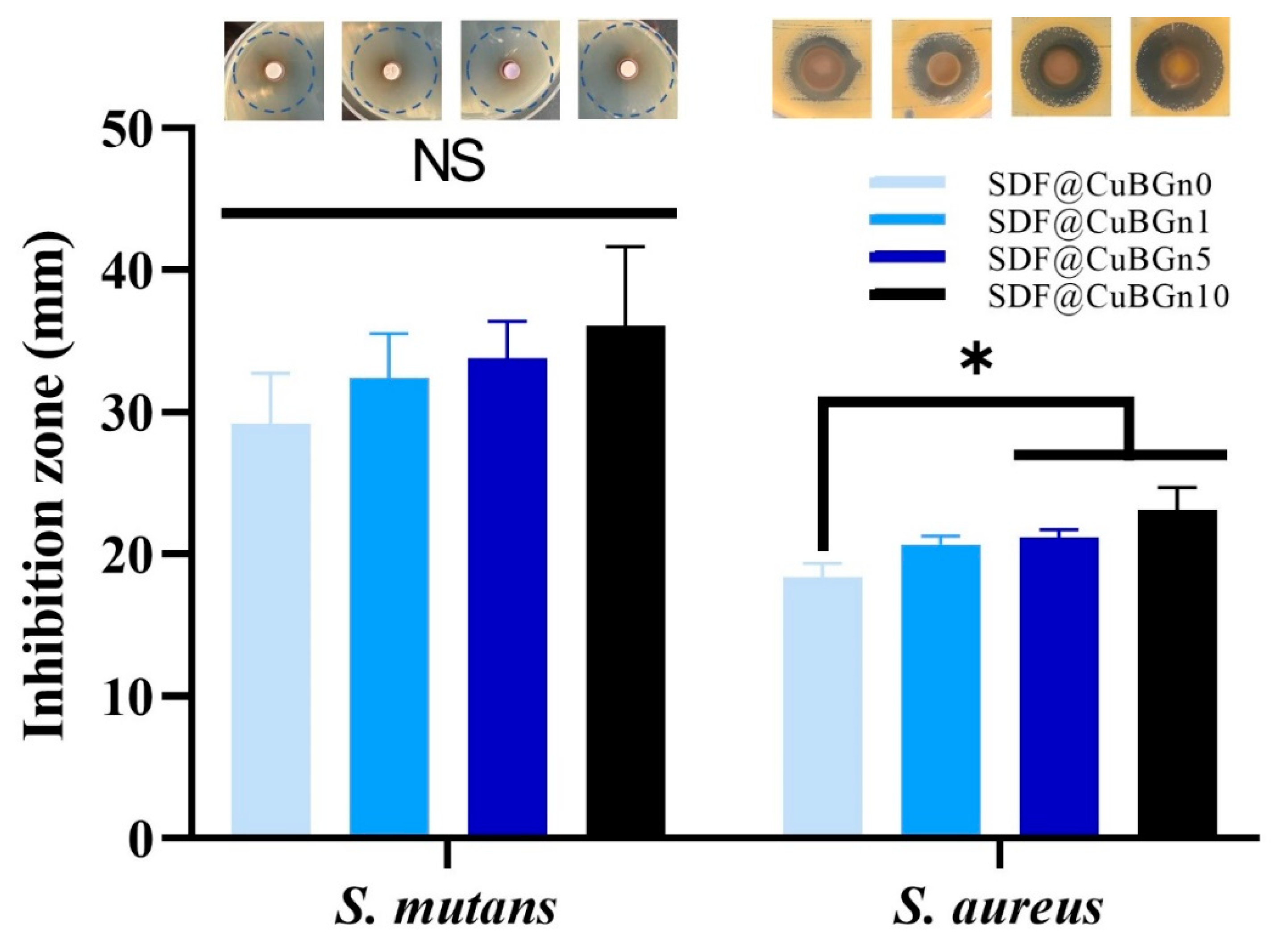
3.3. In Vitro Study of CuBGn Cytotoxicity on Pulp Stem Cells and Antibacterial Effects on Cariogenic Organisms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tinanoff, N.; Douglass, J.M. Clinical decision-making for caries management in primary teeth. J. Dent. Educ. 2001, 65, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Sheiham, A. Impact of dental treatment on the incidence of dental caries in children and adults. Community Dent. Oral Epidemiol. 1997, 25, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Primers 2017, 3, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, J.-Y.; Botelho, M.; Matinlinna, J. Silver compounds used in dentistry for caries management: A review. J. Dent. 2012, 40, 531–541. [Google Scholar] [CrossRef]
- Rosenblatt, A.; Stamford, T.; Niederman, R. Silver diamine fluoride: A caries “silver-fluoride bullet”. J. Dent. Res. 2009, 88, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Bhaskar, V.; Venkatraghavan, K.; Choudhary, P.; Trivedi, K. Silver diamine fluoride: A review and current applications. J. Adv. Oral Res. 2014, 5, 25–35. [Google Scholar] [CrossRef]
- Burgess, J.; Vaghela, P. Silver diamine fluoride: A successful anticarious solution with limits. Adv. Dent. Res. 2018, 29, 131–134. [Google Scholar] [CrossRef] [Green Version]
- Yu, O.Y.; Mei, M.L.; Zhao, I.S.; Li, Q.-L.; Lo, E.C.-M.; Chu, C.-H. Remineralisation of enamel with silver diamine fluoride and sodium fluoride. Dent. Mater. 2018, 34, e344–e352. [Google Scholar] [CrossRef]
- Mei, M.L.; Lo, E.C.; Chu, C.H. Clinical Use of Silver Diamine Fluoride in Dental Treatment. Compend. Contin. Educ. Dent. 2016, 37, 93–98. [Google Scholar]
- Fancher, M.E.; Fournier, S.; Townsend, J.; Lallier, T.E. Cytotoxic effects of silver diamine fluoride. Am. J. Dent. 2019, 32, 152–156. [Google Scholar]
- Hosoya, Y.; Aritomi, K.; Goto, G. Pulpal response to diammine silver fluoride. (2). Application on exposed pulps. Shoni Shikagaku Zasshi 1990, 28, 327–337. [Google Scholar] [PubMed]
- Miller, M.B.; Lopez, L.A.; Quock, R.L. Silver diamine fluoride, potassium iodide, and esthetic perception: An in vitro pilot study. Am. J. Dent. 2016, 29, 248–250. [Google Scholar] [PubMed]
- Ogard, B.; Seppa, L.; Rolla, G. Professional topical fluoride applications--clinical efficacy and mechanism of action. Adv. Dent. Res. 1994, 8, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, S.M.; Moreira, C.D.; Oliveira AC, X.; Oliveira, A.A.; Lemos, E.M.; Pereira, M.M. Bioactive glass nanoparticles for periodontal regeneration and applications in dentistry. In Nanobiomaterials in Clinical Dentistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 351–383. [Google Scholar]
- Clark, A.; Stanley, H.; Hall, M.; King, C.; Colaizzi, F.; Spilman, D.; Hench, L. Clinical-trials of Bioglass implants for alveolar ridge maintenance. J. Dent. Res. 1986, 65, 304. [Google Scholar]
- Elkassas, D.; Arafa, A. The innovative applications of therapeutic nanostructures in dentistry. Nanomedicine 2017, 13, 1543–1562. [Google Scholar] [CrossRef]
- Kim, H.S.; Chen, J.; Wu, L.-P.; Wu, J.; Xiang, H.; Leong, K.W.; Han, J. Prevention of excessive scar formation using nanofibrous meshes made of biodegradable elastomer poly(3-hydroxybutyrate-co-3-hydroxyvalerate). J. Tissue Eng. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Kim, D.-A.; Lee, J.-H.; Jun, S.-K.; Kim, H.-W.; Eltohamy, M.; Lee, H.-H. Sol–gel-derived bioactive glass nanoparticle-incorporated glass ionomer cement with or without chitosan for enhanced mechanical and biomineralization properties. Dent. Mater. 2017, 33, 805–817. [Google Scholar] [CrossRef]
- Misra, S.K.; Mohn, D.; Brunner, T.J.; Stark, W.J.; Philip, S.E.; Roy, I.; Salih, V.; Knowles, J.C.; Boccaccini, A.R. Comparison of nanoscale and microscale bioactive glass on the properties of P (3HB)/Bioglass® composites. Biomaterials 2008, 29, 1750–1761. [Google Scholar] [CrossRef]
- Gisbert-Garzarán, M.; Manzano, M.; Vallet-Regí, M. Mesoporous Silica Nanoparticles for the Treatment of Complex Bone Diseases: Bone Cancer, Bone Infection and Osteoporosis. Pharmaceutics 2020, 12, 83. [Google Scholar] [CrossRef] [Green Version]
- Yoo, S.H.; Kim, H.-W.; Lee, J.H. Restoration of olfactory dysfunctions by nanomaterials and stem cells-based therapies: Current status and future perspectives. J. Tissue Eng. 2022, 13, 1–14. [Google Scholar] [CrossRef]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Montazerian, M.; Hamzehlou, S.; Kim, H.-W.; Baino, F. Mesoporous bioactive glasses: Promising platforms for antibacterial strategies. Acta Biomater. 2018, 81, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Meng, Z.; Wu, Q.; Zeng, D.; Guo, Z.; Yao, J.; Bian, Y.; Gu, Y.; Cheng, S.; Peng, L. Biomimetic and osteogenic 3D silk fibroin composite scaffolds with nano MgO and mineralized hydroxyapatite for bone regeneration. J. Tissue Eng. 2020, 11, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.-Z.; Chakraborty, A.; Lee, J.-H.; Knowles, J.C.; Kim, H.-W. Targeting with nanoparticles for the therapeutic treatment of brain diseases. J. Tissue Eng. 2020, 11, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Ways, T.M.M.; Ng, K.W.; Lau, W.M.; Khutoryanskiy, V.V. Silica Nanoparticles in Transmucosal Drug Delivery. Pharmaceutics 2020, 12, 751. [Google Scholar] [CrossRef]
- Parasuraman, P.; R Y, T.; Shaji, C.; Sharan, A.; Bahkali, A.H.; Al-Harthi, H.F.; Syed, A.; Anju, V.T.; Dyavaiah, M.; Siddhardha, B. Biogenic Silver Nanoparticles Decorated with Methylene Blue Potentiated the Photodynamic Inactivation of Pseudomonas aeruginosa and Staphylococcus aureus. Pharmaceutics 2020, 12, 709. [Google Scholar] [CrossRef]
- Borkow, G.; Gabbay, J. Copper as a biocidal tool. Curr. Med. Chem. 2005, 12, 2163–2175. [Google Scholar] [CrossRef] [Green Version]
- Choe, Y.-E.; Kim, Y.-J.; Jeon, S.-J.; Ahn, J.-Y.; Park, J.-H.; Dashnyam, K.; Mandakhbayar, N.; Knowles, J.C.; Kim, H.-W.; Jun, S.-K. Investigating the mechanophysical and biological characteristics of therapeutic dental cement incorporating copper doped bioglass nanoparticles. Dent. Mater. 2021, 38, 363–375. [Google Scholar] [CrossRef]
- Seo, J.J.; Mandakhbayar, N.; Kang, M.S.; Yoon, J.Y.; Lee, N.H.; Ahn, J.; Lee, H.H.; Lee, J.H.; Kim, H.W. Antibacterial, proangiogenic, and osteopromotive nanoglass paste coordinates regenerative process following bacterial infection in hard tissue. Biomaterials 2021, 268, 120593. [Google Scholar] [CrossRef]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef] [Green Version]
- El-Fiqi, A.; Mandakhbayar, N.; Jo, S.B.; Knowles, J.C.; Lee, J.-H.; Kim, H.-W. Nanotherapeutics for regeneration of degenerated tissue infected by bacteria through the multiple delivery of bioactive ions and growth factor with antibacterial/angiogenic and osteogenic/odontogenic capacity. Bioact. Mater. 2021, 6, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Viswanath, D.S.; Ghosh, T.K.; Prasad, D.H.; Dutt, N.V.; Rani, K.Y. Viscosity of Liquids: Theory, Estimation, Experiment, and Data; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Petford, N. Which effective viscosity? Mineral. Mag. 2009, 73, 167–191. [Google Scholar] [CrossRef]
- Yamaga, R. Diamine silver fluoride and its clinical application. J. Osaka Univ. Dent. Sch. 1972, 12, 1–20. [Google Scholar] [PubMed]
- Jun, S.-K.; Lee, J.-H.; Lee, H.-H. The biomineralization of a bioactive glass-incorporated light-curable pulp capping material using human dental pulp stem cells. Biomed Res. Int. 2017, 2017, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Hori, T.; Kawamura, K.; Kajiwara, T.; Nagao, K.; Isoda, K.; Itsumi, T. Effect of application of 3.8% Ag (NH3) 2F solution as a disinfectant. Dent. Outlook 1975, 46, 495–500. [Google Scholar]
- Suzuki, T. Effects of diammine silver fluoride on tooth enamel. J. Osaka Univ. Dent. Sch. 1974, 14, 61–72. [Google Scholar]
- Chander, S.; Chiao, C.; Fuerstenau, D. Transformation of calcium fluoride for caries prevention. J. Dent. Res. 1982, 61, 403–407. [Google Scholar] [CrossRef]
- Lou, Y.; Botelho, M.; Darvell, B. Reaction of silver diamine fluoride with hydroxyapatite and protein. J. Dent. 2011, 39, 612–618. [Google Scholar] [CrossRef]
- Larsen, M.; Ravnholt, G. Dissolution of various calcium fluoride preparations in inorganic solutions and in stimulated human saliva. Caries Res. 1994, 28, 447–454. [Google Scholar] [CrossRef]
- Saxegaard, E.; Lagerlöf, F.; Rølla, G. Dissolution of calcium fluoride in human saliva. Acta Odontol. Scand. 1988, 46, 355–359. [Google Scholar] [CrossRef]
- Russell, A.; Hugo, W. 7 antimicrobial activity and action of silver. Prog. Med. Chem. 1994, 31, 351–370. [Google Scholar] [PubMed]
- Bragg, P.; Rainnie, D. The effect of silver ions on the respiratory chain of Escherichia coli. Can. J. Microbiol. 1974, 20, 883–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slawson, R.; Lee, H.; Trevors, J. Bacterial interactions with silver. Biol. Met. 1990, 3, 151–154. [Google Scholar] [CrossRef]
- Coward, J.E.; Carr, H.S.; Rosenkranz, H.S. Silver sulfadiazine: Effect on the ultrastructure of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1973, 3, 621–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevenson, M.; Baillie, A.; Richards, R. Antibacterial activity of liposome entrapped chloramphenicol. J. Pharm. Pharmacol. 1981, 33, 31P. [Google Scholar] [CrossRef]
- Hassan, M.; Bakhurji, E.; AlSheikh, R. Application of Er, Cr: YSGG laser versus photopolymerization after silver diamine fluoride in primary teeth. J. Sci. Rep. 2021, 11, 20780. [Google Scholar] [CrossRef]
- Quock, R.; Barros, J.; Yang, S.; Patel, S. Effect of silver diamine fluoride on microtensile bond strength to dentin. J. Oper. Dent. 2012, 37, 610–616. [Google Scholar] [CrossRef] [Green Version]
- Soeno, K.; Taira, Y.; Matsumura, H.; Atsuta, M. Effect of desensitizers on bond strength of adhesive luting agents to dentin. J. Oral Rehabil. 2001, 28, 1122–1128. [Google Scholar] [CrossRef]
- Brookes, S.; Shore, R.; Robinson, C.; Wood, S.; Kirkham, J. Copper ions inhibit the demineralisation of human enamel. Arch. Oral Biol. 2003, 48, 25–30. [Google Scholar] [CrossRef]
- Sierpinska, T.; Konstantynowicz, J.; Orywal, K.; Golebiewska, M.; Szmitkowski, M. Copper deficit as a potential pathogenic factor of reduced bone mineral density and severe tooth wear. Osteoporos. Int. 2014, 25, 447–454. [Google Scholar] [CrossRef] [Green Version]
- Afseth, J.; Amsbaugh, S.; Monell-Torrens, E.; Bowen, W.; Rølla, G.; Brunelle, J.; Dahl, E. Effect of copper applied topically or in drinking water on experimental caries in rats. Caries Res. 1984, 18, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Afseth, J.; Amsbaugh, S.; Monell-Torrens, E.; Bowen, W.; Rölla, G.; Brunelle, J.; Li, S.; Dahl, E. Effect of topical application of copper in combination with fluoride in drinking water on experimental caries in rats. Caries Res. 1984, 18, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Maltz, M.; Emilson, C.G. Effect of copper fluoride and copper sulfate on dental plaque, Streptococcus mutans and caries in hamsters. Eur. J. Oral Sci. 1988, 96, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.; Strafford, S.; Brookes, S.; Duggal, M. The effect of copper on demineralization of dental enamel. J. Dent. Res. 2006, 85, 1011–1015. [Google Scholar] [CrossRef]
- Rosalen, P.; Pearson, S.; Bowen, W. Effects of copper, iron and fluoride co-crystallized with sugar on caries development and acid formation in desalivated rats. Arch. Oral Biol. 1996, 41, 1003–1010. [Google Scholar] [CrossRef]
- Rosalen, P.; Bowen, W.; Pearson, S. Effect of copper co-crystallized with sugar on caries development in desalivated rats. Caries Res. 1996, 30, 367–372. [Google Scholar] [CrossRef]
- Lynch, R.J.; Duckworth, R.M. Microelements: Part I: Zn, Sn, Cu, Fe and I. Monogr. Oral Sci. 2020, 28, 32–47. [Google Scholar]
- Amiri, M.; Etemadifar, Z.; Daneshkazemi, A.; Nateghi, M. Antimicrobial effect of copper oxide nanoparticles on some oral bacteria and candida species. J. Dent. Biomater. 2017, 4, 347. [Google Scholar]
- Covarrubias, C.; Trepiana, D.; Corral, C. Synthesis of hybrid copper-chitosan nanoparticles with antibacterial activity against cariogenic Streptococcus mutans. Dent. Mater. J. 2018, 37, 379–384. [Google Scholar] [CrossRef] [Green Version]
- Sabatini, C.; Mennito, A.S.; Wolf, B.J.; Pashley, D.H.; Renné, W.G. Incorporation of bactericidal poly-acrylic acid modified copper iodide particles into adhesive resins. J. Dent. 2015, 43, 546–555. [Google Scholar] [CrossRef] [Green Version]
- Chu, C.; Lo, E.; Lin, H. Effectiveness of silver diamine fluoride and sodium fluoride varnish in arresting dentin caries in Chinese pre-school children. J. Dent. Res. 2002, 81, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Lo, E.C. Microhardness of dentine in primary teeth after topical fluoride applications. J. Dent. 2008, 36, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Sayed, M.; Matsui, N.; Uo, M.; Nikaido, T.; Oikawa, M.; Burrow, M.F.; Tagami, J. Morphological and elemental analysis of silver penetration into sound/demineralized dentin after SDF application. Dent. Mater. 2019, 35, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Nassar, M.; Tamura, Y.; Hiraishi, N.; Jamleh, A.; Nikaido, T.; Tagami, J. The effect of reduced glutathione on the toxicity of silver diamine fluoride in rat pulpal cells. J. Appl. Oral Sci. 2021, 29, e20200859. [Google Scholar] [CrossRef]
- Michot, B.; Casey, S.M.; Gibbs, J.L. Effects of calcitonin gene-related peptide on dental pulp stem cell viability, proliferation, and differentiation. J. Endod. 2020, 46, 950–956. [Google Scholar] [CrossRef]
- Korwar, A.; Sharma, S.; Logani, A.; Shah, N. Pulp response to high fluoride releasing glass ionomer, silver diamine fluoride, and calcium hydroxide used for indirect pulp treatment: An in-vivo comparative study. Contemp. Clin. Dent. 2015, 6, 288. [Google Scholar]
- Mei, M.L.; Lo, E.C.; Chu, C. Arresting dentine caries with silver diamine fluoride: What’s behind it? J. Dent. Res. 2018, 97, 751–758. [Google Scholar] [CrossRef]
- Lou, Y.; Darvell, B.W.; Botelho, M.G. Antibacterial effect of silver Diammine fluoride on cariogenic organisms. J. Contemp. Dent. Pract. 2018, 19, 591–598. [Google Scholar]
- Abdullah, N.; Al Marzooq, F.; Mohamad, S.; Abd Rahman, N.; Rani KG, A.; Chi Ngo, H.; Samaranayake, L.P. The antibacterial efficacy of silver diamine fluoride (SDF) is not modulated by potassium iodide (KI) supplements: A study on in-situ plaque biofilms using viability real-time PCR with propidium monoazide. PLoS ONE 2020, 15, e0241519. [Google Scholar] [CrossRef]
- Lee, J.-H.; Mandakhbayar, N.; El-Fiqi, A.; Kim, H.-W. Intracellular co-delivery of Sr ion and phenamil drug through mesoporous bioglass nanocarriers synergizes BMP signaling and tissue mineralization. Acta Biomater. 2017, 60, 93–108. [Google Scholar] [CrossRef]
- Jun, S.-K.; Yang, S.-A.; Kim, Y.-J.; El-Fiqi, A.; Mandakhbayar, N.; Kim, D.-S.; Roh, J.; Sauro, S.; Kim, H.-W.; Lee, J.-H. Multi-functional nano-adhesive releasing therapeutic ions for MMP-deactivation and remineralization. Sci. Rep. 2018, 8, 5663. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bang, S.-J.; Jun, S.-K.; Kim, Y.-J.; Ahn, J.-Y.; Vu, H.T.; Mandakhbayar, N.; Han, M.-R.; Lee, J.-H.; Kim, J.-B.; Kim, J.-S.; et al. Characterization of Physical and Biological Properties of a Caries-Arresting Liquid Containing Copper Doped Bioglass Nanoparticles. Pharmaceutics 2022, 14, 1137. https://doi.org/10.3390/pharmaceutics14061137
Bang S-J, Jun S-K, Kim Y-J, Ahn J-Y, Vu HT, Mandakhbayar N, Han M-R, Lee J-H, Kim J-B, Kim J-S, et al. Characterization of Physical and Biological Properties of a Caries-Arresting Liquid Containing Copper Doped Bioglass Nanoparticles. Pharmaceutics. 2022; 14(6):1137. https://doi.org/10.3390/pharmaceutics14061137
Chicago/Turabian StyleBang, Se-Jung, Soo-Kyung Jun, Yu-Jin Kim, Jun-Yong Ahn, Huong Thu Vu, Nandin Mandakhbayar, Mi-Ran Han, Jun-Haeng Lee, Jong-Bin Kim, Jong-Soo Kim, and et al. 2022. "Characterization of Physical and Biological Properties of a Caries-Arresting Liquid Containing Copper Doped Bioglass Nanoparticles" Pharmaceutics 14, no. 6: 1137. https://doi.org/10.3390/pharmaceutics14061137
APA StyleBang, S.-J., Jun, S.-K., Kim, Y.-J., Ahn, J.-Y., Vu, H. T., Mandakhbayar, N., Han, M.-R., Lee, J.-H., Kim, J.-B., Kim, J.-S., Knowles, J. C., Kim, H.-S., Lee, H.-H., Shin, J.-S., & Lee, J.-H. (2022). Characterization of Physical and Biological Properties of a Caries-Arresting Liquid Containing Copper Doped Bioglass Nanoparticles. Pharmaceutics, 14(6), 1137. https://doi.org/10.3390/pharmaceutics14061137







