The Effect of Encapsulated Apigenin Nanoparticles on HePG-2 Cells through Regulation of P53
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Fabrication of Albumin-Folic Acid
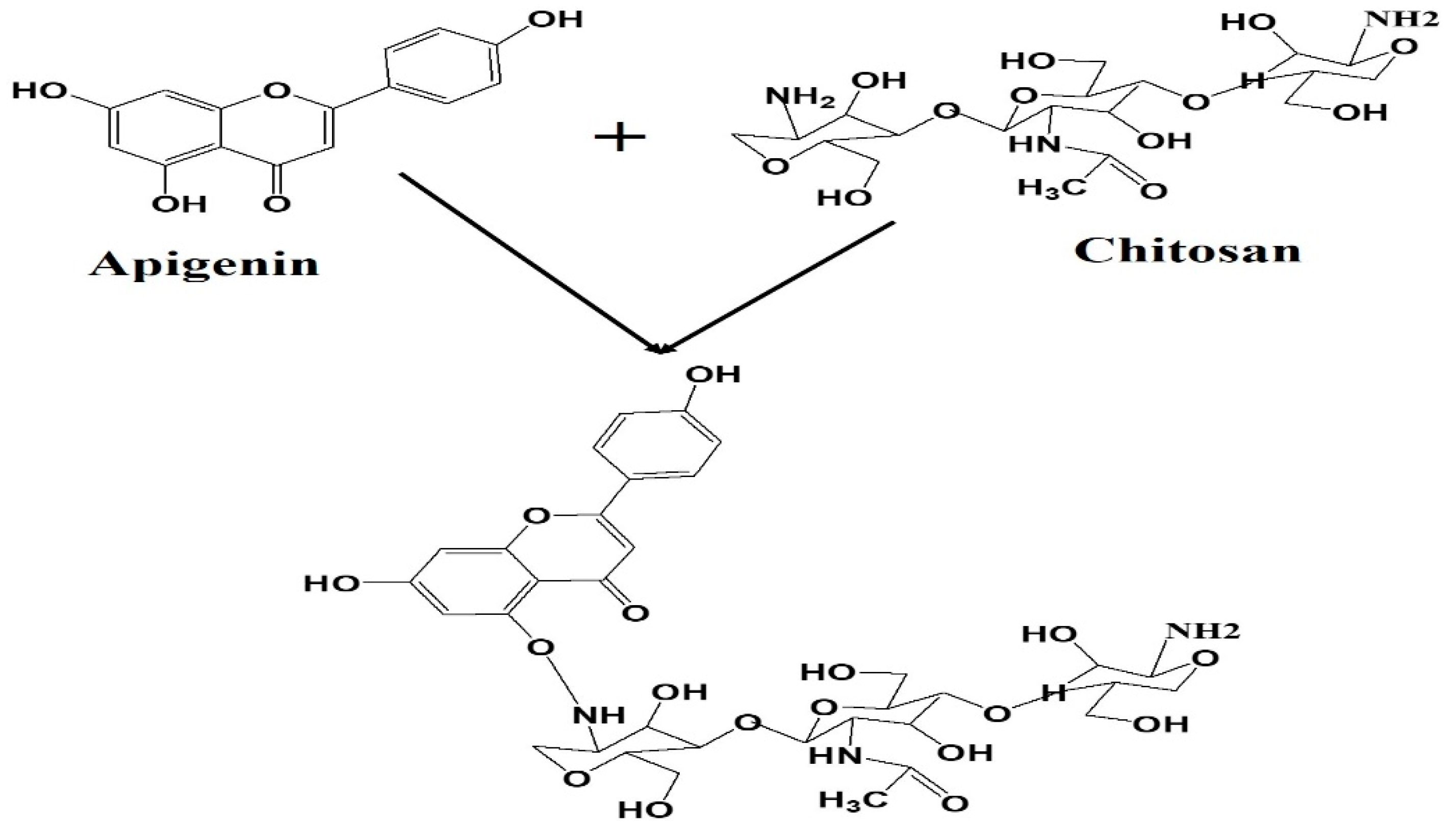
2.3. Fabrication of Apigenin NPs
Characterization
2.4. Determination of Encapsulation Efficiency
2.5. In Vitro Drug Release Study
2.6. Cellular Uptake and Targeting Capacity
2.7. MTT Assay
2.8. Annexin/PI Assay
2.9. Cell Cycle Examination
2.10. Real-Time PCR
2.11. Immunohistochemical Analysis of Bcl-2 Protein
2.12. Antioxidant Assay
2.13. Bio-Statistical Analysis
3. Results
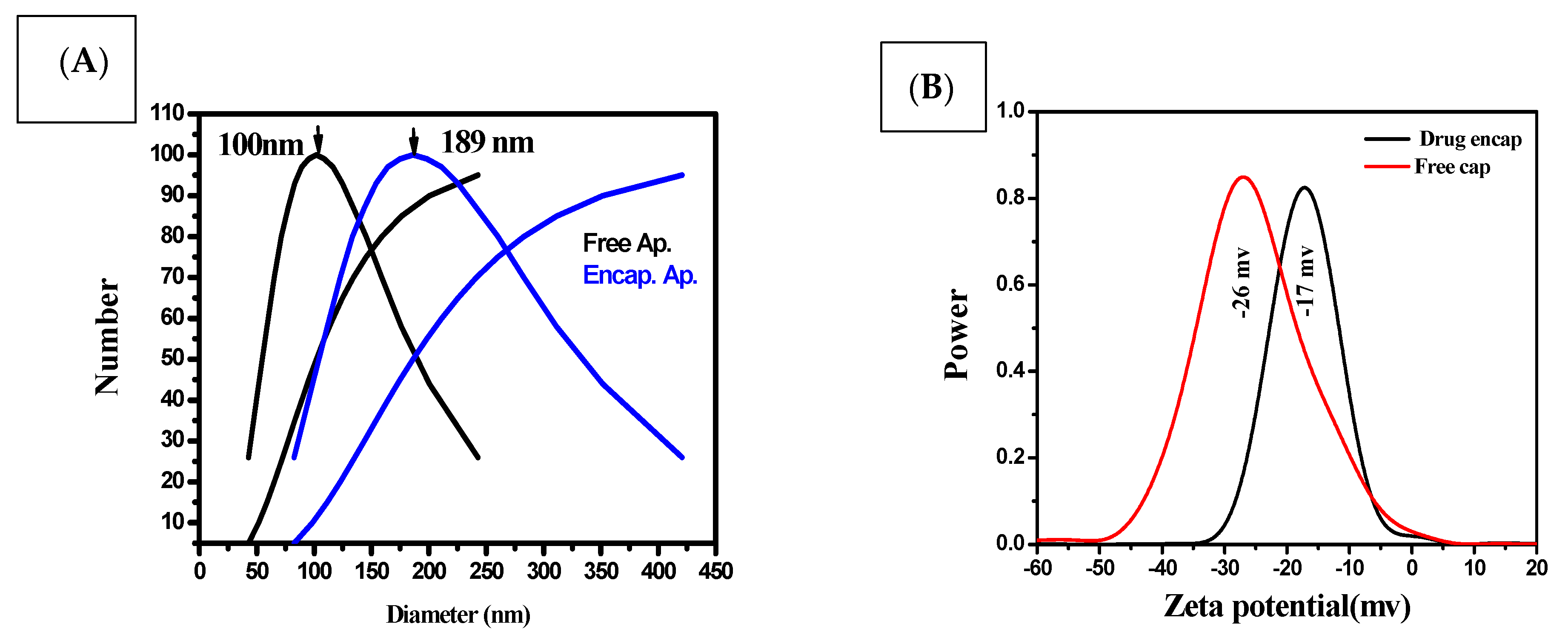
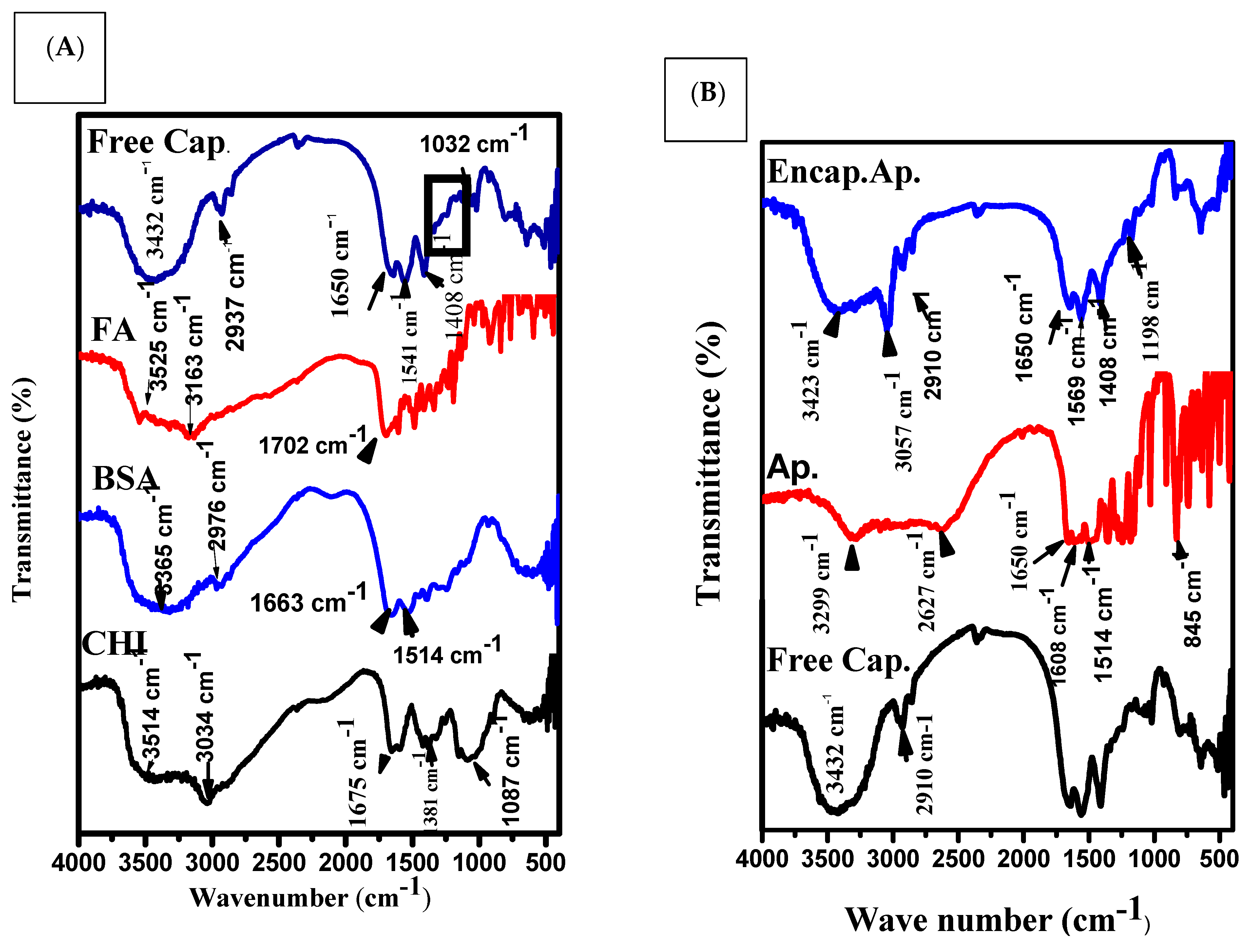
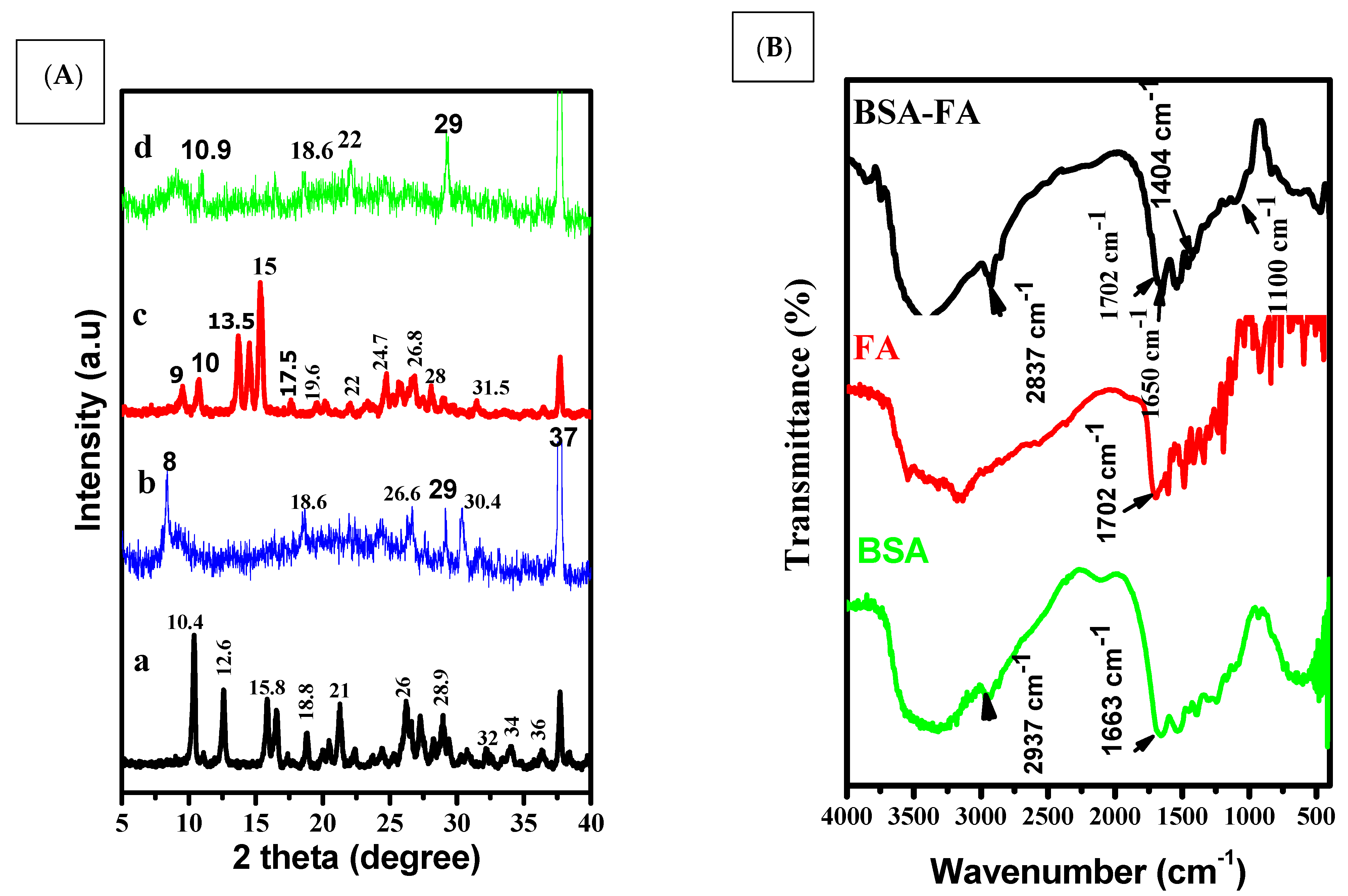
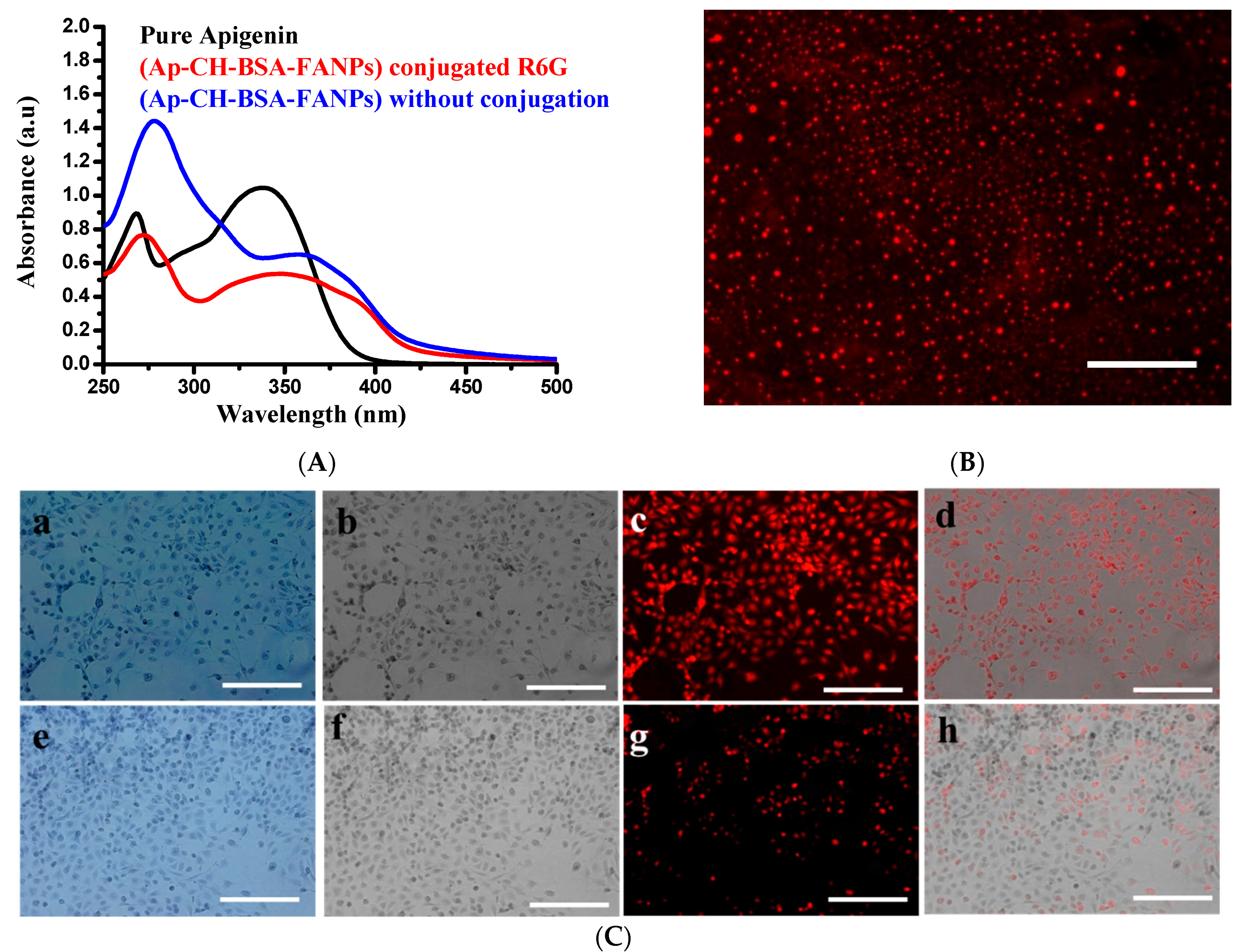
3.1. Size, Shape and Characteristics of the Encapsulated Apigenin
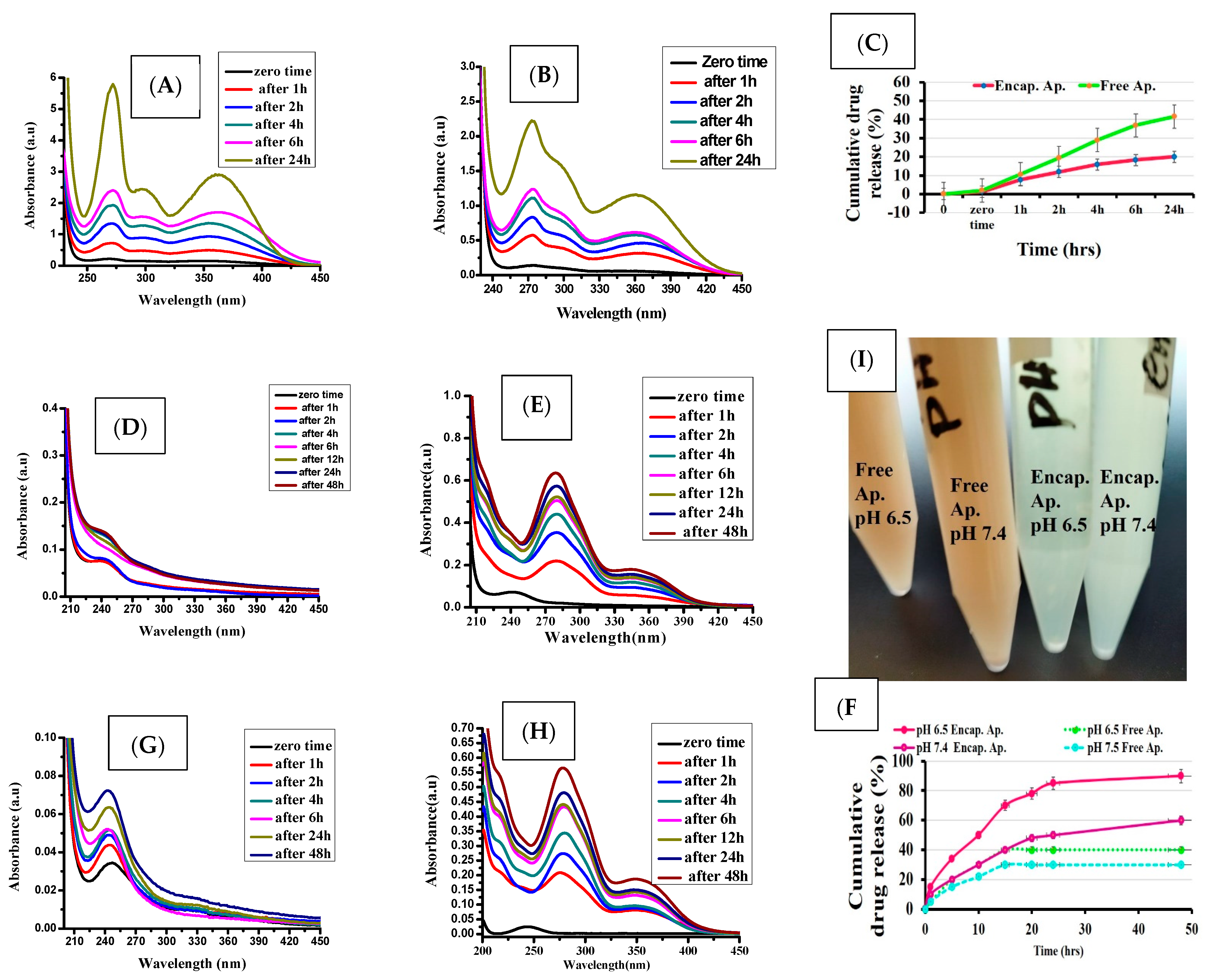
3.2. In Vitro Drug Release
3.3. Demonstration of the Targeting Capacity of the Nanoparticles
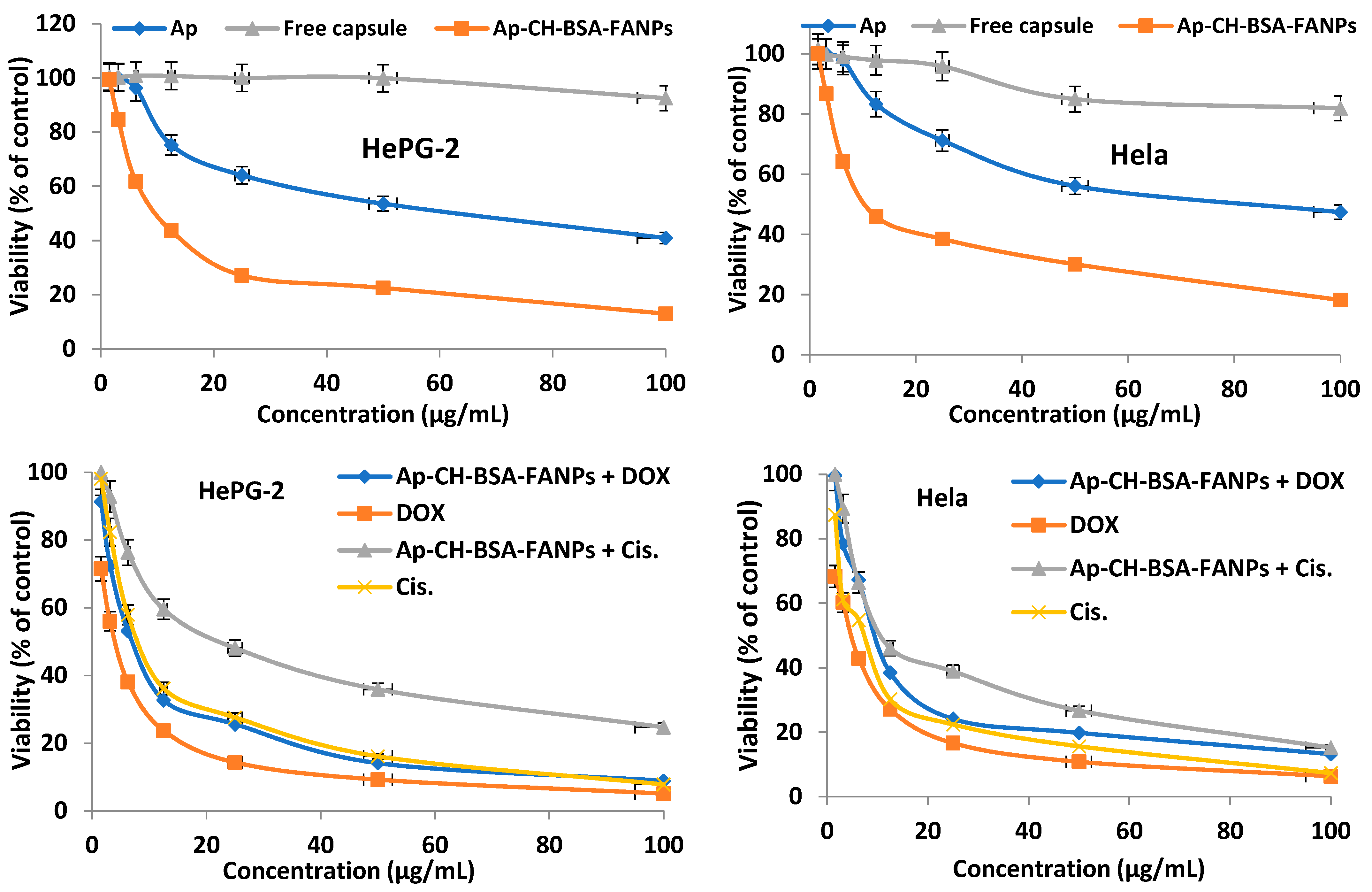
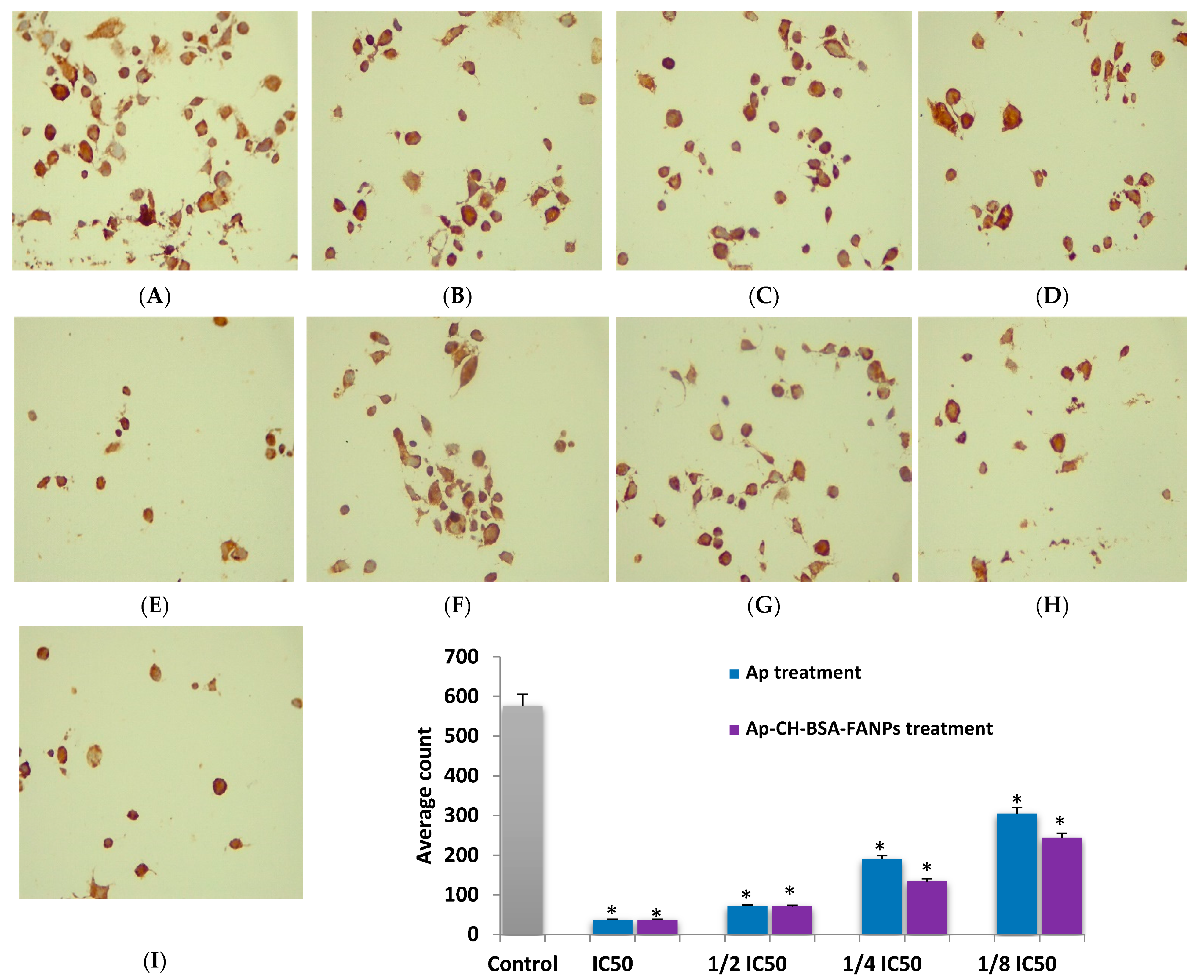
3.4. In Vitro Cytotoxic Activity
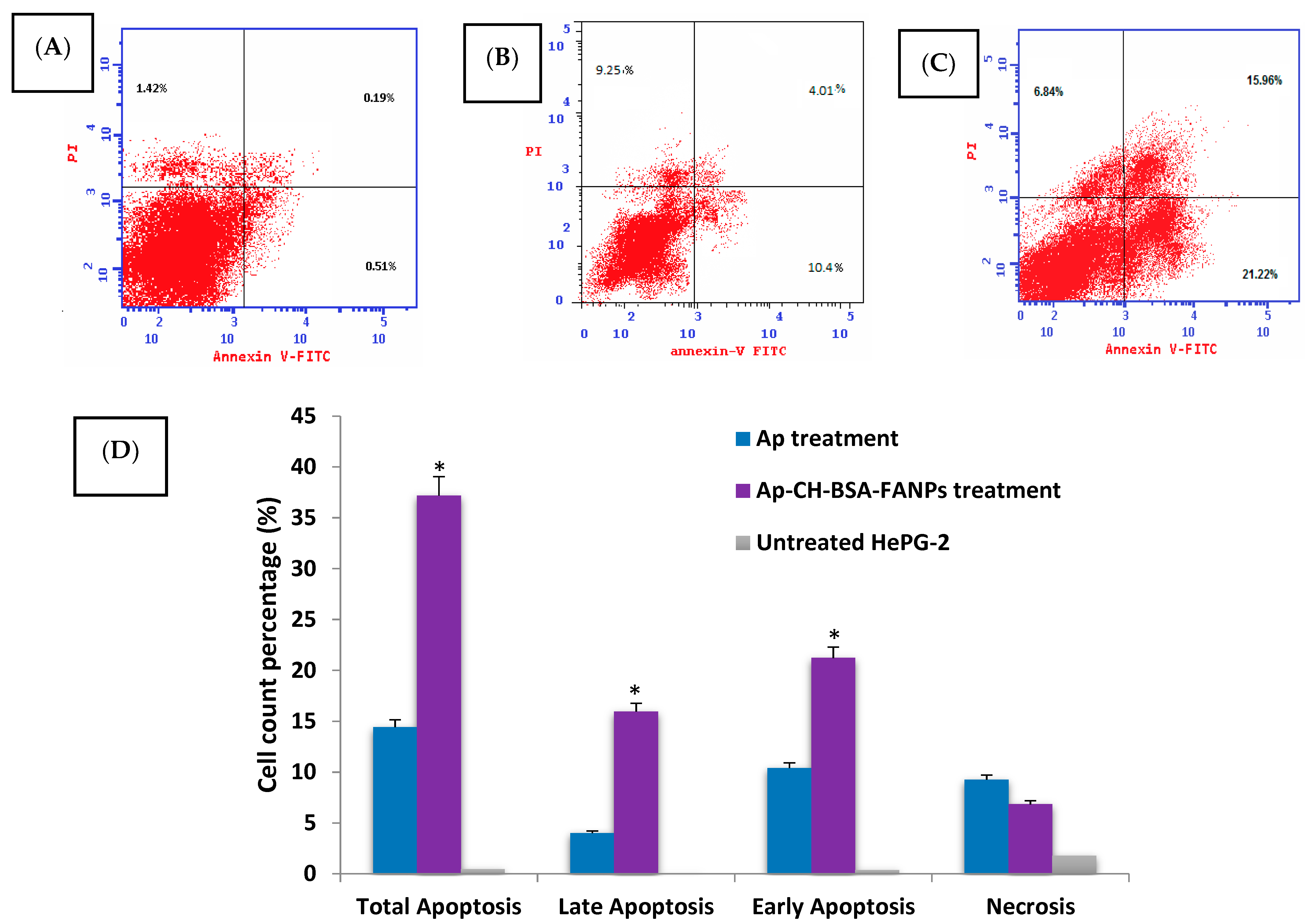
3.5. Ap-CH-BSA-FANPs Treatment Promotes HePG-2 Cell Apoptosis
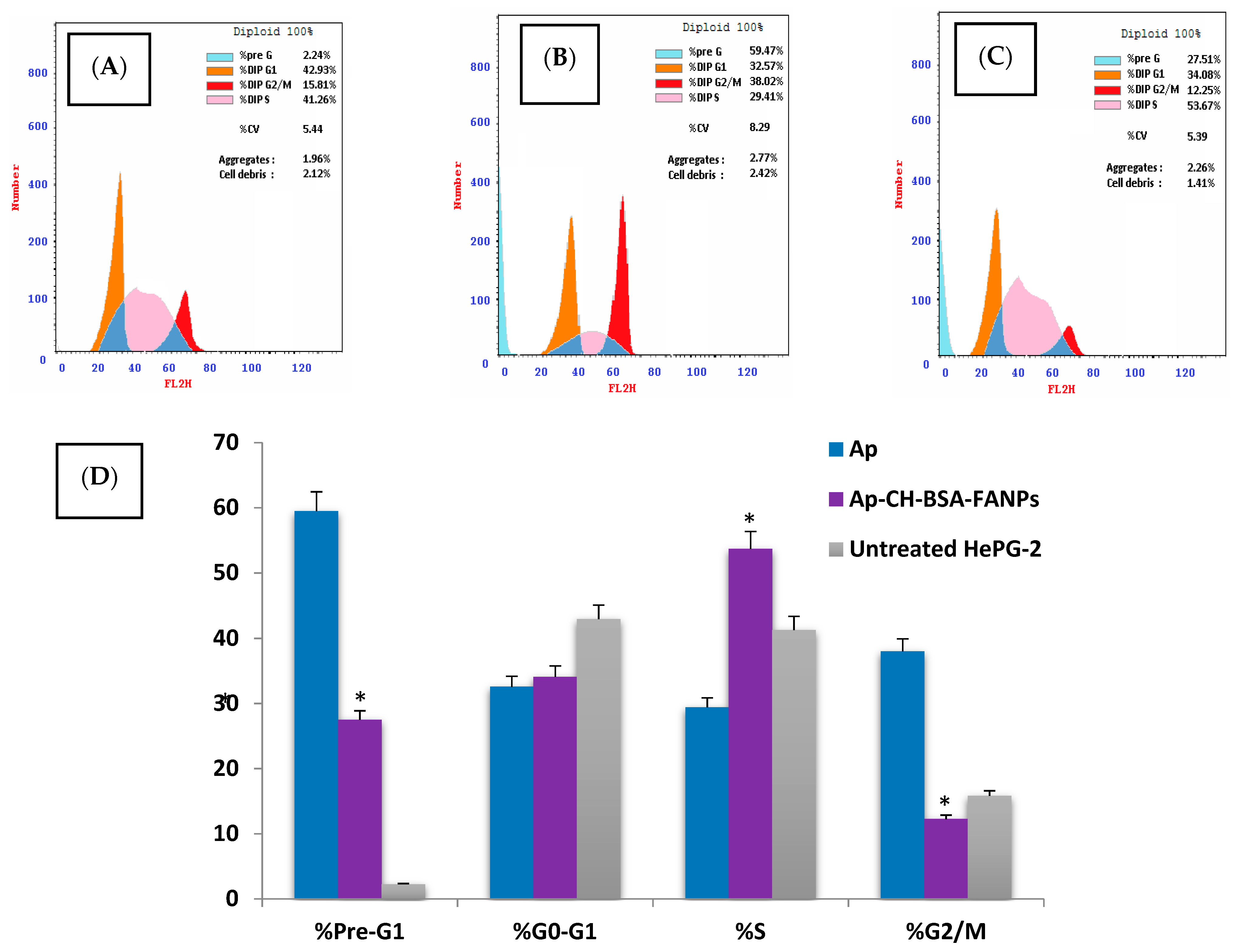
3.6. Ap-CH-BSA-FANPs Induced Cell Cycle Arrest
3.7. Ap-CH-BSA-FANPs Treatment Up-Regulated p53 and Caspase 9 and Down-Regulated MMP9 Genes Expression
3.8. Ap-CH-BSA-FANPs Treatment Down-Regulated Bcl-2 in a Concentration Dependent Manner
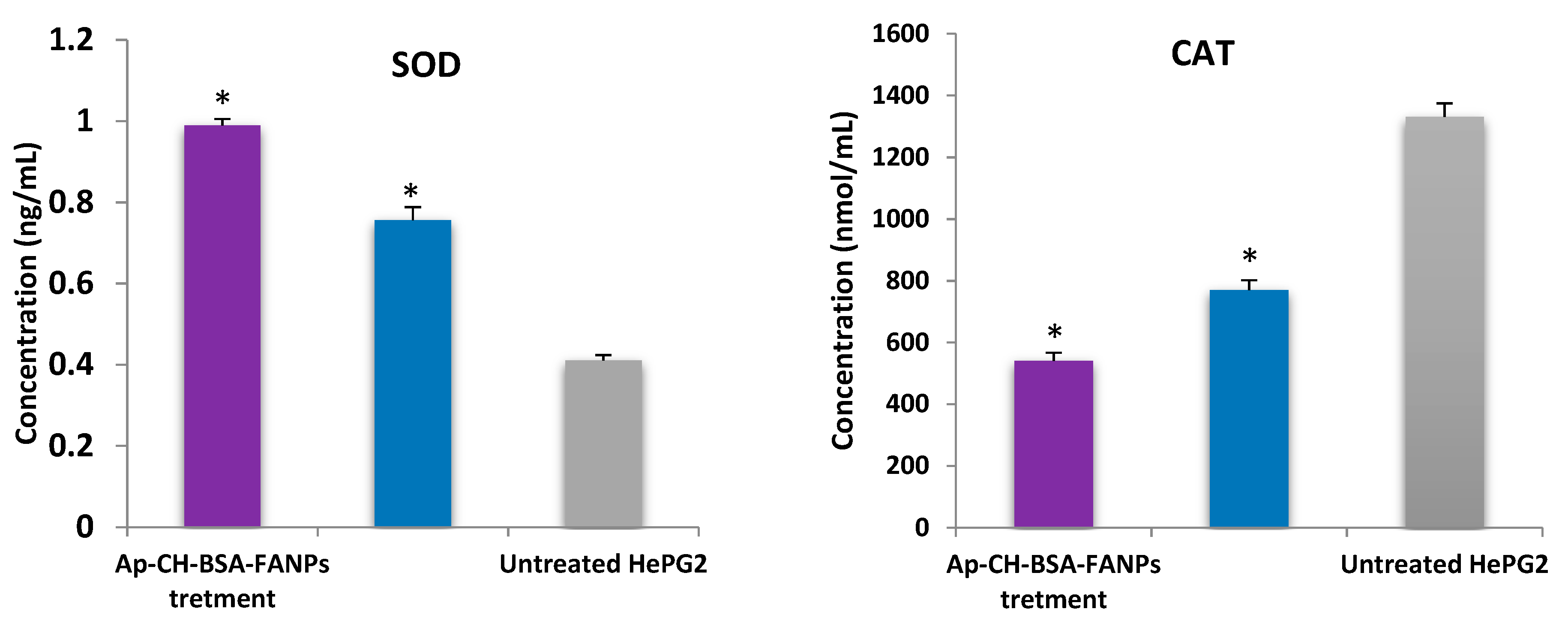
3.9. Ap-CH-BSA-FANPs Treatment Increased SOD Level and Inhibited CAT Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, H.S.; El-Serag, H.B. The Epidemiology of Hepatocellular Carcinoma in the USA. Curr. Gastroenterol. Rep. 2019, 21, 17. [Google Scholar] [CrossRef] [PubMed]
- Pérez, L.M.; López, S.A.; Fajes, J.L.H.; Martín, L.C. Hepatocellular carcinoma. Medicine 2020, 13, 653–665. [Google Scholar] [CrossRef]
- Kim, B.K.; Park, J.Y.; Choi, H.J.; Kim, D.Y.; Ahn, S.H.; Kim, J.K.; Lee, D.Y.; Lee, K.H.; Han, K.H. Long-term clinical outcomes of hepatic arterial infusion chemotherapy with cisplatin with or without 5-fluorouracil in locally advanced hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2011, 137, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Nahon, P.; Vibert, E.; Nault, J.C.; Ganne-Carrié, N.; Ziol, M.; Seror, O. Optimizing curative management of hepatocellular carcinoma. Liver Int. 2020, 40, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Duan, Z.Y.; Cai, G.Y.; Li, J.J.; Chen, X.M. Cisplatin-induced renal toxicity in elderly people. Ther. Adv. Med. Oncol. 2020, 12, 1758835920923430. [Google Scholar] [CrossRef]
- Mitry, M.A.; Edwards, J.G. Doxorubicin induced heart failure: Phenotype and molecular mechanisms. Int. J. Cardiol. Hear. Vasc. 2016, 10, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Mahbub, A.A.; Le Maitre, C.L.; Haywood-Small, S.; Cross, N.A.; Jordan-Mahy, N. Polyphenols enhance the activity of alkylating agents in leukaemia cell lines. Oncotarget 2019, 10, 4570–4586. [Google Scholar] [CrossRef]
- Hanafy, N.A.N. Thesis Development and Production of Multifunctional Bio-Nano-Engineered Drug Delivery Systems Loaded by TGF Inhibitors for Delivering into Hepatocellular Carcinoma Cells; Salento University: Salento, Italy, 2017. [Google Scholar]
- Mahbub, A.; Le Maitre, C.; Haywood-Small, S.; Cross, N.; Jordan-Mahy, N. Polyphenols act synergistically with doxorubicin and etoposide in leukaemia cell lines. Cell Death Discov. 2015, 1, 15043. [Google Scholar] [CrossRef]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The therapeutic potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Qi, M.; Li, P.; Zhan, Y.; Shao, H. Apigenin in cancer therapy: Anti-cancer effects and mechanisms of action. Cell Biosci. 2017, 7, 50. [Google Scholar] [CrossRef] [Green Version]
- Ali, F.; Rahul; Naz, F.; Jyoti, S.; Siddique, Y.H. Health functionality of apigenin: A review. Int. J. Food Prop. 2017, 20, 1197–1238. [Google Scholar] [CrossRef]
- Imran, M.; Aslam Gondal, T.; Atif, M.; Shahbaz, M.; Batool Qaisarani, T.; Hanif Mughal, M.; Salehi, B.; Martorell, M.; Sharifi-Rad, J. Apigenin as an anticancer agent. Phyther. Res. 2020, 34, 1812–1828. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Pi, C.; Wang, G. Inhibition of PI3K/Akt/mTOR pathway by apigenin induces apoptosis and autophagy in hepatocellular carcinoma cells. Biomed. Pharmacother. 2018, 103, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Han, X.; Cheng, W.; Ni, J.; Zhang, Y.; Lin, J.; Song, Z. Apigenin inhibits proliferation and invasion, and induces apoptosis and cell cycle arrest in human melanoma cells. Oncol. Rep. 2017, 37, 2277–2285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madunić, J.; Madunić, I.V.; Gajski, G.; Popić, J.; Garaj-Vrhovac, V. Apigenin: A dietary flavonoid with diverse anticancer properties. Cancer Lett. 2018, 413, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Bakhoda, M.R.; Bahmanpour, Z.; Ilkhani, K.; Zarrabi, A.; Makvandi, P.; Khan, H.; Mazaheri, S.; Darvish, M.; Mirzaei, H. Apigenin as Tumor Suppressor in Cancers: Biotherapeutic Activity, Nanodelivery, and Mechanisms With Emphasis on Pancreatic Cancer. Front. Chem. 2020, 8, 829. [Google Scholar] [CrossRef]
- Wu, W.; Zu, Y.; Zhao, X.; Zhang, X.; Wang, L.; Li, Y.; Wang, L.; Zhang, Y.; Lian, B. Solubility and dissolution rate improvement of the inclusion complex of apigenin with 2-hydroxypropyl-β-cyclodextrin prepared using the liquid antisolvent precipitation and solvent removal combination methods. Drug Dev. Ind. Pharm. 2017, 43, 1366–1377. [Google Scholar] [CrossRef]
- Karim, R.; Palazzo, C.; Laloy, J.; Delvigne, A.S.; Vanslambrouck, S.; Jerome, C.; Lepeltier, E.; Orange, F.; Dogne, J.M.; Evrard, B.; et al. Development and evaluation of injectable nanosized drug delivery systems for apigenin. Int. J. Pharm. 2017, 532, 757–768. [Google Scholar] [CrossRef] [Green Version]
- Ganguly, S.; Dewanjee, S.; Sen, R.; Chattopadhyay, D.; Ganguly, S.; Gaonkar, R.; Debnath, M.C. Apigenin-loaded galactose tailored PLGA nanoparticles: A possible strategy for liver targeting to treat hepatocellular carcinoma. Colloids Surfaces B Biointerfaces 2021, 204, 111778. [Google Scholar] [CrossRef]
- Mahmoudi, S.; Ghorbani, M.; Sabzichi, M.; Ramezani, F.; Hamishehkar, H.; Samadi, N. Targeted hyaluronic acid-based lipid nanoparticle for apigenin delivery to induce Nrf2-dependent apoptosis in lung cancer cells. J. Drug Deliv. Sci. Technol. 2019, 49, 268–276. [Google Scholar] [CrossRef]
- Dianzani, C.; Zara, G.P.; Maina, G.; Pettazzoni, P.; Pizzimenti, S.; Rossi, F.; Gigliotti, C.L.; Ciamporcero, E.S.; Daga, M.; Barrera, G. Drug delivery nanoparticles in skin cancers. Biomed Res. Int. 2014, 2014, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Farooque, F.; Wasi, M.; Mughees, M.M. Liposomes as Drug Delivery System: An Updated Review. J. Drug Deliv. Ther. 2021, 11, 149–158. [Google Scholar] [CrossRef]
- Kosinski, A.M.; Brugnano, J.L.; Seal, B.L.; Knight, F.C.; Panitch, A. Synthesis and characterization of a poly(lactic-co-glycolic acid) core + poly(N-isopropylacrylamide) shell nanoparticle system. Biomatter 2012, 2, 195–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.; Das, J.; Samadder, A.; Paul, A.; Khuda-Bukhsh, A.R. Efficacy of PLGA-loaded apigenin nanoparticles in Benzo[a]pyrene and ultraviolet-B induced skin cancer of mice: Mitochondria mediated apoptotic signalling cascades. Food Chem. Toxicol. 2013, 62, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, B.B.S.; Lasham, A.; Shelling, A.N.; Al-Kassas, R. Development of biodegradable PLGA nanoparticles surface engineered with hyaluronic acid for targeted delivery of paclitaxel to triple negative breast cancer cells. Mater. Sci. Eng. C 2017, 76, 593–600. [Google Scholar] [CrossRef]
- Elsayed, A.M.; Sherif, N.M.; Hassan, N.S.; Althobaiti, F.; Hanafy, N.A.N.; Sahyon, H.A. Novel quercetin encapsulated chitosan functionalized copper oxide nanoparticles as anti-breast cancer agent via regulating p53 in rat model. Int. J. Biol. Macromol. 2021, 185, 134–152. [Google Scholar] [CrossRef]
- Lakshmanan, V.-K.; Snima, K.S.; Bumgardner, J.D.; Nair, S.V.; Jayakumar, R. Chitosan-Based Nanoparticles in Cancer Therapy. In Advances in Polymer Science; Springer: Berlin, Germany, 2011; Volume 243, pp. 55–91. ISBN 9783642231131. [Google Scholar]
- Hanafy, N.A.N.; El-Kemary, M.A. Silymarin/curcumin loaded albumin nanoparticles coated by chitosan as muco-inhalable delivery system observing anti-inflammatory and anti COVID-19 characterizations in oleic acid triggered lung injury and in vitro COVID-19 experiment. Int. J. Biol. Macromol. 2022, 198, 101–110. [Google Scholar] [CrossRef]
- Sahyon, H.A.; Al-Harbi, S.A. Chemoprotective role of an extract of the heart of the Phoenix dactylifera tree on adriamycin-induced cardiotoxicity and nephrotoxicity by regulating apoptosis, oxidative stress and PD-1 suppression. Food Chem. Toxicol. 2020, 135, 111045. [Google Scholar] [CrossRef]
- Castro Nava, A.; Cojoc, M.; Peitzsch, C.; Cirillo, G.; Kurth, I.; Fuessel, S.; Erdmann, K.; Kunhardt, D.; Vittorio, O.; Hampel, S.; et al. Development of novel radiochemotherapy approaches targeting prostate tumor progenitor cells using nanohybrids. Int. J. Cancer 2015, 137, 2492–2503. [Google Scholar] [CrossRef] [Green Version]
- Saremi, S.; Atyabi, F.; Akhlaghi, S.P.; Ostad, S.N.; Dinarvand, R. Thiolated chitosan nanoparticles for enhancing oral absorption of docetaxel: Preparation, in vitro and ex vivo evaluation. Int. J. Nanomed. 2011, 6, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Hashemi, M.; Samadian, N. Effects of Apigenin and Apigenin- Loaded Nanogel on Induction of Apoptosis in Human Chronic Myeloid Leukemia Cells. Galen Med. J. 2018, 7, e1008. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, A.; Davaran, S.; Moosavi-Movahedi, A.A.; Dastmalchi, S. Investigating the interaction of juglone (5-hydroxy-1,4-naphthoquinone) with serum albumins using spectroscopic and in silico methods. J. Iran. Chem. Soc. 2017, 14, 1527–1540. [Google Scholar] [CrossRef]
- Niaz, T.; Imran, M.; Mackie, A. Improving carvacrol bioaccessibility using core–shell carrier-systems under simulated gastrointestinal digestion. Food Chem. 2021, 353, 129505. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Liu, Y.; Gao, L.; Zhang, Y.; Yi, J. Improved chemical stability and cellular antioxidant activity of resveratrol in zein nanoparticle with bovine serum albumin-caffeic acid conjugate. Food Chem. 2018, 261, 283–291. [Google Scholar] [CrossRef]
- Sleep, D. Albumin and its application in drug delivery. Expert Opin. Drug Deliv. 2015, 12, 793–812. [Google Scholar] [CrossRef]
- Caliceti, P.; Salmaso, S.; Semenzato, A.; Carofiglio, T.; Fornasier, R.; Fermeglia, M.; Ferrone, M.; Pricl, S. Synthesis and physicochemical characterization of folate-cyclodextrin bioconjugate for active drug delivery. Bioconjug. Chem. 2003, 14, 899–908. [Google Scholar] [CrossRef]
- Geersing, A.; de Vries, R.H.; Jansen, G.; Rots, M.G.; Roelfes, G. Folic acid conjugates of a bleomycin mimic for selective targeting of folate receptor positive cancer cells. Bioorg. Med. Chem. Lett. 2019, 29, 1922–1927. [Google Scholar] [CrossRef]
- Tagawa, H.; Maruyama, K.; Sasaki, K.; Konoue, N.; Kishimura, A.; Kanai, M.; Mori, T.; Oisaki, K.; Katayama, Y. Induction of ADCC by a folic acid-mAb conjugate prepared by tryptophan-selective reaction toward folate-receptor-positive cancer cells. RSC Adv. 2020, 10, 16727–16731. [Google Scholar] [CrossRef]
- Hanafy, N.A.N.; Quarta, A.; Di Corato, R.; Dini, L.; Nobile, C.; Tasco, V.; Carallo, S.; Cascione, M.; Malfettone, A.; Soukupova, J.; et al. Hybrid polymeric-protein nano-carriers (HPPNC) for targeted delivery of TGFβ inhibitors to hepatocellular carcinoma cells. J. Mater. Sci. Mater. Med. 2017, 28, 120. [Google Scholar] [CrossRef]
- Özbilenler, C.; Altundağ, E.M.; Gazi, M. Synthesis of quercetin-encapsulated alginate beads with their antioxidant and release kinetic studies. J. Macromol. Sci. Part A Pure Appl. Chem. 2020, 58, 22–31. [Google Scholar] [CrossRef]
- Hanafy, N.A.N.; Leporatti, S.; El-Kemary, M.A. Extraction of chlorophyll and carotenoids loaded into chitosan as potential targeted therapy and bio imaging agents for breast carcinoma. Int. J. Biol. Macromol. 2021, 182, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, N.A.N.; Leporatti, S.; El-Kemary, M. Mucoadhesive curcumin crosslinked carboxy methyl cellulose might increase inhibitory efficiency for liver cancer treatment. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 116, 111119. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, G.; Meyyanathan, S.N. Gowramma byran stability-indicating reverse-phase high-performance liquid chromatography method for the simultaneous quantification of apigenin and luteolin from achillea millefolium linn. Asian J. Pharm. Clin. Res. 2019, 12, 169–172. [Google Scholar] [CrossRef]
- Uma Maheswari, P.; Muthappa, R.; Bindhya, K.P.; Meera Sheriffa Begum, K.M. Evaluation of folic acid functionalized BSA-CaFe2O4 nanohybrid carrier for the controlled delivery of natural cytotoxic drugs hesperidin and eugenol. J. Drug Deliv. Sci. Technol. 2021, 61, 102105. [Google Scholar] [CrossRef]
- Boeris, V.; Farruggia, B.; Picó, G. Chitosan-bovine serum albumin complex formation: A model to design an enzyme isolation method by polyelectrolyte precipitation. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 1543–1548. [Google Scholar] [CrossRef]
- Brad, K.; Zhang, Y. Study on extraction and purification of apigenin and the physical and chemical properties of its complex with lecithin. Pharmacogn. Mag. 2018, 14, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Mondal, L.; Mukherjee, B.; Dutta, L.; Ehsan, I.; Debnath, M.C.; Gaonkar, R.H.; Pal, M.M.; Majumdar, S. Apigenin loaded nanoparticle delayed development of hepatocellular carcinoma in rats. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1905–1917. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Wang, W.; Wu, Y.; He, Y.; Wu, T. Oxidative degradation of chitosan to the low molecular water-soluble chitosan over peroxotungstate as chemical scissors. PLoS ONE 2014, 9, e100743. [Google Scholar] [CrossRef]
- Sathivel, A.; Balavinayagamani; Hanumantha Rao, B.R.; Devaki, T. Sulfated polysaccharide isolated from Ulva lactuca attenuates D-galactosamine induced DNA fragmentation and necrosis during liver damage in rats. Pharm. Biol. 2014, 52, 498–505. [Google Scholar] [CrossRef]
- Solanki, R.; Patel, K.; Patel, S. Bovine Serum Albumin Nanoparticles for the Efficient Delivery of Berberine: Preparation, Characterization and In vitro biological studies. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 608, 125501. [Google Scholar] [CrossRef]
- Zhang, C.; Hao, Y.; Wu, L.; Dong, X.; Jiang, N.; Cong, B.; Liu, J.; Zhang, W.; Tang, D.; De Perrot, M.; et al. Curcumin induces apoptosis and inhibits angiogenesis in murine malignant mesothelioma. Int. J. Oncol. 2018, 53, 2531–2541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabrowska, J.; Messyasz, B.; Szyling, J.; Walkowiak, J.; Łęska, B. Isolation of chlorophylls and carotenoids from freshwater algae using different extraction methods. Phycol. Res. 2018, 66, 52–57. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, X.; Zu, Y.; Wang, L.; Deng, Y.; Wu, M.; Wang, H. Enhanced solubility and bioavailability of apigenin via preparation of solid dispersions of mesoporous silica nanoparticles. Iran. J. Pharm. Res. 2019, 18, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Lemasters, J.J.; Qian, T.; Bradham, C.A.; Brenner, D.A.; Cascio, W.E.; Trost, L.C.; Nishimura, Y.; Nieminen, A.L.; Herman, B. Mitochondrial dysfunction in the pathogenesis of necrotic and apoptotic cell death. J. Bioenerg. Biomembr. 1999, 31, 305–319. [Google Scholar] [CrossRef] [PubMed]
- Proskuryakov, S.Y.; Gabai, V.L. Mechanisms of tumor cell necrosis. Curr. Pharm. Des. 2010, 16, 56–68. [Google Scholar] [CrossRef]
- Hanafy, N.; Dini, L.; Citti, C.; Cannazza, G.; Leporatti, S. Inihibition of Glycolysis by Using a Micro/Nano-Lipid Bromopyruvic Chitosan Carrier as a Promising Tool to Improve Treatment of Hepatocellular Carcinoma. Nanomaterials 2018, 8, 34. [Google Scholar] [CrossRef] [Green Version]
- Giannattasio, M.; Branzei, D. S-phase checkpoint regulations that preserve replication and chromosome integrity upon dNTP depletion. Cell. Mol. Life Sci. 2017, 74, 2361–2380. [Google Scholar] [CrossRef] [Green Version]
- Hanafy, N.A.N. Optimally designed theranostic system based folic acids and chitosan as a promising mucoadhesive delivery system for encapsulating curcumin LbL nano-template against invasiveness of breast cancer. Int. J. Biol. Macromol. 2021, 182, 1981–1993. [Google Scholar] [CrossRef]
- Arung, E.T.; Wicaksono, B.D.; Handoko, Y.A.; Kusuma, I.W.; Yulia, D.; Sandra, F. Anti-cancer properties of diethylether extract of wood from Sukun (Artocarpus altilis) in human breast cancer (T47D) cells. Trop. J. Pharm. Res. 2009, 8, 45223. [Google Scholar] [CrossRef] [Green Version]
- O’Connell, M.J.; Walworth, N.C.; Carr, A.M. The G2-phase DNA-damage checkpoint. Trends Cell Biol. 2000, 10, 296–303. [Google Scholar] [CrossRef]
- Chung, T.W.; Lin, S.C.; Su, J.H.; Chen, Y.K.; Lin, C.C.; Chan, H.L. Sinularin induces DNA damage, G2/M phase arrest, and apoptosis in human hepatocellular carcinoma cells. BMC Complement. Altern. Med. 2017, 17, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.; Sung, B.; Kang, Y.J.; Kim, D.H.; Jang, J.Y.; Hwang, S.Y.; Kim, M.; Lim, H.S.; Yoon, J.H.; Chung, H.Y.; et al. Apigenin-induced apoptosis is enhanced by inhibition of autophagy formation in HCT116 human colon cancer cells. Int. J. Oncol. 2014, 44, 1599–1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, T.H.; Chien, M.H.; Lin, W.L.; Wen, Y.C.; Chow, J.M.; Chen, C.K.; Kuo, T.C.; Lee, W.J. Inhibition of MDA-MB-231 breast cancer cell proliferation and tumor growth by apigenin through induction of G2/M arrest and histone H3 acetylation-mediated p21WAF1/CIP1 expression. Environ. Toxicol. 2017, 32, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Zhu, Y.; Li, J.F.; Wang, X.; Liang, Z.; Li, S.Q.; Xu, X.; Chen, H.; Liu, B.; Zheng, X.Y.; et al. Apigenin inhibits renal cell carcinoma cell proliferation. Oncotarget 2017, 8, 19834–19842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, E.J.; Kim, G.H. Apigenin causes G2/M arrest associated with the modulation of p21Cip1 and Cdc2 and activates p53-dependent apoptosis pathway in human breast cancer SK-BR-3 cells. J. Nutr. Biochem. 2009, 20, 285–290. [Google Scholar] [CrossRef]
- Fang, J.; Yang Bao, Y.; Zhou, S.H.; Fan, J. Apigenin inhibits the proliferation of adenoid cystic carcinoma via suppression of glucose transporter-1. Mol. Med. Rep. 2015, 12, 6461–6466. [Google Scholar] [CrossRef] [Green Version]
- Pietenpol, J.A.; Stewart, Z.A. Cell cycle checkpoint signaling: Cell cycle arrest versus apoptosis. Toxicology 2002, 181–182, 475–481. [Google Scholar] [CrossRef]
- Li, T.; Kon, N.; Jiang, L.; Tan, M.; Ludwig, T.; Zhao, Y.; Baer, R.; Gu, W. Tumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and senescence. Cell 2012, 149, 1269–1283. [Google Scholar] [CrossRef] [Green Version]
- Nault, J.C.; Zucman-Rossi, J. Genetics of hepatobiliary carcinogenesis. Semin. Liver Dis. 2011, 31, 173–187. [Google Scholar] [CrossRef]
- Zhang, M.F.; Zhang, Z.Y.; Fu, J.; Yang, Y.F.; Yun, J.P. Correlation between expression of p53, p21/WAF1, and MDM2 proteins and their prognostic significance in primary hepatocellular carcinoma. J. Transl. Med. 2009, 7, 110. [Google Scholar] [CrossRef] [Green Version]
- Yuan, R.H.; Jeng, Y.M.; Hu, R.H.; Lai, P.L.; Lee, P.H.; Cheng, C.C.; Hsu, H.C. Role of p53 and β-catenin mutations in conjunction with CK19 expression on early tumor recurrence and prognosis of hepatocellular carcinoma. J. Gastrointest. Surg. 2011, 15, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.S.; Choi, H.S.; Kim, S.R.; Choi, Y.K.; Woo, S.M.; Shin, I.; Woo, J.K.; Park, S.Y.; Shin, Y.C.; Ko, S.K. Apigenin induces apoptosis via extrinsic pathway, inducing p53 and inhibiting STAT3 and NFκB signaling in HER2-overexpressing breast cancer cells. Mol. Cell. Biochem. 2012, 366, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.W.; Chiang, L.C.; Lin, C.C. Apigenin induced apoptosis through p53-dependent pathway in human cervical carcinoma cells. Life Sci. 2005, 76, 1367–1379. [Google Scholar] [CrossRef]
- Deryugina, E.I.; Quigley, J.P. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006, 25, 9–34. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.D.; Matrisian, L.M. The other side of MMPs: Protective roles in tumor progression. Cancer Metastasis Rev. 2007, 26, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Pellikainen, J.M.; Ropponen, K.M.; Kataja, V.V.; Kellokoski, J.K.; Eskelinen, M.J.; Kosma, V.M. Expression of matrix metalloproteinase (MMP)-2 and MMP-9 in breast cancer with a special reference to activator protein-2, HER2, and prognosis. Clin. Cancer Res. 2004, 10, 7621–7628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zucker, S.; Vacirca, J. Role of matrix metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis Rev. 2004, 23, 101–117. [Google Scholar] [CrossRef]
- Shin, Y.J.; Kim, J.H. The role of EZH2 in the regulation of the activity of matrix metalloproteinases in prostate cancer cells. PLoS ONE 2012, 7, e30393. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Wang, S.; Song, Y.; Yao, J.; Huang, K.; Zhu, X. Apigenin suppresses colorectal cancer cell proliferation, migration and invasion via inhibition of the Wnt/β-catenin signaling pathway. Oncol. Lett. 2016, 11, 3075–3080. [Google Scholar] [CrossRef] [Green Version]
- Hasnat, M.A.; Pervin, M.; Lim, J.H.; Lim, B.O. Apigenin attenuates melanoma cell migration by inducing anoikis through integrin and focal adhesion kinase inhibition. Molecules 2015, 20, 21157–21166. [Google Scholar] [CrossRef]
- Zhou, Z.; Tang, M.; Liu, Y.; Zhang, Z.; Lu, R.; Lu, J. Apigenin inhibits cell proliferation, migration, and invasion by targeting Akt in the A549 human lung cancer cell line. Anticancer. Drugs 2017, 28, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.H.; Chu, J.H.; Kwan, H.Y.; Su, T.; Yu, H.; Cheng, C.Y.; Fu, X.Q.; Guo, H.; Li, T.; Tse, A.K.W.; et al. Inhibition of the STAT3 signaling pathway contributes to apigenin-mediated anti-metastatic effect in melanoma. Sci. Rep. 2016, 6, 21731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saraste, A.; Pulkki, K.; Kallajoki, M.; Henriksen, K.; Parvinen, M.; Voipio-Pulkki, L.-M. Apoptosis in human acute myocardial infarction. Circulation 1997, 95, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.F.R. History of the events leading to the formulation of the apoptosis concept. Toxicology 2002, 181, 471–474. [Google Scholar] [CrossRef]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef] [Green Version]
- Budhraja, A.; Gao, N.; Zhang, Z.; Son, Y.O.; Cheng, S.; Wang, X.; Ding, S.; Hitron, A.; Chen, G.; Luo, J.; et al. Apigenin induces apoptosis in human leukemia cells and exhibits anti-leukemic activity In Vivo. Mol. Cancer Ther. 2012, 11, 132–142. [Google Scholar] [CrossRef] [Green Version]
- Hussain, A.R.; Khan, A.S.; Ahmed, S.O.; Ahmed, M.; Platanias, L.C.; Al-Kuraya, K.S.; Uddin, S. Apigenin induces apoptosis via downregulation of S-phase kinase-associated protein 2-mediated induction of p27Kip1 in primary effusion lymphoma cells. Cell Prolif. 2010, 43, 170–183. [Google Scholar] [CrossRef]
- Sajadimajd, S.; Bahramsoltani, R.; Iranpanah, A.; Kumar Patra, J.; Das, G.; Gouda, S.; Rahimi, R.; Rezaeiamiri, E.; Cao, H.; Giampieri, F.; et al. Advances on Natural Polyphenols as Anticancer Agents for Skin Cancer. Pharmacol. Res. 2020, 151, 104584. [Google Scholar] [CrossRef]
- Ozaki, T.; Nakagawara, A. Role of p53 in cell death and human cancers. Cancers 2011, 3, 994–1013. [Google Scholar] [CrossRef]
- Panieri, E.; Santoro, M.M. Ros homeostasis and metabolism: A dangerous liason in cancer cells. Cell Death Dis. 2016, 7, e2253. [Google Scholar] [CrossRef] [Green Version]
- Ismy, J.; Sugandi, S.; Rachmadi, D.; Hardjowijoto, S.; Mustafa, A. The effect of exogenous superoxide dismutase (SOD) on caspase-3 activation and apoptosis induction in PC-3 prostate cancer cells. Res. Rep. Urol. 2020, 12, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Glorieux, C.; Calderon, P.B. Catalase down-regulation in cancer cells exposed to arsenic trioxide is involved in their increased sensitivity to a pro-oxidant treatment. Cancer Cell Int. 2018, 18, 24. [Google Scholar] [CrossRef] [PubMed]
- Telange, D.R.; Patil, A.T.; Pethe, A.M.; Fegade, H.; Anand, S.; Dave, V.S. Formulation and characterization of an apigenin-phospholipid phytosome (APLC) for improved solubility, in vivo bioavailability, and antioxidant potential. Eur. J. Pharm. Sci. 2017, 108, 36–49. [Google Scholar] [CrossRef] [Green Version]
- Pápay, Z.E.; Kósa, A.; Böddi, B.; Merchant, Z.; Saleem, I.Y.; Zariwala, M.G.; Klebovich, I.; Somavarapu, S.; Antal, I. Study on the Pulmonary Delivery System of Apigenin-Loaded Albumin Nanocarriers with Antioxidant Activity. J. Aerosol. Med. Pulm. Drug Deliv. 2017, 30, 274–288. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.M.; Yang, P.W.; Feng, X.J.; Zhu, Y.W.; Qiu, F.J.; Hu, X.D.; Zhang, X.H. Apigenin Inhibits the Growth of Hepatocellular Carcinoma Cells by Affecting the Expression of microRNA Transcriptome. Front. Oncol. 2021, 11, 657665. [Google Scholar] [CrossRef]
- Hussain, S.P.; Schwank, J.; Staib, F.; Wang, X.W.; Harris, C.C. TP53 mutations and hepatocellular carcinoma: Insights into the etiology and pathogenesis of liver cancer. Oncogene 2007, 26, 2166–2176. [Google Scholar] [CrossRef] [Green Version]
- Dhar, D.; Antonucci, L.; Nakagawa, H.; Kim, J.Y.; Glitzner, E.; Caruso, S.; Shalapour, S.; Yang, L.; Valasek, M.A.; Lee, S.; et al. Liver Cancer Initiation Requires p53 Inhibition by CD44-Enhanced Growth Factor Signaling. Cancer Cell 2018, 33, 1061–1077.e6. [Google Scholar] [CrossRef] [Green Version]
- Guan, Y.; He, Q.; La, Z. Roles of p53 in Carcinogenesis, Diagnosis and Treatment of Hepatocellular Carcinoma. J. Cancer Mol. 2006, 2, 191–197. [Google Scholar]



| Compounds | HePG-2 | Hela |
|---|---|---|
| Apigenin | 57.86 ± 2.9 | 74.71 ± 3.6 |
| Free capsule nanoparticles | 556.3 ± 71.5 *,# | 251.2 ± 29.7 *,# |
| Encapsulated apigenin | 11.49 ± 0.9 * | 15.16 ± 1.2 * |
| Encapsulated apigenin + DOX | 7.76 ± 0.6 * | 10.53 ± 0.9 * |
| Encapsulated apigenin + Cis | 24.63 ± 2.1 *,# | 14.93 ± 1.2 * |
| DOX | 3.93 ± 0.2 *,# | 4.34 ± 0.3 *,# |
| Cis | 9.53 ± 0.7 * | 6.63 ± 0.05 *,# |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mabrouk Zayed, M.M.; Sahyon, H.A.; Hanafy, N.A.N.; El-Kemary, M.A. The Effect of Encapsulated Apigenin Nanoparticles on HePG-2 Cells through Regulation of P53. Pharmaceutics 2022, 14, 1160. https://doi.org/10.3390/pharmaceutics14061160
Mabrouk Zayed MM, Sahyon HA, Hanafy NAN, El-Kemary MA. The Effect of Encapsulated Apigenin Nanoparticles on HePG-2 Cells through Regulation of P53. Pharmaceutics. 2022; 14(6):1160. https://doi.org/10.3390/pharmaceutics14061160
Chicago/Turabian StyleMabrouk Zayed, Mayada Mohamed, Heba A. Sahyon, Nemany A. N. Hanafy, and Maged A. El-Kemary. 2022. "The Effect of Encapsulated Apigenin Nanoparticles on HePG-2 Cells through Regulation of P53" Pharmaceutics 14, no. 6: 1160. https://doi.org/10.3390/pharmaceutics14061160
APA StyleMabrouk Zayed, M. M., Sahyon, H. A., Hanafy, N. A. N., & El-Kemary, M. A. (2022). The Effect of Encapsulated Apigenin Nanoparticles on HePG-2 Cells through Regulation of P53. Pharmaceutics, 14(6), 1160. https://doi.org/10.3390/pharmaceutics14061160







