Predicting the Water Sorption in ASDs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film Preparation
2.3. Water-Sorption Measurements
2.4. Crystallinity Check
3. Modeling
3.1. PC-SAFT
3.2. Calculations of Water-Sorption Isotherms
3.3. Water-Sorption Kinetics
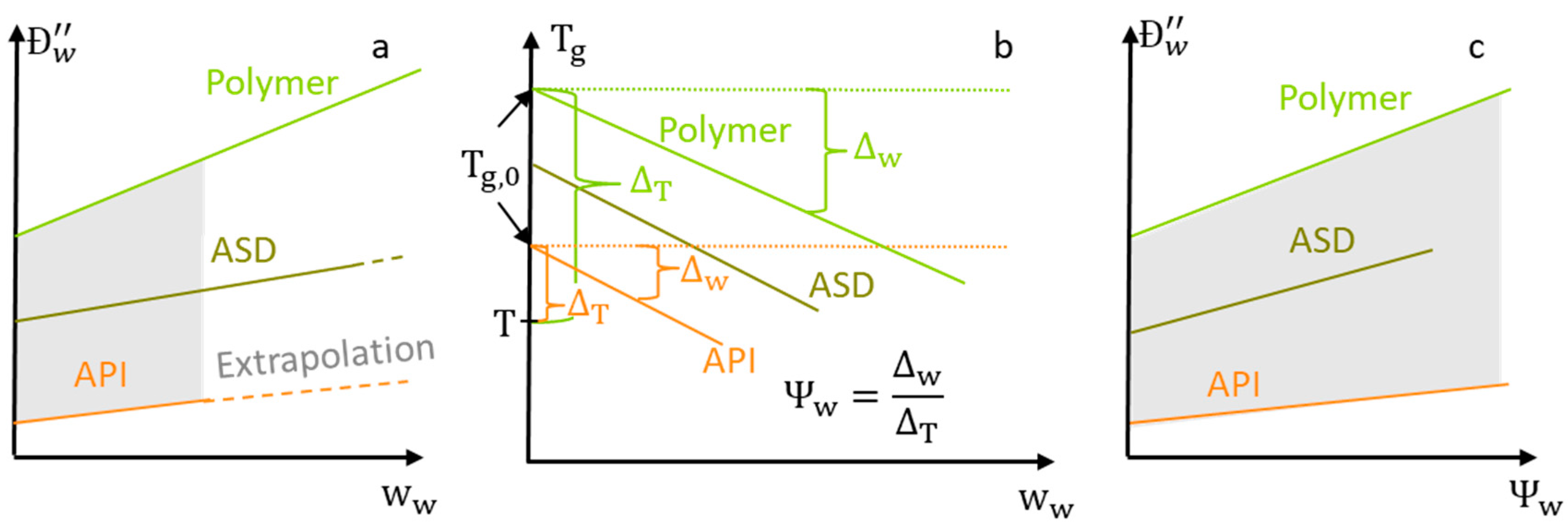
3.4. Water Concentration Dependency of the Water-Diffusion Coefficients
3.5. Model Parameters
| PVP [7] | PVPVA [21] | IND [22] | Water [23] | |
|---|---|---|---|---|
| 25,700 | 65,000 | 357.79 | 18.02 | |
| 0.0407 | 0.0372 | 0.03992 | 0.06687 | |
| 2.71 | 2.947 | 3.535 | 2.7971 | |
| 205.992 | 205.271 | 262.791 | 353.94 | |
| 0 | 0 | 886.44 | 2425.67 | |
| 0.02 | 0.02 | 0.02 | 0.0451 | |
| 231/231 | 653/653 | 3/3 | 1/1 | |
| −0.128 a | −0.128 a | −0.022 b | N.A. | |
| 0.6637 a | 0.7478 a | N.A. | ||
| 0.4279 a | 0.244 a | 0 | N.A. | |
| 1250 | 1190 | 1320 | 997 | |
| 441.51 | 383.9 | 317.6 | 136 | |
| 0.253 c | 0.3 c | 0.11 [24] | N.A. |
| PVP a | PVPVA a | IND b | ||||
|---|---|---|---|---|---|---|
/- | /10−15 m2 s−1 | /- | /10−15 m2 s−1 | /- | /10−15 m2 s−1 | |
| 9.24 | 0.14671 | 255.4 | 0.07363 | 340.7 | 0.05414 | 44.8648 |
| 29.4 | 0.453 | 94.7 | 0.27951 | 301.5 | 0.2415 | 18.5876 |
| 44.5 | 0.70977 | 57.8 | 0.50773 | 75.9 | 0.48449 | 22.2132 |
| 59.9 | 0.91512 | 12.4 | 0.74883 | 106.2 | 0.777 | 58.6949 |
| 73.4 | 1.10884 | 223.6 | 1.01617 | 184.3 | 1.12032 | 125.916 |
| 87.8 | 1.34422 | 345.4 | 1.40619 | 610.6 | 1.58168 | 166.599 |
4. Results and Discussion
4.1. Prediction of Water-Sorption Isotherms
4.2. Experimental Water-Sorption Curves
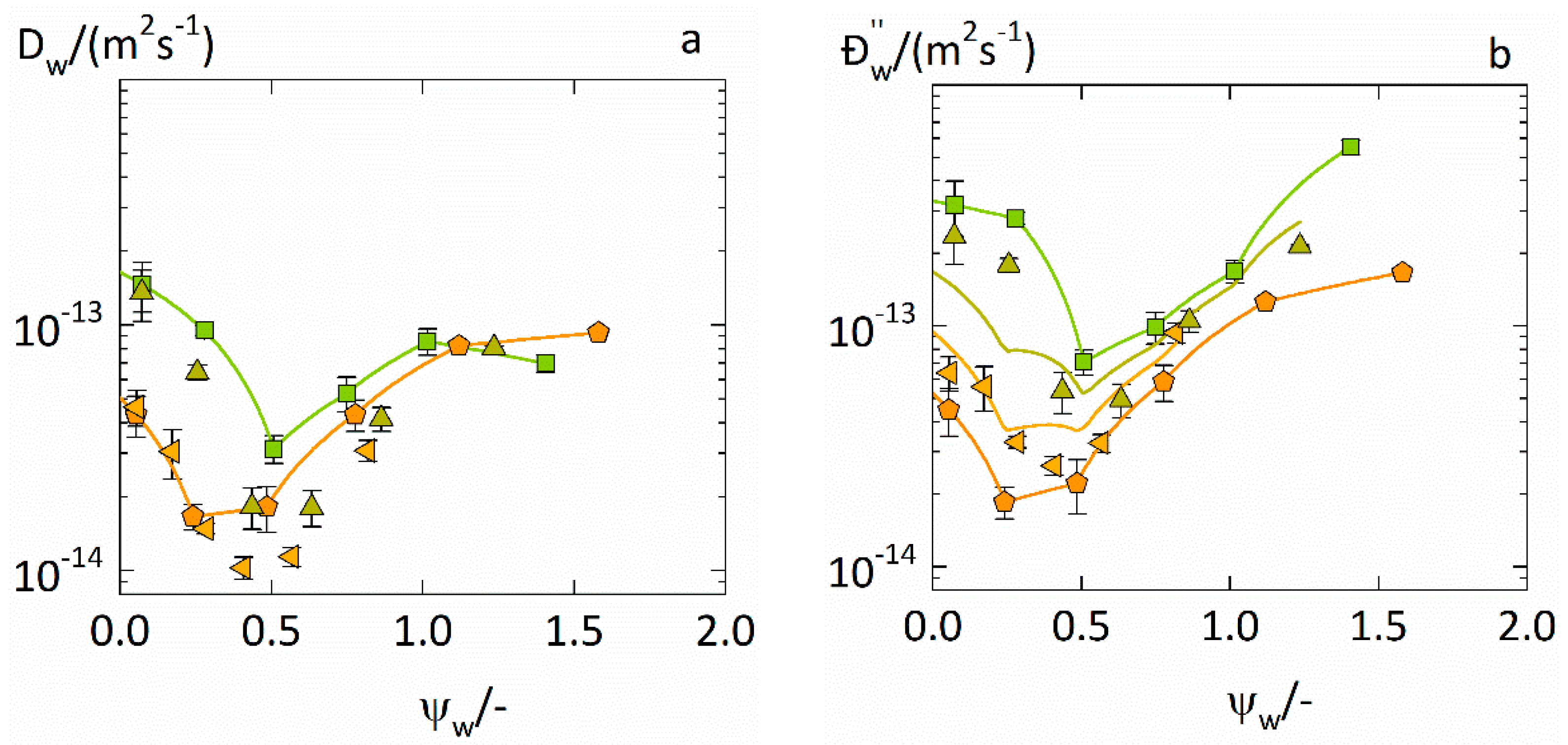
4.3. Prediction of Water-Diffusion Coefficients in ASDs
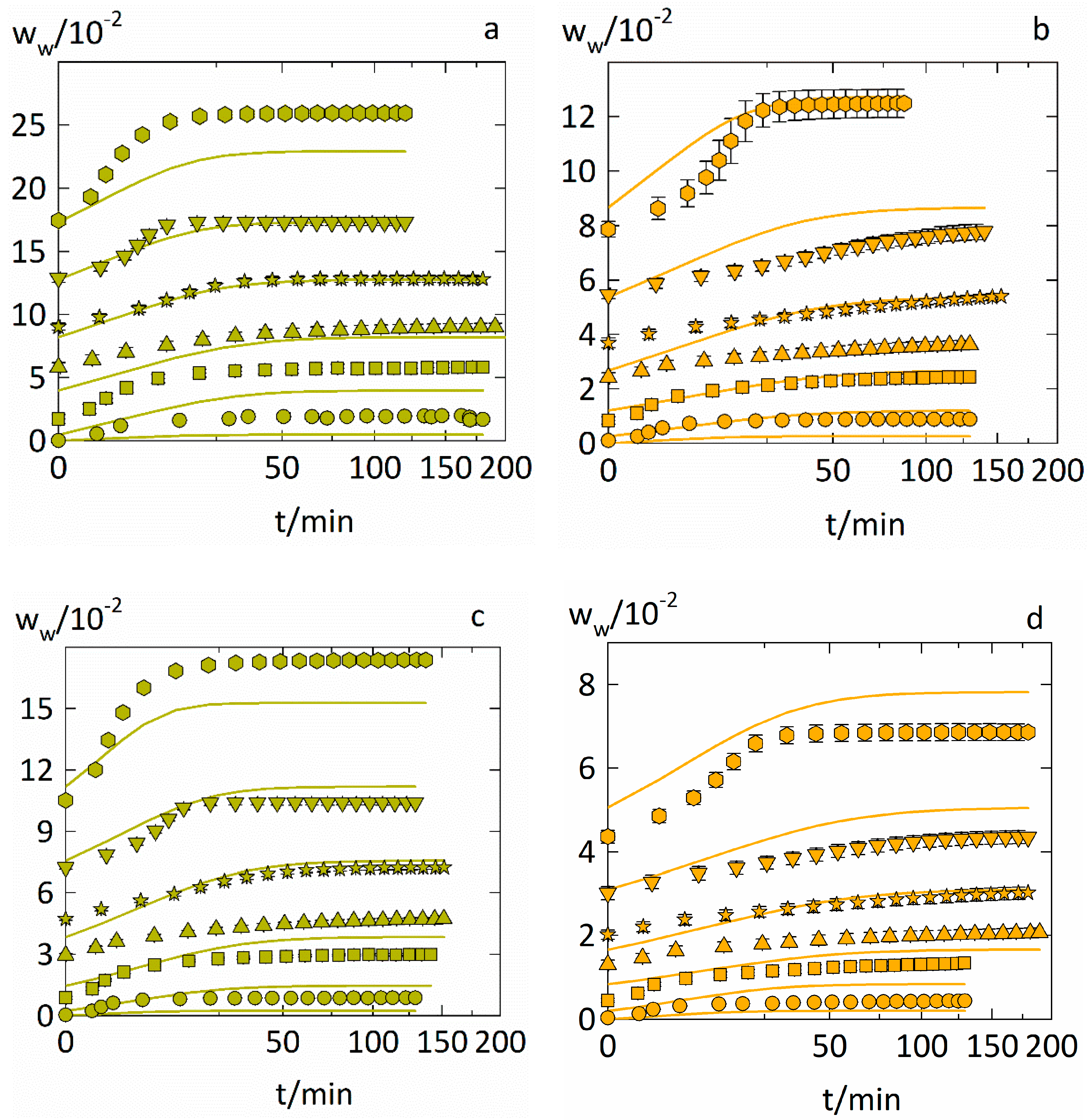
4.4. Prediction of the Water-Sorption Curves
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newman, A.; Zografi, G. An Examination of Water Vapor Sorption by Multicomponent Crystalline and Amorphous Solids and Its Effects on Their Solid-State Properties. J. Pharm. Sci. 2019, 108, 1061–1080. [Google Scholar] [CrossRef] [PubMed]
- Dalton, C.R.; Hancock, B.C. Processing and Storage Effects on Water Vapor Sorption by Some Model Pharmaceutical Solid Dosage Formulations. Int. J. Pharm. 1997, 156, 143–151. [Google Scholar] [CrossRef]
- Zhang, J.; Zografi, G. Water Vapor Absorption into Amorphous Sucrose-Poly(Vinyl Pyrrolidone) and Trehalose-Poly(Vinyl Pyrrolidone) Mixtures. J. Pharm. Sci. 2001, 90, 1375–1385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zografi, G. The Relationship Between “BET” and “Free Volume”-Derived Parameters for Water Vapor Absorption into Amorphous Solids. J. Pharm. Sci. 2000, 89, 1063–1072. [Google Scholar] [CrossRef]
- Vrentas, J.S.; Vrentas, C.M. Sorption in Glassy Polymers. Macromolecules 1991, 24, 2404–2412. [Google Scholar] [CrossRef]
- Crowley, K.J.; Zografi, G. Water Vapor Absorption into Amorphous Hydrophobic Drug/Poly(Vinylpyrrolidone) Dispersions. J. Pharm. Sci. 2002, 91, 2150–2165. [Google Scholar] [CrossRef]
- Prudic, A.; Ji, Y.; Luebbert, C.; Sadowski, G. Influence of Humidity on the Phase Behavior of API/Polymer Formulations. Eur. J. Pharm. Biopharm. 2015, 94, 352–362. [Google Scholar] [CrossRef]
- Lenz, J.; Finke, J.H.; Bunjes, H.; Kwade, A.; Juhnke, M. Tablet Formulation Development Focusing on the Functional Behaviour of Water Uptake and Swelling. Int. J. Pharm. X 2021, 3, 100103. [Google Scholar] [CrossRef]
- Borrmann, D.; Danzer, A.; Sadowski, G. Water Sorption in Glassy Polyvinylpyrrolidone-Based Polymers. Membranes 2022, 12, 434. [Google Scholar] [CrossRef]
- Borrmann, D.; Danzer, A.; Sadowski, G. Measuring and Modeling Water Sorption in Amorphous Indomethacin and Ritonavir. Mol. Pharm. 2022, 19, 998–1007. [Google Scholar] [CrossRef]
- Dengale, S.J.; Ranjan, O.P.; Hussen, S.S.; Krishna, B.S.M.; Musmade, P.B.; Gautham Shenoy, G.; Bhat, K. Preparation and Characterization of Co-Amorphous Ritonavir-Indomethacin Systems by Solvent Evaporation Technique: Improved Dissolution Behavior and Physical Stability without Evidence of Intermolecular Interactions. Eur. J. Pharm. Sci. 2014, 62, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.; Sadowski, G. Perturbed-Chain SAFT: An Equation of State Based on a Perturbation Theory for Chain Molecules. Ind. Eng. Chem. Res. 2001, 40, 1244–1260. [Google Scholar] [CrossRef]
- Wolbach, J.P.; Sandler, S.I. Using Molecular Orbital Calculations to Describe the Phase Behavior of Hydrogen-Bonding Fluids. Ind. Eng. Chem. Res. 1997, 36, 4041–4051. [Google Scholar] [CrossRef]
- De Angelis, M.G.; Sarti, G.C. Solubility of Gases and Liquids in Glassy Polymers. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 97–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grassia, F.; Baschetti, M.G.; Doghieri, F.; Sarti, G.C. Solubility of Gases and Vapors in Glassy Polymer Blends; American Chemical Society: Washington, DC, USA, 2004; pp. 55–73. [Google Scholar]
- Crank, J. The Mathematics of Diffusion; Clarendon Press: Oxford, UK, 1975; ISBN 0198533446. [Google Scholar]
- Sturm, D.R.; Chiu, S.W.; Moser, J.D.; Danner, R.P. Solubility of Water and Acetone in Hypromellose Acetate Succinate, HPMCAS-L. Fluid Phase Equilib. 2016, 429, 227–232. [Google Scholar] [CrossRef]
- Gordon, M.; Taylor, J.S. Ideal Copolymers and the Second-Order Transitions of Synthetic Rubbers. i. Non-Crystalline Copolymers. J. Appl. Chem. 2007, 2, 493–500. [Google Scholar] [CrossRef]
- Vrentas, J.S.; Duda, J.L.; Ni, Y.C. Analysis of Step-Change Sorption Experiments. J. Polym. Sci. Polym. Phys. Ed. 1977, 15, 2039–2045. [Google Scholar] [CrossRef]
- Prudic, A.; Kleetz, T.; Korf, M.; Ji, Y.; Sadowski, G. Influence of Copolymer Composition on the Phase Behavior of Solid Dispersions. Mol. Pharm. 2014, 11, 4189–4198. [Google Scholar] [CrossRef]
- Lehmkemper, K.; Kyeremateng, S.O.; Heinzerling, O.; Degenhardt, M.; Sadowski, G. Long-Term Physical Stability of PVP- and PVPVA-Amorphous Solid Dispersions. Mol. Pharm. 2017, 14, 157–171. [Google Scholar] [CrossRef]
- Prudic, A.; Ji, Y.; Sadowski, G. Thermodynamic Phase Behavior of API/Polymer Solid Dispersions. Mol. Pharm. 2014, 11, 2294–2304. [Google Scholar] [CrossRef]
- Cameretti, L.F.; Sadowski, G. Modeling of Aqueous Amino Acid and Polypeptide Solutions with PC-SAFT. Chem. Eng. Process. Process Intensif. 2008, 47, 1018–1025. [Google Scholar] [CrossRef]
- Andronis, V.; Yoshioka, M.; Zografi, G. Effects of Sorbed Water on the Crystallization of Indomethacin from the Amorphous State. J. Pharm. Sci. 1997, 86, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Simha, R.; Boyer, R.F. On a General Relation Involving the Glass Temperature and Coefficients of Expansion of Polymers. J. Chem. Phys. 1962, 37, 1003–1007. [Google Scholar] [CrossRef]
- Borrmann, D.; Danzer, A.; Sadowski, G. Generalized Diffusion–Relaxation Model for Solvent Sorption in Polymers. Ind. Eng. Chem. Res. 2021, 60, 15766–15781. [Google Scholar] [CrossRef]
- Dohrn, S.; Reimer, P.; Luebbert, C.; Lehmkemper, K.; Kyeremateng, S.O.; Degenhardt, M.; Sadowski, G. Thermodynamic Modeling of Solvent-Impact on Phase Separation in Amorphous Solid Dispersions during Drying. Mol. Pharm. 2020, 17, 2721–2733. [Google Scholar] [CrossRef]
- Fornasiero, F.; Prausnitz, J.M.; Radke, C.J. Multicomponent Diffusion in Highly Asymmetric Systems. An Extended Maxwell–Stefan Model for Starkly Different-Sized, Segment-Accessible Chain Molecules. Macromolecules 2005, 38, 1364–1370. [Google Scholar] [CrossRef]
- Paus, R.; Ji, Y.; Braak, F.; Sadowski, G. Dissolution of Crystalline Pharmaceuticals: Experimental Investigation and Thermodynamic Modeling. Ind. Eng. Chem. Res. 2015, 54, 731–742. [Google Scholar] [CrossRef]
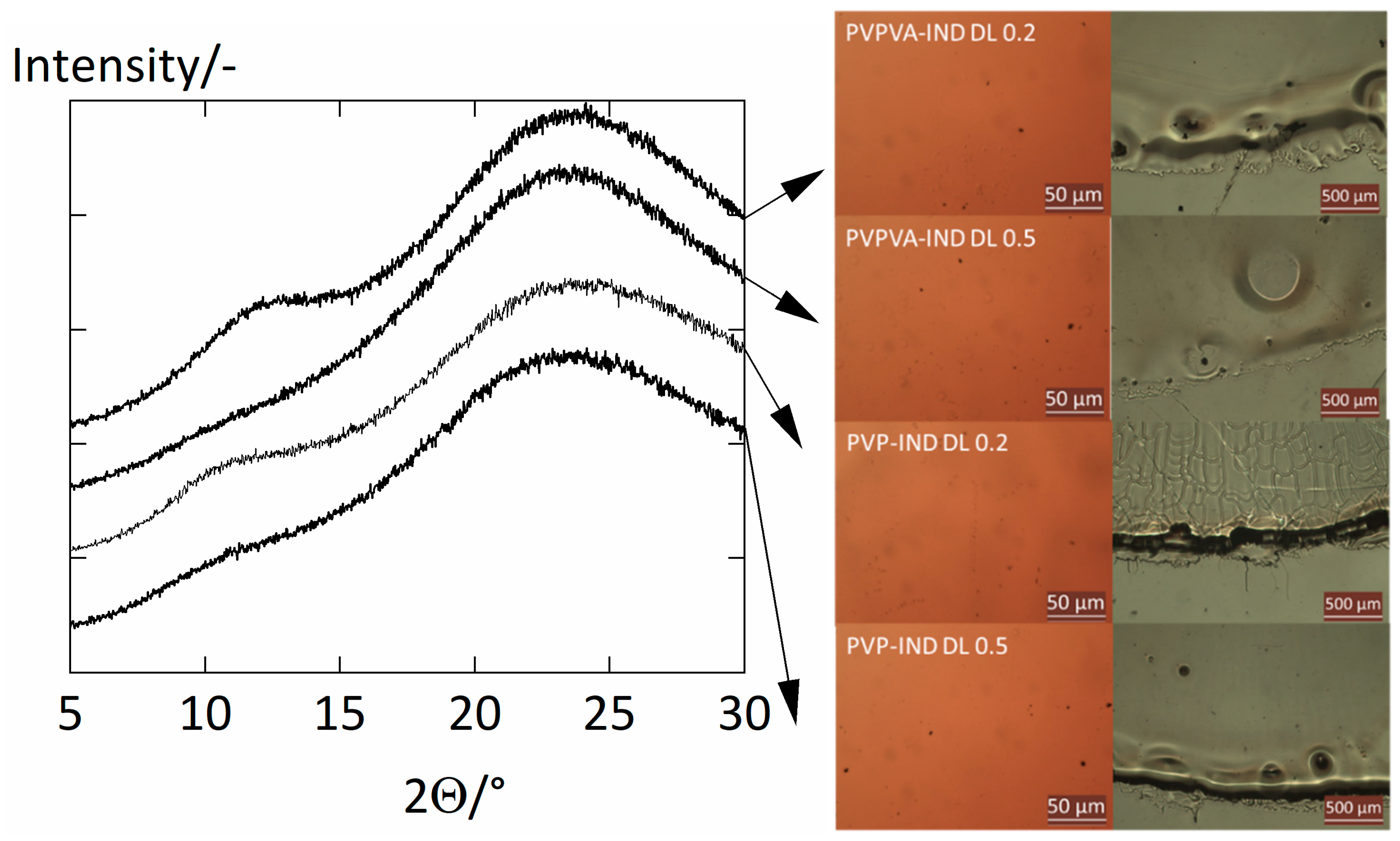


| PVPVA–IND ASDs | PVP–IND ASDs | |||||||
|---|---|---|---|---|---|---|---|---|
| DL | 0.2 | 0.5 | 0.2 | 0.5 | ||||
/10−2 | /10−2 | /10−15 /m2 s−1 | /10−2 | /10−15 /m2 s−1 | /10−2 | /10−15 /m2 s−1 | /10−2 | /10−15 /m2 s−1 |
| 9.24 | 0.88 ± 0.1 | 135.0 ± 30 | 0.47 ± 0.1 | 46.4 ± 25.1 | 1.69 ± 0.4 | 104 ± 55 | 0.86 ± 0.1 | 81.4 ± 2.69 |
| 29.4 | 2.99 ± 0.1 | 64.1 ± 4.15 | 1.35 ± 0.1 | 30.5 ± 6.01 | 5.84 ± 0.4 | 58.9 ± 3.8 | 2.46 ± 0.2 | 27.5 ± 2.89 |
| 44.5 | 4.76 ± 0.1 | 18.2 ± 2.87 | 2.07 ± 0.1 | 14.8 ± 0.634 | 9.02 ± 0.3 | 19.1 ± 0.95 | 3.73 ± 0.2 | 9.67 ± 2.51 |
| 59.9 | 7.24 ± 0.1 | 18.1 ± 2.39 | 3.03 ± 0.2 | 10.3 ± 0.946 | 12.8 ± 0.3 | 29.0 ± 1.82 | 5.46 ± 0.2 | 7.17 ± 1.42 |
| 73.4 | 10.4 ± 0.1 | 41.5 ± 3.48 | 4.36 ± 0.1 | 11.4 ± 0.899 | 17.3 ± 0.3 | 60.4 ± 3.6 | 7.82 ± 0.3 | 7.85 ± 1.62 |
| 87.8 | 17.4 ± 0.2 | 80.8 ± 0.62 | 6.86 ± 0.2 | 30.8 ± 2.52 | 26.0 ± 0.2 | 78.9 ± 2.3 | 12.5 ± 0.5 | 25.2 ± 6.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borrmann, D.; Danzer, A.; Sadowski, G. Predicting the Water Sorption in ASDs. Pharmaceutics 2022, 14, 1181. https://doi.org/10.3390/pharmaceutics14061181
Borrmann D, Danzer A, Sadowski G. Predicting the Water Sorption in ASDs. Pharmaceutics. 2022; 14(6):1181. https://doi.org/10.3390/pharmaceutics14061181
Chicago/Turabian StyleBorrmann, Dominik, Andreas Danzer, and Gabriele Sadowski. 2022. "Predicting the Water Sorption in ASDs" Pharmaceutics 14, no. 6: 1181. https://doi.org/10.3390/pharmaceutics14061181







