Polymeric Micelles with pH-Responsive Cross-Linked Core Enhance In Vivo mRNA Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polymer Synthesis
2.3. In Vitro Transcribed mRNA
2.4. Micelle Preparation
2.5. Micelle Characterization
2.6. Encapsulation Efficiency of mRNA
2.7. Micelle pH Sensitivity
2.8. Micelle Stability against Polyanion
2.9. Stability of mRNA in Micelles in FBS
2.10. Cytotoxicity Assay
2.11. In Vitro Uptake
2.12. In Vitro Cellular Transfection
2.13. In Vivo Transfection
2.14. Statistical Analysis
3. Results and Discussion
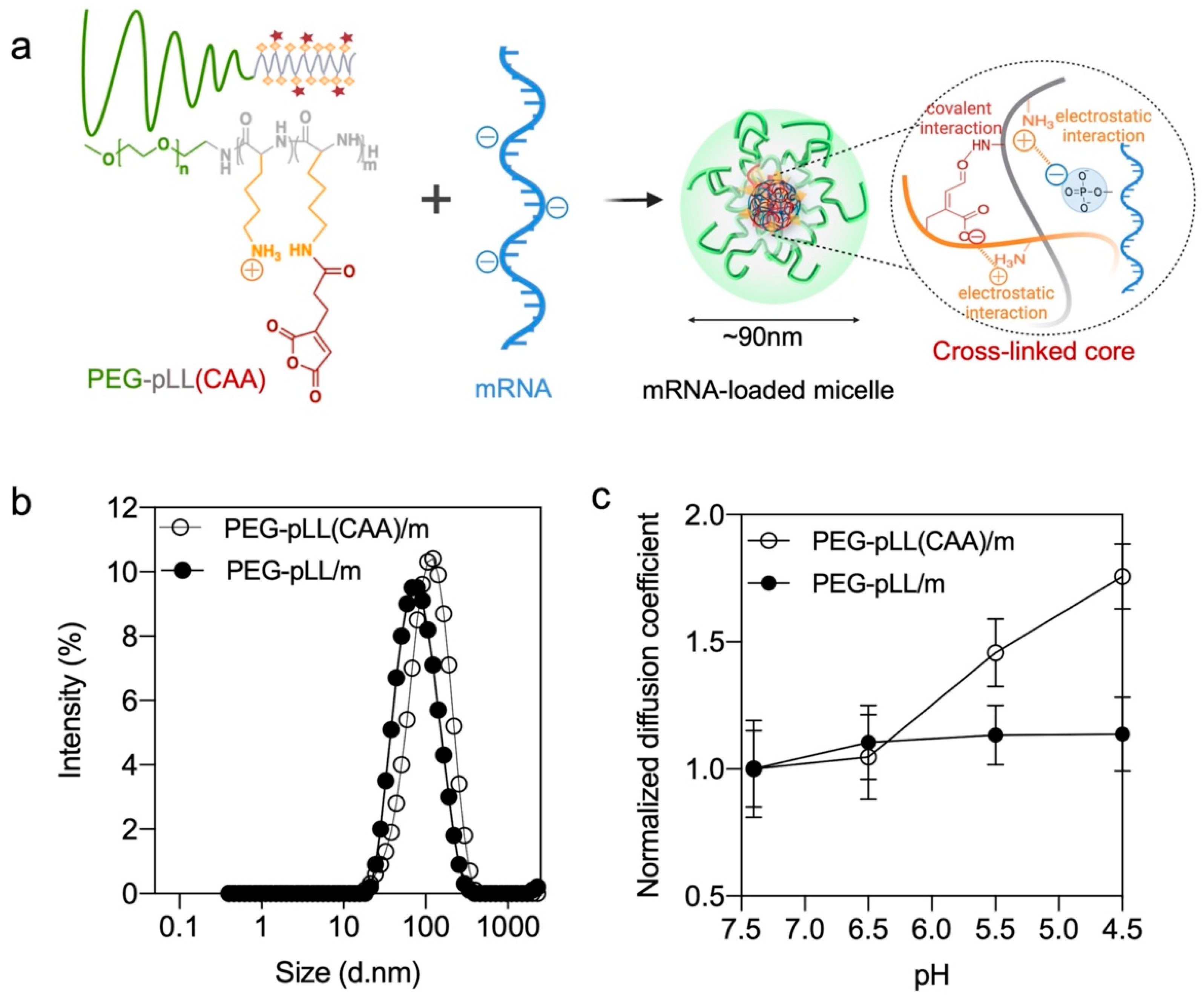
3.1. Synthesis and Characterization of Block Copolymer
3.2. Micelle Formation and Characterization
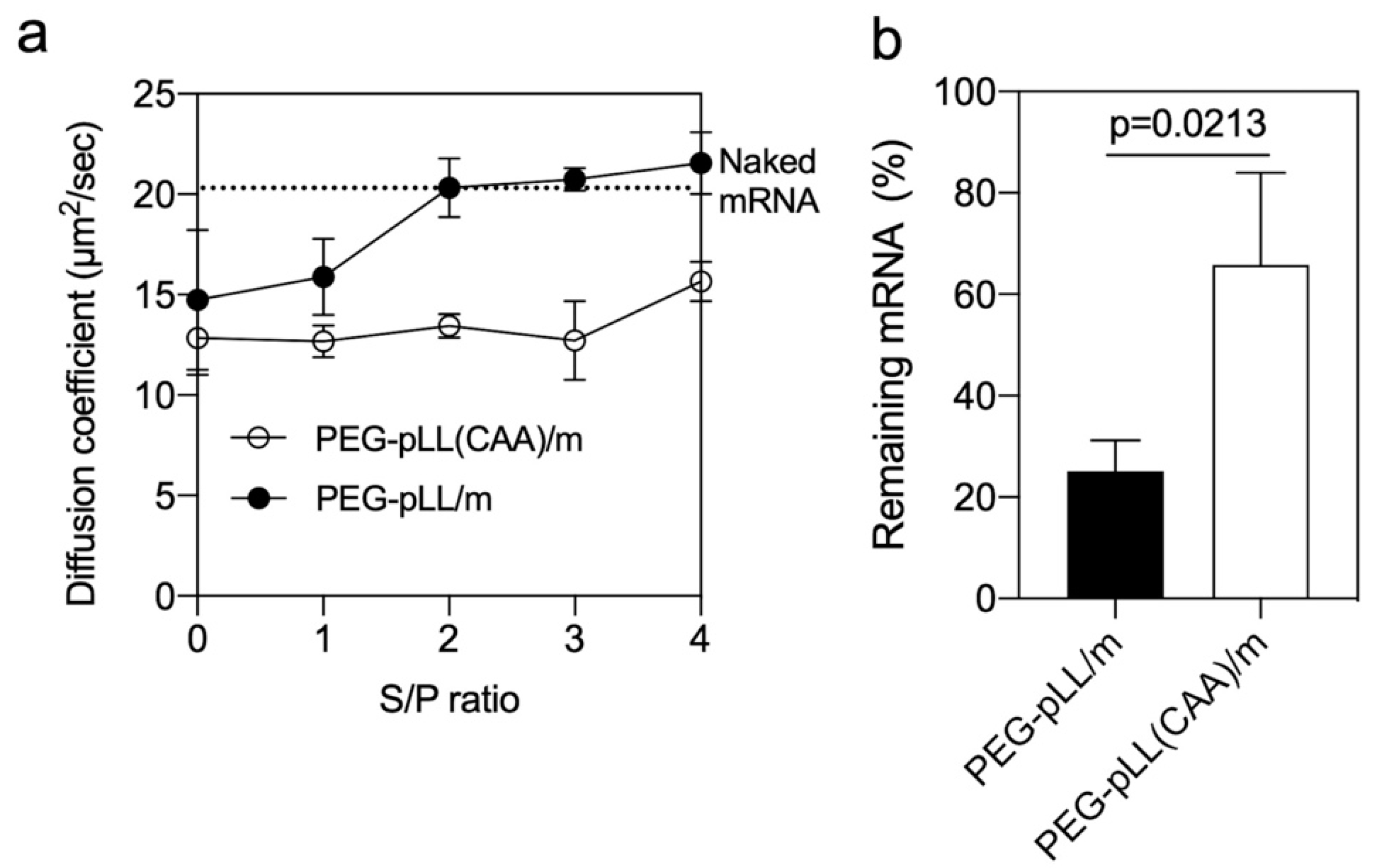
3.3. Stability of the Micelles
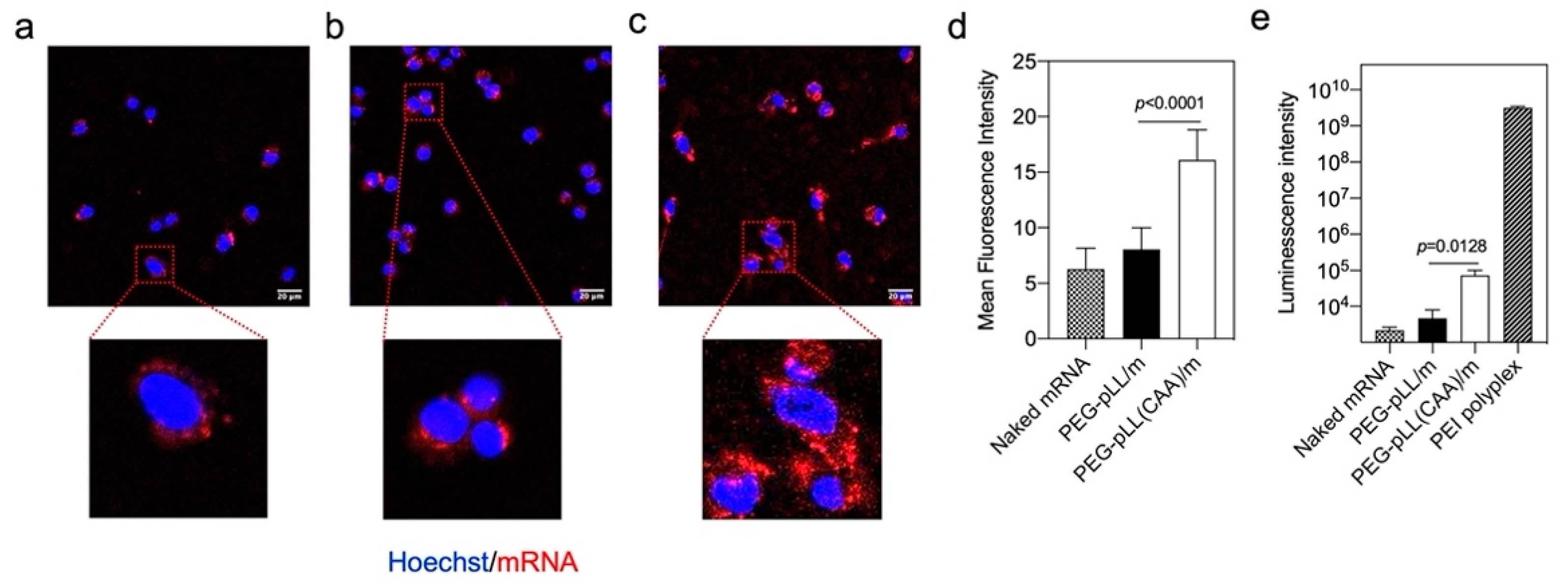
3.4. In Vitro Activity
3.5. In Vivo Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sahin, U.; Kariko, K.; Tureci, O. mRNA-based therapeutics--developing a new class of drugs. Nat. Rev. Drug Discov. 2014, 13, 759–780. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; O’Keeffe-Ahern, J.; Lyu, J.; Pierucci, L.; Zhou, D.; Wang, W. A new developing class of gene delivery: Messenger RNA-based therapeutics. Biomater. Sci. 2017, 5, 2381–2392. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Yang, S.; Hu, X.; Yin, D.; Dai, Y.; Qian, X.; Wang, D.; Pan, X.; Hong, J.; Sun, X.; et al. Lentiviral delivery of co-packaged Cas9 mRNA and a Vegfa-targeting guide RNA prevents wet age-related macular degeneration in mice. Nat. Biomed. Eng. 2021, 5, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [Green Version]
- Kowalski, P.S.; Rudra, A.; Miao, L.; Anderson, D.G. Delivering the Messenger: Advances in Technologies for Therapeutic mRNA Delivery. Mol. Ther. 2019, 27, 710–728. [Google Scholar] [CrossRef] [Green Version]
- Hajj, K.A.; Whitehead, K.A. Tools for translation: Non-viral materials for therapeutic mRNA delivery. Nat. Rev. Mater. 2017, 2, 17056. [Google Scholar] [CrossRef]
- Bessis, N.; GarciaCozar, F.J.; Boissier, M.C. Immune responses to gene therapy vectors: Influence on vector function and effector mechanisms. Gene Ther. 2004, 11 (Suppl. 1), S10–S17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block Copolymer Micelles in Nanomedicine Applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef] [Green Version]
- Uchida, S.; Perche, F.; Pichon, C.; Cabral, H. Nanomedicine-Based Approaches for mRNA Delivery. Mol. Pharm. 2020, 17, 3654–3684. [Google Scholar] [CrossRef]
- Jarak, I.; Pereira-Silva, M.; Santos, A.C.; Veiga, F.; Cabral, H.; Figueiras, A. Multifunctional polymeric micelle-based nucleic acid delivery: Current advances and future perspectives. Appl. Mater. Today 2021, 25, 101217. [Google Scholar] [CrossRef]
- Zuckerman, J.E.; Choi, C.H.; Han, H.; Davis, M.E. Polycation-siRNA nanoparticles can disassemble at the kidney glomerular basement membrane. Proc. Natl. Acad. Sci. USA 2012, 109, 3137–3142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchida, S.; Kinoh, H.; Ishii, T.; Matsui, A.; Tockary, T.A.; Takeda, K.M.; Uchida, H.; Osada, K.; Itaka, K.; Kataoka, K. Systemic delivery of messenger RNA for the treatment of pancreatic cancer using polyplex nanomicelles with a cholesterol moiety. Biomaterials 2016, 82, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, N.; Uchida, S.; Naito, M.; Osada, K.; Cabral, H.; Kataoka, K. Induced packaging of mRNA into polyplex micelles by regulated hybridization with a small number of cholesteryl RNA oligonucleotides directed enhanced in vivo transfection. Biomaterials 2019, 197, 255–267. [Google Scholar] [CrossRef]
- Miyazaki, T.; Uchida, S.; Nagatoishi, S.; Koji, K.; Hong, T.; Fukushima, S.; Tsumoto, K.; Ishihara, K.; Kataoka, K.; Cabral, H. Polymeric Nanocarriers with Controlled Chain Flexibility Boost mRNA Delivery In Vivo through Enhanced Structural Fastening. Adv. Healthc. Mater. 2020, 9, e2000538. [Google Scholar] [CrossRef]
- Miyazaki, T.; Uchida, S.; Hatano, H.; Miyahara, Y.; Matsumoto, A.; Cabral, H. Guanidine-phosphate interactions stabilize polyion complex micelles based on flexible catiomers to improve mRNA delivery. Eur. Polym. J. 2020, 140, 110028. [Google Scholar] [CrossRef]
- Cheng, C.; Convertine, A.J.; Stayton, P.S.; Bryers, J.D. Multifunctional triblock copolymers for intracellular messenger RNA delivery. Biomaterials 2012, 33, 6868–6876. [Google Scholar] [CrossRef] [Green Version]
- Nuhn, L.; Kaps, L.; Diken, M.; Schuppan, D.; Zentel, R. Reductive Decationizable Block Copolymers for Stimuli-Responsive mRNA Delivery. Macromol. Rapid Commun. 2016, 37, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ma, B.; Wang, Y.; Gong, S. A Universal GSH-Responsive Nanoplatform for the Delivery of DNA, mRNA, and Cas9/sgRNA Ribonucleoprotein. ACS Appl. Mater. Interfaces 2018, 10, 18515–18523. [Google Scholar] [CrossRef]
- Dirisala, A.; Uchida, S.; Tockary, T.A.; Yoshinaga, N.; Li, J.; Osawa, S.; Gorantla, L.; Fukushima, S.; Osada, K.; Kataoka, K. Precise tuning of disulphide crosslinking in mRNA polyplex micelles for optimising extracellular and intracellular nuclease tolerability. J. Drug Target. 2019, 27, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, N.; Uchida, S.; Dirisala, A.; Naito, M.; Osada, K.; Cabral, H.; Kataoka, K. mRNA loading into ATP-responsive polyplex micelles with optimal density of phenylboronate ester crosslinking to balance robustness in the biological milieu and intracellular translational efficiency. J. Control. Release 2021, 330, 317–328. [Google Scholar] [CrossRef]
- Hu, Y.B.; Dammer, E.B.; Ren, R.J.; Wang, G. The endosomal-lysosomal system: From acidification and cargo sorting to neurodegeneration. Transl. Neurodegener. 2015, 4, 18. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Miyata, K.; Oba, M.; Ishii, T.; Fukushima, S.; Han, M.; Koyama, H.; Nishiyama, N.; Kataoka, K. Charge-conversion ternary polyplex with endosome disruption moiety: A technique for efficient and safe gene delivery. Angew. Chem. 2008, 47, 5163–5166. [Google Scholar] [CrossRef]
- Kataoka, K.; Togawa, H.; Harada, A.; Yasugi, K.; Matsumoto, T.; Katayose, S. Spontaneous Formation of Polyion Complex Micelles with Narrow Distribution from Antisense Oligonucleotide and Cationic Block Copolymer in Physiological Saline. Macromolecules 1996, 29, 8556–8557. [Google Scholar] [CrossRef]
- Schmitt, S.; Nuhn, L.; Barz, M.; Butt, H.J.; Koynov, K. Shining Light on Polymeric Drug Nanocarriers with Fluorescence Correlation Spectroscopy. Macromol. Rapid. Commun. 2022, e2100892. [Google Scholar] [CrossRef]
- Fu, J.; Schlenoff, J.B. Driving Forces for Oppositely Charged Polyion Association in Aqueous Solutions: Enthalpic, Entropic, but Not Electrostatic. J. Am. Chem. Soc. 2016, 138, 980–990. [Google Scholar] [CrossRef]
- Zintchenko, A.; Rother, G.; Dautzenberg, H. Transition Highly Aggregated Complexes Soluble Complexes via Polyelectrolyte Exchange Reactions: Kinetics, Structural Changes, and Mechanism. Langmuir 2003, 19, 2507–2513. [Google Scholar] [CrossRef]
- Sui, Z.; Salloum, D.; Schlenoff, J.B. Effect of Molecular Weight on the Construction of Polyelectrolyte Multilayers: Stripping versus Sticking. Langmuir 2003, 19, 2491–2495. [Google Scholar] [CrossRef]
- Poon, G.M.K.; Gariépy, J. Cell-surface proteoglycans as molecular portals for cationic peptide and polymer entry into cells. Biochem. Soc. Trans. 2007, 35, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, E.; Hatano, H.; Miyahara, Y.; Uchida, S.; Goda, T.; Cabral, H. A proton/macromolecule-sensing approach distinguishes changes in biological membrane permeability during polymer/lipid-based nucleic acid delivery. J. Mater. Chem. B 2021, 9, 4298–4302. [Google Scholar] [CrossRef]

| Samples | Size (nm) (Mean ± s.d.) (a) | Polydispersity Index (a) | Diffusion Coefficient (μm2/s) (Mean ± s.d.) (b) | Count per Molecule (Mean ± s.d.) (b) | Association mRNA Number in Micelles (Mean ± s.d.) (b) | Encapsulation Efficiency (c) |
|---|---|---|---|---|---|---|
| Naked mRNA | - | - | 19.95 ± 1.98 | 5.82 ± 0.27 | - | - |
| PEG-pLL/m | 72 ± 1 | 0.29 | 12.07 ± 0.87 | 7.48 ± 0.79 | 1.36 | 100% |
| PEG-pLL(CAA)/m | 94 ± 1 | 0.23 | 10.94 ± 2.50 | 9.85 ± 2.10 | 1.78 | 96% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Chen, P.; Boonstra, E.; Hong, T.; Cabral, H. Polymeric Micelles with pH-Responsive Cross-Linked Core Enhance In Vivo mRNA Delivery. Pharmaceutics 2022, 14, 1205. https://doi.org/10.3390/pharmaceutics14061205
Yang W, Chen P, Boonstra E, Hong T, Cabral H. Polymeric Micelles with pH-Responsive Cross-Linked Core Enhance In Vivo mRNA Delivery. Pharmaceutics. 2022; 14(6):1205. https://doi.org/10.3390/pharmaceutics14061205
Chicago/Turabian StyleYang, Wenqian, Pengwen Chen, Eger Boonstra, Taehun Hong, and Horacio Cabral. 2022. "Polymeric Micelles with pH-Responsive Cross-Linked Core Enhance In Vivo mRNA Delivery" Pharmaceutics 14, no. 6: 1205. https://doi.org/10.3390/pharmaceutics14061205
APA StyleYang, W., Chen, P., Boonstra, E., Hong, T., & Cabral, H. (2022). Polymeric Micelles with pH-Responsive Cross-Linked Core Enhance In Vivo mRNA Delivery. Pharmaceutics, 14(6), 1205. https://doi.org/10.3390/pharmaceutics14061205







