Biodegradable Carbonate Apatite Nanoparticle as a Delivery System to Promote Afatinib Delivery for Non-Small Cell Lung Cancer Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
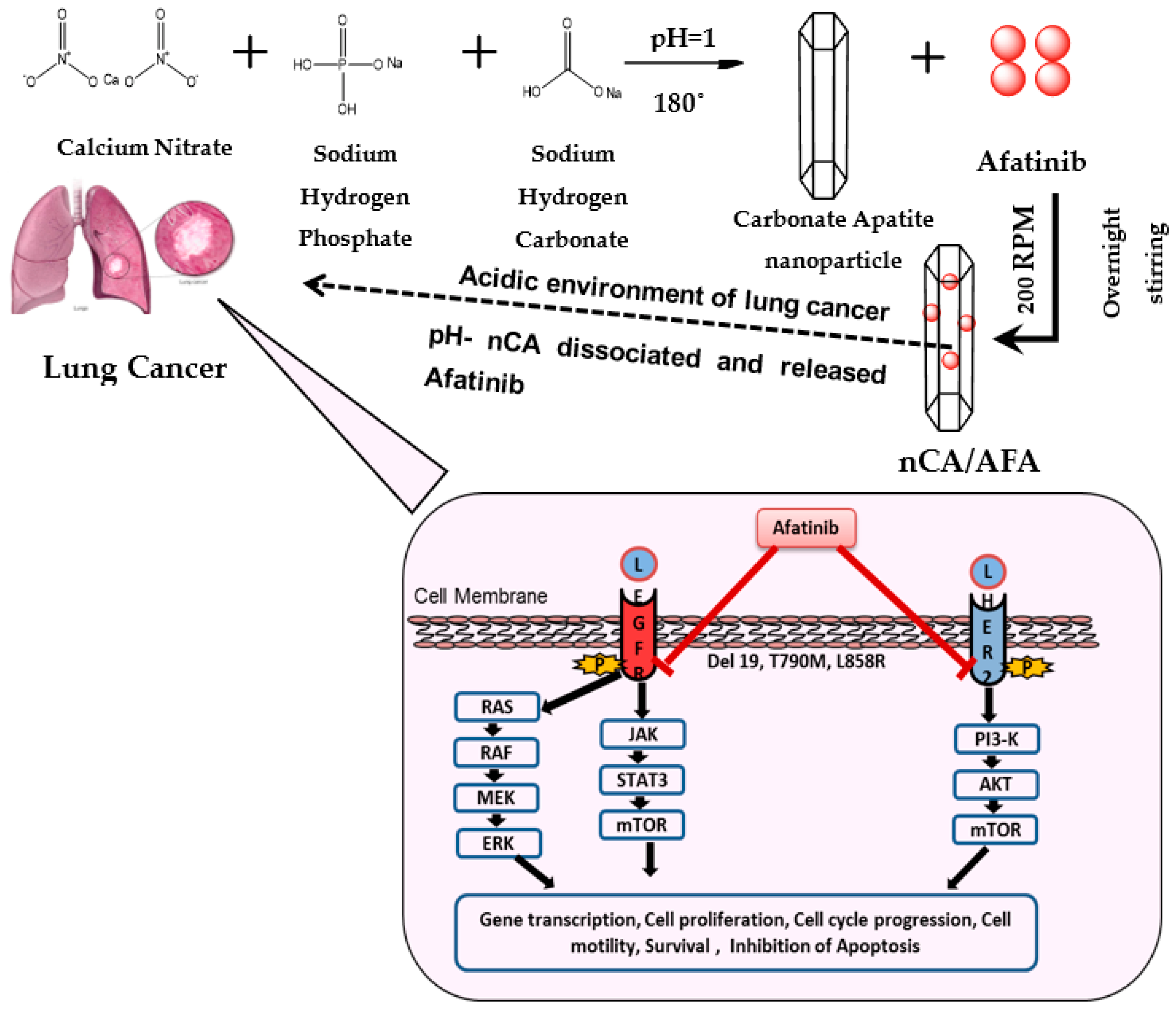
2.2. Preparation of nCA Nanocarriers and nCA/AFA Nanocomposites
2.3. Characterisation of nCA and nCA/AFA
2.4. Yield, Encapsulation Efficiency, and Drug Loading
2.5. Release Study
2.6. Dissolution Analysis of nCA in Biological Media
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterisation of nCA and nCA/AFA
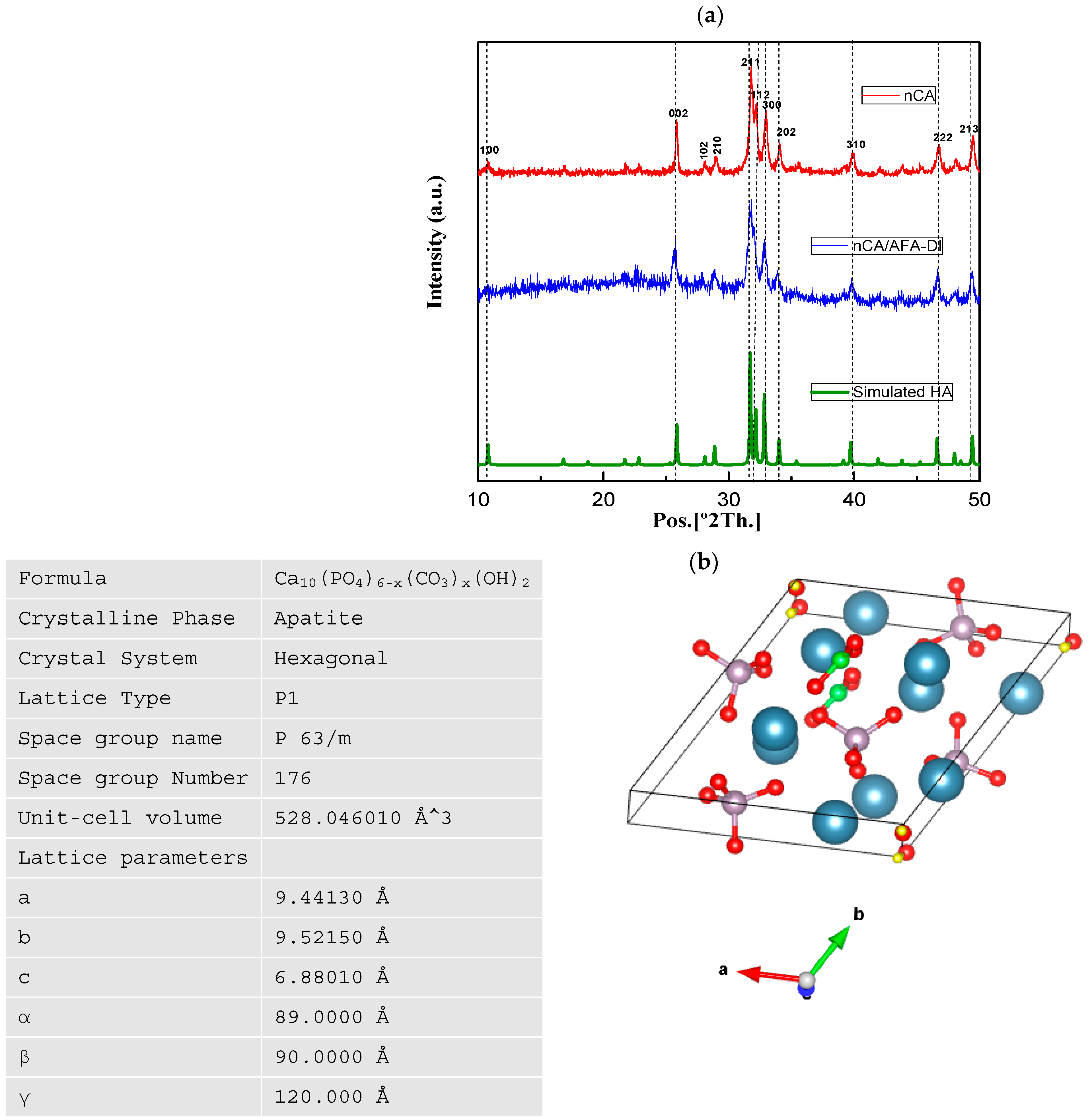
3.1.1. XRD Analysis
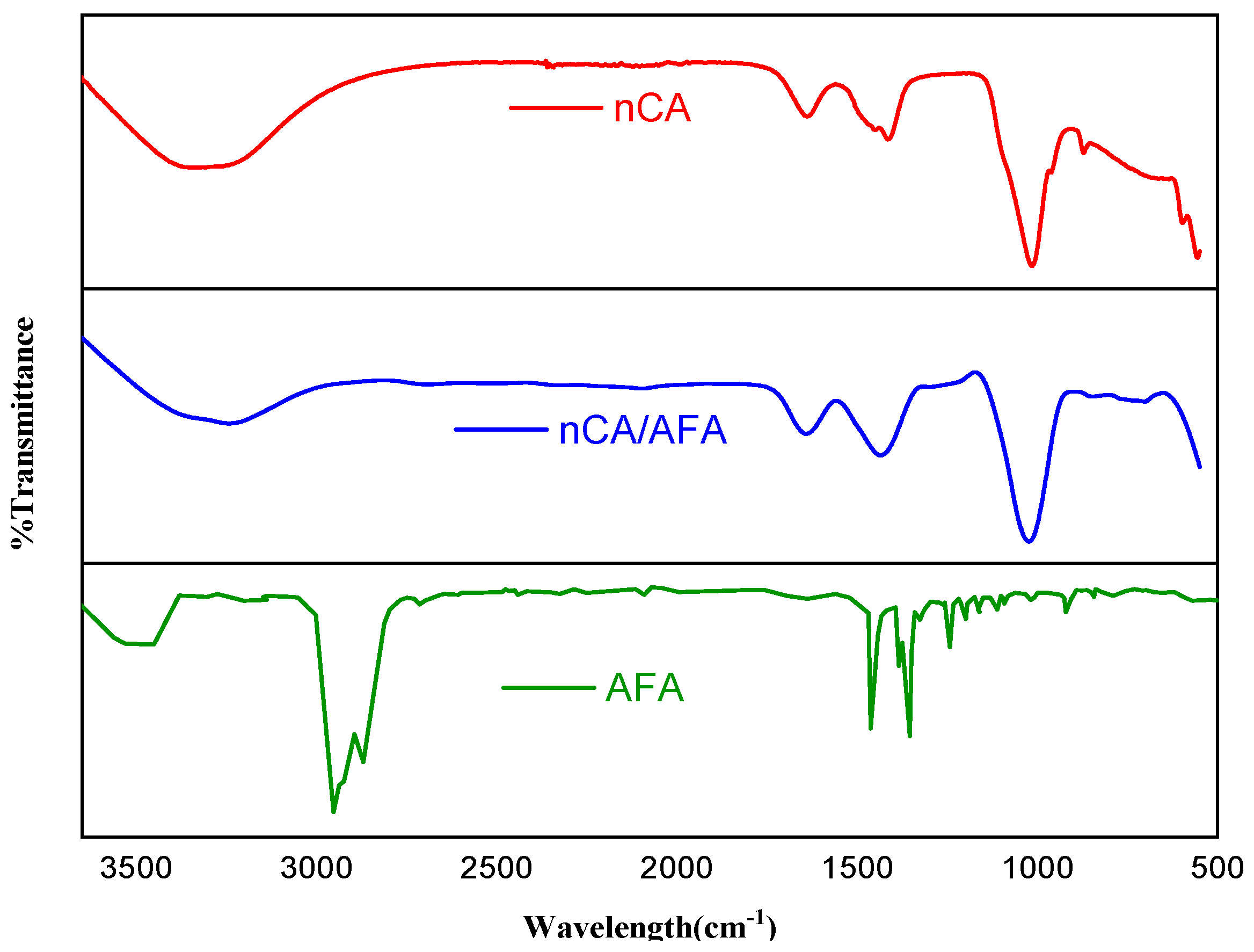
3.1.2. FTIR Analysis
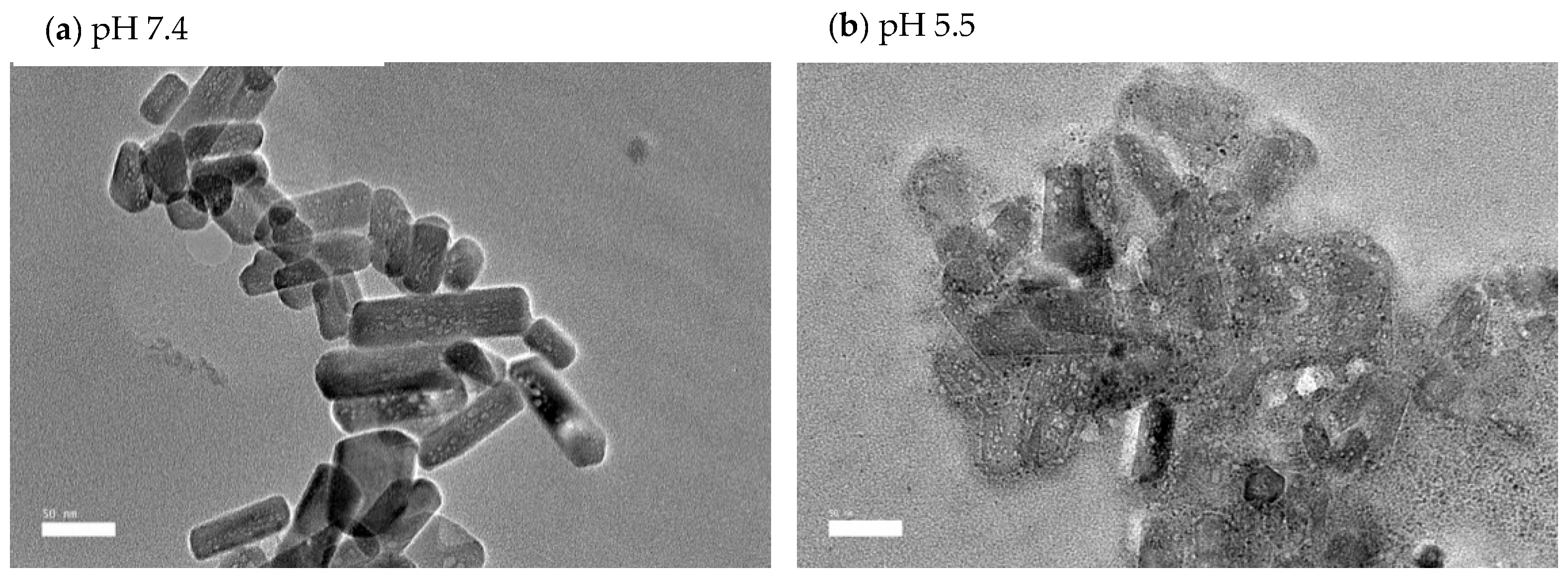
3.1.3. HR-TEM Analysis
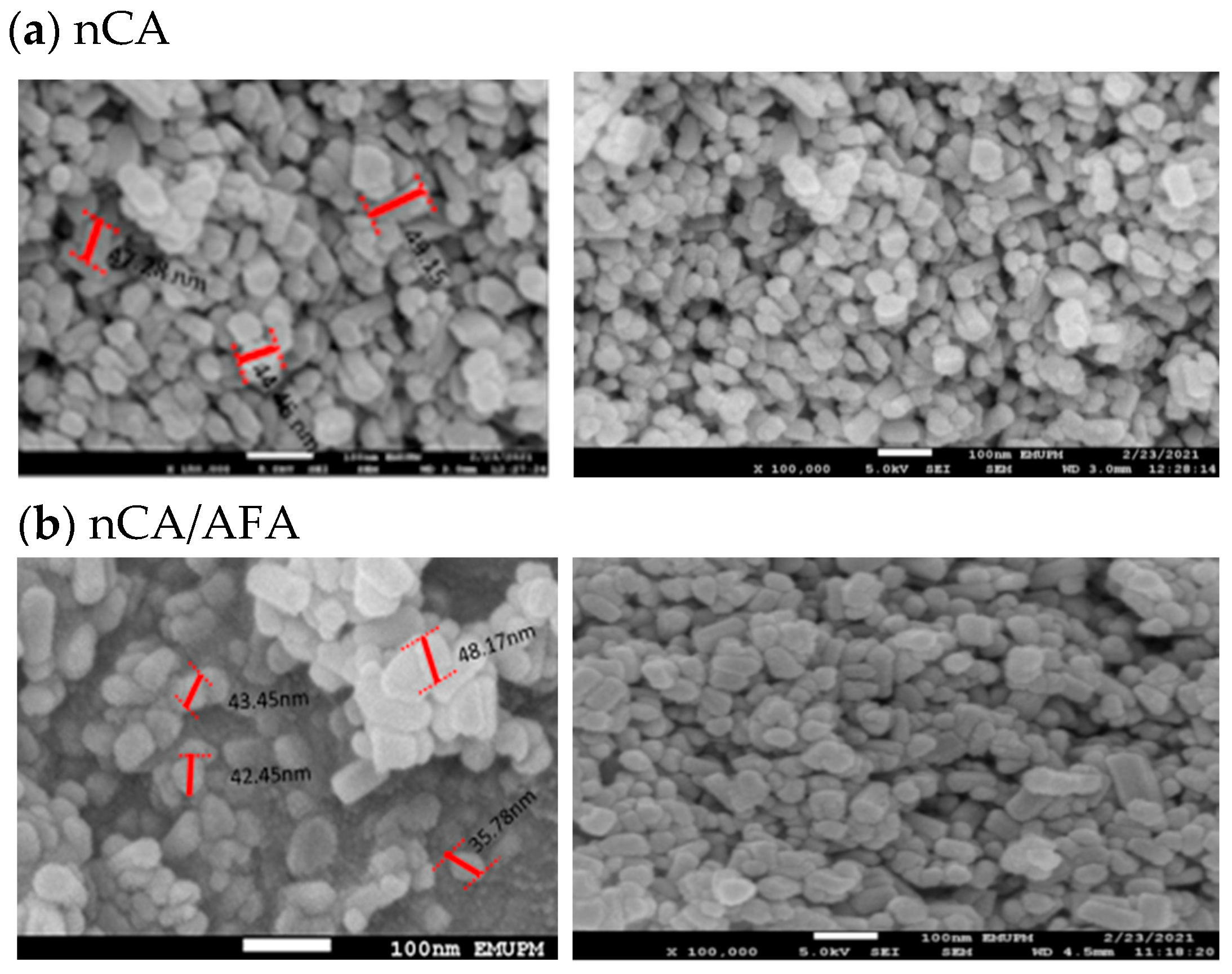
3.1.4. FESEM and Morphology Analysis
3.1.5. AFM and Topography Analysis
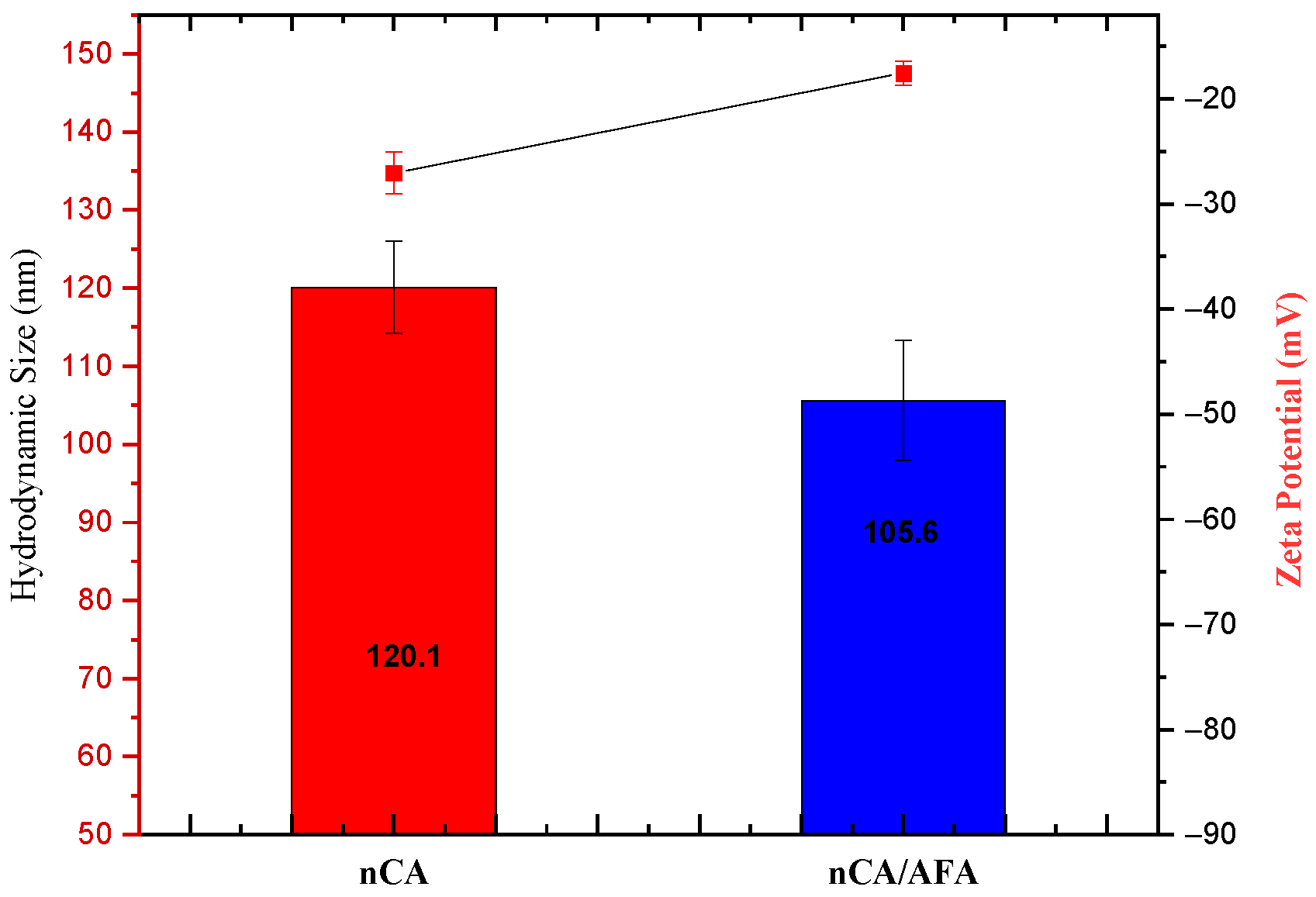
3.1.6. Hydrodynamic Size, Zeta Potential, and Polydispersity Index (PDI)
3.1.7. Surface Area and Porosity
3.2. Yield, Encapsulation Efficiency, and Drug Loading
3.3. pH-Responsive Drug-Release Profile
3.3.1. In Vitro Drug Release
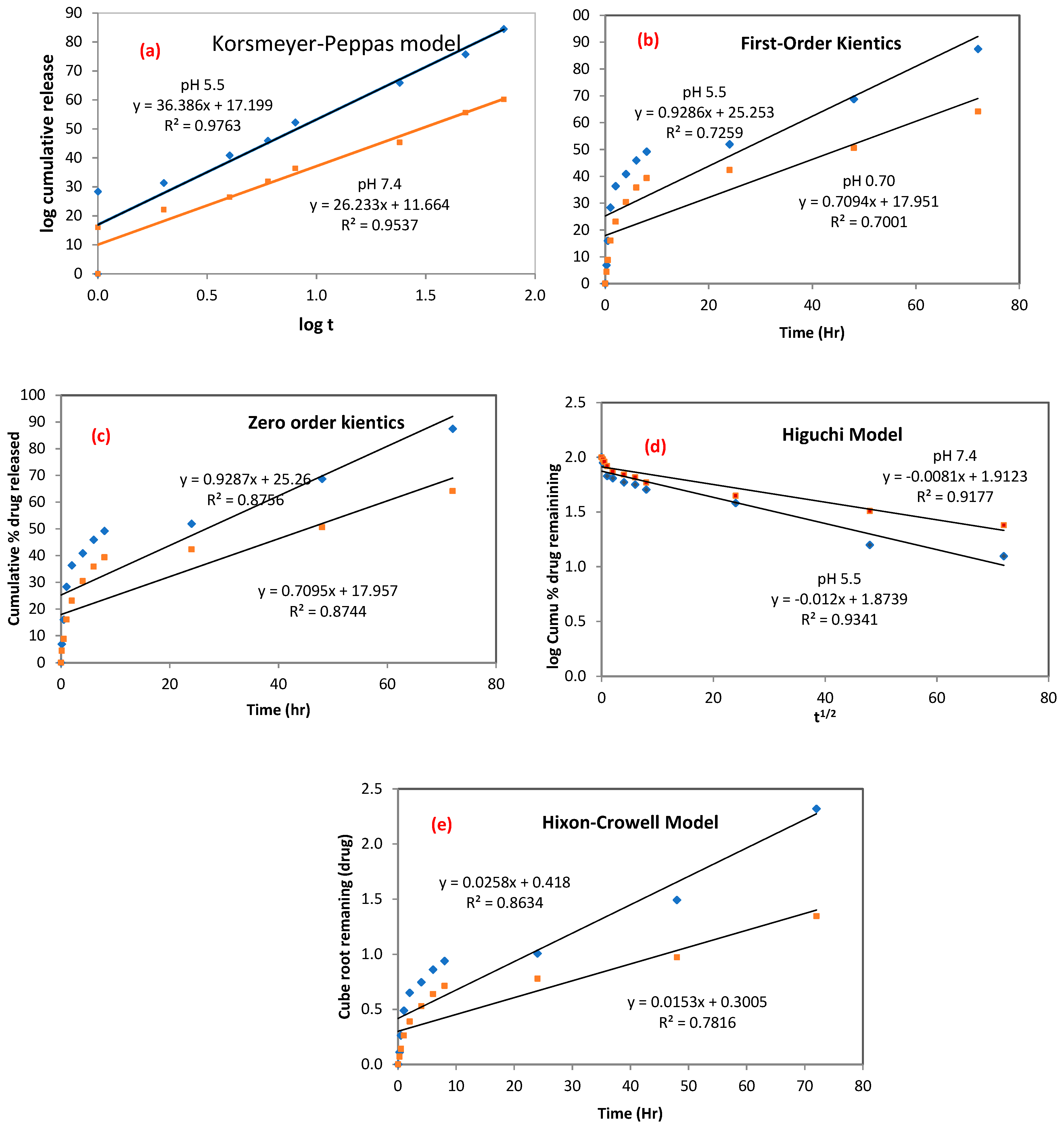
3.3.2. Kinetics Drug Release
3.4. Dissolution Analysis of nCA in Biological Media
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Newman, S.P. Drug Delivery to the Lungs: Challenges and Opportunities. Ther. Deliv. 2017, 8, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Zappa, C.; Mousa, S.A. Non-Small Cell Lung Cancer: Current Treatment and Future Advances. Transl. Lung Cancer Res. 2016, 5, 288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kutkowska, J.; Porębska, I.; Rapak, A. Non-Small Cell Lung Cancer—Mutations, Targeted and Combination Therapy. Postepy Hig. Med. Dosw. (Online) 2017, 71, 431–445. [Google Scholar] [CrossRef]
- Zhu, T.; Bao, X.; Chen, M.; Lin, R.; Zhuyan, J.; Zhen, T.; Xing, K.; Zhou, W.; Zhu, S. Mechanisms and Future of Non-Small Cell Lung Cancer Metastasis. Front. Oncol. 2020, 10, 2441. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P.; Kumar Mahanta, A.; Kumar, S.; Maiti, P. Controlled Drug Delivery Vehicles for Cancer Treatment and Their Performance; Springer Nature: Berlin/Heidelberg, Germany, 2018; Volume 3. [Google Scholar]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled Drug Delivery Vehicles for Cancer Treatment and Their Performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, Properties, and Regulatory Issues. Front. Chem. 2018, 6, 360. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Gao, X.; Chen, Y.; Liu, T. Nanotechnology in Cancer Diagnosis: Progress, Challenges and Opportunities. J. Hematol. Oncol. 2019, 12, 360. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Yamamoto, H.; Nakanishi, H.; Yamamoto, Y.; Inoue, A.; Tei, M.; Hirose, H.; Uemura, M.; Nishimura, J.; Hata, T.; et al. Innovative Delivery of SiRNA to Solid Tumors by Super Carbonate Apatite. PLoS ONE 2015, 10, e0116022. [Google Scholar] [CrossRef] [Green Version]
- Hossain, S.M.; Shetty, J.; Tha, K.K.; Chowdhury, E.H. α-Ketoglutaric Acid-Modified Carbonate Apatite Enhances Cellular Uptake and Cytotoxicity of a Raf- Kinase Inhibitor in Breast Cancer Cells through Inhibition of MAPK and PI-3 Kinase Pathways. Biomedicines 2019, 7, 4. [Google Scholar] [CrossRef] [Green Version]
- Islam, R.A.; Al-Busaidi, H.; Zaman, R.; Abidin, S.A.Z.; Othman, I.; Chowdhury, E.H. Carbonate Apatite and Hydroxyapatite Formulated with Minimal Ingredients to Deliver SiRNA into Breast Cancer Cells in Vitro and in Vivo. J. Funct. Biomater. 2020, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Vermorken, J.B.; Rottey, S.; Ehrnrooth, E.; Pelling, K.; Lahogue, A.; Wind, S.; Machiels, J.P. A Phase Ib, Open-Label Study to Assess the Safety of Continuous Oral Treatment with Afatinib in Combination with Two Chemotherapy Regimens: Cisplatin plus Paclitaxel and Cisplatin plus 5-Fluorouracil, in Patients with Advanced Solid Tumors. Ann. Oncol. 2013, 24, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Maarof, N.N.N.; Alsalahi, A.; Abdulmalek, E.; Fakurazi, S.; Tejo, B.A.; Rahman, M.B.A. Efficacy of Afatinib in the Treatment of Patients with Non-Small Cell Lung Cancer and Head and Neck Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Cancers 2021, 13, 688. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, H.; Kato, T.; Okamoto, I.; Tanaka, H.; Hida, T.; Seto, T.; Kiura, K.; Tian, Y.; Azuma, H.; Yamamoto, N. Therapies after First-Line Afatinib in Patients with EGFRm(+) NSCLC in Japan: Retrospective Analysis of LUX-Lung 3. Future Oncol. 2020, 16, 49–60. [Google Scholar] [CrossRef]
- Reguart, N.; Remon, J. Common EGFR-Mutated Subgroups (Del19/L858R) in Advanced Non-Small-Cell Lung Cancer: Chasing Better Outcomes with Tyrosine Kinase Inhibitors. Future Oncol. (Lond. Engl.) 2015, 11, 1245–1257. [Google Scholar] [CrossRef]
- Engelman, J.A.; Jänne, P.A. Mechanisms of Acquired Resistance to Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Non-Small Cell Lung Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008, 14, 2895–2899. [Google Scholar] [CrossRef] [Green Version]
- Cryer, A.M.; Chan, C.; Eftychidou, A.; Maksoudian, C.; Mahesh, M.; Tetley, T.D.; Spivey, A.C.; Thorley, A.J. Tyrosine Kinase Inhibitor Gold Nanoconjugates for the Treatment of Non-Small Cell Lung Cancer. ACS Appl. Mater. Interfaces 2019, 11, 16336–16346. [Google Scholar] [CrossRef]
- Hirsh, V. Next-Generation Covalent Irreversible Kinase Inhibitors in NSCLC: Focus on Afatinib. BioDrugs Clin. Immunother. Biopharm. Gene Ther. 2015, 29, 167–183. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.C.; Wu, Y.L.; Schuler, M.; Sebastian, M.; Popat, S.; Yamamoto, N.; Zhou, C.; Hu, C.P.; O’Byrne, K.; Feng, J.; et al. Afatinib versus Cisplatin-Based Chemotherapy for EGFR Mutation-Positive Lung Adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): Analysis of Overall Survival Data from Two Randomised, Phase 3 Trials. Lancet Oncol. 2015, 16, 141–151. [Google Scholar] [CrossRef] [Green Version]
- Ichiki, M.; Wataya, I.; Yamada, K.; Tsuruta, N.; Takeoka, H.; Okayama, Y.; Sasaki, J.; Hoshino, T. Preventive Effect of Kampo Medicine (Hangeshashin-to, TJ-14) plus Minocycline against Afatinib-Induced Diarrhea and Skin Rash in Patients with Non-Small Cell Lung Cancer. Oncotargets Ther. 2017, 10, 5107–5113. [Google Scholar] [CrossRef] [Green Version]
- Elbatanony, R.S.; Parvathaneni, V.; Kulkarni, N.S.; Shukla, S.K.; Chauhan, G.; Kunda, N.K.; Gupta, V. Afatinib-Loaded Inhalable PLGA Nanoparticles for Localized Therapy of Non-Small Cell Lung Cancer (NSCLC)-Development and in-Vitro Efficacy. Drug Deliv. Transl. Res. 2021, 11, 927–943. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.T.; Lin, H.; Wang, C.S.; Chang, C.H.; Lin, A.M.Y.; Yang, J.C.H.; Lo, Y.L. Improving the Anticancer Effect of Afatinib and MicroRNA by Using Lipid Polymeric Nanoparticles Conjugated with Dual PH-Responsive and Targeting Peptides. J. Nanobiotechnol. 2019, 17, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T. Characterization Techniques for Nanoparticles: Comparison and Complementarity upon Studying Nanoparticle Properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, F.; Leng, Y.; Ding, Y.; Wang, K. Hydrothermal Growth of Biomimetic Carbonated Apatite Nanoparticles with Tunable Size, Morphology and Ultrastructure. CrystEngComm 2013, 15, 2137–2146. [Google Scholar] [CrossRef]
- Rasuli, H.; Rasuli, R.; Alizadeh, M.; BoonTong, G. Microwave-Assisted Exfoliation and Tearing of Graphene Oxide in the Presence of TiO2 Nanoparticles. Results Phys. 2020, 18, 103200. [Google Scholar] [CrossRef]
- Lu, X.Y.; Liu, S.; Han, M.S.; Yang, X.C.; Sun, K.X.; Wang, H.B.; Mu, H.J.; Du, Y.; Wang, A.P.; Ni, L.; et al. Afatinib-Loaded Immunoliposomes Functionalized with Cetuximab: A Novel Strategy Targeting the Epidermal Growth Factor Receptor for Treatment of Non-Small-Cell Lung Cancer. Int. J. Pharm. 2019, 560, 126–135. [Google Scholar] [CrossRef]
- Chemmalar, S.; Intan-Shameha, A.R.; Abdullah, C.A.C.; Razak, N.A.A.; Yusof, L.M.; Ajat, M.; Gowthaman, N.S.K.; Bakar, M.Z.A. Synthesis and Characterization of Gefitinib and Paclitaxel Mono and Dual Drug-Loaded Blood Cockle Shells (Anadara Granosa)-Derived Aragonite CaCO3 Nanoparticles. Nanomaterials 2021, 11, 1988. [Google Scholar] [CrossRef]
- Lara-Ochoa, S.; Ortega-Lara, W.; Enrique Guerrero-Beltrán, C.; Pinto Ribeiro, V.; Miguel Oliveira, J.; Reis, R.L. Hydroxyapatite Nanoparticles in Drug Delivery: Physicochemistry and Applications. Pharmaceutics 2021, 13, 1642. [Google Scholar] [CrossRef]
- Martínez-Casado, F.J.; Iafisco, M.; Delgado-López, J.M.; Martínez-Benito, C.; Ruiz-Pérez, C.; Colangelo, D.; Oltolina, F.; Prat, M.; Gómez-Morales, J. Bioinspired Citrate-Apatite Nanocrystals Doped with Divalent Transition Metal Ions. Cryst. Growth Des. 2016, 16, 145–153. [Google Scholar] [CrossRef]
- Gómez-Morales, J.; Verdugo-Escamilla, C.; Fernández-Penas, R.; Parra-Milla, C.M.; Drouet, C.; Maube-Bosc, F.; Oltolina, F.; Prat, M.; Fernández-Sánchez, J.F. Luminescent Biomimetic Citrate-Coated Europium-Doped Carbonated Apatite Nanoparticles for Use in Bioimaging: Physico-Chemistry and Cytocompatibility. RSC Adv. 2018, 8, 2385–2397. [Google Scholar] [CrossRef] [Green Version]
- Sari, M.; Kristianto, N.A.; Chotimah; Ana, I.D.; Yusuf, Y. Carbonated Hydroxyapatite-Based Honeycomb Scaffold Coatings on a Titanium Alloy for Bone Implant Application—Physicochemical and Mechanical Properties Analysis. Coatings 2021, 11, 941. [Google Scholar] [CrossRef]
- Mozar, F.S.; Chowdhury, E.H. Surface-Modification of Carbonate Apatite Nanoparticles Enhances Delivery and Cytotoxicity of Gemcitabine and Anastrozole in Breast Cancer Cells. Pharmaceutics 2017, 9, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mozar, F.; Chowdhury, E. Gemcitabine Interacts with Carbonate Apatite with Concomitant Reduction in Particle Diameter and Enhancement of Cytotoxicity in Breast Cancer Cells. Curr. Drug Deliv. 2015, 12, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Syazwan Mohd Noor, M.; Nabilah Afja Mohd Afandi, N.; Mohd Noor, A.-F.; Marliana Baba Ismail, Y.; Marliana Baba, Y. Effect of Carbonate to Phosphate Molar Ratios on the Physico-Chemical Properties of Carbonated Hydroxyapatite Nanopowder. J. Eng. Sci. 2020, 16, 101–110. [Google Scholar] [CrossRef]
- Webb, H.K.; Truong, V.K.; Hasan, J.; Fluke, C.; Crawford, R.J.; Ivanova, E.P. Roughness Parameters for Standard Description of Surface Nanoarchitecture. Scanning 2012, 34, 257–263. [Google Scholar] [CrossRef] [Green Version]
- Hashemzadeh, H.; Allahverdi, A.; Sedghi, M.; Vaezi, Z.; Moghadam, T.T.; Rothbauer, M.; Fischer, M.B.; Ertl, P.; Naderi-Manesh, H. PDMS Nano-Modified Scaffolds for Improvement of Stem Cells Proliferation and Differentiation in Microfluidic Platform. Nanomaterials 2020, 10, 668. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.; Zhu, Y.; Bai, S.; He, C.; Du, G.; Zhang, Y.; Zhong, Y.; Chen, W.; Wang, H.; Sun, X. Nanoparticles with Rough Surface Improve the Therapeutic Effect of Photothermal Immunotherapy against Melanoma. Acta Pharm. Sin. B 2021. [Google Scholar] [CrossRef]
- Aw-Yong, P.Y.; Gan, P.H.; Sasmita, A.O.; Mak, S.T.; Ling, A. (PDF) Nanoparticles as Carriers of Phytochemicals: Recent Applications against Lung Cancer | Andrew O Sasmita—Academia.Edu. Available online: https://www.academia.edu/35949766/Nanoparticles_as_Carriers_of_Phytochemicals_Recent_Applications_Against_Lung_Cancer (accessed on 10 March 2022).
- Maeda, H. Toward a Full Understanding of the EPR Effect in Primary and Metastatic Tumors as Well as Issues Related to Its Heterogeneity. Adv. Drug Deliv. Rev. 2015, 91, 3–6. [Google Scholar] [CrossRef]
- Eaton, P.; Quaresma, P.; Soares, C.; Neves, C.; De Almeida, M.P.; Pereira, E.; West, P. A Direct Comparison of Experimental Methods to Measure Dimensions of Synthetic Nanoparticles. Ultramicroscopy 2017, 182, 179–190. [Google Scholar] [CrossRef]
- Kamal, N.A.M.A.; Abdulmalek, E.; Fakurazi, S.; Cordova, K.E.; Abdul Rahman, M.B. Surface Peptide Functionalization of Zeolitic Imidazolate Framework-8 for Autonomous Homing and Enhanced Delivery of Chemotherapeutic Agent to Lung Tumor Cells. Dalton Trans. 2021, 50, 2375–2386. [Google Scholar] [CrossRef]
- Souza, T.G.F.; Ciminelli, V.S.T.; Mohallem, N.D.S. A Comparison of TEM and DLS Methods to Characterize Size Distribution of Ceramic Nanoparticles; IOP Publishing: Bristol, UK, 2016. [Google Scholar]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, Y.H.; Davis, J.A.; Fujikawa, K.; Ganesh, N.V.; Demchenko, A.V.; Stine, K.J. Surface Area and Pore Size Characteristics of Nanoporous Gold Subjected to Thermal, Mechanical, or Surface Modification Studied Using Gas Adsorption Isotherms, Cyclic Voltammetry, Thermogravimetric Analysis, and Scanning Electron Microscopy. J. Mater. Chem. 2012, 22, 6733. [Google Scholar] [CrossRef] [PubMed]
- Abbasi Aval, N.; Pirayesh Islamian, J.; Hatamian, M.; Arabfirouzjaei, M.; Javadpour, J.; Rashidi, M.R. Doxorubicin Loaded Large-Pore Mesoporous Hydroxyapatite Coated Superparamagnetic Fe3O4 Nanoparticles for Cancer Treatment. Int. J. Pharm. 2016, 509, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Cychosz, K.A.; Thommes, M. Progress in the Physisorption Characterization of Nanoporous Gas Storage Materials. Engineering 2018, 4, 559–566. [Google Scholar] [CrossRef]
- Lo, Y.L.; Lin, H.C.; Hong, S.T.; Chang, C.H.; Wang, C.S.; Lin, A.M.Y. Lipid Polymeric Nanoparticles Modified with Tight Junction-Modulating Peptides Promote Afatinib Delivery across a Blood–Brain Barrier Model. Cancer Nanotechnol. 2021, 12, 13. [Google Scholar] [CrossRef]
- Fosca, M.; Rau, J.V.; Uskoković, V. Factors Influencing the Drug Release from Calcium Phosphate Cements. Bioact. Mater. 2022, 7, 341–363. [Google Scholar] [CrossRef]
- Sambudi, N.S.; Cho, S.; Cho, K. Porous Hollow Hydroxyapatite Microspheres Synthesized by Spray Pyrolysis Using a Microalga Template: Preparation, Drug Delivery, and Bioactivity. RSC Adv. 2016, 6, 43041–43048. [Google Scholar] [CrossRef]
- Hesaraki, S.; Zamanian, M.; Khorami, M. Nano-Structured Apatite Granules as Drug Delivery Device for Bone Infection Treatments. Available online: https://www.researchgate.net/publication/229038005_Nano-Structured_Apatite_Granules_as_Drug_Delivery_Device_for_Bone_Infection_Treatments (accessed on 23 March 2022).
- Unagolla, J.M.; Jayasuriya, A.C. Drug Transport Mechanisms and In Vitro Release Kinetics of Vancomycin Encapsulated Chitosan-Alginate Polyelectrolyte Microparticles as a Controlled Drug Delivery System. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2018, 114, 199. [Google Scholar] [CrossRef]
- Mircioiu, C.; Voicu, V.; Anuta, V.; Tudose, A.; Celia, C.; Paolino, D.; Fresta, M.; Sandulovici, R.; Mircioiu, I. Mathematical Modeling of Release Kinetics from Supramolecular Drug Delivery Systems. Pharmaceutics 2019, 11, 140. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Yuan, W.; Li, D.; Schwendeman, A.; Schwendeman, S.P. Predicting Drug Release Kinetics from Nanocarriers inside Dialysis Bags. J. Control. Release Off. J. Control. Release Soc. 2019, 315, 23–30. [Google Scholar] [CrossRef]
- Ma-Ham, A.; Wu, H.; Wang, J.; Kang, X.; Zhang, Y.; Lin, Y. Apoferritin-Based Nanomedicine Platform for Drug Delivery: Equilibrium Binding Study of Daunomycin with DNA. J. Mater. Chem. 2011, 21, 8700–8708. [Google Scholar] [CrossRef]
- Ishikawa, K.; Hayashi, K. Carbonate Apatite Artificial Bone. Sci. Technol. Adv. Mater. 2021, 22, 683. [Google Scholar] [CrossRef] [PubMed]
- Som, A.; Raliya, R.; Tian, L.; Akers, W.; Ippolito, J.E.; Singamaneni, S.; Biswas, P.; Achilefu, S. Monodispersed Calcium Carbonate Nanoparticles Modulate Local PH and Inhibit Tumor Growth in Vivo. Nanoscale 2016, 8, 12639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cieplik, F.; Rupp, C.M.; Hirsch, S.; Muehler, D.; Enax, J.; Meyer, F.; Hiller, K.A.; Buchalla, W. Ca2+ Release and Buffering Effects of Synthetic Hydroxyapatite Following Bacterial Acid Challenge. BMC Oral Health 2020, 20, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horie, M.; Nishio, K.; Kato, H.; Endoh, S.; Fujita, K.; Nakamura, A.; Kinugasa, S.; Hagihara, Y.; Yoshida, Y.; Iwahashi, H. Evaluation of Cellular Influences Caused by Calcium Carbonate Nanoparticles. Chem.-Biol. Interact. 2014, 210, 64–76. [Google Scholar] [CrossRef] [PubMed]

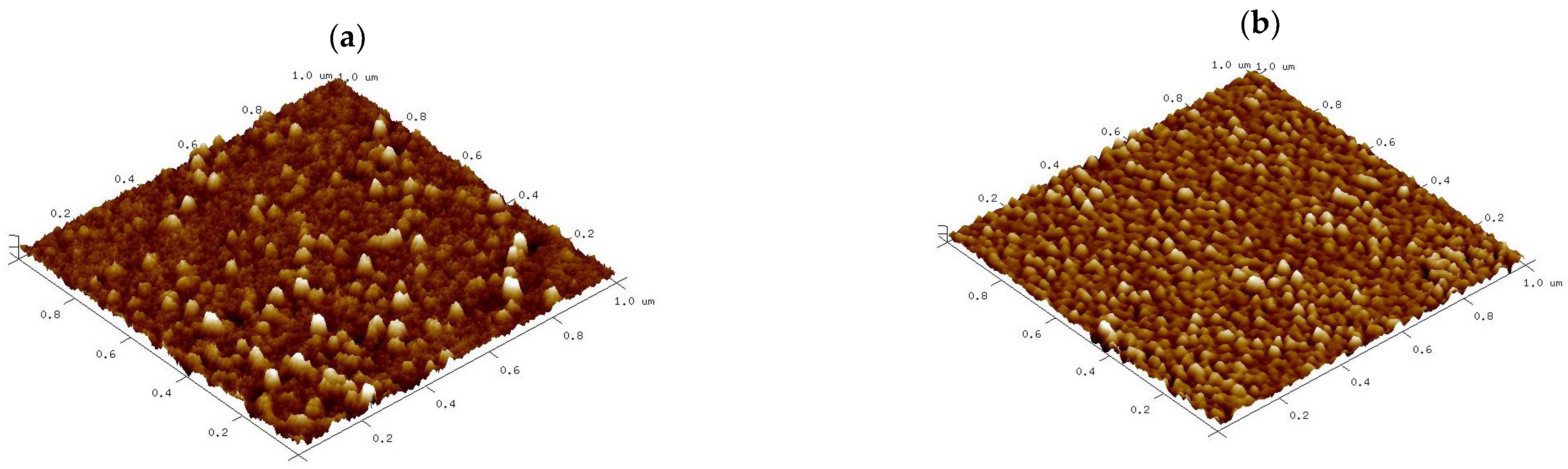



| Sample | Ra (nm) | Rq (nm) | RMax (nm) | Skewness | Kurtosis | Surface Area Difference |
|---|---|---|---|---|---|---|
| nCA | 0.582 | 0.809 | 7.8 | 1.90 | 3.89 | 1.7% |
| nCA/AFA | 0.563 | 1.02 | 7.2 | 0.97 | 3.05 | 1.24% |
| Sample Name | BET Specific Surface Area (m2/g) | BJH Mean Pore Width (nm) | Pore Volume (cm3/g) |
|---|---|---|---|
| nCA | 55.53 | 7.88 | 0.11 |
| nCA/AFA | 52.61 | 7.36 | 0.09 |
| Sample | AFA Weight (µg) | EE (%) | DL (%) | Yield % |
|---|---|---|---|---|
| nCA/AFA1 | 150 | 15.27 ± 0.58 | 0.45 ± 0.47 | 45.38 |
| nCA/AFA2 | 500 | 38.51 ± 0.83 | 3.84 ± 0.38 | 47.87 |
| nCA/AFA3 | 750 | 55.08 ± 1.68 | 8.19 ± 0.52 | 50.81 |
| nCA/AFA4 | 1000 | 41.44 ± 1.09 | 8.08 ± 0.05 | 49.86 |
| Model | Korsmeyer–Peppas | First-Order Kinetics | Zero-Order Kinetics | Higuchi | Hixson–Crowell | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Name and pH of Medium | KKP | R2 | n | K1 | R2 | K0 | R2 | KH | R2 | KHC | R2 |
| nCA/AFA pH 7.4 | 4.7461 | 0.9537 | 0.78 | 0.0705 | 0.7001 | 0.7001 | 0.8744 | 3.2501 | 0.9341 | 0.0304 | 0.8634 |
| nCA/AFA pH 5.5 | 6.6638 | 0.9763 | 0.64 | 0.0742 | 0.7259 | 0.7258 | 0.8756 | 4.5278 | 0.9177 | 0.0291 | 0.7816 |
| Release Exponent (n) | Mechanism | Time |
|---|---|---|
| Fickian diffusion | t0.5 | |
| Non-Fickian diffusion | tn−1 | |
| Case II transport | t | |
| Super case II transport | tn−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maarof, N.N.N.; Abdulmalek, E.; Fakurazi, S.; Rahman, M.B.A. Biodegradable Carbonate Apatite Nanoparticle as a Delivery System to Promote Afatinib Delivery for Non-Small Cell Lung Cancer Treatment. Pharmaceutics 2022, 14, 1230. https://doi.org/10.3390/pharmaceutics14061230
Maarof NNN, Abdulmalek E, Fakurazi S, Rahman MBA. Biodegradable Carbonate Apatite Nanoparticle as a Delivery System to Promote Afatinib Delivery for Non-Small Cell Lung Cancer Treatment. Pharmaceutics. 2022; 14(6):1230. https://doi.org/10.3390/pharmaceutics14061230
Chicago/Turabian StyleMaarof, Nian N. N., Emilia Abdulmalek, Sharida Fakurazi, and Mohd Basyaruddin Abdul Rahman. 2022. "Biodegradable Carbonate Apatite Nanoparticle as a Delivery System to Promote Afatinib Delivery for Non-Small Cell Lung Cancer Treatment" Pharmaceutics 14, no. 6: 1230. https://doi.org/10.3390/pharmaceutics14061230







