Two-Layer Sustained-Release Microneedles Encapsulating Exenatide for Type 2 Diabetes Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Animals
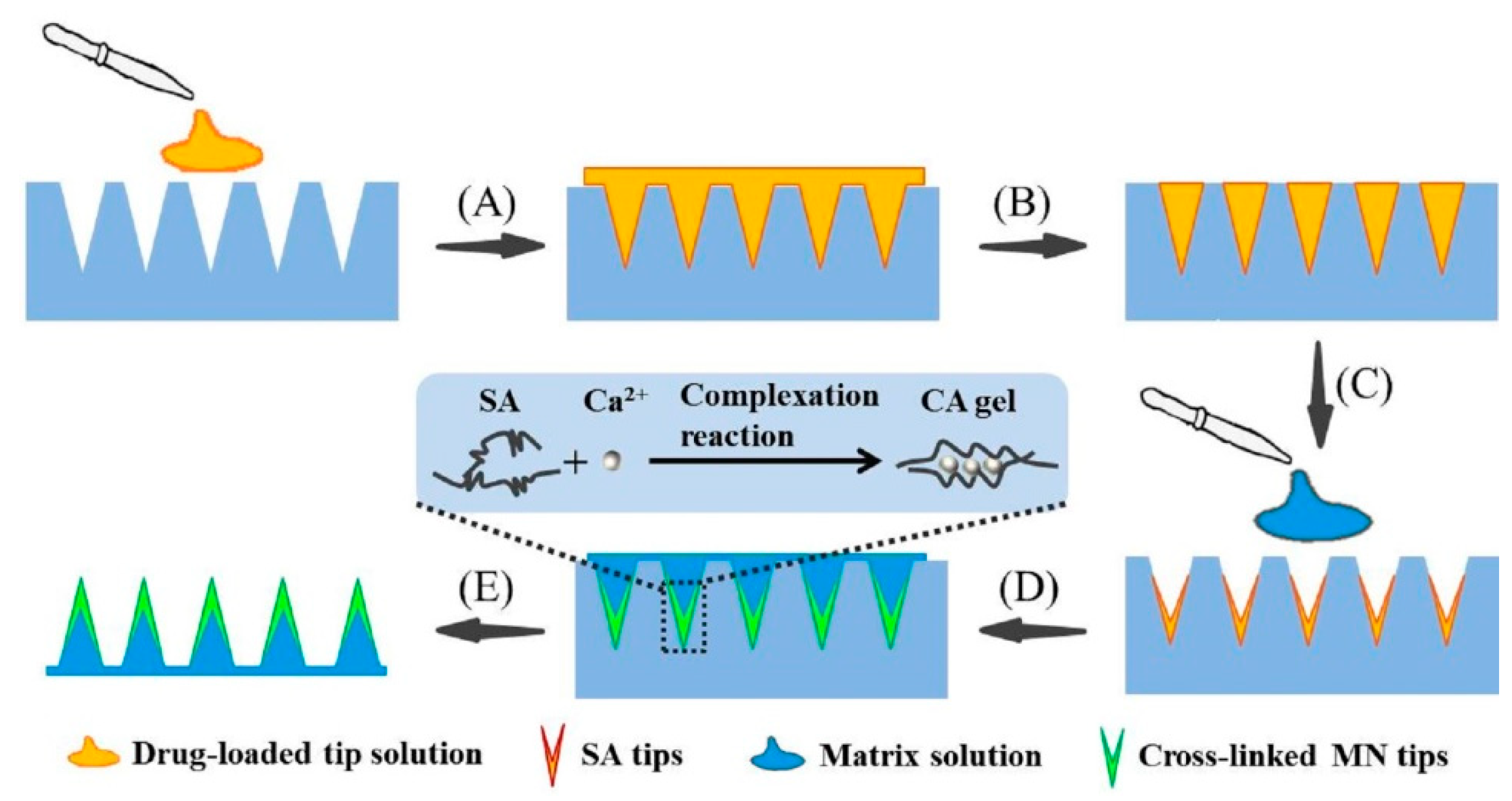
2.2. Fabrication of TS-MNs
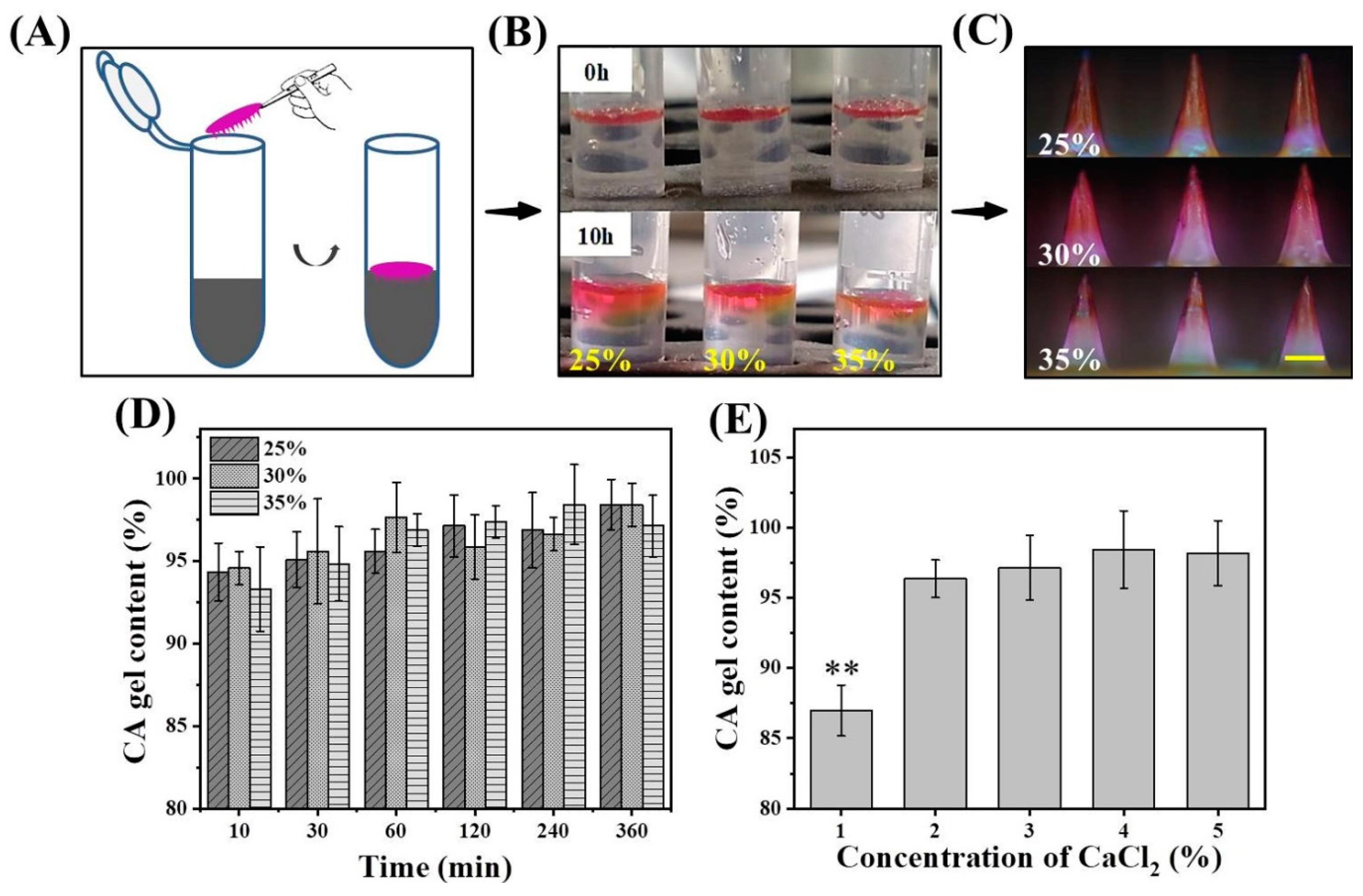
2.3. Effect of Matrix Solution Concentration on Gel Content
2.4. Effect of Matrix Solution Concentration on Drug Diffusion
2.5. Effect of CaCl2 Concentration on Gel Content
2.6. Influence of SA Concentration on Tip Height and Implantation Efficiency
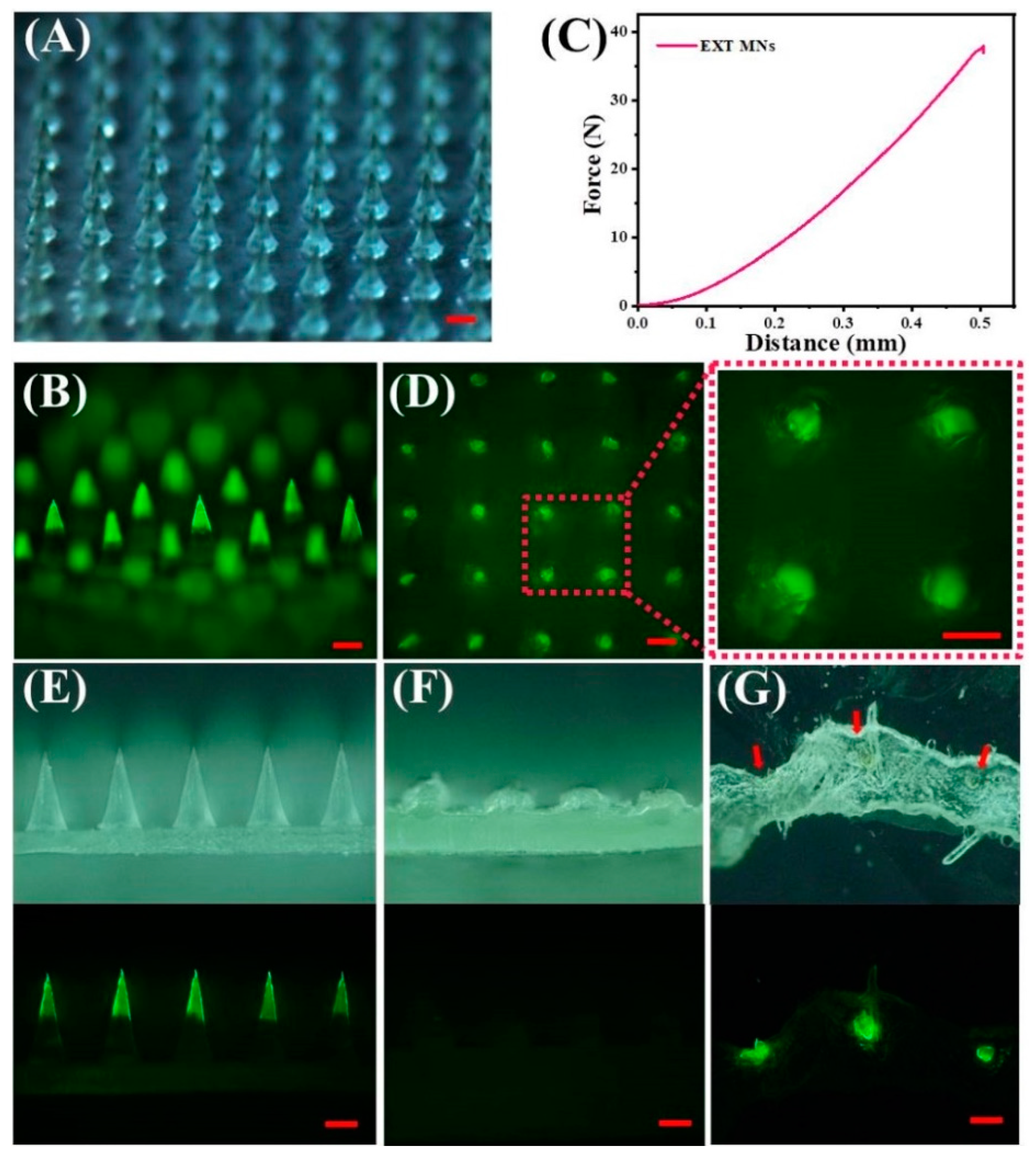
2.7. Morphology Analysis and Skin Insertion Tests
2.7.1. Preparation of FITC-EXT
2.7.2. Morphology Analysis and Skin Insertion Tests
2.8. Quantification of EXT by HPLC
2.9. In Vitro Release and Kinetics Study
2.10. Determination of EXT Activity and MN Storage Stability
2.11. In Vivo Pharmacokinetics
2.12. In Vivo Pharmacodynamics
2.12.1. Establishment of the Type 2 Diabetes Rat Model
2.12.2. Single Administration in Diabetic Rats
2.12.3. Multiple Administrations in Diabetic Rats
2.13. Statistical Analysis
3. Results and Discussion
3.1. Factors Affecting Gel Content and Drug Diffusion
3.2. Influence of SA Concentration on Tip Height and Implantation Efficiency
3.3. Characterization and Insertion Capability of TS-MNs
3.4. In Vitro Release and Kinetics Study
3.5. Activity of EXT after MN Fabrication and Storage Stability of EXT Encapsulated in MNs
3.6. In Vivo Pharmacokinetics
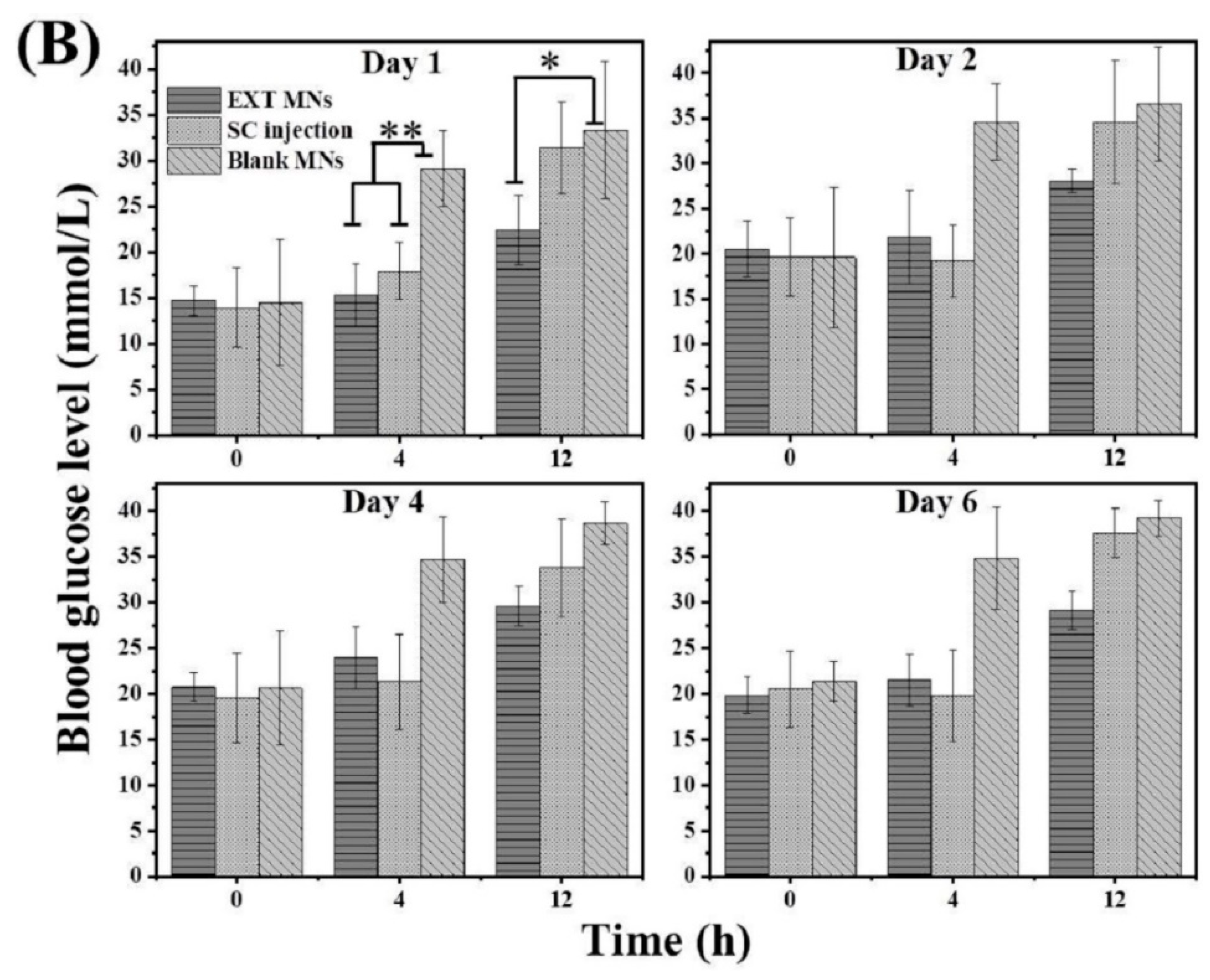
3.7. Hypoglycemic Effect In Vivo
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Wang, J.; Zhang, Y.; Chen, G.; Mao, W.; Ye, Y.; Kahkoska, A.R.; Buse, J.B.; Langer, R.; Gu, Z. Glucose-responsive insulin patch for the regulation of blood glucose in mice and minipigs. Nat. Biomed. Eng. 2020, 4, 499–506. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Hu, Y.; Gui, Z.; Zhou, Y.; Nie, T.; Zhu, J.; Liu, Z.; Chen, K.; Liu, L.; Leong, K.W.; et al. Sustained release of exendin-4 from tannic acid/Fe (III) nanoparticles prolongs blood glycemic control in a mouse model of type II diabetes. J. Control Release 2019, 301, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Fineman, M.S.; Mace, K.F.; Diamant, M.; Darsow, T.; Cirincione, B.B.; Booker Porter, T.K.; Kinninger, L.A.; Trautmann, M.E. Clinical relevance of anti-exenatide antibodies: Safety, efficacy and cross-reactivity with long-term treatment. Diabetes Obes. Metab. 2012, 14, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhuo, X.; Chu, W.; Tang, X. Exenatide-loaded microsphere/thermosensitive hydrogel long-acting delivery system with high drug bioactivity. Int. J. Pharm. 2017, 528, 62–75. [Google Scholar] [CrossRef]
- Song, Y.; Shi, Y.; Zhang, L.; Hu, H.; Zhang, C.; Yin, M.; Chu, L.; Yan, X.; Zhao, M.; Zhang, X.; et al. Synthesis of CSK-DEX-PLGA Nanoparticles for the Oral Delivery of Exenatide to Improve Its Mucus Penetration and Intestinal Absorption. Mol. Pharm. 2019, 16, 518–532. [Google Scholar] [CrossRef]
- Abeer, M.M.; Meka, A.K.; Pujara, N.; Kumeria, T.; Strounina, E.; Nunes, R.; Costa, A.; Sarmento, B.; Hasnain, S.Z.; Ross, B.P.; et al. Rationally Designed Dendritic Silica Nanoparticles for Oral Delivery of Exenatide. Pharmaceutics 2019, 11, 418. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, K.; Kim, K.S.; Bae, Y.H. Long-term oral administration of Exendin-4 to control type 2 diabetes in a rat model. J. Control Release 2019, 294, 259–267. [Google Scholar] [CrossRef]
- Yao, W.; Tao, C.; Zou, J.; Zheng, H.; Zhu, J.; Zhu, Z.; Zhu, J.; Liu, L.; Li, F.; Song, X. Flexible two-layer dissolving and safing microneedle transdermal of neurotoxin: A biocomfortable attempt to treat Rheumatoid Arthritis. Int. J. Pharm. 2019, 563, 91–100. [Google Scholar] [CrossRef]
- Hu, L.; Liao, Z.; Hu, Q.; Maffucci, K.G.; Qu, Y. Novel Bletilla striata polysaccharide microneedles: Fabrication, characterization, and in vitro transcutaneous drug delivery. Int. J. Biol. Macromol. 2018, 117, 928–936. [Google Scholar] [CrossRef]
- Luo, Z.; Sun, W.; Fang, J.; Lee, K.; Li, S.; Gu, Z.; Dokmeci, M.R.; Khademhosseini, A. Biodegradable Gelatin Methacryloyl Microneedles for Transdermal Drug Delivery. Adv. Healthc. Mater. 2019, 8, 1801054. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.R.; Dobson, L.J.; Pattanayek, S.K.; Das, D.B. Swellable microneedles based transdermal drug delivery: Mathematical model development and numerical experiments. Chem. Eng. Sci. 2022, 247, 117005. [Google Scholar] [CrossRef]
- Kim, J.H.; Shin, J.U.; Kim, S.H.; Noh, J.Y.; Kim, H.R.; Lee, I.; Chu, H.; Jeong, K.Y.; Park, K.H.; Kim, J.D.; et al. Successful transdermal allergen delivery and allergen-specific immunotherapy using biodegradable microneedle patches. Biomaterials 2018, 150, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Park, T.Y.; Jeon, E.Y.; Joo, K.I.; Cha, H.J. Double-layered adhesive microneedle bandage based on biofunctionalized mussel protein for cardiac tissue regeneration. Biomaterials 2021, 278, 121171. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Tian, R.; Xu, C.; Yung, B.C.; Wang, G.; Liu, Y.; Ni, Q.; Zhang, F.; Zhou, Z.; Wang, J.; et al. Microneedle-array patches loaded with dual mineralized protein/peptide particles for type 2 diabetes therapy. Nat. Commun. 2017, 8, 1777. [Google Scholar] [CrossRef]
- Li, W.; Terry, R.N.; Tang, J.; Feng, M.R.; Schwendeman, S.P.; Prausnitz, M.R. Rapidly separable microneedle patch for the sustained release of a contraceptive. Nat. Biomed. Eng. 2019, 3, 220–229. [Google Scholar] [CrossRef]
- Chen, M.-C.; Lai, K.-Y.; Ling, M.-H.; Lin, C.-W. Enhancing immunogenicity of antigens through sustained intradermal delivery using chitosan microneedles with a patch-dissolvable design. Acta. Biomater. 2018, 65, 66–75. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Wee, G.S. Protein release from alginate matrices. Adv. Drug. Deliv. Rev. 1998, 31, 267–285. [Google Scholar]
- Uyen, N.T.T.; Hamid, Z.A.A.; Tram, N.X.T.; Ahmad, N. Fabrication of alginate microspheres for drug delivery: A review. Int. J. Biol. Macromol. 2020, 153, 1035–1046. [Google Scholar] [CrossRef]
- Sorasitthiyanukarn, F.N.; Na Bhuket, P.R.; Muangnoi, C.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan/alginate nanoparticles as a promising carrier of novel curcumin diethyl diglutarate. Int. J. Biol. Macromol. 2019, 131, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.P.S.; Lee, W.W.; Han, E.J.; Ahn, G. Alginate-based nanomaterials: Fabrication techniques, properties, and applications. Chem. Eng. J. 2020, 391, 123823. [Google Scholar] [CrossRef]
- Hajifathaliha, F.; Mahboubi, A.; Nematollahi, L.; Mohit, E.; Bolourchian, N. Comparison of different cationic polymers efficacy in fabrication of alginate multilayer microcapsules. Asian J. Pharm. Sci. 2020, 15, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, S.; Yang, G.; Gao, Y. Enhanced delivery efficiency and sustained release of biopharmaceuticals by complexation-based gel encapsulated coated microneedles: rhIFNalpha-1b example. Asian J. Pharm. 2021, 16, 612–622. [Google Scholar] [CrossRef]
- Zuo, Q.; Lu, J.; Hong, A.; Zhong, D.; Xie, S.; Liu, Q.; Huang, Y.; Shi, Y.; He, L.; Xue, W. Preparation and characterization of PEM-coated alginate microgels for controlled release of protein. Biomed. Mater. 2012, 7, 035012. [Google Scholar] [CrossRef]
- Li, W.; Tang, J.; Terry, R.N.; Li, S.; Brunie, A.; Callahan, R.L.; Noel, R.K.; Rodríguez, C.A.; Schwendeman, S.P.; Prausnitz, M.R. Long-acting reversible contraception by effervescent. Sci. Adv. 2019, 5, 8145. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, A.R.; Sohail, M.; Minhas, M.U.; Khaliq, T.; Kousar, M.; Khan, S.; Hussain, Z.; Munir, A. Bioinspired sodium alginate based thermosensitive hydrogel membranes for accelerated wound healing. Int. J. Biol. Macromol. 2020, 155, 751–765. [Google Scholar] [CrossRef]
- Liu, J.L.; Feng, Y.H.; Zhang, X.P.; Zhu, D.D.; Zhang, L.Q.; Guo, X.D. Experimental and theoretical studies of drug-polymer interactions to control the drug distributions in dissolving microneedles. J. Ind. Eng. Chem. 2020, 84, 280–289. [Google Scholar] [CrossRef]
- He, M.; Yang, G.; Zhang, S.; Zhao, X.; Gao, Y. Dissolving Microneedles Loaded with Etonogestrel Microcrystal Particles for Intradermal Sustained Delivery. J. Pharm. Sci. 2018, 107, 1037–1045. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, S.; Yang, G.; Zhou, Z.; Gao, Y. Exploring Trehalose on the Release of Levonorgestrel from Implantable PLGA Microneedles. Polymers (Basel) 2020, 12, 59. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Xing, M.; Zhang, S.; Yang, G.; Gao, Y. Process optimization of Ca2+ cross-linked alginate-based swellable microneedles for enhanced transdermal permeability: More applicable to acidic drugs. Int. J. Pharm. 2022, 618, 121669. [Google Scholar] [CrossRef] [PubMed]
- Alkholief, M.; Kalam, M.A.; Anwer, M.K.; Alshamsan, A. Effect of Solvents, Stabilizers and the Concentration of Stabilizers on the Physical Properties of Poly(d,l-lactide-co-glycolide) Nanoparticles: Encapsulation, In Vitro Release of Indomethacin and Cytotoxicity against HepG2-Cell. Pharmaceutics 2022, 14, 870. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, Z.; Li, L.; Zhou, T.; Lu, W. Pharmacokinetics, in vitro and in vivo correlation, and efficacy of exenatide microspheres in diabetic rats. Drug Deliv. 2015, 22, 86–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Y.; Liu, J.; Zhu, D.; Hao, Y.; Guo, X. Multiscale simulations of drug distributions in polymer dissolvable microneedles. Colloids Surf. B Biointerfaces 2020, 189, 110844. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Li, W.; Tang, I.; Schwendeman, S.P.; Prausnitz, M.R. Immediate detachment of microneedles by interfacial fracture for sustained delivery of a contraceptive hormone in the skin. J. Control Release 2021, 337, 676–685. [Google Scholar] [CrossRef]
- Zhu, Z.; Luo, H.; Lu, W.; Luan, H.; Wu, Y.; Luo, J.; Wang, Y.; Pi, J.; Lim, C.Y.; Wang, H. Rapidly dissolvable microneedle patches for transdermal delivery of exenatide. Pharm. Res. 2014, 31, 3348–3360. [Google Scholar] [CrossRef]
- Wu, D.; Quan, Y.S.; Kamiyama, F.; Kusamori, K.; Katsumi, H.; Sakane, T.; Yamamoto, A. Improvement of Transdermal Delivery of Sumatriptan Succinate Using a Novel Self-dissolving Microneedle Array Fabricated from Sodium Hyaluronate in Rats. Biol. Pharm. Bull. 2015, 38, 365–373. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.-C.; Huang, S.-F.; Lai, K.-Y.; Ling, M.-H. Fully embeddable chitosan microneedles as a sustained release depot for intradermal vaccination. Biomaterials 2013, 34, 3077–3086. [Google Scholar] [CrossRef]
- Houchin, M.L.; Topp, E.M. Chemical degradation of peptides and proteins in PLGA: A review of reactions and mechanisms. J. Pharm. Sci. 2008, 97, 2395–2404. [Google Scholar] [CrossRef]





| Fitting Kinetic Model | Formulations | |||
|---|---|---|---|---|
| SA:EXT = 3:2 | SA:EXT = 3:2 | |||
| Equation | R2 | Equation | R2 | |
| Zero-order | Qt = 1.71 t + 16.27 | 0.7581 | Qt = 1.67 t + 11.96 | 0.8221 |
| First-order | Qt = 84.06 (1 – e−0.08t) | 0.9878 | Qt = 82.40 (1 – e−0.06t) | 0.9893 |
| Higuchi | Qt = 13.59 t1/2 – 1.97 | 0.9177 | Qt = 13.01 t1/2 – 4.86 | 0.9419 |
| Pharmacokinetic Parameters | EXT MN Group | SC Injection Group |
|---|---|---|
| Tmax (h) | 0.5 | 0.5 |
| Cmax (ng/mL) | 13.60 ± 2.95 | 16.68 ± 4.87 |
| AUC0-t (ng·h/mL) | 55.17 | 28.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Zhang, S.; Zhou, Z.; Xing, M.; Gao, Y. Two-Layer Sustained-Release Microneedles Encapsulating Exenatide for Type 2 Diabetes Treatment. Pharmaceutics 2022, 14, 1255. https://doi.org/10.3390/pharmaceutics14061255
Liu H, Zhang S, Zhou Z, Xing M, Gao Y. Two-Layer Sustained-Release Microneedles Encapsulating Exenatide for Type 2 Diabetes Treatment. Pharmaceutics. 2022; 14(6):1255. https://doi.org/10.3390/pharmaceutics14061255
Chicago/Turabian StyleLiu, Han, Suohui Zhang, Zequan Zhou, Mengzhen Xing, and Yunhua Gao. 2022. "Two-Layer Sustained-Release Microneedles Encapsulating Exenatide for Type 2 Diabetes Treatment" Pharmaceutics 14, no. 6: 1255. https://doi.org/10.3390/pharmaceutics14061255
APA StyleLiu, H., Zhang, S., Zhou, Z., Xing, M., & Gao, Y. (2022). Two-Layer Sustained-Release Microneedles Encapsulating Exenatide for Type 2 Diabetes Treatment. Pharmaceutics, 14(6), 1255. https://doi.org/10.3390/pharmaceutics14061255




