Clinical Efficacy of Carbocysteine in COPD: Beyond the Mucolytic Action
Abstract
:1. Introduction
2. Materials and Methods
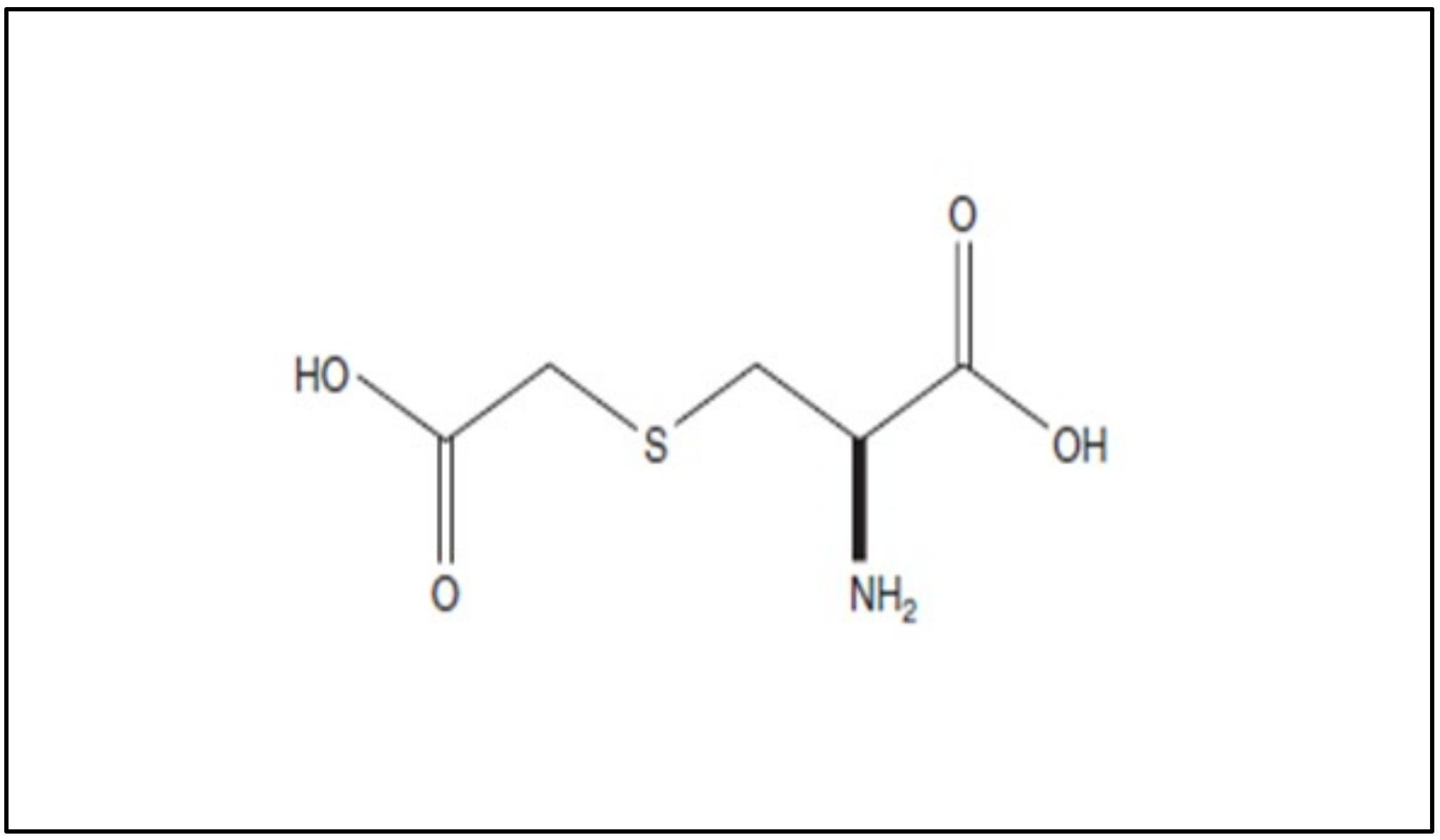
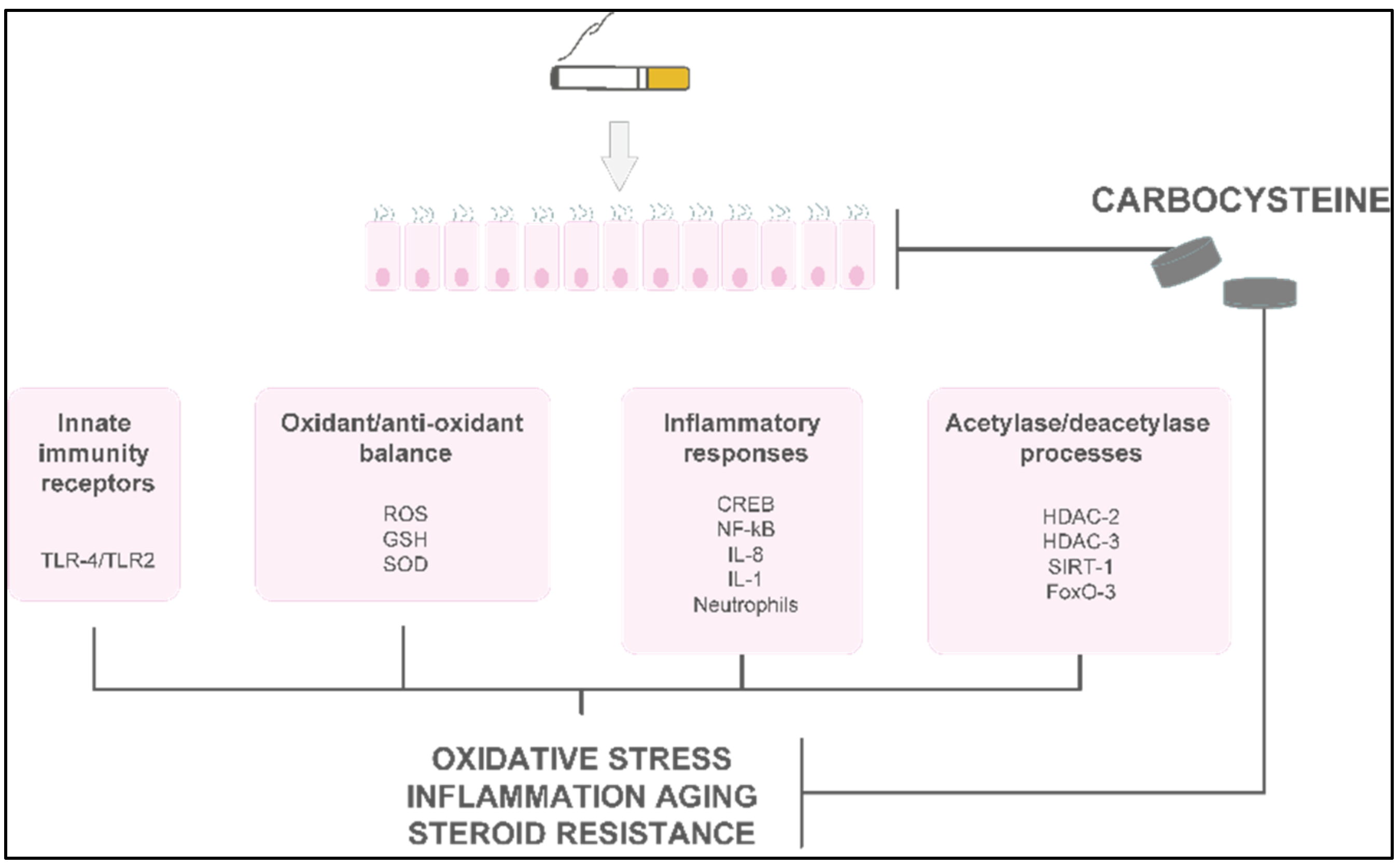
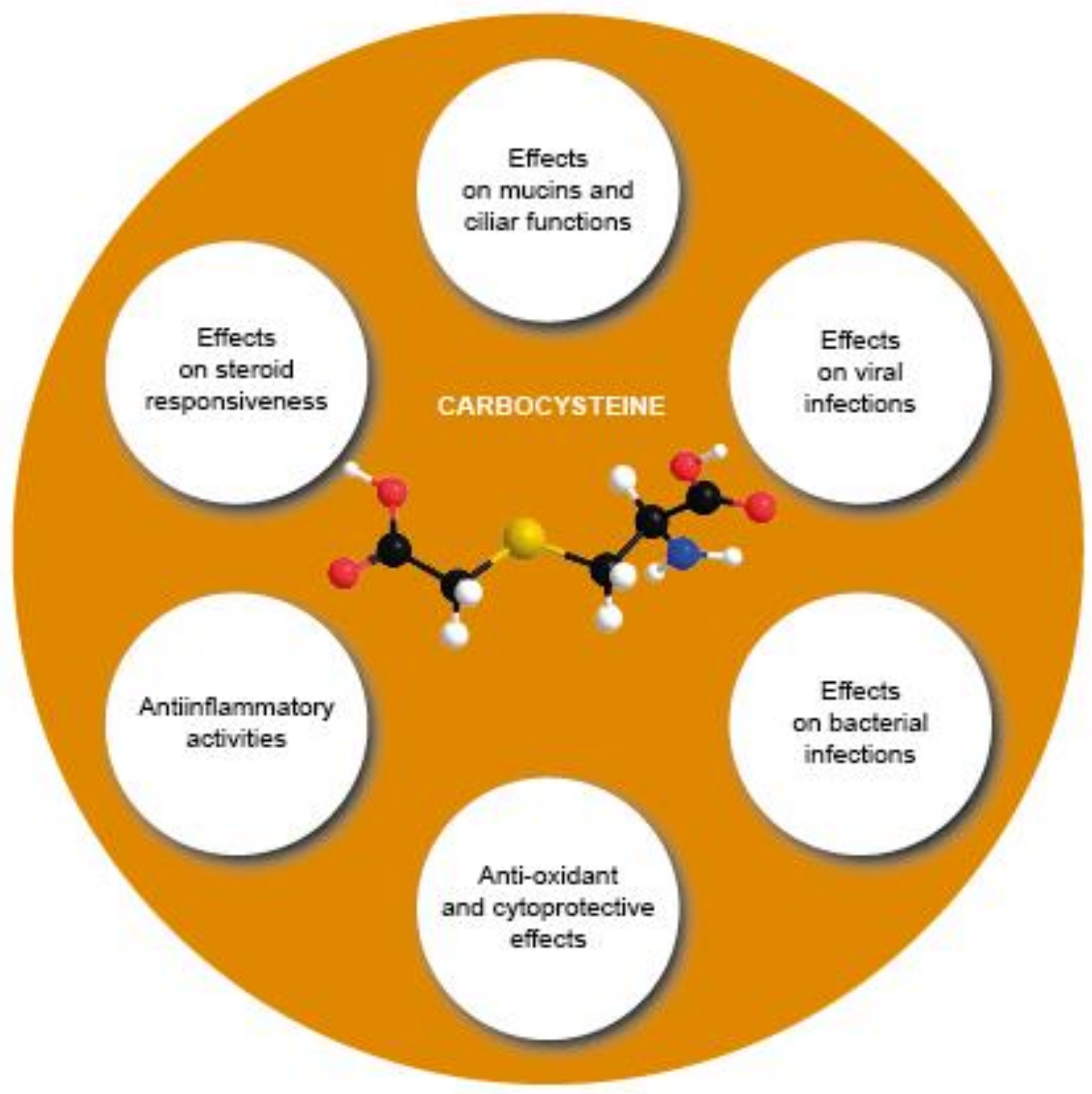
3. Molecular Effects of Carbocysteine
4. Pharmacokinetics
- (1)
- Modulating mucins and ciliary functions;
- (2)
- Counteracting viral infections;
- (3)
- Counteracting bacterial infections;
- (4)
- Counteracting oxidative stress and exerting cytoprotective effects;
- (5)
- Improving steroid responsiveness;
- (6)
- Exerting anti-inflammatory activities.
4.1. Effects of Carbocysteine on Mucins and Ciliary Functions
4.2. Effects of Carbocysteine on Viral Infections
4.3. Effects of Carbocysteine on Bacterial Infections
4.4. Antioxidant and Cytoprotective Effects of Carbocysteine
4.5. Effects of Carbocysteine on Steroid Responsiveness
4.6. Anti-Inflammatory Activities of Carbocysteine
5. In Vitro and In Vivo Studies of Carbocysteine’s Effects
5.1. In Vitro Studies of Carbocysteine’s Effects on Different Cell Lines
5.2. In Vivo Studies of Carbocysteine’s Effects in Animal Models
6. In Vivo Studies of Carbocysteine’s Effects in COPD Patients
Clinical Studies of Carbocysteine’s Effects in COPD
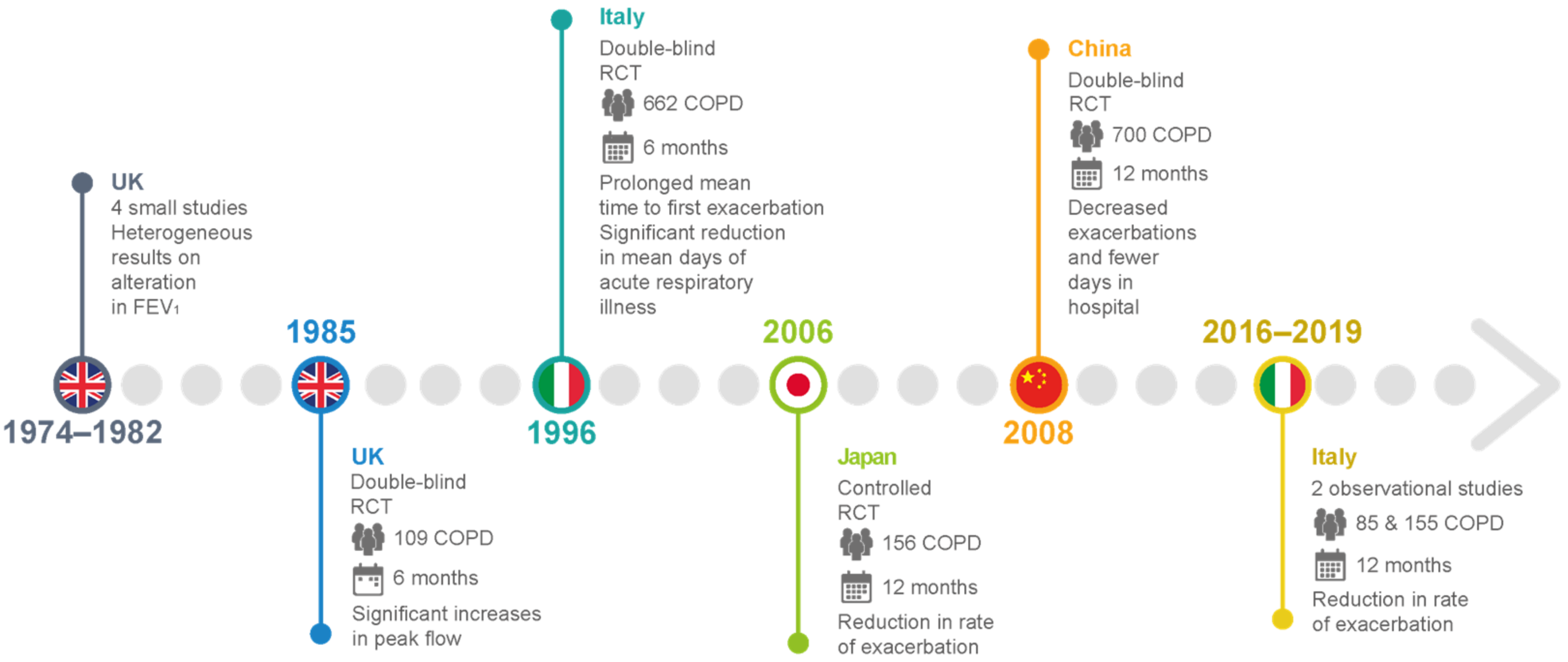
| Clinical Trial | Study Design and Enrolled Patients | Therapeutic Regimen | Outcome |
|---|---|---|---|
| [63,64,65,66] | Small-scale UK studies on patients with chronic bronchitis | 2.25–3.00 g carbocysteine daily vs. placebo | Heterogeneous results for alterations in FEV1, peak flow rate, and dyspnea scores |
| [67] | A double-blind, parallel-group study in the UK of 109 patients with chronic bronchitis over 6 winter months | 750 mg carbocysteine three times daily compared with placebo in terms of peak flow and exacerbation rate. | No significant difference in exacerbation rate. Significant increases in peak flow from baseline in both placebo and intervention groups |
| [26] | Placebo-controlled RCTs in Tokyo for 156 patients with COPD over 12 months | 1.5 g carbocysteine daily with placebo | Significant reduction in the number of common colds and reduction in the rate of exacerbation |
| [68] | An Italian multicenter, prospective, double-blind RCT involving 662 outpatients with chronic bronchitis | 2.7 g SCMC–Lys once daily for 6 months in COPD patients | No significant difference in baseline FEV1 between the groups. Mean time to first exacerbation was significantly prolonged, and significant reduction in mean days of acute respiratory illness per patient. |
| [69] | Multicenter, randomized, double-blind, placebo-controlled, parallel-group study in China involving more than 700 COPD patients with a history of at least two COPD exacerbations within the previous 2 years | 1500 mg/day carbocysteine for one year | Long-term (one year) use of carbocysteine produced a reduction in the numbers of exacerbations in patients with COPD. Decreased exacerbations and fewer days in the hospital. No loss of lung function, and improvement in health-related quality of life. |
| [10] | An Italian observational and prospective study including 85 COPD outpatients with a history of at least 1 COPD exacerbation within the previous year. | 2.7 g of carbocysteine daily for one year | Reduction in the exacerbation rate after 12 months of therapy, completely independent of the use of ICSs. Statistically significant improvement in the quality of life assessed (decrease in SGRQ score) and the distance walked (6MWT), with a significant reduction in the BODE index. No significant improvement in lung function (FEV1, FVC, FEV1/FVC). |
| [72] | Observational prospective study of 155 consecutively enrolled COPD patients with a history of at least 1 COPD exacerbation within the previous year. | 2.7 g of carbocysteine daily for one year | Reduction in the number of exacerbations at 1-year evaluation, irrespective of treatment with or without ICSs. |
7. Limitations and Pitfalls in Carbocysteine Studies
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, X.; Song, Y.; Shen, C.; Xu, W.; Chen, L.; Zhang, J.; Liu, H.; Huang, M.; Lai, G.; Qian, G.; et al. Mucoactive and antioxidant medicines for COPD: Consensus of a group of Chinese pulmonary physicians. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 803–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogelmeier, C.F.; Román Rodríguez, M.; Singh, D.; Han, M.K.; Rodríguez-Roisin, R.; Ferguson, G.T. Goals of COPD treatment: Focus on symptoms and exacerbations. Respir. Med. 2020, 166, 105938. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 DALYs; HALE Collaborators. Global, Regional, and National Disability-Adjusted Life-Years (DALYs) for 359 Diseases and Injuries and Healthy Life Expectancy (HALE) for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1859–1922. [Google Scholar] [CrossRef] [Green Version]
- Decramer, M.; Janssens, W. Mucoactive therapy in COPD. Eur. Respir. Rev. 2010, 19, 134–140. [Google Scholar] [CrossRef]
- Cerveri, I.; Brusasco, V. Revisited role of mucus hypersecretion in the pathogenesis of COPD. Eur. Respir. Rev. 2010, 19, 109–112. [Google Scholar] [CrossRef] [Green Version]
- Flechter, C.M.; Jones, N.L.; Burrows, B.; Niden, A.H. American emphysema and British bronchitis: A standardized comparative study. Am. Rev. Respir. Dis. 1964, 90, 1–13. [Google Scholar]
- Sapey, E.; Bafadhel, M.; Bolton, C.E.; Wilkinson, T.; Hurst, J.R.; Quint, J.K. Building toolkits for COPD exacerbations: Lessons from the past and present. Thorax 2019, 74, 898–905. [Google Scholar] [CrossRef] [Green Version]
- Cazzola, M.; Rogliani, P.; Calzetta, L.; Hanania, N.A.; Matera, M.G. Impact of mucolytic agents on COPD exacerbations: A pair-wise and network meta-analysis. COPD J. Chronic Obstr. Pulm. Dis. 2017, 14, 552–563. [Google Scholar] [CrossRef]
- Poole, P.; Sathananthan, K.; Fortescue, R. Mucolytic agents versus placebo for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2019, 5, CD001287. [Google Scholar] [CrossRef]
- Esposito, A.; Valentino, M.R.; Bruzzese, D.; Bocchino, M.; Ponticiello, A.; Stanziola, A.; Sanduzzi, A. Effect of Carbocysteine in the prevention of exacerbation of chronic obstructive pulmonary disease (CAPRI study): An observational study. Pulm. Pharmacol. Ther. 2016, 37, 85–88. [Google Scholar] [CrossRef] [Green Version]
- Miravitlles, M.; Vogelmeier, C.; Roche, N.; Halpin, D.; Cardoso, J.; Chuchalin, A.G.; Kankaanranta, H.; Sandström, T.; Śliwiński, P.; Zatloukal, J.; et al. A review of national guidelines for management of COPD in Europe. Eur. Respir. J. 2016, 47, 625–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogelmeier, C.F.; Criner, G.J.; Martinez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. Gold Executive Summary. Am. J. Respir. Crit. Care Med. 2017, 195, 557–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Global Strategy for the Diagnosis, Management, and Prevention of 2020 Report. Available online: https:/goldcopd.org (accessed on 7 June 2022).
- Mitchell, S.C.; Steventon, G.B. S-carboxymethyl-L-cysteine. Drug Metab. Rev. 2012, 44, 129–147. [Google Scholar] [CrossRef] [PubMed]
- Colombo, B.; Turconi, P.; Daffonchio, L.; Fedele, G.; Omini, C.; Cremaschi, D. Stimulation of Cl- secretion by the mucoactive drug S-carboxy-methylcysteinelysine salt in the isolated rabbit trachea. Eur. Respir. J. 1994, 7, 1622–1628. [Google Scholar] [CrossRef] [Green Version]
- Brow, D.T. Carbocysteine. Drug Intell. Clin. Pharm. 1988, 22, 603–608. [Google Scholar]
- Servin, A.; Garcet, S.; Huyen, N.; Cohen, Y. Comparative pharmacokinetics of L-cysteine and one of its S-substituted derivatives, S-carboxymethyl cysteine. J. Pharmacol. 1976, 7, 275–286. [Google Scholar]
- Braga, P.C.; Borsa, M.; De Angelis, L.; Bossi, R.; Allegra, L.; Scaglione, F.; Scarpazza, G. Pharmacokinetic behaviour of S-carboxymethyl-cysteine-lys in patients with chronic bronchitis. Clin. Ther. 1982, 4, 480–488. [Google Scholar]
- Waring, R.H.; Mitchell, S.C. The metabolism and elimination of S-carboxymethyl-L-cysteine in man. Drug Metab. Dispos. 1982, 10, 61–62. [Google Scholar]
- Bonser, L.R.; Erle, D.J. Airway Mucus and Asthma: The Role of MUC5AC and MUC5B. J. Clin. Med. 2017, 6, 112. [Google Scholar] [CrossRef] [Green Version]
- Ikeuchi, Y.; Kogiso, H.; Hosogi, S.; Tanaka, S.; Shimamoto, C.; Matsumura, H.; Inui, T.; Marunaka, Y.; Nakahari, T. Carbocysteine stimulated an increase in ciliary bend angle via a decrease in [Cl-]I in mouse airway cilia. Pflügers Arch. 2019, 471, 365–380. [Google Scholar] [CrossRef]
- Sueyoshi, S.; Miyata, Y.; Masumoto, Y.; Ishibashi, Y.; Matsuzawa, S.; Harano, N.; Tsuru, K.; Imai, S. Reduced airway inflammation and remodeling in parallel with mucin 5AC protein expression decreased by s-carboxymethylcysteine, a mucoregulant, in the airways of rats exposed to sulfur dioxide. Int. Arch. Allergy Immunol. 2004, 134, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, Y.; Kobayashi, F.; Idesawa, A.; Taniguchi, A.; Matsuzawa, S. Effects of carbocysteine on altered activities of glycosidase and glycosyltransferase and expression of Muc5ac in SO2-exposed rats. Eur. J. Pharmacol. 2004, 487, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, Y.; Takayama, G.; Inouye, Y.; Taniguchi, A. Carbocysteine normalizes the viscous property of mucus through regulation of fucosylated and sialylated sugar chain on airway mucins. Eur. J. Pharmacol. 2010, 641, 226–228. [Google Scholar] [CrossRef] [PubMed]
- Linden, D.; Guo-Parke, H.; Coyle, P.V.; Fairley, D.; McAuley, D.F.; Taggart, C.C.; Kidney, J. Respiratory viral infection: A potential “missing link” in the pathogenesis of COPD. Eur. Respir. Rev. 2019, 28, 180063. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, H.; Yamaya, M.; Sasaki, T.; Inoue, D.; Nakayama, K.; Yamada, M.; Asada, M.; Yoshida, M.; Suzuki, T.; Nishimura, H.; et al. Carbocisteine inhibits rhinovirus infection in human tracheal epithelial cells. Eur. Respir. J. 2006, 28, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Asada, M.; Yoshida, M.; Hatachi, Y.; Sasaki, T.; Yasuda, H.; Deng, X.; Nishimura, H.; Kubo, H.; Nagatomi, R.; Yamaya, M. l-carbocysteine inhibits respiratory syncytial virus infection in human tracheal epithelial cells. Respir. Physiol. Neurobiol. 2012, 180, 112–118. [Google Scholar] [CrossRef]
- Yamaya, M.; Nishimura, H.; Shinya, K.; Hatachi, Y.; Sasaki, T.; Yasuda, H.; Yoshida, M.; Asada, M.; Fujino, N.; Suzuki, T.; et al. Inhibitory effects of carbocysteine on type A seasonal influenza virus infection in human airway epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010, 299, L160–L168. [Google Scholar] [CrossRef]
- Zheng, C.H.; Ahmed, K.; Rikitomi, N.; Martinez, G.; Nagatake, T. The effects of S-carboxymethylcysteine and N-acetylcysteine on the adherence of Moraxella catarrhalis to human pharyngeal epithelial cells. Microbiol. Immunol. 1999, 43, 107–113. [Google Scholar] [CrossRef]
- Ndour, C.T.; Ahmed, K.; Nakagawa, T.; Nakano, Y.; Ichinose, A.; Tarhan, G.; Aikawa, M.; Nagatake, T. Modulating effects of mucoregulating drugs on the attachment of Haemophilus influenzae. Microb. Pathog. 2001, 30, 121–127. [Google Scholar] [CrossRef]
- Cakan, G.; Turkoz, M.; Turan, T.; Ahmed, K.; Nagatake, T. S-carboxymethyl cysteine inhibits the attachment of Streptococcus pneumoniae to human pharyngeal epithelial cells. Microb. Pathog. 2003, 34, 261–265. [Google Scholar] [CrossRef]
- Sumitomo, T.; Nakata, M.; Yamaguchi, M.; Terao, Y.; Kawabata, S. S-carboxymethyl cysteine inhibits adherence of Streptococcus pneumoniae to human alveolar epithelial cells. J. Med. Microbiol. 2012, 61, 101–108. [Google Scholar] [PubMed] [Green Version]
- Braga, P.C.; Scaglione, F.; Scarpazza, G.; Fraticelli, G.; Roviaro, G.; Varoli, F.; Mariani, C.; Falchi, M.; Fraschini, F. Comparison between penetration of amoxicillin combined with carbocysteine and amoxicillin alone in pathological bronchial secretions and pulmonary tissue. Int. J. Clin. Pharm. Res. 1985, 5, 331–340. [Google Scholar]
- Brusselle, G.G.; Joos, G.F.; Bracke, K.R. New insights into the immunology of chronic obstructive pulmonary disease. Lancet 2011, 378, 1015–1026. [Google Scholar] [CrossRef]
- Garavaglia, M.L.; Bononi, E.; Dossena, S.; Mondini, A.; Bazzini, C.; Lanata, L.; Balsamo, R.; Bagnasco, M.; Conese, M.; Bottà, G.; et al. S-CMC-Lys protective effects on human respiratory cells during oxidative stress. Cell. Physiol. Biochem. 2008, 22, 455–464. [Google Scholar] [CrossRef]
- Guizzardi, F.; Rodighiero, S.; Binelli, A.; Saino, S.; Bononi, E.; Dossena, S.; Garavaglia, M.L.; Bazzini, C.; Bottà, G.; Conese, M.; et al. S-CMC-Lys-dependent stimulation of electrogenic glutathione secretion by human respiratory epithelium. J. Mol. Med. 2006, 84, 97–107. [Google Scholar] [CrossRef]
- Brandolini, L.; Allegretti, M.; Berdini, V.; Cervellera, M.N.; Mascagni, P.; Rinaldi, M.; Melillo, G.; Ghezzi, P.; Mengozzi, M.; Bertini, R. Carbocysteine lysine salt monohydrate (SCMC-LYS) is a selective scavenger of reactive oxygen intermediates (ROIs). Eur. Cytokine Netw. 2003, 14, 20–26. [Google Scholar]
- Bazzini, C.; Rossetti, V.; Civello, D.A.; Sassone, F.; Vezzoli, V.; Persani, L.; Tiberio, L.; Lanata, L.; Bagnasco, M.; Paulmichl, M.; et al. Short- and long-term effects of cigarette smoke exposure on glutathione homeostasis in human bronchial epithelial cells. Cell. Physiol. Biochem. 2013, 32, 129–145. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, M.; Nakayama, K.; Yasuda, H.; Kubo, H.; Kuwano, K.; Arai, H.; Yamaya, M. Carbocysteine inhibits oxidant-induced apoptosis in cultured human airway epithelial cells. Respirology 2009, 14, 1027–1034. [Google Scholar] [CrossRef]
- Hanaoka, M.; Droma, Y.; Chen, Y.; Agatsuma, T.; Kitaguchi, Y.; Voelkel, N.F.; Kubo, K. Carbocysteine protects against emphysema induced by cigarette smoke extract in rats. Chest 2011, 139, 1101–1108. [Google Scholar]
- Pace, E.; Di Vincenzo, S.; Ferraro, M.; Bruno, A.; Dino, P.; Bonsignore, M.R.; Battaglia, S.; Saibene, F.; Lanata, L.; Gjomarkaj, M. Carbocysteine counteracts the effects of cigarette smoke on cell growth and the SIRT1/FoxO3 axis in bronchial epithelial cells. Exp. Gerontol. 2016, 81, 119–128. [Google Scholar] [CrossRef]
- Hakim, A.; Barnes, P.J.; Adcock, I.M.; Usmani, O.S. Importin-7 mediates glucocorticoid receptor nuclear import and is impaired by oxidative stress, leading to glucocorticoid insensitivity. FASEB J. 2013, 27, 4510–4519. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Adcock, I.M. Glucocorticoid resistance in inflammatory diseases. Lancet 2009, 373, 1905–1917. [Google Scholar] [CrossRef]
- Barnes, P.J. Glucocorticosteroids: Current and future directions. Br. J. Pharmacol. 2011, 163, 29–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.; Lu, H.Z.; Xu, J.R.; Wang, X.L.; Zhou, W.; Hou, L.N.; Zhu, L.; Yu, Z.H.; Chen, H.Z.; Cui, Y.Y. Carbocysteine restores steroid sensitivity by targeting histone deacetylase 2 in a thiol/GSH-dependent manner. Pharmacol. Res. 2015, 91, 88–98. [Google Scholar] [PubMed]
- Song, Y.; Yu, P.; Lu, J.J.; Lu, H.Z.; Zhu, L.; Yu, Z.H.; Chen, H.Z.; Cui, Y.Y. A mucoactive drug carbocysteine ameliorates steroid resistance in the rat COPD model. Pulm. Pharmacol. Ther. 2016, 39, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Pace, E.; Ferraro, M.; Di Vincenzo, S.; Cipollina, C.; Gerbino, S.; Cigna, D.; Caputo, V.; Balsamo, R.; Lanata, L.; Gjomarkaj, M. Comparative cytoprotective effects of carbocysteine and fluticasone propionate in cigarette smoke extract-stimulated bronchial epithelial cells. Cell Stress Chaperones 2013, 18, 733–743. [Google Scholar] [CrossRef] [Green Version]
- Pace, E.; Di Vincenzo, S.; Ferraro, M.; Siena, L.; Chiappara, G.; Dino, P.; Vitulo, P.; Bertani, A.; Saibene, F.; Lanata, L.; et al. Effects of Carbocysteine and Beclomethasone on Histone Acetylation/Deacetylation Processes in Cigarette Smoke Exposed Bronchial Epithelial Cells. J. Cell. Physiol. 2017, 232, 2851–2859. [Google Scholar] [CrossRef]
- Aderem, A.; Ulevitch, R.J. Toll-like receptors in the induction of the innate immune response. Nature 2000, 406, 782–787. [Google Scholar] [CrossRef]
- Pace, E.; Ferraro, M.; Siena, L.; Melis, M.; Montalbano, A.M.; Johnson, M.; Bonsignore, M.R.; Bonsignore, G.; Gjomarkaj, M. Cigarette smoke increases Toll-like receptor 4 and modifies lipopolysaccharide-mediated responses in airway epithelial cells. Immunology 2008, 124, 401–411. [Google Scholar] [CrossRef]
- O’Donnell, R.; Breen, D.; Wilson, S.; Djukanovic, R. Inflammatory cells in the airways in COPD. Thorax 2006, 61, 448–454. [Google Scholar] [CrossRef] [Green Version]
- Pace, E.; Ferraro, M.; Siena, L.; Scafidi, V.; Gerbino, S.; Di Vincenzo, S.; Gallina, S.; Lanata, L.; Gjomarkaj, M. Carbocysteine regulates innate immune responses and senescence processes in cigarette smoke stimulated bronchial epithelial cells. Toxicol. Lett. 2013, 223, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Pace, E.; Ferraro, M.; Di Vincenzo, S.; Gerbino, S.; Bruno, A.; Lanata, L.; Gjomarkaj, M. Oxidative stress and innate immunity responses in cigarette smoke stimulated nasal epithelial cells. Toxicol. Vitr. 2014, 28, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Ishii, Y.; Kimura, T.; Morishima, Y.; Mochizuki, M.; Nomura, A.; Sakamoto, T.; Uchida, Y.; Sekizawa, K. S-carboxymethyl cysteine inhibits neutrophil activation mediated by N-formyl-methionyl-leucyl-phenylalanine. Eur. J. Pharmacol. 2002, 449, 183–189. [Google Scholar] [CrossRef]
- Nogawa, H.; Ishibashi, Y.; Ogawa, A.; Masuda, K.; Tsubuki, T.; Kameda, T.; Matsuzawa, S. Carbocysteine can scavenge reactive oxygen species in vitro. Respirology 2009, 14, 53–59. [Google Scholar] [CrossRef]
- Wang, W.; Zheng, J.P.; Zhu, S.X.; Guan, W.J.; Chen, M.; Zhong, N.S. Carbocysteine attenuates hydrogen peroxide-induced inflammatory injury in A549 cells via NF-κB and ERK1/2 MAPK pathways. Int. Immunopharmacol. 2015, 24, 306–313. [Google Scholar] [CrossRef]
- Wang, W.; Guan, W.J.; Huang, R.Q.; Xie, Y.Q.; Zheng, J.P.; Zhu, S.X.; Chen, M.; Zhong, N.S. Carbocysteine attenuates TNF-α-induced inflammation in human alveolar epithelial cells in vitro through suppressing NF-κB and ERK1/2 MAPK signaling pathways. Acta Pharmacol. Sin. 2016, 37, 629–636. [Google Scholar] [CrossRef] [Green Version]
- Asti, C.; Melillo, G.; Caselli, G.F.; Daffonchio, L.; Hernandez, A.; Clavenna, G.; Omini, C. Effectiveness of carbocysteine lysine salt monohydrate on models of airway inflammation and hyperresponsiveness. Pharmacol. Res. 1995, 31, 387–392. [Google Scholar] [CrossRef]
- Inoue, M.; Ishibashi, Y.; Nogawa, H.; Yasue, T. Carbocysteine promotes phagocytosis of apoptotic cells by alveolar macrophages. Eur. J. Pharmacol. 2012, 677, 173–179. [Google Scholar] [CrossRef]
- Song, Y.; Wang, W.; Xie, Y.; Xiang, B.; Huang, X.; Guan, W.; Zheng, J. Carbocysteine inhibits the expression of Muc5b in COPD mouse model. Drug Des. Devel. Ther. 2019, 13, 3259–3268. [Google Scholar] [CrossRef] [Green Version]
- Carpagnano, G.E.; Resta, O.; Foschino-Barbaro, M.P.; Spanevello, A.; Stefano, A.; Di Gioia, G.; Serviddio, G.; Gramiccioni, E. Exhaled Interleukine-6 and 8-isoprostane in chronic obstructive pulmonary disease: Effect of carbocysteine lysine salt monohydrate (SCMC-Lys). Eur. J. Pharmacol. 2004, 505, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Ferraro, M.; Di Vincenzo, S.; Sangiorgi, C.; Leto Barone, S.; Gangemi, S.; Lanata, L.; Pace, E. Carbocysteine Modifies Circulating miR-21, IL-8, sRAGE, and fAGEs Levels in Mild Acute Exacerbated COPD Patients: A Pilot Study. Pharmaceuticals 2022, 15, 218. [Google Scholar] [CrossRef] [PubMed]
- Aylward, M. An assessment of S-carboxymethyl cysteine in the treatment of chronic bronchitis. Curr. Med. Res. Opin. 1974, 2, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Edwards, G.F.; Steel, A.E.; Scott, J.K.; Jordanz, J.W. S-carboxymethyl cysteine in the fluidification of sputum and treatment of chronic airway obstruction. Chest 1976, 70, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Miskoviti, G.; Szule, P.; Mescaros, K. Double blind study of carbocysteine against placebo in chronic bronchitis; Mucoregulation in respiratory tract disorders. Proc. R. Soc. Med. 1982, 5, 1–3. [Google Scholar]
- Puchelle, E.; Girard, F.; Zahm, J.M. Rheology of bronchial secretions and mucociliary transport. Bull. Eur. Physiopathol. Respir. 1976, 12, 771–779. [Google Scholar]
- Grillage, M.; Barnard-Jones, K. Long-term oral carbocysteine therapy in patients with chronic bronchitis. A double-blind trial with placebo control. Br. J. Clin. Pract. 1985, 39, 395–398. [Google Scholar]
- Allegra, L.; Cordaro, C.I.; Grassi, C. Prevention of acute exacerbations of chronic obstructive bronchitis with carbocysteine lysine salt monohydrate: A multicenter, double-blind, placebo-controlled trial. Respiration 1996, 63, 174–180. [Google Scholar] [CrossRef]
- Zheng, J.P.; Kang, J.; Huang, S.G.; Chen, P.; Yao, W.Z.; Yang, L.; Bai, C.X.; Wang, C.Z.; Wang, C.; Chen, B.Y.; et al. Effect of carbocysteine on acute exacerbation of chronic obstructive pulmonary disease (PEACE Study): A randomised placebo-controlled study. Lancet 2008, 371, 2013–2018. [Google Scholar] [CrossRef]
- Decramer, M.; Rutten-van Molken, M.; Dekhuijzen, P.N.; Troosters, T.; van Herwaarden, C.; Pellegrino, R.; van Schayck, C.P.O.; Olivieri, D.; Del Donno, M.; De Backer, W.; et al. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): A randomised placebo-controlled trial. Lancet 2005, 365, 1552–1560. [Google Scholar] [CrossRef]
- Celli, B.R.; Cote, C.G.; Marin, J.M.; Casanova, C.; de Oca, M.M.; Mendez, R.A.; Plata, V.P.; Cabral, H.J. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N. Eng. J. Med. 2004, 350, 1005–1012. [Google Scholar] [CrossRef] [Green Version]
- Paone, G.; Lanata, L.; Saibene, F.; Toti, S.; Palermo, P.; Graziani, C.; Flore, M.C.; Ramaccia, M.; Puglisi, G. A prospective study of the effects of carbocysteine lysine salt on frequency of exacerbations in COPD patients treated with or without inhaled steroids. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 2627–2635. [Google Scholar]
- Zeng, Z.; Yang, D.; Huang, X.; Xiao, Z. Effects of carbocysteine on patients with COPD: A systematic review and meta-analysis. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 2277–2283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minov, J.; Stoleski, S.; Petrova, T.; Vasilevska, K.; Mijakoski, D.; Karadzinska-Bislimovska, J. Effects of a Long-Term Use of Carbocysteine on Frequency and Duration of Exacerbations in Patients with Bronchiectasis. Open Access Maced. J. Med. Sci. 2019, 7, 4030–4035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pace, E.; Cerveri, I.; Lacedonia, D.; Paone, G.; Sanduzzi Zamparelli, A.; Sorbo, R.; Allegretti, M.; Lanata, L.; Scaglione, F. Clinical Efficacy of Carbocysteine in COPD: Beyond the Mucolytic Action. Pharmaceutics 2022, 14, 1261. https://doi.org/10.3390/pharmaceutics14061261
Pace E, Cerveri I, Lacedonia D, Paone G, Sanduzzi Zamparelli A, Sorbo R, Allegretti M, Lanata L, Scaglione F. Clinical Efficacy of Carbocysteine in COPD: Beyond the Mucolytic Action. Pharmaceutics. 2022; 14(6):1261. https://doi.org/10.3390/pharmaceutics14061261
Chicago/Turabian StylePace, Elisabetta, Isa Cerveri, Donato Lacedonia, Gregorino Paone, Alessandro Sanduzzi Zamparelli, Rossella Sorbo, Marcello Allegretti, Luigi Lanata, and Francesco Scaglione. 2022. "Clinical Efficacy of Carbocysteine in COPD: Beyond the Mucolytic Action" Pharmaceutics 14, no. 6: 1261. https://doi.org/10.3390/pharmaceutics14061261






