Sorafenib Chemosensitization by Caryophyllane Sesquiterpenes in Liver, Biliary, and Pancreatic Cancer Cells: The Role of STAT3/ABC Transporter Axis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Lines
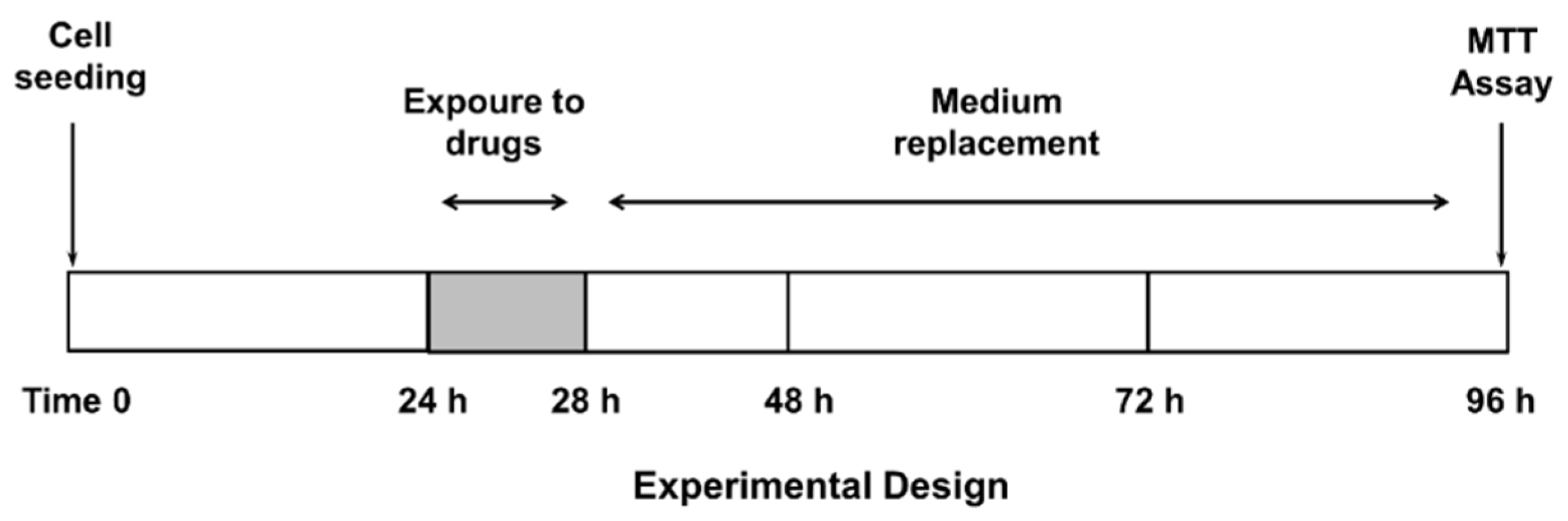
2.3. Treatment Schedule
2.4. Cytotoxicity Assay
2.5. Combination Assay and Analysis of Sesquiterpene-Drug Interactions
2.6. ABC-Mediated Drug Efflux Assay
2.7. Wound Healing Assay
2.8. Western Blotting Analysis
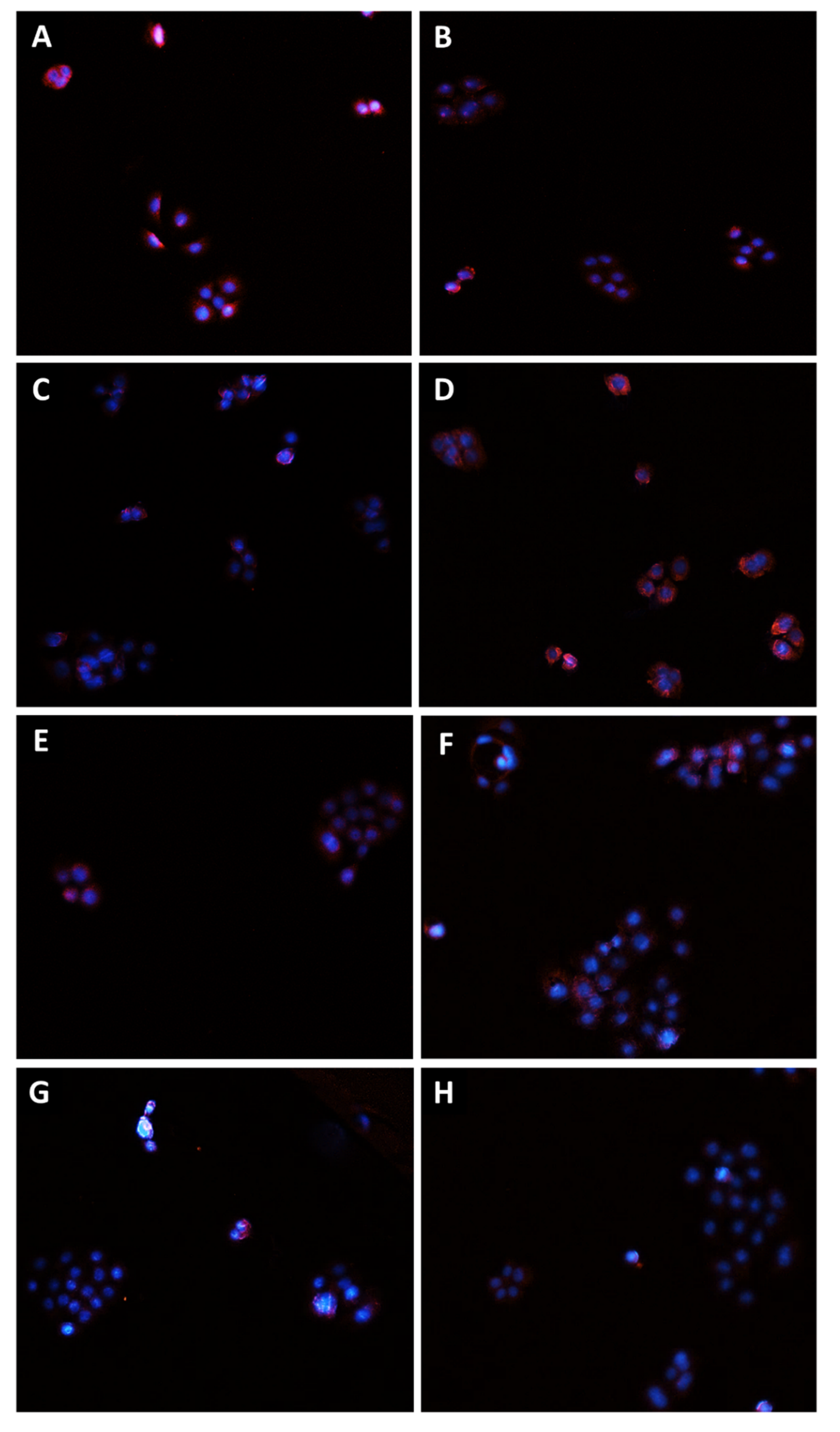
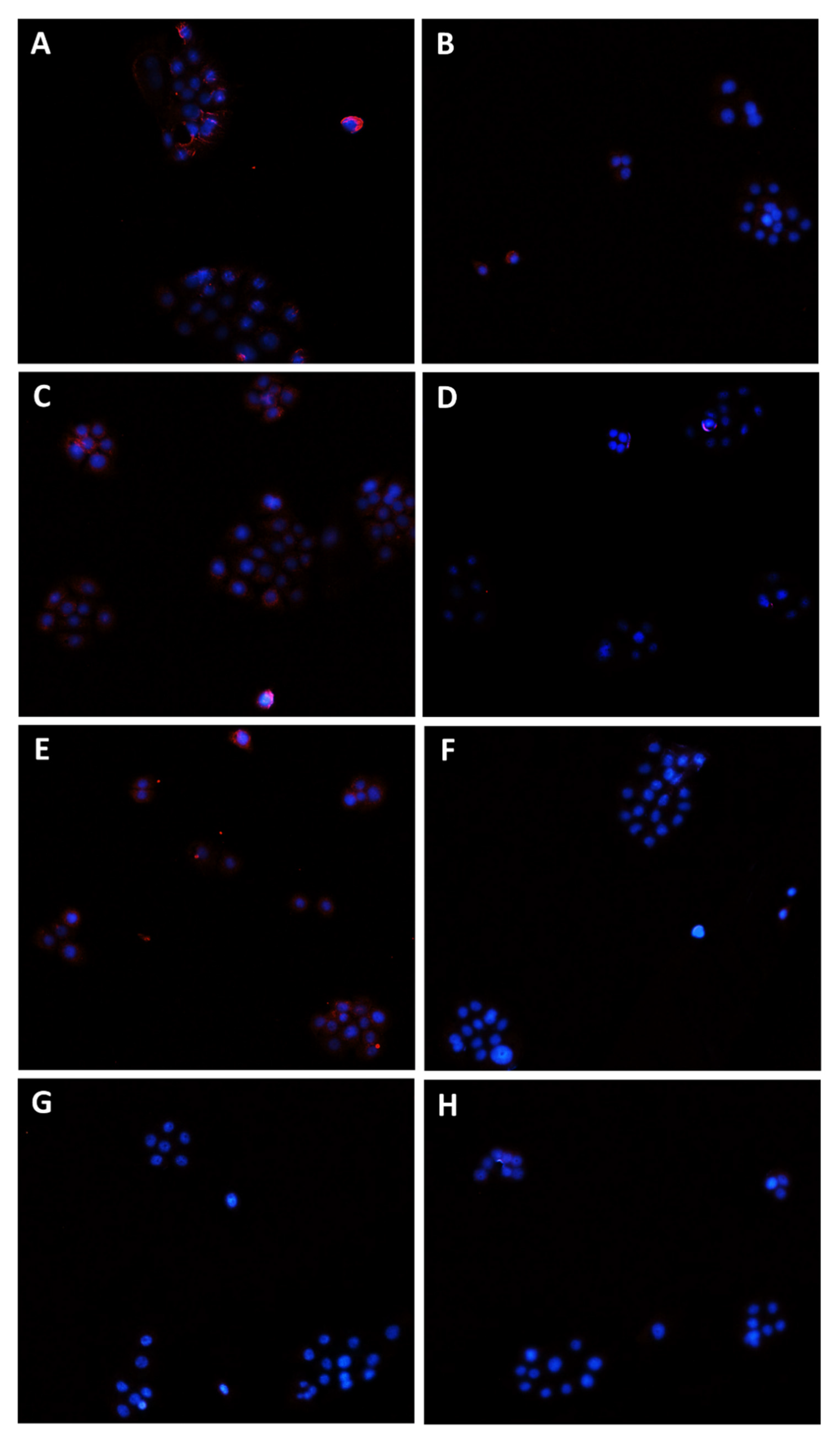
2.9. Immunofluorescence Analysis
2.10. Gene Expression Analysis by RT-qPCR
2.11. Statistical Analysis
3. Results
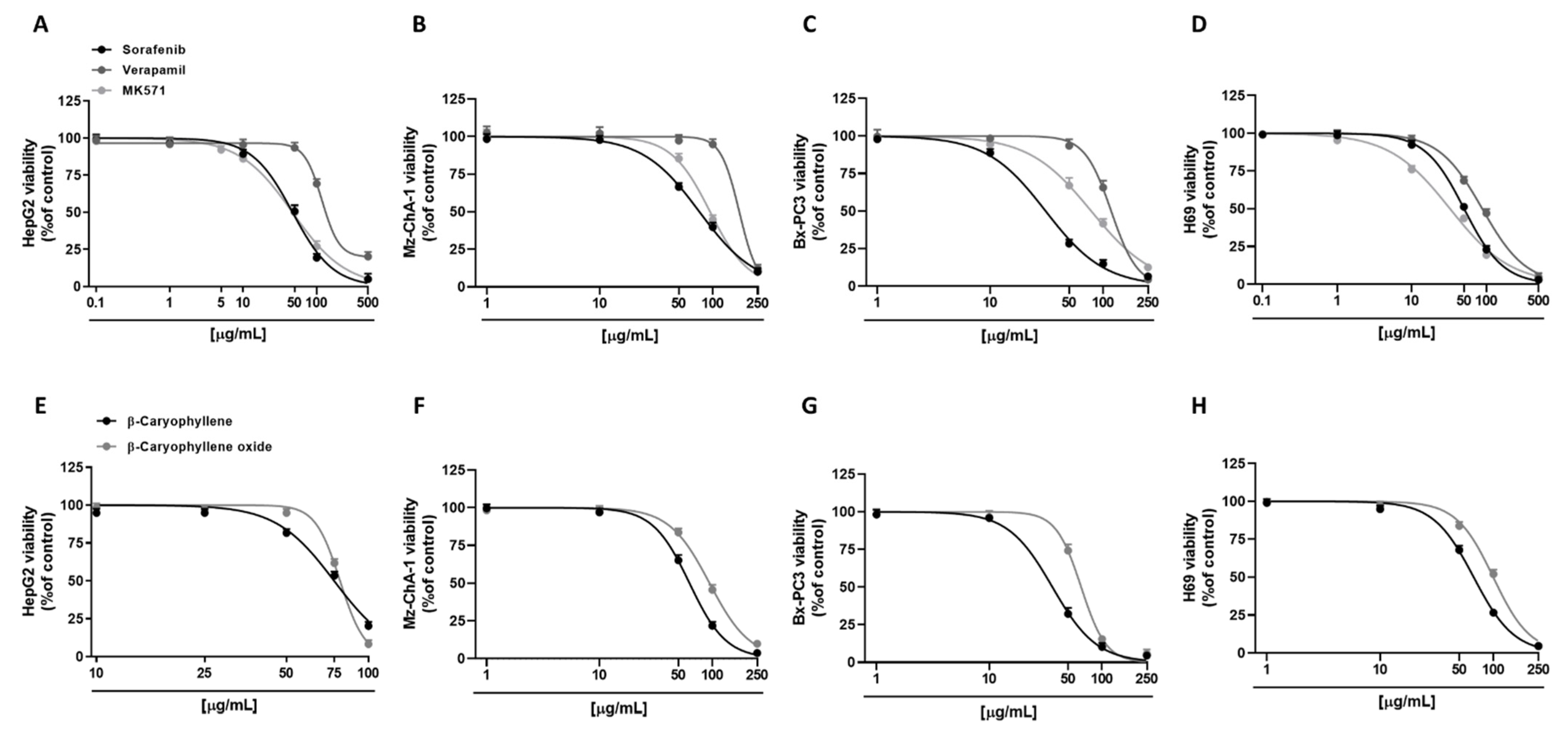
3.1. Cytotoxicity of Sorafenib and Caryophyllane Sesquiterpenes in Human Hepato-Biliary-Pancreatic Cancer Cell Lines
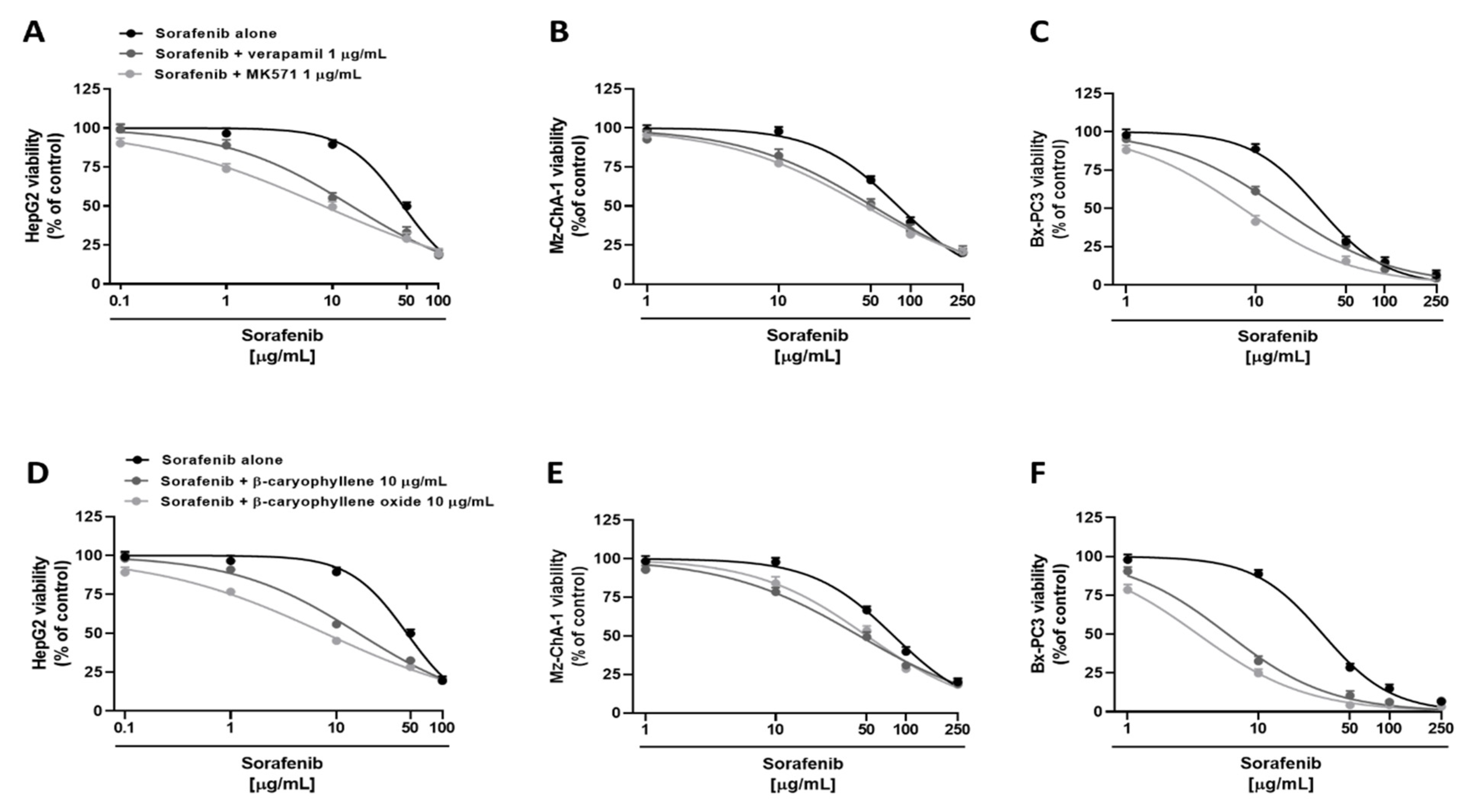
3.2. Chemosensitizing Effects of Caryophyllane Sesquiterpenes in Combination with Sorafenib in Human Hepato-Biliary-Pancreatic Cancer Cell Lines
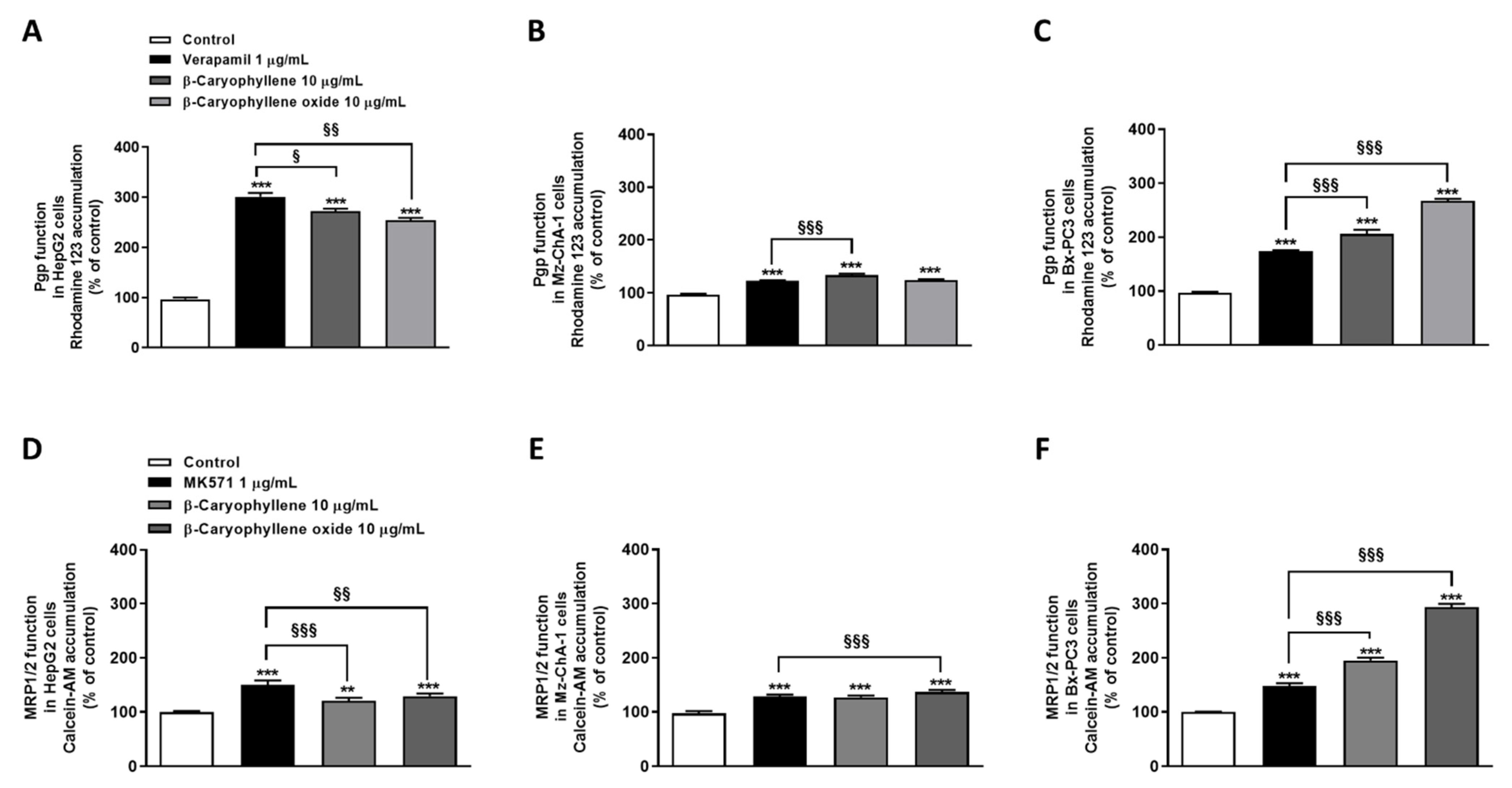
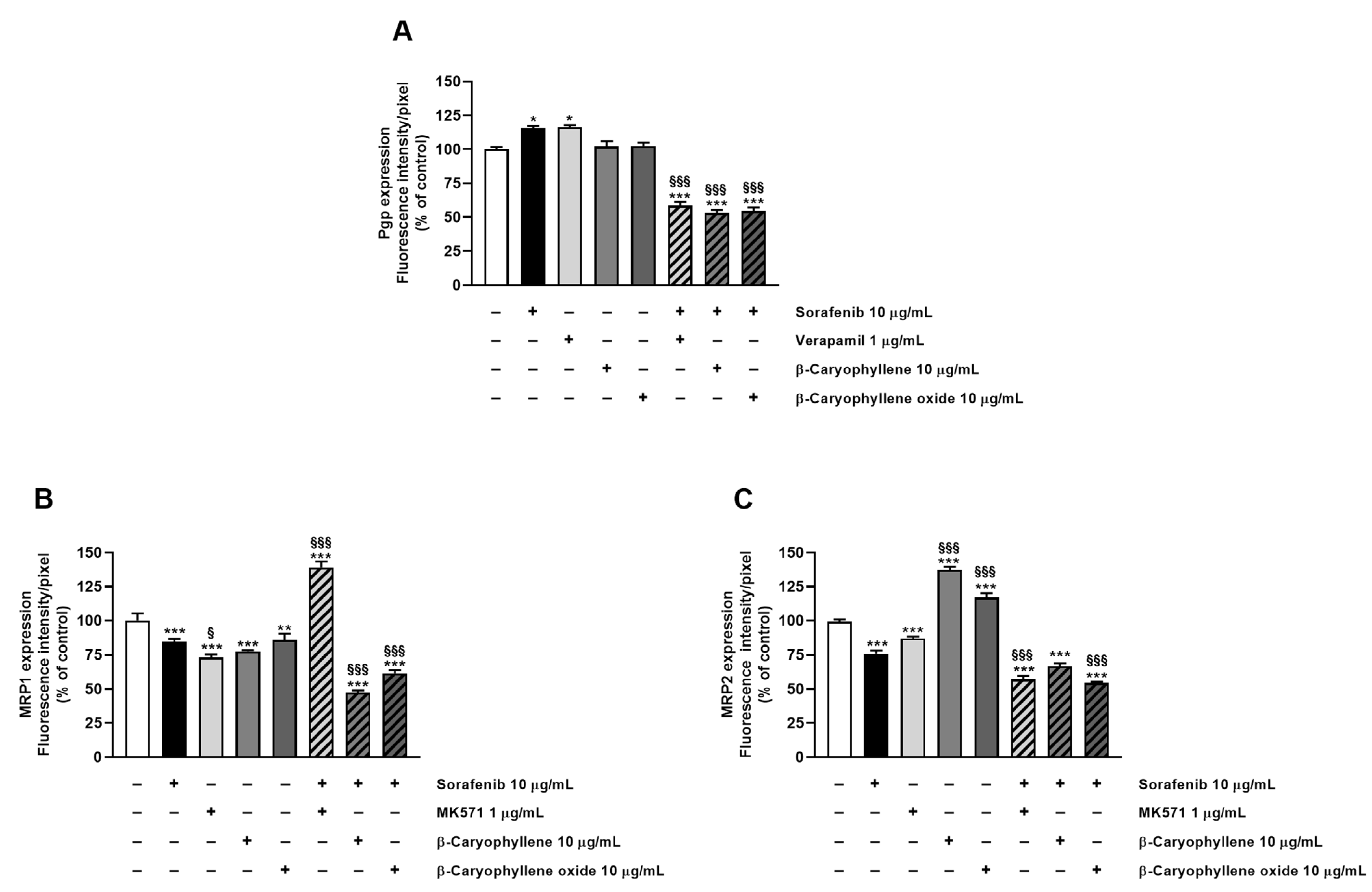
3.3. Caryophyllane Sesquiterpenes Inhibit Pgp and MRP1/2 Transporter Activity in Human Hepato-Biliary-Pancreatic Cancer Cell Lines
3.4. Modulation of MDR1, MRP1, and MRP2 Expression Is Involved in Sorafenib Chemosensitization by Caryophyllane Sesquiterpenes
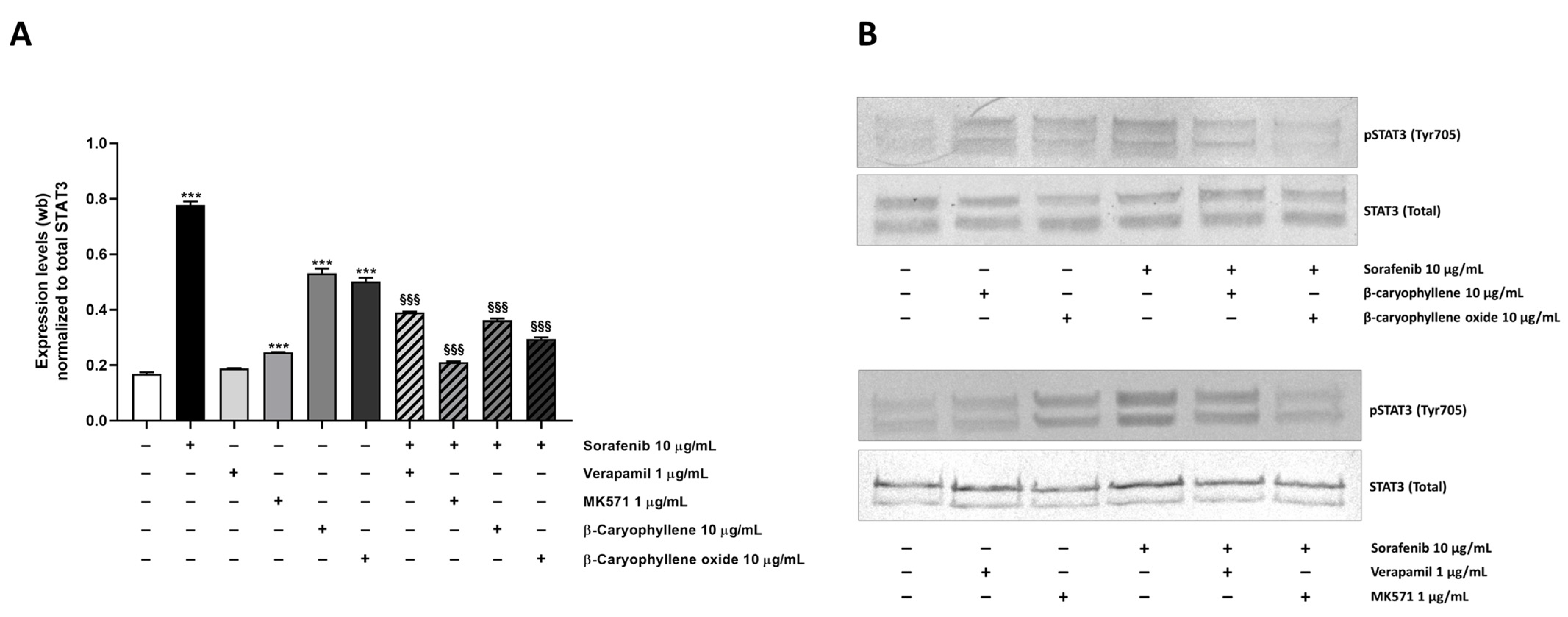
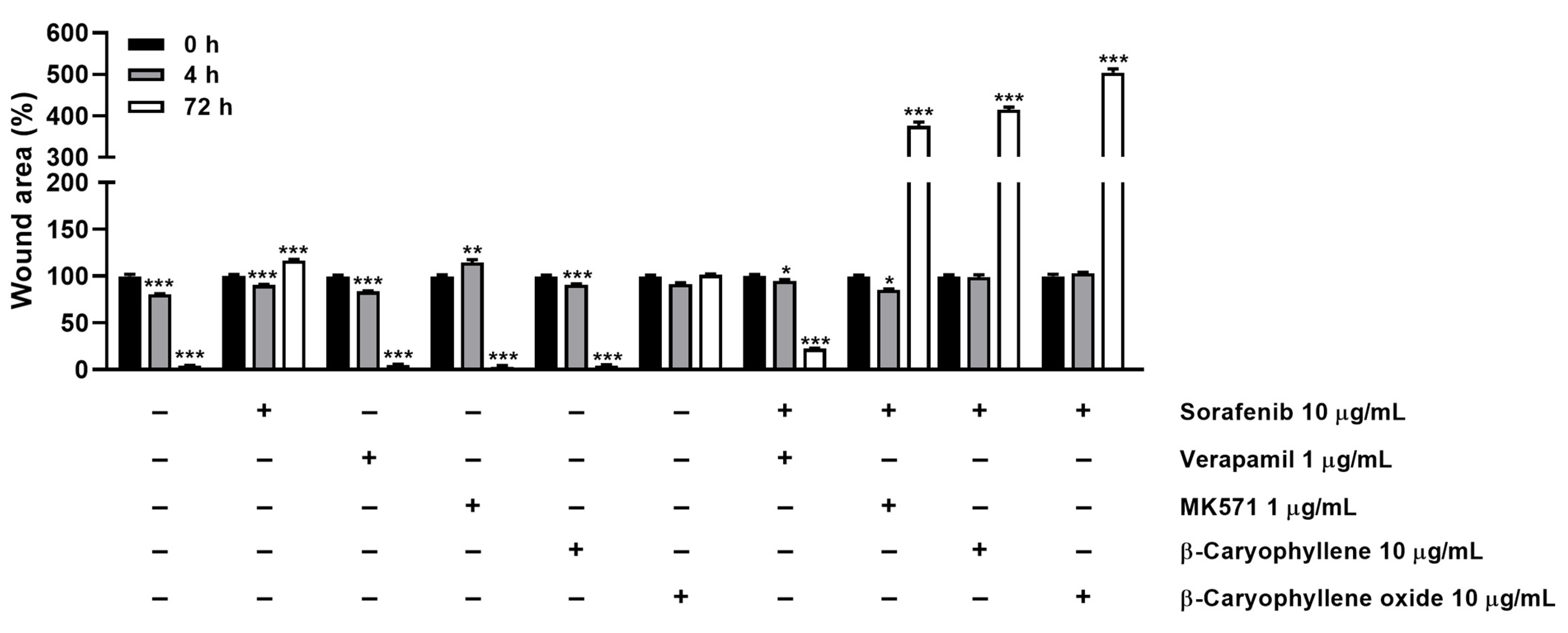
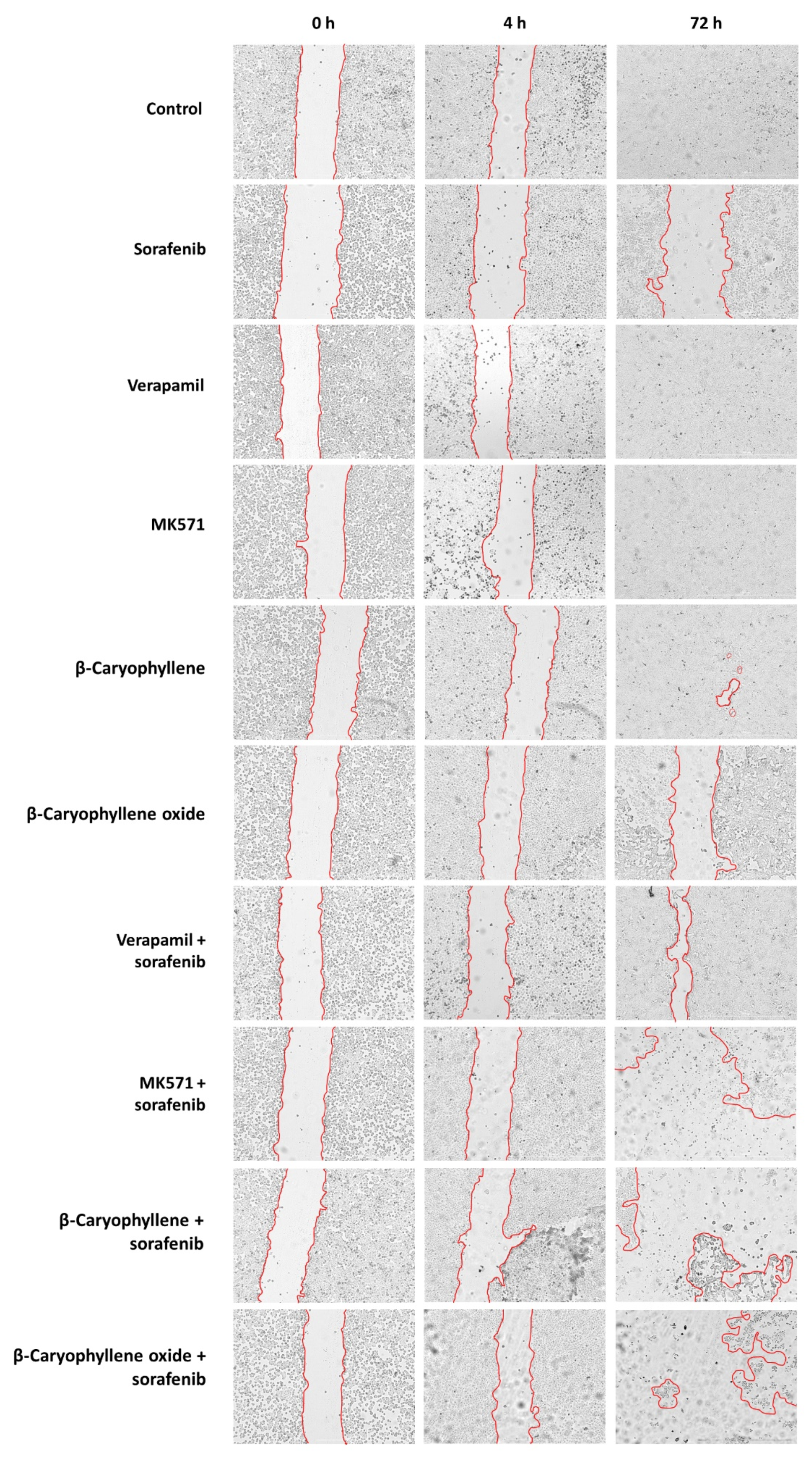
3.5. Modulation of STAT3 Activation and Cell Migration in Sorafenib Chemosensitization by Caryophyllane Sesquiterpenes
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghurburrun, E.; Borbath, I.; Lemaigre, F.P.; Jacquemin, P. Liver and pancreas: Do similar embryonic development and tissue organization lead to similar mechanisms of tumorigenesis? Gene Expr. 2018, 18, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.N.; Tosh, D. Transdifferentiation of pancreatic cells to hepatocytes. Methods Mol. Biol. 2010, 640, 273–280. [Google Scholar] [PubMed]
- Yin, C. Molecular mechanisms of Sox transcription factors during the development of liver, bile duct, and pancreas. Semin. Cell. Dev. Biol. 2017, 63, 68–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.S.; Friedman, J.R.; Fulmer, J.T.; Kaestner, K.H. The initiation of liver development is dependent on Foxa transcription factors. Nature 2005, 435, 944–947. [Google Scholar] [CrossRef]
- Lozano, E.; Macias, R.; Monte, M.J.; Asensio, M.; Del Carmen, S.; Sanchez-Vicente, L.; Alonso-Peña, M.; Al-Abdulla, R.; Munoz-Garrido, P.; Satriano, L.; et al. Causes of hOCT1-Dependent Cholangiocarcinoma resistance to Sorafenib and sensitization by tumor-selective gene therapy. Hepatology 2019, 70, 1246–1261. [Google Scholar] [CrossRef]
- Grasso, C.; Jansen, G.; Giovannetti, E. Drug resistance in pancreatic cancer: Impact of altered energy metabolism. Crit. Rev. Oncol./Hematol. 2017, 114, 139–152. [Google Scholar] [CrossRef]
- Marin, J.; Briz, O.; Herraez, E.; Lozano, E.; Asensio, M.; Di Giacomo, S.; Romero, M.R.; Osorio-Padilla, L.M.; Santos-Llamas, A.I.; Serrano, M.A.; et al. Molecular bases of the poor response of liver cancer to chemotherapy. Clin. Res. Hepatol. Gastroenterol. 2018, 42, 182–192. [Google Scholar] [CrossRef]
- Marin, J.; Lozano, E.; Herraez, E.; Asensio, M.; Di Giacomo, S.; Romero, M.R.; Briz, O.; Serrano, M.A.; Efferth, T.; Macias, R. Chemoresistance and chemosensitization in cholangiocarcinoma. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1444–1453. [Google Scholar] [CrossRef]
- Fouassier, L.; Marzioni, M.; Afonso, M.B.; Dooley, S.; Gaston, K.; Giannelli, G.; Rodrigues, C.; Lozano, E.; Mancarella, S.; Segatto, O.; et al. Signalling networks in cholangiocarcinoma: Molecular pathogenesis, targeted therapies and drug resistance. Liver Int. 2019, 39, 43–62. [Google Scholar] [CrossRef] [Green Version]
- Domenichini, A.; Adamska, A.; Falasca, M. ABC transporters as cancer drivers: Potential functions in cancer development. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 52–60. [Google Scholar] [CrossRef]
- Cheng, Z.; Wei-Qi, J.; Jin, D. New insights on sorafenib resistance in liver cancer with correlation of individualized therapy. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188382. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.R.; Kang, H.; Jo, J.H.; Lee, H.S.; Chung, M.J.; Park, J.Y.; Park, S.W.; Song, S.Y.; An, C.; Park, M.S.; et al. Folfirinox vs. gemcitabine/nab-paclitaxel for treatment of metastatic pancreatic cancer: Single-center cohort study. World. J. Gastrointest. Oncol. 2020, 12, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Di Sotto, A.; Irannejad, H.; Eufemi, M.; Mancinelli, R.; Abete, L.; Mammola, C.L.; Altieri, F.; Mazzanti, G.; Di Giacomo, S. Potentiation of low-dose doxorubicin cytotoxicity by affecting P-Glycoprotein through caryophyllane sesquiterpenes in HepG2 cells: An in vitro and in silico study. Int. J. Mol. Sci. 2020, 21, 633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tagliamonte, M.; Petrizzo, A.; Tornesello, M.L.; Ciliberto, G.; Buonaguro, F.M.; Buonaguro, L. Combinatorial immunotherapy strategies for hepatocellular carcinoma. Curr. Opin. Allergy Clin. Immuno. 2016, 39, 103–113. [Google Scholar] [CrossRef]
- Di Sotto, A.; Mancinelli, R.; Gullì, M.; Eufemi, M.; Mammola, C.L.; Mazzanti, G.; Di Giacomo, S. Chemopreventive potential of caryophyllane sesquiterpenes: An overview of preliminary evidence. Cancers 2020, 12, 3034. [Google Scholar] [CrossRef]
- Rejhová, A.; Opattová, A.; Čumová, A.; Slíva, D.; Vodička, P. Natural compounds and combination therapy in colorectal cancer treatment. Eur. J. Med. Chem. 2018, 144, 582–594. [Google Scholar] [CrossRef]
- Garzoli, S.; Pirolli, A.; Vavala, E.; Di Sotto, A.; Sartorelli, G.; Božović, M.; Angiolella, L.; Mazzanti, G.; Pepi, F.; Ragno, R. Multidisciplinary approach to determine the optimal time and period for extracting the essential oil from Mentha suaveolens Ehrh. Molecules 2015, 20, 9640–9655. [Google Scholar] [CrossRef] [Green Version]
- Mariano, A.; Bigioni, I.; Mattioli, R.; Di Sotto, A.; Leopizzi, M.; Garzoli, S.; Mariani, P.F.; Dalla Vedova, P.; Ammendola, S.; Scotto d’Abusco, A. Harpagophytum procumbens root extract mediates anti-inflammatory effects in osteoarthritis synoviocytes through CB2 Activation. Pharmaceuticals 2022, 15, 457. [Google Scholar] [CrossRef]
- Di Giacomo, S.; Di Sotto, A.; Mazzanti, G.; Wink, M. Chemosensitizing properties of β-Caryophyllene and β-Caryophyllene oxide in combination with doxorubicin in human cancer cells. Anticancer Res. 2017, 37, 1191–1196. [Google Scholar]
- Di Giacomo, S.; Briz, O.; Monte, M.J.; Sanchez-Vicente, L.; Abete, L.; Lozano, E.; Mazzanti, G.; Di Sotto, A.; Marin, J. Chemosensitization of hepatocellular carcinoma cells to sorafenib by β-caryophyllene oxide-induced inhibition of ABC export pumps. Arch. Toxicol. 2019, 93, 623–634. [Google Scholar] [CrossRef]
- Di Sotto, A.; Di Giacomo, S.; Rubini, E.; Macone, A.; Gulli, M.; Mammola, C.L.; Eufemi, M.; Mancinelli, R.; Mazzanti, G. Modulation of STAT3 signaling, cell redox defenses and cell cycle checkpoints by β-Caryophyllene in cholangiocarcinoma cells: Possible mechanisms accounting for doxorubicin chemosensitization and chemoprevention. Cells 2020, 9, 858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Giacomo, S.; Mariano, A.; Gullì, M.; Fraschetti, C.; Vitalone, A.; Filippi, A.; Mannina, L.; Scotto d′Abusco, A.; Di Sotto, A. Role of caryophyllane sesquiterpenes in the entourage effect of Felina 32 hemp inflorescence phytocomplex in triple negative MDA-MB-468 breast cancer cells. Molecules 2021, 26, 6688. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993–5:2009; Biological Evaluation of Medical Devices Part 5: Tests for Invitro Cytotoxicity, Second Edition. International Organization for Standardization/ANSI: Geneva, Switzerland, 2009.
- Facchinetti, R.; Valenza, M.; Bronzuoli, M.R.; Menegoni, G.; Ratano, P.; Steardo, L.; Campolongo, P.; Scuderi, C. Looking for a treatment for the early stage of Alzheimer′s disease: Preclinical evidence with co-ultramicronized Palmitoylethanolamide and Luteolin. Int. J. Mol. Sci. 2020, 27, 3802. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Tsujiuchi, T.; Okui, Y.; Mizutani, A.; Nishi, K.M.; Nakanishi, T.; Nishii, R.; Fukuchi, K.; Tamai, I.; Kawai, K. Different efflux transporter affinity and metabolism of 99mTc-2-Methoxyisobutylisonitrile and 99mTc-Tetrofosmin for multidrug resistance monitoring in cancer. Pharm. Res. 2018, 36, 18. [Google Scholar] [CrossRef]
- Di Giacomo, S.; Di Sotto, A.; El-Readi, M.Z.; Mazzanti, G.; Wink, M. Hexylcinnamaldehyde synergistically increases doxorubicin cytotoxicity towards human cancer cell lines. Anticancer Res. 2016, 36, 3347–3351. [Google Scholar]
- Sharma, P.; Singh, N.; Sharma, S. ATP binding cassette transporters and cancer: Revisiting their controversial role. Pharmacogenomics 2021, 22, 1211–1235. [Google Scholar] [CrossRef]
- Marin, J.; Macias, R.; Cives-Losada, C.; Peleteiro-Vigil, A.; Herraez, E.; Lozano, E. Plasma membrane transporters as biomarkers and molecular targets in cholangiocarcinoma. Cells 2020, 9, 498. [Google Scholar] [CrossRef] [Green Version]
- Adamska, A.; Falasca, M. ATP-binding cassette transporters in progression and clinical outcome of pancreatic cancer: What is the way forward? World J. Gastroenterol. 2018, 24, 3222–3238. [Google Scholar] [CrossRef]
- Guo, H.; Xiao, Y.; Yuan, Z.; Yang, X.; Chen, J.; Chen, C.; Wang, M.; Xie, L.; Chen, Q.; Tong, Y.; et al. Inhibition of STAT3Y705 phosphorylation by Stattic suppresses proliferation and induces mitochondrial-dependent apoptosis in pancreatic cancer cells. Cell Death Discover. 2022, 8, 116. [Google Scholar] [CrossRef]
- Xu, J.; Lin, H.; Wu, G.; Zhu, M.; Li, M. IL-6/STAT3 is a promising therapeutic target for hepatocellular carcinoma. Front. Oncol. 2021, 11, 760971. [Google Scholar] [CrossRef]
- Ploeger, C.; Schreck, J.; Huth, T.; Fraas, A.; Albrecht, T.; Charbel, A.; Ji, J.; Singer, S.; Breuhahn, K.; Pusch, S.; et al. STAT1 and STAT3 exhibit a crosstalk and are associated with increased inflammation in hepatocellular carcinoma. Cancers 2022, 14, 1154. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Farren, M.R.; Ahn, D.; Bekaii-Saab, T.; Lesinski, G.B. Signaling pathways as therapeutic targets in biliary tract cancer. Expert Opin. Ther. Targets 2017, 21, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Q.; Man, Q.W.; Huo, F.Y.; Gao, X.; Lin, H.; Li, S.R.; Wang, J.; Su, F.C.; Cai, L.; Shi, Y.; et al. STAT3 pathway in cancers: Past, present, and future. Med. Commun. 2022, 3, 124. [Google Scholar] [CrossRef] [PubMed]
- Haider, T.; Pandey, V.; Banjare, N.; Gupta, P.N.; Soni, V. Drug resistance in cancer: Mechanisms and tackling strategies. Pharmacol. Rep. 2020, 72, 1125–1151. [Google Scholar] [CrossRef] [PubMed]
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef]
- Wang, J.Q.; Yang, Y.; Cai, C.Y.; Teng, Q.X.; Cui, Q.; Lin, J.; Assaraf, Y.G.; Chen, Z.S. Multidrug resistance proteins (MRPs): Structure, function and the overcoming of cancer multidrug resistance. Drug Resist. Updates 2021, 54, 100743. [Google Scholar] [CrossRef]
- Neul, C.; Schaeffeler, E.; Sparreboom, A.; Laufer, S.; Schwab, M.; Nies, A.T. Impact of Membrane Drug Transporters on Resistance to Small-Molecule Tyrosine Kinase Inhibitors. Trends Pharmacol. Sci. 2016, 37, 904–932. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Zheng, B.; Wang, H.Y.; Chen, L. New knowledge of the mechanisms of sorafenib resistance in liver cancer. Acta Pharmacol. Sin. 2017, 38, 614–622. [Google Scholar] [CrossRef] [Green Version]
- Chien, A.J.; Moasser, M.M. Cellular mechanisms of resistance to anthracyclines and taxanes in cancer: Intrinsic and acquired. Semin. Oncol. 2008, 35, S1–S39. [Google Scholar] [CrossRef]
- Mirzaei, S.; Gholami, M.H.; Hashemi, F.; Zabolian, A.; Farahani, M.V.; Hushmandi, K.; Zarrabi, A.; Goldman, A.; Ashrafizadeh, M.; Orive, G. Advances in understanding the role of P-gp in doxorubicin resistance: Molecular pathways, therapeutic strategies, and prospects. Drug Discov. Today 2022, 27, 436–455. [Google Scholar] [CrossRef]
- Dohse, M.; Scharenberg, C.; Shukla, S.; Robey, R.W.; Volkmann, T.; Deeken, J.F.; Brendel, C.; Ambudkar, S.V.; Neubauer, A.; Bates, S.E. Comparison of ATP-binding cassette transporter interactions with the tyrosine kinase inhibitors imatinib, nilotinib, and dasatinib. Drug Metab. Dispos. 2010, 38, 1371–1380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balabanov, S.; Gontarewicz, A.; Keller, G.; Raddrizzani, L.; Braig, M.; Bosotti, R.; Moll, J.; Jost, E.; Barett, C.; Rohe, I.; et al. Abcg2 overexpression represents a novel mechanism for acquired resistance to the multi-kinase inhibitor Danusertib in BCR-ABL-positive cells in vitro. PLoS ONE 2011, 6, e19164. [Google Scholar] [CrossRef] [PubMed]
- Nobili, S.; Lapucci, A.; Landini, I.; Coronnello, M.; Roviello, G.; Mini, E. Role of ATP-binding cassette transporters in cancer initiation and progression. Semin. Cancer Biol. 2020, 60, 72–95. [Google Scholar] [CrossRef]
- Estevinho, M.M.; Fernandes, C.; Silva, J.C.; Gomes, A.C.; Afecto, E.; Correia, J.; Carvalho, J. Role of ATP-binding cassette transporters in Sorafenib therapy for hepatocellular carcinoma: An overview. Curr. Drug. Targets 2022, 23, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Skarkova, V.; Caltová, K.; Svobodová, H.; Ambroz, M.; Skarka, A.; Murínová, N.; Králová, V.; Tomšík, P.; Skálová, L. The effects of β-caryophyllene oxide and trans -nerolidol on the efficacy of doxorubicin in breast cancer cells and breast tumor-bearing mice. Biomed. Pharmacother. 2017, 95, 828–836. [Google Scholar]
- Ambrož, M.; Šmatová, M.; Šadibolová, M.; Pospíšilová, E.; Hadravská, P.; Kašparová, M.; Skarková, V.H.; Králová, V.; Skálová, L. Sesquiterpenes α-humulene and β-caryophyllene oxide enhance the efficacy of 5-fluorouracil and oxaliplatin in colon cancer cells. Acta Pharm. 2019, 69, 121–128. [Google Scholar] [CrossRef] [Green Version]
- Al-Taee, H.; Azimullah, S.; Meeran, M.N.; Almheiri, M.K.A.; Al Jasmi, R.A.; Tariq, S.; Ab Khan, M.; Adeghate, E.; Ojha, S.K.; Almehiri, M.K.A. β-caryophyllene, a dietary phytocannabinoid attenuates oxidative stress, inflammation, apoptosis and prevents structural alterations of the myocardium against doxorubicin-induced acute cardiotoxicity in rats: An in vitro and in vivo study. Eur. J. Pharmacol. 2019, 858, 172467. [Google Scholar] [CrossRef]
- Tomonari, T.; Takeishi, S.; Taniguchi, T.; Tanaka, T.; Tanaka, H.; Fujimoto, S.; Kimura, T.; Okamoto, K.; Miyamoto, H.; Muguruma, N.; et al. MRP3 as a novel resistance factor for sorafenib in hepatocellular carcinoma. Oncotarget 2016, 7, 7207–7215. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Qian, Z.; Zhao, H.; Zhang, X.; Che, S.; Zhang, H.; Shang, H.; Bao, J.; Hao, C.; Liu, J.; et al. CSN5 silencing reverses sorafenib resistance of human hepatocellular carcinoma HepG2 cells. Mol. Med. Rep. 2015, 12, 3902–3908. [Google Scholar] [CrossRef]
- Fouquet, G.; Marié, C.; Collet, L.; Vilpoux, C.; Ouled-Haddou, H.; Nguyen-Khac, E.M.; Bayry, J.; Naassila, M.; Marcq, I.; Bouhlal, H. Rescuing SLAMF3 expression restores sorafenib response in hepatocellular carcinoma cells through the Induction of mesenchymal-to-epithelial transition. Cancers 2022, 14, 910. [Google Scholar] [CrossRef]
- Gana, C.C.; Hanssen, K.M.; Yu, D.M.T.; Flemming, C.L.; Wheatley, M.S.; Conseil, G.; Cole, S.P.C.; Norris, M.D.; Haber, M.; Fletcher, J.I. MRP1 modulators synergize with buthionine sulfoximine to exploit collateral sensitivity and selectively kill MRP1-expressing cancer cells. Biochem. Pharmacol. 2019, 168, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Vasilogianni, A.M.; Al-Majdoub, Z.M.; Achour, B.; Peters, S.A.; Rostami-Hodjegan, A.; Barber, J. Proteomics of colorectal cancer liver metastasis: A quantitative focus on drug elimination and pharmacodynamics effects. Br. J. Clin. Pharmacol. 2022, 88, 1811–1823. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, M.; Hatozaki, D.; Shima, D.; Yokota, H.; Furukubo, T.; Izumi, S.; Yamakawa, T.; Minegaki, T.; Nishiguchi, K. Influence of serum in hemodialysis patients on the expression of intestinal and hepatic transporters for the excretion of pravastatin. Ther. Apher. Dial. 2012, 16, 580–587. [Google Scholar] [CrossRef]
- Drozdzik, M.; Szelag-Pieniek, S.; Post, M.; Zeair, S.; Wrzesinski, M.; Kurzawski, M.; Prieto, J.; Oswald, S. Protein Abundance of Hepatic Drug Transporters in Patients with Different Forms of Liver Damage. Clin. Pharmacol. Ther. 2020, 107, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Noma, B.; Sasaki, T.; Fujimoto, Y.; Serikawa, M.; Kobayashi, K.; Inoue, M.; Itsuki, H.; Kamigaki, M.; Minami, T.; Chayama, K. Expression of multidrug resistance-associated protein 2 is involved in chemotherapy resistance in human pancreatic cancer. Int. J. Oncol. 2008, 33, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, S.; Shiraha, H.; Nagahara, T.; Kataoka, J.; Iwamuro, M.; Matsubara, M.; Nishina, S.; Kato, H.; Takaki, A.; Nouso, K.; et al. Loss of runt-related transcription factor 3 induces gemcitabine resistance in pancreatic cancer. Mol. Oncol. 2013, 7, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, K.; Franz, C.; Xiao, Z.; Mohr, E.; Serba, S.; Büchler, M.W.; Schemmer, P. Sorafenib modulates the gene expression of multi-drug resistance mediating ATP-binding cassette proteins in experimental hepatocellular carcinoma. Anticancer Res. 2010, 30, 4503–4508. [Google Scholar]
- Xiao, Z.; Ding, N.; Xiao, G.; Wang, S.; Wu, Y.; Tang, L. Reversal of multidrug resistance by gefitinib via RAF1/ERK pathway in pancreatic cancer cell line. Anat. Rec. 2012, 295, 2122–2128. [Google Scholar] [CrossRef]
- Wu, P.; Wu, D.; Zhao, L.; Huang, L.; Shen, G.; Huang, J.; Chai, Y. Prognostic role of STAT3 in solid tumors: A systematic review and meta-analysis. Oncotarget 2016, 7, 19863–19883. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, N.; Hirano, K.; Shichi, Y.; Gomi, F.; Yoshimura, H.; Matsushita, A.; Toyoda, M.; Ishiwata, T. Gp130-Mediated STAT3 Activation Contributes to the Aggressiveness of Pancreatic Cancer through H19 Long Non-Coding RNA Expression. Cancers 2022, 14, 2055. [Google Scholar] [CrossRef]
- Fang, Z.; Chen, W.; Yuan, Z.; Liu, X.; Jiang, H. LncRNA-MALAT1 contributes to the cisplatin-resistance of lung cancer by upregulating MRP1 and MDR1 via STAT3 activation. Biomed. Pharmacother. 2018, 101, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, G.; Han, Z.; Cheng, H.; Qiao, L.; Li, Y. IL-6/STAT3 Signaling Contributes to Sorafenib Resistance in Hepatocellular Carcinoma Through Targeting Cancer Stem Cells. Onco Targets Ther. 2020, 13, 9721–9730. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Wang, X.; Peng, R.; Zhang, B.; Han, Q.; Lin, J.; Wang, J.; Lin, J.; Jiang, M.; Liu, H.; et al. Induction of IL-6Rα by ATF3 enhances IL-6 mediated sorafenib and regorafenib resistance in hepatocellular carcinoma. Cancer Lett. 2022, 524, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Sinicrope, F.A. Sorafenib inhibits STAT3 activation to enhance TRAIL-mediated apoptosis in human pancreatic cancer cells. Mol. Cancer Ther. 2010, 9, 742–750. [Google Scholar] [CrossRef] [Green Version]
- Teng, Y.; Ross, J.L.; Cowell, J.K. The involvement of JAK-STAT3 in cell motility, invasion, and metastasis. JAKSTAT 2014, 3, e28086. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, A.; Wang, S.C.; Morris, J.P., 4th; Folias, A.E.; Liou, A.; Kim, G.E.; Akira, S.; Boucher, K.M.; Firpo, M.A.; Mulvihill, S.J.; et al. Stat3 and MMP7 contribute to pancreatic ductal adenocarcinoma initiation and progression. Cancer Cell 2011, 19, 441–455. [Google Scholar] [CrossRef] [Green Version]
- Nagathihalli, N.S.; Castellanos, J.A.; Shi, C.; Beesetty, Y.; Reyzer, M.L.; Caprioli, R.; Chen, X.; Walsh, A.J.; Skala, M.C.; Moses, H.L.; et al. Signal Transducer and Activator of Transcription 3, Mediated Remodeling of the Tumor Microenvironment Results in Enhanced Tumor Drug Delivery in a Mouse Model of Pancreatic Cancer. Gastroenterology 2015, 149, 1932–1943.e9. [Google Scholar] [CrossRef] [Green Version]
- Pasello, M.; Giudice, A.M.; Scotlandi, K. The ABC subfamily A transporters: Multifaceted players with incipient potentialities in cancer. Semin. Cancer Biol. 2020, 60, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Salaroglio, I.C.; Mungo, E.; Gazzano, E.; Kopecka, J.; Riganti, C. ERK is a Pivotal Player of Chemo-Immune-Resistance in Cancer. Int. J. Mol. Sci. 2019, 20, 2505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.; Zhou, X.; Qu, H.; Ma, Y.; Yue, Z.; Shang, W.; Wang, P.; Xie, S.; Li, Y.; Sun, Y. TRIB2 knockdown as a regulator of chemotherapy resistance and proliferation via the ERK/STAT3 signaling pathway in human chronic myelogenous leukemia K562/ADM cells. Oncol. Rep. 2018, 39, 1910–1918. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Gene | Brand | Primer (5′ → 3′) | Annealing (°C) | Efficiency (%) | R2 | |
|---|---|---|---|---|---|---|
| MDR1 | Bio-Rad | Forward | N/A (Cod. qHsaCED0056970) | 60 | 99.0 | 0.999 |
| Reverse | ||||||
| MRP1 | Bio-Rad | Forward | N/A (Cod. qHsaCID0016624) | 60 | 98.0 | 0.998 |
| Reverse | ||||||
| MRP2 | Bio-Rad | Forward | N/A (Cod. qHsaCID0008411) | 60 | 99.0 | 0.999 |
| Reverse | ||||||
| GAPDH | Bio-Rad | Forward | N/A (Cod. qHsaCED0038674) | 60 | 97.0 | 0.999 |
| Reverse | ||||||
| Compound | IC50 [µg/mL] (CL) | |||
|---|---|---|---|---|
| HepG2 | Mz-ChA-1 | Bx-PC3 | H69 | |
| Sorafenib | 47.2 (43.7–51.0) | 76.1 (72.2–80.2) | 31.0 (28.4–33.7) | 50.3 (45.0–55.58) |
| β-Caryophyllene | 74.8 (71.6–78.2) *** | 62.7 (60.3–65.2) ** | 36.3 (33.6–39.1) | 67.0 (62.6–71.7) * |
| β-Caryophyllene oxide | 78.7 (76.8–80.5) *** | 94.5 (90.1–99.1) ** | 65.3 (62.1–68.7) *** | 99.7 (93.1–106.9) *** |
| Verapamil | 117.5 (107.6–128.3) *** | 171.5 (159.3–188.9) *** | 117.9 (112.3–124.3) *** | 87.9 (78.7–98.71) ** |
| MK571 | 46.8 (43.8–50.1) | 94.6 (89.3–100.4) ** | 78.5 (73.0–84.3) *** | 32.6 (27.9–37.8) * |
| Compound | IC50 [µg/mL] (CL) RR a | ||
|---|---|---|---|
| HepG2 | CCA | Bx-PC3 | |
| Sorafenib | 47.2 (43.7–51.0) - | 76.1 (72.2–80.2) - | 31.0 (28.4–33.7) - |
| + β-Caryophyllene | 16.1 (14.6–17.7) *** 2.9 | 43.9 (39.4–48.7) *** 1.7 | 5.8 (4.7–7.2) *** 5.3 |
| + β-Caryophyllene oxide | 7.8 (6.8–8.9) *** 6.0 | 50.1 (43.7–56.8) ** 1.5 | 3.4 (2.8–4.2) *** 9.1 |
| + Verapamil | 15.2 (11.8–19.3) *** 3.1 | 51.5 (46.1–57.3) ** 1.5 | 15.6 (13.9–17.6) ** 2.0 |
| + MK571 | 8.3 (6.1–11.2) *** 5.7 | 45.1 (37.5–53.8) ** 1.7 | 7.9 (6.5–9.5) *** 3.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Giacomo, S.; Gullì, M.; Facchinetti, R.; Minacori, M.; Mancinelli, R.; Percaccio, E.; Scuderi, C.; Eufemi, M.; Di Sotto, A. Sorafenib Chemosensitization by Caryophyllane Sesquiterpenes in Liver, Biliary, and Pancreatic Cancer Cells: The Role of STAT3/ABC Transporter Axis. Pharmaceutics 2022, 14, 1264. https://doi.org/10.3390/pharmaceutics14061264
Di Giacomo S, Gullì M, Facchinetti R, Minacori M, Mancinelli R, Percaccio E, Scuderi C, Eufemi M, Di Sotto A. Sorafenib Chemosensitization by Caryophyllane Sesquiterpenes in Liver, Biliary, and Pancreatic Cancer Cells: The Role of STAT3/ABC Transporter Axis. Pharmaceutics. 2022; 14(6):1264. https://doi.org/10.3390/pharmaceutics14061264
Chicago/Turabian StyleDi Giacomo, Silvia, Marco Gullì, Roberta Facchinetti, Marco Minacori, Romina Mancinelli, Ester Percaccio, Caterina Scuderi, Margherita Eufemi, and Antonella Di Sotto. 2022. "Sorafenib Chemosensitization by Caryophyllane Sesquiterpenes in Liver, Biliary, and Pancreatic Cancer Cells: The Role of STAT3/ABC Transporter Axis" Pharmaceutics 14, no. 6: 1264. https://doi.org/10.3390/pharmaceutics14061264
APA StyleDi Giacomo, S., Gullì, M., Facchinetti, R., Minacori, M., Mancinelli, R., Percaccio, E., Scuderi, C., Eufemi, M., & Di Sotto, A. (2022). Sorafenib Chemosensitization by Caryophyllane Sesquiterpenes in Liver, Biliary, and Pancreatic Cancer Cells: The Role of STAT3/ABC Transporter Axis. Pharmaceutics, 14(6), 1264. https://doi.org/10.3390/pharmaceutics14061264








