Comparison of Choi, RECIST and Somatostatin Receptor PET/CT Based Criteria for the Evaluation of Response and Response Prediction to PRRT
Abstract
:1. Introduction
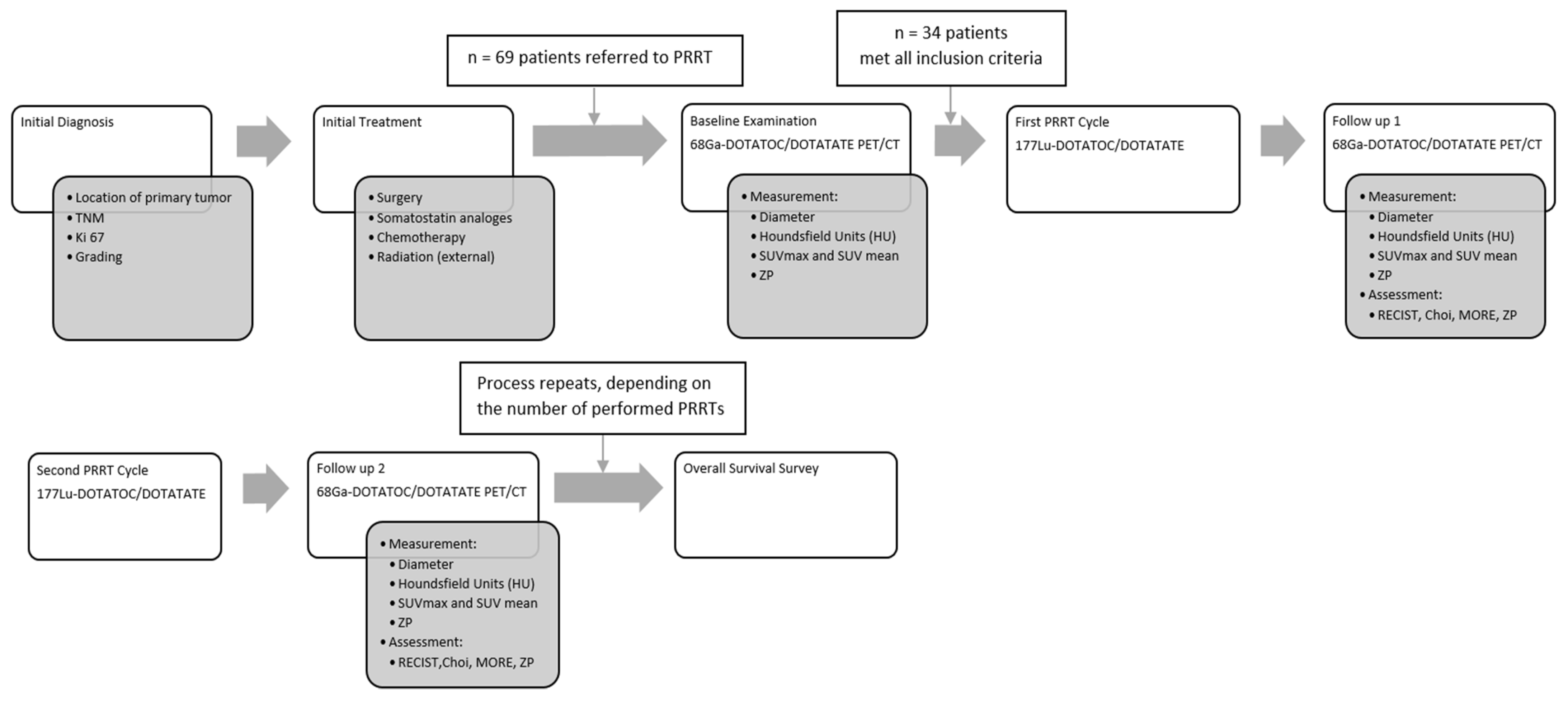
2. Patients and Methods
2.1. Study Design and Ethical Clearance
2.2. Radiopeptide Treatment
2.3. Somatostatin Receptor PET/CT
2.4. Image Analysis
2.4.1. PET
2.4.2. CT
2.4.3. ZP Parameter
2.5. Statistical Analyses
3. Results
3.1. Demography, Histopathology, Previous Therapy
3.2. PET/CT
3.3. Response (RECIST 1.1, Choi, MORE, ZP)
3.4. Prediction of Response to PRRT
3.5. Pseudoprogression
3.6. Overall Survival
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hicks, R.J.; Kwekkeboom, D.J.; Krenning, E.; Bodei, L.; Grozinsky-Glasberg, S.; Arnold, R.; Borbath, I.; Cwikla, J.; Toumpanakis, C.; Kaltsas, G.; et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Neoplasia: Peptide Receptor Radionuclide Therapy with Radiolabeled Somatostatin Analogues. Neuroendocrinology 2017, 105, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.R.; Caplin, M.E.; Kunz, P.L.; Ruszniewski, P.B.; Bodei, L.; Hendifar, A.E.; Mittra, E.; Wolin, E.M.; Yao, J.C.; Pavel, M.E.; et al. Final overall survival in the phase 3 NETTER-1 study of lutetium-177-DOTATATE in patients with midgut neuroendocrine tumors. J. Clin. Oncol. 2021, 39, 4112. [Google Scholar] [CrossRef]
- Prasad, V.; Bodei, L.; Kidd, M.; Modlin, I.M. Whither peptide receptor radionuclide therapy for neuroendocrine tumors: An Einsteinian view of the facts and myths. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1825–1830. [Google Scholar] [CrossRef] [Green Version]
- Ilan, E.; Sandström, M.; Wassberg, C.; Sundin, A.; Garske-Román, U.; Eriksson, B.; Granberg, D.; Lubberink, M. Dose response of pancreatic neuroendocrine tumors treated with peptide receptor radionuclide therapy using 177Lu-DOTATATE. J. Nucl. Med. 2015, 56, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Kwekkeboom, D.J.; de Herder, W.W.; Kam, B.L.; van Eijck, C.H.; van Essen, M.; Kooij, P.P.; Feelders, R.A.; van Aken, M.O.; Krenning, E.P. Treatment with the radiolabeled somatostatin analog [177Lu-DOTA0,Tyr3]octreotate: Toxicity, efficacy, and survival. J. Clin. Oncol. 2008, 26, 2124–2130. [Google Scholar] [CrossRef] [Green Version]
- Haug, A.R.; Auernhammer, C.J.; Wängler, B.; Schmidt, G.P.; Uebleis, C.; Göke, B.; Cumming, P.; Bartenstein, P.; Tiling, R.; Hacker, M. 68Ga-DOTATATE PET/CT for the early prediction of response to somatostatin receptor-mediated radionuclide therapy in patients with well-differentiated neuroendocrine tumors. J. Nucl. Med. 2010, 51, 1349–1356. [Google Scholar] [CrossRef] [Green Version]
- Gabriel, M.; Oberauer, A.; Dobrozemsky, G.; Decristoforo, C.; Putzer, D.; Kendler, D.; Uprimny, C.; Kovacs, P.; Bale, R.; Virgolini, I.J. 68Ga-DOTA-Tyr3-octreotide PET for assessing response to somatostatin-receptor-mediated radionuclide therapy. J. Nucl. Med. 2009, 50, 1427–1434. [Google Scholar] [CrossRef] [Green Version]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.; Charnsangavej, C.; Faria, S.C.; Macapinlac, H.A.; Burgess, M.A.; Patel, S.R.; Chen, L.L.; Podoloff, D.A.; Benjamin, R.S. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: Proposal of new computed tomography response criteria. J. Clin. Oncol. 2007, 25, 1753–1759. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, J.; Huang, K.; Lin, Y.; Chen, M.; Xu, L.; Li, Z.-P.; Feng, S.-T. Early evaluation of sunitinib for the treatment of advanced gastroenteropancreatic neuroendocrine neoplasms via CT imaging: RECIST 1.1 or Choi Criteria? BMC Cancer 2017, 17, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhernosekov, K.P.; Filosofov, D.V.; Baum, R.P.; Aschoff, P.; Bihl, H.; Razbash, A.A.; Jahn, M.; Jennewein, M.; Rösch, F. Processing of generator-produced 68Ga for medical application. J. Nucl. Med. 2007, 48, 1741–1748. [Google Scholar] [CrossRef] [PubMed]
- Velikyan, I.; Sundin, A.; Sörensen, J.; Lubberink, M.; Sandström, M.; Garske-Román, U.; Lundqvist, H.; Granberg, D.; Eriksson, B. Quantitative and qualitative intrapatient comparison of 68Ga-DOTATOC and 68Ga-DOTATATE: Net uptake rate for accurate quantification. J. Nucl. Med. 2014, 55, 204–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virgolini, I.; Ambrosini, V.; Bomanji, J.B.; Baum, R.P.; Fanti, S.; Gabriel, M.; Papathanasiou, N.D.; Pepe, G.; Oyen, W.; de Cristoforo, C.; et al. Procedure guidelines for PET/CT tumour imaging with 68Ga-DOTA-conjugated peptides: 68Ga-DOTA-TOC, 68Ga-DOTA-NOC, 68Ga-DOTA-TATE. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 2004–2010. [Google Scholar] [CrossRef]
- Ruf, J.; Schiefer, J.; Furth, C.; Kosiek, O.; Kropf, S.; Heuck, F.; Denecke, T.; Pavel, M.; Pascher, A.; Wiedenmann, B.; et al. 68Ga-DOTATOC PET/CT of neuroendocrine tumors: Spotlight on the CT phases of a triple-phase protocol. J. Nucl. Med. 2011, 52, 697–704. [Google Scholar] [CrossRef] [Green Version]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Young, H.; Baum, R.; Cremerius, U.; Herholz, K.; Hoekstra, O.; Lammertsma, A.A.; Pruim, J.; Price, P. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: Review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur. J. Cancer 1999, 35, 1773–1782. [Google Scholar] [CrossRef]
- Verburg, F.A.; Luster, M.; Giovanella, L.; Lassmann, M.; Chiesa, C.; Chouin, N.; Flux, G.D. The “reset button” revisited: Why high activity 131I therapy of advanced differentiated thyroid cancer after dosimetry is advantageous for patients. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 915–917. [Google Scholar] [CrossRef] [Green Version]
- Jahn, U.; Ilan, E.; Sandström, M.; Lubberink, M.; Garske-Román, U.; Sundin, A. Peptide Receptor Radionuclide Therapy (PRRT) with 177Lu-DOTATATE; Differences in Tumor Dosimetry, Vascularity and Lesion Metrics in Pancreatic and Small Intestinal Neuroendocrine Neoplasms. Cancers 2021, 13, 962. [Google Scholar] [CrossRef]
- Roth, D.; Gustafsson, J.R.; Warfvinge, C.F.; Sundlöv, A.; Åkesson, A.; Tennvall, J.; Sjögreen Gleisner, K. Dosimetric quantities of neuroendocrine tumors over treatment cycles with 177Lu-DOTA-TATE. J. Nucl. Med. 2021, 63, 399–405. [Google Scholar] [CrossRef]
- Brabander, T.; van der Zwan, W.A.; Teunissen, J.J.M.; Kam, B.L.R.; de Herder, W.W.; Feelders, R.A.; Krenning, E.P.; Kwekkeboom, D.J. Pitfalls in the response evaluation after peptide receptor radionuclide therapy with [177Lu-DOTA0,Tyr3]octreotate. Endocr. Relat. Cancer 2017, 24, 243–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graf, J.; Pape, U.F.; Jann, H.; Denecke, T.; Arsenic, R.; Brenner, W.; Pavel, M.; Prasad, V. Prognostic Significance of Somatostatin Receptor Heterogeneity in Progressive Neuroendocrine Tumor Treated with Lu-177 DOTATOC or Lu-177 DOTATATE. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 881–894. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; Valle, J.W.; Eriksson, B.; Rinke, A.; Caplin, M.; Chen, J.; Costa, F.; Falkerby, J.; Fazio, N.; Gorbounova, V.; et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Neoplasms: Systemic Therapy—Biotherapy and Novel Targeted Agents. Neuroendocrinology 2017, 105, 266–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavel, M.; Baudin, E.; Couvelard, A.; Krenning, E.; Öberg, K.; Steinmüller, T.; Anlauf, M.; Wiedenmann, B.; Salazar, R. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology 2012, 95, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Prasad, V.; Fetscher, S.; Baum, R.P. Changing role of somatostatin receptor targeted drugs in NET: Nuclear Medicine’s view. J. Pharm. Pharm. Sci. 2007, 10, 321s–337s. [Google Scholar]






| WHO | RECIST | Choi | MORE | ZP | ||
|---|---|---|---|---|---|---|
| Non PD (non-progressive disease) | CR (complete response) | Complete disappearance of all disease manifestations at an interval of at least 4 weeks | Disappearance of all lesions and no new lesions | Disappearance of all lesions and no new lesions | Complete disappearance of uptake in all lesions | No lesions detectable in CT or PET |
| PR (partial remission) | Greater than or equal to 50% decrease in tumor size | ≥30% reduction in the sum of the greatest diameter and no new lesions | ≥10% decrease in the greatest diameter or a ≥15% decrease in tumor density (HU) and no new lesions | ≥25% reduction in the sum of SUVmax after more than one cycle of treatment | ≥25% reduction in the product of SUVmean and HU | |
| SD (stable disease) | Increase or decrease in tumor size of less than 25% | Does not meet the criteria for complete response (CR), partial response (PR), and progressive disease (PD) | Does not meet the criteria for complete response (CR), partial response (PR), and progressive disease (PD) | Does not meet the criteria for complete response (CR), partial response (PR), and progressive disease (PD) | Does not meet the criteria for complete response (CR), partial response (PR), and progressive disease (PD) | |
| PD (progressive disease) | PD (progressive disease) | Greater than 25% increase in tumor lesions and/or appearance of new foci of tumor | ≥20% increase in the sum of the greatest diameters or at least one new lesion | ≥10% increase in the greatest diameter and does not meet the criteria for partial response (PR) Or at least one new lesion | ≥25% increase in the sum of SUVmax or at least one new lesions | ≥25% increase in the product of SUVmean and HU |
| Patients Characteristics (n = 34) | |
|---|---|
| Age in years, mean (SD) | 61.19 (11.98) |
| Sex, male: female ratio | 23:11 |
| Median time interval between first diagnosis and first PRRT in months, mean (range) | 32.35 (1.3–165.4) |
| Target lesions per patient, mean (range) | 2.2 (1–4) |
| PRRT | |
| Mean number of therapy cycles (range) | 3 (2–6) |
| Radiopeptide used (177 Lu-DOTATOC/DOTATATE) | 29/72 |
| PET/CT Acquisition Data | |
| Total number of PET/CT analyzed | 130 (2–8 per patient) |
| Number of lesions evaluated | 77 |
| Time interval between PRRT and PET/CT in months, median (range) | 3.2 (2.6–6.2) |
| Injected activity in GBq, mean (SD) | 6.58 (0.87) |
| Time interval between tracer injection and acquisition in min, mean (SD) | 60.3 (6.2) |
| Follow-up | |
| Median duration of follow-up from first PRRT in months, mean (range) | 63.88 (6.2–86.2) |
| Median duration of follow-up after last PRRT in months, mean (range) | 50.33 (1.9–69.0) |
| nonPD | PD | ||
|---|---|---|---|
| RECIST 1.1 | 29 | 5 | |
| CR | 0 | ||
| PR | 20 | ||
| SD | 9 | ||
| Choi | 22 | 12 | |
| CR | 0 | ||
| PR | 12 | ||
| SD | 10 | ||
| MORE | 21 | 13 | |
| CR | 0 | ||
| PR | 8 | ||
| SD | 13 | ||
| ZP | 24 | 10 | |
| CR | 0 | ||
| PR | 10 | ||
| SD | 14 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zwirtz, K.; Hardt, J.; Acker, G.; Baur, A.D.J.; Pavel, M.; Huang, K.; Brenner, W.; Prasad, V. Comparison of Choi, RECIST and Somatostatin Receptor PET/CT Based Criteria for the Evaluation of Response and Response Prediction to PRRT. Pharmaceutics 2022, 14, 1278. https://doi.org/10.3390/pharmaceutics14061278
Zwirtz K, Hardt J, Acker G, Baur ADJ, Pavel M, Huang K, Brenner W, Prasad V. Comparison of Choi, RECIST and Somatostatin Receptor PET/CT Based Criteria for the Evaluation of Response and Response Prediction to PRRT. Pharmaceutics. 2022; 14(6):1278. https://doi.org/10.3390/pharmaceutics14061278
Chicago/Turabian StyleZwirtz, Kevin, Juliane Hardt, Güliz Acker, Alexander D. J. Baur, Marianne Pavel, Kai Huang, Winfried Brenner, and Vikas Prasad. 2022. "Comparison of Choi, RECIST and Somatostatin Receptor PET/CT Based Criteria for the Evaluation of Response and Response Prediction to PRRT" Pharmaceutics 14, no. 6: 1278. https://doi.org/10.3390/pharmaceutics14061278
APA StyleZwirtz, K., Hardt, J., Acker, G., Baur, A. D. J., Pavel, M., Huang, K., Brenner, W., & Prasad, V. (2022). Comparison of Choi, RECIST and Somatostatin Receptor PET/CT Based Criteria for the Evaluation of Response and Response Prediction to PRRT. Pharmaceutics, 14(6), 1278. https://doi.org/10.3390/pharmaceutics14061278







