Pre-Clinical Evaluation of Tenofovir and Tenofovir Alafenamide for HIV-1 Pre-Exposure Prophylaxis in Foreskin Tissue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Viral Isolate
2.2. Human Tissue
2.3. Cell and Foreskin Tissue Explants Culture
2.4. Infectivity and Inhibition Assays
2.5. Bioanalysis
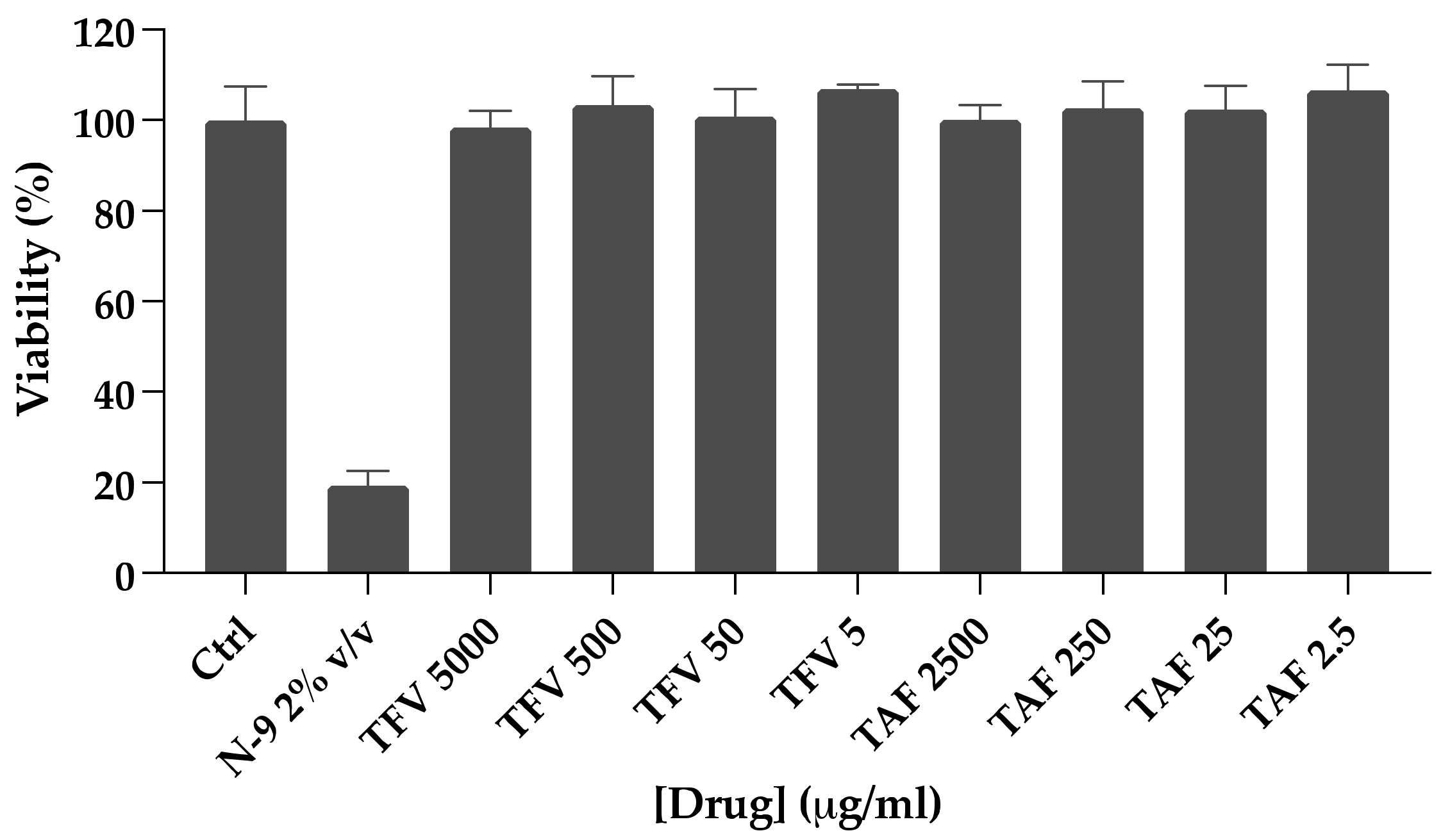
2.6. Viability Assay
2.7. Statistical Analysis
3. Results
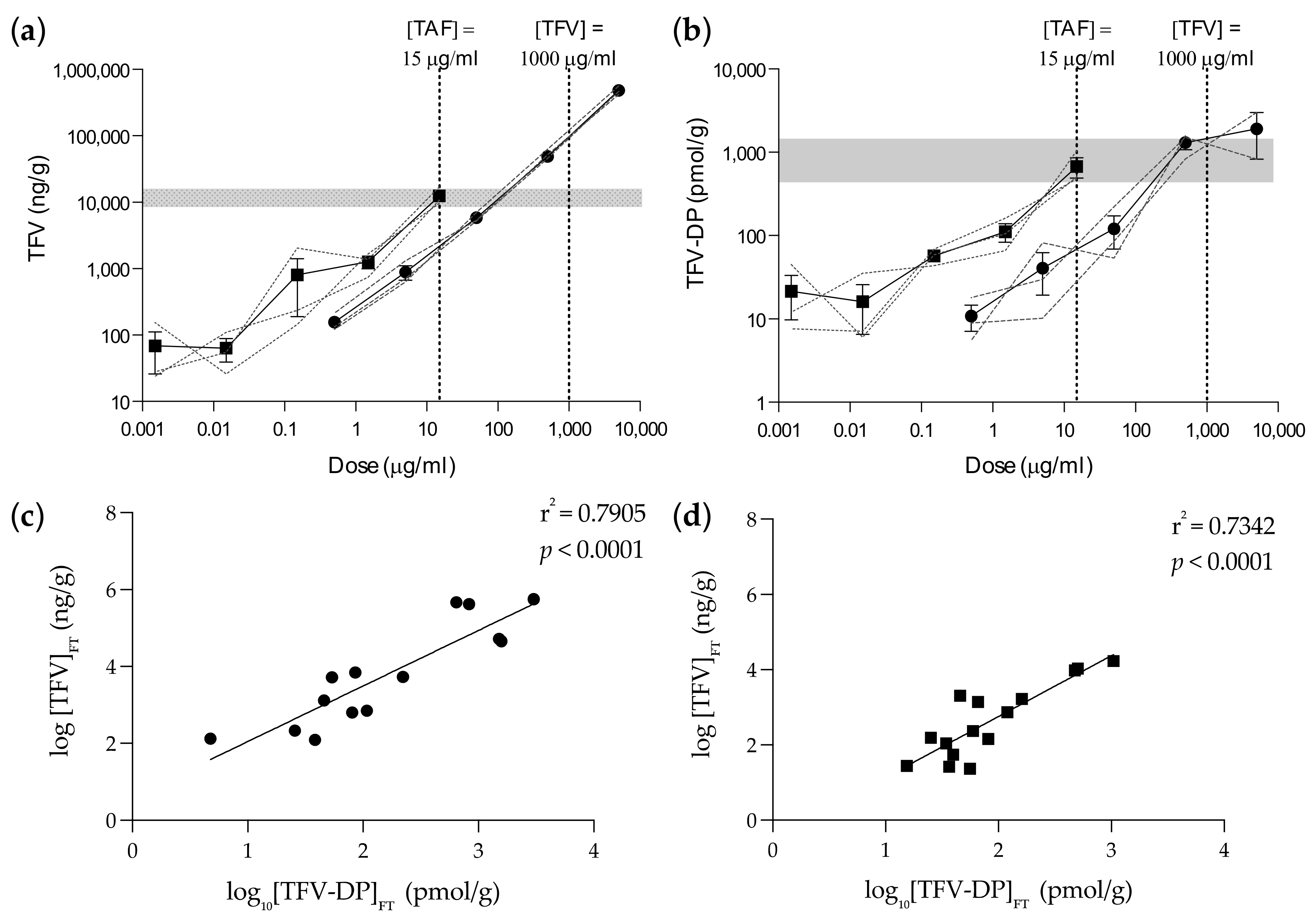
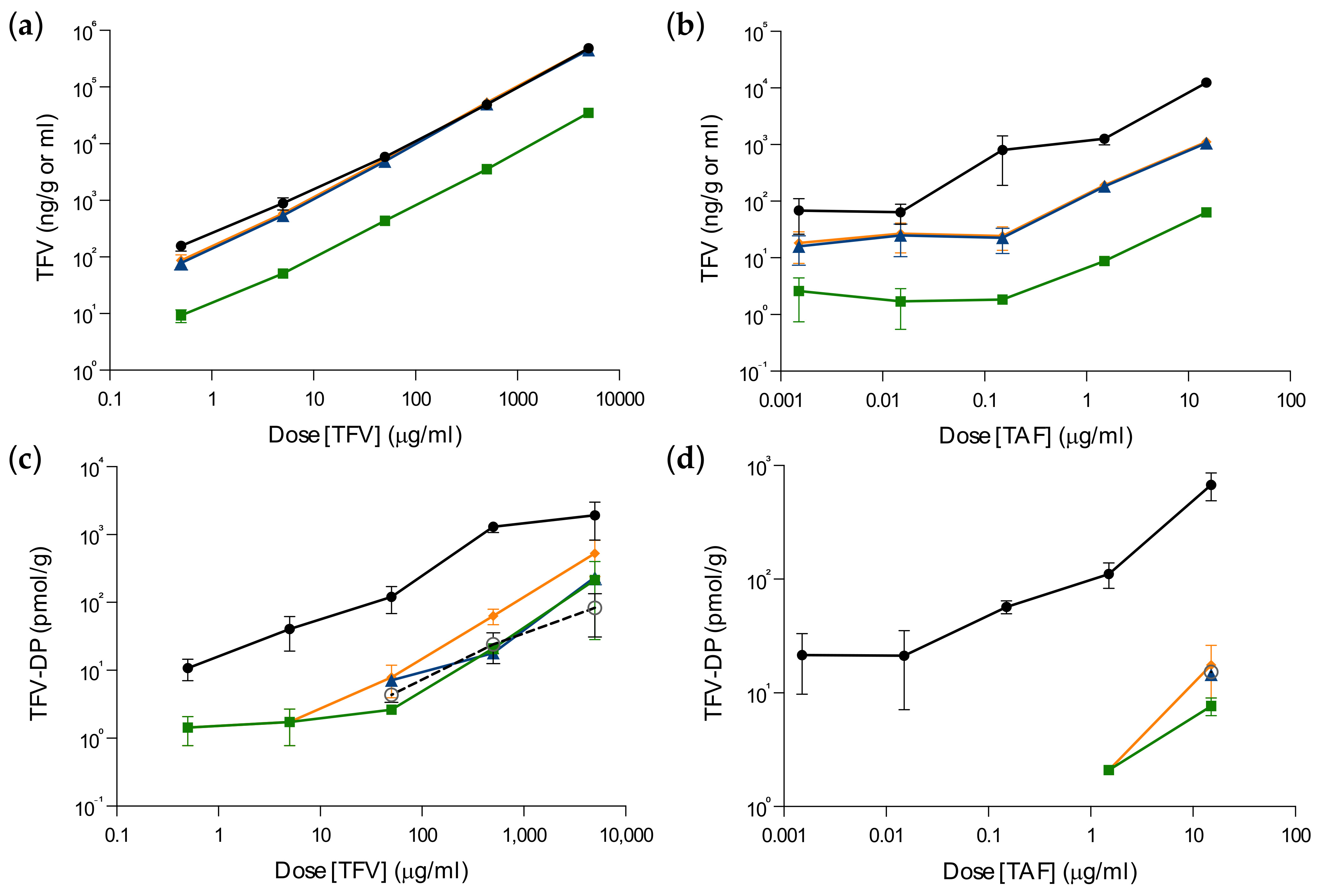
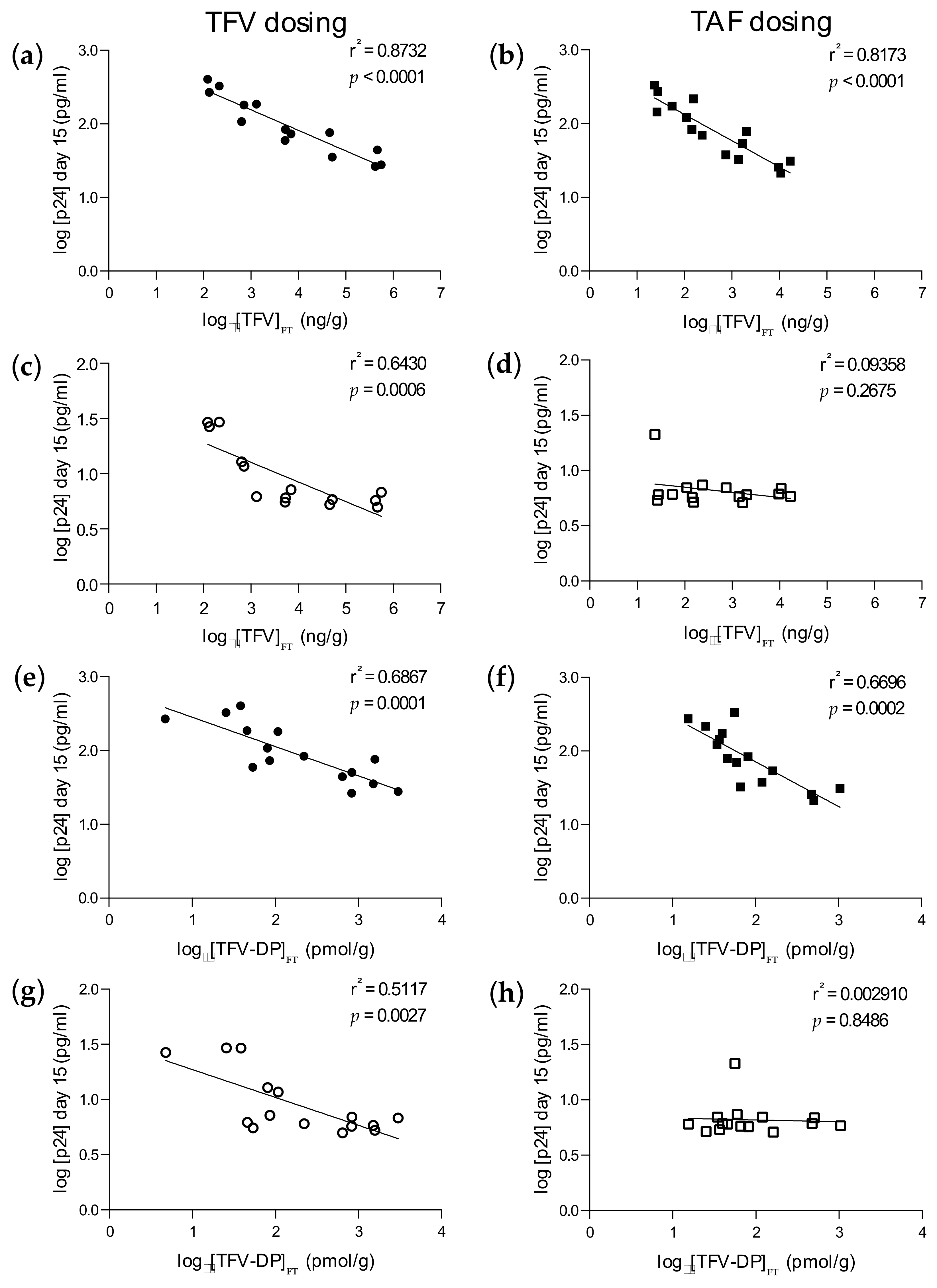
3.1. Ex Vivo PK Equivalency in Foreskin Explants
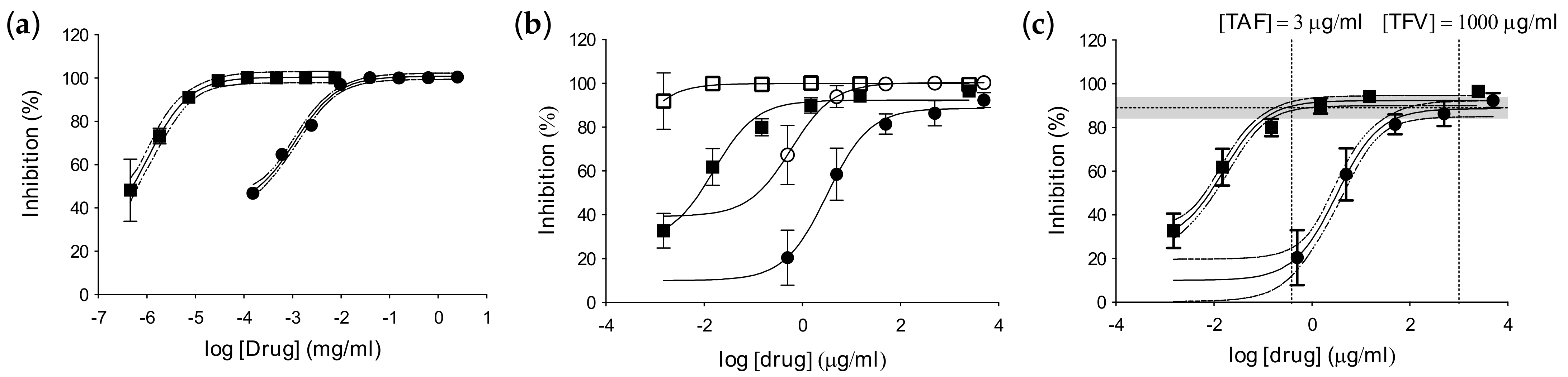
3.2. Inhibitory Activity in Foreskin Explants
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Molina, J.-M.; Capitant, C.; Spire, B.; Pialoux, G.; Cotte, L.; Charreau, I.; Tremblay, C.; Le Gall, J.-M.; Cua, E.; Pasquet, A.; et al. On-Demand Preexposure Prophylaxis in Men at High Risk for HIV-1 Infection. N. Engl. J. Med. 2015, 373, 2237–2246. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.H.; Molina, J.M.; Thompson, M.A.; Anderson, P.L.; Mounzer, K.C.; De Wet, J.J.; DeJesus, E.; Jessen, H.; Grant, R.M.; Ruane, P.J.; et al. Emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV pre-exposure prophylaxis (DISCOVER): Primary results from a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet 2020, 396, 239–254. [Google Scholar] [CrossRef]
- Ogbuagu, O.; Ruane, P.J.; Podzamczer, D.; Salazar, L.C.; Henry, K.; Asmuth, D.M.; Wohl, D.; Gilson, R.; Shao, Y.; Ebrahimi, R.; et al. Long-term safety and efficacy of emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV-1 pre-exposure prophylaxis: Week 96 results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet HIV 2021, 8, e397–e407. [Google Scholar] [CrossRef]
- Nash, S.; Dietrich, J.; Ssemata, A.S.; Herrera, C.; O’Hagan, K.; Else, L.; Chiodi, F.; Kelly, C.; Shattock, R.; Chirenje, M.; et al. Combined HIV Adolescent Prevention Study (CHAPS): Comparison of HIV pre-exposure prophylaxis regimens for adolescents in sub-Saharan Africa-study protocol for a mixed-methods study including a randomised controlled trial. Trials 2020, 21, 900. [Google Scholar] [CrossRef]
- Lee, W.A.; He, G.X.; Eisenberg, E.; Cihlar, T.; Swaminathan, S.; Mulato, A.; Cundy, K.C. Selective intracellular activation of a novel prodrug of the human immunodeficiency virus reverse transcriptase inhibitor tenofovir leads to preferential distribution and accumulation in lymphatic tissue. Antimicrob. Agents Chemother. 2005, 49, 1898–1906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callebaut, C.; Stepan, G.; Tian, Y.; Miller, M.D. In Vitro Virology Profile of Tenofovir Alafenamide, a Novel Oral Prodrug of Tenofovir with Improved Antiviral Activity Compared to That of Tenofovir Disoproxil Fumarate. Antimicrob. Agents Chemother. 2015, 59, 5909–5916. [Google Scholar] [CrossRef] [Green Version]
- Ruane, P.J.; DeJesus, E.; Berger, D.; Markowitz, M.; Bredeek, U.F.; Callebaut, C.; Zhong, L.; Ramanathan, S.; Rhee, M.S.; Fordyce, M.W.; et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of tenofovir alafenamide as 10-day monotherapy in HIV-1-positive adults. J. Acquir. Immune Defic. Syndr. 2013, 63, 449–455. [Google Scholar] [CrossRef] [Green Version]
- Sax, P.E.; Wohl, D.; Yin, M.T.; Post, F.; DeJesus, E.; Saag, M.; Pozniak, A.; Thompson, M.; Podzamczer, D.; Molina, J.M.; et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: Two randomised, double-blind, phase 3, non-inferiority trials. Lancet 2015, 385, 2606–2615. [Google Scholar] [CrossRef]
- Sax, P.E.; Zolopa, A.; Brar, I.; Elion, R.; Ortiz, R.; Post, F.; Wang, H.; Callebaut, C.; Martin, H.; Fordyce, M.W.; et al. Tenofovir alafenamide vs. tenofovir disoproxil fumarate in single tablet regimens for initial HIV-1 therapy: A randomized phase 2 study. J. Acquir. Immune Defic. Syndr. 2014, 67, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Cottrell, M.L.; Garrett, K.L.; Prince, H.M.A.; Sykes, C.; Schauer, A.; Emerson, C.W.; Peery, A.; Rooney, J.F.; McCallister, S.; Gay, C.; et al. Single-dose pharmacokinetics of tenofovir alafenamide and its active metabolite in the mucosal tissues. J. Antimicrob. Chemother. 2017, 72, 1731–1740. [Google Scholar] [CrossRef]
- Cottrell, M.L.; Yang, K.H.; Prince, H.M.; Sykes, C.; White, N.; Malone, S.; Dellon, E.S.; Madanick, R.D.; Shaheen, N.J.; Hudgens, M.G.; et al. A Translational Pharmacology Approach to Predicting Outcomes of Preexposure Prophylaxis Against HIV in Men and Women Using Tenofovir Disoproxil Fumarate with or Without Emtricitabine. J. Infect. Dis. 2016, 214, 55–64. [Google Scholar] [CrossRef]
- Thurman, A.R.; Schwartz, J.L.; Cottrell, M.L.; Brache, V.; Chen, B.A.; Cochon, L.; Ju, S.; McGowan, I.; Rooney, J.F.; McCallister, S.; et al. Safety and Pharmacokinetics of a Tenofovir Alafenamide Fumarate-Emtricitabine based Oral Antiretroviral Regimen for Prevention of HIV Acquisition in Women: A Randomized Controlled Trial. EClinicalMedicine 2021, 36, 100893. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Garcia, M.; Patel, M.V.; Shen, Z.; Bodwell, J.; Rossoll, R.M.; Wira, C.R. Tenofovir Inhibits Wound Healing of Epithelial Cells and Fibroblasts from the Upper and Lower Human Female Reproductive Tract. Sci. Rep. 2017, 8, 45725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Z.; Rodriguez-Garcia, M.; Patel, M.V.; Bodwell, J.; Wira, C.R. Epithelial Cells and Fibroblasts from the Human Female Reproductive Tract Accumulate and Release TFV and TAF to Sustain Inhibition of HIV Infection of CD4+ T cells. Sci. Rep. 2019, 9, 1864. [Google Scholar] [CrossRef] [PubMed]
- Fischetti, L.; Barry, S.M.; Hope, T.J.; Shattock, R.J. HIV-1 infection of human penile explant tissue and protection by candidate microbicides. AIDS 2009, 23, 319–328. [Google Scholar] [CrossRef]
- Gartner, S.; Markovits, P.; Markovitz, D.M.; Kaplan, M.H.; Gallo, R.C.; Popovic, M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science 1986, 233, 215–219. [Google Scholar] [CrossRef]
- Gordon, C.J.; Muesing, M.A.; Proudfoot, A.E.; Power, C.A.; Moore, J.P.; Trkola, A. Enhancement of human immunodeficiency virus type 1 infection by the CC-chemokine RANTES is independent of the mechanism of virus-cell fusion. J. Virol. 1999, 73, 684–694. [Google Scholar] [CrossRef] [Green Version]
- Derdeyn, C.A.; Decker, J.M.; Sfakianos, J.N.; Wu, X.; O’Brien, W.A.; Ratner, L.; Kappes, J.C.; Shaw, G.M.; Hunter, E. Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 is modulated by coreceptor specificity defined by the V3 loop of gp120. J. Virol. 2000, 74, 8358–8367. [Google Scholar] [CrossRef] [Green Version]
- Platt, E.J.; Wehrly, K.; Kuhmann, S.E.; Chesebro, B.; Kabat, D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J. Virol. 1998, 72, 2855–2864. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Decker, J.M.; Liu, H.; Zhang, Z.; Arani, R.B.; Kilby, J.M.; Saag, M.S.; Wu, X.; Shaw, G.M.; Kappes, J.C. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob. Agents Chemother. 2002, 46, 1896–1905. [Google Scholar] [CrossRef] [Green Version]
- Herrera, C.; Cranage, M.; McGowan, I.; Anton, P.; Shattock, R.J. Reverse transcriptase inhibitors as potential colorectal microbicides. Antimicrob. Agents Chemother. 2009, 53, 1797–1807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slater, T.F.; Sawyer, B.; Straeuli, U. Studies on Succinate-Tetrazolium Reductase Systems. Iii. Points of Coupling of Four Different Tetrazolium Salts. Biochim. Biophys. Acta 1963, 77, 383–393. [Google Scholar] [CrossRef]
- Mesquita, P.M.; Wilson, S.S.; Manlow, P.; Fischetti, L.; Keller, M.J.; Herold, B.C.; Shattock, R.J. Candidate microbicide PPCM blocks human immunodeficiency virus type 1 infection in cell and tissue cultures and prevents genital herpes in a murine model. J. Virol. 2008, 82, 6576–6584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fichorova, R.N.; Tucker, L.D.; Anderson, D.J. The molecular basis of nonoxynol-9-induced vaginal inflammation and its possible relevance to human immunodeficiency virus type 1 transmission. J. Infect. Dis. 2001, 184, 418–428. [Google Scholar] [CrossRef] [Green Version]
- Agrahari, V.; Putty, S.; Mathes, C.; Murowchick, J.B.; Youan, B.B. Evaluation of degradation kinetics and physicochemical stability of tenofovir. Drug Test. Anal. 2015, 7, 207–213. [Google Scholar] [CrossRef] [Green Version]
- Golla, V.M.; Kurmi, M.; Shaik, K.; Singh, S. Stability behaviour of antiretroviral drugs and their combinations. 4: Characterization of degradation products of tenofovir alafenamide fumarate and comparison of its degradation and stability behaviour with tenofovir disoproxil fumarate. J. Pharm. Biomed. Anal. 2016, 131, 146–155. [Google Scholar] [CrossRef]
- Delahunty, T.; Bushman, L.; Robbins, B.; Fletcher, C.V. The simultaneous assay of tenofovir and emtricitabine in plasma using LC/MS/MS and isotopically labeled internal standards. J. Chromatogr. B 2009, 877, 1907–1914. [Google Scholar] [CrossRef] [Green Version]
- Haaland, R.E.; Fountain, J.; Dinh, C.; Lupo, L.D.; Martin, A.; Conway-Washington, C.; Hall, L.; Kelley, C.F.; Garcia-Lerma, J.G.; Heneine, W. Antiretroviral drug exposure in urethral and glans surface sampling of the penis. J. Antimicrob. Chemother. 2021, 76, 2368–2374. [Google Scholar] [CrossRef]
- Berry, N.; Herrera, C.; Cranage, M. Detection, quantification, and characterisation of HIV/SIV. Methods Mol. Biol. 2011, 665, 133–160. [Google Scholar] [CrossRef]
- Grivel, J.C.; Margolis, L. Use of human tissue explants to study human infectious agents. Nat. Protoc. 2009, 4, 256–269. [Google Scholar] [CrossRef]
- Introini, A.; Vanpouille, C.; Grivel, J.C.; Margolis, L. An ex vivo Model of HIV-1 Infection in Human Lymphoid Tissue and Cervico-vaginal Tissue. Bio-Protocol 2014, 4, e1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arakelyan, A.; Fitzgerald, W.; Grivel, J.C.; Vanpouille, C.; Margolis, L. Histocultures (tissue explants) in human retrovirology. Methods Mol. Biol. 2014, 1087, 233–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fletcher, P.S.; Elliott, J.; Grivel, J.C.; Margolis, L.; Anton, P.; McGowan, I.; Shattock, R.J. Ex vivo culture of human colorectal tissue for the evaluation of candidate microbicides. AIDS 2006, 20, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Herrera, C.; McRaven, M.D.; Laing, K.G.; Dennis, J.; Hope, T.J.; Shattock, R.J. Early Colorectal Responses to HIV-1 and Modulation by Antiretroviral Drugs. Vaccines 2021, 9, 231. [Google Scholar] [CrossRef]
- Calenda, G.; Villegas, G.; Reis, A.; Millen, L.; Barnable, P.; Mamkina, L.; Kumar, N.; Roberts, K.; Kalir, T.; Martinelli, E.; et al. Mucosal Susceptibility to Human Immunodeficiency Virus Infection in the Proliferative and Secretory Phases of the Menstrual Cycle. AIDS Res. Hum. Retrovir. 2019, 35, 335–347. [Google Scholar] [CrossRef]
- Saba, E.; Origoni, M.; Taccagni, G.; Ferrari, D.; Doglioni, C.; Nava, A.; Lisco, A.; Grivel, J.C.; Margolis, L.; Poli, G. Productive HIV-1 infection of human cervical tissue ex vivo is associated with the secretory phase of the menstrual cycle. Mucosal Immunol. 2013, 6, 1081–1090. [Google Scholar] [CrossRef]
- Nicol, M.R.; Brewers, L.M.; Kashuba, A.D.M.; Sykes, C. The role of menopause in tenofovir diphosphate and emtricitabine triphosphate concentrations in cervical tissue. AIDS 2018, 32, 11–15. [Google Scholar] [CrossRef]
- Fahrbach, K.M.; Barry, S.M.; Anderson, M.R.; Hope, T.J. Enhanced cellular responses and environmental sampling within inner foreskin explants: Implications for the foreskin’s role in HIV transmission. Mucosal Immunol. 2010, 3, 410–418. [Google Scholar] [CrossRef]
- Penn, M.L.; Grivel, J.C.; Schramm, B.; Goldsmith, M.A.; Margolis, L. CXCR4 utilization is sufficient to trigger CD4+ T cell depletion in HIV-1-infected human lymphoid tissue. Proc. Natl. Acad. Sci. USA 1999, 96, 663–668. [Google Scholar] [CrossRef] [Green Version]
- Herrera, C.; Shattock, R.J. Candidate microbicides and their mechanisms of action. Curr. Top. Microbiol. Immunol. 2014, 383, 1–25. [Google Scholar]
- Anton, P.A.; Cranston, R.D.; Kashuba, A.; Hendrix, C.W.; Bumpus, N.N.; Richardson-Harman, N.; Elliott, J.; Janocko, L.; Khanukhova, E.; Dennis, R.; et al. RMP-02/MTN-006: A phase 1 rectal safety, acceptability, pharmacokinetic, and pharmacodynamic study of tenofovir 1% gel compared with oral tenofovir disoproxil fumarate. AIDS Res. Hum. Retrovir. 2012, 28, 1412–1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, J.; Tiraboschi, J.M.; Herrera, C.; Else, L.; Egan, D.; Dickinson, L.; Jackson, A.; Olejniczak, N.; Back, D.; Khoo, S.; et al. Brief Report: Pharmacokinetic/Pharmacodynamic Investigation of Single-Dose Oral Maraviroc in the Context of HIV-1 Pre-exposure Prophylaxis. J. Acquir. Immune Defic. Syndr. 2016, 73, 252–257. [Google Scholar] [CrossRef] [PubMed]
- McGowan, I.; Cranston, R.D.; Duffill, K.; Siegel, A.; Engstrom, J.C.; Nikiforov, A.; Jacobson, C.; Rehman, K.K.; Elliott, J.; Khanukhova, E.; et al. A Phase 1 Randomized, Open Label, Rectal Safety, Acceptability, Pharmacokinetic, and Pharmacodynamic Study of Three Formulations of Tenofovir 1% Gel (the CHARM-01 Study). PLoS ONE 2015, 10, e0125363. [Google Scholar] [CrossRef] [PubMed]
- Richardson-Harman, N.; Hendrix, C.W.; Bumpus, N.N.; Mauck, C.; Cranston, R.D.; Yang, K.; Elliott, J.; Tanner, K.; McGowan, I.; Kashuba, A.; et al. Correlation between compartmental tenofovir concentrations and an ex vivo rectal biopsy model of tissue infectibility in the RMP-02/MTN-006 phase 1 study. PLoS ONE 2014, 9, e111507. [Google Scholar]
- Richardson-Harman, N.; Mauck, C.; McGowan, I.; Anton, P. Dose-response relationship between tissue concentrations of UC781 and explant infectibility with HIV type 1 in the RMP-01 rectal safety study. AIDS Res. Hum. Retrovir. 2012, 28, 1422–1433. [Google Scholar] [CrossRef] [PubMed]
- Herrera, C.; Lwanga, J.; Lee, M.; Mantori, S.; Amara, A.; Else, L.; Penchala, S.D.; Egan, D.; Challenger, E.; Dickinson, L.; et al. Pharmacokinetic/pharmacodynamic investigation of raltegravir with or without lamivudine in the context of HIV-1 pre-exposure prophylaxis (PrEP). J. Antimicrob. Chemother. 2021, 76, 2129–2136. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.A.; Panther, L.; Marzinke, M.A.; Hendrix, C.W.; Hoesley, C.J.; van der Straten, A.; Husnik, M.J.; Soto-Torres, L.; Nel, A.; Johnson, S.; et al. Phase 1 Safety, Pharmacokinetics, and Pharmacodynamics of Dapivirine and Maraviroc Vaginal Rings: A Double-Blind Randomized Trial. J. Acquir. Immune Defic. Syndr. 2015, 70, 242–249. [Google Scholar] [CrossRef] [Green Version]
- McGowan, I.; Dezzutti, C.S.; Siegel, A.; Engstrom, J.; Nikiforov, A.; Duffill, K.; Shetler, C.; Richardson-Harman, N.; Abebe, K.; Back, D.; et al. Long-acting rilpivirine as potential pre-exposure prophylaxis for HIV-1 prevention (the MWRI-01 study): An open-label, phase 1, compartmental, pharmacokinetic and pharmacodynamic assessment. Lancet HIV 2016, 3, e569–e578. [Google Scholar] [CrossRef] [Green Version]
- Robinson, J.A.; Marzinke, M.A.; Bakshi, R.P.; Fuchs, E.J.; Radebaugh, C.L.; Aung, W.; Spiegel, H.M.; Coleman, J.S.; Rohan, L.C.; Hendrix, C.W. Comparison of Dapivirine Vaginal Gel and Film Formulation Pharmacokinetics and Pharmacodynamics (FAME 02B). AIDS Res. Hum. Retrovir. 2017, 33, 339–346. [Google Scholar] [CrossRef]
- Fonsart, J.; Saragosti, S.; Taouk, M.; Peytavin, G.; Bushman, L.; Charreau, I.; Hance, A.; Goldwirt, L.; Morel, S.; Mammano, F.; et al. Single-dose pharmacokinetics and pharmacodynamics of oral tenofovir and emtricitabine in blood, saliva and rectal tissue: A sub-study of the ANRS IPERGAY trial. J. Antimicrob. Chemother. 2017, 72, 478–485. [Google Scholar] [CrossRef]
- Sekabira, R.; McGowan, I.; Yuhas, K.; Brand, R.M.; Marzinke, M.A.; Manabe, Y.C.; Frank, I.; Eron, J.; Landovitz, R.J.; Anton, P.; et al. Higher colorectal tissue HIV infectivity in cisgender women compared with MSM before and during oral preexposure prophylaxis. AIDS 2021, 35, 1585–1595. [Google Scholar] [CrossRef] [PubMed]
- McGowan, I.; Wilkin, T.; Landovitz, R.J.; Wu, C.; Chen, Y.; Marzinke, M.A.; Hendrix, C.W.; Richardson, P.; Eshleman, S.H.; Andrade, A.; et al. The pharmacokinetics, pharmacodynamics, and mucosal responses to maraviroc-containing pre-exposure prophylaxis regimens in MSM. AIDS 2019, 33, 237–246. [Google Scholar] [CrossRef] [PubMed]
- McGowan, I.M.; Kunjara Na Ayudhya, R.P.; Brand, R.M.; Marzinke, M.A.; Hendrix, C.W.; Johnson, S.; Piper, J.; Holtz, T.H.; Curlin, M.E.; Chitwarakorn, A.; et al. An Open-Label Pharmacokinetic and Pharmacodynamic Assessment of Tenofovir Gel and Oral Emtricitabine/Tenofovir Disoproxil Fumarate. AIDS Res. Hum. Retrovir. 2022, 38, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Leyva, F.; Fuchs, E.J.; Bakshi, R.; Carballo-Dieguez, A.; Ventuneac, A.; Yue, C.; Caffo, B.; Du, Y.; Torbenson, M.; Li, L.; et al. Simultaneous Evaluation of Safety, Acceptability, Pericoital Kinetics, and Ex Vivo Pharmacodynamics Comparing Four Rectal Microbicide Vehicle Candidates. AIDS Res. Hum. Retrovir. 2015, 31, 1089–1097. [Google Scholar] [CrossRef] [Green Version]
- Anton, P.A.; Saunders, T.; Elliott, J.; Khanukhova, E.; Dennis, R.; Adler, A.; Cortina, G.; Tanner, K.; Boscardin, J.; Cumberland, W.G.; et al. First phase 1 double-blind, placebo-controlled, randomized rectal microbicide trial using UC781 gel with a novel index of ex vivo efficacy. PLoS ONE 2011, 6, e23243. [Google Scholar] [CrossRef] [Green Version]
- Richardson-Harman, N.; Lackman-Smith, C.; Fletcher, P.S.; Anton, P.A.; Bremer, J.W.; Dezzutti, C.S.; Elliott, J.; Grivel, J.C.; Guenthner, P.; Gupta, P.; et al. Multisite comparison of anti-human immunodeficiency virus microbicide activity in explant assays using a novel endpoint analysis. J. Clin. Microbiol. 2009, 47, 3530–3539. [Google Scholar] [CrossRef] [Green Version]
- Herrera, C.; Cottrell, M.L.; Prybylski, J.; Kashuba, A.D.M.; Veazey, R.S.; García-Pérez, J.; Olejniczak, N.; McCoy, C.F.; Ziprin, P.; Richardson-Harman, N.; et al. The ex vivo pharmacology of HIV-1 antiretrovirals differs between macaques and humans. iScience 2022, 25, 104409. [Google Scholar] [CrossRef]
- Abraha, A.; Nankya, I.L.; Gibson, R.; Demers, K.; Tebit, D.M.; Johnston, E.; Katzenstein, D.; Siddiqui, A.; Herrera, C.; Fischetti, L.; et al. CCR5- and CXCR4-tropic subtype C human immunodeficiency virus type 1 isolates have a lower level of pathogenic fitness than other dominant group M subtypes: Implications for the epidemic. J. Virol. 2009, 83, 5592–5605. [Google Scholar] [CrossRef] [Green Version]
- Dinh, M.H.; Anderson, M.R.; McRaven, M.D.; Cianci, G.C.; McCoombe, S.G.; Kelley, Z.L.; Gioia, C.J.; Fought, A.J.; Rademaker, A.W.; Veazey, R.S.; et al. Visualization of HIV-1 interactions with penile and foreskin epithelia: Clues for female-to-male HIV transmission. PLoS Pathog. 2015, 11, e1004729. [Google Scholar] [CrossRef]
- Hladik, F.; Hope, T.J. HIV infection of the genital mucosa in women. Curr. HIV/AIDS Rep. 2009, 6, 20–28. [Google Scholar] [CrossRef]
| TFV | TAF | ||||
|---|---|---|---|---|---|
| Model | IC50 (µg/mL) | IC90 (µg/mL) | IC50 (µg/mL) | IC90 (µg/mL) | |
| TZM-bl cells | 0.203 (0.069) | 5.157 (0.537) | 0.0006 (0.0003) ** | 0.007 (0.002) **** | |
| Foreskin explants | HVT | 3.69 (0.61) | 435.60 (115.44) | 0.018 (0.004) **** | 0.90 (0.26) *** |
| LVT | N/A | 3.17 (0.75) | N/A | 0.005 (0.001) * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Else, L.; Penchala, S.D.; Pillay, A.-D.; Seiphetlo, T.B.; Lebina, L.; Callebaut, C.; Minhas, S.; Morley, R.; Rashid, T.; Martinson, N.; et al. Pre-Clinical Evaluation of Tenofovir and Tenofovir Alafenamide for HIV-1 Pre-Exposure Prophylaxis in Foreskin Tissue. Pharmaceutics 2022, 14, 1285. https://doi.org/10.3390/pharmaceutics14061285
Else L, Penchala SD, Pillay A-D, Seiphetlo TB, Lebina L, Callebaut C, Minhas S, Morley R, Rashid T, Martinson N, et al. Pre-Clinical Evaluation of Tenofovir and Tenofovir Alafenamide for HIV-1 Pre-Exposure Prophylaxis in Foreskin Tissue. Pharmaceutics. 2022; 14(6):1285. https://doi.org/10.3390/pharmaceutics14061285
Chicago/Turabian StyleElse, Laura, Sujan D. Penchala, Azure-Dee Pillay, Thabiso B. Seiphetlo, Limakatso Lebina, Christian Callebaut, Suks Minhas, Roland Morley, Tina Rashid, Neil Martinson, and et al. 2022. "Pre-Clinical Evaluation of Tenofovir and Tenofovir Alafenamide for HIV-1 Pre-Exposure Prophylaxis in Foreskin Tissue" Pharmaceutics 14, no. 6: 1285. https://doi.org/10.3390/pharmaceutics14061285
APA StyleElse, L., Penchala, S. D., Pillay, A.-D., Seiphetlo, T. B., Lebina, L., Callebaut, C., Minhas, S., Morley, R., Rashid, T., Martinson, N., Fox, J., Khoo, S., & Herrera, C., on behalf of the CHAPS Consortium. (2022). Pre-Clinical Evaluation of Tenofovir and Tenofovir Alafenamide for HIV-1 Pre-Exposure Prophylaxis in Foreskin Tissue. Pharmaceutics, 14(6), 1285. https://doi.org/10.3390/pharmaceutics14061285






