Chitosans and Nanochitosans: Recent Advances in Skin Protection, Regeneration, and Repair
Abstract
:1. A Brief Overview of Biopolymers
2. Chitin and Chitosan: Availability, Preparation, Physicochemical Characteristics, and Novel Pharmaceutical Uses
3. Chemical Modifications on Chitin and Chitosan Surfaces: Developing Novel Pharmaceuticals
4. Chitosan Can Be Combined with Other Polymers to Build Broader Drug Carriers
4.1. Alginate and Chitosan Composites
4.2. Chitosan and Agarose Composites
4.3. Chitosan and Collagen Composites
5. Chitsosan and Nanochitosan Skin-Targeted Products: Novel Cosmetics, Cosmeceuticals, and Pharmaceuticals
| Chitosan or Chitosan Composite | Type of Assay | Biological Effect | Experimental Conditions | Ref. |
|---|---|---|---|---|
| Chitin nanofibril-hyaluronan block copolymeric nanoparticles (CN-HA) | In vitro In vivo (healthy women) | Antiaging activity | Eye cream applied twice a day and serum: 2/3 drops twice a day, three times a week for 60 days | [94] |
| Chitin nanofibers and nanocrystals | In vitro | Skin protective effects | Nine tested conditions using nanofibers and nanocrystals at 4, 12, and 24 h post-application | [108] |
| Chitosan-alginate nanoparticles | In vitro | Antimicrobial and anti-inflammatory activity | 1%, 0.5%, 0.2%, and 0.1% of chitosan-alginate nanoparticle/4 h | [110] |
| Chitosan nanoparticles | In vitro | Antimicrobial activity | 0.5 and 1 mg/mL chitosan derivatives/24 h | [61] |
| Silver-nanoparticle-incorporated chitosan-based membranes | In vitro In vivo (rat) | Antibacterial efficacy and wound-healing ability | 12 mg and 60 mg silver nitrate/chitosan-based membranes for 7 and 28 days | [116] |
| Chitosan nanoparticle-containing dexamethasone sodium phosphate | In vitro | Anti-inflammatory activity | 4.19, 10.65, and 43.06% of dexamethasone/5 mg chitosan nanoparticles for 35 days | [100] |
| Phosphatidylcholine hyaluronic acid chitin–nanofibrils complex | In vivo (volunteer patients) | Antiaging activity | Single injection (1 mL solution with 10 µg/mL block-polymer) every 7 days for 10 weeks | [111] |
| Chitin nanofibril-hyaluronan nanoparticles (CN-HA) | In vitro and in vivo studies (women) | Antioxidant and anti-inflammatory activities | 2 mg/mL CN-HA nanoparticles for 60 days | [103] |
| Carvacrol and eugenol chitosan nanoparticles | In vitro | Antioxidant activity | 0.125 mg/mL to 1 mg/mL | [117] |
| Chitosan nanoparticles | In vitro | Drug delivery applications | 30% w/w (protein based on chitosan)/12 mL of phosphate buffer | [101] |
| Chitosan nanofibers | In vivo (mice) | Antileishmanial wound | 20 wt% nanofibers as wound dressings, daily for 30 days | [104] |
| Chitin nanofibrils | In vivo (rat) | Wound healing | 2 g/L chitin nanofibril for 15 days | [109] |
| Chitin nanofibril-hyaluronan block copolymeric nanoparticles (CN-HA) | In vitro In vivo (healthy women) | Antiaging activity | Eye cream: applied two times a day; serum: 2/3 drops two times a day, three times a week (60 days) | [94] |
| Chitin nanofibers and nanocrystals | In vitro | Skin protective effects | Nine tested conditions using nanofibers and nanocrystals at 4, 12, and 24 h post-application | [108] |
| Chitosan-alginate nanoparticles | In vitro | Antimicrobial and anti-inflammatory activities | 1%, 0.5%, 0.2%, and 0.1% of chitosan-alginate nanoparticles/4 h | [110] |
| Chitosan nanoparticles | In vitro | Antimicrobial activity | 0.5 and 1 mg/mL chitosan derivatives/24 h | [61] |
| PVA/Chitosan hydrogel dressing loaded with PHMB | 1: In vitro 2: In vivo (dog) | 1: Growth inactivation of S. aureus and S. epidermidis 2: Faster wound recovery | 5% PVA/chitosan (1:1) + PHMD 8.12 µg/mg dry sample Daily topical application for 21 days | [118] |
| Chitosan dressing–loaded iturin-AgNPs (CS-AgNPs) | In vivo (mice) | Faster wound healing and reduced E. coli colonies | Wounds covered with 0.02 g/mL CS and iturin-AgNPs 10 μg/mL | [119] |
| 2 chitosan-dialdehyde cellulose (2CS-DC) composite foam sponge | In vivo (rabbit) | Reduced hemostasis time by 79.5% or 47.7% | Amputated tail covered with 2:1 CS-DAC 0.02 g Femoral vein excision covered with 2:1 CS-DAC 1.0 g | [120] |
| Oligo-chitosan (O-C) scaffold | Ex vivo (blood of (vWD) patients) | Increased release of PDGF and TGF-β1 by 29.8% and 23%, respectively; platelet activation, adhesion, and aggregation promotion | O-C 75–95% DDA applied to the blood | [121] |
| Chitosan/titanium dioxide (CS/TiO2) composite membrane | In vitro | Fibroblast proliferation; increased cytokine expression; S. aureus growth inactivation | CS/TiO2 membrane incorporated with 025% TiO2 | [122] |
| Chitosan-PVA soft membranes plasticized with glycerol | In vivo (rabbit) In vitro | Burn wound healing in second-degree burns; inhibition of P. aeruginosa, E. coli, and B. subtillis | Chitosan 80%, PVA 20%, and glycerol 2% | [123] |
| Chitosan mesh membrane wound dressing | Clinical trial (skin donor patients) | Faster wound healing with no scar formation on the 10th day | 1% chitosan mesh membrane covering the wound for two months | [124] |
| Chitosan sheet wound dressing | Clinical trial (skin donor patients) | Faster wound healing on the 11th day | No posology informed; wounds treated with chitosan sheets for six months | [125] |
| Heparin-chitosan membrane wound dressing | Clinical trial (skin donor patients) | Wound-healing acceleration on the 12th day | Heparin 7% in 1% chitosan membrane covering the wound with 15-day follow up | [126] |
| Chitosan-capped silver nanoparticles | In vivo (rat) | Burn wound healing | Ch/AgNPs to 50 mg/wound of the 1% silver sulfadiazine for 28 days | [70] |
| Silver chitosan nanocomposites | In vitro In vivo (mice) | Antifungal | Nanocomposites: 0.06 to 16 μg/mL 3 to 5 μg/mL of nanocomposites for 4 days | [59] |
| Chitosan-gentamicin (CS-GS) film | In vitro | Antibacterial—S. aureus and E. coli | CS-GS films immersed in PBS for 1, 3, 5, and 7 days were covered with 0.5 mL Log-phase bacteria suspension for 24 h | [65] |
| Chitosan/glycosaminoglycan scaffolds-Ag Nanoparticles | In vitro | Antimicrobial—S. aureus and E. coli Human fibroblast proliferation | Scaffold portion with Ag 385 μg/mL added to bacteria suspension for 24 h; 0.36 cm2 scaffold portions added to fibroblast culture for 3 and 6 days | [67] |
| Silver nanoparticles/chitosan oligosaccharide/PVA nanofibers | In vivo (rat) | Wound healing | 5% nanofibers for 18 days | [69] |
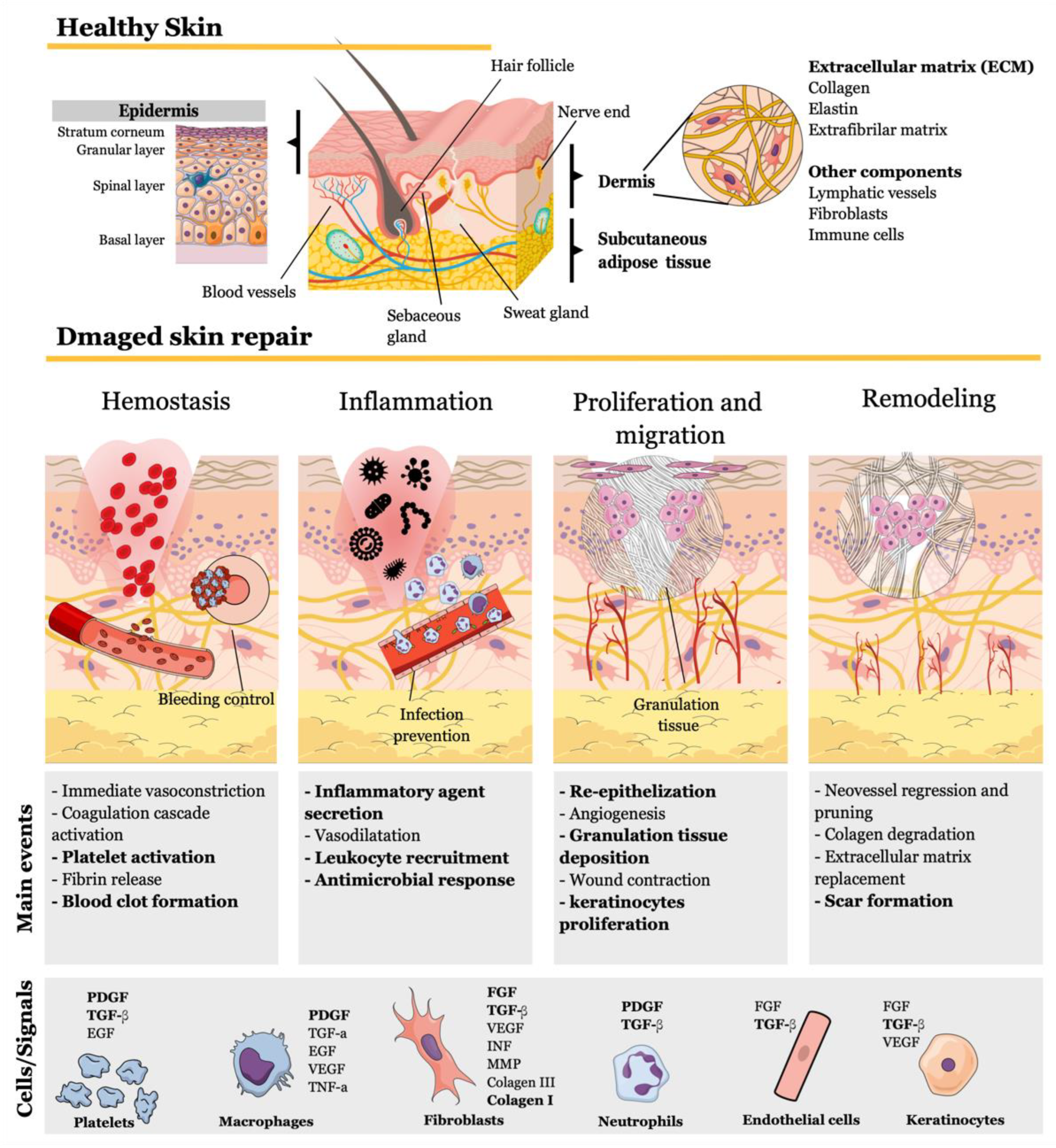
6. Molecular and Cellular Skin Repair Mechanisms of Chitosan-Based Wound Dressings
7. Conclusions
8. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Christy, P.N.; Basha, S.K.; Kumari, V.S.; Bashir, A.; Maaza, M.; Kaviyarasu, K.; Arasu, M.V.; Al-Dhabi, N.A.; Ignacimuthu, S. Biopolymeric nanocomposite scaffolds for bone tissue engineering—A review. J. Drug Deliv. Sci. Technol. 2020, 55, 101452. [Google Scholar] [CrossRef]
- Kargozar, S.; Ramakrishna, S.; Mozafari, M. Chemistry of biomaterials: Future prospects. Curr. Opin. Biomed. Eng. 2019, 10, 181–190. [Google Scholar] [CrossRef]
- George, A.; Sanjay, M.R.; Srisuk, R.; Parameswaranpillai, J.; Siengchin, S. A comprehensive review on chemical properties and applications of biopolymers and their composites. Int. J. Biol. Macromol. 2020, 154, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Sahana, T.G.; Rekha, P.D. Biopolymers: Applications in wound healing and skin tissue engineering. Mol. Biol. Rep. 2018, 45, 2857–2867. [Google Scholar] [CrossRef] [PubMed]
- Mohan, K.; Ganesan, A.R.; Muralisankar, T.; Jayakumar, R.; Sathishkumar, P.; Uthayakumar, V.; Chandirasekar, R.; Revathi, N. Recent insights into the extraction, characterization, and bioactivities of chitin and chitosan from insects. Trends Food Sci. Technol. 2020, 105, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [Green Version]
- Ju, S.; Zhang, F.; Duan, J.; Jiang, J. Characterization of bacterial cellulose composite films incorporated with bulk chitosan and chitosan nanoparticles: A comparative study. Carbohydr. Polym. 2020, 237, 116167. [Google Scholar] [CrossRef]
- Shen, L.; Worrell, E.; Patel, M. Present and future development in plastics from biomass. Biofuels Bioprod. Biorefin. 2010, 4, 25–40. [Google Scholar] [CrossRef]
- Jha, A.; Kumar, A. Biobased technologies for the efficient extraction of biopolymers from waste biomass. Bioprocess. Biosyst. Eng. 2019, 42, 1893–1901. [Google Scholar] [CrossRef]
- Braconnot, H. Sur la nature des champibnons. Ann. Chi. 1881, 79, 265–304. [Google Scholar]
- Shintani, T. Food Industrial Production of Monosaccharides Using Microbial, Enzymatic, and Chemical Methods. Fermentation 2019, 5, 47. [Google Scholar] [CrossRef] [Green Version]
- Yen, M.-T.; Tseng, Y.-H.; Li, R.-C.; Mau, J.-L. Antioxidant properties of fungal chitosan from shiitake stipes. LWT 2007, 40, 255–261. [Google Scholar] [CrossRef]
- Liu, X.-Q.; Zhao, X.-X.; Liu, Y.; Zhang, T.-A. Review on preparation and adsorption properties of chitosan and chitosan composites. Polym. Bull. 2022, 79, 2633–2665. [Google Scholar] [CrossRef]
- Gomes, L.P.; Paschoalin, V.; Del Aguila, E.M. Chitosan Nanoparticles: Production, Physicochemical Characteristics and Nutraceutical Applications. Rev. Virtual Quim. 2017, 9, 387–409. [Google Scholar] [CrossRef]
- Bissett, D.L. Glucosamine: An ingredient with skin and other benefits. J. Cosmet. Dermatol. 2006, 5, 309–315. [Google Scholar] [CrossRef]
- Lichtenthaler, F.W.; Peters, S. Carbohydrates as green raw materials for the chemical industry. Comptes Rendus. Chim. 2004, 7, 65–90. [Google Scholar] [CrossRef]
- BeMiller, J.N. Carbohydrate Chemistry for Food Scientists; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Yadav, M.; Goswami, P.; Paritosh, K.; Kumar, M.; Pareek, N.; Vivekanand, V. Seafood waste: A source for preparation of commercially employable chitin/chitosan materials. Bioresour. Bioprocess. 2019, 6, 8. [Google Scholar] [CrossRef]
- Gomes, L.P.; Andrade, C.T.; Silva, J.T.; Del Aguila, E.M.; Paschoalin, V.M.F. Green Synthesis and Physical and Chemical Characterization of Chitosans with a High Degree of Deacetylation, Produced by a Binary Enzyme System. J. Life Sci. 2014, 8, 276–282. [Google Scholar]
- Ifuku, S.; Saimoto, H. Chitin nanofibers: Preparations, modifications, and applications. Nanoscale 2012, 4, 3308–3318. [Google Scholar] [CrossRef]
- Gomes, L.P.; Souza, H.K.S.; Campiña, J.M.; Andrade, C.T.; Paschoalin, V.M.F.; Silva, A.F.; Gonçalves, M.P. Tweaking the mechanical and structural properties of colloidal chitosans by sonication. Food Hydrocoll. 2016, 56, 29–40. [Google Scholar] [CrossRef]
- EL Knidri, H.; Dahmani, J.; Addaou, A.; Laajeb, A.; Lahsini, A. Rapid and efficient extraction of chitin and chitosan for scale-up production: Effect of process parameters on deacetylation degree and molecular weight. Int. J. Biol. Macromol. 2019, 139, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Furuike, T.; Nair, S.V.; Jayakumar, R. Biomedical applications of chitin hydrogel membranes and scaffolds. Carbohydr. Polym. 2011, 84, 820–824. [Google Scholar] [CrossRef]
- Madhumathi, K.; Binulal, N.S.; Nagahama, H.; Tamura, H.; Shalumon, K.T.; Selvamurugan, N.; Nair, S.V.; Jayakumar, R. Preparation and characterization of novel β-chitin–hydroxyapatite composite membranes for tissue engineering applications. Int. J. Biol. Macromol. 2009, 44, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Reis, R.L.; Mano, J.F. Phosphorous Containing Chitosan Beads for Controlled Oral Drug Delivery. J. Bioact. Compat. Polym. 2006, 21, 327–340. [Google Scholar] [CrossRef] [Green Version]
- Omanović-Mikličanin, E.; Badnjević, A.; Kazlagić, A.; Hajlovac, M. Nanocomposites: A brief review. Health Technol. 2020, 10, 51–59. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Brasuel, M.; Wise, K. The current state of engineered nanomaterials in consumer goods and waste streams: The need to develop nanoproperty-quantifiable sensors for monitoring engineered nanomaterials. Nanotechnol. Sci. Appl. 2011, 4, 73–86. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Shan, J.; Zhang, W.; Su, S.; Yuwen, L.; Wang, L. Recent Advances in Synthesis and Biomedical Applications of Two-Dimensional Transition Metal Dichalcogenide Nanosheets. Small 2017, 13, 1602660. [Google Scholar] [CrossRef]
- Mushi, N.E. A review on native well-preserved chitin nanofibrils for materials of high mechanical performance. Int. J. Biol. Macromol. 2021, 178, 591–606. [Google Scholar] [CrossRef]
- Kurita, K. Controlled functionalization of the polysaccharide chitin. Prog. Polym. Sci. 2001, 26, 1921–1971. [Google Scholar] [CrossRef]
- Del Aguila, E.M.; Gomes, L.P.; Andrade, C.T.; Silva, J.T.; Paschoalin, V.M.F. Biocatalytic production of chitosan polymers from shrimp shells, using a recombinant enzyme produced by pichia pastoris. Am. J. Mol. Biol. 2012, 2, 341–350. [Google Scholar] [CrossRef] [Green Version]
- Gopal, J.; Muthu, M.; Dhakshanamurthy, T.; Kim, K.J.; Hasan, N.; Kwon, S.J.; Chun, S. Sustainable ecofriendly phytoextract mediated one pot green recovery of chitosan. Sci. Rep. 2019, 9, 1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franca, E.F.; Freitas, L.C.G.; Lins, R.D. Chitosan molecular structure as a function of N-acetylation. Biopolymers 2011, 95, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Bukzem, A.L.; Signini, R.; dos Santos, D.M.; Lião, L.M.; Ascheri, D.P.R. Optimization of carboxymethyl chitosan synthesis using response surface methodology and desirability function. Int. J. Biol. Macromol. 2016, 85, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, L.; Singh, J.; Agarwal, V.; Tewari, R.P. Biomedical applications of carboxymethyl chitosans. Carbohydr. Polym. 2013, 91, 452–466. [Google Scholar] [CrossRef]
- Elsoud, M.M.A.; El Kady, E.M. Current trends in fungal biosynthesis of chitin and chitosan. Bull. Natl. Res. Cent. 2019, 43, 59. [Google Scholar] [CrossRef] [Green Version]
- Gomes, L.P.; Anjo, S.I.; Manadas, B.; Coelho, A.V.; Paschoalin, V.M.F. Proteomic Analyses Reveal New Insights on the Antimicrobial Mechanisms of Chitosan Biopolymers and Their Nanosized Particles against Escherichia coli. Int. J. Mol. Sci. 2020, 21, 225. [Google Scholar] [CrossRef] [Green Version]
- Ngo, D.-H.; Kim, S.-K. Chapter Two–Antioxidant Effects of Chitin, Chitosan, and Their Derivatives. Adv. Food Nutr. Res. 2014, 73, 15–31. [Google Scholar]
- Arancibia, M.Y.; Alemán, A.; Calvo, M.M.; López-Caballero, M.E.; Montero, P.; Gómez-Guillén, M.C. Antimicrobial and antioxidant chitosan solutions enriched with active shrimp (Litopenaeus vannamei) waste materials. Food Hydrocoll. 2014, 35, 710–717. [Google Scholar] [CrossRef] [Green Version]
- Yen, M.-T.; Yang, J.-H.; Mau, J.-L. Antioxidant properties of chitosan from crab shells. Carbohydr. Polym. 2008, 74, 840–844. [Google Scholar] [CrossRef]
- Kerch, G. The Potential of Chitosan and Its Derivatives in Prevention and Treatment of Age-Related Diseases. Mar. Drugs 2015, 13, 2158–2182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, X.; Zhou, J.; Zhao, C.-N.; Gan, R.-Y.; Li, H.-B. Health Benefits and Molecular Mechanisms of Resveratrol: A Narrative Review. Foods 2020, 9, 340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fogacci, F.; Tocci, G.; Presta, V.; Fratter, A.; Borghi, C.; Cicero, A.F.G. Effect of resveratrol on blood pressure: A systematic review and meta-analysis of randomized, controlled, clinical trials. Crit. Rev. Food Sci. Nutr. 2019, 59, 1605–1618. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, D.C.F.; Rangel, L.P.; Martins-Dinis, M.M.D.D.C.; Ferretti, G.D.D.S.; Ferreira, V.F.; Silva, J.L. Anticancer Potential of Resveratrol, β-Lapachone and Their Analogues. Molecules 2020, 25, 893. [Google Scholar] [CrossRef] [Green Version]
- Silva, J.; Costa, D.; Campos, N.; Costa, C.; Rangel, L. Understanding the p53 Aggregation Process through its Activators Resveratrol and PRIMA. FASEB J. 2015, 29, LB151. [Google Scholar] [CrossRef]
- Filardo, S.; Di Pietro, M.; Mastromarino, P.; Sessa, R. Therapeutic potential of resveratrol against emerging respiratory viral infections. Pharmacol. Ther. 2020, 214, 107613. [Google Scholar] [CrossRef]
- Ratz-Łyko, A.; Arct, J. Resveratrol as an active ingredient for cosmetic and dermatological applications: A review. J. Cosmet. Laser Ther. 2019, 21, 84–90. [Google Scholar] [CrossRef]
- Pastor, C.; Sánchez-González, L.; Chiralt, A.; Cháfer, M.; González-Martínez, C. Physical and antioxidant properties of chitosan and methylcellulose based films containing resveratrol. Food Hydrocoll. 2013, 30, 272–280. [Google Scholar] [CrossRef] [Green Version]
- Zu, Y.; Zhang, Y.; Wang, W.; Zhao, X.; Han, X.; Wang, K.; Ge, Y. Preparation and in vitro/in vivo evaluation of resveratrol-loaded carboxymethyl chitosan nanoparticles. Drug Deliv. 2016, 23, 971–981. [Google Scholar] [CrossRef]
- Frémont, L. Biological effects of resveratrol. Life Sci. 2000, 66, 663–673. [Google Scholar] [CrossRef]
- Cho, A.R.; Chun, Y.G.; Kim, B.-K.; Park, D.J. Preparation of Chitosan-TPP Microspheres as Resveratrol Carriers. J. Food Sci. 2014, 79, E568–E576. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Tan, W.; Wang, G.; Li, Q.; Dong, F.; Guo, Z. The antioxidant and antifungal activity of chitosan derivatives bearing Schiff bases and quaternary ammonium salts. Carbohydr. Polym. 2019, 226, 115256. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.F.; Facchi, S.P.; Follmann, H.D.M.; Pereira, A.G.B.; Rubira, A.F.; Muniz, E.C. Antimicrobial Activity of Chitosan Derivatives Containing N-Quaternized Moieties in Its Backbone: A Review. Int. J. Mol. Sci. 2014, 15, 20800–20832. [Google Scholar] [CrossRef] [PubMed]
- Ying, G.-Q.; Xiong, W.-Y.; Wang, H.; Sun, Y.; Liu, H.-Z. Preparation, water solubility and antioxidant activity of branched-chain chitosan derivatives. Carbohydr. Polym. 2011, 83, 1787–1796. [Google Scholar] [CrossRef]
- Omidi, S.; Kakanejadifard, A. Modification of chitosan and chitosan nanoparticle by long chain pyridinium compounds: Synthesis, characterization, antibacterial, and antioxidant activities. Carbohydr. Polym. 2019, 208, 477–485. [Google Scholar] [CrossRef]
- Duan, S.; Wang, R. Bimetallic nanostructures with magnetic and noble metals and their physicochemical applications. Prog. Nat. Sci. 2013, 23, 113–126. [Google Scholar] [CrossRef] [Green Version]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. Rsc Adv. 2014, 4, 3974–3983. [Google Scholar] [CrossRef] [Green Version]
- Bonilla, J.J.A.; Honorato, L.; de Oliveira, D.F.C.; Gonçalves, R.A.; Guimarães, A.; Miranda, K.; Nimrichter, L. Silver chitosan nanocomposites as a potential treatment for superficial candidiasis. Med. Mycol. 2021, 59, 993–1005. [Google Scholar] [CrossRef]
- Ganan, M.; Carrascosa, A.V.; Martínez-Rodríguez, A.J. Antimicrobial Activity of Chitosan against Campylobacter spp. and Other Microorganisms and Its Mechanism of Action. J. Food Prot. 2009, 72, 1735–1738. [Google Scholar] [CrossRef]
- Gomes, L.P.; Andrade, C.T.; Del Aguila, E.M.; Alexander, C.; Paschoalin, V. Assessing the antimicrobial activity of chitosan nanoparticles by fluorescence-labeling. Int. J. Biotechnol. Bioeng. 2018, 12, 111–117. [Google Scholar]
- Tan, H.; Ma, R.; Lin, C.; Liu, Z.; Tang, T. Quaternized Chitosan as an Antimicrobial Agent: Antimicrobial Activity, Mechanism of Action and Biomedical Applications in Orthopedics. Int. J. Mol. Sci. 2013, 14, 1854–1869. [Google Scholar] [CrossRef]
- Xing, Y.; Xu, Q.; Li, X.; Chen, C.; Ma, L.; Li, S.; Che, Z.; Lin, H. Chitosan-Based Coating with Antimicrobial Agents: Preparation, Property, Mechanism, and Application Effectiveness on Fruits and Vegetables. Int. J. Polym. Sci. 2016, 2016, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Ly, N.T.K.; Shin, H.; Gupta, K.C.; Kang, I.K.; Yu, W. Bioactive Antibacterial Modification of Orthodontic Microimplants Using Chitosan Biopolymer. Macromol. Res. 2019, 27, 504–510. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, H.; Qin, Y.; Deng, L.; Wang, Y. Modification of chitosan film via surface grafting of gentamicin molecular to improve the antibacterial property. J. Control. Release 2017, 259, e166. [Google Scholar] [CrossRef]
- Dananjaya, S.; Kulatunga, D.; Godahewa, G.; Nikapitiya, C.; Oh, C.; Edussuriya, M.; Lee, J.; De Zoysa, M. Preparation, characterization, and antimicrobial properties of chitosan–silver nanocomposites films against fish pathogenic bacteria and fungi. Indian J. Microbiol. 2017, 57, 427–437. [Google Scholar] [CrossRef]
- Sandri, G.; Miele, D.; Faccendini, A.; Bonferoni, M.C.; Rossi, S.; Grisoli, P.; Taglietti, A.; Ruggeri, M.; Bruni, G.; Vigani, B.; et al. Chitosan/Glycosaminoglycan Scaffolds: The Role of Silver Nanoparticles to Control Microbial Infections in Wound Healing. Polymers 2019, 11, 1207. [Google Scholar] [CrossRef] [Green Version]
- Mei, M.L.; Li, Q.; Chu, C.H.; Yiu, C.K.; Lo, E.C. The inhibitory effects of silver diamine fluoride at different concentrations on matrix metalloproteinases. Dent. Mater. 2012, 28, 903–908. [Google Scholar] [CrossRef]
- Li, C.-W.; Wang, Q.; Li, J.; Hu, M.; Shi, S.-J.; Li, Z.-W.; Wu, G.-L.; Cui, H.-H.; Li, Y.-Y.; Zhang, Q. Silver nanoparticles/chitosan oligosaccharide/poly (vinyl alcohol) nanofiber promotes wound healing by activating TGFβ1/Smad signaling pathway. Int. J. Nanomed. 2016, 11, 373. [Google Scholar]
- Oryan, A.; Alemzadeh, E.; Tashkhourian, J.; Ana, S.F.N. Topical delivery of chitosan-capped silver nanoparticles speeds up healing in burn wounds: A preclinical study. Carbohydr. Polym. 2018, 200, 82–92. [Google Scholar] [CrossRef]
- Kim, E.-K.; Je, J.-Y.; Lee, S.-J.; Kim, Y.-S.; Hwang, J.-W.; Sung, S.-H.; Moon, S.-H.; Jeon, B.-T.; Kim, S.-K.; Jeon, Y.-J.; et al. Chitooligosaccharides induce apoptosis in human myeloid leukemia HL-60 cells. Bioorg. Med. Chem. Lett. 2012, 22, 6136–6138. [Google Scholar] [CrossRef]
- Gibot, L.; Chabaud, S.; Bouhout, S.; Bolduc, S.; Auger, F.A.; Moulin, V.J. Anticancer properties of chitosan on human melanoma are cell line dependent. Int. J. Biol. Macromol. 2015, 72, 370–379. [Google Scholar] [CrossRef]
- Kaya, M.; Akyuz, B.; Bulut, E.; Sargin, I.; Tan, G.; Erdonmez, D.; Maheta, M.; Satkauskas, S.; Mickevičius, S. DNA interaction, antitumor and antimicrobial activities of three-dimensional chitosan ring produced from the body segments of a diplopod. Carbohydr. Polym. 2016, 146, 80–89. [Google Scholar] [CrossRef]
- Subhapradha, N.; Shanmugam, A. Fabrication of β-chitosan nanoparticles and its anticancer potential against human hepatoma cells. Int. J. Biol. Macromol. 2017, 94, 194–201. [Google Scholar] [CrossRef]
- Salah, R.; Michaud, P.; Mati, F.; Harrat, Z.; Lounici, H.; Abdi, N.; Drouiche, N.; Mameri, N. Anticancer activity of chemically prepared shrimp low molecular weight chitin evaluation with the human monocyte leukaemia cell line, THP-1. Int. J. Biol. Macromol. 2013, 52, 333–339. [Google Scholar] [CrossRef]
- Schneible, J.D.; Singhal, A.; Lilova, R.L.; Hall, C.K.; Grafmüller, A.; Menegatti, S. Tailoring the Chemical Modification of Chitosan Hydrogels to Fine-Tune the Release of a Synergistic Combination of Chemotherapeutics. Biomacromolecules 2019, 20, 3126–3141. [Google Scholar] [CrossRef]
- Oliveira, M.Z.F.d.S.; Fernandes, T.S.M.; Carvalho, T.V. Síntese e caracterização de beads de quitosana comercial reticulados com glutaraldeído. Matéria 2021, 26. [Google Scholar] [CrossRef]
- Sun, M.; Yuan, L.; Yang, X.; Shao, L. Preparation and Modification of Chitosan-Based Membrane. ES Mater. Manuf. 2020, 9, 40–47. [Google Scholar] [CrossRef]
- Sivanesan, I.; Muthu, M.; Gopal, J.; Hasan, N.; Ali, S.K.; Shin, J.; Oh, J.-W. Nanochitosan: Commemorating the Metamorphosis of an ExoSkeletal Waste to a Versatile Nutraceutical. Nanomaterials 2021, 11, 821. [Google Scholar] [CrossRef]
- Kadhum, W.N.; Zaidan, I.A. The Synergistic Effects of Chitosan-Alginate Nanoparticles Loaded with Doxycycline Antibiotic Against Multidrug Resistant Proteus Mirabilis, Escherichia coli and Enterococcus Faecalis. Iraqi J. Sci. 2020, 61, 3187–3199. [Google Scholar]
- Sorasitthiyanukarn, F.N.; Muangnoi, C.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan-alginate nanoparticles as effective oral carriers to improve the stability, bioavailability, and cytotoxicity of curcumin diethyl disuccinate. Carbohydr. Polym. 2021, 256, 117426. [Google Scholar] [CrossRef]
- Ferreira, V.F.; Nicoletti, C.D.; Ferreira, P.G.; Futuro, D.O.; Silva, F.D.C.D. Strategies for Increasing the Solubility and Bioavailability of Anticancer Compounds: β-Lapachone and Other Naphthoquinones. Curr. Pharm. Des. 2016, 22, 5899–5914. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hadisi, Z.; Ismail, A.F.; Aziz, M.; Akbari, M.; Berto, F.; Chen, X.B. In vitro and in vivo evaluation of chitosan-alginate/gentamicin wound dressing nanofibrous with high antibacterial performance. Polym. Test. 2020, 82, 106298. [Google Scholar] [CrossRef]
- Thai, H.; Nguyen, C.T.; Thach, L.T.; Tran, M.T.; Mai, H.D.; Nguyen, T.T.T.; Le, G.D.; Van Can, M.; Tran, L.D.; Bach, G.L.; et al. Characterization of chitosan/alginate/lovastatin nanoparticles and investigation of their toxic effects in vitro and in vivo. Sci. Rep. 2020, 10, 909–915. [Google Scholar] [CrossRef]
- Zamora-Mora, V.; Velasco, D.; Hernandez, R.; Mijangos, C.; Kumacheva, E. Chitosan/agarose hydrogels: Cooperative properties and microfluidic preparation. Carbohydr. Polym. 2014, 111, 348–355. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Hong, P.; Liao, M.; Kong, S.; Huang, N.; Ou, C.; Li, S. Preparation and Characterization of Chitosan—Agarose Composite Films. Materials 2016, 9, 816. [Google Scholar] [CrossRef] [Green Version]
- Garakani, S.S.; Khanmohammadi, M.; Atoufi, Z.; Kamrava, S.K.; Setayeshmehr, M.; Alizadeh, R.; Faghihi, F.; Bagher, Z.; Davachi, S.M.; Abbaspourrad, A. Fabrication of chitosan/agarose scaffolds containing extracellular matrix for tissue engineering applications. Int. J. Biol. Macromol. 2020, 143, 533–545. [Google Scholar] [CrossRef]
- Sionkowska, A.; Wisniewski, M.; Skopinska, J.; Kennedy, C.J.; Wess, T.J. Molecular interactions in collagen and chitosan blends. Biomaterials 2004, 25, 795–801. [Google Scholar] [CrossRef]
- Irastorza, A.; Zarandona, I.; Andonegi, M.; Guerrero, P.; de la Caba, K. The versatility of collagen and chitosan: From food to biomedical applications. Food Hydrocoll. 2021, 116, 106633. [Google Scholar] [CrossRef]
- Suo, H.; Zhang, J.; Xu, M.; Wang, L. Low-temperature 3D printing of collagen and chitosan composite for tissue engineering. Mater. Sci. Eng. C 2021, 123, 111963. [Google Scholar] [CrossRef]
- Socrates, R.; Prymak, O.; Loza, K.; Sakthivel, N.; Rajaram, A.; Epple, M.; Kalkura, S.N. Biomimetic fabrication of mineralized composite films of nanosilver loaded native fibrillar collagen and chitosan. Mater. Sci. Eng. C 2019, 99, 357–366. [Google Scholar] [CrossRef]
- Sionkowska, A.; Kaczmarek, B.; Lewandowska, K. Modification of collagen and chitosan mixtures by the addition of tannic acid. J. Mol. Liq. 2014, 199, 318–323. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Tan, F.-J.; Marra, K.G.; Jan, S.-S.; Liu, D.-C. Synthesis and characterization of collagen/hyaluronan/chitosan composite sponges for potential biomedical applications. Acta Biomater. 2009, 5, 2591–2600. [Google Scholar] [CrossRef]
- Morganti, P.; Palombo, M.; Fabrizi, G.; Guarneri, F.; Svolacchia, F.; Cardillo, A.; Del Ciotto, P.; Francesco, C.; Gianluca, M. New Insights on Anti-Aging Activity of Chitin Nanofibril-Hyaluronan Block Copolymers Entrapping Active Ingredients: In Vitro and In Vivo Study. J. Appl. Cosmetol. 2013, 31, 1–29. [Google Scholar]
- Morganti, P.; Febo, P.; Cardillo, M.R.; Donnarumma, G.; Baroni, A. Chitin Nanofibril and Nanolignin: Natural Polymers of Biomedical Interest. J. Clin. Cosmet. Dermatol. 2017, 1. [Google Scholar] [CrossRef]
- Triunfo, M.; Tafi, E.; Guarnieri, A.; Scieuzo, C.; Hahn, T.; Zibek, S.; Salvia, R.; Falabella, P. Insect Chitin-Based Nanomaterials for Innovative Cosmetics and Cosmeceuticals. Cosmetics 2021, 8, 40. [Google Scholar] [CrossRef]
- Fytianos, G.; Rahdar, A.; Kyzas, G.Z. Nanomaterials in Cosmetics: Recent Updates. Nanomaterials 2020, 10, 979. [Google Scholar] [CrossRef]
- Morganti, P.; Morganti, G.; Coltelli, M.-B. Smart and Sustainable Hair Products Based on Chitin-Derived Compounds. Cosmetics 2021, 8, 20. [Google Scholar] [CrossRef]
- Ahmad, S.I.; Ahmad, R.; Khan, M.S.; Kant, R.; Shahid, S.; Gautam, L.; Hasan, G.M.; Hassan, I. Chitin and its derivatives: Structural properties and biomedical applications. Int. J. Biol. Macromol. 2020, 164, 526–539. [Google Scholar] [CrossRef]
- Behl, G.; Iqbal, J.; O’Reilly, N.J.; McLoughlin, P.; Fitzhenry, L. Synthesis and Characterization of Poly(2-hydroxyethylmethacrylate) Contact Lenses Containing Chitosan Nanoparticles as an Ocular Delivery System for Dexamethasone Sodium Phosphate. Pharm. Res. 2016, 33, 1638–1648. [Google Scholar] [CrossRef]
- Grenha, A.; Gomes, M.E.; Rodrigues, M.; Santo, V.E.; Mano, J.F.; Neves, N.M.; Reis, R.L. Development of new chitosan/carrageenan nanoparticles for drug delivery applications. J. Biomed. Mater. Res. A 2010, 92, 1265–1272. [Google Scholar] [CrossRef] [Green Version]
- Casadidio, C.; Peregrina, D.V.; Gigliobianco, M.R.; Deng, S.; Censi, R.; Di Martino, P. Chitin and Chitosans: Characteristics, Eco-Friendly Processes, and Applications in Cosmetic Science. Mar. Drugs 2019, 17, 369. [Google Scholar] [CrossRef] [Green Version]
- Morganti, P.; Palombo, M.; Tishchenko, G.; Yudin, V.E.; Guarneri, F.; Cardillo, M.; Del Ciotto, P.; Carezzi, F.; Morganti, G.; Fabrizi, G. Chitin-Hyaluronan Nanoparticles: A Multifunctional Carrier to Deliver Anti-Aging Active Ingredients through the Skin. Cosmetics 2014, 1, 140–158. [Google Scholar] [CrossRef] [Green Version]
- Tabaei, S.J.S.; Rahimi, M.; Akbaribazm, M.; Ziai, S.A.; Sadri, M.; Shahrokhi, S.R.; Rezaei, M.S. Chitosan-based nano-scaffolds as antileishmanial wound dressing in BALB/c mice treatment: Characterization and design of tissue regeneration. Iran. J. Basic Med. Sci. 2020, 23, 788–799. [Google Scholar] [CrossRef]
- Goossens, A. Contact-Allergic Reactions to Cosmetics. J. Allergy 2011, 2011, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Morganti, P.; Morganti, G. Chitin nanofibrils for advanced cosmeceuticals. Clin. Dermatol. 2008, 26, 334–340. [Google Scholar] [CrossRef]
- Ishida, K.; Yokota, S.; Kondo, T. Emulsifying Properties of α-Chitin Nanofibrils Prepared by Aqueous Counter Collision. J. Fiber Sci. Technol. 2021, 77, 203–212. [Google Scholar] [CrossRef]
- Ito, I.; Osaki, T.; Ifuku, S.; Saimoto, H.; Takamori, Y.; Kurozumi, S.; Imagawa, T.; Azuma, K.; Tsuka, T.; Okamoto, Y.; et al. Evaluation of the effects of chitin nanofibrils on skin function using skin models. Carbohydr. Polym. 2014, 101, 464–470. [Google Scholar] [CrossRef]
- Mattioli-Belmonte, M.; Zizzi, A.; Lucarini, G.; Giantomassi, F.; Biagini, G.; Tucci, G.; Orlando, F.; Provinciali, M.; Carezzi, F.; Morganti, P. Chitin Nanofibrils Linked to Chitosan Glycolate as Spray, Gel, and Gauze Preparations for Wound Repair. J. Bioact. Compat. Polym. 2007, 22, 525–538. [Google Scholar] [CrossRef]
- Friedman, A.; Phan, J.; Schairer, D.O.; Champer, J.; Qin, M.; Pirouz, A.; Blecher-Paz, K.; Oren, A.; Liu, P.T.; Modlin, R.; et al. Antimicrobial and Anti-Inflammatory Activity of Chitosan–Alginate Nanoparticles: A Targeted Therapy for Cutaneous Pathogens. J. Investig. Dermatol. 2013, 133, 1231–1239. [Google Scholar] [CrossRef] [Green Version]
- Morganti, P.; Guevara, L.; Palombo, P.; Cardillo, A.; Mezzana, P.; Fabrizi, G.; Svolacchia, F. A phosphatidylcholine hyaluronic acid chitin–nanofibrils complex for a fast skin remodeling and a rejuvenating look. Clin. Cosmet. Investig. Dermatol. 2012, 5, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Morganti, P.; Carezzi, F.; Del Ciotto, P.; Tishchenko, G.; Chianese, A.; Yudin, V.E. A Green Multifunctional Polymer from Discarded Material: Chitin Nanofibrils. Br. J. Appl. Sci. Technol. 2014, 4, 4175–4190. [Google Scholar] [CrossRef]
- Chang, J.; Liu, W.; Han, B.; Peng, S.; He, B.; Gu, Z. Investigation of the skin repair and healing mechanism of N-carboxymethyl chitosan in second-degree burn wounds. Wound Repair Regen. 2012, 21, 113–121. [Google Scholar] [CrossRef]
- Morganti, P.; Coltelli, M.-B. A New Carrier for Advanced Cosmeceuticals. Cosmetics 2019, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- Morganti, P.; Morganti, G.; Coltelli, M.B. Chitin Nanomaterials and Nanocomposites for Tissue Repair. In Marine-Derived Biomaterials for Tissue Engineering Applications; Choi, A.H., Ben-Nissan, B., Eds.; Springer: Singapore, 2019; pp. 523–544. [Google Scholar] [CrossRef]
- Shao, J.; Wang, B.; Li, J.; Jansen, J.A.; Walboomers, X.F.; Yang, F. Antibacterial effect and wound healing ability of silver nanoparticles incorporation into chitosan-based nanofibrous membranes. Mater. Sci. Eng. C 2019, 98, 1053–1063. [Google Scholar] [CrossRef]
- Mondéjar-López, M.; López-Jimenez, A.J.; Martínez, J.C.G.; Ahrazem, O.; Gómez-Gómez, L.; Niza, E. Comparative evaluation of carvacrol and eugenol chitosan nanoparticles as eco-friendly preservative agents in cosmetics. Int. J. Biol. Macromol. 2022, 206, 288–297. [Google Scholar] [CrossRef]
- Massarelli, E.; Silva, D.; Pimenta, A.; Fernandes, A.; Mata, J.; Armês, H.; Salema-Oom, M.; Saramago, B.; Serro, A. Polyvinyl alcohol/chitosan wound dressings loaded with antiseptics. Int. J. Pharm. 2021, 593, 120110. [Google Scholar] [CrossRef]
- Zhou, L.; Zhao, X.; Li, M.; Yan, L.; Lu, Y.; Jiang, C.; Liu, Y.; Pan, Z.; Shi, J. Antibacterial and wound healing–promoting effect of sponge-like chitosan-loaded silver nanoparticles biosynthesized by iturin. Int. J. Biol. Macromol. 2021, 181, 1183–1195. [Google Scholar] [CrossRef]
- Wei, X.; Ding, S.; Liu, S.; Yang, K.; Cai, J.; Li, F.; Wang, C.; Lin, S.; Tian, F. Polysaccharides-modified chitosan as improved and rapid hemostasis foam sponges. Carbohydr. Polym. 2021, 264, 118028. [Google Scholar] [CrossRef]
- Periayah, M.H.; Halim, A.S.; Saad, A.Z.M.; Yaacob, N.S.; Hussein, A.R.; Karim, F.A.; Rashid, A.H.A.; Ujang, Z. Chitosan scaffold enhances growth factor release in wound healing in von Willebrand disease. Int. J. Clin. Exp. Med. 2015, 8, 15611–15620. [Google Scholar]
- Behera, S.S.; Das, U.; Kumar, A.; Bissoyi, A.; Singh, A.K. Chitosan/TiO2 composite membrane improves proliferation and survival of L929 fibroblast cells: Application in wound dressing and skin regeneration. Int. J. Biol. Macromol. 2017, 98, 329–340. [Google Scholar] [CrossRef]
- Bano, I.; Arshad, M.; Yasin, T.; Ghauri, M.A. Preparation, characterization and evaluation of glycerol plasticized chitosan/PVA blends for burn wounds. Int. J. Biol. Macromol. 2019, 124, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.K.; Sermsintham, N.; Chandrkrachang, S.; Stevens, W.F. Chitosan membrane as a wound-healing dressing: Characterization and clinical application. J. Biomed. Mater. Res. B Appl. Biomater. 2004, 69, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Stone, C.A.; Wright, H.; Devaraj, V.S.; Clarke, T.; Powell, R. Healing at skin graft donor sites dressed with chitosan. Br. J. Plast. Surg. 2000, 53, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Kratz, G.; Back, M.; Arnander, C.; Larm, O. Immobilised heparin accelerates the healing of human wounds in vivo. Scand. J. Plast. Reconstr. Surg. Hand Surg. 1998, 32, 381–386. [Google Scholar] [CrossRef]
- Francesko, A.; Tzanov, T. Chitin, Chitosan and Derivatives for Wound Healing and Tissue Engineering. Adv. Biochem. Eng. Biotechnol. 2011, 125, 1–27. [Google Scholar] [CrossRef]
- Farhadihosseinabadi, B.; Zarebkohan, A.; Eftekhary, M.; Heiat, M.; Moghaddam, M.M.; Gholipourmalekabadi, M. Crosstalk between chitosan and cell signaling pathways. Cell. Mol. Life Sci. 2019, 76, 2697–2718. [Google Scholar] [CrossRef]
- Feng, P.; Luo, Y.; Ke, C.; Qiu, H.; Wang, W.; Zhu, Y.; Hou, R.; Xu, L.; Wu, S. Chitosan-Based Functional Materials for Skin Wound Repair: Mechanisms and Applications. Front. Bioeng. Biotechnol. 2021, 9, 650598. [Google Scholar] [CrossRef]
- Cañedo-Dorantes, L.; Cañedo-Ayala, M. Skin Acute Wound Healing: A Comprehensive Review. Int. J. Inflamm. 2019, 2019, 37063. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Zomer, H.D.; Trentin, A.G. Skin wound healing in humans and mice: Challenges in translational research. J. Dermatol. Sci. 2018, 90, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; DiPietro, L.A. Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.; Cullen, M.; Chambers, H.; Carroll, M.; Walker, J. Elements affecting wound healing time: An evidence based analysis. Wound Repair Regen. 2015, 23, 550–556. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, D.-Y.; Lu, S.-T.; Li, P.-W.; Li, S.-D. Chitosan-Based Composite Materials for Prospective Hemostatic Applications. Mar. Drugs 2018, 16, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patrulea, V.; Ostafe, V.; Borchard, G.; Jordan, O. Chitosan as a starting material for wound healing applications. Eur. J. Pharm. Biopharm. 2015, 97, 417–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lord, M.S.; Cheng, B.; McCarthy, S.J.; Jung, M.; Whitelock, J.M. The modulation of platelet adhesion and activation by chitosan through plasma and extracellular matrix proteins. Biomaterials 2011, 32, 6655–6662. [Google Scholar] [CrossRef]
- Wang, Q.Z.; Chen, X.G.; Li, Z.X.; Wang, S.; Liu, C.; Meng, X.H.; Liu, C.G.; Lv, Y.H.; Yu, L.J. Preparation and blood coagulation evaluation of chitosan microspheres. J. Mater. Sci. Mater. Med. 2007, 19, 1371–1377. [Google Scholar] [CrossRef]
- He, Q.; Gong, K.; Ao, Q.; Ma, T.; Yan, Y.; Gong, Y.; Zhang, X. Positive charge of chitosan retards blood coagulation on chitosan films. J. Biomater. Appl. 2013, 27, 1032–1045. [Google Scholar] [CrossRef]
- Howling, G.I.; Dettmar, P.W.; Goddard, P.A.; Hampson, F.C.; Dornish, M.; Wood, E.J. The effect of chitin and chitosan on the proliferation of human skin fibroblasts and keratinocytes in vitro. Biomaterials 2001, 22, 2959–2966. [Google Scholar] [CrossRef]
- Chatelet, C.; Damour, O.; Domard, A. Influence of the degree of acetylation on some biological properties of chitosan films. Biomaterials 2001, 22, 261–268. [Google Scholar] [CrossRef]
- Clinical Trials. Available online: https://www.clinicaltrials.gov/ct2/home (accessed on 27 March 2022).
- Shivakumar, P.; Gupta, M.S.; Jayakumar, R.; Gowda, D.V. Prospection of chitosan and its derivatives in wound healing: Proof of patent analysis (2010–2020). Int. J. Biol. Macromol. 2021, 184, 701–712. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, P.G.; Ferreira, V.F.; da Silva, F.d.C.; Freitas, C.S.; Pereira, P.R.; Paschoalin, V.M.F. Chitosans and Nanochitosans: Recent Advances in Skin Protection, Regeneration, and Repair. Pharmaceutics 2022, 14, 1307. https://doi.org/10.3390/pharmaceutics14061307
Ferreira PG, Ferreira VF, da Silva FdC, Freitas CS, Pereira PR, Paschoalin VMF. Chitosans and Nanochitosans: Recent Advances in Skin Protection, Regeneration, and Repair. Pharmaceutics. 2022; 14(6):1307. https://doi.org/10.3390/pharmaceutics14061307
Chicago/Turabian StyleFerreira, Patricia Garcia, Vitor Francisco Ferreira, Fernando de Carvalho da Silva, Cyntia Silva Freitas, Patricia Ribeiro Pereira, and Vania Margaret Flosi Paschoalin. 2022. "Chitosans and Nanochitosans: Recent Advances in Skin Protection, Regeneration, and Repair" Pharmaceutics 14, no. 6: 1307. https://doi.org/10.3390/pharmaceutics14061307
APA StyleFerreira, P. G., Ferreira, V. F., da Silva, F. d. C., Freitas, C. S., Pereira, P. R., & Paschoalin, V. M. F. (2022). Chitosans and Nanochitosans: Recent Advances in Skin Protection, Regeneration, and Repair. Pharmaceutics, 14(6), 1307. https://doi.org/10.3390/pharmaceutics14061307










