Abstract
Drug-induced cardiotoxicity not only leads to the attrition of drugs during development, but also contributes to the high morbidity and mortality rates of cardiovascular diseases. Comprehensive testing for proarrhythmic risks of drugs has been applied in preclinical cardiac safety assessment for over 15 years. However, other mechanisms of cardiac toxicity have not received such attention. Of them, mitochondrial impairment is a common form of cardiotoxicity and is known to account for over half of cardiovascular adverse-event-related black box warnings imposed by the U.S. Food and Drug Administration. Although it has been studied in great depth, mitochondrial toxicity assessment has not yet been incorporated into routine safety tests for cardiotoxicity at the preclinical stage. This review discusses the main characteristics of mitochondria in cardiomyocytes, drug-induced mitochondrial toxicities, and high-throughput screening strategies for cardiomyocytes, as well as their proposed integration into preclinical safety pharmacology. We emphasize the advantages of using adult human primary cardiomyocytes for the evaluation of mitochondrial morphology and function, and the need for a novel cardiac safety testing platform integrating mitochondrial toxicity and proarrhythmic risk assessments in cardiac safety evaluation.
1. Introduction
The heart, our central dispatcher of oxygen, nutrients, and drugs, is itself particularly susceptible to drug-induced toxicity. Cardiotoxicity is defined as the side effects of drugs that cause impairment of myocardial performance, which includes myocardial damage, abnormal electrical conduction, and secondary toxicity caused by drug effects on the vascular system or heart valves [1,2]. Cardiovascular toxicities due to therapeutic drugs comprise the group of toxicities with the highest incidence and severity among adverse drug reactions (ADRs) [3,4,5]. For example, 17% of drugs are halted at the preclinical stage due to cardiovascular toxicity [6]. In another report, drug discontinuation in non-clinical or clinical development related to cardiotoxicity reached 27–34% [1]. Despite increasing awareness of the variety of drug toxicities affecting the heart, their incidence in marketed drugs is 15–35% [1]. Notably, nearly 2000 marketed drugs have been associated with cardiovascular side effects, including ones with cardiovascular and noncardiovascular indications [7,8]. Sixty-nine drugs were withdrawn from the market due to serious cardiovascular ADRs between 1950 and December 2014 (Table 1).
The major clinical manifestations of cardiotoxicity are systolic or diastolic dysfunction and arrhythmia, the latter including abnormal cardiac rhythm disturbances in QT interval, bradycardia, and tachycardia [1,5,9,10]. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines, including ICH S7B [9] and ICH E14 [10], were enacted to develop clinical and preclinical cardiotoxicity screening approaches in 2005, which significantly lowered the proportion of drugs with QT prolongation from 60% in 2005 to 10% in 2012 [11]. However, another 17 cardiotoxic drugs were withdrawn from the market following their implementation, including benfluorex (2009), rosiglitazone (2011), celecoxib (2011), ponatinib (2013), and etoricoxib, which have been reported to cause mitochondria dysfunction [12,13,14,15,16]. Thus far, 29% of withdrawn drugs have been reported to exhibit mitochondrial toxicity (Table 1). All current guidelines for standardizing the detection of cardiotoxicity, however, are still directed at arrhythmic risks.
Mitochondria are the metabolic centers of cells, performing fatty acid oxidation, the tricarboxylic acid (TCA) cycle, oxidative phosphorylation (OXPHOS) for ATP synthesis, heme biosynthesis, and amino acid metabolism. In addition, they also play an important role in the regulation of intracellular homeostasis, such as calcium homeostasis, biologic redox equilibrium, hormonal signaling, and apoptosis [17]. Cardiomyocyte, harboring up to 6000 mitochondria [18] that occupy 30–40% of the cell volume, is one of the highest ATP-consuming cell types. Much of the generated energy is used to sustain contraction [19] to supply blood through the circulatory system [20]. It is this high reliance on energy production that render cardiomyocytes particularly vulnerable to mitochondrial toxicants [21]. Mitochondrial toxicants are compounds that interrupt normal mitochondrial functions, resulting in compromised mitochondrial homeostasis, including disruption of oxidative phosphorylation, permeability transition, and generation of mitochondrial oxidative stress, contributing to energy supply disorder, aberrant intracellular signaling, toxic substances accumulation, autophagy or mitophagy disturbances, and programmed cell death, ultimately decreasing cardiac function [22,23,24,25]. At the organ level, many cardiac abnormalities are induced via these mechanisms, including cardiomyopathy [26,27,28], coronary heart disease [29,30], arrhythmias [31,32], ischemia reperfusion [33,34], and heart failure [35,36]. Mitochondrial impairment can adversely impact cardiomyocyte electrical excitability through mitochondrial gene expression alteration [37], mitochondrial membrane potential (MMP) collapse [38], excessive ROS generation [39], and ATP depletion [40], resulting in cardiac arrhythmias [41,42]. Therefore, mitochondria play important roles in the pathogenesis and development of various heart diseases and are common targets in promoting cardiotoxicity in both animal and cell culture [22,43,44,45]. Understanding and monitoring drug-induced mitochondrial cardiotoxicity constitutes a major part of cardiac safety and is critical to modern drug development. It is possible that a standardized approach for assessing non-arrhythmic toxicities, such as mitochondrial toxicity, might mitigate the occurrence of unexpected cardiotoxicity.
In this review, we introduce the many aspects of mitochondrial biology and representative drugs that have been associated with cardiac toxicity. We elaborate on currently used detection methods of mitochondrial toxicity and discuss how these assays could be multiplexed in a high-throughput manner in cardiomyocytes. Finally, we propose ways to enhance our ability to identify mitochondrial liabilities of drugs as early as possible in the drug discovery process.

Table 1.
List of drugs withdrawn from the market due to cardiovascular toxicity and their association with mitochondrial toxicity.
Table 1.
List of drugs withdrawn from the market due to cardiovascular toxicity and their association with mitochondrial toxicity.
| Medicinal Product | Class | Mechanism of Action | Launch Date | Year Withdrawn | Side Effects on Cardiac Function | Mitohondrial Toxicity |
|---|---|---|---|---|---|---|
| Amfepramone | Psychostimulant | Norepinephrine-releasing agent | 1957 | 1975 | - | Unknown |
| Benfluorex | Psychostimulant, anorectic, and hypolipidemic | Blocking of 5-HT2B | 1976 | 2009 | Valvular heart disease | Decrease in CPT I expression [46] |
| Emetine (ipecac syrup) | Emetic | Stimulation of the CTZ, local irritation | 1912 | 1982 | - | Unknown |
| Mephenesin | Muscle relaxant | Spinal reflex inhibition | 1948 | 1976 | - | Unknown |
| Rofecoxib | NSAID | COX-2 inhibitor | 1999 | 2004 | MI, cardiovascular thrombotic events, sudden death | Unknown |
| Adenosine phosphate | Antiarrhythmic | Direct nodal inhibition | 1930 | 1973 | - | Unknown |
| Alphacetylmethadol | Analgesic | OP1 receptor agonist | 1993 | 2003 | - | Unknown |
| Bepridil (Bepridil Hydrochlonde) | Antiarrhythmic, antianginal | Calcium channel blockers | 1981 | 2004 | Prolonged QT, TdP | Unknown |
| Budipine | Antiparkinsonian | Muscarinic and NMDA receptor antagonist | 1979 | 2000 | - | Unknown |
| Cliobutinol | Antitussive | Unclear | 1961 | 2007 | - | Unknown |
| Dofetilide | Antiarrhythmic | Inhibition of KCNH2, KCNK2, KCNJ12 | 1999 | 2004 | QT prolongation, TdP | Unknown |
| Dolansetron | Propulsive | 5-HT3 receptor antagonist | 1997 | 2011 | - | Unknown |
| Encainide | Antiarrhythmic | Na channel blocker | 1985 | 1991 | QT prolongation, TdP | Unknown |
| Grepafloxacin (Grepafloxacin Hydrochloride) | Antimicrobial | Inhibition of DNA gyrase | 1997 | 1999 | QT prolongation | Unknown |
| Indoramin | Vasodilator | Alpha-1 adrenoceptor antagonist | 1981 | 2011 | - | Unknown |
| Isoprenaline | Cardiac stimulant | Non-selective beta-adrenergic agonist | 1949 | 1992 | - | mPTP opening [47] |
| Inhibition of OXPHOS [48] | ||||||
| Levacetylmethadol | Antidote | Mu-opioid receptor agonist, nicotinic acetylcholine receptor antagonist | 1995 | 2001 | - | Unknown |
| Nifedipine (10 mg) | Antihypertensive, antiemetic | Calcium channel blockers | 1975 | 1996 | Hypertension, angina, MI, CHF | Inhibition of ATP synthase [48] |
| Orciprenaline (metaprotenerol) | Bronchodilator | β2 adrenoceptor agonist | 1961 | 2009 | Tachycardia, palpitations | Unknown |
| Pergolide Mesylate | Anti-parkinsonian | Dopamine receptor agonist | 2002 | 2007 | Valvular heart disease | Unknown |
| Rosiglitazone | Hypoglycemic | Gluconeogenesis decrease | 1999 | 2011 | CHF, MI | Inhibition of ETC [48] |
| Increase in mitochondrial oxidative stress, impairment of mitochondrial bioenergetics [13] | ||||||
| Inhibition of complex I; uncoupling of OXPHOS [13] | ||||||
| Sibutramine (Sibutramine Hydrochlonde Hydrate) | Psychostimulant | Serotonin-norepinephrine reuptake inhibitor | 2001 | 2002 | Myocardial infarction | Increase in ROS formation [49] |
| Technetium (99mTc) fanolesomab | Radiography | Radioisotope | 2004 | 2005 | Cardiopulmonary arrest | Unknown |
| Tegaserod (Tegaserod Maleate) | Antispasmodic | 5-HT4 receptor agonist | 2002 | 2007 | HF, ischemia | Unknown |
| Terodiline | Antispasmodic | Calcium channel blockade, blocks cholinergic receptor | 1965 | 1991 | Ventricular tachycardia, cardiac death | Unknown |
| Sertindole | Antipsychotic | 5HT and D2 receptor antagonist/blocking of DRD2,HTR2A, HTR2C, HTR6 | 1996 | 1998 | QT prolongation, TdP, sudden cardiac death | Unknown |
| Cloforex | Psychostimulant | Similar to amphetamine | 1965 | 1967 | - | Unknown |
| Astemizole | Antihistamine | H1-receptor antagonist, inhibition of KCNH2 | 1977 | 1987 | long QT syndrome, TdP | Unknown |
| Cisapride monohydrate | Prokinetic agent | 5-HT4 receptor agonist; inhibition of KCNH2 | 1993 | 2000 | Ventricular arrhythmia, QT prolongation, TdP, cardiac arrest | Unknown |
| Tranylcypromine | Antidepressant | MAOI | 1961 | 1964 | - | Unknown |
| Bromocriptine mesylate | Anti-lactation | D2 and D3 agonist | 1976 | 1989 | - | Swollen mitochondria [50] |
| Domperidone (injectable) | Propulsive | Dopamine receptor antagonist | 1979 | 1985 | - | Unknown |
| Mepazine | Antiepileptic | Unclear | 1955 | 1970 | - | Unknown |
| Clozapine | Antipsychotic | Blocking of DRD2, HTR2A, DRD1, DRD3, DRD4, HTR1A, HTR1B, HTR1D, HTR1E, HTR2C, HTR3A, HTR6, HTR7, HRH1, HRH4, ADRA1A, ADRA1B, ADRA2A, ADRA2B, ADRA2C, CHRM1, CHRM2, CHRM3, CHRM4, CHRM5 | 1972 | 1975 | Cardiomyopathy, MI, myocarditis, arrhythmia, Prolonged QT, TdP, cardiomyopathy | Inhibition of the ETC [51] |
| Increase in ROS formation, GSH depletion, mitochondrial dysfunction, and swelling [52] | ||||||
| Vincamine | Nootropic | Unclear | 1955 | 1980 | - | Unknown |
| Lysine amidotriazoate | Radiography | - | 1975 | 1995 | - | Unknown |
| Terfenadine | Antihistamine | H1-receptor antagonist | 1985 | 1997 | QT prolongation, TdP | Increase in mtROS formation [53] |
| MMP collapse [54] | ||||||
| Naftidrofuryl oxalate (IV) | Vasodilator | 5HT2 receptor antagonist | 1974 | 1992 | - | Unknown |
| Cobalt | Hematinic | As cobalamin | 1951 | 1967 | - | Interruption of TCA and interference with the MRC enzymes [54] |
| MMP collapse [55] | ||||||
| Chloroform (trichloromethane) | Anesthetic | Depression of the respiratory centres | 1847 | 1976 | - | MMP collapse [56] |
| Megamitochondria [57] | ||||||
| Dithiazanine iodide | Antihelminth | Interruption of glucose uptake in cells | 1959 | 1964 | Prolonged QT, TdP | Unknown |
| Epinephrine (topical) | Anesthetic | Vasoconstriction | 1899 | 2004 | - | Unknown |
| Methylhexanamine (DMAA) | Nasal decongestant | Norepinephrine and dopamine transporter blockade | 1948 | 1983 | - | Unknown |
| Dexfenfluramine | Psychostimulant | Serotonin receptor agonist | 1995 | 1997 | Valvular heart disease, cardiac fibrosis | Unknown |
| Fenfluramine | Psychostimulant | Serotonin receptor antagonist | 1973 | 1997 | valvular heart disease | Mitochondrial fragmentation [58] |
| Parecoxib | Analgesic | COX-2 inhibitor | 2002 | 2005 | - | - |
| Prenylamine | Antianginal | Calcium channel blocker | 1960 | 1989 | QT prolongation, sudden cardiac death, ventricular tachycardia, TdP | Inhibition of FAO [59] |
| Probucol | Antioxidant | Inductor of LDL catabolism | 1980 | 1989 | QT prolongation, arrhythmias | Unknown |
| Droperidol | Antipsychotic | Dopamine 2 receptor antagonist | 1970 | 2001 | - | Unknown |
| Valdecoxib | NSAID | COX-2 inhibitor | 2001 | 2005 | Cardiomyopathy, CHF, hypertension, angina, arrhythmia | Inhibition of OXPHOS, mPTP opening [16] |
| Celecoxib (Onsenal) | NSAID | COX-2 inhibitor | 2003 | 2011 | - | Decrease in mitochondrial complex IV activity and induces oxidative stress [14] |
| Increase in ROS formation, MMP collapse, mitochondrial swelling, ATP depletion [60] | ||||||
| Suppression of mitochondrial function [61] | ||||||
| Bismuth salts | Antidyspepsia | Unclear. Forms insoluble complexes | 1875 | 1978 | - | Unknown |
| Levarterenol | Vasopressor | L-norepinephrine analogue | 1904 | 1973 | - | Unknown |
| Pipradrol | Psychostimulant | Norepinephrine-dopamine reuptake inhibitor | 1953 | 1982 | - | Unknown |
| Pseudoephedrine | Sympathomimetic | Direct action on adrenergic receptors | 1959 | 2008 | - | Unknown |
| Gallopamil | Antiarrhythmic | Calcium channel blockers | 1983 | 2001 | - | Decrease in mitochondrial biogenesis and mass [62] |
| Chlorphentermine | Psychostimulant | TAAR1 agonist, blocking of 5-HTs | 1962 | 1969 | Pulmonary heart disease | Inhibition of OXPHOS, uncoupling of OXPHOS [63] |
| Thioridazine | Antipsychotic | 5HT2 receptor antagonist | 1959 | 2000 | QT prolongation, TdP, sudden cardiac death | mPTP opening [64] |
| MMP collapse [65] | ||||||
| Buflomedil | Vasodilator | A-adrenergic blockade | 1970 | 2006 | QT prolongation, cardiac arrest | Unknown |
| Ponatinib Hydrochloride | Antineoplastic | Multi-target kinase inhibitor | 2012 | 2013 | - | Impairment of respiratory chain, increase in ROS formation, MMP collapse, mitochondrial fission [66] |
| Levomethadyl acetate | Analgesic (central nervous system agents) | Activation of OPRM1 | 1993 | 2002 | QT prolongation, TdP | Unknown |
| Mesoridazine Besylate | Antipsychotic | 1970 | - | - | Unknown | |
| Clobutinol Hydrochloride | Antitussive | Inhibition of GABA receptors | 1961 | 2007 | QT prolongation | Unknown |
| Phentermine | Central nervous system agents | Inhibition of SLC6A2, SLC6A3, SLC6A4; blockingof MAOA, MAOB | 1959 | 1997 | Valvular heart disease | Unknown |
| Mibefradil | Antihypertensive | Calcium channel blockers | 1997 | 1998 | QT prolongation | Unknown |
| Sparfloxacin | Antibiotics | Inhibits DNA gyrase | 1997 | 2001 | QT prolongation | MMP collapse [67] |
| Etoricoxib | Anti-inflammatory agents | Inhibition of COX-2 | 2002 | 2007 | thrombotic events | Inhibition of OXPHOS [16] |
| Propoxyphene | Central nervous system agents | Activation of OP1, OP2, OP3 | 1957 | 2010 | QT prolongation, TdP | Unknown |
| Lidoflazine | Cardiovascular agents | Blocking of calcium channels | 1973 | 1989 | QT prolongation | Unknown |
2. Main Properties of Mitochondria and Drug-Induced Mitochondrial Toxicity in Cardiomyocytes
2.1. Morphology, Classification, and Structural Features of Mitochondria
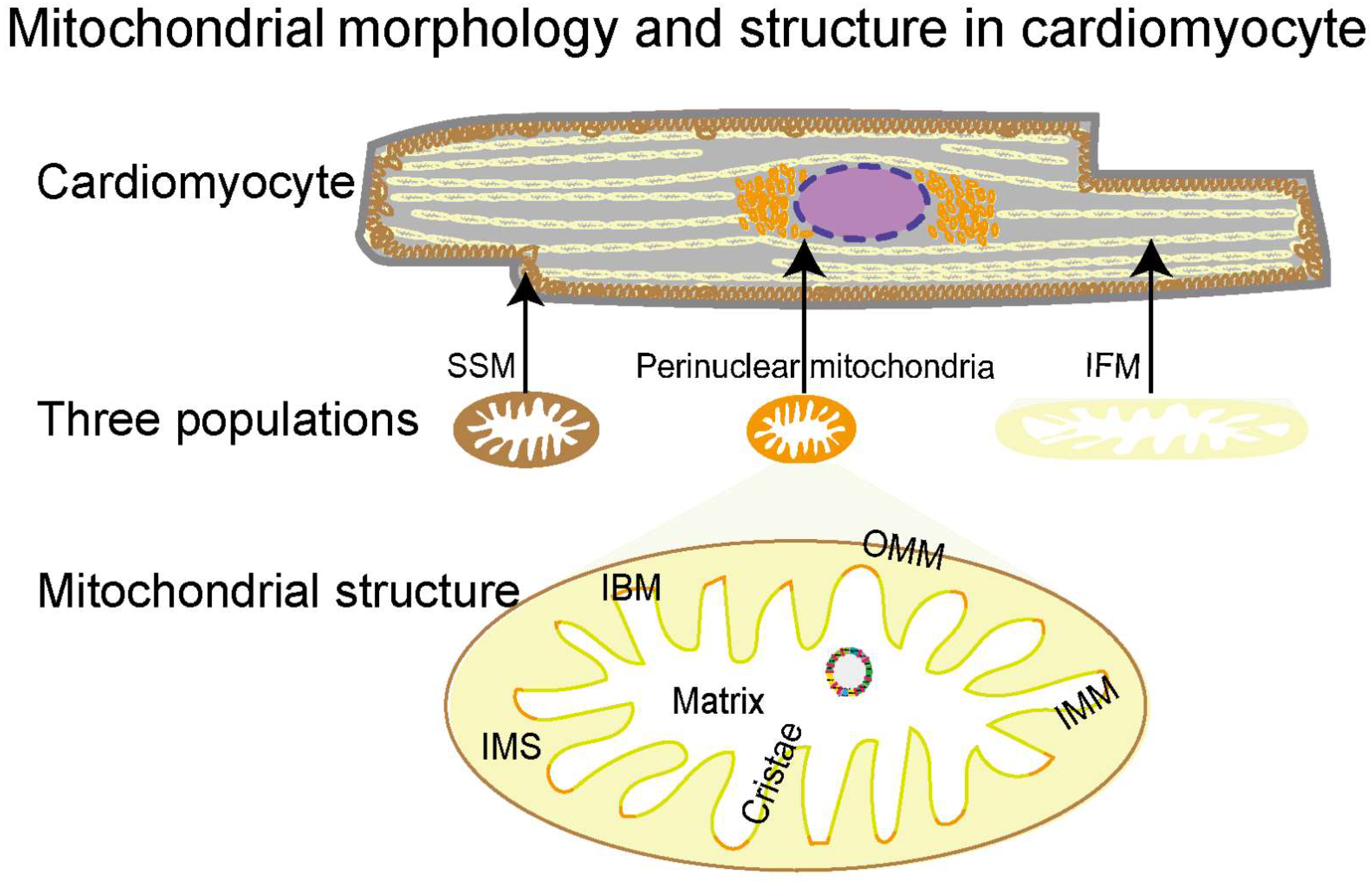
The densely packed mitochondria in the cardiomyocyte provide over 95% of the energy for the heart to pump blood around the body [68,69]. They are highly dynamic organelles that quickly adjust their morphology, protein expression, and activity in response to the cellular environment [70,71,72]. Based on intracellular localizations, mitochondria in adult cardiomyocytes are classified into three populations: perinuclear, subsarcolemmal mitochondria (SSMs), and interfibrillar mitochondria (IFMs) (Figure 1) [68]. With distinctive locations, the three populations present unique morphologies and functions for the nucleus, cellular functions, and myofibril contraction, respectively [73,74,75,76]. Mitochondria are double-membrane organelles, consisting of structurally and functionally different membranes, that is, the outer mitochondrial membrane (OMM) and the inner mitochondrial membrane (IMM) (Figure 1). The OMM and IMM establish five compartments to provide unique biochemical environments for different functions, including the OMM, intermembrane space (IMS), IMM, cristae, and matrix [77,78]. The OMM forms a unique biochemical environment for diverse functions including coordination of protein import, mitochondrial dynamics, and inter-organellar communication. The IMS, the small volume between OMM and IMM, facilitates the translocation, folding, and post-translational events of nuclear DNA-encoding proteins into the matrix. Unlike the OMM permeabilizing molecules of up to 5 kDa [79], the IMM, a highly selective membrane, tightly controls the exchange of ions and metabolites through specialized molecular machinery. The IMM is essential for electron transport since it is necessary for the development of the proton motive force used for ATP generation. The cristae are formed by extensive inward folding of the IMM, increasing the membrane surface 1.5–2 fold to accommodate multi-enzyme complexes for ATP generation, especially the five bioenergetic complexes constituting the OXPHOS system [80]. The mitochondrial matrix is a complex mixture harboring several metabolic processes, including the tricarboxylic acid cycle (TCA), mitochondrial fatty acid oxidation (mtFAO), OXPHOS, Fe-s cluster biogenesis, heme synthesis, and many others. In addition, the matrix also hosts the mitochondrial genome (mtDNA), RNA, and ribosomes. Together, these five compartments coordinate with each other to perform mitochondrial functions, and their structural integrity is essential for healthy mitochondria.
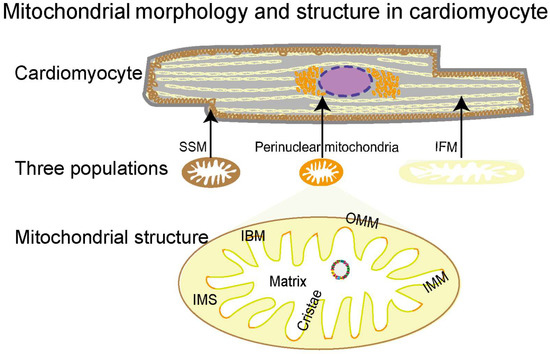
Figure 1.
Mitochondrial morphology and structure in cardiomyocytes. Mitochondria in cardiomyocytes can be categorized into three subtypes, subsarcolemmal mitochondria (SSMs) (in brown), interfibrillar mitochondria (IFMs) (in yellow), and perinuclear mitochondria (in orange), according to their distribution, size, and shape. The mitochondrial double-membrane consists of an outer mitochondrial membrane (OMM) (brown) and an inner mitochondrial membrane (IMM) (green and orange). The space between the OMM and the IMM is the IMS, and inside the IMM is the matrix. The IMM consists of the inner boundary membrane (orange) and cristae (green), the latter of which are formed by extensive inward folding of the IMM.
Ultrastructural analysis by transmission electron microscopy (TEM) is a traditional method for the assessment of mitochondrial architecture. In addition, MitoTracker or fluorescently labeled mitochondrial antibodies have been applied to automated high-content imaging of mitochondria, and the resulting mitochondrial scores correlated well with cytotoxicity [81]. These methods are efficient at detecting several structural abnormalities induced by mitochondrial toxicants, including both oncological and non-oncological drugs. Of the non-oncological drugs, isoproterenol induces mitochondrial swelling, cristae disappearance, and matrix cavitation in cardiomyocytes [82,83]. Mitochondrial swelling and mitochondrial membrane rupture occurred in cardiomyocytes of As2O3-treated mice [84]. Diclofenac [85] and remdesivir [86] treatment also result in mitochondrial damages, as indicated by MitoTracker. Based on immunofluorescence analysis of TOM20, mitochondrial distribution and mitochondrial network disruption, as well as mitophagy, were revealed in nicotine-treated neonatal rat ventricular myocytes [87,88]. Oncological drugs have been frequently reported to cause structural damage to mitochondria. TEM revealed mitochondrial swelling, electron-dense matrix deposits, and matrix clearout in rats given sunitinib, and disrupted mitochondrial cristae in rats given sorafenib [89]. Regorafenib was also reported to induce electron-dense mitochondria and matrix cavitation [90]. MitoTracker indicated mitochondrial damage in cardiomyocytes induced by imatinib [91] and sunitinib [92]. The high sensitivity of mitochondrial structure to functional aberrations makes structural abnormality one of the most commonly observed manifestations of mitotoxicity.
The use of DOX, a commonly used chemotherapeutic anti-cancer drug of the anthracycline family, has been greatly limited because one-fourth of patients have experienced DOX-induced cardiotoxicity, including CHF, decreased LVEF, sinus tachycardia, myocarditis, and cardiomyopathy [93]. Numerous animal- and cardiomyocyte-based studies have revealed DOX-induced abnormal mitochondrial morphology and structure. Abnormal mitochondrial morphology, including mitochondrial swelling, cristae disappearance, and matrix cavitation, was found in doxorubicin (DOX) [94,95,96]. Microscopic evaluation by MitoTracker staining of mitochondria pinpointed the distribution of daunorubicin and DOX [97,98]. Based on immunofluorescence analysis of TOM20, mitochondrial distribution and mitochondrial network disruption, as well as mitophagy, were revealed in DOX-treated neonatal rat ventricular myocytes [87,88]. To mimic human anticancer therapy, the administration schedule was conducted by multiple administrations at separate time points, including 3 mg/kg every other day for a cumulative dose of 9 mg/kg [99], twice a week for three weeks to attain a 9 mg/kg cumulative dose [100], 6 doses of 3 mg/kg [101], 2–2.5 mg/kg/48 h over 12 days [94], 2 mg/kg for 10 consecutive days [102], 5 mg/kg once a week to a total of 20 mg/kg [103], or two doses of 10 mg/kg [104]. Of note, a cumulative dose of 20 mg/kg in adult mice roughly corresponds to 120 mg/m2 in humans, which is much lower than the maximum lifelong dose of 400–550 mg/m2 [105]. Yet even at these low dosages, cardiomyocytes had swollen mitochondria, loss of mitochondrial membrane integrity and cristae, cristae disarrangement, and/or clear matrix, suggesting the strong mitochondrial toxicity of DOX. DOX has been reported to cause acute cardiotoxicity when administered at a dose of 15 mg/kg or greater [106]. Mitochondria showed vacuolization, or even complete loss of the cristae, 48 h after a single dose of 20 mg/kg DOX injection into rats [107]. Fourteen days’ administration of DOX (20 mg/kg, single dose) in C57BL/6 mice resulted in mitochondrial oedema [108]. To investigate chronic cardiotoxicity, five doses (3 mg/kg each, bi-weekly) of DOX were given to C57BL/6 mice. Three months after the first dose, the authors observed hyperproliferation of mitochondria in cardiomyocytes [109]. It is evident from these studies that, even with different treatment schedules and dosages, mitochondrial structure is a stable indication for mitotoxicant assessment. Additional details, including maximum serum concentration (Cmax), cardiotoxicity manifestations, experimental models, and so on, of drugs affecting mitochondrial morphology and structure are listed in Table 2.

Table 2.
Drugs affecting mitochondrial morphology, structure, MQC, their clinical manifestations, and relevant in vitro and in vivo studies.
2.2. Substrate Catabolism and OXPHOS
The heart consumes about 6 kg of ATP per day, which is mainly generated through mitochondrial OXPHOS from the catabolism of lipids and carbohydrates [133,134,135]. Glucose, lactate, and fatty acids are oxidized in the mitochondrion and produce a common end product (i.e., acetyl-CoA), which then goes through eight enzymatic steps of the Krebs cycle, where electrons are extracted from TCA intermediates in the form of reducing equivalents (nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FADH2)) (Figure 2A). The OXPHOS system links substrate oxidation to ATP generation (Figure 2B). It is made up of mitochondrial respiratory chain (MRC) complexes, including NADH:ubichinone oxidoreductase (NADH-dehydrogenase, complex I), succinate dehydrogenase (SDH, complex II), cytochrome c-reductase (complex III), cytochrome c oxidase (complex IV), and ATP synthase (complex V) [136]. Using NADH as a substrate for complex I and succinic acid for complex II, the MRC complexes transfer electrons from NADH and FADH2 to convert O2 to water (complex IV). The energy liberated during this mitochondrial electron transport is used to establish a highly electronegative potential (−140 to −180 mV), termed the MMP, across the IMM by extruding protons at complexes I, III, and IV into the IMS. When intracellular ADP is high, protons are channeled back into the matrix through the F0 portion of ATP synthase, fueling the conversion of ADP into ATP by the F1 portion of this enzyme. This process is tightly regulated, with close coupling of electron transport, membrane potential, and ATP synthesis. ATP is then moved from the mitochondrial matrix to the cytoplasm by the adenine nucleotide transporter (ANT), finally allowing energy to be available for cellular activity [137]. Substrate catabolism and OXPHOS are central to the energy homeostasis of mitochondria, and hence critical for cardiomyocyte functions.
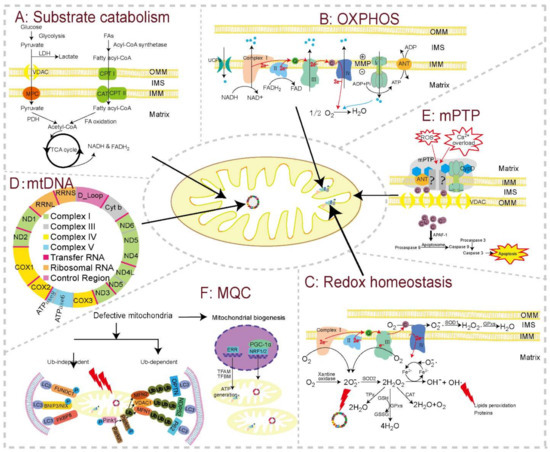
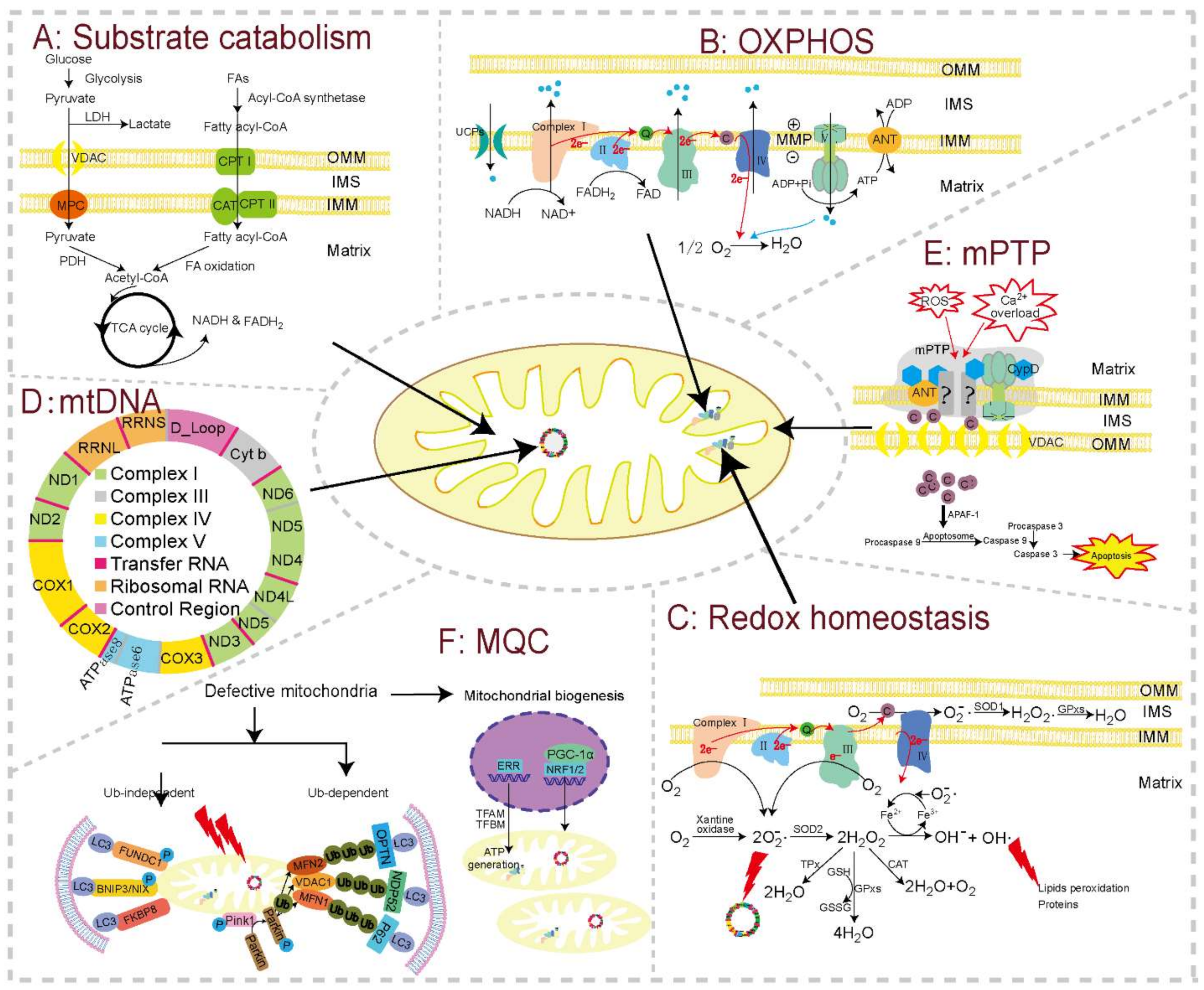
Figure 2.
The basic modules of mitochondrial function and major toxicity targets in cardiomyocytes. Substrate catabolism, oxidative phosphorylation (OXPHOS), redox homeostasis, mitochondrial genome (mtDNA), mitochondrial permeability transition pore (mPTP), and mitochondrial quality control (MQC) constitute the major functional units and toxicity targets in cardiomyocytes. (A) Substrate catabolism. Fatty acids, esterificated by fatty acyl-CoA synthase enzymes, are taken up through CPT I, CPT II, and CAT, and are then oxidized into acetyl-CoA inside the mitochondrion. Pyruvate from glycolysis is also oxidized into acetyl-CoA by PDH in the mitochondrion. Acetyl-CoA then goes through TCA cycle to generate reducing equivalents (NADH and FADH2), which fuel OXPHOS to produce ATP. This bioenergetic process can be disturbed by downregulated expression or decreased activities of carriers and enzymes for the TCA cycle and fatty acids β-oxidation. (B) OXPHOS. Electrons are extracted by complex Ⅰ and II from reducing equivalents, and move through ETC complexes, pumping protons into the IMS to generate MMP. MMP in turn drives proton flow back into the mitochondrial matrix through complex V, releasing this energy to generate ATP. Mitochondrial toxicants can reduce the expression and/or activity of ETC complexes, uncouple ETC from ATP synthesis, and impair MMP. (C) Redox homeostasis. mtROS produced in physiological state can be cleared by series antioxidant enzymes, such as GSH, SOD, and CAT. Drugs with mitochondrial toxicity can overproduce mtROS by inhibiting ETC complexes (especially complex Ⅰ and III) or decreasing the levels or activity of antioxidant enzymes, or there may be a combination of both mechanisms. (D) Map of mtDNA. The mitochondrion possesses its own genome, mtDNA, within the matrix, which can be replicated, transcribed, and translated into some of the MRC complexes. mtDNA, topoisomerase II for mtDNA repair, and DNA polymerase for mtDNA replication are all reported targets for toxicants. (E) mPTP. mPTP is a channel whose components have not been fully elucidated. The normal closed state can be triggered into an open state by a series of stresses, especially Ca2+ overload and oxidative stress. mPTP opening induces cytochrome c releasing into the cytoplasm, resulting in the initiation of apoptosis. (F) MQC. Defective mitochondria can be regulated by MQC, mainly including mitochondrial biogenesis and mitophagy. Damaged mitochondria can be cleared by mitophagy though ubiquitin-dependent or -independent pathways. In cardiomyocytes, ubiquitin-dependent pathway (Pink1-Parkin-mediated mitophagy) is induced by MMP depolarization, while the ubiquitin-independent pathway can be directly induced by LIR containing mitophagy receptors located on OMM in cardiomyocytes. Energy depletion after mitophagy activates genome-encoded transcriptional elements, which directly express mitochondrial proteins or regulate mtDNA to express related proteins for the assembly of new mitochondria. Toxicants may influence mitophagy or biogenesis to disturb MQC. Abbreviations: ANT: adenine nucleotide transporter; APAF: apoptotic peptidase activating factor; BNIP3: BCL2 interacting protein 3; C: cytochrome c; CAT: catalase; CPT: carnitine palmitoyltransferase; Complex I: NADH dehydrogenase; Complex II: succinate dehydrogenase; Complex III: cytochrome c reductase; Complex IV: cytochrome c oxidase; Complex V: ATP synthase; CypD: cyclophilin D; ERR: estrogen-related receptor; ETC: electron transport chain; FAs: fatty acids; FADH2: flavin adenine dinucleotide. FUNDC1: FUN14 domain-containing protein 1; FKBP8: FK506 binding protein 8; GPxs: glutathione peroxidase; GSH: glutathione; GSSG: glutathione disulfide; IBM: inner boundary membrane; IFMs: interfibrillar mitochondria; IMM: the inner mitochondrial membrane; IMS: intermembrane space; LC3: light chain 3; LDH: lactic dehydrogenase; LIR: LC3-interacting region; MFN1/2: mitofusin 1/2; MMP: mitochondrial 6membrane potential; MPC: mitochondrial pyruvate carrier; mPTP: mitochondrial permeability transition pore; NADH: nicotinamide adenine dinucleotide; NRFs: nuclear respiratory factors; OMM: outer mitochondrial membrane; OXPHOS: oxidative phosphorylation; PCMs: primary cardiomyocyte; PDH: pyruvate dehydrogenase; PGC-1α: peroxisome proliferator-activated receptorγ (PPARγ) coactivator 1α; Q: coenzyme Q; SOD: superoxide dismutase; SOD1: Cu/ZnSOD, copper- and zinc-dependent SOD; SOD2: MnSOD, manganese-dependent SOD; SSMs: subsarcolemmal mitochondria; TCA cycle: tricarboxylic acid cycle; TPx: thioredoxin peroxidase; UCP: mitochondrial uncoupling proteins; VDAC: voltage-dependent anion channel.
Many drugs are known inhibitors of the MRC, interfering with one or more of the complexes (Table 3). Inhibition can be caused by directly inhibiting the activity of MRC complexes. For example, zoniporide [64], naproxen [60,138], dronedarone [139], and mubritinib [140] inhibit complex I; propranolol and atenolol disrupt complex II [119]; celecoxib suppresses complex IV [14]; and As2O3 inhibits complex I, III, and IV [141]. OXPHOS may also be blocked by inhibition of the expression of MRC complexes, such as by mitoxantrone [100]. Additionally, uncoupling electron transport from ATP synthesis by tenidap [64] and nonsteroidal anti-inflammatory drugs (NSAIDs) (e.g., nimesulide, meloxicam, and acetylsalicylate) [142] is yet another way to inhibit OXPHOS. Lipophilic drugs can damage phospholipids on the IMM, especially cardiolipin [143], or activate the mitochondrial permeability transition pore (mPTP), which are mechanisms [144,145] that lead to pathologic uncoupling of respiration [72]. Bupivacaine has been suggested to interact with phospholipids in the IMM, which often result in increased membrane permeability, electron transport chain disruption, and calcium accumulation [146]. These inhibitory mechanisms of MRC complexes may cause a series of deleterious consequences. Firstly, OXPHOS inhibition can results in MMP dissipation and diminishment, or even abolishment, of ATP production [147]. Another important consequence of MRC inhibition is the secondary impairment of mitochondrial β-oxidation and the TCA cycle. Furthermore, blocking the flow of electrons favors reactive oxygen species (ROS) overproduction, leading to oxidative stress [148,149,150]. The majority of drugs with known mitochondrial liabilities display impairment of MRC function, highlighting it as a key indicator of mitochondrial health. The drugs listed in Table 4 are reported to downregulate the expression of proteins or to directly inhibit enzyme activity in FA oxidation and the TCA cycle.

Table 3.
Drugs affecting OXPHOS, MMP and mPTP, their clinical manifestations, and relevant in vitro and in vivo studies.

Table 4.
Drugs affecting FA oxidation and TCA cycle, their clinical manifestations, and relevant in vitro and in vivo studies.
2.3. Mitochondrial ROS (mtROS)
Mitochondria are the center of cellular aerobic metabolism and are thus also the major source of cellular ROS [191]. Electrons leaked from various sites (mainly complex I and III) of the MRC react with O2 and subsequently form a series of mtROS (Figure 2C) [191,192]. Nine of the eleven types of ROS are found in mitochondria [193]. In addition, studies revealed that extra-mitochondrial ROS could transduce signals into mitochondria and induce the production of mtROS. mtROS can be partly eliminated through antioxidant enzymes and antioxidant molecules, as well as glutathione and thioredoxin [194]. Oxidative stress is induced in response to a decreased level of antioxidant enzymes and increased production of ROS. Mitochondria, while being a primary source of ROS themselves, also suffer from ROS-mediated damages caused by peroxidation of macromolecules including proteins, lipids, and DNA [135,195,196,197]. Therefore, the balance between mtROS production and elimination is critical for mitochondria.
The balance of mtROS can be destroyed by toxic drugs decreasing antioxidant enzyme levels, inhibiting antioxidant enzyme activity, or producing mtROS (Table 5). The mitochondrial toxicity of some drugs, including cisplatin [112], azidothymidine [198], cannabinoids, glycosides [36], and pioglitazone [122], seems to be mediated by increases in the production of free radicals. DOX not only induces ROS production by Fe2+/Fe3+ cycling, but also inhibits antioxidant enzymes, including glutathione, glutathione peroxidase, and glutathione reductase [199,200]. Furthermore, ROS levels are elevated when OXPHOS complexes are inhibited [201,202] by drugs such as As2O3 [141]. Such free radicals can directly cause a diverse range of macromolecular damage, resulting in mitochondrial dysfunction. For example, ROS produced by DOX peroxidates cardiolipin, inducing cytochrome c release [188]. Hence, excessive mtROS production is a very common mechanism of mitochondrial dysfunction induced by mitotoxicants.

Table 5.
Drugs affecting mitochondrial redox, their clinical manifestations, and relevant in vitro and in vivo studies.
2.4. Replication, Translation, and Transcription of mtDNA
One cardiomyocyte contains up to 6000 mitochondria, each equipped with its own copies of mtDNA. Maintaining the stability, proper replication, transcription, and translation of mtDNA are critical for mitochondrial health [195]. The mtDNA encodes for 2 rRNAs, 22 tRNAs, and 13 proteins, which serve as essential subunits of the MRC complexes (Figure 2D) [78]. mtDNA is characterized by high gene density without introns or only separated by single noncoding nucleotides, thus requiring great accuracy to ensure the functionality of the resulting transcripts. mtDNA is transcribed at a high rate, especially in the highly energetically active heart [212]. The majority of the subunits and proteins in mitochondria are encoded by nDNA, whereas mtDNA only encodes 13 polypeptides of MRC complexes I, III, IV, and V. Translation of these 13 genes is accomplished via the mitochondria’s transcription and translation machinery, which comprises both nuclear-encoded protein factors, mitochondrial encoded RNA components, and mitoribosomes [213]. Critically, unlike nuclear DNA, mtDNA is not tightly packed into nucleosomes and proximal to ROS production sites [214], rendering it particularly susceptible to toxicants.
mtDNA can be interfered with by the inhibition of mitochondrial DNA polymerase and topoisomerase II (Table 6). DOX [158], mitoxantrone [215], and daunorubicin [130,207] inhibit DNA topoisomerase II β and disturb mtDNA stability and expression. DOX also decreases mtDNA and mtDNA-encoded subunit COX I in complex IV [96]. In addition, antiviral agents, such as zalcitabine, didanosine, and stavudine are specific inhibitors of the mitochondrial DNA polymerase-gamma, and therefore impair mtDNA replication [216]. It was later observed that nucleoside reverse transcriptase inhibitor-mediated mitochondrial toxicity can also occur as a result of direct inhibition of mtDNA-encoded protein synthesis [217,218]. Furthermore, antibiotics impair mtDNA-encoded protein synthesis due to the structural similarity between bacterial and mitochondrial ribosomes [219]. The mechanisms that deplete mtDNA-encoded protein levels eventually lead to decreases in ATP levels [202,220]. Clearly, mtDNA stability and expression are common targets of mitotoxic drugs, including anthracyclines, antiviral agents, and antibiotics.
2.5. Mitochondrial Membrane Potential (MMP) and mPTP
MMP is both a chief function and key sentinel of the mitochondrial network, indicating the functional metabolic status of mitochondria. Through the establishment of MMP, ADP and inorganic phosphate (Pi) converts to ATP. Besides ATP generation, MMP is broadly required for both ion homeostasis and protein import into the mitochondrial network [221]. MMP dissipation can be dependently or independently induced by mitochondrial permeability transition pore (MPTP) opening [222]. Mitochondria are master regulators of cell survival. Ca2+ overload, excessive ROS production, MMP dissipation, fatty acid, and adenine nucleotide pool depletion have all been reported to trigger mPTP opening [223,224]. The opening of mPTP, a non-selective pore, is defined as a sudden increase in the permeability of IMM for small molecules and ions (<1.5 kDa), leading to cellular apoptosis and the occurrence and development of cardiovascular diseases [224,225]. However, the precise molecular composition of the mPTP is currently unknown [223]. ATP synthase, ANT, and cyclophilin D (CypD) have all been recognized as key components of the mPTP (Figure 2E) [226,227,228]. Normal MMP and closed mPTP are essential to healthy mitochondrial respiration and are therefore sentinels of mitochondrial impairment and cell survival.
Drugs inducing cardiotoxicity by targeting mitochondria invariably proceed to MMP collapse and mPTP opening (Table 3). Antineoplastic agents, including DOX [73,81,164], As2O3 [84,229], and imatinib [91]; β adrenergic receptor blockers, such as propranolol and atenolol [119]; antiarrhythmic drugs dronedarone and amiodarone [139]; antibiotics erythromycin and clarithromycin; NSAIDs such as naproxen, diclofenac, and celecoxib [60]; and diabetes drug pioglitazone [122] have all been reported to cause these harmful effects. mPTP opening and MMP decrease consequently induce loss of respiratory control and imbalance in ATP production, and loss of mitochondrial components such as ATP, NAD+, and glutathione, leading to water accumulation in the matrix, which causes mitochondrial osmotic swelling, IMM unfolding, and the rupture of the OMM [230,231]. As a gatekeeper of cellular death, the opening of the mPTP eventually results in the release of pro-apoptotic proteins from the IMS into the cytosol, thus stimulating both caspase-dependent and caspase-independent apoptosis [202]. Due to the universality of MMP collapse and mPTP opening induced by mitotoxicants, evaluating their status has become one of the most basic and routine tests in the assessment of drug-induced mitochondrial dysfunction.

Table 6.
Drugs affecting mitochondrial carriers and mtDNA, their clinical manifestations, and relevant in vitro and in vivo studies.
Table 6.
Drugs affecting mitochondrial carriers and mtDNA, their clinical manifestations, and relevant in vitro and in vivo studies.
| Modules | Alterations | Pharmacology | Drugs | Clinical Manifestations | Cmax | Models | Dose | Time | References |
|---|---|---|---|---|---|---|---|---|---|
| Carrier | Downregulation of CPT I expression | Alkylating agent | Cyclophosphamide | HMC, CMP | 143 μM | Male Wistar rats (IP) | 200 mg/kg | 10 d | [189] |
| Carrier | Downregulation of CPT I expression | Anesthesia | Propofol | HF, arrhythmia | 30.13 μM | HiPSC-CMs | 10 µg/mL | 48 h | [163] |
| Carrier | Downregulation of CPT I expression | TKIs | Sunitinib | Decreased LVEF, QT prolongation, TdP, hypertension, HF, CMP | 0.25 μM | Rats (oral) | 25 mg/kg/d | 28 d | [173] |
| Carrier | Inhibition of CPT1 activity | Anti-arrhythmic drug | Dronedarone | AF, HF | 0.15–0.26 μM | Isolated rat heart mitochondria | IC50 = 40 µM | 20 min | [139] |
| Carrier | loss of carnitine | Co-catalyst | Pivalic acid | CMP | [232] | ||||
| Carrier | Inhibition of ANT | NSAIDs | Diclofenac | Hypertension, arrhythmias | 7.9 µM | Submitochondrial particles | 314 nM/mg protein diminished 76% | [142] | |
| Nimesulide | Submitochondrial particles | 259 nM/mg protein diminished 60% | [142] | ||||||
| Carrier | Inhibition of ANT | NRTIs | Zidovudine | CMP | 4 μM | [233] | |||
| mtDNA | Inhibition of mitochondrial DNA polymerase | NRTIs | Zidovudine | CMP | 4 μM | Cardiac DNA pol-γ | 1 µM | [234] | |
| mtDNA | Inhibition of topoisomerase II | Anthracyclines | DOX | CHF, decreased LVEF, ST, myocarditis, CMP | 15.3 μM | - | - | - | [235] |
| Daunorubicin | CMP, MI, CHF, VA, pericarditis, myocarditis | 89 μM | - | - | - | [207] | |||
| Idarubicin | CMP, MI, CHF, VA, decreased LVEF | 23.22 μM | - | - | - | [207] | |||
| mtDNA | Inhibition of topoisomerase II | Chemotherapeutic agents | Mitoxantrone | CHF, CMP, decreased LVEF, arrhythmia | 3.3 μM | - | - | - | [215] |
| mtDNA | mtDNA content decreasing | Anthracyclines | DOX | CHF, decreased LVEF, ST, myocarditis, CMP | 15.3 μM | Male Wistar rats (IV) | 1 mg/kg/w | 7 w (started at 11 w, observed at 48 w) | [96] |
| mtDNA | mtDNA content decreasing | TKIs | Regorafenib | MI; hypertension | 8.08 μM | H9c2 | 5 μM | 72 h | [90] |
Abbreviations: NRTIs: nucleoside reverse transcriptase inhibitors; NSAIDs: nonsteroidal anti-inflammatory drugs; TKIs: tyrosine kinase inhibitors; ANT: mitochondrial ADP/ATP transport; TCA: tricarboxylic acid; DOX: doxorubicin; CHF: congestive heart failure; LVEF: left ventricular ejection fraction; HF: heart failure; TdP: torsades de pointes; MI: myocardial infarction; AF: atrial fibrillation; CMP: cardiomyopathy; ST: sinus tachycardia; HMC: hemorrhagic myocarditis; VA: ventricular arrhythmia; HA: heart attack; IP: intraperitoneal; IV: intravenously; BID: twice daily; w: week; d: day; h: hours.
2.6. Mitochondrial Carriers
Mitochondrial function (including the TCA cycle; fatty acid oxidation; OXPHOS; amino acid degradation; biosynthesis of amino acid, iron sulfide, urea, heme, and cholesterol; and dissipation of proton gradient for heat production) relies on the exchange of a very diverse set of solutes and metabolites across the IMM and OMM. Mitochondrial carriers located on the IMM ensure that massive transport processes can occur between the mitochondrial matrix and the cytoplasm. These specific carriers are a superfamily of nuclear-encoded proteins including the solute carrier (SLC) family, the sideroflexin family, the mitochondrial pyruvate carrier (MPC1/2), ATP-binding cassette transporter (ABCB) isoforms, and splice variants of other solute carriers, as well as recently identified mitochondrial amino acid carriers [236]. Consequently, mitochondrial carriers are fundamental for various mitochondrial functions.
Mitochondrial toxicity can occur as a result of inhibition of carriers, such as adenine nucleotide translocator (ANT) for exchanging ADP in for ATP out [237,238,239] and carnitine palmitoyltransferase (CPT) I and II for fatty acyl-CoA transfer into the matrix (Table 6) [202]. Zidovudine impairs ANT activity, which is one of the biochemical processes responsible for cardiomyopathy [233]. Inhibiting the export of ATP causes MRC inhibition, compromising cell function due to energy deficiency [216]. Pivalic-acid-induced cardiomyopathy is the result of CPT activity inhibition, which is responsible for fatty acid oxidation in mitochondria [36,232]. In addition, sunitinib significantly decreased the level of CPT I expression [173]. In addition to ANT and CPT, other carriers potentially targeted by drugs are worth investigation.
2.7. Mitochondrial Quality Control (MQC)
Cardiomyocytes require healthy mitochondrial homeostasis to provide sufficient ATP for maintaining the pump function of the heart. Mitochondrial structure and function are tightly regulated by the MQC system, which is fundamental for sustaining mitochondrial bioenergetics demand and metabolic functions [240]. MQC is a series of processes comprising quality control of mitochondrial proteins, mitochondrial biogenesis, mitochondrial dynamics (fission and fusion), and mitophagy (Figure 2F) [241]. MQC repair or remove dysfunctional and damaged mitochondria; promote mitochondrial regeneration; improve mitochondrial biochemical processes and signaling transduction; maintain mitochondrial morphology, quantity, and function; and promote cell survival [242]. The vast majority of unrepaired damaged proteins are removed through the cytosolic ubiquitin/26S proteasome system (UPS), which has been shown to be crucial in the quality control of mitochondrial proteins [243]. In cardiomyocytes, mitophagy pathways include ubiquitin-dependent pathways, such as the PTEN-induced kinase 1/E3 ubiquitin ligase parkin (PINK1/Parkin) pathway, and ubiquitin-independent pathways, such as the BCL2 and adenovirus E1B 19 kDa-interacting protein 3 and BNIP3-like (Bnip3/Nix) pathway [244]. Due to its critical role in maintaining the mitochondrial network, MQC has recently been found to be vulnerable to unfavorable factors, including disease and toxicants [245].
UPS, mitochondrial biogenesis and dynamics, and mitophagy are reported targets for drug-induced cardiotoxicity (Table 2). Trastuzumab inhibits mitochondrial biogenesis, possibly through Her2-dependent estrogen-related receptor alpha activation [246]. Etoposide [169], zidovudine [131], and remdesivir were reported to disrupt mitochondrial dynamics, promoting mitochondria fragmentation [86]. Mitophagy mediates the clearance of damaged mitochondria with excessive mtROS marked by MitoSOX or/and decreased MMP indicated by JC-1 [247,248]. Mitophagy can be visualized in vivo using mitophagy-associated fluorescence proteins, such as mt-keima, mito-QC, and RFP/GFP-LC3 [249]. Colocalization of mitochondria (marked by MitoTracker or mitochondrial-specific fluorescent antibody) and autophagosomes (indicated by GFP-LC3) or lysosomes (dansylcadaverine, LysoTracker, or lysosome-specific fluorescent antibody) under fluorescence microscope, as well as immunoblotting of Parkin, LC3II/I, ubiquitin, Atg5, Beclin1, and p62 are widely used methods for mitophagy detection in vitro [247,250,251,252,253]. Based on these methods, sunitinib and sorafenib were shown to impair mitophagy via inhibition of ribosomal S6 kinase and AMPK. As2O3 induced parkin-dependent UPS activation [129]. Excessive mitophagy induced by the Parkin/PINK pathway contributed to DOX-induced toxicity [127,128]. On the other hand, mitigation of BNIP3-dependent mitophagy by aconitine induced cardiomyocyte damage [121]. Recently, increasing numbers of drugs interrupting MQC have been reported, indicating MQC impairment as a potentially critical criterion for mitotoxicant identification.
2.8. Other Mitotoxicants and Their Targets
Most mitochondria-related toxins and their targets are searchable in MitoTox [51]. In addition to the above-mentioned targets for mitotoxicity, several other toxicological mechanisms have been implicated in mitochondrial dysfunction but require further interrogation in cardiomyocytes. Ion channels and transporters on the IMM are associated with redox regulation and electrical and contractile dysfunction of cardiomyocytes [254]. H+/Na+ antiporters can be inhibited by amiloride analogs [255]. Inner membrane anion channels that are permeable to a variety of anions and anionic metabolites can be blocked by amiodarone, dibucaine, propranolol, amitryptiline, and clonazepam [254,256]. However, not all of these ion channels and transporters have been confirmed as a mechanism mediating mitochondrial toxicity in cardiomyocytes. The mitochondrial unfolded protein response (mtUPR) that is induced upon stress serves an important protective role in the heart by ameliorating mitochondrial dysfunction [257]. Although activated mtUPR resists statin toxicity in C. elegans [258], there is still a lack of evidence for the association between mtUPR and mitochondrial toxicants, especially in cardiomyocytes. Many other emerging molecular processes are gaining attention as mechanisms underlying mitochondrial dysfunction, and thus could be potential mediators of toxicity. Protein post-translational modifications (PTMs) were found to contribute to heart failure progression by regulating mitochondrial function [259]. Of the many proteins regulated by PTMs, mPTP is gaining attention as a potential mechanism underlying mitochondrial dysfunction and has as many as 55 PTM sites [260]. It is, therefore, possible that PTM of mPTP may contribute to mitochondrial toxicity in the heart.
3. Limitations of Current Preclinical Models for Assessing Cardiotoxicity
The successful development of a new drug takes about 14 years, at an average cost of USD 403 million, with roughly one-third spent on preclinical studies and the rest on clinical trials [261,262]. As a leading cause of attrition, drug-induced toxicity appears at all stages of drug development [263]. Even when drugs pass through the series of preclinical evaluations for drug safety, only 11% make it through clinical studies [264]. More strikingly, 462 marketed drugs were withdrawn due to ADRs from 1953 to 2013 [264]. Among all types of drug toxicity, cardiovascular toxicity constitutes 30% of all organ-toxicity-caused attritions [265], as well as 14% of all drug-toxicity-related withdrawals. Still, there remains 15–35% of drugs in the market with cardiovascular ADRs, which may contribute to the high and still increasing morbidity and mortality rates of CVDs, which claimed over 17.6 million lives in 2016 alone worldwide [266]. Therefore, drug-induced cardiac toxicity has caused serious financial losses for the pharmaceutical industry, as well as harm to patients’ well-being. The high rate of cardiotoxicity-related drug attrition, withdrawal, and ADRs are attributed to the insufficient cardiac safety evaluation system. Given that a 10% improvement in toxicity prediction at the preclinical stage could save $100 million per drug [263], improvements in early cardiotoxicity identification may have a profound impact on costly late attrition. It also helps to avoid unexpected ADRs that may threaten patients’ lives [263].
3.1. Limitations in the Current Workflow of Cardiac Safety Testing
Currently, drug-induced arrhythmia, especially QT prolongation leading to life-threatening complications including torsade de pointes, ventricular tachycardia, and sudden cardiac death, is the predominant concern during drug development. Arrhythmia can be induced by a wide range of drug classes including both non-antiarrhythmic drugs and, paradoxically, antiarrhythmic drugs [267]. In order to identify proarrhythmic drugs, the nonclinical ICH S7B (in vitro human ether-a-go-go-related (hERG) current and in vivo QT assays) and clinical ICH E14 (thorough QT study) guidelines were issued in 2005, which effectively precluded drugs with QT prolongation effects from further development [268]. According to these guidelines, in vitro hERG current measurement is mostly performed in immortalized heterologous cell lines stably expressing hERG channels using the patch-clamp technique, while in vivo QT assays are performed in animal models via electrocardiography. However, QT prolongation is not a robust surrogate for arrhythmogenic risk, and drugs blocking the hERG current do not always cause arrhythmias, resulting in nearly 60% of promising compounds being mistakenly eliminated during development [269]. In August 2020, ICH released an updated guideline combining S7B and E14 Questions and Answers (Q&As) to define an appropriate and efficient assessment of drug-induced corrected QT interval prolongation. The comprehensive in vitro proarrhythmia assay (CiPA) is a new strategy to determine the arrhythmogenic effects of drugs by evaluating a variety of cardiac repolarization-related currents in heterologous expression systems, reconstructing cardiac electrophysiologic activity in silico, followed by validations in human induced pluripotent-stem-cell-derived cardiomyocytes (hiPSC-CMs) and supplementation with data from phase I clinical trials. Although these guidelines have proven effective at pro-arrhythmic risk assessment, the complexity of drug-induced cardiotoxicity goes far beyond abnormal heart rhythm. Mitochondrial toxicity is increasingly implicated in drug-induced cardiotoxicity. Over 50% of clinical drugs causing cardiovascular adverse events, eliciting black box warnings from the U.S. Food and Drug Administration (FDA), are caused by mitochondrial liabilities [147]. However, mitochondrial toxicity testing has not been incorporated into routine safety testing procedures during drug development.
Another caveat of the current cardiac safety evaluation system is the failure to identify hidden cardiotoxicity. Hidden cardiotoxicity is a type of toxicity that goes undetected in healthy individuals, and only reveals itself under disease conditions [262]. Diseased hearts are loaded with function alterations involving ion channels, mitochondria, and electro-mechanical coupling, and are often more vulnerable to drugs. For example, cardiac arrhythmias may only be revealed in preclinical models of cardiovascular disease (e.g., myocardial infarction) [270]. This could be one of the reasons for the high rate of cardiotoxicity-related drug attrition, withdrawal, and ADRs. Although these aspects are important, given the focus of this article, we will only discuss viable measures to detect mitochondrial toxicity preclinically.
3.2. In Vitro Models for Cardiac Toxicity Assessment
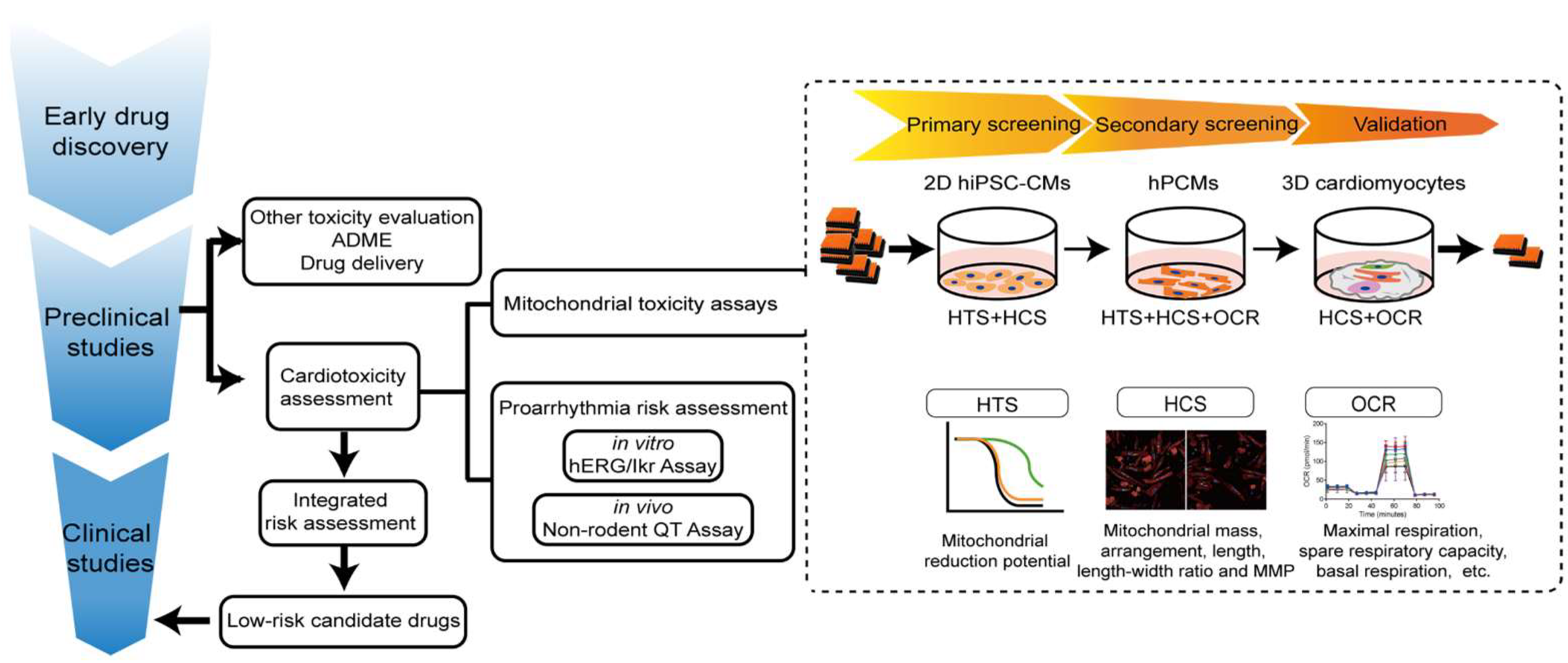
Understanding the advantages and limitations of cardiac models is critical for proper cardiac risk assessment. To assess and confirm mitochondrial toxicity, physiological relevance ranked from high to low is as follows: human data, animal models, organ models, cell models, and organelle models. However, animal and organ models are not only low-throughput, expensive, and time-consuming, but also frequently incapable of directly reporting mitochondrial impairment. Isolated mitochondria provide a useful in vitro experimental system for mechanistic analysis, with the advantage of allowing extensive control over experimental conditions, such as measuring toxic effects on mitochondrial oxygen consumption without interference from other physiological processes [271]. Mitochondria subtypes, including SSMs and IFMs, can be either jointly or individually [272,273] isolated from cultured cardiomyocytes and heart tissues. However, their low yield, lack of physiological environment, and biased extraction of healthier ones from the sample [271] make isolated mitochondria not suitable for HTS. In contrast, intact cardiomyocytes exhibit several advantages over other models for evaluating drug-induced cardiotoxicity. In contrast with whole-heart preparations and tissue slices, cardiomyocyte cultures rule out signal contamination from other cell types, thereby identifying cell-type-specific toxicity. Unlike isolated mitochondria, cardiomyocytes provide a more physiologically relevant cellular environment, including materials for mitochondrial import and export, normal subcellular arrangements and structures of mitochondria, all mitochondrial subtypes with specific subcellular localizations, and so on. At present, three cell models are used according to the new E14/S7B draft guideline, including cell lines stably expressing hERG channels, hiPSC-CMs, and human primary cardiomyocytes (hPCMs). Heterologous hERG-expressing cell lines, while useful for single-channel screening, do not recapitulate the complex electrical activities seen in cardiomyocytes. By contrast, intact cardiomyocytes are more physiologically relevant for evaluating drug-induced cardiotoxicity. Currently used cardiomyocyte models include H9c2 cardiomyoblasts, stem-cell-derived cardiomyocytes, and primary cardiomyocytes (PCMs).
3.2.1. H9c2 Cardiomyoblasts
The H9c2, derived from the ventricular part of the embryonic BDIX rat heart [274], is an immortalized myoblast cell line used as cardiomyocytes due to its biochemical, electrophysiological, and hormonal signaling properties [274,275]. By sequential selective passaging and culturing with all-trans retinoic acid and 1% serum media, they may overexpress L-type calcium channels, mimicking the adult cardiac muscle phenotype [276,277]. H9c2 cardiomyoblasts have been used for mitochondrial toxicity assessment of DOX, and demonstrated increased mitochondrial swelling [278], mtROS [279], mitochondrial fission [280], decreased MMP [281], and OCR and ATP production [282], whereas trastuzumab led to increased mtROS and decreased MMP [185]. Treatment of H9c2 cells with tyrosine kinase inhibitors, such as imatinib, sorafenib, and sunitinib, resulted in mitochondrial swelling, cristae loss, MMP reduction, inhibition of MRC complexes, superoxide accumulation, and cellular GSH depletion [283,284,285]. Similar mitochondrial impairments were also observed with other toxicants, such as As2O3 [286] and simvastatin [151]. H9c2 was utilized in HTS to identify compounds potentially conferring protection from DOX-induced damage [287]. However, H9c2 cardiomyoblasts have a lower predictive value than hESC-CMs with regard to ATP levels, MMP, Ca2+ mobilization, and endoplasmic reticulum integrity with therapeutic concentrations of toxic drugs [288]. Furthermore, drug responses may vary with differentiation state [289,290]. Ultimately, H9c2 cells are not of human origin, with differences including mitochondrial content and metabolism potentially affecting their translational capability, thus limiting their use as a model targeting mitochondrial toxicity [69].
3.2.2. Stem-Cell-Derived Cardiomyocytes
Stem-cell-derived cardiomyocytes, including human embryonic stem-cell-derived cardiomyocytes (hESC-CMs) and hiPSC-CMs, are derived from blastocysts or reprogrammed somatic cells, respectively, with a series of differentiation processes [264]. They resemble in vivo cardiomyocytes in terms of ultrastructure [291], electrophysiology, and contraction [3], and are widely used as surrogates for native human cardiomyocytes [3], providing a promising platform for cardiotoxicity assessment [292]. However, hESC-CMs are limited by ethical concerns and regulatory restrictions. hiPSC-CMs, while circumventing these problems, face other challenges, the most prominent of which is immaturity [293]. Fortunately, many techniques are being developed that aim at enhancing cardiomyocyte maturation [294,295,296,297,298], thus improving drug response [299,300,301]. Other efforts are directed at the mass production of hiPSC-CMs for screening purposes. Approximately 1.5–2.8 billion cardiomyocytes per bioprocess can be generated by two-dimensional (2D) and 3D culture systems [302,303], and this number can be increased a hundred-fold through the inhibition of the glycogen synthase kinase-3β (GSK-3β) pathway [301], meeting the demands of HTS [304,305]. Downstream screening, and advances in computational methods, such as artificial intelligence (AI) algorithms, are being developed to more accurately define endpoints, as has already been implemented for Ca2+ transients [306] and cardiomyocyte structure [307]. hiPSC-CMs, a human-based cardiomyocyte model with patient- and disease-specific characteristics, are versatile tools for phenotypic or target-based screening in lead compound discovery, as well as preclinical arrhythmia detection as required by CiPA. 2D hiPSC-CMs achieved 90% sensitivity, 74% specificity, and 82% accuracy in detecting drugs blocking ion channels and contraction [308]. Similarly good performance was also reached when profiling drugs using metabolic and viability endpoints [309]. Toxicities induced by anticancer therapies, including anthracyclines and tyrosine kinase inhibitors, were also successfully recapitulated in 2D hiPSC-CMs [301]. Chronic cardiotoxicity usually emerges between one month and decades after administration of treatment doses and might only be discovered during post-market monitoring [8]. Cardiotoxicity studies performed with single and relatively short exposure periods (up to 48 h) do not reflect the true mechanisms of chronic cardiotoxicity [310]. Given that delayed-onset cardiotoxicity may take longer to detect [21], long-term cultured cells with long-term recording are required [311]. hiPSC-CMs can beat spontaneously with stable baseline functions for months [312,313], rendering them more suited for assessing chronic toxicity. Notably, hiPSC-CMs have been widely used as a cellular model for evaluating the chronic effects of anticancer drugs and nucleoside analogs [23,293,295,297,298]. However, there is increasing awareness of the differences between hiPSC-CMs and their primary counterparts (i.e., hPCMs) concerning metabolism, structure, and function [293,314]. hiPSC-CMs remain similar to other cell lines in terms of their mitochondrial morphology, distribution, and function. In hiPSC-CMs, mitochondria occupy only about 5% of the cell volume and are located around the nucleus. Morphologically, they assume a rounded shape with poor cristae. Metabolically, they mostly rely on glucose for ATP production (~85%) [314]. All of these result in resistance to mitochondrial toxicity measurements [315].
3.2.3. hPCMs
PCMs are directly harvested from the native tissue and are considered to possess all properties of normal cardiomyocytes in the heart. Therefore, they are well suited for pharmacological evaluation of cellular morphology, function (e.g., electrophysiology, calcium handling, contraction), and subcellular structures, such as mitochondria [316,317]. Additionally, they are exceptional tools with regard to disease modeling, because they can be directly isolated from target animals or patients, eliminating the need for external manipulations, as is routine with all other biological models [3]. PCMs can be isolated from the embryonic, neonatal, or adult stages of animals and humans. However, owing to species differences, PCMs derived from humans and animals vary significantly in their functional and molecular characteristics [318]. hPCMs probably bear the highest degree of resemblance to native cardiomyocytes and are thus well suited for cardiotoxicity assessment. Preserving the donor’s genetic background, clinical manifestation, and medical history is a particular advantage of hPCMs, as drugs may induce unexpected effects in an old, diseased, and susceptible heart that is hidden in the healthy one [262]. Most of the current cardiotoxicity detection platforms for measuring contractility, calcium transient, membrane potential, and mitochondrial functions in hiPSC-CMs can also be applied to hPCMs [293,319]. Twenty-six inotropes (17 positive, 9 negative) were identified in adult hPCMs based on contractility transients [320]. It is worth noting that compared with hPCMs, hiPSC-CMs exhibited higher rates of false-positive and negative results for 33 multi-ion channel-blocking drugs [321]. Except for limited availability and technical challenges in handling, hPCMs are ideal tools for high-throughput assays examining the effects of compounds on mitochondria, due to their abundance, subtype distribution, shape, and substrate utilization [299,305,308]. Mitochondria occupy 30% of the hPCM cell volume and are distributed between myofibrils, under sarcolemma, and at the two longitudinal poles of the nucleus. They are also more reliant on fatty acids (80%) as the metabolic substrate, whose oxidation process for ATP production is reliant upon mature mitochondria [314]. Based on higher mitochondrial content, an elevated ROS level after doxorubicin treatment in more mature cardiomyocytes was detected compared to immature cardiomyocytes [322]. However, despite recent progress in hPCM isolation [323,324,325] and culture [326,327], they have not been used for mitochondrial toxicity screening to date.
3.2.4. 3D Cardiomyocyte Models
3D cardiac models are a promising class of models in that they are of human origin, reflect in vivo cardiomyocyte physiology and function, comprise multiple cell types, are suited for evaluation of both acute and chronic toxicities, and are available in high-throughput format [328,329]. Cardiac spheroids are a type of self-assembled ball shape structure comprising one or multiple cell types. They have been used to verify the cardiotoxicity of DOX, sunitinib, verapamil, and quinidine at clinically relevant concentrations, assessed by cell viability, contractility, MMP, and endoplasmic reticulum integrity [283,317,318]. Currently, cardiac spheroids can be easily generated in microscale platforms, such as 96/384-well plates [300,330], to decrease the number of cardiac cells. Cardiac organoids are hollow 3D structures of relevant cardiac cells, including cardiomyocytes, endothelial cells, fibroblasts, and so on, in the presence of extracellular matrix proteins. Cardiac organoids resemble the human heart by exhibiting similar ultrastructure and physiology, including oxidative metabolism, force-frequency relationship, and calcium handling [298]. Transcriptomic analysis revealed that cardiac organoids share the highest degree of similarity with human adult myocardium compared with 2D, 3D hiPSC-CMs, and fetal myocardium [331]. Small-size engineered heart tissue platforms have also been described [332]. Measurements using cardiac organoids can also be conducted in a high-throughput manner and are compatible with the detection of electrophysiological abnormalities, contractile dysfunction, and structural toxicity [333,334]. More importantly, cardiac organoids showed drug evaluation results similar to the adult heart [316,323,324]. The reactivity of cardiac organoids induced by clinical compounds, including antibiotic, antidiabetic, and anticancer drugs, was shown to be more physiologically relevant compared with 2D-cultured hiPSC-CMs [335,336]. Based on a panel of eight metrics, cardiac organoids responded appropriately to pro-arrhythmic stimuli and effectively differentiated between high- and low-risk hERG-inhibiting compounds, meeting the critical demand in pro-arrhythmic cardiotoxicity prediction [337]. In addition to electrophysiology, cardiac organoids are also sensitive to drugs affecting cardiac contractility and can be applied in HTS format using a customized image acquisition workflow and optical flow analysis methods [329,335]. Structural parameters, including cell membrane permeability, MMP, endoplasmic reticulum integrity, and cellular viability, can be measured in cardiac organoids using high-throughput assays as well [332,334]. Therefore, 3D cardiomyocyte models hold great promise for cardiotoxicity screening. However, the technical challenges are still relatively high. As a 3D structure, organoids frequently present a necrotic core owing to the heterogeneous diffusion of nutrients. Similarly, drugs not evenly distributed by diffusion in the cardiac organoids also influence the accuracy of toxicity prediction [328]. Furthermore, the production of sufficiently large quantities and sufficient uniformity of generated organoids for high-throughput assays is a challenging task. Thus, additional work is needed to make these models available to the pharmaceutical industry.
4. Proposed Preclinical Model of Cardiomyocytes for Assessment of Drug-Induced Mitochondria Toxicity
4.1. In Vitro Cell Culture for Cardiotoxicity Assays
H9c2 can be either self-differentiated or purchased from cell banks (e.g., American Tissue Culture Collection (Manassas, VA, USA) [185,279] and Cell Bank of the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China) [280,282]) and cultured with Dulbecco’s modified Eagle’s Medium (DMEM; Gibco) containing 10% fetal bovine serum (FBS; Gibco), 100 U/mL penicillin, and 100 μg/mL streptomycin. In addition, 2D hiPSC-CMs can be self-differentiated and cultured with RPMI + B27 with insulin [338,339], or purchased from biotechnology companies (e.g., Cellular Dynamics International (Madison, WI, USA) [308] and FUJIFILM Cellular Dynamics, Inc (FCDI, Madison, WI, USA) [309]), and maintained in culture according to their protocols. As for 3D cardiomyocyte models, hiPSC-CMs are the most common type of cardiomyocytes used in cardiac organoids. Other non-cardiomyocytes can be either induced from hiPSCs [332] or isolated from human tissue. Cardiac organoids are cultured with 50% cardiomyocytes maintenance medium and 50% endothelial basal medium when endothelial cells are included in organoids [300,329]. Alternatively, a 100% cardiomyocytes maintenance medium is used when cardiac organoids only consist of cardiomyocytes and fibroblasts [298]. hPCMs are isolated from human heart samples during surgical procedures such as coronary artery bypass surgery, valve replacement, and so on, and may be cultured with ACCITT3 culture medium [327].
The carbon source in the culture media of cardiomyocytes is one of the most critical determinants of reliable mitochondrial toxicity evaluation. Cells grown in media containing glucose may cause the ‘Crabtree effect’ [340], allowing high levels of glycolysis with minimal OXPHOS, and altered mitochondrial physiology. This artificial shift in metabolism undermines the effectiveness of HTS assays examining mitochondrial toxicity. On the contrary, when grown under conditions of low-glucose or glucose-free media with abundant oxygen supply, cardiomyocytes are forced to use OXPHOS for ATP production [340], exhibiting mitochondrial respiration comparable to in vivo conditions [341]. Cardiomyocytes, including H9c2 [283,284,285,342], hESC-CMs [140], and hiPSC-CMs [343], grown in galactose become susceptible to mitochondrial toxicants [344,345]. None of the H9c2 cells cultured in galactose survived troglitazone treatment, whereas those in high-glucose medium were unaffected 24 h post-treatment [346]. Owing to the 2–3 orders of magnitude higher sensitivity to various mitotoxicants with galactose culture [345], the differential sensitivities of glucose- versus galactose-grown cells were therefore used as an identifier of specific drug-induced mitochondrial impairment. Specifically, a ratio of half-maximal inhibitory concentration (IC50) of a drug, based on ATP production as a readout, in glucose- and galactose-grown cells (IC50 Glucose: IC50 Galactose), of >3 is taken as an indication of mitochondrial toxicity [345,346]. Mitochondrial liabilities for members of the biguanide family as well as certain antidepressants (nefazodone) were identified in this manner [347], and the approach has since found widespread use across the pharmaceutical industry. Of note, this method worked particularly well for inhibitors of ETC complexes I and III, but was useless for uncouplers [345] and other toxic effects, including mitochondrial ion channels inhibition and DNA damage [267].
4.2. Mitochondrial Target as Readouts in Cardiotoxicity Assays
4.2.1. Mitochondrial Morphology, Structure
Mitochondria toxicity can be detected by its abundance, arrangement, and morphology alterations. Traditionally, these changes are detected by transmission electron microscopy [348], a method providing only a snapshot in a specific space and time. Nowadays, changes in mitochondrial dynamics can be visualized directly by high-content screening (HCS) in 96- and 384-well plates. Multiple fluorescent probes, mitochondria-tagged fluorescent proteins, or immuno-labelling with antibodies can indicate mitochondrial abundance, arrangement, morphology (e.g., swollen, punctate, etc.). Fluorescent probes, such as nonyl-acridine orange (NAO), measuring mitochondrial cardiolipin content, and Mito Tracker, which determines MMP, are used to characterize subpopulations of mitochondria by HCS [349]. Mito Tracker is retained in fixed mitochondria and is therefore compatible with antibody-based imaging [350]. Constructs expressing fluorescent proteins (i.e., GFP, RFP, YFP) fused with specific sequences are also used for mitochondrial analysis by targeting the OMM, IMM, or matrix [351,352]. Immuno-labeled antibodies targeting specific proteins, such as MRC complexes or TOM20 on the OMM, can also be used for HCS [353]. Systematic image analysis software now makes it possible to quantify mitochondria in cardiomyocytes in a high-throughput manner [307].
4.2.2. Oxygen Consumption Rate (OCR)
Oxygen consumption, one of the classic end points of assessing the metabolic implications of drug treatment, provides direct information on the activity of OXPHOS. OCR is highly sensitive to perturbations in mitochondrial function [354]. Traditionally, OCR measurements on isolated mitochondria are performed using Clark-type oxygen electrodes [355,356]. Today, Seahorse Bioanalyzers represent a significant advance in OCR assessment, improving both throughput and sensitivity [357,358,359]. By orderly injection of chemical probes including oligomycin, FCCP, rotenone, and antimycin A, a series of readouts, including basal respiration, proton leak, non-mitochondrial oxygen consumption, maximal respiration, ATP production, spare respiratory capacity, and coupling efficiency, can be calculated to reveal OXPHOS damage. Reductions in OCR can result from altered control mechanisms (e.g., MMP decline), diminishments in the supply of reducing equivalents, inhibition of individual MRC complexes, or ANT inhibition. Therefore, the primary mechanism for such reduction needs to be identified as the next step. When necessary, the activity of individual complexes can be interrogated through the use of specific respiratory substrates and inhibitors [345,360].
4.2.3. ATP
Determining cellular ATP levels is an effective and robust way to assess compound toxicity [361]. Since mitochondria are the sites for ATP production in cells, decreases in ATP levels indicate impaired mitochondrial function. During apoptosis, reductions in ATP are usually accompanied by decreases in the MMP. As a secondary measurement for mitochondrial function [362], ATP content is frequently used as an indicator of cellular viability in HTS [339]. ATP content can be measured by colorimetry, fluorescence, luminescence, or isotopes. Photoluminescence measurement on microplate readers is the most popular method at present [363,364,365,366], due to its superior detection sensitivity and operational convenience. A rhodamine-based spirolactam ATP sensor was developed to specifically monitor mitochondrial ATP in real time and has already been applied to human and mouse skin fibroblasts [367].
4.2.4. Redox Homeostasis
The balance between the generation and neutralization of ROS is another important determinant of mitochondrial health. Therefore, the functional state of mitochondria can be reflected by detecting the level of ROS, especially mtROS [362]. ROS is adapted to HTS platforms with probes including MitoPY1 [365], MitoSox [368], and CellRox [369]. MitoSOX, a mitochondrially targeted fluorescent dye [370], is widely used for the measurement of O2 formation in active mitochondria. Another ROS probe, Amplex UltraRed, is oxidized by H2O2 to form a fluorescent product and is specifically used in monitoring H2O2 production [371,372]. Upon detection of aberrantly high ROS levels, dysfunction of the antioxidant system is frequently interrogated as a potential mechanism. For example, SOD activity can be determined by pyrogallol autoxidation, while glutathione levels can be assessed through its oxidation by 2-nitrobenzoic acid. Furthermore, downstream of ROS overproduction, ROS-mediated damage-induced peroxidation of macromolecules is also an indicator of mitochondrial toxicity. For example, the levels of malondialdehyde, one of the final products of polyunsaturated fatty acids peroxidation, can rise as a consequence of an increase in free radicals. Its reaction with thiobarbituric acid provides a colorimetric approach to evaluating lipid peroxidation [373].
4.2.5. MMP
Assays using fluorescent probes to quantify disruption of MMP have been validated as an effective method for assessing mitochondrial toxicity and have been adopted for HTS [374]. Although cell models vary in their responses to mitochondrial toxicants, MMP is a steady criterion to indicate mitochondrial dysfunction [375]. MMP-dependent lipophilic and cationic dyes, including rhodamine 123, tetramethylrhodamine methyl (TMRM) [376], tetramethylrhodamine ethyl ester (TMRE), 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolycarbocyanine iodide (JC-1), and JC-10 [375] are widely used to assess MMP [377]. The widely used probe JC-1 and its modified, water-soluble version, JC-9, accumulate in mitochondria MMP-dependently and exhibit a shift in emission wavelength from green (monomers) to red (J-aggregates), providing a readout of the potential difference across the IMM. Although MitoTracker MMP-dependently labels mitochondria [378], it is more generally used as a mitochondrion-specific probe to track mitochondria, for example in colocalization experiments with ROS indicators [379,380,381] or lysosomes indicators [382]. However, MMP quantification alone cannot distinguish whether such loss is due to inhibition of MRC complexes, uncoupling, or mitochondrial permeability transition, so complementary assays are required to determine the underlying causes [362].
4.3. High-Throughput Assessment of Mitochondrial Toxicity
Owing to the diverse range of drugs that can cause cardiac mitochondrial toxicity (Table 2, Table 3, Table 4, Table 5 and Table 6), and due to the varying degrees and types of toxic manifestations, it would be desirable to screen for such toxicity in a high-throughput manner. As discussed above, mitochondrial toxicities are classified into several categories (i.e., effects on ROS production, MMP depolarization) that are likely intertwined. Therefore, HTS for mitochondrial liabilities of drugs provides a means of accurate classification of such toxicities, which may prove critical to safety pharmacology. HTS techniques for mitochondrial liability detection usually include a self-defined combination of the following assays: HCS for mitochondrial content, arrangement, shape, MMP, and so on; microplate reader-based assays for ATP and ROS detection; and Seahorse assay for OCR measurement. HTS for mitochondrial liabilities is widely applied in a variety of cell types [354,365,368,383,384,385]. However, the use of HTS to detect cardiac mitochondrial toxicity is still in its infancy. Multiparametric analyses were performed by HCS to show the effects of the drugs on mitochondria in hiPSC-CMs [81]. An antibody against translocase of outer mitochondrial membrane 20 (TOM20) indicated mitochondrial changes similar to sarcomeres and nuclei induced by aspirin, doxorubicin, erlotinib, and sorafenib. Most notably, mitochondrial structure changes were detected at lower concentrations compared to the loss of contractility and cell count [386]. Furthermore, concentration–effect profiles of mitochondria-related changes correlated well with cell viability induced by cardiotoxic drugs [387]. Twenty-three cardiotoxicants were identified in 69 environmental hazards based on MMP evaluation by JC-10 staining in hiPSC-CMs [388]. Mitochondrial respiration analyzed by Seahorse assay was found to be a very sensitive and robust means of detecting mitotoxicity in hiPSC-CMs, and thus can be used both as a screening method and validation tool [307].
Several technical details are worth paying attention to when planning an HTS for mitotoxicity. Drug concentration and incubation time are critical for the identification of mitochondrial toxicity, and distinguishing them from cytotoxicity, in HTS assays. However, the existing literature does not fully distinguish between mitotoxicity and cytotoxicity, as evidenced by the frequent use of mitochondrial parameters as a surrogate of cellular conditions. Therefore, no consensus has yet been reached concerning the threshold separating these two entities. For example, 80% of drugs with hepatocyte toxicity were identified at a concentration of 100 μM or 30-fold of Cmax with 3 days of incubation. TMRM staining indicated that 70% of those with cytotoxicity exhibited mitochondrial toxicity [383]. In cardiotoxicity drugs, the percentage of mitochondrial toxic drugs increased with increasing concentrations of drugs, ranging from 1 to 100 fold of Cmax, as evaluated by the glucose/galactose model in rat liver mitochondria [64], an insensitive mitotoxicity measurement [345]. Exposure of up to 100 fold of Cmax over a period of 72 h was found to be essential for cytotoxicity examination of slower-acting toxicants in HCS [389]. On the contrary, the combined use of four metabolic biomarkers of toxicity (three of which were pertinent to mitochondria) achieved 90% sensitivity and 79% specificity in an assay using 10 fold of Cmax in hiPSC-CMs [309]. Therefore, a concentration lower than that used to induce cytotoxicity would be useful for identifying compounds with primary actions on mitochondria. A shorter incubation time of 1 to 6 h [357,390] or 24 h (if requiring metabolism for mitochondrial toxicity) [359] also helps to distinguish mitochondrial toxicity from cytotoxicity. It is noteworthy that when the concentration of drug needed to induce mitochondrial toxicity is not significantly lower than that needed to induce cytotoxicity (IC50 ratio ≤ 3), it is difficult to determine whether mitochondrial toxicity is a primary or secondary effect of drug action, and further validation is therefore required to dissect the underlying mechanisms [390]. More detailed information on drug metabolism is also worthy of attention. Drugs, especially prodrugs, may be metabolized into active forms in cells, which do not easily diffuse back into the extracellular matrix and are thus accumulated in the cytoplasm, inducing a higher drug concentration than Cmax [391]. This type of toxicity, including both mitochondrial toxicity and cytotoxicity, may not be discovered by exposing cells to the Cmax concentration.
4.4. Proposed Integrated Assays for Drug-Induced Mitochondria Toxicity of Cardiomyocytes
The prevalence of drug-induced mitochondrial cardiotoxicity warrants a more rigorous, systematic, and comprehensive evaluation of compounds early in the drug discovery process. HTS is a commonly used method for drug screening, and thus can also be utilized for the detection of mitochondrial liabilities of drugs. Since arrhythmia and mitochondrial dysfunction exist as two distinct entities in cardiotoxicity, we suggest an independent screening module that can be performed in parallel for proarrhythmic risk assessments to enhance predictive capabilities for cardiotoxicity (Figure 3). The choice of cellular model is pivotal to HTS. As discussed above, 2D and 3D hiPSC-derived cardiomyocytes and hPCMs models each have their advantages and drawbacks. On one hand, 2D hiPSC-CMs have been widely used due to their ease of scaled production, but do not sufficiently resemble the in vivo condition; on the other, hPCMs, while of native origin, can face practical problems, including tissue availability, isolation quality, and compatibility with HTS. According to our unpublished data, approximately three million hPCMs can be isolated from one milligram of heart tissue, and they can be further cultured and cryopreserved without morphological and functional alterations, indicating their compatibility with HTS. While 3D cardiomyocyte model assemblies are structurally and functionally advanced, however, their uniformity and scalability still need optimization.
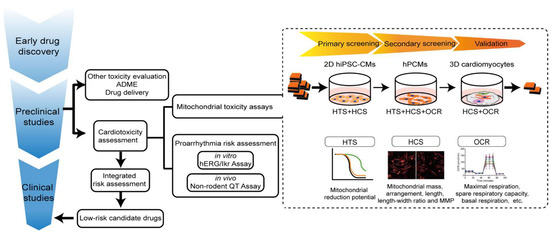
Figure 3.
Proposed workflow of mitochondrial toxicity evaluation during preclinical cardiotoxicity profiling. Mitochondrial toxicity assays can be conducted in parallel with the existing proarrhythmic risk assessments to aid the selection of safer drugs for subsequent clinical studies. In our proposed workflow, 2D hiPSC-CMs, hPCMs, and 3D hiPSC-derived cardiomyocytes models can be cultured in 96- or 384-well assay plates, and treated with candidate drugs for primary screening, secondary screening, and subsequent validation, respectively. Primary assays can be performed firstly by PrestoBlue staining for measurement of reduction potential. Then, the fluorescent images can be captured in a high-content manner after MitoTracker and TMRM staining. Secondary screening combines microplate reading, HCS, and OCR measurement. Validation can be conducted with HCS and OCR measurement. These readouts (reduction potential, mitochondrial mass, distribution, morphology, MMP, and OCR) can be subsequently quantified to identify mitochondrially safe drugs. Abbreviations: ADME: absorption, distribution, metabolism, and excretion; hERG: human ether-a-go-go-related current; 2D hiPSC-CMs: two-dimensional human induced pluripotent-stem-cell-derived cardiomyocytes; HCS: high-content screening; HTS: high-throughput screening; OCR: oxygen consumption rate.
An HTS approach for drug-induced mitochondrial toxicity can incorporate many of the aforementioned parameters. For example, primary screening can be performed by 2D hiPSC-CMs combining microplate reading and HCS of a variety of readouts, including mitochondrial reduction potential, mass, arrangement, length, length-to-width ratio, and MMP. In particular, cardiomyocytes cultured in 96- or 384-well assay plates can be firstly tested by PrestoBlue for reduction potential, then detected by HCS for the rest of the readouts (MitoTracker for mitochondrial mass and morphology, TMRM for MMP). Secondary screening and subsequent validation can be conducted in the hPCM and 3D cardiomyocyte models, respectively, by combining microplate reading, HCS, and OCR measurement. A mitochondrial toxicity index can be calculated as a weighted average of these readouts and can guide ranking of the cardiomyocyte-specific mitochondrial toxicity of compounds, and when combined with data from proarrhythmic risk assessment, can provide evidence for decisions regarding further development.
Even if mitochondrial toxicity does not reach the level of discontinuation of drug development, the resultant data will provide an early warning sign of potential adverse reactions in a clinical setting, and may indicate measures for monitoring potential adverse events, such as lipoatrophy and peripheral neuropathy [392], and inform patient care. The combined preclinical cardiotoxicity assessments may also be useful for dissecting mechanisms of toxicity, such as the relationship between mitochondrial toxicity and excitation-contraction coupling or arrhythmias. Another benefit of screening for mitochondrial toxicity early in the drug discovery process is the early identification of structure–toxicity relationships to minimize or circumvent this liability from a chemical perspective. The recently identified 1,3-nitrogen motif in anticancer drugs was shown to inhibit MRC complex I in cardiomyocytes [140].
5. Conclusions and Future Perspectives
Mitochondria in cardiomyocytes ensure the proper functioning of the heart by producing energy and regulating redox balance, calcium homeostasis, and cell death [393,394]. Due to their mass and their central roles in cellular function, mitochondria in cardiomyocytes are particularly vulnerable to mitochondrial toxicants [22]. Cancer therapies, antiviral compounds, antibiotics, antidiabetic drugs, nonsteroidal anti-inflammatory agents, local anesthetics, and many other therapeutics often impair mitochondrial function [22]. Mitochondrial dysfunction is known to cause a broad spectrum of CVDs, including cardiomyopathies, arrhythmias, and abnormalities of the conduction system [395]. Therefore, cardiomyopathy, arrhythmias, and heart failure are the most common presentations of mitochondrial cardiotoxicity [36]. Up to 26% of patients treated with DOX exhibit cardiotoxicity with symptoms of cardiomyopathy [396], arrhythmia [397], and heart failure [398]. In addition, existing CVDs can also be aggravated by mitochondrion-toxic agents [36]. Despite the prevalence of mitochondrial toxicity and its impact on the heart, current clinical assessments of cardiac function are not able to detect subclinical myocardial dysfunction, let alone the underlying pathophysiology (e.g., mitochondrial toxicity) [399]. Nuclear imaging-based strategies with mitochondrial-potential- and ROS-targeted tracers for mitotoxicity in vivo have not yet achieved the desired sensitivity and molecular specificity for clinical assessments, but have the potential for future translation [400]. Here, we proposed a preclinical screening model for drug-induced mitochondrial toxicity of cardiomyocytes in HTS format, which can be performed in parallel with current proarrhythmic risk assessments for cardiac safety. Although this proposed workflow potentially improves and perfects the cardiac safety screening system, it is not intended to provide solid evidence of human cardiac toxicity, or lack thereof, in areas that exceed the scope of such screening (e.g., chronic toxicity).
While the mechanisms of drug toxicity are heavily studied in animal hearts, the real effect and mechanisms in human cardiomyocytes are less well understood [36], which prompted the development of HTS for mitochondrial toxicants in human-relevant platforms. In addition to drug-induced mitochondrial toxicity, accumulating studies have pointed out that environmental toxins, including various pesticides and heavy metals, may also induce cardiotoxicity [401,402]. Hence, HTS may be useful in applications beyond the regular drug discovery pipeline. In addition to cardiac safety assessment, HTS can be utilized to search for cardioprotective drugs and provide clues to their pharmacological actions. By applying mitochondrial toxicants with distinct mechanisms of action, the screen is capable of identifying different categories of cardioprotectancts. On the other hand, unexpected hits from such screens may be indicative of previously unknown drug actions. In a similar vein, since mitochondrial dysfunction is a common feature of many CVDs, HTS is a viable approach to finding mitotherapeutics for disease treatment, such as cardiomyopathy [403,404]. Screens intended to determine the mitochondrial liability of drugs may also reveal inter-relations of different toxicity phenotypes. For example, mitochondrial impairment by cardiotoxins was found to be an underlying cause of structural cardiotoxicity in hESC-CMs and H9c2 cells [288]. Given that Ca2+ handling, ATP production, and ROS signaling in mitochondria have all been shown to play important roles in arrhythmia, such as atrial fibrillation [401], it might be worth deciphering the relationship between drug-induced mitochondrial dysfunction and drug-induced arrhythmia. Furthermore, it is crucial to determine whether mitochondrial toxicants affect non-cardiovascular organ systems or the heart, particularly cardiomyocytes. For example, sertraline caused hepatotoxicity by uncoupling OXPHOS and inhibiting MRC complexes I and V [405]. Whether they also exert the same effects in cardiomyocytes is unclear. Screening for potential cardiac mitochondrial toxicity will contribute to identifying hidden cardiotoxicity and guiding clinical medication.
Author Contributions
X.T.: writing—original draft preparation; Z.W., S.H. and B.Z.: writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC: No. 82070287 to Bingying Zhou) and the CAMS Initiative for Innovative Medicine Program (grant 2021-1-I2M-006).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no competing interest.
References
- Stummann, T.C.; Beilmann, M.; Duker, G.; Dumotier, B.; Fredriksson, J.M.; Jones, R.L.; Hasiwa, M.; Kang, Y.J.; Mandenius, C.F.; Meyer, T.; et al. Report and recommendations of the workshop of the European Centre for the Validation of Alternative Methods for Drug-Induced Cardiotoxicity. Cardiovasc. Toxicol. 2009, 9, 107–125. [Google Scholar] [CrossRef] [PubMed]
- Albakri, A. Drugs-related cardiomyopathy: A systematic review and pooled analysis of pathophysiology, diagnosis and clinical management. Intern. Med. Care 2019, 3, 1–19. [Google Scholar] [CrossRef]
- Savoji, H.; Mohammadi, M.H.; Rafatian, N.; Toroghi, M.K.; Wang, E.Y.; Zhao, Y.; Korolj, A.; Ahadian, S.; Radisic, M. Cardiovascular disease models: A game changing paradigm in drug discovery and screening. Biomaterials 2019, 198, 3–26. [Google Scholar] [CrossRef]
- Potter, E.; Marwick, T.H. Assessment of Left Ventricular Function by Echocardiography: The Case for Routinely Adding Global Longitudinal Strain to Ejection Fraction. JACC Cardiovasc. Imaging 2018, 11, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Yuan, C.; Wang, L.; Chen, R.; Li, X.; Zhang, Y.; Liu, C.; Liu, X.; Liang, W.; Xing, Y. The Beneficial Effects of Saffron Extract on Potential Oxidative Stress in Cardiovascular Diseases. Oxid. Med. Cell. Longev. 2021, 2021, 6699821. [Google Scholar] [CrossRef]
- Cook, D.; Brown, D.; Alexander, R.; March, R.; Morgan, P.; Satterthwaite, G.; Pangalos, M.N. Lessons learned from the fate of AstraZeneca’s drug pipeline: A five-dimensional framework. Nat. Rev. Drug Discov. 2014, 13, 419–431. [Google Scholar] [CrossRef]
- Kuhn, M.; Letunic, I.; Jensen, L.J.; Bork, P. The SIDER database of drugs and side effects. Nucleic Acids Res. 2016, 44, D1075–D1079. [Google Scholar] [CrossRef]
- Magdy, T.; Schuldt, A.J.T.; Wu, J.C.; Bernstein, D.; Burridge, P.W. Human Induced Pluripotent Stem Cell (hiPSC)-Derived Cells to Assess Drug Cardiotoxicity: Opportunities and Problems. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 83–103. [Google Scholar] [CrossRef]
- ICH, S.B. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Steering Committee. The Non Clinical Evaluation of the Potential for Delayed Ventricular Repolarization (QT prolongation) by Human Pharmaceuticals S7B. Available online: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Safety/S7B/Step4/S7B_Guideline.pdf (accessed on 12 May 2005).
- International Conference on Harmonisation. Guidance for Industry: E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs. Available online: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E14/E14_Guideline.pdf (accessed on 1 November 2005).
- Park, E.; Willard, J.; Bi, D.; Fiszman, M.; Kozeli, D.; Koerner, J. The impact of drug-related QT prolongation on FDA regulatory decisions. Int. J. Cardiol. 2013, 168, 4975–4976. [Google Scholar] [CrossRef]
- Geelen, M.J. Mechanisms responsible for the inhibitory effects of benfluorex on hepatic intermediary metabolism. Biochem. Pharmacol. 1983, 32, 1765–1772. [Google Scholar] [CrossRef]
- He, H.; Tao, H.; Xiong, H.; Duan, S.Z.; McGowan, F.X., Jr.; Mortensen, R.M.; Balschi, J.A. Rosiglitazone causes cardiotoxicity via peroxisome proliferator-activated receptor gamma-independent mitochondrial oxidative stress in mouse hearts. Toxicol. Sci. 2014, 138, 468–481. [Google Scholar] [CrossRef] [PubMed]
- Atashbar, S.; Jamali, Z.; Khezri, S.; Salimi, A. Celecoxib decreases mitochondrial complex IV activity and induces oxidative stress in isolated rat heart mitochondria: An analysis for its cardiotoxic adverse effect. J. Biochem. Mol. Toxicol. 2021, 36, e22934. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, M.E.; Chun, B.; Moya, R.; Saucerman, J.J. Computational model of cardiomyocyte apoptosis identifies mechanisms of tyrosine kinase inhibitor-induced cardiotoxicity. J. Mol. Cell. Cardiol. 2021, 155, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Syed, M.; Skonberg, C.; Hansen, S.H. Mitochondrial toxicity of selective COX-2 inhibitors via inhibition of oxidative phosphorylation (ATP synthesis) in rat liver mitochondria. Toxicol. In Vitro 2016, 32, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Nunnari, J.; Suomalainen, A. Mitochondria: In sickness and in health. Cell 2012, 148, 1145–1159. [Google Scholar] [CrossRef]
- Cao, Y.P.; Zheng, M. Mitochondrial dynamics and inter-mitochondrial communication in the heart. Arch. Biochem. Biophys. 2019, 663, 214–219. [Google Scholar] [CrossRef]
- Bround, M.J.; Wambolt, R.; Luciani, D.S.; Kulpa, J.E.; Rodrigues, B.; Brownsey, R.W.; Allard, M.F.; Johnson, J.D. Cardiomyocyte ATP production, metabolic flexibility, and survival require calcium flux through cardiac ryanodine receptors in vivo. J. Biol. Chem. 2013, 288, 18975–18986. [Google Scholar] [CrossRef]
- Barry, S.P.; Townsend, P.A. What Causes a Broken Heart—Molecular Insights into Heart Failure. Int. Rev. Cell Mol. Biol. 2010, 284, 113–179. [Google Scholar]
- Gintant, G.; Burridge, P.; Gepstein, L.; Harding, S.; Herron, T.; Hong, C.; Jalife, J.; Wu, J.C. Use of Human Induced Pluripotent Stem Cell–Derived Cardiomyocytes in Preclinical Cancer Drug Cardiotoxicity Testing: A Scientific Statement From the American Heart Association. Circ. Res. 2019, 125, e75–e92. [Google Scholar] [CrossRef]
- Varga, Z.V.; Ferdinandy, P.; Liaudet, L.; Pacher, P. Drug-induced mitochondrial dysfunction and cardiotoxicity. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1453–H1467. [Google Scholar] [CrossRef]
- He, L.; Liu, F.; Li, J. Mitochondrial Sirtuins and Doxorubicin-induced Cardiotoxicity. Cardiovasc. Toxicol. 2021, 21, 179–191. [Google Scholar] [CrossRef]
- Wallace, K.B.; Sardão, V.A.; Oliveira, P.J. Mitochondrial Determinants of Doxorubicin-Induced Cardiomyopathy. Circ. Res. 2020, 126, 926–941. [Google Scholar] [CrossRef]
- Yin, Y.; Shen, H. Advances in Cardiotoxicity Induced by Altered Mitochondrial Dynamics and Mitophagy. Front. Cardiovasc. Med. 2021, 8, 739095. [Google Scholar] [CrossRef] [PubMed]
- Hantson, P. Mechanisms of toxic cardiomyopathy. Clin. Toxicol. 2019, 57, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kanniah, G.; Kumar, S. Clozapine associated cardiotoxicity: Issues, challenges and way forward. Asian J. Psychiatr. 2020, 50, 101950. [Google Scholar] [CrossRef] [PubMed]
- Arangalage, D.; Pavon, A.G.; Hugelshofer, S.; Desgraz, B.; Tzimas, G.; Delyon, J.; Muller, O.; Obeid, M.; Ribi, C.; Michielin, O.; et al. Cardiotoxicity of immune checkpoint inhibitors used in cancer treatment. Rev. Med. Suisse 2020, 16, 1165–1168. [Google Scholar] [PubMed]
- Grivicich, I.; Regner, A.; da Rocha, A.B.; Grass, L.B.; Alves, P.A.; Kayser, G.B.; Schwartsmann, G.; Henriques, J.A. Irinotecan/5-fluorouracil combination induces alterations in mitochondrial membrane potential and caspases on colon cancer cell lines. Oncol. Res. 2005, 15, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wu, Y. Role of Mitophagy in Coronary Heart Disease: Targeting the Mitochondrial Dysfunction and Inflammatory Regulation. Front. Cardiovasc. Med. 2022, 9, 819454. [Google Scholar] [CrossRef]
- Tantawy, M.; Pamittan, F.G.; Singh, S.; Gong, Y. Epigenetic Changes Associated With Anthracycline-Induced Cardiotoxicity. Clin. Transl. Sci. 2021, 14, 36–46. [Google Scholar] [CrossRef]
- Ma, W.; Liu, M.; Liang, F.; Zhao, L.; Gao, C.; Jiang, X.; Zhang, X.; Zhan, H.; Hu, H.; Zhao, Z. Cardiotoxicity of sorafenib is mediated through elevation of ROS level and CaMKII activity and dysregulation of calcium homoeostasis. Basic Clin. Pharmacol. Toxicol. 2020, 126, 166–180. [Google Scholar] [CrossRef]
- Nishinaka, Y.; Sugiyama, S.; Yokota, M.; Saito, H.; Ozawa, T. The effects of a high dose of ascorbate on ischemia-reperfusion-induced mitochondrial dysfunction in canine hearts. Heart Vessel. 1992, 7, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, L.; Pratt, K.; Gavin, J. Endothelin-3-induced microvascular incompetence and mitochondrial damage in rat myocardium. Clin. Exp. Pharmacol. Physiol. 1992, 19, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Cheng, R.K.; Tian, R. Combat Doxorubicin Cardiotoxicity With the Power of Mitochondria Transfer. JACC CardioOncol. 2021, 3, 441–443. [Google Scholar] [CrossRef]
- Finsterer, J.; Ohnsorge, P. Influence of mitochondrion-toxic agents on the cardiovascular system. Regul. Toxicol. Pharmacol. 2013, 67, 434–445. [Google Scholar] [CrossRef]
- Szendrei, L.; Turoczi, T.; Kovacs, P.; Vecsernyes, M.; Das, D.K.; Tosaki, A. Mitochondrial gene expression and ventricular fibrillation in ischemic/reperfused nondiabetic and diabetic myocardium. Biochem. Pharmacol. 2002, 63, 543–552. [Google Scholar] [CrossRef]
- Aon, M.A.; Cortassa, S.; Akar, F.G.; O’Rourke, B. Mitochondrial criticality: A new concept at the turning point of life or death. Biochim. Biophys. Acta 2006, 1762, 232–240. [Google Scholar] [CrossRef]
- Tosaki, A. ArrhythmoGenoPharmacoTherapy. Front. Pharmacol. 2020, 11, 616. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, W.; Biermans, G.; Callewaert, G.; Vereecke, J.; Opie, L.; Carmeliet, E. The effect of inhibition of mitochondrial energy metabolism on the transient inward current of isolated guinea-pig ventricular myocytes. J. Mol. Cell. Cardiol. 1988, 20, 181–185. [Google Scholar] [CrossRef]
- Yang, K.C.; Bonini, M.G.; Dudley, S.C., Jr. Mitochondria and arrhythmias. Free Radic. Biol. Med. 2014, 71, 351–361. [Google Scholar] [CrossRef]
- Song, J.; Yang, R.; Yang, J.; Zhou, L. Mitochondrial Dysfunction-Associated Arrhythmogenic Substrates in Diabetes Mellitus. Front. Physiol. 2018, 9, 1670. [Google Scholar] [CrossRef]
- Yarmohammadi, F.; Rezaee, R.; Haye, A.W.; Karimi, G. Endoplasmic reticulum stress in doxorubicin-induced cardiotoxicity may be therapeutically targeted by natural and chemical compounds: A review. Pharmacol. Res. 2021, 164, 105383. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.W.; Choi, K.C. Effects of anticancer drugs on the cardiac mitochondrial toxicity and their underlying mechanisms for novel cardiac protective strategies. Life Sci. 2021, 277, 119607. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.J.; Zhang, J.; Xiao, M.; Wang, S.; Wang, B.J.; Guo, Y.; Tang, Y.; Gu, J. Molecular mechanisms of doxorubicin-induced cardiotoxicity: Novel roles of sirtuin 1-mediated signaling pathways. Cell. Mol. Life Sci. 2021, 78, 3105–3125. [Google Scholar] [CrossRef] [PubMed]
- Kohl, C.; Ravel, D.; Girard, J.; Pegorier, J.P. Effects of benfluorex on fatty acid and glucose metabolism in isolated rat hepatocytes: From metabolic fluxes to gene expression. Diabetes 2002, 51, 2363–2368. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lewis, M.J.; Khaliulin, I.; Hall, K.; Suleiman, M.S. Cardioprotection of Immature Heart by Simultaneous Activation of PKA and Epac: A Role for the Mitochondrial Permeability Transition Pore. Int. J. Mol. Sci. 2022, 23, 1720. [Google Scholar] [CrossRef]
- Krestinin, R.; Baburina, Y.; Odinokova, I.; Kruglov, A.; Fadeeva, I.; Zvyagina, A.; Sotnikova, L.; Krestinina, O. Isoproterenol-Induced Permeability Transition Pore-Related Dysfunction of Heart Mitochondria Is Attenuated by Astaxanthin. Biomedicines 2020, 8, 437. [Google Scholar] [CrossRef]
- Morikawa, Y.; Shibata, A.; Sasajima, Y.; Suenami, K.; Sato, K.; Takekoshi, Y.; Endo, S.; Ikari, A.; Matsunaga, T. Sibutramine facilitates apoptosis and contraction of aortic smooth muscle cells through elevating production of reactive oxygen species. Eur. J. Pharmacol. 2018, 841, 113–121. [Google Scholar] [CrossRef]
- Zhang, S.L.; Tang, H.B.; Hu, J.T.; Zang, Z.L.; Ding, X.; Li, S.; Yang, H. PGAM5-CypD pathway is involved in bromocriptine-induced RIP3/MLKL-dependent necroptosis of prolactinoma cells. Biomed. Pharm. 2019, 111, 638–648. [Google Scholar] [CrossRef]
- Lin, Y.T.; Lin, K.H.; Huang, C.J.; Wei, A.C. MitoTox: A comprehensive mitochondrial toxicity database. BMC Bioinform. 2021, 22, 369. [Google Scholar] [CrossRef]
- Hafez, A.A.; Jamali, Z.; Khezri, S.; Salimi, A. Thymoquinone reduces mitochondrial damage and death of cardiomyocytes induced by clozapine. Naunyn Schmiedebergs Arch. Pharm. 2021, 394, 1675–1684. [Google Scholar] [CrossRef]
- Nicolau-Galmes, F.; Asumendi, A.; Alonso-Tejerina, E.; Perez-Yarza, G.; Jangi, S.M.; Gardeazabal, J.; Arroyo-Berdugo, Y.; Careaga, J.M.; Diaz-Ramon, J.L.; Apraiz, A.; et al. Terfenadine induces apoptosis and autophagy in melanoma cells through ROS-dependent and -independent mechanisms. Apoptosis 2011, 16, 1253–1267. [Google Scholar] [CrossRef] [PubMed]
- Jangi, S.M.; Diaz-Perez, J.L.; Ochoa-Lizarralde, B.; Martin-Ruiz, I.; Asumendi, A.; Perez-Yarza, G.; Gardeazabal, J.; Diaz-Ramon, J.L.; Boyano, M.D. H1 histamine receptor antagonists induce genotoxic and caspase-2-dependent apoptosis in human melanoma cells. Carcinogenesis 2006, 27, 1787–1796. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.L.; Li, Z.Q.; Zhao, Y.J.; Zhao, S.M.; Zhu, L.; Li, T.; Fu, Y.; Li, H.J. Ginsenoside Rb1 protects cardiomyocytes against CoCl2-induced apoptosis in neonatal rats by inhibiting mitochondria permeability transition pore opening. Acta Pharmacol. Sin. 2010, 31, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Hartig, S.; Fries, S.; Balcarcel, R.R. Reduced mitochondrial membrane potential and metabolism correspond to acute chloroform toxicity of in vitro hepatocytes. J. Appl. Toxicol. 2005, 25, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Guastadisegni, C.; Balduzzi, M.; Mancuso, M.T.; Di Consiglio, E. Liver mitochondria alterations in chloroform-treated Sprague-Dawley rats. J. Toxicol. Environ. Health A 1999, 57, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Mu, W.; Zeifman, A.; Lofti, M.; Remillard, C.V.; Makino, A.; Perkins, D.L.; Garcia, J.G.; Yuan, J.X.; Zhang, W. Fenfluramine-induced gene dysregulation in human pulmonary artery smooth muscle and endothelial cells. Pulm. Circ. 2011, 1, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Loo, G.; Berlin, E.; Smith, J.T. Inhibition of mitochondrial palmitate oxidation by calmodulin antagonists. Int. J. Biochem. 1990, 22, 631–634. [Google Scholar] [CrossRef]
- Salimi, A.; Neshat, M.R.; Naserzadeh, P.; Pourahmad, J. Mitochondrial Permeability Transition Pore Sealing Agents and Antioxidants Protect Oxidative Stress and Mitochondrial Dysfunction Induced by Naproxen, Diclofenac and Celecoxib. Drug Res. 2019, 69, 598–605. [Google Scholar] [CrossRef]
- Tatematsu, Y.; Fujita, H.; Hayashi, H.; Yamamoto, A.; Tabata, A.; Nagamune, H.; Ohkura, K. Effects of the Nonsteroidal Anti-inflammatory Drug Celecoxib on Mitochondrial Function. Biol. Pharm. Bull. 2018, 41, 319–325. [Google Scholar] [CrossRef]
- Beaufils, F.; Esteves, P.; Enaud, R.; Germande, O.; Celle, A.; Marthan, R.; Trian, T.; Fayon, M.; Berger, P. Mitochondria are involved in bronchial smooth muscle remodeling in severe preschool wheezers. J. Allergy Clin. Immunol. 2021, 148, 645–651.e11. [Google Scholar] [CrossRef]
- Zychlinski, L. Mitochondrial alterations in the brain of the rat caused by chlorphentermine. Neuropharmacology 1986, 25, 1111–1117. [Google Scholar] [CrossRef]
- Rana, P.; Aleo, M.D.; Gosink, M.; Will, Y. Evaluation of in Vitro Mitochondrial Toxicity Assays and Physicochemical Properties for Prediction of Organ Toxicity Using 228 Pharmaceutical Drugs. Chem. Res. Toxicol. 2019, 32, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, A.; Ahmadian, E.; Azarmi, Y.; Parvizpur, A.; Fard, J.K.; Eghbal, M.A. Mechanistic Approach for Thioridazine-Induced Hepatotoxicity and Potential Benefits of Melatonin and/or Coenzyme Q10 on Freshly Isolated Rat Hepatocytes. Iran J. Pharm. Res. 2018, 17, 1465–1475. [Google Scholar]
- Paech, F.; Mingard, C.; Grunig, D.; Abegg, V.F.; Bouitbir, J.; Krahenbuhl, S. Mechanisms of mitochondrial toxicity of the kinase inhibitors ponatinib, regorafenib and sorafenib in human hepatic HepG2 cells. Toxicology 2018, 395, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Dwivedi, A.; Ray, L.; Chopra, D.; Dubey, D.; Srivastva, A.K.; Kumari, S.; Yadav, R.K.; Amar, S.K.; Haldar, C.; et al. PLGA nanoformulation of sparfloxacin enhanced antibacterial activity with photoprotective potential under ambient UV-R exposure. Int. J. Pharm. 2018, 541, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, C. The importance of drug discovery for treatment of cardiovascular diseases. Future Med. Chem. 2013, 5, 355–357. [Google Scholar] [CrossRef]
- Barth, E.; Stammler, G.; Speiser, B.; Schaper, J. Ultrastructural quantitation of mitochondria and myofilaments in cardiac muscle from 10 different animal species including man. J. Mol. Cell. Cardiol. 1992, 24, 669–681. [Google Scholar] [CrossRef]
- Croston, T.L.; Thapa, D.; Holden, A.A.; Tveter, K.J.; Lewis, S.E.; Shepherd, D.L.; Nichols, C.E.; Long, D.M.; Olfert, I.M.; Jagannathan, R.; et al. Functional deficiencies of subsarcolemmal mitochondria in the type 2 diabetic human heart. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H54–H65. [Google Scholar] [CrossRef]
- Dabkowski, E.R.; Williamson, C.L.; Bukowski, V.C.; Chapman, R.S.; Leonard, S.S.; Peer, C.J.; Callery, P.S.; Hollander, J.M. Diabetic cardiomyopathy-associated dysfunction in spatially distinct mitochondrial subpopulations. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H359–H369. [Google Scholar] [CrossRef]
- Lesnefsky, E.J.; Chen, Q.; Hoppel, C.L. Mitochondrial Metabolism in Aging Heart. Circ. Res. 2016, 118, 1593–1611. [Google Scholar] [CrossRef]
- Shimada, T.; Horita, K.; Murakami, M.; Ogura, R. Morphological studies of different mitochondrial populations in monkey myocardial cells. Cell. Tissue Res. 1984, 238, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Fernandez-Sanz, C.; Sheu, S.S. Regulation of mitochondrial bioenergetics by the non-canonical roles of mitochondrial dynamics proteins in the heart. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1991–2001. [Google Scholar] [CrossRef] [PubMed]
- Boengler, K.; Lochnit, G.; Schulz, R. Mitochondria “THE” target of myocardial conditioning. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1215–H1231. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.B.; Kalkhoran, S.B.; Hernandez-Resendiz, S.; Samangouei, P.; Ong, S.G.; Hausenloy, D.J. Mitochondrial-Shaping Proteins in Cardiac Health and Disease—The Long and the Short of It! Cardiovasc. Drugs Ther. 2017, 31, 87–107. [Google Scholar] [CrossRef] [PubMed]
- Jakobs, S.; Stephan, T.; Ilgen, P.; Bruser, C. Light Microscopy of Mitochondria at the Nanoscale. Annu. Rev. Biophys. 2020, 49, 289–308. [Google Scholar] [CrossRef]
- Chipuk, J.E.; Mohammed, J.N.; Gelles, J.D.; Chen, Y. Mechanistic connections between mitochondrial biology and regulated cell death. Dev. Cell. 2021, 56, 1221–1233. [Google Scholar] [CrossRef]
- Shoshan-Barmatz, V.; De Pinto, V.; Zweckstetter, M.; Raviv, Z.; Keinan, N.; Arbel, N. VDAC, a multi-functional mitochondrial protein regulating cell life and death. Mol. Asp. Med. 2010, 31, 227–285. [Google Scholar] [CrossRef]
- Vogel, F.; Bornhovd, C.; Neupert, W.; Reichert, A.S. Dynamic subcompartmentalization of the mitochondrial inner membrane. J. Cell. Biol. 2006, 175, 237–247. [Google Scholar] [CrossRef]
- Portella, D.C.N.; Rossi, E.A.; Paredes, B.D.; Bastos, T.M.; Meira, C.S.; Nonaka, C.V.K.; Silva, D.N.; Improta-Caria, A.; Moreira, D.R.M.; Leite, A.C.L.; et al. A Novel High-Content Screening-Based Method for Anti-Trypanosoma cruzi Drug Discovery Using Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Stem Cells Int. 2021, 2021, 2642807. [Google Scholar] [CrossRef]
- Punithavathi, V.R.; Shanmugapriya, K.; Prince, P.S. Protective effects of rutin on mitochondrial damage in isoproterenol-induced cardiotoxic rats: An in vivo and in vitro study. Cardiovasc. Toxicol. 2010, 10, 181–189. [Google Scholar] [CrossRef]
- Devika, P.T.; Stanely Mainzen Prince, P. (-)Epigallocatechin-gallate (EGCG) prevents mitochondrial damage in isoproterenol-induced cardiac toxicity in albino Wistar rats: A transmission electron microscopic and in vitro study. Pharmacol. Res. 2008, 57, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Wang, M.; Wang, R.Y.; Sun, X.; Du, Y.Y.; Ye, J.X.; Sun, G.B.; Sun, X.B. Salvianolic Acid A Ameliorates Arsenic Trioxide-Induced Cardiotoxicity Through Decreasing Cardiac Mitochondrial Injury and Promotes Its Anticancer Activity. Front. Pharmacol. 2018, 9, 487. [Google Scholar] [CrossRef] [PubMed]
- Brandolini, L.; Antonosante, A.; Giorgio, C.; Bagnasco, M.; d’Angelo, M.; Castelli, V.; Benedetti, E.; Cimini, A.; Allegretti, M. NSAIDs-dependent adaption of the mitochondria-proteasome system in immortalized human cardiomyocytes. Sci. Rep. 2020, 10, 18337. [Google Scholar] [CrossRef] [PubMed]
- Kwok, M.; Lee, C.; Li, H.S.; Deng, R.; Tsoi, C.; Ding, Q.; Tsang, S.Y.; Leung, K.T.; Yan, B.P.; Poon, E.N. Remdesivir induces persistent mitochondrial and structural damage in human induced pluripotent stem cell derived cardiomyocytes. Cardiovasc. Res. 2021. [Google Scholar] [CrossRef]
- Antonucci, S.; Di Sante, M.; Tonolo, F.; Pontarollo, L.; Scalcon, V.; Alanova, P.; Menabo, R.; Carpi, A.; Bindoli, A.; Rigobello, M.P.; et al. The Determining Role of Mitochondrial Reactive Oxygen Species Generation and Monoamine Oxidase Activity in Doxorubicin-Induced Cardiotoxicity. Antioxid. Redox. Signal 2021, 34, 531–550. [Google Scholar] [CrossRef]
- Jia, G.; Meng, Z.; Liu, C.; Ma, X.; Gao, J.; Liu, J.; Guo, R.; Yan, Z.; Christopher, T.; Lopez, B.; et al. Nicotine induces cardiac toxicity through blocking mitophagic clearance in young adult rat. Life Sci. 2020, 257, 118084. [Google Scholar] [CrossRef]
- French, K.J.; Coatney, R.W.; Renninger, J.P.; Hu, C.X.; Gales, T.L.; Zhao, S.; Storck, L.M.; Davis, C.B.; McSurdy-Freed, J.; Chen, E.; et al. Differences in effects on myocardium and mitochondria by angiogenic inhibitors suggest separate mechanisms of cardiotoxicity. Toxicol. Pathol. 2010, 38, 691–702. [Google Scholar] [CrossRef]
- Boran, T.; Akyildiz, A.G.; Jannuzzi, A.T.; Alpertunga, B. Extended regorafenib treatment can be linked with mitochondrial damage leading to cardiotoxicity. Toxicol. Lett. 2021, 336, 39–49. [Google Scholar] [CrossRef]
- Kerkela, R.; Grazette, L.; Yacobi, R.; Iliescu, C.; Patten, R.; Beahm, C.; Walters, B.; Shevtsov, S.; Pesant, S.; Clubb, F.J.; et al. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat. Med. 2006, 12, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.M.; Kim, H.; Yeon, J.H.; Lee, J.H.; Park, H.O. Identification of a Mitochondrial DNA Polymerase Affecting Cardiotoxicity of Sunitinib Using a Genome-Wide Screening on S. pombe Deletion Library. Toxicol. Sci. 2016, 149, 4–14. [Google Scholar] [CrossRef][Green Version]
- Swain, S.M.; Whaley, F.S.; Ewer, M.S. Congestive heart failure in patients treated with doxorubicin: A retrospective analysis of three trials. Cancer 2003, 97, 2869–2879. [Google Scholar] [CrossRef] [PubMed]
- Babaei, H.; Razmaraii, N.; Assadnassab, G.; Mohajjel Nayebi, A.; Azarmi, Y.; Mohammadnejad, D.; Azami, A. Ultrastructural and Echocardiographic Assessment of Chronic Doxorubicin-Induced Cardiotoxicity in Rats. Arch. Razi. Inst. 2020, 75, 55–62. [Google Scholar] [CrossRef]
- Gnanapragasam, A.; Yogeeta, S.; Subhashini, R.; Ebenezar, K.K.; Sathish, V.; Devaki, T. Adriamycin induced myocardial failure in rats: Protective role of Centella asiatica. Mol. Cell. Biochem. 2007, 294, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Lebrecht, D.; Kirschner, J.; Geist, A.; Haberstroh, J.; Walker, U.A. Respiratory chain deficiency precedes the disrupted calcium homeostasis in chronic doxorubicin cardiomyopathy. Cardiovasc. Pathol. 2010, 19, e167–e174. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Zhong, L.; Han, X.; Wang, H.; Zhong, J.; Xuan, Z. Astragalus membranaceus prevents daunorubicin-induced apoptosis of cultured neonatal cardiomyocytes: Role of free radical effect of Astragalus membranaceus on daunorubicin cardiotoxicity. Phytother. Res. 2009, 23, 761–767. [Google Scholar] [CrossRef]
- Jean, S.R.; Tulumello, D.V.; Riganti, C.; Liyanage, S.U.; Schimmer, A.D.; Kelley, S.O. Mitochondrial Targeting of Doxorubicin Eliminates Nuclear Effects Associated with Cardiotoxicity. ACS Chem. Biol. 2015, 10, 2007–2015. [Google Scholar] [CrossRef]
- Sun, J.; Sun, G.; Meng, X.; Wang, H.; Luo, Y.; Qin, M.; Ma, B.; Wang, M.; Cai, D.; Guo, P.; et al. Isorhamnetin protects against doxorubicin-induced cardiotoxicity in vivo and in vitro. PLoS ONE 2013, 8, e64526. [Google Scholar] [CrossRef]
- Brandao, S.R.; Reis-Mendes, A.; Domingues, P.; Duarte, J.A.; Bastos, M.L.; Carvalho, F.; Ferreira, R.; Costa, V.M. Exploring the aging effect of the anticancer drugs doxorubicin and mitoxantrone on cardiac mitochondrial proteome using a murine model. Toxicology 2021, 459, 152852. [Google Scholar] [CrossRef]
- Khuanjing, T.; Ongnok, B.; Maneechote, C.; Siri-Angkul, N.; Prathumsap, N.; Arinno, A.; Chunchai, T.; Arunsak, B.; Chattipakorn, S.C.; Chattipakorn, N. Acetylcholinesterase inhibitor ameliorates doxorubicin-induced cardiotoxicity through reducing RIP1-mediated necroptosis. Pharmacol. Res. 2021, 173, 105882. [Google Scholar] [CrossRef]
- Yao, Y.F.; Liu, X.; Li, W.J.; Shi, Z.W.; Yan, Y.X.; Wang, L.F.; Chen, M.; Xie, M.Y. (-)-Epigallocatechin-3-gallate alleviates doxorubicin-induced cardiotoxicity in sarcoma 180 tumor-bearing mice. Life Sci. 2017, 180, 151–159. [Google Scholar] [CrossRef]
- Bose, C.; Awasthi, S.; Sharma, R.; Benes, H.; Hauer-Jensen, M.; Boerma, M.; Singh, S.P. Sulforaphane potentiates anticancer effects of doxorubicin and attenuates its cardiotoxicity in a breast cancer model. PLoS ONE 2018, 13, e0193918. [Google Scholar] [CrossRef]
- Liu, D.; Ma, Z.; Di, S.; Yang, Y.; Yang, J.; Xu, L.; Reiter, R.J.; Qiao, S.; Yuan, J. AMPK/PGC1alpha activation by melatonin attenuates acute doxorubicin cardiotoxicity via alleviating mitochondrial oxidative damage and apoptosis. Free Radic. Biol. Med. 2018, 129, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Reis-Mendes, A.; Dores-Sousa, J.L.; Padrao, A.I.; Duarte-Araujo, M.; Duarte, J.A.; Seabra, V.; Goncalves-Monteiro, S.; Remiao, F.; Carvalho, F.; Sousa, E.; et al. Inflammation as a Possible Trigger for Mitoxantrone-Induced Cardiotoxicity: An In Vivo Study in Adult and Infant Mice. Pharmaceuticals 2021, 14, 510. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.R.; Sharma, A.; Lytwyn, M.; Bohonis, S.; Thliveris, J.; Singal, P.K.; Jassal, D.S. The cardioprotective role of probucol against anthracycline and trastuzumab-mediated cardiotoxicity. J. Am. Soc. Echocardiogr. 2011, 24, 699–705. [Google Scholar] [CrossRef]
- Al-Harthi, S.E.; Alarabi, O.M.; Ramadan, W.S.; Alaama, M.N.; Al-Kreathy, H.M.; Damanhouri, Z.A.; Khan, L.M.; Osman, A.M. Amelioration of doxorubicininduced cardiotoxicity by resveratrol. Mol. Med. Rep. 2014, 10, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ni, J.; Li, M.; Chen, J.; Han, L.; Zhu, Y.; Kong, D.; Mao, J.; Wang, Y.; Zhang, B.; et al. Ginsenoside Rg3 micelles mitigate doxorubicin-induced cardiotoxicity and enhance its anticancer efficacy. Drug Deliv. 2017, 24, 1617–1630. [Google Scholar] [CrossRef] [PubMed]
- Henninger, C.; Huelsenbeck, S.; Wenzel, P.; Brand, M.; Huelsenbeck, J.; Schad, A.; Fritz, G. Chronic heart damage following doxorubicin treatment is alleviated by lovastatin. Pharmacol. Res. 2015, 91, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Kalender, S.; Kalender, Y.; Ates, A.; Yel, M.; Olcay, E.; Candan, S. Protective role of antioxidant vitamin E and catechin on idarubicin-induced cardiotoxicity in rats. Braz. J. Med. Biol. Res. 2002, 35, 1379–1387. [Google Scholar] [CrossRef]
- Sudharsan, P.T.; Mythili, Y.; Selvakumar, E.; Varalakshmi, P. Lupeol and its ester exhibit protective role against cyclophosphamide-induced cardiac mitochondrial toxicity. J. Cardiovasc. Pharmacol. 2006, 47, 205–210. [Google Scholar] [CrossRef]
- Ma, H.; Jones, K.R.; Guo, R.; Xu, P.; Shen, Y.; Ren, J. Cisplatin compromises myocardial contractile function and mitochondrial ultrastructure: Role of endoplasmic reticulum stress. Clin. Exp. Pharmacol. Physiol. 2010, 37, 460–465. [Google Scholar] [CrossRef]
- Laird-Fick, H.S.; Tokala, H.; Kandola, S.; Kehdi, M.; Pelosi, A.; Wang, L.; Grondahl, B. Early morphological changes in cardiac mitochondria after subcutaneous administration of trastuzumab in rabbits: Possible prevention with oral selenium supplementation. Cardiovasc. Pathol. 2020, 44, 107159. [Google Scholar] [CrossRef] [PubMed]
- Force, T.; Kolaja, K.L. Cardiotoxicity of kinase inhibitors: The prediction and translation of preclinical models to clinical outcomes. Nat. Rev. Drug Discov. 2011, 10, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Khezri, S.; Atashbar, S.; Azizian, S.; Shaikhgermchi, Z.; Kurdpour, P.; Salimi, A. Calcitriol Reduces Adverse Effects of Diclofenac on Mitochondrial Function in Isolated Rat Heart Mitochondria. Drug Res. 2020, 70, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Zhu, Z.N.; Wang, J.Z.; Huang, S.M.; Feng, X.M.; Li, A.Y.; Yang, D.L.; Wang, B.J. Assessment of mitochondrial toxicity induced by zidovudine and adefovir dipivoxil in rats. Chin. J. Hepatol. 2012, 20, 794–797. [Google Scholar] [CrossRef]
- Botelho, A.F.M.; Santos-Miranda, A.; Joca, H.C.; Mattoso, C.R.S.; de Oliveira, M.S.; Pierezan, F.; Cruz, J.S.; Soto-Blanco, B.; Melo, M.M. Hydroalcoholic extract from Nerium oleander L. (Apocynaceae) elicits arrhythmogenic activity. J. Ethnopharmacol. 2017, 206, 170–177. [Google Scholar] [CrossRef]
- Sharmila Queenthy, S.; Stanely Mainzen Prince, P.; John, B. Diosmin Prevents Isoproterenol-Induced Heart Mitochondrial Oxidative Stress in Rats. Cardiovasc. Toxicol. 2018, 18, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Seydi, E.; Tabbati, Y.; Pourahmad, J. Toxicity of Atenolol and Propranolol on Rat Heart Mitochondria. Drug Res. 2020, 70, 151–157. [Google Scholar] [CrossRef]
- Salimi, A.; Eybagi, S.; Seydi, E.; Naserzadeh, P.; Kazerouni, N.P.; Pourahmad, J. Toxicity of macrolide antibiotics on isolated heart mitochondria: A justification for their cardiotoxic adverse effect. Xenobiotica 2016, 46, 82–93. [Google Scholar] [CrossRef]
- Peng, F.; Zhang, N.; Wang, C.; Wang, X.; Huang, W.; Peng, C.; He, G.; Han, B. Aconitine induces cardiomyocyte damage by mitigating BNIP3-dependent mitophagy and the TNFalpha-NLRP3 signalling axis. Cell. Prolif. 2020, 53, e12701. [Google Scholar] [CrossRef]
- Seydi, E.; Servati, T.; Samiei, F.; Naserzadeh, P.; Pourahmad, J. Toxicity of Pioglitazone on Mitochondria Isolated from Brain and Heart: An Analysis for Probable Drug-Induced Neurotoxicity and Cardiotoxicity. Drug Res. 2020, 70, 112–118. [Google Scholar] [CrossRef]
- Liu, Y.; Nguyen, P.; Baris, T.Z.; Poirier, M.C. Molecular analysis of mitochondrial compromise in rodent cardiomyocytes exposed long term to nucleoside reverse transcriptase inhibitors (NRTIs). Cardiovasc. Toxicol. 2012, 12, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Mythili, Y.; Sudharsan, P.T.; Varalakshmi, P. dl-alpha-lipoic acid ameliorates cyclophosphamide induced cardiac mitochondrial injury. Toxicology 2005, 215, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, S.; Kawano, H.; Hayashi, T.; Satoh, O.; Yonekura, T.; Eguchi, M.; Takeno, M.; Tsuneto, A.; Koide, Y.; Jo, T.; et al. Cyclophosphamide-induced cardiotoxicity with a prolonged clinical course diagnosed on an endomyocardial biopsy. Intern. Med. 2013, 52, 2311–2315. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.B.; Tani, Y.; Witt, K.; Johnson, J.A.; Peddada, S.; Dunnick, J.; Nyska, A. Mitochondrial damage revealed by morphometric and semiquantitative analysis of mouse pup cardiomyocytes following in utero and postnatal exposure to zidovudine and lamivudine. Toxicol. Sci. 2004, 81, 512–517. [Google Scholar] [CrossRef]
- Yin, J.; Guo, J.; Zhang, Q.; Cui, L.; Zhang, L.; Zhang, T.; Zhao, J.; Li, J.; Middleton, A.; Carmichael, P.L.; et al. Doxorubicin-induced mitophagy and mitochondrial damage is associated with dysregulation of the PINK1/parkin pathway. Toxicol. In Vitro 2018, 51, 1–10. [Google Scholar] [CrossRef]
- Gharanei, M.; Hussain, A.; Janneh, O.; Maddock, H. Attenuation of doxorubicin-induced cardiotoxicity by mdivi-1: A mitochondrial division/mitophagy inhibitor. PLoS ONE 2013, 8, e77713. [Google Scholar] [CrossRef]
- Watanabe, M.; Funakoshi, T.; Unuma, K.; Aki, T.; Uemura, K. Activation of the ubiquitin-proteasome system against arsenic trioxide cardiotoxicity involves ubiquitin ligase Parkin for mitochondrial homeostasis. Toxicology 2014, 322, 43–50. [Google Scholar] [CrossRef]
- Mamoshina, P.; Rodriguez, B.; Bueno-Orovio, A. Toward a broader view of mechanisms of drug cardiotoxicity. Cell. Rep. Med. 2021, 2, 100216. [Google Scholar] [CrossRef]
- Nomura, R.; Sato, T.; Sato, Y.; Medin, J.A.; Kushimoto, S.; Yanagisawa, T. Azidothymidine-triphosphate impairs mitochondrial dynamics by disrupting the quality control system. Redox Biol. 2017, 13, 407–417. [Google Scholar] [CrossRef]
- Sivakumar, A.; Shanmugarajan, S.; Subbiah, R.; Balakrishnan, R. Cardiac Mitochondrial PTEN-L determines cell fate between apoptosis and survival during chronic alcohol consumption. Apoptosis 2020, 25, 590–604. [Google Scholar] [CrossRef]
- Sorrentino, V.; Menzies, K.J.; Auwerx, J. Repairing Mitochondrial Dysfunction in Disease. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 353–389. [Google Scholar] [CrossRef] [PubMed]
- Martin-Fernandez, B.; Gredilla, R. Mitochondria and oxidative stress in heart aging. Age 2016, 38, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Alford, J.; Qiu, H. Structural and Functional Remodeling of Mitochondria in Cardiac Diseases. Int. J. Mol. Sci. 2021, 22, 4167. [Google Scholar] [CrossRef]
- Marin, W.; Marin, D.; Ao, X.; Liu, Y. Mitochondria as a therapeutic target for cardiac ischemiareperfusion injury (Review). Int. J. Mol. Med. 2021, 47, 485–499. [Google Scholar] [CrossRef]
- Yehualashet, A.S.; Belachew, T.F.; Kifle, Z.D.; Abebe, A.M. Targeting Cardiac Metabolic Pathways: A Role in Ischemic Management. Vasc. Health Risk Manag. 2020, 16, 353–365. [Google Scholar] [CrossRef]
- Ghosh, R.; Hwang, S.M.; Cui, Z.; Gilda, J.E.; Gomes, A.V. Different effects of the nonsteroidal anti-inflammatory drugs meclofenamate sodium and naproxen sodium on proteasome activity in cardiac cells. J. Mol. Cell. Cardiol. 2016, 94, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Karkhanis, A.; Leow, J.W.H.; Hagen, T.; Chan, E.C.Y. Dronedarone-Induced Cardiac Mitochondrial Dysfunction and Its Mitigation by Epoxyeicosatrienoic Acids. Toxicol. Sci. 2018, 163, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, Z.A.; Harvey, R.F.; Pryde, K.R.; Mistry, S.; Hardy, R.E.; Serreli, R.; Chung, I.; Allen, T.E.; Stoneley, M.; MacFarlane, M.; et al. Identification of a novel toxicophore in anti-cancer chemotherapeutics that targets mitochondrial respiratory complex I. Elife 2020, 9, e55845. [Google Scholar] [CrossRef] [PubMed]
- Vineetha, V.P.; Soumya, R.S.; Raghu, K.G. Phloretin ameliorates arsenic trioxide induced mitochondrial dysfunction in H9c2 cardiomyoblasts mediated via alterations in membrane permeability and ETC complexes. Eur. J. Pharmacol. 2015, 754, 162–172. [Google Scholar] [CrossRef]
- Moreno-Sanchez, R.; Bravo, C.; Vasquez, C.; Ayala, G.; Silveira, L.H.; Martinez-Lavin, M. Inhibition and uncoupling of oxidative phosphorylation by nonsteroidal anti-inflammatory drugs: Study in mitochondria, submitochondrial particles, cells, and whole heart. Biochem. Pharmacol. 1999, 57, 743–752. [Google Scholar] [CrossRef]
- Hoch, F.L. Cardiolipins and biomembrane function. Biochim. Biophys. Acta 1992, 1113, 71–133. [Google Scholar] [CrossRef] [PubMed]
- Petronilli, V.; Penzo, D.; Scorrano, L.; Bernardi, P.; Di Lisa, F. The mitochondrial permeability transition, release of cytochrome c and cell death. Correlation with the duration of pore openings in situ. J. Biol. Chem. 2001, 276, 12030–12034. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.N.; Korge, P.; Honda, H.M.; Ping, P. Role of the mitochondrial permeability transition in myocardial disease. Circ. Res. 2003, 93, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Sztark, F.; Nouette-Gaulain, K.; Malgat, M.; Dabadie, P.; Mazat, J.P. Absence of stereospecific effects of bupivacaine isomers on heart mitochondrial bioenergetics. Anesthesiology 2000, 93, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Dykens, J.A.; Will, Y. The significance of mitochondrial toxicity testing in drug development. Drug Discov. Today 2007, 12, 777–785. [Google Scholar] [CrossRef]
- Li, N.; Ragheb, K.; Lawler, G.; Sturgis, J.; Rajwa, B.; Melendez, J.A.; Robinson, J.P. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J. Biol. Chem. 2003, 278, 8516–8525. [Google Scholar] [CrossRef]
- Tsutsui, H.; Kinugawa, S.; Matsushima, S. Oxidative stress and heart failure. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H2181–H2190. [Google Scholar] [CrossRef]
- Hu, H.; Lin, Y.; Xu, X.; Lin, S.; Chen, X.; Wang, S. The alterations of mitochondrial DNA in coronary heart disease. Exp. Mol. Pathol. 2020, 114, 104412. [Google Scholar] [CrossRef]
- Bonifacio, A.; Mullen, P.J.; Mityko, I.S.; Navegantes, L.C.; Bouitbir, J.; Krahenbuhl, S. Simvastatin induces mitochondrial dysfunction and increased atrogin-1 expression in H9c2 cardiomyocytes and mice in vivo. Arch. Toxicol. 2016, 90, 203–215. [Google Scholar] [CrossRef]
- Liu, Y.; Shim, E.; Nguyen, P.; Gibbons, A.T.; Mitchell, J.B.; Poirier, M.C. Tempol protects cardiomyocytes from nucleoside reverse transcriptase inhibitor-induced mitochondrial toxicity. Toxicol. Sci. 2014, 139, 133–141. [Google Scholar] [CrossRef]
- Grundmanova, M.; Jarkovska, D.; Suss, A.; Tuma, Z.; Markova, M.; Grundman, Z.; El-Kadi, A.; Cedikova, M.; Stengl, M.; Kuncova, J. Propofol-induced mitochondrial and contractile dysfunction of the rat ventricular myocardium. Physiol. Res. 2016, 65, S601–S609. [Google Scholar] [CrossRef] [PubMed]
- Hanley, P.J.; Ray, J.; Brandt, U.; Daut, J. Halothane, isoflurane and sevoflurane inhibit NADH:ubiquinone oxidoreductase (complex I) of cardiac mitochondria. J. Physiol. 2002, 544, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Acosta, D., Jr. Effect of cocaine on mitochondrial electron transport chain evaluated in primary cultures of neonatal rat myocardial cells and in isolated mitochondrial preparations. Drug Chem. Toxicol. 2000, 23, 339–348. [Google Scholar] [CrossRef] [PubMed]
- BB, N.G.; Sanchez, H.; Zoll, J.; Ribera, F.; Dufour, S.; Lampert, E.; Kindo, M.; Geny, B.; Ventura-Clapier, R.; Mettauer, B. Oxidative capacities of cardiac and skeletal muscles of heart transplant recipients: Mitochondrial effects of cyclosporin-A and its vehicle Cremophor-EL. Fundam. Clin. Pharmacol. 2014, 28, 151–160. [Google Scholar] [CrossRef]
- Ghosh, R.; Goswami, S.K.; Feitoza, L.; Hammock, B.; Gomes, A.V. Diclofenac induces proteasome and mitochondrial dysfunction in murine cardiomyocytes and hearts. Int. J. Cardiol. 2016, 223, 923–935. [Google Scholar] [CrossRef]
- Pointon, A.V.; Walker, T.M.; Phillips, K.M.; Luo, J.; Riley, J.; Zhang, S.D.; Parry, J.D.; Lyon, J.J.; Marczylo, E.L.; Gant, T.W. Doxorubicin in vivo rapidly alters expression and translation of myocardial electron transport chain genes, leads to ATP loss and caspase 3 activation. PLoS ONE 2010, 5, e12733. [Google Scholar] [CrossRef]
- Nowis, D.; Maczewski, M.; Mackiewicz, U.; Kujawa, M.; Ratajska, A.; Wieckowski, M.R.; Wilczynski, G.M.; Malinowska, M.; Bil, J.; Salwa, P.; et al. Cardiotoxicity of the anticancer therapeutic agent bortezomib. Am. J. Pathol. 2010, 176, 2658–2668. [Google Scholar] [CrossRef]
- Rossato, L.G.; Costa, V.M.; Dallegrave, E.; Arbo, M.; Silva, R.; Ferreira, R.; Amado, F.; Dinis-Oliveira, R.J.; Duarte, J.A.; de Lourdes Bastos, M.; et al. Mitochondrial cumulative damage induced by mitoxantrone: Late onset cardiac energetic impairment. Cardiovasc. Toxicol. 2014, 14, 30–40. [Google Scholar] [CrossRef]
- Dzimiri, N. Effects of procainamide, tocainide and phenytoin on guinea pig cardiac mitochondrial ATPase activity. Res. Commun. Chem. Pathol. Pharmacol. 1993, 80, 121–124. [Google Scholar]
- Hu, C.; Ge, F.; Hyodo, E.; Arai, K.; Iwata, S.; Lobdell, H.t.; Walewski, J.L.; Zhou, S.; Clugston, R.D.; Jiang, H.; et al. Chronic ethanol consumption increases cardiomyocyte fatty acid uptake and decreases ventricular contractile function in C57BL/6J mice. J. Mol. Cell. Cardiol. 2013, 59, 30–40. [Google Scholar] [CrossRef]
- Kido, K.; Ito, H.; Yamamoto, Y.; Makita, K.; Uchida, T. Cytotoxicity of propofol in human induced pluripotent stem cell-derived cardiomyocytes. J. Anesth. 2018, 32, 120–131. [Google Scholar] [CrossRef]
- Wang, H.; Sheehan, R.P.; Palmer, A.C.; Everley, R.A.; Boswell, S.A.; Ron-Harel, N.; Ringel, A.E.; Holton, K.M.; Jacobson, C.A.; Erickson, A.R.; et al. Adaptation of Human iPSC-Derived Cardiomyocytes to Tyrosine Kinase Inhibitors Reduces Acute Cardiotoxicity via Metabolic Reprogramming. Cell. Syst. 2019, 8, 412–426.e7. [Google Scholar] [CrossRef] [PubMed]
- Nulton-Persson, A.C.; Szweda, L.I.; Sadek, H.A. Inhibition of cardiac mitochondrial respiration by salicylic acid and acetylsalicylate. J. Cardiovasc. Pharmacol. 2004, 44, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Hiller, N.; Mirtschink, P.; Merkel, C.; Knels, L.; Oertel, R.; Christ, T.; Deussen, A.; Koch, T.; Stehr, S.N. Myocardial accumulation of bupivacaine and ropivacaine is associated with reversible effects on mitochondria and reduced myocardial function. Anesth. Analg. 2013, 116, 83–92. [Google Scholar] [CrossRef]
- Branca, D.; Vincenti, E.; Scutari, G. Influence of the anesthetic 2,6-diisopropylphenol (propofol) on isolated rat heart mitochondria. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1995, 110, 41–45. [Google Scholar] [CrossRef]
- Graf, B.M. The cardiotoxicity of local anesthetics: The place of ropivacaine. Curr. Top Med. Chem. 2001, 1, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Nemade, H.; Chaudhari, U.; Acharya, A.; Hescheler, J.; Hengstler, J.G.; Papadopoulos, S.; Sachinidis, A. Cell death mechanisms of the anti-cancer drug etoposide on human cardiomyocytes isolated from pluripotent stem cells. Arch. Toxicol. 2018, 92, 1507–1524. [Google Scholar] [CrossRef] [PubMed]
- Asiri, Y.A. Probucol attenuates cyclophosphamide-induced oxidative apoptosis, p53 and Bax signal expression in rat cardiac tissues. Oxid. Med. Cell. Longev. 2010, 3, 308–316. [Google Scholar] [CrossRef]
- Nagi, M.N.; Al-Shabanah, O.A.; Hafez, M.M.; Sayed-Ahmed, M.M. Thymoquinone supplementation attenuates cyclophosphamide-induced cardiotoxicity in rats. J. Biochem. Mol. Toxicol. 2011, 25, 135–142. [Google Scholar] [CrossRef]
- Gorini, S.; De Angelis, A.; Berrino, L.; Malara, N.; Rosano, G.; Ferraro, E. Chemotherapeutic Drugs and Mitochondrial Dysfunction: Focus on Doxorubicin, Trastuzumab, and Sunitinib. Oxid. Med. Cell. Longev. 2018, 2018, 7582730. [Google Scholar] [CrossRef]
- Sayed-Ahmed, M.M.; Alrufaiq, B.I.; Alrikabi, A.; Abdullah, M.L.; Hafez, M.M.; Al-Shabanah, O.A. Carnitine Supplementation Attenuates Sunitinib-Induced Inhibition of AMP-Activated Protein Kinase Downstream Signals in Cardiac Tissues. Cardiovasc. Toxicol. 2019, 19, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.D.; Babiarz, J.E.; Abrams, R.M.; Guo, L.; Kameoka, S.; Chiao, E.; Taunton, J.; Kolaja, K.L. Use of human stem cell derived cardiomyocytes to examine sunitinib mediated cardiotoxicity and electrophysiological alterations. Toxicol. Appl. Pharmacol. 2011, 257, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Thai, P.N.; Ren, L.; Xu, W.; Overton, J.; Timofeyev, V.; Nader, C.E.; Haddad, M.; Yang, J.; Gomes, A.V.; Hammock, B.D.; et al. Chronic Diclofenac Exposure Increases Mitochondrial Oxidative Stress, Inflammatory Mediators, and Cardiac Dysfunction. Cardiovasc. Drugs Ther. 2021. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Shin, J.S.; Park, S.J.; Jung, E.; Park, Y.G.; Lee, J.; Kim, S.J.; Park, H.J.; Lee, J.H.; Park, S.M.; et al. Antiviral activity and safety of remdesivir against SARS-CoV-2 infection in human pluripotent stem cell-derived cardiomyocytes. Antivir. Res. 2020, 184, 104955. [Google Scholar] [CrossRef]
- Martins, M.J.; Roque Bravo, R.; Enea, M.; Carmo, H.; Carvalho, F.; Bastos, M.L.; Dinis-Oliveira, R.J.; Dias da Silva, D. Ethanol addictively enhances the in vitro cardiotoxicity of cocaine through oxidative damage, energetic deregulation, and apoptosis. Arch. Toxicol. 2018, 92, 2311–2325. [Google Scholar] [CrossRef] [PubMed]
- Vergeade, A.; Mulder, P.; Vendeville-Dehaudt, C.; Estour, F.; Fortin, D.; Ventura-Clapier, R.; Thuillez, C.; Monteil, C. Mitochondrial impairment contributes to cocaine-induced cardiac dysfunction: Prevention by the targeted antioxidant MitoQ. Free Radic. Biol. Med. 2010, 49, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Vergeade, A.; Mulder, P.; Vendeville, C.; Ventura-Clapier, R.; Thuillez, C.; Monteil, C. Xanthine oxidase contributes to mitochondrial ROS generation in an experimental model of cocaine-induced diastolic dysfunction. J. Cardiovasc. Pharmacol. 2012, 60, 538–543. [Google Scholar] [CrossRef]
- Liu, Y.; Shim, E.; Crespo-Mejias, Y.; Nguyen, P.; Gibbons, A.; Liu, D.; Shide, E.; Poirier, M.C. Cardiomyocytes are Protected from Antiretroviral Nucleoside Analog-Induced Mitochondrial Toxicity by Overexpression of PGC-1alpha. Cardiovasc. Toxicol. 2015, 15, 224–231. [Google Scholar] [CrossRef]
- Lange, L.G.; Sobel, B.E. Mitochondrial dysfunction induced by fatty acid ethyl esters, myocardial metabolites of ethanol. J. Clin. Investig. 1983, 72, 724–731. [Google Scholar] [CrossRef]
- Jyoti, S.; Tandon, S. Disruption of mitochondrial membrane potential coupled with alterations in cardiac biomarker expression as early cardiotoxic signatures in human ES cell-derived cardiac cells. Hum. Exp. Toxicol. 2019, 38, 1111–1124. [Google Scholar] [CrossRef]
- Zhao, L. Protective effects of trimetazidine and coenzyme Q10 on cisplatin-induced cardiotoxicity by alleviating oxidative stress and mitochondrial dysfunction. Anatol. J. Cardiol. 2019, 22, 232–239. [Google Scholar] [CrossRef]
- Vineetha, V.P.; Prathapan, A.; Soumya, R.S.; Raghu, K.G. Arsenic trioxide toxicity in H9c2 myoblasts--damage to cell organelles and possible amelioration with Boerhavia diffusa. Cardiovasc. Toxicol. 2013, 13, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, M.; Pinto, A.; Popolo, A. Trastuzumab-induced cardiotoxicity and role of mitochondrial connexin43 in the adaptive response. Toxicol. In Vitro 2020, 67, 104926. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.Q.; Yu, Y.; Chen, H.; Li, M.; Ihsan, A.; Tong, H.Y.; Huang, X.J.; Gao, Y. Sweroside Alleviated Aconitine-Induced Cardiac Toxicity in H9c2 Cardiomyoblast Cell Line. Front. Pharmacol. 2018, 9, 1138. [Google Scholar] [CrossRef] [PubMed]
- Sudheesh, N.P.; Ajith, T.A.; Janardhanan, K.K. Ganoderma lucidum ameliorate mitochondrial damage in isoproterenol-induced myocardial infarction in rats by enhancing the activities of TCA cycle enzymes and respiratory chain complexes. Int. J. Cardiol. 2013, 165, 117–125. [Google Scholar] [CrossRef]
- Pereira, G.C.; Pereira, S.P.; Tavares, L.C.; Carvalho, F.S.; Magalhaes-Novais, S.; Barbosa, I.A.; Santos, M.S.; Bjork, J.; Moreno, A.J.; Wallace, K.B.; et al. Cardiac cytochrome c and cardiolipin depletion during anthracycline-induced chronic depression of mitochondrial function. Mitochondrion 2016, 30, 95–104. [Google Scholar] [CrossRef]
- Sayed-Ahmed, M.M.; Aldelemy, M.L.; Al-Shabanah, O.A.; Hafez, M.M.; Al-Hosaini, K.A.; Al-Harbi, N.O.; Al-Sharary, S.D.; Al-Harbi, M.M. Inhibition of gene expression of carnitine palmitoyltransferase I and heart fatty acid binding protein in cyclophosphamide and ifosfamide-induced acute cardiotoxic rat models. Cardiovasc. Toxicol. 2014, 14, 232–242. [Google Scholar] [CrossRef]
- Mihailovic, D.; Nikolic, J.; Bjelakovic, B.B.; Stankovic, B.N.; Bjelakovic, G. Morphometric and biochemical characteristics of short-term effects of ethanol on rat cardiac muscle. Exp. Toxicol. Pathol. 1999, 51, 545–547. [Google Scholar] [CrossRef]
- Figueira, T.R.; Barros, M.H.; Camargo, A.A.; Castilho, R.F.; Ferreira, J.C.; Kowaltowski, A.J.; Sluse, F.E.; Souza-Pinto, N.C.; Vercesi, A.E. Mitochondria as a source of reactive oxygen and nitrogen species: From molecular mechanisms to human health. Antioxid. Redox Signal. 2013, 18, 2029–2074. [Google Scholar] [CrossRef]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, Y.; Saredy, J.; Wang, X.; Drummer Iv, C.; Shao, Y.; Saaoud, F.; Xu, K.; Liu, M.; Yang, W.Y.; et al. ROS systems are a new integrated network for sensing homeostasis and alarming stresses in organelle metabolic processes. Redox Biol. 2020, 37, 101696. [Google Scholar] [CrossRef] [PubMed]
- Berndt, C.; Lillig, C.H.; Holmgren, A. Thiol-based mechanisms of the thioredoxin and glutaredoxin systems: Implications for diseases in the cardiovascular system. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H1227–H1236. [Google Scholar] [CrossRef] [PubMed]
- Wisnovsky, S.; Lei, E.K.; Jean, S.R.; Kelley, S.O. Mitochondrial Chemical Biology: New Probes Elucidate the Secrets of the Powerhouse of the Cell. Cell. Chem. Biol. 2016, 23, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Torrealba, N.; Aranguiz, P.; Alonso, C.; Rothermel, B.A.; Lavandero, S. Mitochondria in Structural and Functional Cardiac Remodeling. Adv. Exp. Med. Biol. 2017, 982, 277–306. [Google Scholar] [CrossRef]
- Li, A.; Zheng, N.; Ding, X. Mitochondrial abnormalities: A hub in metabolic syndrome-related cardiac dysfunction caused by oxidative stress. Heart Fail. Rev. 2022, 27, 1387–1394. [Google Scholar] [CrossRef]
- Gao, R.Y.; Mukhopadhyay, P.; Mohanraj, R.; Wang, H.; Horvath, B.; Yin, S.; Pacher, P. Resveratrol attenuates azidothymidine-induced cardiotoxicity by decreasing mitochondrial reactive oxygen species generation in human cardiomyocytes. Mol. Med. Rep. 2011, 4, 151–155. [Google Scholar] [CrossRef]
- Myers, C. The role of iron in doxorubicin-induced cardiomyopathy. Semin. Oncol. 1998, 25, 10–14. [Google Scholar]
- Gustafson, D.L.; Swanson, J.D.; Pritsos, C.A. Modulation of glutathione and glutathione dependent antioxidant enzymes in mouse heart following doxorubicin therapy. Free Radic. Res. Commun. 1993, 19, 111–120. [Google Scholar] [CrossRef]
- Adam-Vizi, V.; Chinopoulos, C. Bioenergetics and the formation of mitochondrial reactive oxygen species. Trends Pharmacol. Sci. 2006, 27, 639–645. [Google Scholar] [CrossRef]
- Nadanaciva, S.; Will, Y. New insights in drug-induced mitochondrial toxicity. Curr. Pharm. Des. 2011, 17, 2100–2112. [Google Scholar] [CrossRef]
- Oz, E.; Erbas, D.; Surucu, H.S.; Duzgun, E. Prevention of doxorubicin-induced cardiotoxicity by melatonin. Mol. Cell. Biochem. 2006, 282, 31–37. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Li, G.Y.; Liu, Y.; Chai, L.M.; Chen, J.X.; Zhang, Y.; Du, Z.M.; Lu, Y.J.; Yang, B.F. Resveratrol protects against arsenic trioxide-induced cardiotoxicity in vitro and in vivo. Br. J. Pharmacol. 2008, 154, 105–113. [Google Scholar] [CrossRef] [PubMed]
- de la Asuncion, J.G.; Del Olmo, M.L.; Gomez-Cambronero, L.G.; Sastre, J.; Pallardo, F.V.; Vina, J. AZT induces oxidative damage to cardiac mitochondria: Protective effect of vitamins C and E. Life Sci. 2004, 76, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.J.; Doroshow, J.H. Redox cycling of anthracyclines by cardiac mitochondria. I. Anthracycline radical formation by NADH dehydrogenase. J. Biol. Chem. 1986, 261, 3060–3067. [Google Scholar] [CrossRef]
- Bloom, M.W.; Hamo, C.E.; Cardinale, D.; Ky, B.; Nohria, A.; Baer, L.; Skopicki, H.; Lenihan, D.J.; Gheorghiade, M.; Lyon, A.R.; et al. Cancer Therapy-Related Cardiac Dysfunction and Heart Failure: Part 1: Definitions, Pathophysiology, Risk Factors, and Imaging. Circ. Heart Fail. 2016, 9, e002661. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Bai, Z.; Lv, D.; Liu, H.; Li, X.; Chen, X. Rescue effect of lipid emulsion on bupivacaine-induced cardiac toxicity in cardiomyocytes. Mol. Med. Rep. 2015, 12, 3739–3747. [Google Scholar] [CrossRef] [PubMed]
- Guven, A.; Yavuz, O.; Cam, M.; Ercan, F.; Bukan, N.; Comunoglu, C. Melatonin protects against epirubicin-induced cardiotoxicity. Acta Histochem. 2007, 109, 52–60. [Google Scholar] [CrossRef]
- Serrano, J.; Palmeira, C.M.; Kuehl, D.W.; Wallace, K.B. Cardioselective and cumulative oxidation of mitochondrial DNA following subchronic doxorubicin administration. Biochim. Biophys. Acta 1999, 1411, 201–205. [Google Scholar] [CrossRef]
- Wu, Z.J.; Yu, J.; Fang, Q.J.; Lian, J.B.; Wang, R.X.; He, R.L.; Lin, M.J. Sodium ferulate protects against daunorubicin-induced cardiotoxicity by inhibition of mitochondrial apoptosis in juvenile rats. J. Cardiovasc. Pharmacol. 2014, 63, 360–368. [Google Scholar] [CrossRef]
- Mercer, T.R.; Neph, S.; Dinger, M.E.; Crawford, J.; Smith, M.A.; Shearwood, A.M.; Haugen, E.; Bracken, C.P.; Rackham, O.; Stamatoyannopoulos, J.A.; et al. The human mitochondrial transcriptome. Cell 2011, 146, 645–658. [Google Scholar] [CrossRef]
- Barshad, G.; Marom, S.; Cohen, T.; Mishmar, D. Mitochondrial DNA Transcription and Its Regulation: An Evolutionary Perspective. Trends Genet. 2018, 34, 682–692. [Google Scholar] [CrossRef]
- Franci, L.; Tubita, A.; Bertolino, F.M.; Palma, A.; Cannino, G.; Settembre, C.; Rasola, A.; Rovida, E.; Chiariello, M. MAPK15 protects from oxidative stress-dependent cellular senescence by inducing the mitophagic process. Aging Cell 2022, e13620. [Google Scholar] [CrossRef]
- Douarre, C.; Sourbier, C.; Dalla Rosa, I.; Brata Das, B.; Redon, C.E.; Zhang, H.; Neckers, L.; Pommier, Y. Mitochondrial topoisomerase I is critical for mitochondrial integrity and cellular energy metabolism. PLoS ONE 2012, 7, e41094. [Google Scholar] [CrossRef]
- Setzer, B.; Schlesier, M.; Thomas, A.K.; Walker, U.A. Mitochondrial toxicity of nucleoside analogues in primary human lymphocytes. Antivir. Ther. 2005, 10, 327–334. [Google Scholar] [CrossRef] [PubMed]
- McKee, E.E.; Ferguson, M.; Bentley, A.T.; Marks, T.A. Inhibition of mammalian mitochondrial protein synthesis by oxazolidinones. Antimicrob. Agents Chemother. 2006, 50, 2042–2049. [Google Scholar] [CrossRef] [PubMed]
- Nagiec, E.E.; Wu, L.; Swaney, S.M.; Chosay, J.G.; Ross, D.E.; Brieland, J.K.; Leach, K.L. Oxazolidinones inhibit cellular proliferation via inhibition of mitochondrial protein synthesis. Antimicrob. Agents Chemother. 2005, 49, 3896–3902. [Google Scholar] [CrossRef] [PubMed]
- Bottger, E.C.; Springer, B.; Prammananan, T.; Kidan, Y.; Sander, P. Structural basis for selectivity and toxicity of ribosomal antibiotics. EMBO Rep. 2001, 2, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Dykens, J.A.; Marroquin, L.D.; Will, Y. Strategies to reduce late-stage drug attrition due to mitochondrial toxicity. Expert Rev. Mol. Diagn. 2007, 7, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Herrmann, J.M.; Becker, T. Quality control of the mitochondrial proteome. Nat. Rev. Mol. Cell. Biol. 2021, 22, 54–70. [Google Scholar] [CrossRef] [PubMed]
- Gyulkhandanyan, A.V.; Mutlu, A.; Freedman, J.; Leytin, V. Mitochondrial permeability transition pore (MPTP)-dependent and -independent pathways of mitochondrial membrane depolarization, cell shrinkage and microparticle formation during platelet apoptosis. Br. J. Haematol. 2015, 169, 142–145. [Google Scholar] [CrossRef]
- Strubbe-Rivera, J.O.; Schrad, J.R.; Pavlov, E.V.; Conway, J.F.; Parent, K.N.; Bazil, J.N. The mitochondrial permeability transition phenomenon elucidated by cryo-EM reveals the genuine impact of calcium overload on mitochondrial structure and function. Sci. Rep. 2021, 11, 1037. [Google Scholar] [CrossRef] [PubMed]
- Ramaccini, D.; Montoya-Uribe, V.; Aan, F.J.; Modesti, L.; Potes, Y.; Wieckowski, M.R.; Krga, I.; Glibetic, M.; Pinton, P.; Giorgi, C.; et al. Mitochondrial Function and Dysfunction in Dilated Cardiomyopathy. Front. Cell Dev. Biol. 2020, 8, 624216. [Google Scholar] [CrossRef] [PubMed]
- Bonora, M.; Wieckowski, M.R.; Chinopoulos, C.; Kepp, O.; Kroemer, G.; Galluzzi, L.; Pinton, P. Molecular mechanisms of cell death: Central implication of ATP synthase in mitochondrial permeability transition. Oncogene 2015, 34, 1475–1486. [Google Scholar] [CrossRef] [PubMed]
- Bonora, M.; Morganti, C.; Morciano, G.; Pedriali, G.; Lebiedzinska-Arciszewska, M.; Aquila, G.; Giorgi, C.; Rizzo, P.; Campo, G.; Ferrari, R.; et al. Mitochondrial permeability transition involves dissociation of F1FO ATP synthase dimers and C-ring conformation. EMBO Rep. 2017, 18, 1077–1089. [Google Scholar] [CrossRef]
- Morciano, G.; Bonora, M.; Giorgi, C.; Pinton, P. Other bricks for the correct construction of the mitochondrial permeability transition pore complex. Cell Death Dis. 2017, 8, e2698. [Google Scholar] [CrossRef] [PubMed]
- Bonora, M.; Pinton, P. A New Current for the Mitochondrial Permeability Transition. Trends Biochem. Sci. 2019, 44, 559–561. [Google Scholar] [CrossRef]
- Zhou, J.; Peng, F.; Cao, X.; Xie, X.; Chen, D.; Yang, L.; Rao, C.; Peng, C.; Pan, X. Risk Compounds, Preclinical Toxicity Evaluation, and Potential Mechanisms of Chinese Materia Medica-Induced Cardiotoxicity. Front. Pharmacol. 2021, 12, 578796. [Google Scholar] [CrossRef]
- Marroquin, L.; Swiss, R.; Will, Y. Identifying Compounds that Induce Opening of the Mitochondrial Permeability Transition Pore in Isolated Rat Liver Mitochondria. Curr. Protoc. Toxicol. 2014, 60, 25.4.1–25.4.17. [Google Scholar] [CrossRef]
- Pessayre, D.; Mansouri, A.; Berson, A.; Fromenty, B. Mitochondrial involvement in drug-induced liver injury. Handb. Exp. Pharmacol. 2010, 311–365. [Google Scholar] [CrossRef]
- Broderick, T.L. Hypocarnitinaemia induced by sodium pivalate in the rat is associated with left ventricular dysfunction and impaired energy metabolism. Drugs R D 2006, 7, 153–161. [Google Scholar] [CrossRef]
- Barile, M.; Valenti, D.; Passarella, S.; Quagliariello, E. 3′-Azido-3′-deoxythmidine uptake into isolated rat liver mitochondria and impairment of ADP/ATP translocator. Biochem. Pharmacol. 1997, 53, 913–920. [Google Scholar] [CrossRef]
- Lewis, W.; Simpson, J.F.; Meyer, R.R. Cardiac mitochondrial DNA polymerase-gamma is inhibited competitively and noncompetitively by phosphorylated zidovudine. Circ. Res. 1994, 74, 344–348. [Google Scholar] [CrossRef]
- Mitry, M.A.; Edwards, J.G. Doxorubicin induced heart failure: Phenotype and molecular mechanisms. Int. J. Cardiol. Heart Vasc. 2016, 10, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Hewton, K.G.; Johal, A.S.; Parker, S.J. Transporters at the Interface between Cytosolic and Mitochondrial Amino Acid Metabolism. Metabolites 2021, 11, 112. [Google Scholar] [CrossRef] [PubMed]
- Aquila, H.; Misra, D.; Eulitz, M.; Klingenberg, M. Complete amino acid sequence of the ADP/ATP carrier from beef heart mitochondria. Hoppe. Seylers Z Physiol. Chem. 1982, 363, 345–349. [Google Scholar] [PubMed]
- Hu, W.J.; Chen, X.M.; Meng, H.D.; Meng, Z.H. Fermented corn flour poisoning in rural areas of China. III. Isolation and identification of main toxin produced by causal microorganisms. Biomed. Environ. Sci. 1989, 2, 65–71. [Google Scholar]
- Stewart, M.J.; Steenkamp, V. The biochemistry and toxicity of atractyloside: A review. Ther. Drug Monit. 2000, 22, 641–649. [Google Scholar] [CrossRef]
- Tahrir, F.G.; Langford, D.; Amini, S.; Mohseni Ahooyi, T.; Khalili, K. Mitochondrial quality control in cardiac cells: Mechanisms and role in cardiac cell injury and disease. J. Cell. Physiol. 2019, 234, 8122–8133. [Google Scholar] [CrossRef]
- Chang, X.; Zhang, W.; Zhao, Z.; Ma, C.; Zhang, T.; Meng, Q.; Yan, P.; Zhang, L.; Zhao, Y. Regulation of Mitochondrial Quality Control by Natural Drugs in the Treatment of Cardiovascular Diseases: Potential and Advantages. Front. Cell Dev. Biol. 2020, 8, 616139. [Google Scholar] [CrossRef]
- Suliman, H.B.; Piantadosi, C.A. Mitochondrial Quality Control as a Therapeutic Target. Pharmacol. Rev. 2016, 68, 20–48. [Google Scholar] [CrossRef]
- Tatsuta, T.; Langer, T. Quality control of mitochondria: Protection against neurodegeneration and ageing. EMBO J. 2008, 27, 306–314. [Google Scholar] [CrossRef]
- Koleini, N.; Kardami, E. Autophagy and mitophagy in the context of doxorubicin-induced cardiotoxicity. Oncotarget 2017, 8, 46663–46680. [Google Scholar] [CrossRef]
- Oh, C.M.; Ryu, D.; Cho, S.; Jang, Y. Mitochondrial Quality Control in the Heart: New Drug Targets for Cardiovascular Disease. Korean Circ. J. 2020, 50, 395–405. [Google Scholar] [CrossRef]
- Chang, C.Y.; Kazmin, D.; Jasper, J.S.; Kunder, R.; Zuercher, W.J.; McDonnell, D.P. The metabolic regulator ERRalpha, a downstream target of HER2/IGF-1R, as a therapeutic target in breast cancer. Cancer Cell 2011, 20, 500–510. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Peugnet, V.; Chwastyniak, M.; Mulder, P.; Lancel, S.; Bultot, L.; Fourny, N.; Renguet, E.; Bugger, H.; Beseme, O.; Loyens, A.; et al. Mitochondrial-Targeted Therapies Require Mitophagy to Prevent Oxidative Stress Induced by SOD2 Inactivation in Hypertrophied Cardiomyocytes. Antioxidants 2022, 11, 723. [Google Scholar] [CrossRef]
- Beak, J.Y.; Kang, H.S.; Huang, W.; Aghajanian, A.; Gerrish, K.; Jetten, A.M.; Jensen, B.C. The nuclear receptor RORα preserves cardiomyocyte mitochondrial function by regulating caveolin-3-mediated mitophagy. J. Biol. Chem. 2020, 297. [Google Scholar] [CrossRef]
- Morales, P.E.; Arias-Duran, C.; Avalos-Guajardo, Y.; Aedo, G.; Verdejo, H.E.; Parra, V.; Lavandero, S. Emerging role of mitophagy in cardiovascular physiology and pathology. Mol. Asp. Med. 2020, 71, 100822. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Ma, Z.; An, D.; Liu, Z.; Cai, W.; Bai, Y.; Zhan, Q.; Lai, W.; Zeng, Q.; Ren, H.; et al. Mitofusin 2 Participates in Mitophagy and Mitochondrial Fusion Against Angiotensin II-Induced Cardiomyocyte Injury. Front. Physiol. 2019, 10, 411. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Wang, L.; Qiao, Y.; Yang, B.; Yin, D.; He, M. Epigallocatechin-3-gallate pretreatment alleviates doxorubicin-induced ferroptosis and cardiotoxicity by upregulating AMPKalpha2 and activating adaptive autophagy. Redox Biol. 2021, 48, 102185. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Wan, K.; Yin, M.; Hu, P.; Que, Y.; Zhou, X.; Zhang, L.; Li, T.; Du, Y.; Xu, G.; et al. RIPK3 Induces Cardiomyocyte Necroptosis via Inhibition of AMPK-Parkin-Mitophagy in Cardiac Remodelling after Myocardial Infarction. Oxid. Med. Cell. Longev. 2021, 2021, 6635955. [Google Scholar] [CrossRef]
- Ramirez-Sagredo, A.; Quiroga, C.; Garrido-Moreno, V.; Lopez-Crisosto, C.; Leiva-Navarrete, S.; Norambuena-Soto, I.; Ortiz-Quintero, J.; Diaz-Vesga, M.C.; Perez, W.; Hendrickson, T.; et al. Polycystin-1 regulates cardiomyocyte mitophagy. FASEB J. 2021, 35, e21796. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, B.; Cortassa, S.; Aon, M.A. Mitochondrial ion channels: Gatekeepers of life and death. Physiology 2005, 20, 303–315. [Google Scholar] [CrossRef]
- Bernardi, P. Mitochondrial transport of cations: Channels, exchangers, and permeability transition. Physiol. Rev. 1999, 79, 1127–1155. [Google Scholar] [CrossRef] [PubMed]
- Beavis, A.D. Properties of the inner membrane anion channel in intact mitochondria. J. Bioenerg. Biomembr. 1992, 24, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Smyrnias, I.; Gray, S.P.; Okonko, D.O.; Sawyer, G.; Zoccarato, A.; Catibog, N.; Lopez, B.; Gonzalez, A.; Ravassa, S.; Diez, J.; et al. Cardioprotective Effect of the Mitochondrial Unfolded Protein Response During Chronic Pressure Overload. J. Am. Coll. Cardiol. 2019, 73, 1795–1806. [Google Scholar] [CrossRef] [PubMed]
- Rauthan, M.; Ranji, P.; Aguilera Pradenas, N.; Pitot, C.; Pilon, M. The mitochondrial unfolded protein response activator ATFS-1 protects cells from inhibition of the mevalonate pathway. Proc. Natl. Acad. Sci. USA 2013, 110, 5981–5986. [Google Scholar] [CrossRef] [PubMed]
- Marquez, J.; Lee, S.R.; Kim, N.; Han, J. Post-Translational Modifications of Cardiac Mitochondrial Proteins in Cardiovascular Disease: Not Lost in Translation. Korean Circ. J. 2016, 46, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Alves-Figueiredo, H.; Silva-Platas, C.; Lozano, O.; Vazquez-Garza, E.; Guerrero-Beltran, C.E.; Zarain-Herzberg, A.; Garcia-Rivas, G. A systematic review of post-translational modifications in the mitochondrial permeability transition pore complex associated with cardiac diseases. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 165992. [Google Scholar] [CrossRef]
- DiMasi, J.A.; Hansen, R.W.; Grabowski, H.G. The price of innovation: New estimates of drug development costs. J. Health Econ. 2003, 22, 151–185. [Google Scholar] [CrossRef]
- Ferdinandy, P.; Baczko, I.; Bencsik, P.; Giricz, Z.; Gorbe, A.; Pacher, P.; Varga, Z.V.; Varro, A.; Schulz, R. Definition of hidden drug cardiotoxicity: Paradigm change in cardiac safety testing and its clinical implications. Eur. Heart J. 2019, 40, 1771–1777. [Google Scholar] [CrossRef]
- Kramer, J.A.; Sagartz, J.E.; Morris, D.L. The application of discovery toxicology and pathology towards the design of safer pharmaceutical lead candidates. Nat. Rev. Drug. Discov. 2007, 6, 636–649. [Google Scholar] [CrossRef]
- Lin, X.; Tang, J.; Lou, Y.R. Human Pluripotent Stem-Cell-Derived Models as a Missing Link in Drug Discovery and Development. Pharmaceuticals 2021, 14, 525. [Google Scholar] [CrossRef] [PubMed]
- Arrowsmith, J.; Miller, P. Trial watch: Phase II and phase III attrition rates 2011–2012. Nat. Rev. Drug Discov. 2013, 12, 569. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Gwathmey, J.K.; Tsaioun, K.; Hajjar, R.J. Cardionomics: A new integrative approach for screening cardiotoxicity of drug candidates. Expert Opin. Drug Metab. Toxicol. 2009, 5, 647–660. [Google Scholar] [CrossRef]
- Sager, P.T.; Gintant, G.; Turner, J.R.; Pettit, S.; Stockbridge, N. Rechanneling the cardiac proarrhythmia safety paradigm: A meeting report from the Cardiac Safety Research Consortium. Am. Heart J. 2014, 167, 292–300. [Google Scholar] [CrossRef]
- Clark, M. Prediction of clinical risks by analysis of preclinical and clinical adverse events. J. Biomed. Inform. 2015, 54, 167–173. [Google Scholar] [CrossRef]
- Brenner, G.B.; Makkos, A.; Nagy, C.T.; Onodi, Z.; Sayour, N.V.; Gergely, T.G.; Kiss, B.; Gorbe, A.; Saghy, E.; Zadori, Z.S.; et al. Hidden Cardiotoxicity of Rofecoxib Can be Revealed in Experimental Models of Ischemia/Reperfusion. Cells 2020, 9, 551. [Google Scholar] [CrossRef]
- Piper, H.M.; Sezer, O.; Schleyer, M.; Schwartz, P.; Hutter, J.F.; Spieckermann, P.G. Development of ischemia-induced damage in defined mitochondrial subpopulations. J. Mol. Cell. Cardiol. 1985, 17, 885–896. [Google Scholar] [CrossRef]
- Palmer, J.W.; Tandler, B.; Hoppel, C.L. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J. Biol. Chem. 1977, 252, 8731–8739. [Google Scholar] [CrossRef]
- O’Shea, K.M.; Khairallah, R.J.; Sparagna, G.C.; Xu, W.; Hecker, P.A.; Robillard-Frayne, I.; Des Rosiers, C.; Kristian, T.; Murphy, R.C.; Fiskum, G.; et al. Dietary omega−3 fatty acids alter cardiac mitochondrial phospholipid composition and delay Ca2+-induced permeability transition. J. Mol. Cell. Cardiol. 2009, 47, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Kimes, B.W.; Brandt, B.L. Properties of a clonal muscle cell line from rat heart. Exp. Cell. Res. 1976, 98, 367–381. [Google Scholar] [CrossRef]
- Hescheler, J.; Meyer, R.; Plant, S.; Krautwurst, D.; Rosenthal, W.; Schultz, G. Morphological, biochemical, and electrophysiological characterization of a clonal cell (H9c2) line from rat heart. Circ. Res. 1991, 69, 1476–1486. [Google Scholar] [CrossRef] [PubMed]
- Menard, C.; Pupier, S.; Mornet, D.; Kitzmann, M.; Nargeot, J.; Lory, P. Modulation of L-type calcium channel expression during retinoic acid-induced differentiation of H9C2 cardiac cells. J. Biol. Chem. 1999, 274, 29063–29070. [Google Scholar] [CrossRef] [PubMed]
- Branco, A.F.; Pereira, S.P.; Gonzalez, S.; Gusev, O.; Rizvanov, A.A.; Oliveira, P.J. Gene Expression Profiling of H9c2 Myoblast Differentiation towards a Cardiac-Like Phenotype. PLoS ONE 2015, 10, e0129303. [Google Scholar] [CrossRef]
- Sardao, V.A.; Oliveira, P.J.; Holy, J.; Oliveira, C.R.; Wallace, K.B. Morphological alterations induced by doxorubicin on H9c2 myoblasts: Nuclear, mitochondrial, and cytoskeletal targets. Cell Biol. Toxicol. 2009, 25, 227–243. [Google Scholar] [CrossRef]
- Sacks, B.; Onal, H.; Martorana, R.; Sehgal, A.; Harvey, A.; Wastella, C.; Ahmad, H.; Ross, E.; Pjetergjoka, A.; Prasad, S.; et al. Mitochondrial targeted antioxidants, mitoquinone and SKQ1, not vitamin C, mitigate doxorubicin-induced damage in H9c2 myoblast: Pretreatment vs. co-treatment. BMC Pharmacol. Toxicol. 2021, 22, 49. [Google Scholar] [CrossRef]
- Shi, Y.; Li, F.; Shen, M.; Sun, C.; Hao, W.; Wu, C.; Xie, Y.; Zhang, S.; Gao, H.; Yang, J.; et al. Luteolin Prevents Cardiac Dysfunction and Improves the Chemotherapeutic Efficacy of Doxorubicin in Breast Cancer. Front. Cardiovasc. Med. 2021, 8, 750186. [Google Scholar] [CrossRef]
- Helal, M.; Alcorn, J.; Bandy, B. Doxorubicin Cytotoxicity in Differentiated H9c2 Cardiomyocytes: Evidence for Acute Mitochondrial Superoxide Generation. Cardiovasc. Toxicol. 2021, 21, 152–161. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, Z.; Lu, D.; Wu, Y.; Fan, M.; Qian, J.; Ge, J. Overexpression of COX5A protects H9c2 cells against doxorubicin-induced cardiotoxicity. Biochem. Biophys. Res. Commun. 2020, 524, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Bouitbir, J.; Alshaikhali, A.; Panajatovic, M.; Abegg, V.; Paech, F.; Krahenbuhl, S. Mechanisms of Cardiotoxicity Associated with Tyrosine Kinase Inhibitors in H9c2 Cells and Mice. Eur. Cardiol. 2020, 15, e33. [Google Scholar] [CrossRef] [PubMed]
- Bouitbir, J.; Alshaikhali, A.; Panajatovic, M.V.; Abegg, V.F.; Paech, F.; Krahenbuhl, S. Mitochondrial oxidative stress plays a critical role in the cardiotoxicity of sunitinib: Running title: Sunitinib and oxidative stress in hearts. Toxicology 2019, 426, 152281. [Google Scholar] [CrossRef] [PubMed]
- Will, Y.; Dykens, J.A.; Nadanaciva, S.; Hirakawa, B.; Jamieson, J.; Marroquin, L.D.; Hynes, J.; Patyna, S.; Jessen, B.A. Effect of the multitargeted tyrosine kinase inhibitors imatinib, dasatinib, sunitinib, and sorafenib on mitochondrial function in isolated rat heart mitochondria and H9c2 cells. Toxicol. Sci. 2008, 106, 153–161. [Google Scholar] [CrossRef]
- Vineetha, R.C.; Binu, P.; Arathi, P.; Nair, R.H. L-ascorbic acid and alpha-tocopherol attenuate arsenic trioxide-induced toxicity in H9c2 cardiomyocytes by the activation of Nrf2 and Bcl2 transcription factors. Toxicol. Mech. Methods 2018, 28, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Gergely, S.; Hegedus, C.; Lakatos, P.; Kovacs, K.; Gaspar, R.; Csont, T.; Virag, L. High Throughput Screening Identifies a Novel Compound Protecting Cardiomyocytes from Doxorubicin-Induced Damage. Oxid. Med. Cell. Longev. 2015, 2015, 178513. [Google Scholar] [CrossRef][Green Version]
- Pointon, A.; Abi-Gerges, N.; Cross, M.J.; Sidaway, J.E. Phenotypic profiling of structural cardiotoxins in vitro reveals dependency on multiple mechanisms of toxicity. Toxicol. Sci. 2013, 132, 317–326. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Branco, A.F.; Pereira, S.L.; Moreira, A.C.; Holy, J.; Sardao, V.A.; Oliveira, P.J. Isoproterenol cytotoxicity is dependent on the differentiation state of the cardiomyoblast H9c2 cell line. Cardiovasc. Toxicol. 2011, 11, 191–203. [Google Scholar] [CrossRef]
- Branco, A.F.; Sampaio, S.F.; Moreira, A.C.; Holy, J.; Wallace, K.B.; Baldeiras, I.; Oliveira, P.J.; Sardao, V.A. Differentiation-dependent doxorubicin toxicity on H9c2 cardiomyoblasts. Cardiovasc. Toxicol. 2012, 12, 326–340. [Google Scholar] [CrossRef]
- Gherghiceanu, M.; Barad, L.; Novak, A.; Reiter, I.; Itskovitz-Eldor, J.; Binah, O.; Popescu, L.M. Cardiomyocytes derived from human embryonic and induced pluripotent stem cells: Comparative ultrastructure. J. Cell. Mol. Med. 2011, 15, 2539–2551. [Google Scholar] [CrossRef]
- Mummery, C.L.; Zhang, J.; Ng, E.S.; Elliott, D.A.; Elefanty, A.G.; Kamp, T.J. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: A methods overview. Circ. Res. 2012, 111, 344–358. [Google Scholar] [CrossRef]
- Pang, L.; Sager, P.; Yang, X.; Shi, H.; Sannajust, F.; Brock, M.; Wu, J.C.; Abi-Gerges, N.; Lyn-Cook, B.; Berridge, B.R.; et al. Workshop Report: FDA Workshop on Improving Cardiotoxicity Assessment With Human-Relevant Platforms. Circ. Res. 2019, 125, 855–867. [Google Scholar] [CrossRef]
- Beauchamp, P.; Jackson, C.B.; Ozhathil, L.C.; Agarkova, I.; Galindo, C.L.; Sawyer, D.B.; Suter, T.M.; Zuppinger, C. 3D Co-culture of hiPSC-Derived Cardiomyocytes With Cardiac Fibroblasts Improves Tissue-Like Features of Cardiac Spheroids. Front. Mol. Biosci. 2020, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.C.; Ting, S.; Lee, Y.K.; Ng, K.M.; Zhang, J.; Chen, Z.; Siu, C.W.; Oh, S.K.; Tse, H.F. Electrical stimulation promotes maturation of cardiomyocytes derived from human embryonic stem cells. J. Cardiovasc. Transl. Res. 2013, 6, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Feyen, D.A.M.; McKeithan, W.L.; Bruyneel, A.A.N.; Spiering, S.; Hormann, L.; Ulmer, B.; Zhang, H.; Briganti, F.; Schweizer, M.; Hegyi, B.; et al. Metabolic Maturation Media Improve Physiological Function of Human iPSC-Derived Cardiomyocytes. Cell. Rep. 2020, 32, 107925. [Google Scholar] [CrossRef] [PubMed]
- Parikh, S.S.; Blackwell, D.J.; Gomez-Hurtado, N.; Frisk, M.; Wang, L.; Kim, K.; Dahl, C.P.; Fiane, A.; Tonnessen, T.; Kryshtal, D.O.; et al. Thyroid and Glucocorticoid Hormones Promote Functional T-Tubule Development in Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Circ. Res. 2017, 121, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Ronaldson-Bouchard, K.; Ma, S.P.; Yeager, K.; Chen, T.; Song, L.; Sirabella, D.; Morikawa, K.; Teles, D.; Yazawa, M.; Vunjak-Novakovic, G. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 2018, 556, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Karbassi, E.; Fenix, A.; Marchiano, S.; Muraoka, N.; Nakamura, K.; Yang, X.; Murry, C.E. Cardiomyocyte maturation: Advances in knowledge and implications for regenerative medicine. Nat. Rev. Cardiol. 2020, 17, 341–359. [Google Scholar] [CrossRef]
- Archer, C.R.; Sargeant, R.; Basak, J.; Pilling, J.; Barnes, J.R.; Pointon, A. Characterization and Validation of a Human 3D Cardiac Microtissue for the Assessment of Changes in Cardiac Pathology. Sci. Rep. 2018, 8, 10160. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; Shenoy, S.; Sayed, N. Building Multi-Dimensional Induced Pluripotent Stem Cells-Based Model Platforms to Assess Cardiotoxicity in Cancer Therapies. Front. Pharmacol. 2021, 12, 607364. [Google Scholar] [CrossRef] [PubMed]
- Correia, C.; Serra, M.; Espinha, N.; Sousa, M.; Brito, C.; Burkert, K.; Zheng, Y.; Hescheler, J.; Carrondo, M.J.; Saric, T.; et al. Combining hypoxia and bioreactor hydrodynamics boosts induced pluripotent stem cell differentiation towards cardiomyocytes. Stem Cell Rev. Rep. 2014, 10, 786–801. [Google Scholar] [CrossRef]
- Tohyama, S.; Fujita, J.; Fujita, C.; Yamaguchi, M.; Kanaami, S.; Ohno, R.; Sakamoto, K.; Kodama, M.; Kurokawa, J.; Kanazawa, H.; et al. Efficient Large-Scale 2D Culture System for Human Induced Pluripotent Stem Cells and Differentiated Cardiomyocytes. Stem Cell Rep. 2017, 9, 1406–1414. [Google Scholar] [CrossRef]
- Paik, D.T.; Chandy, M.; Wu, J.C. Patient and Disease-Specific Induced Pluripotent Stem Cells for Discovery of Personalized Cardiovascular Drugs and Therapeutics. Pharmacol. Rev. 2020, 72, 320–342. [Google Scholar] [CrossRef]
- Foldes, G.; Mioulane, M.; Wright, J.S.; Liu, A.Q.; Novak, P.; Merkely, B.; Gorelik, J.; Schneider, M.D.; Ali, N.N.; Harding, S.E. Modulation of human embryonic stem cell-derived cardiomyocyte growth: A testbed for studying human cardiac hypertrophy? J. Mol. Cell. Cardiol. 2011, 50, 367–376. [Google Scholar] [CrossRef]
- Juhola, M.; Joutsijoki, H.; Penttinen, K.; Aalto-Setala, K. Detection of genetic cardiac diseases by Ca(2+) transient profiles using machine learning methods. Sci. Rep. 2018, 8, 9355. [Google Scholar] [CrossRef] [PubMed]
- Grafton, F.; Ho, J.; Ranjbarvaziri, S.; Farshidfar, F.; Budan, A.; Steltzer, S.; Maddah, M.; Loewke, K.E.; Green, K.; Patel, S.; et al. Deep learning detects cardiotoxicity in a high-content screen with induced pluripotent stem cell-derived cardiomyocytes. Elife 2021, 10, e68714. [Google Scholar] [CrossRef] [PubMed]
- Scott, C.W.; Zhang, X.; Abi-Gerges, N.; Lamore, S.D.; Abassi, Y.A.; Peters, M.F. An impedance-based cellular assay using human iPSC-derived cardiomyocytes to quantify modulators of cardiac contractility. Toxicol. Sci. 2014, 142, 331–338. [Google Scholar] [CrossRef]
- Palmer, J.A.; Smith, A.M.; Gryshkova, V.; Donley, E.L.R.; Valentin, J.P.; Burrier, R.E. A Targeted Metabolomics-Based Assay Using Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes Identifies Structural and Functional Cardiotoxicity Potential. Toxicol. Sci. 2020, 174, 218–240. [Google Scholar] [CrossRef] [PubMed]
- Louisse, J.; Wust, R.C.I.; Pistollato, F.; Palosaari, T.; Barilari, M.; Macko, P.; Bremer, S.; Prieto, P. Assessment of acute and chronic toxicity of doxorubicin in human induced pluripotent stem cell-derived cardiomyocytes. Toxicol. In Vitro 2017, 42, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Habeler, W.; Pouillot, S.; Plancheron, A.; Puceat, M.; Peschanski, M.; Monville, C. An in vitro beating heart model for long-term assessment of experimental therapeutics. Cardiovasc. Res. 2009, 81, 253–259. [Google Scholar] [CrossRef]
- Kopljar, I.; De Bondt, A.; Vinken, P.; Teisman, A.; Damiano, B.; Goeminne, N.; Van den Wyngaert, I.; Gallacher, D.J.; Lu, H.R. Chronic drug-induced effects on contractile motion properties and cardiac biomarkers in human induced pluripotent stem cell-derived cardiomyocytes. Br. J. Pharmacol. 2017, 174, 3766–3779. [Google Scholar] [CrossRef]
- Dias, T.P.; Pinto, S.N.; Santos, J.I.; Fernandes, T.G.; Fernandes, F.; Diogo, M.M.; Prieto, M.; Cabral, J.M.S. Biophysical study of human induced Pluripotent Stem Cell-Derived cardiomyocyte structural maturation during long-term culture. Biochem. Biophys. Res. Commun. 2018, 499, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hua, Y.; Miyagawa, S.; Zhang, J.; Li, L.; Liu, L.; Sawa, Y. hiPSC-Derived Cardiac Tissue for Disease Modeling and Drug Discovery. Int. J. Mol. Sci. 2020, 21, 8893. [Google Scholar] [CrossRef]
- Clements, M.; Millar, V.; Williams, A.S.; Kalinka, S. Bridging Functional and Structural Cardiotoxicity Assays Using Human Embryonic Stem Cell-Derived Cardiomyocytes for a More Comprehensive Risk Assessment. Toxicol. Sci. 2015, 148, 241–260. [Google Scholar] [CrossRef] [PubMed]
- Gilsbach, R.; Preissl, S.; Gruning, B.A.; Schnick, T.; Burger, L.; Benes, V.; Wurch, A.; Bonisch, U.; Gunther, S.; Backofen, R.; et al. Dynamic DNA methylation orchestrates cardiomyocyte development, maturation and disease. Nat. Commun. 2014, 5, 5288. [Google Scholar] [CrossRef]
- Oh, J.G.; Kho, C.; Hajjar, R.J.; Ishikawa, K. Experimental models of cardiac physiology and pathology. Heart Fail. Rev. 2019, 24, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Kistamas, K.; Hezso, T.; Horvath, B.; Nanasi, P.P. Late sodium current and calcium homeostasis in arrhythmogenesis. Channels 2021, 15, 1–19. [Google Scholar] [CrossRef]
- Sala, L.; van Meer, B.J.; Tertoolen, L.G.J.; Bakkers, J.; Bellin, M.; Davis, R.P.; Denning, C.; Dieben, M.A.E.; Eschenhagen, T.; Giacomelli, E.; et al. MUSCLEMOTION: A Versatile Open Software Tool to Quantify Cardiomyocyte and Cardiac Muscle Contraction In Vitro and In Vivo. Circ. Res. 2018, 122, e5–e16. [Google Scholar] [CrossRef]
- Abi-Gerges, N.; Indersmitten, T.; Truong, K.; Nguyen, W.; Ratchada, P.; Nguyen, N.; Page, G.; Miller, P.E.; Ghetti, A. Multiparametric Mechanistic Profiling of Inotropic Drugs in Adult Human Primary Cardiomyocytes. Sci. Rep. 2020, 10, 7692. [Google Scholar] [CrossRef]
- Nguyen, N.; Nguyen, W.; Nguyenton, B.; Ratchada, P.; Page, G.; Miller, P.E.; Ghetti, A.; Abi-Gerges, N. Adult Human Primary Cardiomyocyte-Based Model for the Simultaneous Prediction of Drug-Induced Inotropic and Pro-arrhythmia Risk. Front. Physiol. 2017, 8, 1073. [Google Scholar] [CrossRef]
- Cui, N.; Wu, F.; Lu, W.J.; Bai, R.; Ke, B.; Liu, T.; Li, L.; Lan, F.; Cui, M. Doxorubicin-induced cardiotoxicity is maturation dependent due to the shift from topoisomerase IIalpha to IIbeta in human stem cell derived cardiomyocytes. J. Cell. Mol. Med. 2019, 23, 4627–4639. [Google Scholar] [CrossRef]
- Guo, G.R.; Chen, L.; Rao, M.; Chen, K.; Song, J.P.; Hu, S.S. A modified method for isolation of human cardiomyocytes to model cardiac diseases. J. Transl. Med. 2018, 16, 288. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yu, P.; Zhou, B.; Song, J.; Li, Z.; Zhang, M.; Guo, G.; Wang, Y.; Chen, X.; Han, L.; et al. Single-cell reconstruction of the adult human heart during heart failure and recovery reveals the cellular landscape underlying cardiac function. Nat. Cell Biol. 2020, 22, 108–119. [Google Scholar] [CrossRef]
- Shamsaldeen, Y.A.; Culliford, L.; Clout, M.; James, A.F.; Ascione, R.; Hancox, J.C.; Marrion, N.V. Role of SK channel activation in determining the action potential configuration in freshly isolated human atrial myocytes from the SKArF study. Biochem. Biophys. Res. Commun. 2019, 512, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Benardeau, A.; Hatem, S.N.; Rucker-Martin, C.; Tessier, S.; Dinanian, S.; Samuel, J.L.; Coraboeuf, E.; Mercadier, J.J. Primary culture of human atrial myocytes is associated with the appearance of structural and functional characteristics of immature myocardium. J. Mol. Cell. Cardiol. 1997, 29, 1307–1320. [Google Scholar] [CrossRef] [PubMed]
- Bistola, V.; Nikolopoulou, M.; Derventzi, A.; Kataki, A.; Sfyras, N.; Nikou, N.; Toutouza, M.; Toutouzas, P.; Stefanadis, C.; Konstadoulakis, M.M. Long-term primary cultures of human adult atrial cardiac myocytes: Cell viability, structural properties and BNP secretion in vitro. Int. J. Cardiol. 2008, 131, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Saleem, U.; van Meer, B.J.; Katili, P.A.; Mohd Yusof, N.A.N.; Mannhardt, I.; Garcia, A.K.; Tertoolen, L.; de Korte, T.; Vlaming, M.L.H.; McGlynn, K.; et al. Blinded, Multicenter Evaluation of Drug-induced Changes in Contractility Using Human-induced Pluripotent Stem Cell-derived Cardiomyocytes. Toxicol. Sci. 2020, 176, 103–123. [Google Scholar] [CrossRef] [PubMed]
- Pointon, A.; Pilling, J.; Dorval, T.; Wang, Y.; Archer, C.; Pollard, C. From the Cover: High-Throughput Imaging of Cardiac Microtissues for the Assessment of Cardiac Contraction during Drug Discovery. Toxicol. Sci. 2017, 155, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Ravenscroft, S.M.; Pointon, A.; Williams, A.W.; Cross, M.J.; Sidaway, J.E. Cardiac Non-myocyte Cells Show Enhanced Pharmacological Function Suggestive of Contractile Maturity in Stem Cell Derived Cardiomyocyte Microtissues. Toxicol. Sci. 2016, 152, 99–112. [Google Scholar] [CrossRef]
- Kerr, C.M.; Richards, D.; Menick, D.R.; Deleon-Pennell, K.Y.; Mei, Y. Multicellular Human Cardiac Organoids Transcriptomically Model Distinct Tissue-Level Features of Adult Myocardium. Int. J. Mol. Sci. 2021, 22, 8482. [Google Scholar] [CrossRef]
- Branco, M.A.; Cabral, J.M.S.; Diogo, M.M. From Human Pluripotent Stem Cells to 3D Cardiac Microtissues: Progress, Applications and Challenges. Bioengineering 2020, 7, 92. [Google Scholar] [CrossRef]
- Cho, S.; Lee, C.; Skylar-Scott, M.A.; Heilshorn, S.C.; Wu, J.C. Reconstructing the heart using iPSCs: Engineering strategies and applications. J. Mol. Cell. Cardiol. 2021, 157, 56–65. [Google Scholar] [CrossRef]
- Fonoudi, H.; Burridge, P.W. Cellular model systems to study cardiovascular injury from chemotherapy. J. Thromb. Thrombolysis 2021, 51, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.F.; Leong, M.F.; Lim, T.C.; Chua, Y.P.; Lim, J.K.; Du, C.; Wan, A.C.A. Engineering a functional three-dimensional human cardiac tissue model for drug toxicity screening. Biofabrication 2017, 9, 025011. [Google Scholar] [CrossRef] [PubMed]
- Mills, R.J.; Parker, B.L.; Quaife-Ryan, G.A.; Voges, H.K.; Needham, E.J.; Bornot, A.; Ding, M.; Andersson, H.; Polla, M.; Elliott, D.A.; et al. Drug Screening in Human PSC-Cardiac Organoids Identifies Pro-proliferative Compounds Acting via the Mevalonate Pathway. Cell Stem Cell 2019, 24, 895–907.e6. [Google Scholar] [CrossRef] [PubMed]
- Kofron, C.M.; Kim, T.Y.; Munarin, F.; Soepriatna, A.H.; Kant, R.J.; Mende, U.; Choi, B.R.; Coulombe, K.L.K. A predictive in vitro risk assessment platform for pro-arrhythmic toxicity using human 3D cardiac microtissues. Sci. Rep. 2021, 11, 10228. [Google Scholar] [CrossRef]
- Sharma, A.; Marceau, C.; Hamaguchi, R.; Burridge, P.W.; Rajarajan, K.; Churko, J.M.; Wu, H.; Sallam, K.I.; Matsa, E.; Sturzu, A.C.; et al. Human induced pluripotent stem cell-derived cardiomyocytes as an in vitro model for coxsackievirus B3-induced myocarditis and antiviral drug screening platform. Circ. Res. 2014, 115, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Burridge, P.W.; McKeithan, W.L.; Serrano, R.; Shukla, P.; Sayed, N.; Churko, J.M.; Kitani, T.; Wu, H.; Holmstrom, A.; et al. High-throughput screening of tyrosine kinase inhibitor cardiotoxicity with human induced pluripotent stem cells. Sci. Transl. Med. 2017, 9, eaaf2584. [Google Scholar] [CrossRef] [PubMed]
- Mot, A.I.; Liddell, J.R.; White, A.R.; Crouch, P.J. Circumventing the Crabtree Effect: A method to induce lactate consumption and increase oxidative phosphorylation in cell culture. Int. J. Biochem. Cell. Biol. 2016, 79, 128–138. [Google Scholar] [CrossRef]
- Beeson, C.C.; Beeson, G.C.; Schnellmann, R.G. A high-throughput respirometric assay for mitochondrial biogenesis and toxicity. Anal. Biochem. 2010, 404, 75–81. [Google Scholar] [CrossRef]
- Deus, C.M.; Zehowski, C.; Nordgren, K.; Wallace, K.B.; Skildum, A.; Oliveira, P.J. Stimulating basal mitochondrial respiration decreases doxorubicin apoptotic signaling in H9c2 cardiomyoblasts. Toxicology 2015, 334, 1–11. [Google Scholar] [CrossRef]
- Rana, P.; Anson, B.; Engle, S.; Will, Y. Characterization of human-induced pluripotent stem cell-derived cardiomyocytes: Bioenergetics and utilization in safety screening. Toxicol. Sci. 2012, 130, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Sekine, S.; Song, B.; Ito, K. Use of Primary Rat Hepatocytes for Prediction of Drug-Induced Mitochondrial Dysfunction. Curr. Protoc. Toxicol. 2017, 72, 14.16.1–14.16.10. [Google Scholar] [CrossRef] [PubMed]
- Delp, J.; Funke, M.; Rudolf, F.; Cediel, A.; Bennekou, S.H.; van der Stel, W.; Carta, G.; Jennings, P.; Toma, C.; Gardner, I.; et al. Development of a neurotoxicity assay that is tuned to detect mitochondrial toxicants. Arch. Toxicol. 2019, 93, 1585–1608. [Google Scholar] [CrossRef]
- Rana, P.; Nadanaciva, S.; Will, Y. Mitochondrial membrane potential measurement of H9c2 cells grown in high-glucose and galactose-containing media does not provide additional predictivity towards mitochondrial assessment. Toxicol. In Vitro 2011, 25, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Dykens, J.A.; Jamieson, J.D.; Marroquin, L.D.; Nadanaciva, S.; Xu, J.J.; Dunn, M.C.; Smith, A.R.; Will, Y. In vitro assessment of mitochondrial dysfunction and cytotoxicity of nefazodone, trazodone, and buspirone. Toxicol. Sci. 2008, 103, 335–345. [Google Scholar] [CrossRef]
- Hom, J.; Sheu, S.S. Morphological dynamics of mitochondria—A special emphasis on cardiac muscle cells. J. Mol. Cell. Cardiol. 2009, 46, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Saunders, J.E.; Beeson, C.C.; Schnellmann, R.G. Characterization of functionally distinct mitochondrial subpopulations. J. Bioenerg. Biomembr. 2013, 45, 87–99. [Google Scholar] [CrossRef]
- Kepiro, M.; Varkuti, B.H.; Davis, R.L. High Content, Phenotypic Assays and Screens for Compounds Modulating Cellular Processes in Primary Neurons. Methods Enzymol. 2018, 610, 219–250. [Google Scholar] [CrossRef]
- Rizzuto, R.; Brini, M.; De Giorgi, F.; Rossi, R.; Heim, R.; Tsien, R.Y.; Pozzan, T. Double labelling of subcellular structures with organelle-targeted GFP mutants in vivo. Curr. Biol. 1996, 6, 183–188. [Google Scholar] [CrossRef]
- Legros, F.; Lombes, A.; Frachon, P.; Rojo, M. Mitochondrial fusion in human cells is efficient, requires the inner membrane potential, and is mediated by mitofusins. Mol. Biol. Cell. 2002, 13, 4343–4354. [Google Scholar] [CrossRef]
- Fogo, G.M.; Anzell, A.R.; Maheras, K.J.; Raghunayakula, S.; Wider, J.M.; Emaus, K.J.; Bryson, T.D.; Bukowski, M.J.; Neumar, R.W.; Przyklenk, K.; et al. Machine learning-based classification of mitochondrial morphology in primary neurons and brain. Sci. Rep. 2021, 11, 5133. [Google Scholar] [CrossRef] [PubMed]
- Hallinger, D.R.; Lindsay, H.B.; Paul Friedman, K.; Suarez, D.A.; Simmons, S.O. Respirometric Screening and Characterization of Mitochondrial Toxicants Within the ToxCast Phase I and II Chemical Libraries. Toxicol. Sci. 2020, 176, 175–192. [Google Scholar] [CrossRef] [PubMed]
- Chance, B.; Williams, G.R. The respiratory chain and oxidative phosphorylation. Adv. Enzymol. Relat. Subj. Biochem. 1956, 17, 65–134. [Google Scholar] [CrossRef]
- Lanza, I.R.; Nair, K.S. Functional assessment of isolated mitochondria in vitro. Methods Enzymol. 2009, 457, 349–372. [Google Scholar] [CrossRef] [PubMed]
- Attene-Ramos, M.S.; Huang, R.; Sakamuru, S.; Witt, K.L.; Beeson, G.C.; Shou, L.; Schnellmann, R.G.; Beeson, C.C.; Tice, R.R.; Austin, C.P.; et al. Systematic study of mitochondrial toxicity of environmental chemicals using quantitative high throughput screening. Chem. Res. Toxicol. 2013, 26, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Wills, L.P.; Beeson, G.C.; Trager, R.E.; Lindsey, C.C.; Beeson, C.C.; Peterson, Y.K.; Schnellmann, R.G. High-throughput respirometric assay identifies predictive toxicophore of mitochondrial injury. Toxicol. Appl. Pharmacol. 2013, 272, 490–502. [Google Scholar] [CrossRef]
- Wills, L.P.; Beeson, G.C.; Hoover, D.B.; Schnellmann, R.G.; Beeson, C.C. Assessment of ToxCast Phase II for Mitochondrial Liabilities Using a High-Throughput Respirometric Assay. Toxicol. Sci. 2015, 146, 226–234. [Google Scholar] [CrossRef]
- Hynes, J.; Marroquin, L.D.; Ogurtsov, V.I.; Christiansen, K.N.; Stevens, G.J.; Papkovsky, D.B.; Will, Y. Investigation of drug-induced mitochondrial toxicity using fluorescence-based oxygen-sensitive probes. Toxicol. Sci. 2006, 92, 186–200. [Google Scholar] [CrossRef]
- Wagner, B.K.; Kitami, T.; Gilbert, T.J.; Peck, D.; Ramanathan, A.; Schreiber, S.L.; Golub, T.R.; Mootha, V.K. Large-scale chemical dissection of mitochondrial function. Nat. Biotechnol. 2008, 26, 343–351. [Google Scholar] [CrossRef]
- Wills, L.P. The use of high-throughput screening techniques to evaluate mitochondrial toxicity. Toxicology 2017, 391, 34–41. [Google Scholar] [CrossRef]
- Rosenke, K.; Hansen, F.; Schwarz, B.; Feldmann, F.; Haddock, E.; Rosenke, R.; Barbian, K.; Meade-White, K.; Okumura, A.; Leventhal, S.; et al. Orally delivered MK-4482 inhibits SARS-CoV-2 replication in the Syrian hamster model. Nat. Commun. 2021, 12, 2295. [Google Scholar] [CrossRef]
- Nehdi, A.; Samman, N.; Mashhour, A.; Alhallaj, A.; Trivilegio, T.; Gul, S.; Reinshagen, J.; Alaskar, A.; Gmati, G.; Abuelgasim, K.A.; et al. A Drug Repositioning Approach Identifies a Combination of Compounds as a Potential Regimen for Chronic Lymphocytic Leukemia Treatment. Front. Oncol. 2021, 11, 579488. [Google Scholar] [CrossRef]
- Naia, L.; Pinho, C.M.; Dentoni, G.; Liu, J.; Leal, N.S.; Ferreira, D.M.S.; Schreiner, B.; Filadi, R.; Fao, L.; Connolly, N.M.C.; et al. Neuronal cell-based high-throughput screen for enhancers of mitochondrial function reveals luteolin as a modulator of mitochondria-endoplasmic reticulum coupling. BMC Biol. 2021, 19, 57. [Google Scholar] [CrossRef] [PubMed]
- Bakowski, M.A.; Beutler, N.; Wolff, K.C.; Kirkpatrick, M.G.; Chen, E.; Nguyen, T.H.; Riva, L.; Shaabani, N.; Parren, M.; Ricketts, J.; et al. Drug repurposing screens identify chemical entities for the development of COVID-19 interventions. Nat. Commun. 2021, 12, 3309. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente-Herreruela, D.; Gonzalez-Charro, V.; Almendro-Vedia, V.G.; Moran, M.; Martin, M.A.; Lillo, M.P.; Natale, P.; Lopez-Montero, I. Rhodamine-based sensor for real-time imaging of mitochondrial ATP in living fibroblasts. Biochim. Biophys. Acta Bioenerg. 2017, 1858, 999–1006. [Google Scholar] [CrossRef]
- Tolosa, L.; Jimenez, N.; Perez, G.; Castell, J.V.; Gomez-Lechon, M.J.; Donato, M.T. Customised in vitro model to detect human metabolism-dependent idiosyncratic drug-induced liver injury. Arch. Toxicol. 2018, 92, 383–399. [Google Scholar] [CrossRef]
- Wilson, J.; Berntsen, H.F.; Zimmer, K.E.; Frizzell, C.; Verhaegen, S.; Ropstad, E.; Connolly, L. Effects of defined mixtures of persistent organic pollutants (POPs) on multiple cellular responses in the human hepatocarcinoma cell line, HepG2, using high content analysis screening. Toxicol. Appl. Pharmacol. 2016, 294, 21–31. [Google Scholar] [CrossRef]
- Kuznetsov, A.V.; Kehrer, I.; Kozlov, A.V.; Haller, M.; Redl, H.; Hermann, M.; Grimm, M.; Troppmair, J. Mitochondrial ROS production under cellular stress: Comparison of different detection methods. Anal. Bioanal. Chem. 2011, 400, 2383–2390. [Google Scholar] [CrossRef] [PubMed]
- Luczak, E.D.; Wu, Y.; Granger, J.M.; Joiner, M.A.; Wilson, N.R.; Gupta, A.; Umapathi, P.; Murphy, K.R.; Reyes Gaido, O.E.; Sabet, A.; et al. Mitochondrial CaMKII causes adverse metabolic reprogramming and dilated cardiomyopathy. Nat. Commun. 2020, 11, 4416. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.L.; Teixeira, A.F.; Pidde, G.; Ching, A.T.C.; Tambourgi, D.V.; Nascimento, A.; Herwald, H. Leptospira interrogans outer membrane protein LipL21 is a potent inhibitor of neutrophil myeloperoxidase. Virulence 2018, 9, 414–425. [Google Scholar] [CrossRef]
- Cali, T.; Ottolini, D.; Brini, M. Mitochondria, calcium, and endoplasmic reticulum stress in Parkinson’s disease. Biofactors 2011, 37, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.Y.; Turner, N.; Li, Y.Y.; Gu, M.; Huang, M.W.; Wu, F.; Pang, T.; Nan, F.J.; Ye, J.M.; Li, J.Y.; et al. High-throughput assay for modulators of mitochondrial membrane potential identifies a novel compound with beneficial effects on db/db mice. Diabetes 2010, 59, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Xue, L.; Zhu, Y.; Qian, X.; Zou, L.; Jin, Q.; Wang, D.; Ge, G. Differences in susceptibility of HT-29 and A549 cells to statin-induced toxicity: An investigation using high content screening. J. Biochem. Mol. Toxicol. 2021, 35, e22699. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Lu, Q.; Ding, Y.; Wu, Y.; Qiu, Y.; Wang, P.; Mao, X.; Huang, K.; Xie, Z.; Zou, M.H. Hyperglycemia-Driven Inhibition of AMP-Activated Protein Kinase alpha2 Induces Diabetic Cardiomyopathy by Promoting Mitochondria-Associated Endoplasmic Reticulum Membranes In Vivo. Circulation 2019, 139, 1913–1936. [Google Scholar] [CrossRef] [PubMed]
- Chazotte, B. Labeling mitochondria with MitoTracker dyes. Cold Spring Harb. Protoc. 2011, 2011, 990–992. [Google Scholar] [CrossRef]
- Perry, S.W.; Norman, J.P.; Barbieri, J.; Brown, E.B.; Gelbard, H.A. Mitochondrial membrane potential probes and the proton gradient: A practical usage guide. Biotechniques 2011, 50, 98–115. [Google Scholar] [CrossRef]
- Huang, L.; Su, W.; Zhao, Y.; Zhan, J.; Lin, W. Synthesis, molecular docking calculation, fluorescence and bioimaging of mitochondria-targeted ratiometric fluorescent probes for sensing hypochlorite in vivo. J. Mater. Chem. B 2021, 9, 2666–2673. [Google Scholar] [CrossRef]
- Salvatorelli, E.; Guarnieri, S.; Menna, P.; Liberi, G.; Calafiore, A.M.; Mariggio, M.A.; Mordente, A.; Gianni, L.; Minotti, G. Defective one-or two-electron reduction of the anticancer anthracycline epirubicin in human heart. Relative importance of vesicular sequestration and impaired efficiency of electron addition. J. Biol. Chem. 2006, 281, 10990–11001. [Google Scholar] [CrossRef]
- Korga, A.; Jozefczyk, A.; Zgorka, G.; Homa, M.; Ostrowska, M.; Burdan, F.; Dudka, J. Evaluation of the phytochemical composition and protective activities of methanolic extracts of Centaurea borysthenica and Centaurea daghestanica (Lipsky) Wagenitz on cardiomyocytes treated with doxorubicin. Food Nutr. Res. 2017, 61, 1344077. [Google Scholar] [CrossRef]
- Feng, X.; Yin, W.; Wang, J.; Feng, L.; Kang, Y.J. Mitophagy promotes the stemness of bone marrow-derived mesenchymal stem cells. Exp. Biol. Med. 2021, 246, 97–105. [Google Scholar] [CrossRef]
- O’Brien, P.J.; Irwin, W.; Diaz, D.; Howard-Cofield, E.; Krejsa, C.M.; Slaughter, M.R.; Gao, B.; Kaludercic, N.; Angeline, A.; Bernardi, P.; et al. High concordance of drug-induced human hepatotoxicity with in vitro cytotoxicity measured in a novel cell-based model using high content screening. Arch. Toxicol. 2006, 80, 580–604. [Google Scholar] [CrossRef]
- Tilmant, K.; Gerets, H.; De Ron, P.; Hanon, E.; Bento-Pereira, C.; Atienzar, F.A. In vitro screening of cell bioenergetics to assess mitochondrial dysfunction in drug development. Toxicol. In Vitro 2018, 52, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Biesemann, N.; Ried, J.S.; Ding-Pfennigdorff, D.; Dietrich, A.; Rudolph, C.; Hahn, S.; Hennerici, W.; Asbrand, C.; Leeuw, T.; Strubing, C. High throughput screening of mitochondrial bioenergetics in human differentiated myotubes identifies novel enhancers of muscle performance in aged mice. Sci. Rep. 2018, 8, 9408. [Google Scholar] [CrossRef] [PubMed]
- Maddah, M.; Mandegar, M.A.; Dame, K.; Grafton, F.; Loewke, K.; Ribeiro, A.J.S. Quantifying drug-induced structural toxicity in hepatocytes and cardiomyocytes derived from hiPSCs using a deep learning method. J. Pharmacol. Toxicol. Methods 2020, 105, 106895. [Google Scholar] [CrossRef] [PubMed]
- Grimm, F.A.; Iwata, Y.; Sirenko, O.; Bittner, M.; Rusyn, I. High-Content Assay Multiplexing for Toxicity Screening in Induced Pluripotent Stem Cell-Derived Cardiomyocytes and Hepatocytes. Assay Drug. Dev. Technol. 2015, 13, 529–546. [Google Scholar] [CrossRef] [PubMed]
- Sirenko, O.; Grimm, F.A.; Ryan, K.R.; Iwata, Y.; Chiu, W.A.; Parham, F.; Wignall, J.A.; Anson, B.; Cromwell, E.F.; Behl, M.; et al. In vitro cardiotoxicity assessment of environmental chemicals using an organotypic human induced pluripotent stem cell-derived model. Toxicol. Appl. Pharmacol. 2017, 322, 60–74. [Google Scholar] [CrossRef]
- O’Brien, P.J.; Edvardsson, A. Validation of a Multiparametric, High-Content-Screening Assay for Predictive/Investigative Cytotoxicity: Evidence from Technology Transfer Studies and Literature Review. Chem. Res. Toxicol. 2017, 30, 804–829. [Google Scholar] [CrossRef]
- Attene-Ramos, M.S.; Huang, R.; Michael, S.; Witt, K.L.; Richard, A.; Tice, R.R.; Simeonov, A.; Austin, C.P.; Xia, M. Profiling of the Tox21 chemical collection for mitochondrial function to identify compounds that acutely decrease mitochondrial membrane potential. Env. Health Perspect. 2015, 123, 49–56. [Google Scholar] [CrossRef]
- Nabati, M.; Parsaee, H. Potential Cardiotoxic Effects of Remdesivir on Cardiovascular System: A Literature Review. Cardiovasc. Toxicol. 2021, 22, 268–272. [Google Scholar] [CrossRef]
- McComsey, G.; Lonergan, J.T. Mitochondrial dysfunction: Patient monitoring and toxicity management. J. Acquir. Immune Defic. Syndr. 2004, 37 (Suppl. S1), S30–S35. [Google Scholar] [CrossRef]
- Zhou, B.; Tian, R. Mitochondrial dysfunction in pathophysiology of heart failure. J. Clin. Investig. 2018, 128, 3716–3726. [Google Scholar] [CrossRef] [PubMed]
- Bertero, E.; Maack, C. Metabolic remodelling in heart failure. Nat. Rev. Cardiol. 2018, 15, 457–470. [Google Scholar] [CrossRef]
- Limongelli, G.; Masarone, D.; Pacileo, G. Mitochondrial disease and the heart. Heart 2017, 103, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Renu, K.; Abilash, V.G.; Tirupathi Pichiah, P.B.; Arunachalam, S. Molecular mechanism of doxorubicin-induced cardiomyopathy—An update. Eur. J. Pharmacol. 2018, 818, 241–253. [Google Scholar] [CrossRef]
- Benjanuwattra, J.; Siri-Angkul, N.; Chattipakorn, S.C.; Chattipakorn, N. Doxorubicin and its proarrhythmic effects: A comprehensive review of the evidence from experimental and clinical studies. Pharmacol. Res. 2020, 151, 104542. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.M.; Cho, S.; Jang, J.Y.; Kim, H.; Chun, S.; Choi, M.; Park, S.; Ko, Y.G. Cardioprotective Potential of an SGLT2 Inhibitor Against Doxorubicin-Induced Heart Failure. Korean Circ. J. 2019, 49, 1183–1195. [Google Scholar] [CrossRef]
- Tong, D.; Zaha, V.G. Metabolic Imaging in Cardio-oncology. J. Cardiovasc. Transl. Res. 2020, 13, 357–366. [Google Scholar] [CrossRef]
- Sivapackiam, J.; Sharma, M.; Schindler, T.H.; Sharma, V. PET Radiopharmaceuticals for Imaging Chemotherapy-Induced Cardiotoxicity. Curr. Cardiol. Rep. 2020, 22, 62. [Google Scholar] [CrossRef]
- Mason, F.E.; Pronto, J.R.D.; Alhussini, K.; Maack, C.; Voigt, N. Cellular and mitochondrial mechanisms of atrial fibrillation. Basic Res. Cardiol. 2020, 115, 72. [Google Scholar] [CrossRef]
- Yang, Y.; Wei, S.; Zhang, B.; Li, W. Recent Progress in Environmental Toxins-Induced Cardiotoxicity and Protective Potential of Natural Products. Front. Pharmacol. 2021, 12, 699193. [Google Scholar] [CrossRef]
- Behjati, M.; Sabri, M.R.; Etemadi Far, M.; Nejati, M. Cardiac complications in inherited mitochondrial diseases. Heart Fail. Rev. 2020, 26, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Liu, Z.; Cao, N. Human pluripotent stem cell-based cardiovascular disease modeling and drug discovery. Pflugers Arch. 2021, 473, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Couch, L.; Higuchi, M.; Fang, J.L.; Guo, L. Mitochondrial dysfunction induced by sertraline, an antidepressant agent. Toxicol. Sci. 2012, 127, 582–591. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).