The Preparation of Gen-NH2-MCM-41@SA Nanoparticles and Their Anti-Rotavirus Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Chemical Experimental Materials
2.1.2. Biological Cell Experimental Materials
2.1.3. Animal Experimental Materials
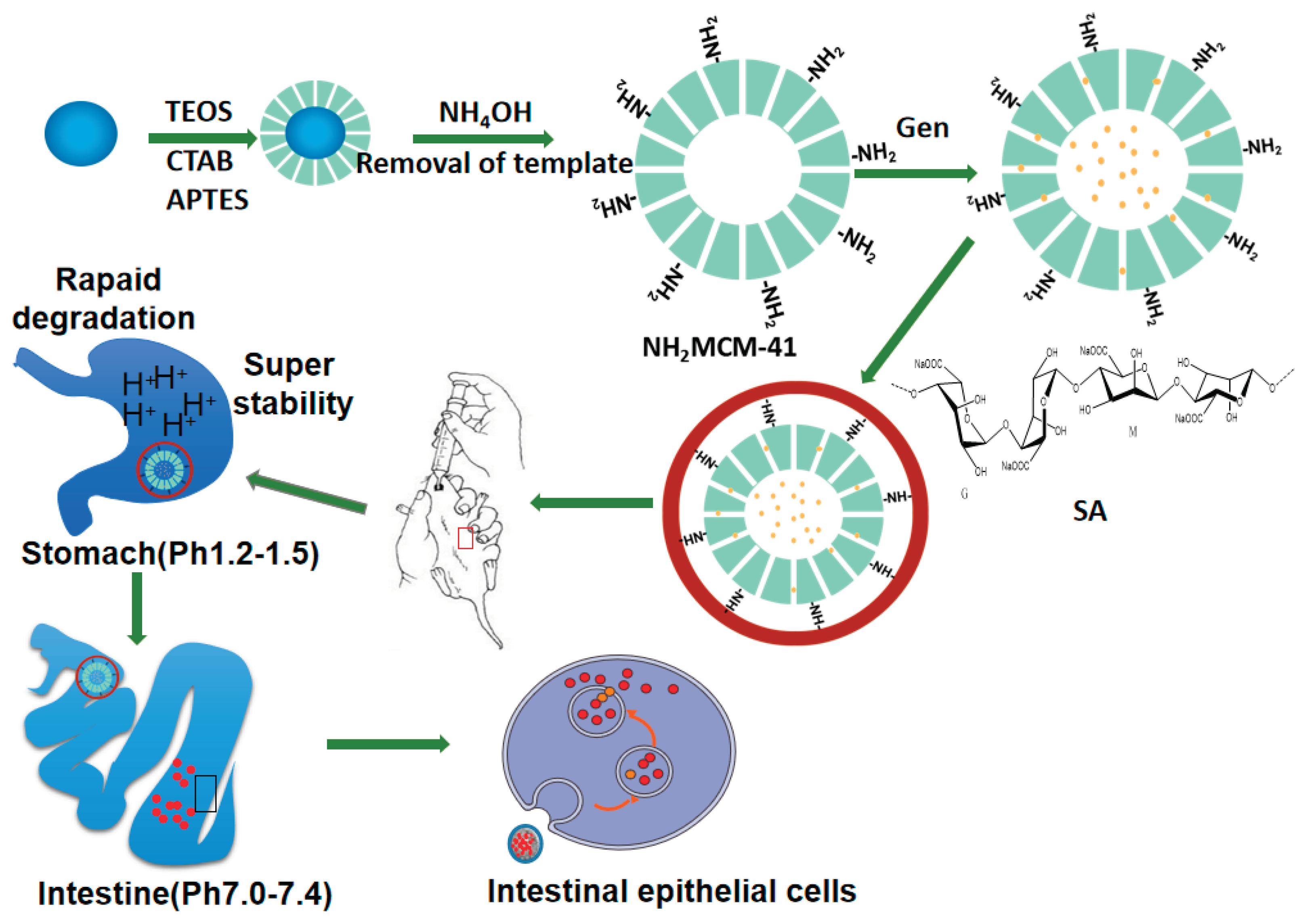
2.2. Synthesis
2.2.1. Preparation of MCM-41
2.2.2. Preparation of NH2-MCM-41
2.3. Sample Preparation and Analysis
2.3.1. NH2-MCM-41 Loading with Gen
2.3.2. SA Packaging of Gen-NH2-MCM-41
2.3.3. Gen-NH2-MCM-41@SA In Vitro Release Performance Test
2.3.4. Gen-NH2-MCM-41@SA Drug Release Model Fitting
2.4. Physico-Chemical Characterization
2.4.1. XRD
2.4.2. Nitrogen Adsorption–Desorption (BET)
2.4.3. Fourier Infrared Spectroscopy (FT-IR)
2.4.4. Zeta Potential
2.4.5. Scanning Electron Microscopy (SEM)
2.4.6. Differential Scanning Thermal Analysis (DSC)
2.4.7. Adsorption Performance and Drug Loading Test (UV)
2.5. Administration of Gen-NH2-MCM-41@SA, NH2-MCM-41, SA, and Gen against RV Infection in Caco-2 Cells
2.5.1. Cell Culture
2.5.2. Amplification and Titer Determination of RV in MA104 Cells
2.5.3. The Cytotoxic Effect of Gen-NH2-MCM-41@SA
2.5.4. Anti-RV Effects of Gen-NH2-MCM-41@SA Nanoparticles
Inhibition of RV Attachment of Gen-NH2-MCM-41@SA Nanoparticles and Gen
Effects of Gen-NH2-MCM-41@SA Nanoparticles and Gen on the Direct Inactivation of RV
Effects of Gen-NH2-MCM-41@SA Nanoparticles and Gen on Inhibiting RV Replication
2.6. Administration of Gen-NH2-MCM-41@SA and Gen against RV Infection in Neonatal Mice
2.6.1. Culture of Suckling Mice of Kunming Species
2.6.2. Study on Modeling and Drug Administration in Kunming Species Mice
2.6.3. HE Staining of Histopathological Sections
2.7. Statistical Analysis
3. Results and Discussion
3.1. Physico-Chemical Characterization
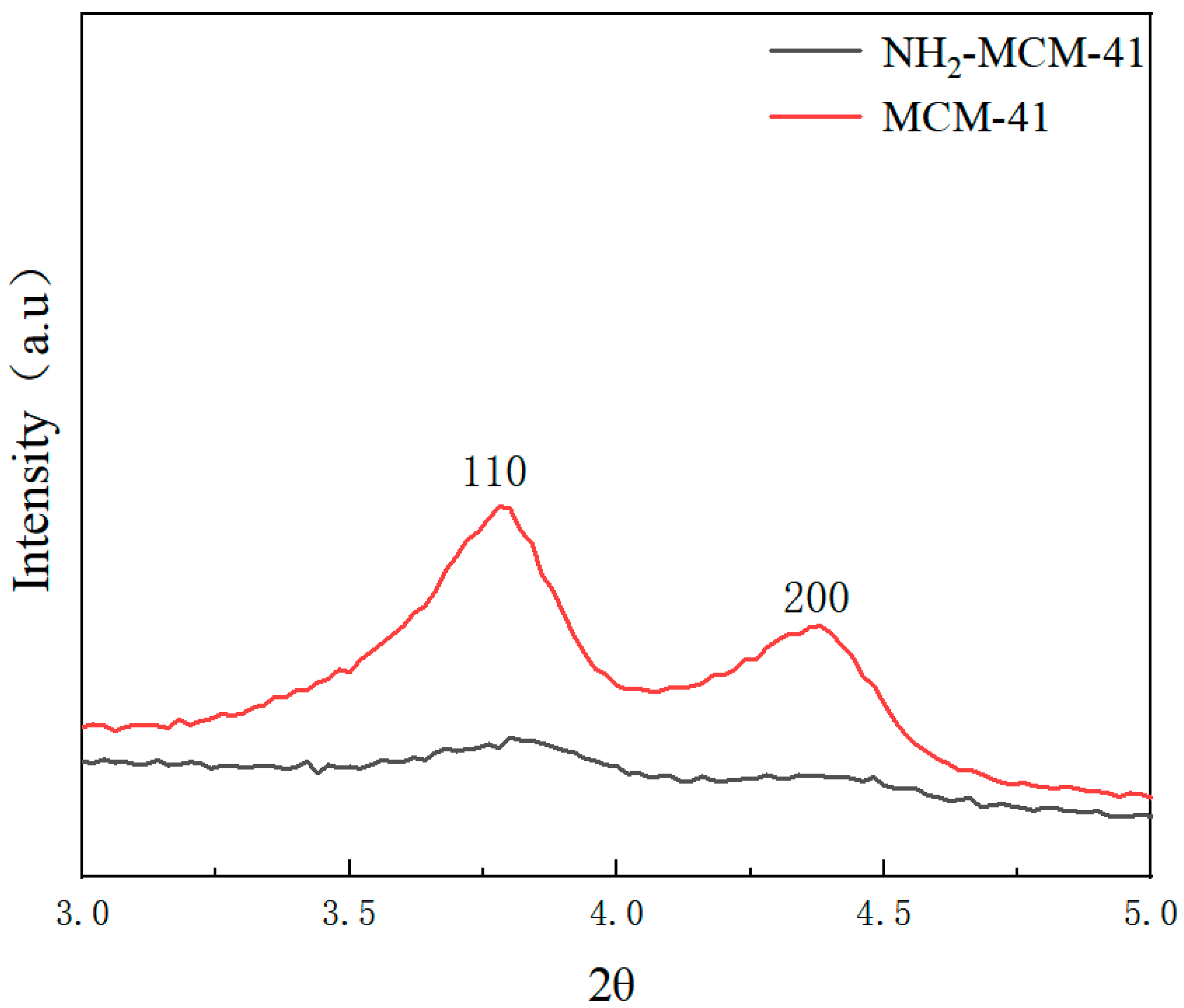
3.1.1. X-ray (Small Angle) Diffraction
3.1.2. Nitrogen Adsorption–Desorption (BET) Analysis of MCM-41 and NH2-MCM-41
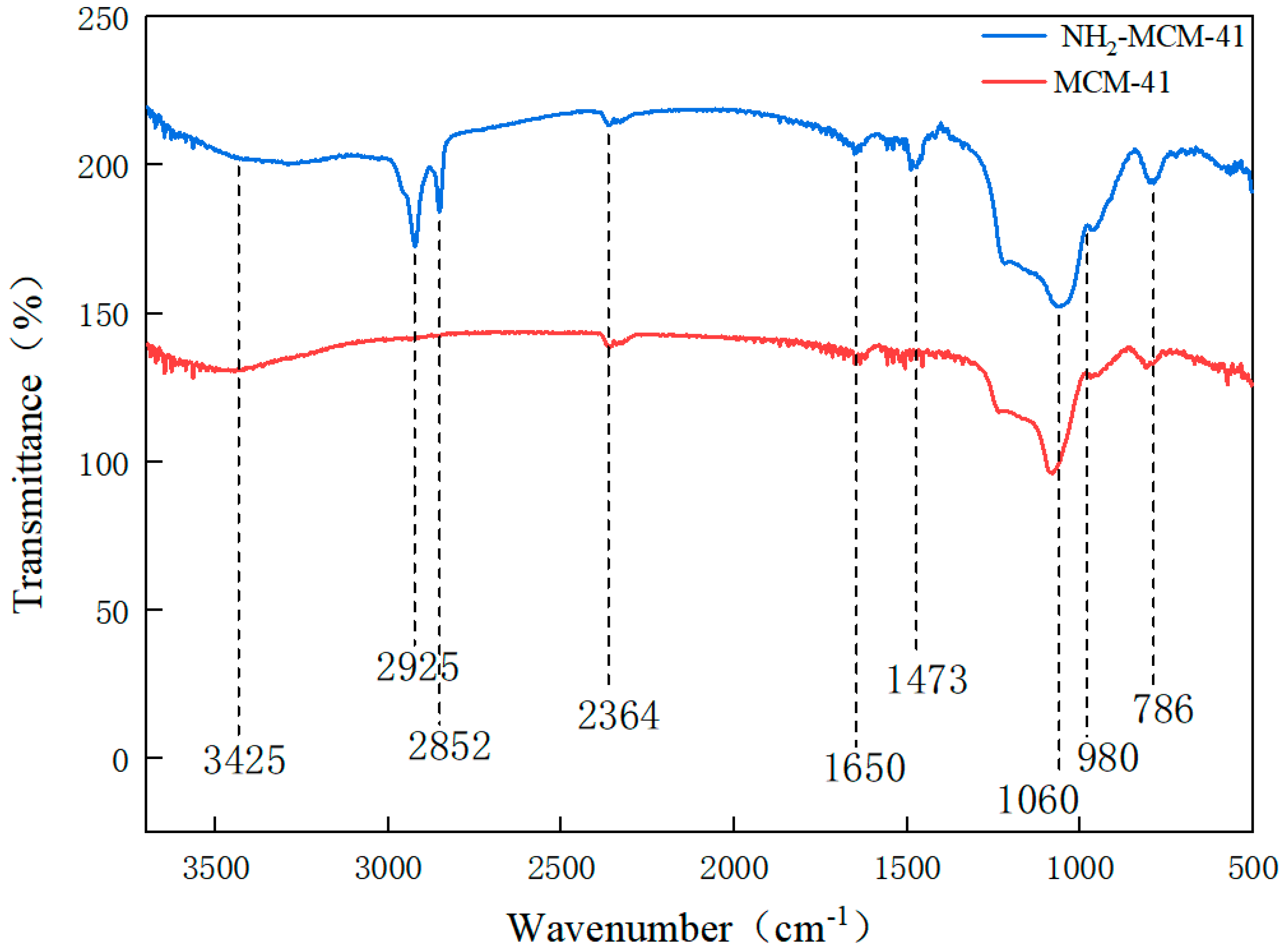
3.1.3. Fourier Infrared Spectroscopy (FT-IR) Analysis of MCM-41 and NH2-MCM-41
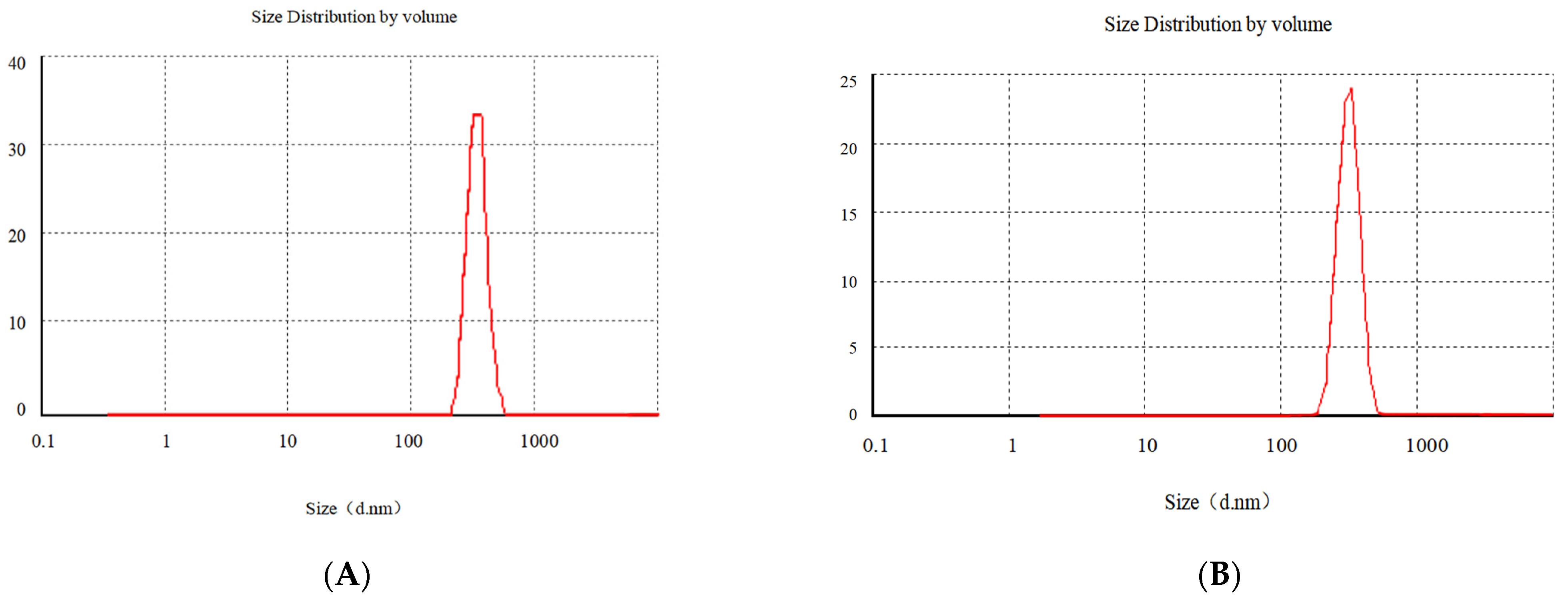
3.1.4. Particle Size Analysis and Zeta Potential Measurement
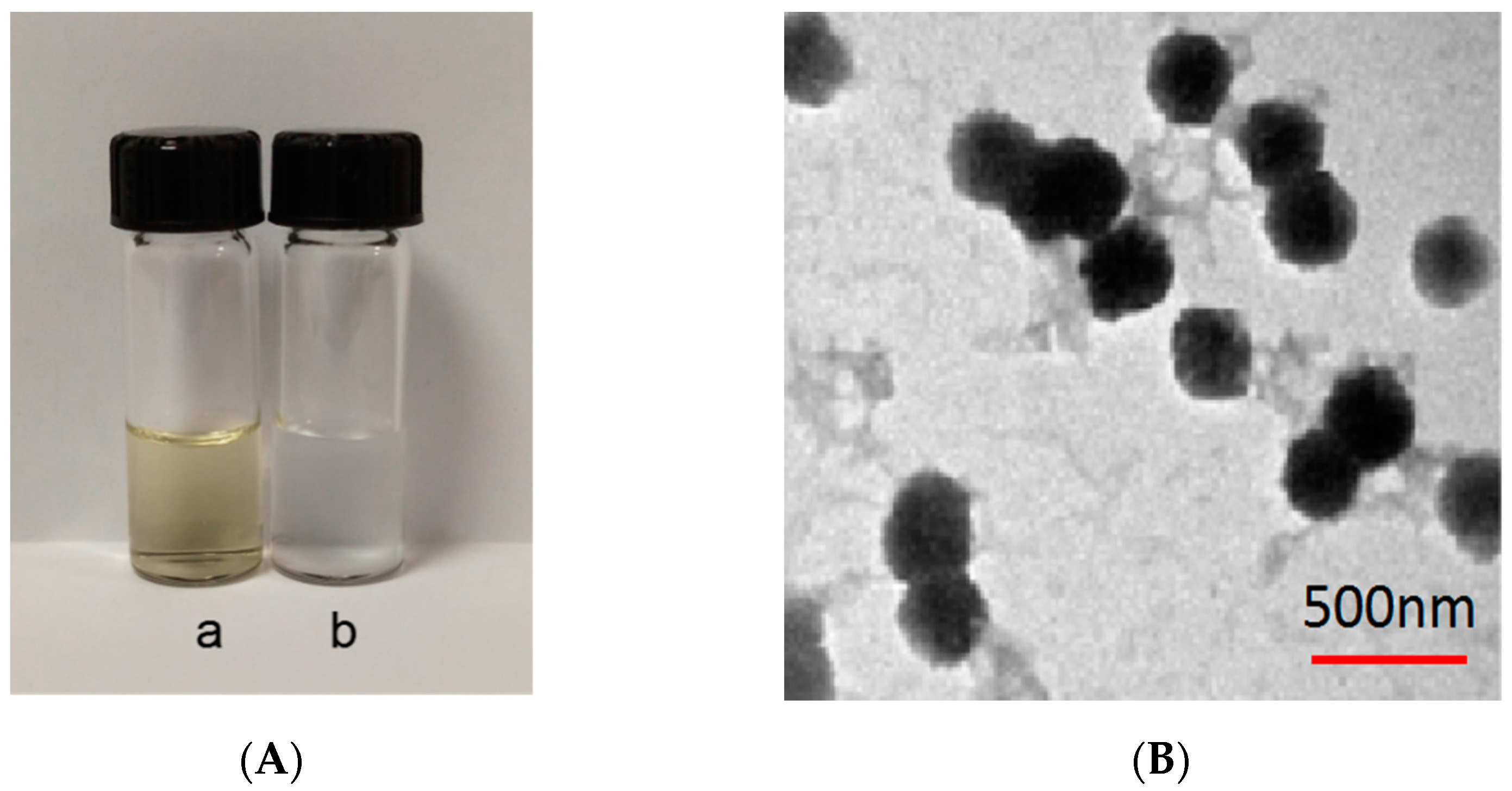
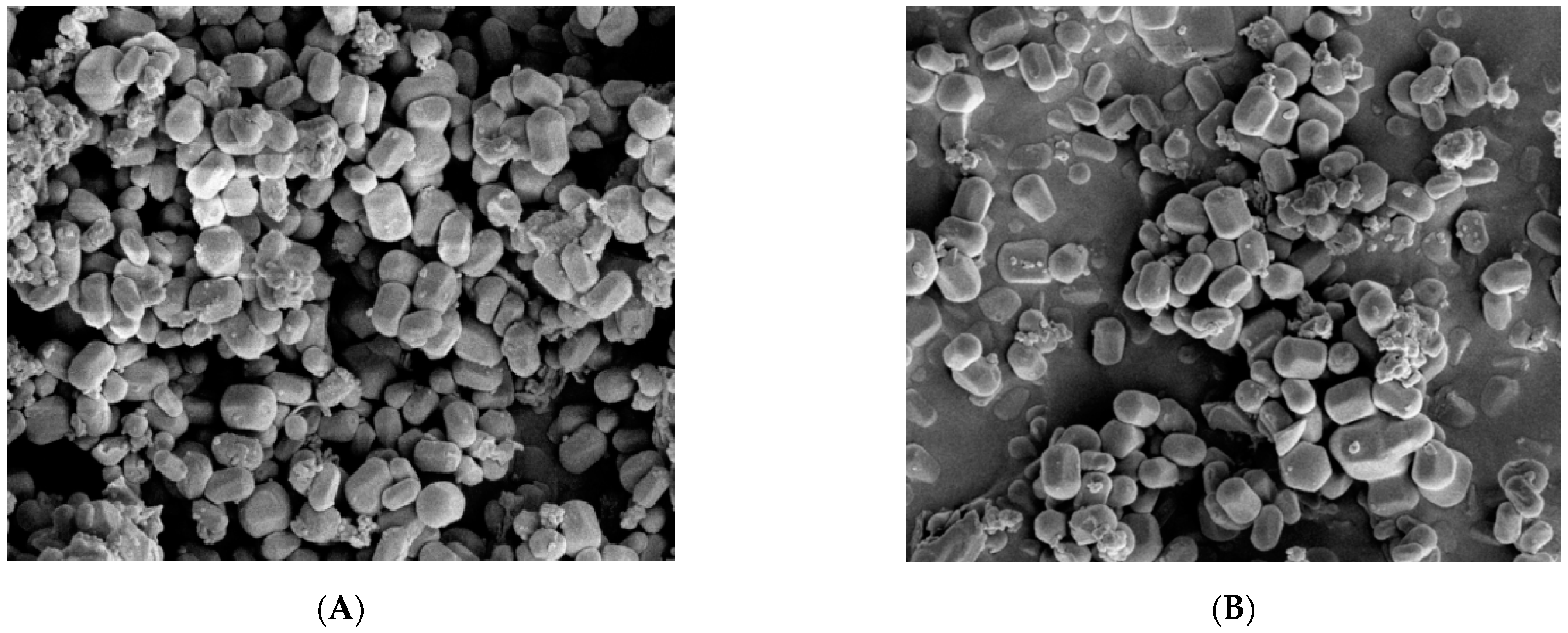
3.1.5. SEM Analysis of MCM-41 and NH2-MCM-41
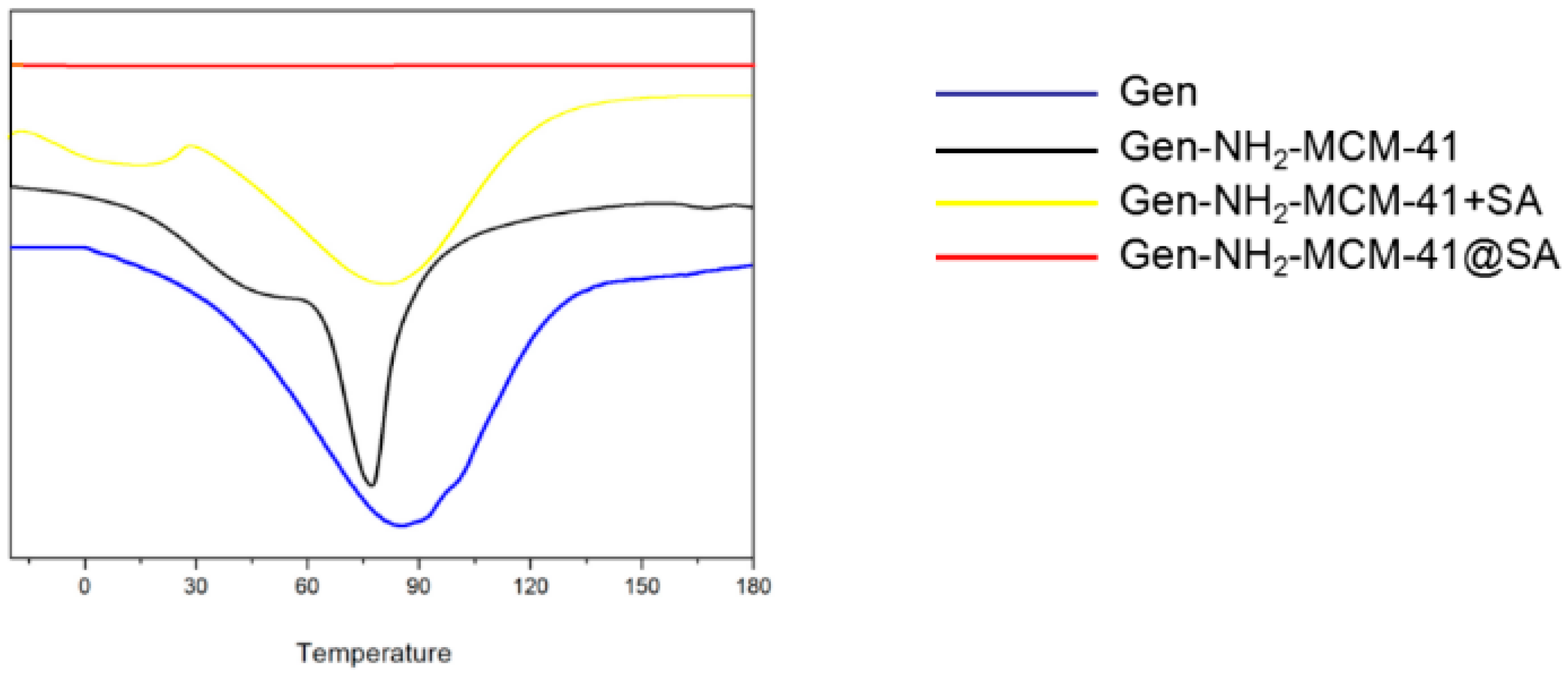
3.1.6. Differential Scanning Thermal Analysis (DSC)
3.1.7. Adsorption Performance and Drug Loading
3.1.8. Gen-NH2-MCM- 41@SA Analysis of Sustained-Release Properties of Nanoparticles In Vitro
3.1.9. Gen-NH2-MCM-41@SA Drug Release Model Fitting under Different pH Conditions
3.2. In Vitro Cell Experiment
3.2.1. CCK-8 Detected the Cytotoxicity of NH2-MCM-41, SA, and Gen in Caco-2
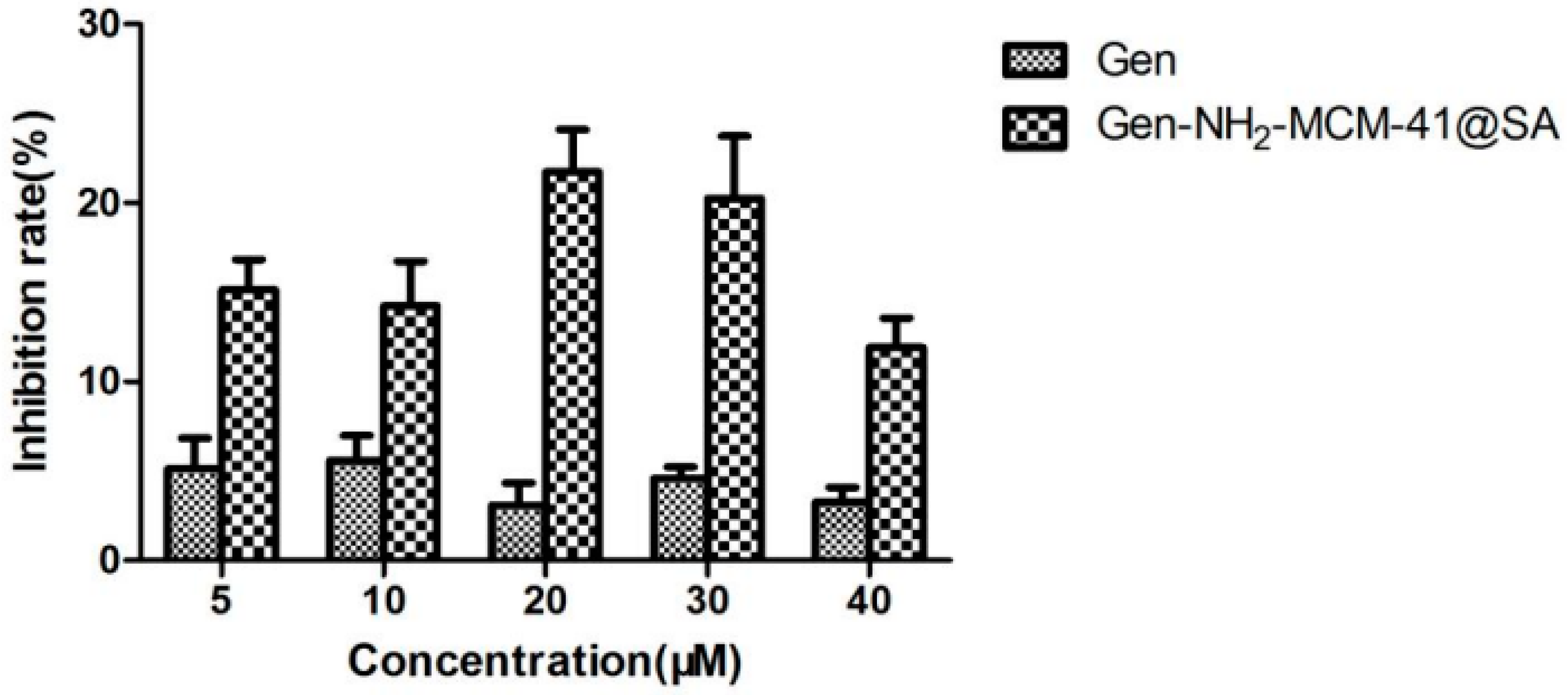
3.2.2. Gen-NH2-MCM-41@SA and Gen Anti-RV-WA Adsorption
3.2.3. Gen-NH2-MCM-41@SA and Gen Can Directly Inactivate RV-WA
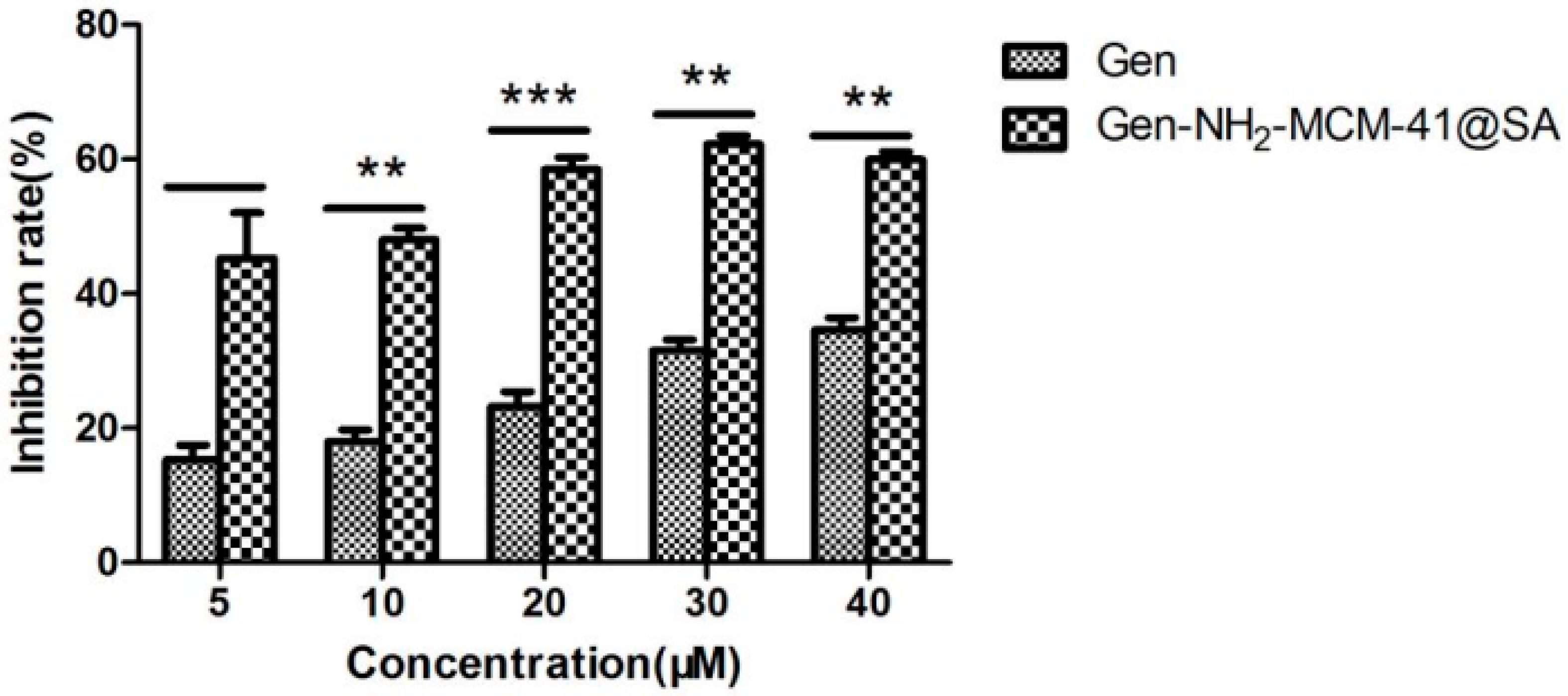
3.2.4. Gen-NH2-MCM-41@SA and Gen against RV-WA Biosynthesis
3.3. Effect of Gen-NH2-MCM-41@SA Nanoparticles on RV-SA Infection in Suckling Mice
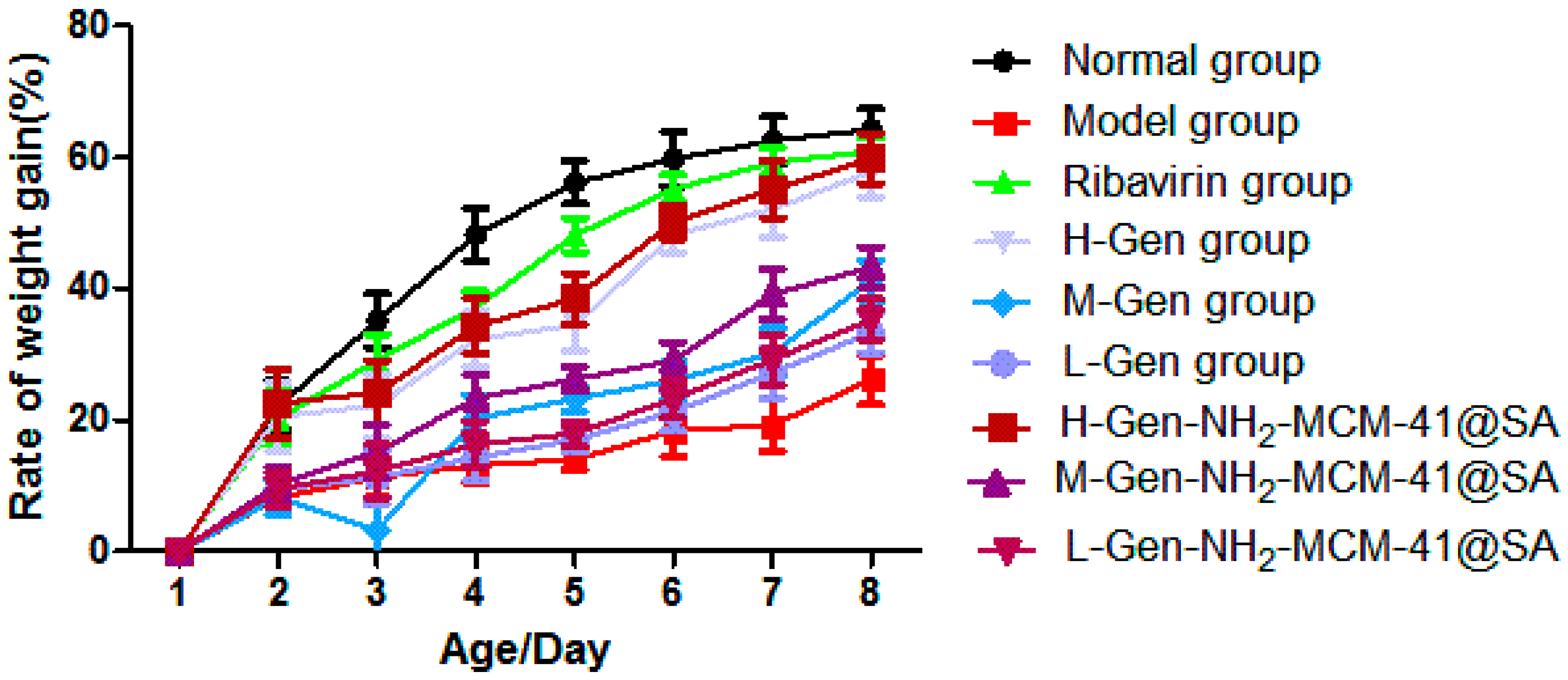
3.3.1. Changes in Body Weight of Suckling Mice after Drug Administration
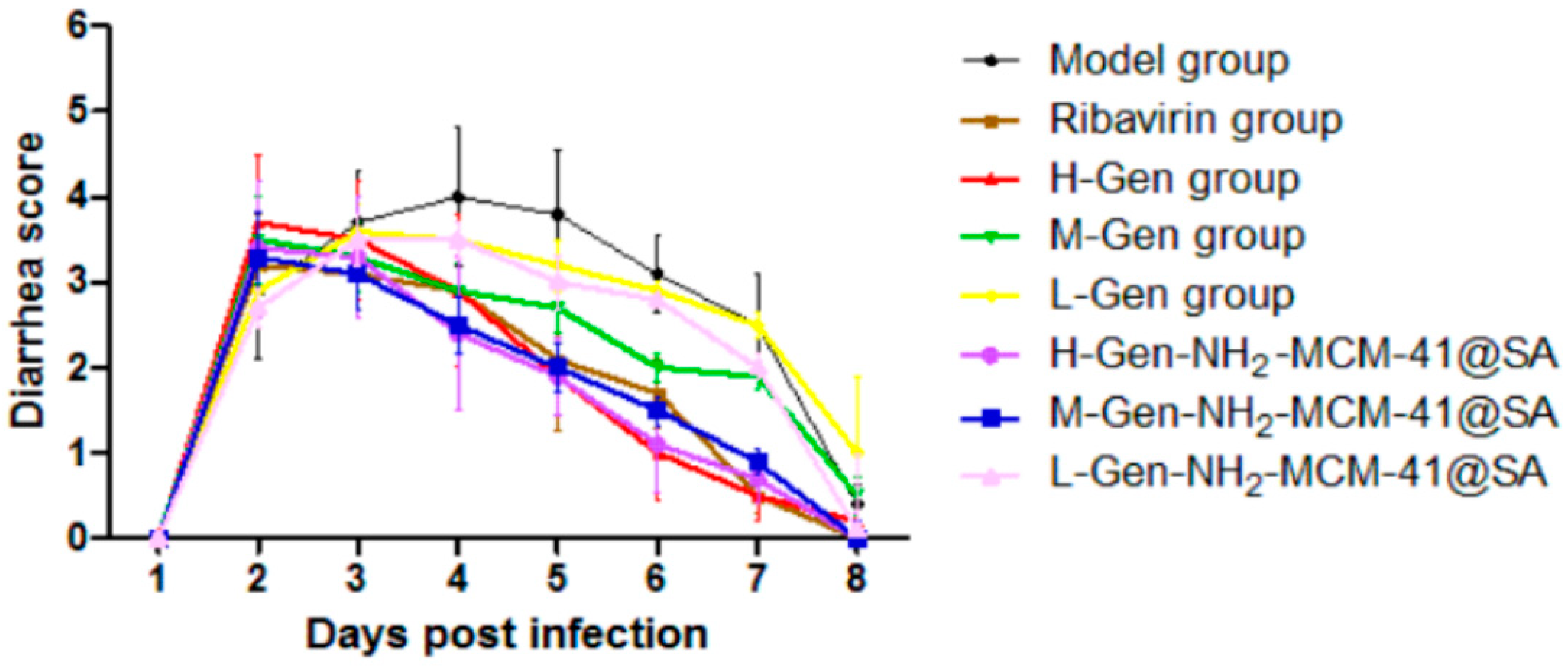
3.3.2. Diarrhea Score of Suckling Mice after Administration and Diarrhea Degree after RV-SA Infection in Suckling Mice
3.3.3. Pathological Changes in Intestinal Tissue on the Third Day after Administration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mazokopakis, E.E. Ecballium Elaterium for Gastrointestinal Cancer. Asian Pac. J. Cancer Prev. 2018, 19, 583. [Google Scholar] [PubMed]
- Hu, T.; Li, Z.; Gao, C.Y.; Cho, C.H. Mechanisms of drug resistance in colon cancer and its therapeutic strategies. World J. Gastroenterol. 2016, 22, 6876–6889. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Ding, J. Research progress of structure modification and activity of genistein. Fine Chem. 2018, 35, 1441–1448.1465. [Google Scholar]
- Fu, Y.; Du, P.; Zhao, J.; Qin, Y.; Huang, G. Gastric Cancer Stem Cells: Mechanisms and Therapeutic Approaches. Yonsei Med. J. 2018, 59, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Matthies, A.; Clavel, T.; Gütschow, M.; Engst, W.; Haller, D.; Blaut, M.; Braune, A. Conversion of daidzein and genistein by an anaerobic bacterium newly isolated from the mouse intestine. Appl. Environ. Microbiol. 2008, 74, 4847–4852. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Song, L.; Li, G.; Chen, W.; Zhao, S.; Zhou, R.; Zhao, W. Human rotavirus strain Wa downregulates NHE1 and NHE6 expressions in rotavirus-infected Caco-2 cells. Virus Genes 2017, 53, 367–376. [Google Scholar] [CrossRef]
- Chen, W.; Song, L.; Zhao, W. Research progress of aquaporin in diarrhea. Med. Rev. 2018, 24, 1041–1046. [Google Scholar]
- Berlier, G.; Gastaldi, L.; Sapino, S.; Miletto, I.; Bottinelli, E.; Chirio, D.; Ugazio, E. MCM-41 as a useful vector for rutin topical formulations: Synthesis, characterization and testing. Int. J. Pharm. 2013, 457, 177–186. [Google Scholar] [CrossRef]
- Zong, W.X.; Rabinowitz, J.D.; White, E. Mitochondria and Cancer. Mol. Cell 2016, 61, 667–676. [Google Scholar] [CrossRef] [Green Version]
- Yousefnia, S.; Momenzadeh, S.; Forootan, F.S.; Ghaedi, K.; Esfahani MH, N. The influence of peroxisome proliferator-activated receptor γ (PPARγ) ligands on cancer cell tumorigenicity. Gene 2018, 649, 14–22. [Google Scholar] [CrossRef]
- Yun, S.H.; Han, S.H.; Park, J.I. Peroxisome Proliferator-Activated Receptor gamma and PGC-1alpha in Cancer: Dual Actions as Tumor Promoter and Suppressor. PPAR Res. 2018, 2018, 6727421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Liu, L.; Huang, T.; Wang, Q.; Fang, Y. Bubble template fabrication of chitosan/poly (vinyl alcohol) sponges for wound dressing applications. Int. J. Biol. Macromol. 2013, 62, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Nagai, Y.; Fukui, Y.; Tamiya-Koizumi, K.; Iwata, H.; Watanabe, T.; Hamaguchi, M. Expression and tyrosine phosphorylation of phosphatidyl-inositol-3 kinase in human gastric-cancer cells—its correlation with cell-growth. Int. J. Oncol. 1995, 6, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.F.; Yeung, K.L.; McKay, G. A rational approach in the design of selective mesoporous adsorbents. Langmuir The ACS J. Surf. Colloids 2006, 22, 9632–9641. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.F.; Yeung, K.L.; McKay, G. An investigation of gold adsorption from a binary mixture with selective mesoporous silica adsorbents. J. Phys. Chem. B 2006, 110, 2187–2194. [Google Scholar] [CrossRef]
- Yang, C.; Guo, W.; Cui, L.; An, N.; Zhang, T.; Guo, G.; Lin, H.; Qu, F. Fe3O4@mSiO2 core-shell nanocomposite capped with disulfide gatekeepers for enzyme-sensitive controlled release of anti-cancer drugs. J. Mater. Chem. B 2015, 3, 1010–1019. [Google Scholar] [CrossRef]
- Putz, A.-M.; Ciopec, M.; Negrea, A.; Grad, O.; Ianăşi, C.; Ivankov, O.; Milanović, M.; Stijepović, I.; Almásy, L. Comparison of Structure and Adsorption Properties of Mesoporous Silica Functionalized with Aminopropyl Groups by the Co-Condensation and the Post Grafting Methods. Materials 2021, 14, 628. [Google Scholar] [CrossRef]
- Huang, S.; Fu, X. Naturally derived materials-based cell and drug delivery systems in skin regeneration. J. Control. Release Off. J. Control. Release Soc. 2010, 142, 149–159. [Google Scholar] [CrossRef]
- Ovington, L.G. The evolution of wound management: Ancient origins and advances of the past 20 years. Home Healthc. Now 2002, 20, 652–656. [Google Scholar] [CrossRef]
- Annabi, N.; Rana, D.; Sani, E.S.; Portillo-Lara, R.; Gifford, J.L.; Fares, M.M.; Mithieux, S.M.; Weiss, A.S. Engineering a sprayable and elastic hydrogel adhesive with antimicrobial properties for wound healing. Biomaterials 2017, 139, 229–243. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, P.; Soni, S.; Mittal, G.; Bhatnagar, A. Role of Polymeric Biomaterials as Wound Healing Agents. Int. J. Low. Extrem. Wounds 2014, 13, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Mukund, V.; Mukund, D.; Sharma, V.; Mannarapu, M.; Alam, A. Genistein: Its role in metabolic diseases and cancer. Crit. Rev. Oncol. Hematol. 2017, 119, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wan, C.; Luo, Q.; Huang, Z.; Luo, Q. Genistein-inhibited cancer stem cell-like properties and reduced chemoresistance of gastric cancer. Int. J. Mol. Sci. 2014, 15, 3432–3443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farruggio, S.; Raina, G.; Cocomazzi, G.; Librasi, C.; Mary, D.; Gentilli, S.; Grossini, E. Genistein improves viability, proliferation and mitochondrial function of cardiomyoblasts cultured in physiologic and peroxidative conditions. Int. J. Mol. Med. 2019, 44, 2298–2310. [Google Scholar] [CrossRef]
- Akimoto, T.; Nonaka, T.; Ishikawa, H.; Sakurai, H.; Saitoh, J.I.; Takahashi, T.; Mitsuhashi, N. Genistein, a tyrosine kinase inhibitor, enhanced radiosensitivity in human esophageal cancer cell lines in vitro: Possible involvement of inhibition of survival signal transduction pathways. Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 195–201. [Google Scholar] [CrossRef]
- Adams, N.R. Detection of the effects of phytoestrogens on sheep and cattle. J. Anim. Sci. 1995, 73, 1509–1515. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [Green Version]
- Adlercreutz, H.; Mazur, W. Phyto-oestrogens and Western diseases. Ann. Med. 1997, 29, 95–120. [Google Scholar] [CrossRef]
- Ravanan, P.; Srikumar, I.F.; Talwar, P. Autophagy: The spotlight for cellular stress responses. Life Sci. 2017, 188, 53–67. [Google Scholar] [CrossRef]
- Jin, S.; Zhang, Q.Y.; Kang, X.M.; Wang, J.X.; Zhao, W.H. Daidzein induces MCF-7 breast cancer cell apoptosis via the mitochondrial pathway. Ann. Oncol. 2010, 21, 263–268. [Google Scholar] [CrossRef]
- Qi, W.; Weber, C.R.; Wasland, K.; Savkovic, S.D. Genistein inhibits proliferation of colon cancer cells by attenuating a negative effect of epidermal growth factor on tumor suppressor FOXO3 activity. BMC Cancer 2011, 11, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, X.; Lin, M.; Zhang, F.; Hu, Y.; Xu, X.; Li, Y.; Yao, J. Dietary flavonoid genistein induces Nrf2 and phase II detoxification gene expression via ERKs and PKC pathways and protects against oxidative stress in Caco-2 cells. Mol. Nutr. Food Res. 2013, 57, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Yanagihara, K.; Takigahira, M.; Mihara, K.; Kubo, T.; Morimoto, C.; Morita, Y.; Seyama, T. Inhibitory effects of isoflavones on tumor growth and cachexia in newly established cachectic mouse models carrying human stomach cancers. Nutr. Cancer 2013, 65, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Jing, X.; Li, H.; Zhao, X.; Wang, D. Soy isoflavone consumption and colorectal cancer risk: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 1–9. [Google Scholar]
- Adams, J.M.; Cory, S. The Bcl-2 protein family: Arbiters of cell survival. Science 1998, 281, 1322–1326. [Google Scholar] [CrossRef]
- Koch, U.; Radtke, F. Notch and cancer: A double-edged sword. Cell Mol. Life Sci. 2007, 64, 2746–2762. [Google Scholar] [CrossRef] [Green Version]
- Krüger-Genge, A.; Blocki, A.; Franke, R.P.; Jung, F. Vascular Endothelial Cell Biology: An Update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef] [Green Version]
- Kulling, S.E.; Rosenberg, B.; Jacobs, E.; Metzler, M. The phytoestrogens coumoestrol and genistein induce structural chromosomal aberrations in cultured human peripheral blood lymphocytes. Arch. Toxicol. 1999, 73, 50–54. [Google Scholar] [CrossRef]
- Mayo, B.; Vázquez, L.; Flórez, A.B. Equol: A Bacterial Metabolite from The Daidzein Isoflavone and Its Presumed Beneficial Health Effects. Nutrients 2019, 11, 2231. [Google Scholar] [CrossRef] [Green Version]
- Park, C.E.; Yun, H.; Lee, E.B.; Min, B.I.; Bae, H.; Choe, W.; Ha, J. The antioxidant effects of genistein are associated with AMP-activated protein kinase activation and PTEN induction in prostate cancer cells. J. Med. Food 2010, 13, 815–820. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, C.A.; Cai, P.Z.; Xu, F.P.; Zhu, W.J.; Wang, W.W.; Jiang, H.P. Omega-3PUFA Attenuates MNU-Induced Colorectal Cancer in Rats by Blocking PI3K/AKT/Bcl-2 Signaling. Onco Targets Ther. 2020, 13, 1953–1965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smit, G.; Puvanesarajah, V.; Carlson, R.W.; Barbour, W.M.; Stacey, G. Bradyrhizobium japonicum nodD1 can be specifically induced by soybean flavonoids that do not induce the nodYABCSUIJ operon. J. Biol. Chem. 1992, 267, 310–318. [Google Scholar] [CrossRef]
- Nagaraju, G.P.; Zafar, S.F.; El-Rayes, B.F. Pleiotropic effects of genistein in metabolic, inflammatory, and malignant diseases. Nutr. Rev. 2013, 71, 562–572. [Google Scholar] [CrossRef] [PubMed]









| Sample Name | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Aperture (nm) |
|---|---|---|---|
| MCM-41 | 804 | 0.795 | 2.95 |
| NH2-MCM-41 | 452 | 0.408 | 1.88 |
| Gen: NH2-MCM-41 | Adsorption Capacity (mg/g ± SD) | Drug Loading (mg/g ± SD) |
|---|---|---|
| 1:0.5 | 13.15 ± 0.89% | 12.65 ± 1.53% |
| 1:1 | 9.14 ± 0.71% | 8.97 ± 1.28% |
| 1:2 | 8.1 ± 1.76% | 6.96 ± 1.10% |
| Pharmacokinetic Model | Fitted Equation | pH | Correlation Coefficient/R2 | |
|---|---|---|---|---|
| Zero-order | 0.00272t + 0.4758 | 1.0 | 0.61442 | |
| 0.00285t + 0.05282 | 6.8 | 0.69925 | ||
| 0.001475t + 0.00122 | 7.4 | 0.71021 | ||
| First-order | −0.0049t − 0.1251 | 1.0 | 0.64511 | |
| −0.00412t − 0.11241 | 6.8 | 0.7945 | ||
| −0.000421t − 0.00714 | 7.4 | 0.8147 | ||
| Higuchi | 0.00659t1/2 + 0.14915 | 1.0 | 0.62584 | |
| 0.00124t1/2 + 0.01548 | 6.8 | 0.61591 | ||
| 0.0000987t1/2 + 0.00126 | 7.4 | 0.79948 | ||
| Korsmeyer–Peppas | 0.1301t0.25124 | 1.0 | 0.98101 | |
| 0.09801t0.27874 | 6.8 | 0.92156 | ||
| 0.00051402t0.41211 | 7.4 | 0.95641 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, L.; Chen, J.; Feng, Y.; Zhou, Y.; Li, F.; Dai, G.; Yuan, Y.; Yi, H.; Qian, Y.; Yang, S.; et al. The Preparation of Gen-NH2-MCM-41@SA Nanoparticles and Their Anti-Rotavirus Effects. Pharmaceutics 2022, 14, 1337. https://doi.org/10.3390/pharmaceutics14071337
Song L, Chen J, Feng Y, Zhou Y, Li F, Dai G, Yuan Y, Yi H, Qian Y, Yang S, et al. The Preparation of Gen-NH2-MCM-41@SA Nanoparticles and Their Anti-Rotavirus Effects. Pharmaceutics. 2022; 14(7):1337. https://doi.org/10.3390/pharmaceutics14071337
Chicago/Turabian StyleSong, Lijun, Jiabo Chen, Yuxuan Feng, Yujing Zhou, Feng Li, Guiqin Dai, Yue Yuan, Haosen Yi, Yupei Qian, Siyan Yang, and et al. 2022. "The Preparation of Gen-NH2-MCM-41@SA Nanoparticles and Their Anti-Rotavirus Effects" Pharmaceutics 14, no. 7: 1337. https://doi.org/10.3390/pharmaceutics14071337
APA StyleSong, L., Chen, J., Feng, Y., Zhou, Y., Li, F., Dai, G., Yuan, Y., Yi, H., Qian, Y., Yang, S., Chen, Y., & Zhao, W. (2022). The Preparation of Gen-NH2-MCM-41@SA Nanoparticles and Their Anti-Rotavirus Effects. Pharmaceutics, 14(7), 1337. https://doi.org/10.3390/pharmaceutics14071337






