Nitric Oxide-Releasing Platforms for Treating Cardiovascular Disease
Abstract
:1. Introduction
2. The Mechanism of NO in CVD and NO-Related Drugs
2.1. Hypertension
2.2. Myocardial Infarction
2.3. Left Ventricular Hypertrophy
2.4. Heart Failure
3. Application of NO-Releasing Biomaterial Platforms in CVD Treatment
3.1. Cardiovascular Stents
3.2. Medical Catheter
3.3. Vascular Grafts
3.4. Nanoparticles
3.5. Hydrogel
4. Methods to Improve the Efficacy of NO-Based Therapy for CVD
4.1. Synergistic Gas Therapy Based on NO and H2S
4.2. Synergistic Therapy Based on Stem Cells and NO
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report from the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar]
- Vergara, P. Age-dependent changes in nitric oxide synthase activity and protein expression in striata of mice transgenic for the Huntington’s disease mutation. Brain Res. 2002, 951, 36–42. [Google Scholar]
- Ignarro, L.J.; Byrns, R.E.; Buga, G.M.; Wood, K.S.; Chaudhuri, G. Pharmacological evidence that endothelium-derived relaxing factor is nitric oxide: Use of pyrogallol and superoxide dismutase to study endothelium-dependent and nitric oxide-elicited vascular smooth muscle relaxation. J. Pharmacol. Exp. Ther. 1988, 244, 181–189. [Google Scholar] [PubMed]
- Dillon, K.M.; Carrazzone, R.J.; Matson, J.B.; Kashfi, K. The evolving landscape for cellular nitric oxide and hydrogen sulfide delivery systems: A new era of customized medications. Biochem. Pharmacol. 2020, 176, 113931. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, P.M.; Shimokawa, H.; Feletou, M.; Tang, E.H. Endothelial dysfunction and vascular disease—a 30th anniversary update. Acta Physiol. 2017, 219, 22–96. [Google Scholar] [CrossRef]
- Chen, J.Y.; Ye, Z.X.; Wang, X.F.; Chang, J.; Yang, M.W.; Zhong, H.H.; Hong, F.F.; Yang, S.L. Nitric oxide bioavailability dysfunction involves in atherosclerosis. Biomed. Pharmacother. 2018, 97, 423–428. [Google Scholar] [CrossRef]
- Lee, J.; Bae, E.H.; Ma, S.K.; Kim, S.W. Altered Nitric Oxide System in Cardiovascular and Renal Diseases. Chonnam Med. J. 2016, 52, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Restini, C.B.A.; Gonçalves, L. Nitric Oxide and Related Aspects Underlying Angina. Open Cardiovasc. Med. J. 2017, 11, 33–46. [Google Scholar] [CrossRef] [Green Version]
- Takimoto, E.; Champion, H.C.; Li, M.; Ren, S.; Rodriguez, E.R.; Tavazzi, B.; Lazzarino, G.; Paolocci, N.; Gabrielson, K.L.; Wang, Y. Oxidant stress from nitric oxide synthase–3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J. Clin. Investig. 2005, 115, 1221–1231. [Google Scholar] [CrossRef] [Green Version]
- Nichols, S.P.; Storm, W.L.; Koh, A.; Schoenfisch, M.H. Local delivery of nitric oxide: Targeted delivery of therapeutics to bone and connective tissues. Adv. Drug Deliv. Rev. 2012, 64, 1177–1188. [Google Scholar] [CrossRef] [Green Version]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Shah, A.M. ROS signalling between endothelial cells and cardiac cells. Cardiovasc. Res. 2014, 102, 249–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landmesser, U.; Dikalov, S.; Price, S.R.; McCann, L.; Fukai, T.; Holland, S.M.; Mitch, W.E.; Harrison, D.G. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J. Clin. Investig. 2003, 111, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Gao, Z.; Liu, Y.; Zhao, J.; Ou, H.; Xu, F.; Ding, D. Polymeric Nitric Oxide Delivery Nanoplatforms for Treating Cancer, Cardiovascular Diseases, and Infection. Adv. Healthc. Mater. 2021, 10, e2001550. [Google Scholar] [CrossRef] [PubMed]
- Midgley, A.C.; Wei, Y.; Li, Z.; Kong, D.; Zhao, Q. Nitric-Oxide-Releasing Biomaterial Regulation of the Stem Cell Microenvironment in Regenerative Medicine. Adv. Mater. 2020, 32, e1805818. [Google Scholar] [CrossRef]
- Taylor, T.H.; Styles, M.; Lamming, A.J. Sodium Nitroprusside as a Hypotensive Agent in General Anaesthesia. Brit. J. Anaesth. 1970, 42, 859–864. [Google Scholar] [CrossRef] [Green Version]
- Hottinger, D.G.; Beebe, D.S.; Kozhimannil, T.; Prielipp, R.C.; Belani, K.G. Sodium nitroprusside in 2014: A clinical concepts review. J. Anaesthesiol. Clin. Pharmacol. 2014, 30, 462–471. [Google Scholar]
- O’Neill, S.K.; Dutta, S.; Triggle, C.R. Computerized data acquisition and analysis applied to chemiluminescence detection of nitric oxide in headspace gas. J. Pharmacol. Toxicol. Met. 1993, 29, 217–221. [Google Scholar] [CrossRef]
- Rao, D.N.; Elguindi, S.; O’Brien, P.J. Reductive metabolism of nitroprusside in rat hepatocytes and human erythrocytes. Arch. Biochem. Biophys. 1991, 286, 30–37. [Google Scholar] [CrossRef]
- Ma, T.; Zhang, Z.; Chen, Y.; Su, H.; Deng, X.; Liu, X.; Fan, Y. Delivery of Nitric Oxide in the Cardiovascular System: Implications for Clinical Diagnosis and Therapy. Int. J. Mol. Sci. 2021, 22, 12166. [Google Scholar] [CrossRef]
- Yu, H.; Cui, L.X.; Huang, N.; Yang, Z.L. Recent developments in nitric oxide-releasing biomaterials for biomedical applications. Med. Gas Res. 2019, 9, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, N.; de Mel, A.; Alavijeh, O.S.; Cousins, B.G.; Seifalian, A.M. Nitric oxide donors for cardiovascular implant applications. Small 2013, 9, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Riccio, D.A.; Schoenfisch, M.H. Nitric oxide release: Part I. Macromolecular scaffolds. Chem. Soc. Rev. 2012, 41, 3731–3741. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.G.; Xian, M.; Tang, X.; Wu, X.; Wen, Z.; Cai, T.; Janczuk, A.J. Nitric oxide donors: Chemical activities and biological applications. Chem. Rev. 2002, 102, 1091–1134. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Meyerhoff, M.E. Polyurethane with tethered copper(II)-cyclen complex: Preparation, characterization and catalytic generation of nitric oxide from S-nitrosothiols. Biomaterials 2008, 29, 2443–2452. [Google Scholar] [CrossRef] [Green Version]
- Ford, P.C. Photochemical delivery of nitric oxide. Nitric Oxide 2013, 34, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.; Nagae, O.; Kato, Y.; Nakagawa, H.; Fukuhara, K.; Miyata, N. Photoinduced nitric oxide release from nitrobenzene derivatives. J. Am. Chem. Soc. 2005, 127, 11720–11726. [Google Scholar] [CrossRef]
- Cebová, M.; Košútová, M.; Pecháňová, O. Cardiovascular effects of gasotransmitter donors. Physiol. Res. 2016, 65, S291–S307. [Google Scholar] [CrossRef]
- Ibarra-Lara, L.; Del Valle-Mondragón, L.; Soria-Castro, E.; Torres-Narváez, J.C.; Pérez-Severiano, F.; Sánchez-Aguilar, M.; Ramírez-Ortega, M.; Cervantes-Pérez, L.G.; Pastelín-Hernández, G.S.; Oidor-Chan, V.H.; et al. Peroxisome prolifera-tor-activated receptor-α stimulation by clofibrate favors an antioxidant and vasodilator environment in a stressed left ven-tricle. Pharmacol. Rep. 2016, 68, 692–702. [Google Scholar] [CrossRef]
- Burov, O.N.; Kletskii, M.E.; Kurbatov, S.V.; Lisovin, A.V.; Fedik, N.S. Mechanisms of nitric oxide generation in living systems. Nitric Oxide 2022, 118, 1–16. [Google Scholar] [CrossRef]
- Gatzke, N.; Hillmeister, P.; Dülsner, A.; Güc, N.; Dawid, R.; Smith, K.H.; Pagonas, N.; Bramlage, P.; Gorath, M.; Buschmann, I.R. Nitroglycerin application and coronary arteriogenesis. PLoS ONE 2018, 13, e0201597. [Google Scholar] [CrossRef] [PubMed]
- Münzel, T.; Daiber, A. Inorganic nitrite and nitrate in cardiovascular therapy: A better alternative to organic nitrates as nitric oxide donors? Vascul. Pharmacol. 2018, 102, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Noack, E.; Feelisch, M. Molecular mechanisms of nitrovasodilator bioactivation. Basic Res. Cardiol. 1991, 86 (Suppl. 2), 37–50. [Google Scholar]
- Roth, L.; Van der Donckt, C.; Emini Veseli, B.; Van Dam, D.; De Deyn, P.P.; Martinet, W.; Herman, A.G.; De Meyer, G.R.Y. Nitric oxide donor molsidomine favors features of atherosclerotic plaque stability and reduces myocardial infarction in mice. Vascul. Pharmacol. 2019, 118–119, 106561. [Google Scholar] [CrossRef]
- Reinero, M.; Beghetti, M.; Tozzi, P.; Segesser, L.K.V.; Samaja, M.; Milano, G. Nitric Oxide-cGMP Pathway Modulation in an Experimental Model of Hypoxic Pulmonary Hypertension. J. Cardiovasc. Pharmacol. Ther. 2021, 26, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Polvani, S.; Tarocchi, M.; Galli, A. PPARγ and Oxidative Stress: Con(β) Catenating NRF2 and FOXO. PPAR Res. 2012, 2012, 641087. [Google Scholar] [CrossRef] [Green Version]
- Vanhoutte, P.M.; Gao, Y. Beta blockers, nitric oxide, and cardiovascular disease. Curr. Opin. Pharmacol. 2013, 13, 265–273. [Google Scholar] [CrossRef]
- Sorrentino, S.A.; Doerries, C.; Manes, C.; Speer, T.; Dessy, C.; Lobysheva, I.; Mohmand, W.; Akbar, R.; Bahlmann, F.; Besler, C.; et al. Nebivolol exerts beneficial effects on endothelial function, early endothelial progenitor cells, myocardial neovas-cularization, and left ventricular dysfunction early after myocardial infarction beyond conventional β1-blockade. J. Am. Coll. Cardiol. 2011, 57, 601–611. [Google Scholar] [CrossRef] [Green Version]
- Dandona, P.; Ghanim, H.; Brooks, D.P. Antioxidant activity of carvedilol in cardiovascular disease. Am. J. Hypertens. 2007, 25, 731–741. [Google Scholar] [CrossRef]
- Arozal, W.; Watanabe, K.; Veeraveedu, P.T.; Ma, M.; Thandavarayan, R.A.; Sukumaran, V.; Suzuki, K.; Kodama, M.; Aizawa, Y. Protective effect of carvedilol on daunorubicin-induced cardiotoxicity and nephrotoxicity in rats. Toxicology 2010, 274, 18–26. [Google Scholar] [CrossRef]
- Ma, L.; Gul, R.; Habibi, J.; Yang, M.; Pulakat, L.; Whaley-Connell, A.; Ferrario, C.M.; Sowers, J.R. Nebivolol improves dias-tolic dysfunction and myocardial remodeling through reductions in oxidative stress in the transgenic (mRen2) rat. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H2341–H2351. [Google Scholar] [CrossRef] [PubMed]
- Shantsila, E.; Wrigley, B.J.; Blann, A.D.; Gill, P.S.; Lip, G.Y. A contemporary view on endothelial function in heart failure. Eur. J. Heart Fail. 2012, 14, 873–881. [Google Scholar] [CrossRef]
- Maccallini, C.; Mollica, A.; Amoroso, R. The Positive Regulation of eNOS Signaling by PPAR Agonists in Cardiovascular Diseases. Am. J. Cardiovasc. Drugs 2017, 17, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Goya, K.; Sumitani, S.; Xu, X.; Kitamura, T.; Yamamoto, H.; Kurebayashi, S.; Saito, H.; Kouhara, H.; Kasayama, S.; Kawase, I. Peroxisome proliferator-activated receptor alpha agonists increase nitric oxide synthase expression in vascular endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 658–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Lu, C.; Li, F.; Wang, H.; He, L.; Hao, Y.; Chen, A.F.; An, H.; Wang, X.; Hong, T.; et al. PPAR-α Agonist Fenofibrate Upregulates Tetrahydrobiopterin Level through Increasing the Expression of Guanosine 5′-Triphosphate Cyclohydrolase-I in Human Umbilical Vein Endothelial Cells. PPAR Res. 2011, 2011, 523520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilahur, G.; Segalés, E.; Casaní, L.; Badimon, L. A novel anti-ischemic nitric oxide donor inhibits thrombosis without mod-ifying haemodynamic parameters. Thromb. Haemost. 2004, 91, 1035–1043. [Google Scholar] [CrossRef]
- Paulis, L.; Matuskova, J.; Adamcova, M.; Pelouch, V.; Simko, J.; Krajcirovicova, K.; Potacova, A.; Hulin, I.; Janega, P.; Pechanova, O.; et al. Regression of left ventricular hypertrophy and aortic remodelling in NO-deficient hypertensive rats: Effect of L-arginine and spironolactone. Acta Physiol. 2008, 194, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.; Benincasa, G.; Lucchese, R.; Infante, T.; Nicoletti, G.F.; Napoli, C. Effect of nitric oxide reduction on arterial thrombosis. Scand. Cardiovasc. J. 2019, 53, 1–8. [Google Scholar] [CrossRef]
- Matsuoka, H.; Nakata, M.; Kohno, K.; Koga, Y.; Nomura, G.; Toshima, H.; Imaizumi, T. Chronic L-arginine administration attenuates cardiac hypertrophy in spontaneously hypertensive rats. Hypertension 1996, 27, 14–18. [Google Scholar] [CrossRef]
- Kalla, M.; Chotalia, M.; Coughlan, C.; Hao, G.; Crabtree, M.J.; Tomek, J.; Bub, G.; Paterson, D.J.; Herring, N. Protection against ventricular fibrillation via cholinergic receptor stimulation and the generation of nitric oxide. J. Physiol. 2016, 594, 3981–3992. [Google Scholar] [CrossRef] [Green Version]
- Touyz, R.M. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: What is the clinical significance? Hypertension 2004, 44, 248–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iadecola, C.; Davisson, R.L. Hypertension and Cerebrovascular Dysfunction. Cell Metab. 2008, 7, 476–484. [Google Scholar] [CrossRef] [Green Version]
- Dinh, Q.N.; Drummond, G.R.; Sobey, C.G.; Chrissobolis, S. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. BioMed Res. Int. 2014, 2014, 406960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, S.; Kevil, C.G. Nitric Oxide and Hydrogen Sulfide Regulation of Ischemic Vascular Remodeling. Microcirculation 2016, 23, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, R.H.; Drummond, G.R.; Sobey, C.G.; De Silva, T.M.; Kemp-Harper, B.K. The opposing roles of NO and oxidative stress in cardiovascular disease. Pharmacol. Res. 2017, 116, 57–69. [Google Scholar] [CrossRef]
- Chen, R.; Liang, F.; Moriya, J.; Yamakawa, J.; Takahashi, T.; Shen, L.; Kanda, T. Peroxisome proliferator-activated receptors (PPARs) and their agonists for hypertension and heart failure: Are the reagents beneficial or harmful? Int. J. Cardiol. 2008, 130, 131–139. [Google Scholar] [CrossRef]
- Van Hove, C.; Carreer-Bruhwyler, F.; Géczy, J.; Herman, A.G. Long-term treatment with the NO-donor molsidomine re-duces circulating ICAM-1 levels in patients with stable angina. Arteriosclerosis 2005, 180, 399–405. [Google Scholar] [CrossRef]
- Guimarães, S.; Moura, D. Vascular adrenoceptors: An update. Pharmacol. Rev. 2001, 53, 319–356. [Google Scholar]
- Figueroa, X.F.; Poblete, I.; Fernández, R.; Pedemonte, C.; Cortés, V.; Huidobro-Toro, J.P. NO production and eNOS phos-phorylation induced by epinephrine through the activation of beta-adrenoceptors. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H134–H143. [Google Scholar] [CrossRef]
- Manoury, B.; Montiel, V.; Balligand, J.L. Nitric oxide synthase in post-ischaemic remodelling: New pathways and mecha-nisms. Cardiovasc. Res. 2012, 94, 304–315. [Google Scholar] [CrossRef] [Green Version]
- Bernátová, I.; Pechánová, O.; Simko, F. Captopril prevents NO-deficient hypertension and left ventricular hypertrophy without affecting nitric oxide synthase activity in rats. Physiol. Res. 1996, 45, 311–316. [Google Scholar]
- Rizzoni, D.; Muiesan, M.L.; Porteri, E.; Castellano, M.; Zulli, R.; Bettoni, G.; Salvetti, M.; Monteduro, C.; Agabiti-Rosei, E. Effects of long-term antihypertensive treatment with lisinopril on resistance arteries in hypertensive patients with left ven-tricular hypertrophy. Am. J. Hypertens. 1997, 15, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, H.; Raij, L. The link among nitric oxide synthase activity, endothelial function, and aortic and ventricular hy-pertrophy in hypertension. Hypertension 1997, 29, 235–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frohlich, E.D. Hemodynamics and other determinants in development of left ventricular hypertrophy. Fed. Proc. 1983, 42, 2709–2715. [Google Scholar]
- Palazzuoli, A.; Giannotti, G.; Capobianco, S.; Nuti, R. Left Ventricular Hypertrophy Beyond Hemodynamics: Genetic, Metabolic and Hormonal Factors. Curr. Hypertens. Rev. 2005, 1, 217–221. [Google Scholar] [CrossRef]
- Simko, F.; Simko, J. The potential role of nitric oxide in the hypertrophic growth of the left ventricle. Physiol. Res. 2000, 49, 37–46. [Google Scholar] [PubMed]
- Ahmad, A.; Sattar, M.A.; Rathore, H.A.; Abdulla, M.H.; Khan, S.A.; Abdullah, N.A.; Johns, E.J. Enhanced expression of endothelial nitric oxide synthase in the myocardium ameliorates the progression of left ventricular hypertrophy in L-arginine treated Wistar-Kyoto rats. J. Physiol. Pharmacol. 2016, 67, 31–44. [Google Scholar] [PubMed]
- Ahmad, A.; Dempsey, S.K.; Daneva, Z.; Azam, M.; Li, N.; Li, P.L.; Ritter, J.K. Role of Nitric Oxide in the Cardiovascular and Renal Systems. Systems. Int. J. Mol. Sci. 2018, 19, 2605. [Google Scholar] [CrossRef] [Green Version]
- Couto, G.K.; Britto, L.R.; Mill, J.G.; Rossoni, L.V. Enhanced nitric oxide bioavailability in coronary arteries prevents the onset of heart failure in rats with myocardial infarction. J. Mol. Cell. Cardiol. 2015, 86, 110–120. [Google Scholar] [CrossRef]
- Drexler, H.; Kästner, S.; Strobel, A.; Studer, R.; Brodde, O.E.; Hasenfuss, G. Expression, activity and functional significance of inducible nitric oxide synthase in the failing human heart. J. Am. Coll. Cardiol. 1998, 32, 955–963. [Google Scholar] [CrossRef]
- Poulos, T.L. Soluble guanylate cyclase. Curr. Opin. Struct. Biol. 2006, 16, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, Y.; Arakawa, T.; Nawata, Y.; Shimada, S.; Oishi, S.; Fujii, N.; Nishimura, S.; Hattori, A.; Kakeya, H. Design, synthesis, and structure-activity relationships of 1-ethylpyrazole-3-carboxamide compounds as novel hypoxia-inducible factor (HIF)-1 inhibitors. Bioorg. Med. Chem. 2015, 23, 1776–1787. [Google Scholar] [CrossRef] [PubMed]
- Ghofrani, H.A.; D’Armini, A.M.; Grimminger, F.; Hoeper, M.M.; Jansa, P.; Kim, N.H.; Mayer, E.; Simonneau, G.; Wilkins, M.R.; Fritsch, A.; et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N. Engl. J. Med. 2013, 369, 319–329. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, P.W.; Roessig, L.; Patel, M.J.; Anstrom, K.J.; Butler, J.; Voors, A.A.; Lam, C.S.P.; Ponikowski, P.; Temple, T.; Pieske, B.; et al. A Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial of the Efficacy and Safety of the Oral Soluble Guanylate Cyclase Stimulator: The VICTORIA Trial. JACC Heart Fail. 2018, 6, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, P.W.; Pieske, B.; Anstrom, K.J.; Ezekowitz, J.; Hernandez, A.F.; Butler, J.; Lam, C.S.P.; Ponikowski, P.; Voors, A.A.; Jia, G.; et al. Vericiguat in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2020, 382, 1883–1893. [Google Scholar] [CrossRef]
- Lombardi, C.M.; Cimino, G.; Pagnesi, M.; Dell’Aquila, A.; Tomasoni, D.; Ravera, A.; Inciardi, R.; Carubelli, V.; Vizzardi, E.; Nodari, S.; et al. Vericiguat for Heart Failure with Reduced Ejection Fraction. Curr. Cardiol. Rep. 2021, 23, 144. [Google Scholar] [CrossRef]
- Veres, G.; Bai, Y.; Stark, K.A.; Schmidt, H.; Radovits, T.; Loganathan, S.; Korkmaz-Icöz, S.; Szabó, G. Pharmacological acti-vation of soluble guanylate cyclase improves vascular graft function. Interac. Cardiovasc. Thorac. Surg. 2021, 32, 803–811. [Google Scholar] [CrossRef]
- Doshi, R.; Shah, J.; Jauhar, V.; Decter, D.; Jauhar, R.; Meraj, P. Comparison of drug eluting stents (DESs) and bare metal stents (BMSs) with STEMI: Who received BMS in the era of 2nd generation DES? Heart Lung 2018, 47, 231–236. [Google Scholar] [CrossRef]
- Hertault, A.; Chai, F.; Maton, M.; Sobocinski, J.; Woisel, P.; Maurel, B.; Lyskawa, J.; Blanchemain, N. In vivo evaluation of a pro-healing polydopamine coated stent through an in-stent restenosis rat model. Biomater. Sci. 2021, 9, 212–220. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, Y.; Zhao, Q.; Xie, Y.; Luo, R.; Yang, P.; Weng, Y. Immobilization of nano Cu-MOFs with polydopamine coating for adaptable gasotransmitter generation and copper ion delivery on cardiovascular stents. Biomaterials 2019, 204, 36–45. [Google Scholar] [CrossRef]
- Pinto, R.V.; Wang, S.; Tavares, S.R.; Pires, J.; Antunes, F.; Vimont, A.; Clet, G.; Daturi, M.; Maurin, G.; Serre, C.; et al. Tuning Cellular Biological Functions Through the Controlled Release of NO from a Porous Ti-MOF. Angew. Chem. Int. Ed. 2020, 59, 5135–5143. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, N.; Salimi, A.; Sham, T.-K. CuO/Cu-MOF nanocomposite for highly sensitive detection of nitric oxide released from living cells using an electrochemical microfluidic device. Mikrochim. Acta 2021, 188, 240. [Google Scholar] [CrossRef] [PubMed]
- Garren, M.; Maffe, P.; Melvin, A.; Griffin, L.; Wilson, S.; Douglass, M.; Reynolds, M.; Handa, H. Surface-Catalyzed Nitric Oxide Release via a Metal Organic Framework Enhances Antibacterial Surface Effects. ACS Appl. Mater. Interfaces 2021, 13, 56931–56943. [Google Scholar] [CrossRef] [PubMed]
- Lyu, N.; Du, Z.; Qiu, H.; Gao, P.; Yao, Q.; Xiong, K.; Tu, Q.; Li, X.; Chen, B.; Wang, M.; et al. Mimicking the Nitric Oxide-Releasing and Glycocalyx Functions of Endothelium on Vascular Stent Surfaces. Adv. Sci. 2020, 7, 2002330. [Google Scholar] [CrossRef]
- Li, X.; Qiu, H.; Gao, P.; Yang, Y.; Yang, Z.; Huang, N. Synergetic coordination and catecholamine chemistry for catalytic generation of nitric oxide on vascular stents. NPG Asia Mater. 2018, 10, 482–496. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Q.; Li, X.; Huang, N.; Zhao, X.; Yang, Z. Mussel-inspired dopamine-Cu(II) coatings for sustained in situ generation of nitric oxide for prevention of stent thrombosis and restenosis. Biomaterials 2019, 194, 117–129. [Google Scholar] [CrossRef]
- Ma, Q.; Shi, X.; Tan, X.; Wang, R.; Xiong, K.; Maitz, M.F.; Cui, Y.; Hu, Z.; Tu, Q.; Huang, N.; et al. Durable endothelium-mimicking coating for surface bioengineering cardiovascular stents. Bioact. Mater. 2021, 6, 4786–4800. [Google Scholar] [CrossRef]
- Cheng, Q.; Shafiq, M.; Rafique, M.; Shen, L.; Mo, X.; Wang, K. Extracellular matrix and nitric oxide based functional coatings for vascular stents. Eng. Regen. 2022, 3, 149–153. [Google Scholar] [CrossRef]
- Zhu, T.; Gao, W.; Fang, D.; Liu, Z.; Wu, G.; Zhou, M.; Wan, M.; Mao, C. Bifunctional polymer brush-grafted coronary stent for anticoagulation and endothelialization. Mater. Sci. Eng. C 2020, 120, 111725. [Google Scholar] [CrossRef]
- Brisbois, E.J.; Davis, R.P.; Jones, A.M.; Major, T.C.; Bartlett, R.H.; Meyerhoff, M.E.; Handa, H. Reduction in Thrombosis and Bacterial Adhesion with 7 Day Implantation of S-Nitroso-N-acetylpenicillamine (SNAP)-Doped Elast-eon E2As Catheters in Sheep. J. Mater. Chem. B 2015, 3, 1639–1645. [Google Scholar] [CrossRef] [Green Version]
- Goudie, M.J.; Brisbois, E.J.; Pant, J.; Thompson, A.; Potkay, J.A.; Handa, H. Characterization of an S-nitroso-N-acetylpenicillamine-based nitric oxide releasing polymer from a translational perspective. Int. J. Polym. Mater. 2016, 65, 769–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brisbois, E.J.; Major, T.C.; Goudie, M.J.; Meyerhoff, M.E.; Bartlett, R.H.; Handa, H. Attenuation of thrombosis and bacterial infection using dual function nitric oxide releasing central venous catheters in a 9day rabbit model. Acta Biomater. 2016, 44, 304–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, T.; Zelikin, A.N.; Chandrawati, R. Progress and Promise of Nitric Oxide-Releasing Platforms. Adv. Sci. 2018, 5, 1701043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCabe, M.M.; Hala, P.; Rojas-Pena, A.; Lautner-Csorba, O.; Major, T.C.; Ren, H.; Bartlett, R.H.; Brisbois, E.J.; Meyerhoff, M.E. Enhancing analytical accuracy of intravascular electrochemical oxygen sensors via nitric oxide release using S-nitroso-N-acetyl-penicillamine (SNAP) impregnated catheter tubing. Talanta 2019, 205, 120077. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, S.P.; Pant, J.; Goudie, M.J.; Schmiedt, C.; Handa, H. Achieving Long-Term Biocompatible Silicone via Covalently Immobilized S-Nitroso- N-acetylpenicillamine (SNAP) That Exhibits 4 Months of Sustained Nitric Oxide Release. ACS Appl. Mater. Interfaces 2018, 10, 27316–27325. [Google Scholar] [CrossRef]
- Chung, K.K.; Schumacher, J.F.; Sampson, E.M.; Burne, R.A.; Antonelli, P.J.; Brennan, A.B. Impact of engineered surface microtopography on biofilm formation of Staphylococcus aureus. Biointerphases 2007, 2, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Pant, J.; Goudie, M.J.; Chaji, S.M.; Johnson, B.W.; Handa, H. Nitric oxide releasing vascular catheters for eradicating bacterial infection. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2849–2857. [Google Scholar] [CrossRef]
- Wo, Y.; Brisbois, E.J.; Wu, J.; Li, Z.; Major, T.C.; Mohammed, A.; Wang, X.; Colletta, A.; Bull, J.L.; Matzger, A.J.; et al. Reduction of Thrombosis and Bacterial Infection via Controlled Nitric Oxide (NO) Release from S-Nitroso-N-acetylpenicillamine (SNAP) Impregnated CarboSil Intravascular Catheters. ACS Biomater. Sci. Eng. 2017, 3, 349–359. [Google Scholar] [CrossRef]
- Wo, Y.; Li, Z.; Colletta, A.; Wu, J.; Xi, C.; Matzger, A.J.; Brisbois, E.J.; Bartlett, R.H.; Meyerhoff, M.E. Study of crystal formation and nitric oxide (NO) release mechanism from S-nitroso-N-acetylpenicillamine (SNAP)-doped CarboSil polymer composites for potential antimicrobial applications. Compos. B. Eng. 2017, 121, 23–33. [Google Scholar] [CrossRef]
- Xu, L.C.; Meyerhoff, M.E.; Siedlecki, C.A. Blood coagulation response and bacterial adhesion to biomimetic polyurethane biomaterials prepared with surface texturing and nitric oxide release. Acta Biomater. 2019, 84, 77–87. [Google Scholar] [CrossRef]
- Li, P.; Jin, D.; Dou, J.; Wang, L.; Wang, Y.; Jin, X.; Han, X.; Kang, I.K.; Yuan, J.; Shen, J.; et al. Nitric oxide-releasing poly(ε-caprolactone)/S-nitrosylated keratin biocomposite scaffolds for potential small-diameter vascular grafts. Int. J. Biol. Macromol. 2021, 189, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, J.; Feng, G.; Shen, J.; Kong, D.; Zhao, Q. Nitric Oxide-Releasing Biomaterials for Biomedical Applications. Curr. Med. Chem. 2016, 23, 2579–2601. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Chen, S.; Hou, D.; Gao, J.; Jiang, L.; Shi, J.; Liang, Q.; Kong, D.; Wang, S. Regulation of macrophage polarization and promotion of endothelialization by NO generating and PEG-YIGSR modified vascular graft. Mater. Sci. Eng. C 2018, 84, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lu, Y.; Qin, K.; Wu, Y.; Tian, Y.; Wang, J.; Zhang, J.; Hou, J.; Cui, Y.; Wang, K.; et al. Enzyme-functionalized vascular grafts catalyze in-situ release of nitric oxide from exogenous NO prodrug. J. Control. Release 2015, 210, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Pan, Y.; Zhu, D.; Fan, Y.; Feng, G.; Wei, Y.; Wang, H.; Qin, K.; Zhao, T.; Yang, Q.; et al. Targeted delivery of nitric oxide via a ‘bump-and-hole’-based enzyme–prodrug pair. Nat. Chem. Biol. 2019, 15, 151–160. [Google Scholar] [CrossRef]
- Saraiva, J.; Marotta-Oliveira, S.S.; Cicillini, S.A.; Eloy, J.D.O.; Marchetti, J.M. Nanocarriers for Nitric Oxide Delivery. J. Drug Deliv. 2011, 2011, 936438. [Google Scholar] [CrossRef] [Green Version]
- Seabra, A.; Lima, R.; Calderón, M. Nitric Oxide Releasing Nanomaterials for Cancer Treatment: Current Status and Perspectives. Curr. Top. Med. Chem. 2015, 15, 298–308. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Z.; Wen, F.; Ren, L.; Li, J.; Teoh, S.H.; Thian, E.S. Gelatin–siloxane nanoparticles to deliver nitric oxide for vascular cell regulation: Synthesis, cytocompatibility, and cellular responses. J. Biomed. Mater. Res. A 2015, 103, 929–938. [Google Scholar] [CrossRef]
- Rink, J.S.; Sun, W.; Misener, S.; Wang, J.J.; Zhang, Z.J.; Kibbe, M.R.; Dravid, V.P.; Venkatraman, S.S.; Thaxton, C.S. Nitric Oxide-Delivering High-Density Lipoprotein-like Nanoparticles as a Biomimetic Nanotherapy for Vascular Diseases. ACS Appl. Mater. 2018, 10, 6904–6916. [Google Scholar] [CrossRef]
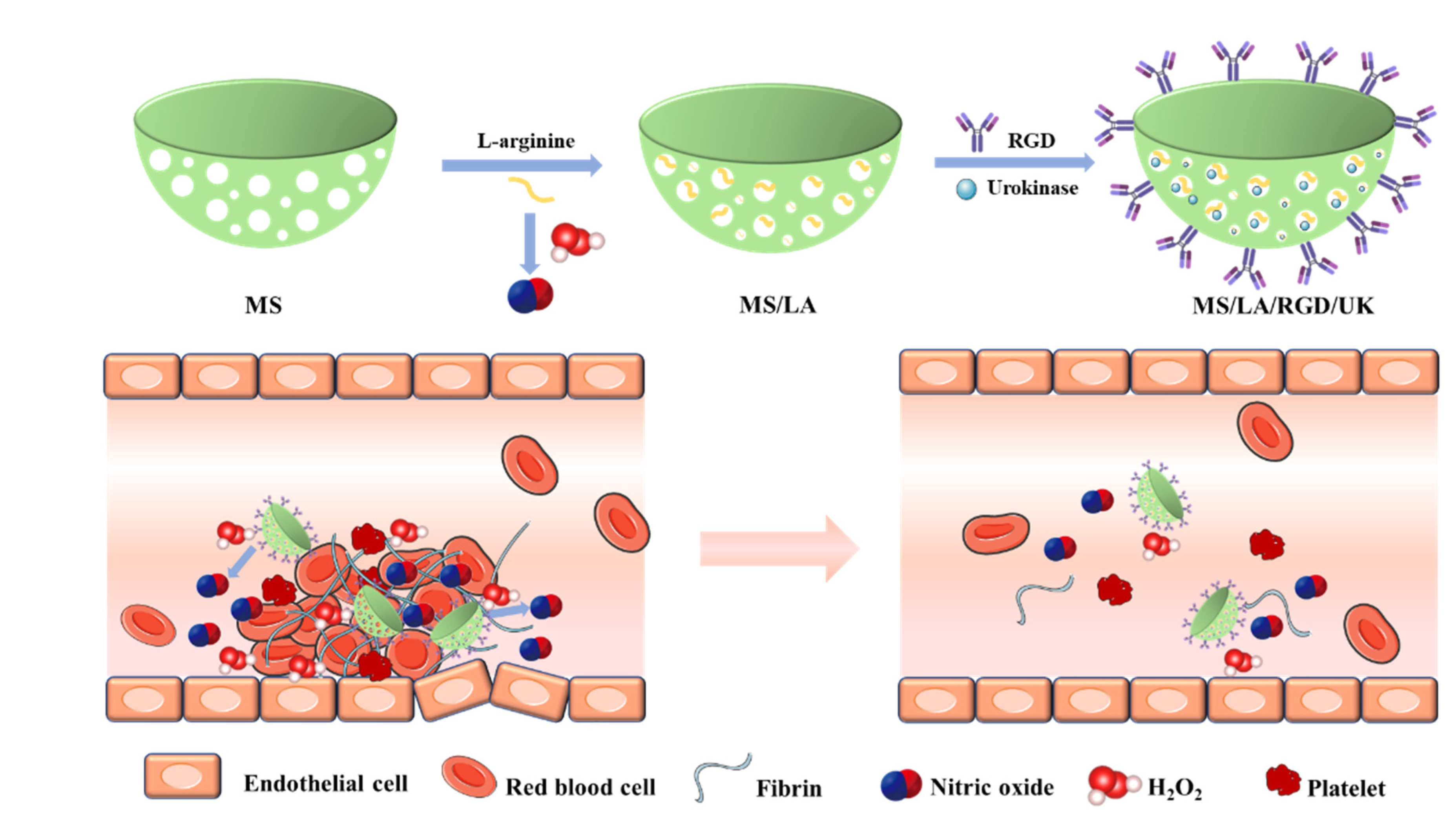
- Tao, Y.; Li, X.; Wu, Z.; Chen, C.; Tan, K.; Wan, M.; Zhou, M.; Mao, C. Nitric oxide-driven nanomotors with bowl-shaped mesoporous silica for targeted thrombolysis. J. Colloid Interface Sci. 2022, 611, 61–70. [Google Scholar] [CrossRef]
- Vong, L.B.; Bui, T.Q.; Tomita, T.; Sakamoto, H.; Hiramatsu, Y.; Nagasaki, Y. Novel angiogenesis therapeutics by redox injectable hydrogel—Regulation of local nitric oxide generation for effective cardiovascular therapy. Biomaterials 2018, 167, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gao, P.; Huang, L.; Tan, X.; Zhou, N.; Yang, T.; Qiu, H.; Dai, X.; Michael, S.; Tu, Q.; et al. A tough nitric oxide-eluting hydrogel coating suppresses neointimal hyperplasia on vascular stent. Nat. Commun. 2021, 12, 7079. [Google Scholar] [CrossRef]
- Wang, R. Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol. Rev. 2012, 92, 791–896. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Aponte, A.M.; Menazza, S.; Gucek, M.; Steenbergen, C.; Murphy, E. Additive cardioprotection by pharmacological postconditioning with hydrogen sulfide and nitric oxide donors in mouse heart: S-sulfhydration vs. S-nitrosylation. Cardiovasc. Res. 2016, 110, 96–106. [Google Scholar] [CrossRef] [Green Version]
- Yong, Q.C.; Lee, S.W.; Foo, C.S.; Neo, K.L.; Chen, X.; Bian, J.S. Endogenous hydrogen sulphide mediates the cardioprotection induced by ischemic postconditioning. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H1330–H1340. [Google Scholar] [CrossRef] [Green Version]
- Ansari, S.B.; Kurian, G.A. Hydrogen sulfide modulates sub-cellular susceptibility to oxidative stress induced by myocardial ischemic reperfusion injury. Chem. Biol. Interact. 2016, 252, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.C.; Sohn, E.H.; Lee, S.R. Hydrogen Sulfide as a Potential Alternative for the Treatment of Myocardial Fibrosis. Oxid. Med. Cell. Longev. 2020, 2020, 4105382. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Wu, L.; Liang, W.; Wang, R. Direct stimulation of K(ATP) channels by exogenous and endogenous hydrogen sulfide in vascular smooth muscle cells. Mol. Pharmacol. 2005, 68, 1757–1764. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Wang, Y.; Li, Y.; Li, L.; Xu, S.; Feng, X.; Liu, S. Hydrogen Sulfide (H2S)-Releasing Compounds: Therapeutic Potential in Cardiovascular Diseases. Front. Pharmacol. 2018, 9, 1066. [Google Scholar] [CrossRef] [Green Version]
- Predmore, B.L.; Julian, D.; Cardounel, A.J. Hydrogen sulfide increases nitric oxide production from endothelial cells by an akt-dependent mechanism. Front. Physiol. 2011, 2, 104. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Zhang, J.; Lu, Y.; Wang, R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 2001, 20, 6008–6016. [Google Scholar] [CrossRef] [Green Version]
- Monti, M.; Hyseni, I.; Pacini, A.; Monzani, E.; Casella, L.; Morbidelli, L. Cross-talk between endogenous H2S and NO accounts for vascular protective activity of the metal-nonoate Zn(PipNONO)Cl. Biochem. Pharmacol. 2018, 152, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Ke, X.; Zhang, T.; Chen, Y.; Huang, Q.; Deng, B.; Xie, S.; Wang, J.; Nie, R. Hydrogen sulfide improves vascular repair by promoting endothelial nitric oxide synthase-dependent mobilization of endothelial progenitor cells. Am. J. Hypertens. 2019, 37, 972–984. [Google Scholar] [CrossRef]
- Szabo, C. Hydrogen sulfide, an enhancer of vascular nitric oxide signaling: Mechanisms and implications. Am. J. Physiol. Cell. Physiol. 2017, 312, C3–C15. [Google Scholar] [CrossRef]
- Kodela, R.; Chattopadhyay, M.; Kashfi, K. NOSH-Aspirin: A Novel Nitric Oxide–Hydrogen Sulfide-Releasing Hybrid: A New Class of Anti-inflammatory Pharmaceuticals. ACS Med. Chem. Lett. 2012, 3, 257–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kodela, R.; Chattopadhyay, M.; Kashfi, K. Synthesis and biological activity of NOSH-naproxen (AVT-219) and NOSH-sulindac (AVT-18A) as potent anti-inflammatory agents with chemotherapeutic potential. MedChemComm 2013, 4, 1472–1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kodela, R.; Chattopadhyay, M.; Velázquez-Martínez, C.A.; Kashfi, K. NOSH-aspirin (NBS-1120), a novel nitric oxide- and hydrogen sulfide-releasing hybrid has enhanced chemo-preventive properties compared to aspirin, is gastrointestinal safe with all the classic therapeutic indications. Biochem. Pharmacol. 2015, 98, 564–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Q.; Wu, D.; Ma, F.; Yang, S.; Tan, B.; Xin, H.; Gu, X.; Chen, X.; Chen, S.; Mao, Y.; et al. Novel Angiogenic Activity and Molecular Mechanisms of ZYZ-803, a Slow-Releasing Hydrogen Sulfide-Nitric Oxide Hybrid Molecule. Antioxid. Redox Signal. 2016, 25, 498–514. [Google Scholar] [CrossRef]
- Wu, D.; Hu, Q.; Ma, F.; Zhu, Y.Z. Vasorelaxant Effect of a New Hydrogen Sulfide-Nitric Oxide Conjugated Donor in Isolated Rat Aortic Rings through cGMP Pathway. Oxidative Med. Cell. Longev. 2016, 2016, 7075682. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Hu, Q.; Xiong, Y.; Zhu, D.; Mao, Y.; Zhu, Y.Z. Novel H2S-NO hybrid molecule (ZYZ-803) promoted synergistic effects against heart failure. Redox Biol. 2018, 15, 243–252. [Google Scholar] [CrossRef]
- Xiong, Y.; Chang, L.L.; Tran, B.; Dai, T.; Zhong, R.; Mao, Y.C.; Zhu, Y.Z. ZYZ-803, a novel hydrogen sulfide-nitric oxide conjugated donor, promotes angiogenesis via cross-talk between STAT3 and CaMKII. Acta Pharmacol. Sin. 2020, 41, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Guo, X.; Chen, S.Y. Function and Therapeutic Potential of Mesenchymal Stem Cells in Atherosclerosis. Front. Cardiovasc. Med. 2017, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Salvolini, E.; Orciani, M.; Vignini, A.; Mattioli-Belmonte, M.; Mazzanti, L.; Di Primio, R. Skin-derived mesenchymal stem cells (S-MSCs) induce endothelial cell activation by paracrine mechanisms. Exp. Dermatol. 2010, 19, 848–850. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Kawi, S.H.; Hashem, K.S. Possible Therapeutic Effect of Stem Cell in Atherosclerosis in Albino Rats. A Histological and Immunohistochemical Study. Int. J. Stem Cells 2015, 8, 200–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.; Chen, L.; Zhu, X.; Li, Q.; Hu, L.; Li, H. Mesenchymal stem cell-derived exosomal miR-21a-5p promotes M2 macrophage polarization and reduces macrophage infiltration to attenuate atherosclerosis. Acta Biochim. Biophys. Sin. 2021, 53, 1227–1236. [Google Scholar] [CrossRef]
- Ruggiero, A.D.; Key, C.-C.C.; Kavanagh, K. Adipose Tissue Macrophage Polarization in Healthy and Unhealthy Obesity. Front. Nutr. 2021, 8, 625331. [Google Scholar] [CrossRef]
- Yang, W.; Yin, R.; Zhu, X.; Yang, S.; Wang, J.; Zhou, Z.; Pan, X.; Ma, A. Mesenchymal stem-cell-derived exosomal miR-145 inhibits atherosclerosis by targeting JAM-A. Mol. Ther. Nucl. Acids 2021, 23, 119–131. [Google Scholar] [CrossRef]
- Moghaddam, A.S.; Afshari, J.T.; Esmaeili, S.-A.; Saburi, E.; Joneidi, Z.; Momtazi-Borojeni, A.A. Cardioprotective microRNAs: Lessons from stem cell-derived exosomal microRNAs to treat cardiovascular disease. Atherosclerosis 2019, 285, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Bonafe, F.; Guarnieri, C.; Muscari, C. Nitric oxide regulates multiple functions and fate of adult progenitor and stem cells. J. Physiol. Biochem. 2015, 71, 141–153. [Google Scholar] [CrossRef]
- Du, W.; Zhang, K.; Zhang, S.; Wang, R.; Nie, Y.; Tao, H.; Han, Z.; Liang, L.; Wang, D.; Liu, J.; et al. Enhanced proangiogenic potential of mesenchymal stem cell-derived exosomes stimulated by a nitric oxide releasing polymer. Biomaterials 2017, 133, 70–81. [Google Scholar] [CrossRef]
- Shi, C.Z.; Zhang, X.P.; Lv, Z.W.; Zhang, H.L.; Xu, J.Z.; Yin, Z.F.; Yan, Y.Q.; Wang, C.Q. Adipose tissue-derived stem cells embedded with eNOS restore cardiac function in acute myocardial infarction model. Int. J. Cardiol. 2012, 154, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Janeczek, A.; Zimna, A.; Rozwadowska, N.; Fraczek, M.; Kucharzewska, P.; Rucinski, M.; Mietkiewski, T.; Kolanowski, T.; Malcher, A.; Kurpisz, M. Genetically modified human myoblasts with eNOS may improve regenerative ability of myogenic stem cells to infarcted heart. Kardiol. Pol. 2013, 71, 1048–1058. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Varga, M.; Wang, X.; Haddad, D.J.; An, S.; Medzikovic, L.; Derakhshandeh, R.; Kostyushev, D.S.; Zhang, Y.; Clifford, B.T.; et al. Overexpression of Nitric Oxide Synthase Restores Circulating Angiogenic Cell Function in Patients with Coronary Artery Disease: Implications for Autologous Cell Therapy for Myocardial Infarction. J. Am. Heart Assoc. 2016, 5, e002257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Zhang, Y.; Tao, L.; Yang, Z.; Wang, L. Mesenchymal Stem Cells with eNOS Over-Expression Enhance Cardiac Repair in Rats with Myocardial Infarction. Cardiovasc. Drugs Ther. 2017, 31, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.K.; Wulur, I.; Alfonso, Z.; Hedrick, M.H. Fat tissue: An underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006, 24, 150–154. [Google Scholar] [CrossRef]
- Ikegame, Y.; Yamashita, K.; Hayashi, S.; Mizuno, H.; Tawada, M.; You, F.; Yamada, K.; Tanaka, Y.; Egashira, Y.; Nakashima, S. Comparison of mesenchymal stem cells from adipose tissue and bone marrow for ischemic stroke therapy. Cytotherapy 2011, 13, 675–685. [Google Scholar] [CrossRef]
- Uysal, C.A.; Tobita, M.; Hyakusoku, H.; Mizuno, H. The Effect of Bone-Marrow-Derived Stem Cells and Adipose-Derived Stem Cells on Wound Contraction and Epithelization. Adv. Wound Care 2014, 3, 405–413. [Google Scholar] [CrossRef]
- Ma, T.; Sun, J.; Zhao, Z.; Lei, W.; Chen, Y.; Wang, X.; Yang, J.; Shen, Z. A brief review: Adipose-derived stem cells and their therapeutic potential in cardiovascular diseases. Stem Cell Res. Ther. 2017, 8, 124. [Google Scholar] [CrossRef] [Green Version]
- Burdick, J.A.; Mauck, R.L.; Gerecht, S. To Serve and Protect: Hydrogels to Improve Stem Cell-Based Therapies. Cell Stem Cell 2016, 18, 13–15. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Liu, Y.; Gao, J.; Yang, L.; Mao, D.; Stefanitsch, C.; Li, Y.; Zhang, J.; Ou, L.; Kong, D.; et al. Nitric oxide releasing hydrogel enhances the therapeutic efficacy of mesenchymal stem cells for myocardial infarction. Biomaterials 2015, 60, 130–140. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, J.; Song, L.; Ji, Q.; Yao, Y.; Cui, Y.; Shen, J.; Wang, P.G.; Kong, D. Polysaccharide-based biomaterials with on-demand nitric oxide releasing property regulated by enzyme catalysis. Biomaterials 2013, 34, 8450–8458. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Zhang, K.; Zhang, S.; Wang, D.; Han, Z.; Che, Y.; Kong, D.; Zhao, Q.; Han, Z.; He, Z.X.; et al. Nitric oxide releasing hydrogel promotes endothelial differentiation of mouse embryonic stem cells. Acta Biomater. 2017, 63, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Tan, B.; Chen, J.; Bao, R.; Zhang, X.; Liang, S.; Shang, Y.; Liang, W.; Cui, Y.; Fan, G.; et al. An injectable conductive hydrogel encapsulating plasmid DNA-eNOs and ADSCs for treating myocardial infarction. Biomaterials 2018, 160, 69–81. [Google Scholar] [CrossRef] [PubMed]









| Name | Essence | Feature | Application | Refs. |
|---|---|---|---|---|
| Sodium nitroprusside | NO donor | A direct NO donor independent of the endothelium. | Rapid blood pressure reduction | [28] |
| Clofibrate | PPARα agonist (indirectly increases NO prodution) | Increases eNOS protein expression and enzyme activity in the left ventricle. | Treatment of hypertension | [29] |
| Nitroglycerin | NO donor | Suppresses ST segment elevation and reduce infarct size by promoting the development of collateral coronary circulation | Controls angina pectoris and treats myocardial infarction | [30,31] |
| Amyl nitrite | NO donor | Release NO after the formation of S-nitrosothiol intermediate by interaction with sulfhydryl groups | Lowers blood pressure and treats angina pectoris | [32,33] |
| Molsidomine | NO donor | Regulate NO-cGMP signaling via multiple pathways in vivo | Maintains atherosclerotic plaque stability and prevents myocardial infarction and congestive heart failure | [34,35] |
| Rosiglitazone | PPARγ agonist (indirectly increases NO by activating the eNOS) | Upregulates nuclear factor erythroid-2-related factor 2 (Nrf2) in the positive feedback loop to maintain transcription factor expression | Treatment of spontaneous hypertension in youth | [36] |
| Nebivolol | Third-generation β-receptor blocker (indirectly increases NO prodution) | The β-adrenergic antagonist that causes vasorelaxation primarily by activating eNOS | Treatment of LV dysfunction of myocardial infarction; antioxidant properties, preventing NOS uncoupling; improving LV function in chronic heart failure | [37,38,39,40,41,42] |
| Fenofibrate | PPARα agonist (indirectly increases NO prodution) | Increases endothelial eNOS expression; prevents endothelial dysfunction; reconnects eNOS | Protecting atherosclerosis and endothelial dysfunction, and finally preventing myocardial infarction | [43,44,45] |
| LA419 | NO donor | The effects of anti-ischemia, anti-thrombosis and anti-atherosclerosis | Treatment of myocardial infarction; the prevention of the progression of inadaptable cardiac hypertrophy | [28,46] |
| L-arginine | A precursor of NO production | Reversing several markers in LV hypertrophy and reducing blood pressure and interacting with renin–angiotension–aldosterone system and sympathetic nervous system and other systems | Prevents the progression of LV hypertrophy, anti-hypertrophic effect | [47,48,49] |
| BRL37344 | β3-ARs agonists (indirectly increases NO prodution) | Increases nNOS protein expression and NO production, inhibits superoxide anion generation | Improves LV systolic and diastolic dysfunction, used in the treatment of cardiac hypertrophy | [37] |
| Carbachol | M-cholinergic agonist (indirectly increases NO prodution) | Mediates the production of NO from nNOS | Prevents heart failure | [50] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, M.; Wang, D.; Xu, Y.; Jiang, F.; Zheng, J.; Feng, Y.; Cao, J.; Zhou, X. Nitric Oxide-Releasing Platforms for Treating Cardiovascular Disease. Pharmaceutics 2022, 14, 1345. https://doi.org/10.3390/pharmaceutics14071345
He M, Wang D, Xu Y, Jiang F, Zheng J, Feng Y, Cao J, Zhou X. Nitric Oxide-Releasing Platforms for Treating Cardiovascular Disease. Pharmaceutics. 2022; 14(7):1345. https://doi.org/10.3390/pharmaceutics14071345
Chicago/Turabian StyleHe, Mingyue, Deping Wang, Yumei Xu, Fangying Jiang, Jian Zheng, Yanlin Feng, Jimin Cao, and Xin Zhou. 2022. "Nitric Oxide-Releasing Platforms for Treating Cardiovascular Disease" Pharmaceutics 14, no. 7: 1345. https://doi.org/10.3390/pharmaceutics14071345
APA StyleHe, M., Wang, D., Xu, Y., Jiang, F., Zheng, J., Feng, Y., Cao, J., & Zhou, X. (2022). Nitric Oxide-Releasing Platforms for Treating Cardiovascular Disease. Pharmaceutics, 14(7), 1345. https://doi.org/10.3390/pharmaceutics14071345






