A Mathematical Approach Using Strat-M® to Predict the Percutaneous Absorption of Chemicals under Finite Dose Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. In Vitro Skin Permeation Experiment under Infinite Dose Conditions
2.3. In Vitro Percutaneous Absorption Experiment under Finite Dose Conditions
2.4. Water Evaporation Experiment
2.5. Quantification Method Using HPLC
2.6. Theoretical
2.6.1. Determination of Permeation Parameters
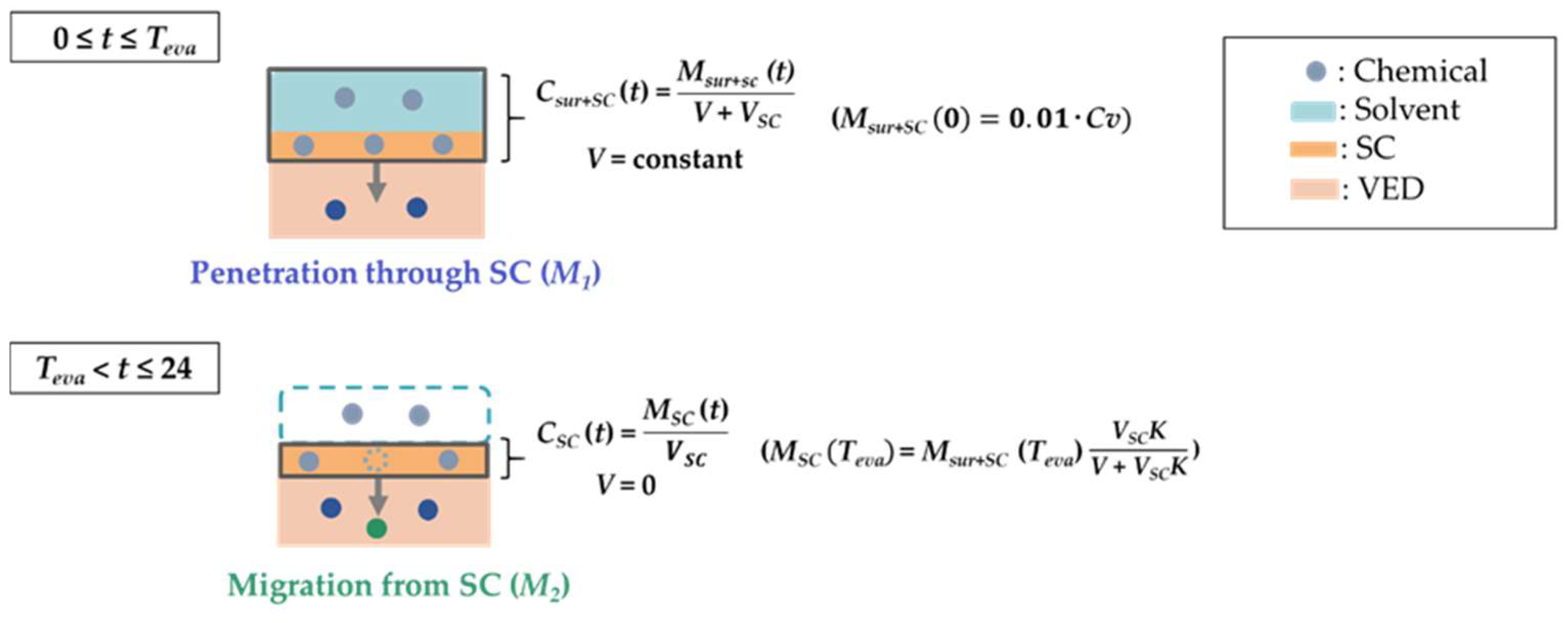
2.6.2. Prediction of the Chemical Amount Permeated through the SC until the Complete Evaporation Time for the Solvent (M1)
2.6.3. Prediction of the Chemical Amount Permeated through the SC from Teva to 24 h (M2)
2.7. Statistical Analysis
3. Results
3.1. Permeation Parameters Obtained from Infinite Dose Experiments
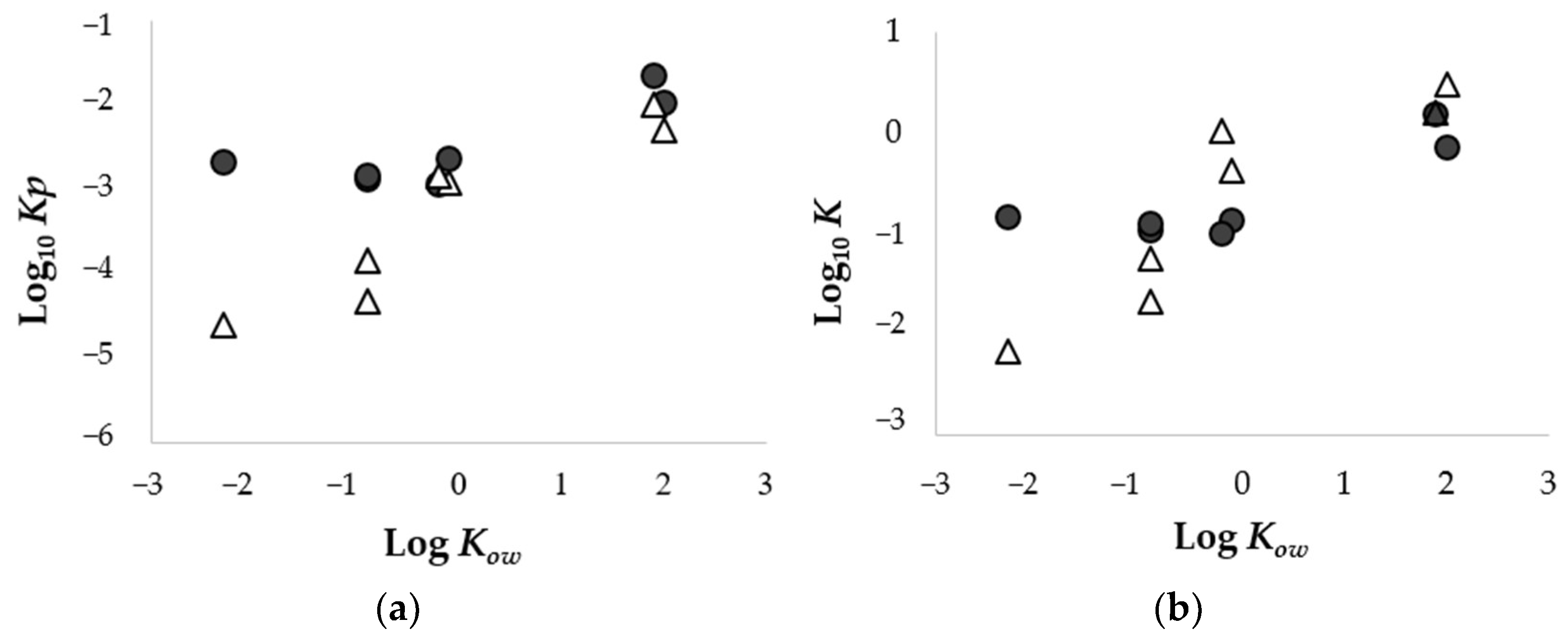
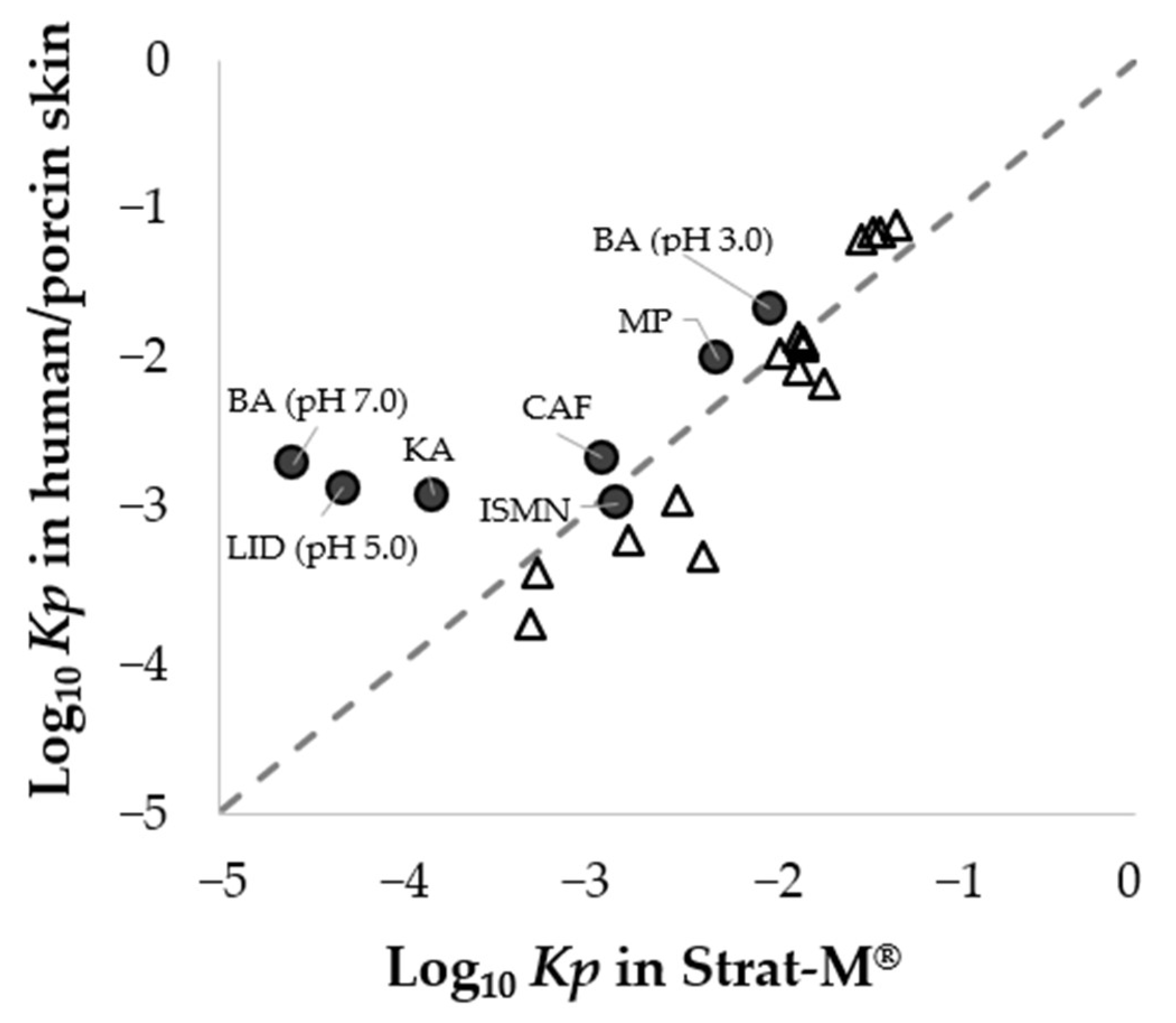
3.2. Relationship in Permeation Parameters between Porcine Skin and Strat-M®
3.3. Solvent Evaporation from the Applied Solution
3.4. Application of a Mathematical Model for Predicting Percutaneous Absorption
3.5. Prediction of Percutaneous Absorption Using Strat-M®
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scientific Committee on Consumer Safety. SCCS/1628/21, the SCCS’s Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation; 11th Revision; Scientific Committee on Consumer Safety: Brussels, Belgium, 2021. [Google Scholar]
- OECD. Organization for Economic Cooperation and Development. In Guidance Notes on Dermal Absorption; Series on Testing and Assessment number 156; OECD Press: Paris, France, 2011. [Google Scholar]
- Neupane, R.; Boddu, S.H.S.; Renukuntla, J.; Babu, R.J.; Tiwari, A.K. Alternatives to biological skin in permeation studies: Current trends and possibilities. Pharmaceutics 2020, 12, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchida, T.; Kadhum, W.R.; Kanai, S.; Todo, H.; Oshizaka, T.; Sugibayashi, K. Prediction of skin permeation by chemical compounds using the artificial membrane, Strat-MTM. Eur. J. Pharm. Sci. 2015, 67, 113–118. [Google Scholar] [CrossRef] [Green Version]
- Haq, A.; Goodyear, B.; Ameen, D.; Joshi, V.; Michniak-Kohn, B. Strat-M® synthetic membrane: Permeability comparison to human cadaver Skin. Int. J. Pharm. 2018, 547, 432–437. [Google Scholar] [CrossRef]
- Simon, A.; Amaro, M.; Healy, A.; Cabral, L.; de Sousa, V. Comparative evaluation of rivastigmine permeation from a percutaneous system in the Franz cell using synthetic membranes and porcine ear skin with in vivo-in vitro correlation. Int. J. Phram. 2016, 512, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Potts, R.O.; Guy, R.H. Predicting skin permeability. Pharm. Res. 1992, 9, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Lian, G.; Chen, L.; Han, L. An evaluation of mathematical models for predicting skin permeability. J. Pharm. Sci. 2008, 97, 584–598. [Google Scholar] [CrossRef]
- Okamoto, H.; Hashida, M.; Sezaki, H. Structure-activity relationship of 1-alkyl- or 1-alkenylazacycloalkanone derivatives as percutaneous penetration enhancers. J. Pharm. Sci. 1988, 77, 418–424. [Google Scholar] [CrossRef]
- Uchida, T.; Nishioka, K.; Motoki, A.; Yakumaru, M.; Sano, T.; Todo, H.; Sugibayashi, K. Effect of esters on the permeation of chemicals with different polarities through synthetic artificial membranes using a high throughput diffusion cell array. Chem. Pharm. Bull. 2016, 64, 1597–1606. [Google Scholar] [CrossRef] [Green Version]
- Flynn, G.L.; Yalkowsky, S.H.; Roseman, T.J. Mass transport phenomena and models: Theoretical concepts. J. Pharm. Sci. 1974, 63, 479–510. [Google Scholar] [CrossRef]
- Arce, F., Jr.; Asano, N.; See, G.L.; Oshizaka, T.; Itakura, S.; Todo, H.; Sugibayashi, K. Prediction of skin permeation and concentration of rhododendrol applied as finite dose from complex cosmetic vehicles. Int. J. Pharm. 2020, 578, 119186. [Google Scholar] [CrossRef]
- Buist, H.E.; van Burgsteden, J.A.; Freidig, A.P.; Maas, W.J.; van de Sandt, J.J. New in vitro dermal absorption database and the prediction of dermal absorption under finite conditions for risk assessment purposes. Regul. Toxicol. Pharmacol. 2010, 57, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, N.; Grégoire, S.; Cubberley, R.; Duplan, H.; Eilstein, J.; Ellison, C.; Lester, C.; Fabian, E.; Fernandez, J.; Géniès, C.; et al. Measurement of the penetration of 56 cosmetic relevant chemicals into and through human skin using a standardized protocol. J. Appl. Toxicol. 2020, 40, 403–415. [Google Scholar] [CrossRef] [Green Version]
- Gomaa, Y.; Morrow, D.I.; Garland, M.J.; Donnelly, R.F.; El-Khordagui, L.K.; Meidan, V.M. Effects of microneedle length, density, insertion time and multiple applications on human skin barrier function: Assessments by transepidermal water loss. Toxicol. Vitro 2010, 24, 1971–1978. [Google Scholar] [CrossRef] [PubMed]
- Anissimov, Y.G. Mathematical models for different exposure conditions. In Dermal Absorption and Toxicity Assessment, 2nd ed.; Roberts, M.S., Walters, K.A., Eds.; Informa Healthcare: New York, NY, USA, 2008; pp. 271–286. [Google Scholar]
- Guy, R.H.; Hadgraft, J. A theoretical description relating skin penetration to the thickness of the applied medicament. Int. J. Pharm. 1980, 6, 321–332. [Google Scholar] [CrossRef]
- Roberts, M.S.; Anissimov, Y.G. Mathematical Models in Percutaneous Absorption. Cutan. Ocul. Toxicol. 2001, 20, 221–270. [Google Scholar] [CrossRef]
- Barry, B.W. Novel mechanisms and devices to enable successful transdermal drug delivery. Eur. J. Pharm. Sci. 2001, 14, 101–114. [Google Scholar] [CrossRef]
- Ruela, A.; Perissinato, A.; de Sousa Lino, M.; Mudrik, P.; Pereira, G. Evaluation of skin absorption of drugs from topical and percutaneous formulations. Braz. J. Pharm. Sci. 2016, 52, 527–544. [Google Scholar] [CrossRef] [Green Version]
- Todo, H.; Kimura, E.; Yasuno, H.; Tokudome, Y.; Hashimoto, F.; Ikarashi, Y.; Sugibayashi, K. Permeation pathway of macromolecules and nanospheres through skin. Biol. Pharm. Bull. 2010, 33, 1394–1399. [Google Scholar] [CrossRef] [Green Version]
- Horita, D.; Yoshimoto, M.; Todo, H.; Sugibayashi, K. Analysis of hair follicle penetration of lidocaine and fluorescein isothiocyanate-dextran 4 kDa using hair follicle-plugging method. Drug Dev. Ind. Pharm. 2014, 40, 345–351. [Google Scholar] [CrossRef] [Green Version]
- Mangelsdorf, S.; Vergou, T.; Sterry, W.; Lademann, J.; Patzelt, A. Comparative study of hair follicle morphology in eight mammalian species and humans. Skin Res. Technol. 2014, 20, 147–154. [Google Scholar] [CrossRef]
- Moser, K.; Kriwet, K.; Naik, A.; Kalia, Y.N.; Guy, R.H. Passive skin penetration enhancement and its quantification in vitro. Eur. J. Pharm. Biopharm. 2001, 52, 103–112. [Google Scholar] [CrossRef]
- Wiechers, J.; Watkinson, A.; Cross, S.; Roberts, M. Predicting skin penetration of actives from complex cosmetic formulations: An evaluation of inter formulation and inter active effects during formulation optimization for percutaneous delivery. Int. J. Cosmet. Sci. 2012, 34, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Abd, E.; Namjoshi, S.; Mohammed, Y.H.; Roberts, M.S.; Grice, J.E. Synergistic skin penetration enhancer and nanoemulsion formulations promote the human epidermal permeation of caffeine and naproxen. J. Pharm. Sci. 2016, 105, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Lundborg, M.; Wennberg, C.L.; Narangifard, A.; Lindahl, E.; Norlén, L. Predicting drug permeability through skin using molecular dynamics simulation. J. Control. Release. 2018, 283, 269–279. [Google Scholar] [CrossRef]
- Jameel, B.; Huynh, A.; Chadha, A.; Pandey, S.; Duncan, J.; Chandler, M.; Baki, G. Computer-based formulation design and optimization using Hansen solubility parameters to enhance the delivery of ibuprofen through the skin. Int. J. Pharm. 2019, 569, 118549. [Google Scholar] [CrossRef]
- Arce, F.J., Jr.; Asano, N.; See, G.L.; Itakura, S.; Todo, H.; Sugibayashi, K. Usefulness of artificial membrane, Strat-M®, in the assessment of drug permeation from complex vehicles in finite dose conditions. Pharmaceutics 2020, 12, 173. [Google Scholar] [CrossRef] [Green Version]
- Riviere, J.E.; Brooks, J.D. Predicting skin permeability from complex chemical mixtures: Dependency of quantitative structure permeation relationships on biology of skin model used. Toxicol. Sci. 2011, 119, 224–232. [Google Scholar] [CrossRef]
- Karadzovska, D.; Brooks, J.; Monteiro-Riviere, N.; Riviere, J. Predicting skin permeability from complex vehicles. Adv. Drug Deliv. Rev. 2013, 65, 265–277. [Google Scholar] [CrossRef]
- Riviere, J.E.; Brooks, J.D.; Collard, W.T.; Deng, J.; de Rose, G.; Mahabir, S.P.; Merritt, D.A.; Marchiondo, A.A. Prediction of formulation effects on dermal absorption of topically applied ectoparasiticides dosed in vitro on canine and porcine skin using a mixture-adjusted quantitative structure permeability relationship. J. Vet. Pharmacol. Ther. 2014, 37, 435–444. [Google Scholar] [CrossRef]
- Baba, H.; Takahara, J.; Yamashita, F.; Hashida, M. Modeling and prediction of solvent effect on human skin permeability using support vector regression and random forest. Pharm. Res. 2015, 32, 3604–3617. [Google Scholar] [CrossRef]
- Baba, H.; Takahara, J.; Mamitsuka, H. In silico predictions of human skin permeability using nonlinear quantitative structure–property relationship models. Pharm. Res. 2015, 32, 2360–2371. [Google Scholar] [CrossRef] [PubMed]
- Atobe, T.; Mori, M.; Yamashita, F.; Hashida, M.; Kouzuki, H. Artificial neural network analysis for predicting human percutaneous absorption taking account of vehicle properties. J. Toxicol. Sci. 2015, 40, 277–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grégoire, S.; Ribaud, C.; Benech, F.; Meunier, J.R.; Garrigues-Mazert, A.; Guy, R.H. Prediction of chemical absorption into and through the skin from cosmetic and dermatological formulations. Br. J. Dermatol. 2009, 160, 80–91. [Google Scholar] [CrossRef] [PubMed]




| Chemicals | Abbreviations | MW | Log Kow | Cv (µg/mL) | Solvent |
|---|---|---|---|---|---|
| Kojic acid | KA | 142.11 | −0.9 | 1.04 × 104 | Water |
| Caffeine | CAF | 194.19 | −0.1 | 1.00 × 104 | Water |
| Isosorbide 5-mononitrate | ISMN | 191.13 | −0.2 | 1.10 × 104 | Water |
| Lidocaine | LID (pH 5.0) | 234.34 | −0.9 | 9.56 × 103 | pH 5.0 citrate buffer |
| Sodium benzoate | BA (pH 3.0) | 122.12 | 1.9 | 1.83 × 103 | pH 3.0 citrate buffer |
| BA (pH 7.0) | 121.12 | −2.3 | 1.08 × 104 | pH 7.0 PBS | |
| Methyl paraben | MP | 152.15 | 2.0 | 1.39 × 103 | Water |
| Chemicals | Porcine Skin | Strat-M® | ||
|---|---|---|---|---|
| Kp (cm/h) | K (−) | Kp (cm/h) | K (−) | |
| KA | (1.30 ± 0.37) × 10−3 | (1.08 ± 0.15) × 10−1 | (1.46 ± 0.30) × 10−4 | (5.77 ± 2.32) × 10−2 |
| CAF | (2.32 ± 0.12) × 10−3 | (1.36 ± 0.62) × 10−1 | (1.23 ± 0.26) × 10−3 | (4.22 ± 0.91) × 10−1 |
| ISMN | (1.15 ± 0.32) × 10−3 | (1.01 ± 0.26) × 10−1 | (1.49 ± 0.48) × 10−3 | 1.04 ± 0.37 |
| LID (pH5.0) | (1.44 ± 0.69) × 10−3 | (1.25 ± 0.83) × 10−1 | (4.72 ± 2.43) × 10−5 | (2.15 ± 1.71) × 10−2 |
| BA (pH3.0) | (2.27 ± 0.07) × 10−2 | 1.51 ± 0.09 | (1.01 ± 0.03) × 10−2 | 1.57 ± 0.20 |
| BA (pH7.0) | (2.11 ± 2.10) × 10−3 | (1.44 ± 1.31) × 10−1 | (2.51 ± 0.25) × 10−5 | (6.73 ± 1.04) × 10−3 |
| MP | (1.06 ± 0.15) × 10−2 | (7.08 ± 2.90) × 10−1 | (5.20 ± 1.08) × 10−3 | 3.08 ± 0.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunita, R.; Nishijima, T.; Todo, H.; Sugibayashi, K.; Sakaguchi, H. A Mathematical Approach Using Strat-M® to Predict the Percutaneous Absorption of Chemicals under Finite Dose Conditions. Pharmaceutics 2022, 14, 1370. https://doi.org/10.3390/pharmaceutics14071370
Kunita R, Nishijima T, Todo H, Sugibayashi K, Sakaguchi H. A Mathematical Approach Using Strat-M® to Predict the Percutaneous Absorption of Chemicals under Finite Dose Conditions. Pharmaceutics. 2022; 14(7):1370. https://doi.org/10.3390/pharmaceutics14071370
Chicago/Turabian StyleKunita, Ryoki, Takafumi Nishijima, Hiroaki Todo, Kenji Sugibayashi, and Hitoshi Sakaguchi. 2022. "A Mathematical Approach Using Strat-M® to Predict the Percutaneous Absorption of Chemicals under Finite Dose Conditions" Pharmaceutics 14, no. 7: 1370. https://doi.org/10.3390/pharmaceutics14071370
APA StyleKunita, R., Nishijima, T., Todo, H., Sugibayashi, K., & Sakaguchi, H. (2022). A Mathematical Approach Using Strat-M® to Predict the Percutaneous Absorption of Chemicals under Finite Dose Conditions. Pharmaceutics, 14(7), 1370. https://doi.org/10.3390/pharmaceutics14071370








