Nanomaterial-Based Drug Delivery System Targeting Lymph Nodes
Abstract
:1. Introduction
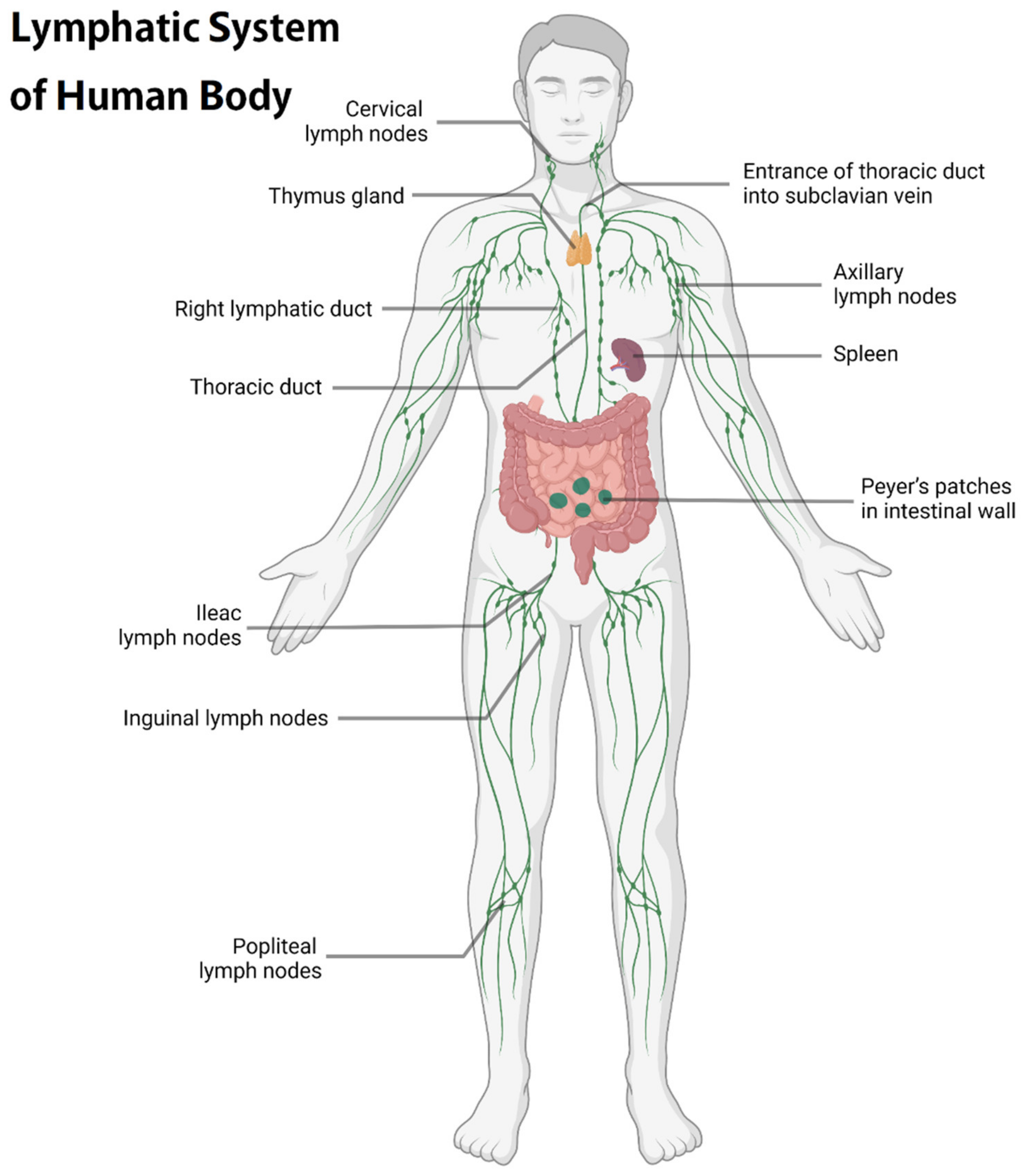
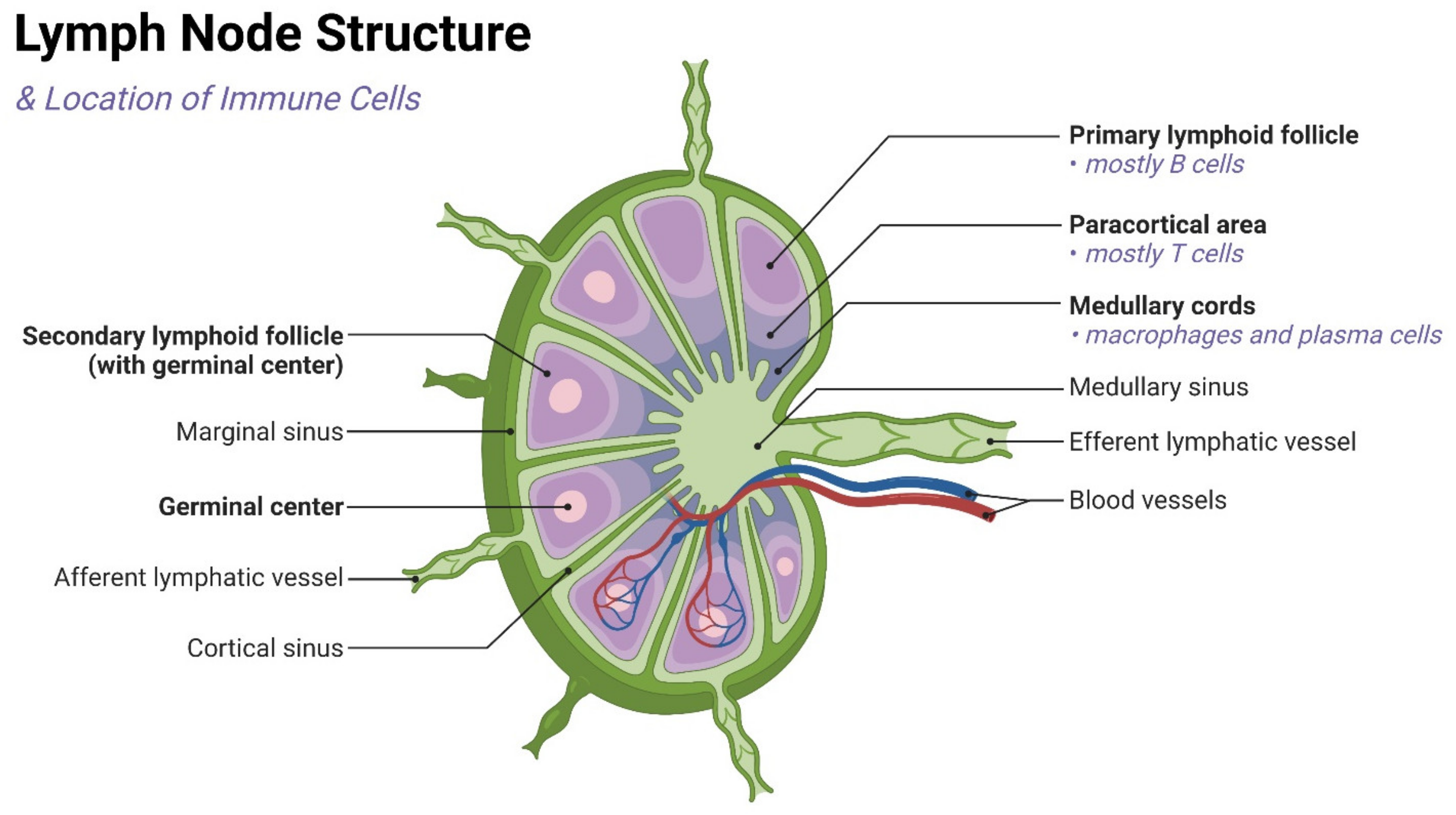
2. Physiological Function and Importance of LNs
3. Interaction between Nanoparticles and Innate Immune System
4. Nano-Drug Delivery Platform System Targeting Lymph Nodes

4.1. Liposome-Related Nano-Drug Delivery Design
4.2. Micellar-Based Nano Drug Delivery Platform
4.3. Inorganic Nanoparticles-Based Delivery Systems
4.3.1. Gold Nanoparticles
4.3.2. Iron Oxide Nanoparticles
4.3.3. Mesoporous Silica Nanoparticles
4.3.4. Carbon Nanoparticles

4.3.5. Other Inorganic Nanoparticles
4.4. Nano-Drug Delivery System Based on Hydrogels
4.5. New Type of High-Efficiency Drug Delivery Nanocapsules
4.6. Endogenous Nanocarriers for Targeted Therapy
4.7. Other Novel Targeted Delivery Nanoparticles
5. Nanomaterial-Based Drug Delivery Systems Targeting T Cells
6. Stimuli-Responsive Nanomaterials for Lymphatic System Drug Delivery in Tumor Therapy
6.1. pH Stimuli
6.2. Redox Stimuli
6.3. Magnetic Responsive
6.4. Light Responsive
6.5. Ultrasound Responsive
7. Clinical Applications of Nano-Drugs
8. Perspectives and Disscussion
9. Conclusions
| Type of Nano-Drug Delivery System | Combined Nanomaterials/Applied Targeting Molecules | Advantages | Therapeutic Agents | Application | Therapeutic Performance | Ref. |
|---|---|---|---|---|---|---|
| Liposome | DDAB and TDB | Lower potential safety risks | N/A | Vaccine adjuvants | Induce a robust CD8+ T-cell response | [159] |
| PEG phospholipid derivatives and new peptides | Activate tumor-specific T-cell immune response more effectively | Anti-PD1 antibody or Treg inhibitory peptide P60 | Melanoma | Tumor immunotherapy | [123] | |
| SsPalm | Activated by pH change | N/A | Tumor and protozoa infection | Induce strong anti-tumor or antiprotozoal effect | [164] | |
| N/A | Promote gene silencing in DCs | siRNA | Tumor | Enhance tumor immunotherapy | [165] | |
| N/A | Direct adjuvant to draining LNs | Cyclic dinucleotides | Vaccine adjuvants | Enhance the efficacy of the adjuvant significantly | [166] | |
| PS | Accumulate and retain effectively in sentinel LNs | N/A | Probe for selective detection | Tumor immunotherapy | [167] | |
| Mannose | Increase the uptake of macrophages | N/A | HIV | Increase the absorption of in the lymphatic system | [119] | |
| Cholesterol | Improve transportation ability and safety | 1V209 (a TLR7 agonist) | Tumor | Induce safe and durable anti-tumor immunity response | [168] | |
| Micelle | mPEG-DSPE | Cause less tissue damage | Adriamycin | Tumor | Increase the uptake of Adriamycin | [176] |
| mPEG-PLA and PLGA/mPEG-PLA | Have no toxicity to immune cells | N/A | Tumor | Act as an agonist for TLR7 | [177] | |
| N/A | Deliver effective immunostimulatory small molecules | N/A | Tumor | Inhibit systemic inflammation and stimulate the strong immune activity | [178] | |
| Polyethylene glycol-b-polyaspartic acid | Have pH-triggered drug release | Epirubicin | Breast cancer | Inhibit tumor growth and axillary lymph node metastasis | [180] | |
| PEG-PE and PSA | Increase uptake and prolong the retention of APCs in LNs | Trp2 peptides and CpGODN | Cancer vaccines therapy | Expand antigen-specific cytotoxic T lymphocytes | [124] | |
| MPDA and PVP | Improve lymphatic drainage, transport and retention ability | Toll-like receptor 7 agonist imiquimod (R837) | Melanoma | Active effective DC and CD8+ T-cell response | [182] | |
| PluronicF-127 | Lower risk of LN metastasis | N/A | Tumor | Increase CD8+ T cells in LNs and slow down tumor growth | [184] | |
| N/A | Change the pharmacokinetic characteristics of drugs | Toll-like receptor 7/8 agonists | N/A | Achieve effective lymphatic transport | [185] | |
| Micelle | Pluronic and PPS | Activate complement cascades and produce danger signals | N/A | N/A | Activate DCs effectively | [186] |
| PCL-PEI and PCL-PEG | Have low toxicity | Trp2 peptides and CpG oligonucleotides | N/A | Have high efficacy on DCs | [189] | |
| N/A | Target tumor lymphatic vessels and gather near blood vessels | LYP-1 | Tumor | Have the better anti-tumor effect in vitro | [126] | |
| PEG-PLGA | Better distribution | LYP-1 | Tumor | Achieve better anti-tumor effects | [127] | |
| poly (lactide-glycolide) | Prolong the residence time and activate DCs more persistently | PolyIC | Therapeutic or prophylactic vaccines | Produce a certain immune enhancement effect | [120] | |
| Inorganic nanoparticle | Neutral polyethylene glycol polyalloy nanorods | Achieve local photothermal therapy | N/A | Tumor | Have clear inhibitory effects on tumor metastasis of LNs | [196] |
| AuNP with octyl mercaptan and 11-mercaptoundecane sulfonic acid | Inhibited the growth of large tumors and prolong the survival time | TLR7 ligands | Tumor | Cause local immune activation and stimulate the response of cytotoxic T cells | [121] | |
| AuNP with escherichia coli membrane | Induce and regulate immune response | N/A | Antibacterial vaccine | Result in a strong antibody response | [199] | |
| Inorganic nanoparticle | Au-SGSH | Target DNA vaccine to APCs | N/A | Tumor | Generate long-term immune response | [122] |
| Lauric acid and HSA | Achieve site-specific drug delivery under the action of a localized external magnetic field | Mitoxantrone | Tumor | Have strengthen stability and linear drug release kinetics | [212] | |
| LHRHR and uPAR | Have small hydrodynamic diameter and high drug loading | Paclitaxel | Prostate cancer | Increase the cytotoxicity of cancer cells and reduce the concentration required for free drugs by ten times | [128] | |
| USPIO | Pass through lymphatic vessels faster | N/A | N/A | Gather in sentinel LNs earlier | [215] | |
| PEGylated DOX-Fe2+ complexes | Achieve pH-dependent drug release | Doxorubicin | Tumor | Facilitate the penetration into tumors, become less susceptible to MDR than the free drug and increase therapeutic effect | [216] | |
| Chitosan | Temperature-controlled drug release | Doxorubicin | N/A | Enhance therapeutic effects | [217] | |
| Phospholipid-PEG | Generate heat itself and benefit hyperthermia | Adriamycin | Tumor | Strengthen the effect of chemotherapy and hyperthermia in the treatment of cancer | [218] | |
| XL-MSN | Have high biomolecule loading | TLR9 agonist | Tumor | Enhance antigen presentation ability and increase pro-inflammatory cytokine secretion | [219] | |
| Inorganic nanoparticle | GDMON | Change the intracellular microenvironment and ROS levels | Antigenic proteins OVA and TLR9 agonists | Tumor | Promote cytotoxic T lymphocyte proliferation and inhibit tumor growth | [220] |
| RGO-PEG | Adapt to a variety of personalized new antigen peptides and transport efficiently | N/A | Nano-vaccine | Generate reactive oxygen species in DCs and induce new antigen-specific T-cell responses | [224] | |
| Magnetic multi-walled carbon nanotubes | Reduce lymphatic metastasis | Gemcitabine | N/A | Achieve more effective drug delivery | [225] | |
| Zinc phosphate and monophospholipids | Make it more difficult for tumors to escape immune surveillance | H-2kb-restricted peptides Trp2180-188 and H-2DB-restricted peptides Hgp10025-33 | Subcutaneous melanoma and lung metastatic melanoma | Induce CD8+ T-cell response and inhibit tumor growth | [130] | |
| OVA modified α-alumina nanoparticles | Induce effective autophagy-dependent cross-presentation | N/A | N/A | Induce strong anti-tumor response | [228] | |
| Zinc-loaded ferromagnetic nano-phospholipid | Activate the immune response through TLR connection directly | PolyIC and imiquimod (R837) | Invasive B16-F10 melanoma | Induce a potent innate immune response in LNs | [229] | |
| Hydrogel | Cholesterol pullulan nanogels | Specifically absorbed by macrophages located in the medulla | Synthetic long peptide antigens | Tumor | Present CD8+T cell antigen and inhibit tumor growth | [240] |
| N/A | Have higher specificity and controlled release properties | Gemcitabine | Lung cancer | Reduce the toxicity and inhibit mediastinal metastasis | [242] | |
| Imidazoline | Improve the therapeutic benefit of local application | TLR7/8 agonist | Tumor | Induce better antibody and T-cell response and greatly reduce systemic inflammatory response | [243] | |
| Polyethylene glycol poly (L-valine) | Prolong the time of antigen at the injection site and increase the number of LNs | Polyinosine:polycytidine monophosphate | Melanoma | Induce cytotoxic T lymphocyte reaction and increase the number of CD8+ T cells in draining LNs | [131] | |
| Polyethylene glycol | Target multiple immune cell subsets in LNs | N/A | Cancer vaccines | Improve the ability of antigen presentation | [244] | |
| N/A | Affect the presence of immune cells in draining LNs | GM-CSF | Type I diabetic | Increase antigen-specific CD4+ T cells | [132] | |
| Nanocapsule | Polysaccharide shells | Form a repository at the injection site | Docetaxel | Tumor | Have better biodistribution and faster access to lymphatic vessels | [246] |
| N/A | Load antigens and adjuvants easily | Protein or peptide antigens | Tumor vaccines and prophylactic virus vaccines | Promote the uptake of APCs and the transport of APCs to draining LNs | [247] | |
| Nanocapsule | N/A | Improve the oral bioavailability of insoluble drugs | Docetaxel | N/A | Increase in exposure time | [248] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIDS | acquired immune deficiency syndrome |
| APCs | antigen-presenting cells |
| AuNPs | gold nanoparticles |
| CTL | cytotoxic T lymphocytes |
| DCs | dendritic cells |
| DDA | dimethyldioctadecylammonium |
| DDAB | dimethyldioctadecylammonium bromide |
| DDS | drug-delivery systems |
| DOX | doxorubicin |
| EPR | enhanced permeability and retention |
| GDMON | glutathione-depleted dendritic mesoporous organosilica nanoparticles |
| IONs | iron oxide nanoparticles |
| LHRHR | luteinizing hormone-releasing hormone receptor |
| LNs | lymph nodes |
| MDR | multidrug resistance |
| MHC x | major histocompatibility complex |
| mPEG-DSPE | methyl polyethylene glycol distearyl phosphatidylethanolamine |
| MRI | magnetic resonance imaging |
| MSNs | mesoporous silica nanoparticles |
| NK cells | natural killer cells |
| OND | oxyboradiene |
| OVA | ovalbumin |
| PDT | photothermal therapy |
| PEG | polyethylene glycol |
| PLA | polylactic acid |
| PLGA | poly (Dmuryl L-lactic acid-glycolic acid) |
| PolyIC | poly (inosinic acid: cytidine) |
| PS | 1-dioleoyl-n-glycerophosphate serine |
| PTT | photothermal therapy |
| PTX | paclitaxel |
| PVP | polyvinylpyrrolidone |
| uPAR | urokinase type plasminogen activator receptor |
References
- Margaris, K.N.; Black, R.A. Modelling the lymphatic system: Challenges and opportunities. J. R. Soc. Interface 2012, 9, 601–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, T.J. Structure and function of lymphatics. J. Investig. Dermatol. 1989, 93, 18s–24s. [Google Scholar] [CrossRef] [PubMed]
- Cote, B.; Rao, D.; Alany, R.G.; Kwon, G.S.; Alani, A.W.G. Lymphatic changes in cancer and drug delivery to the lymphatics in solid tumors. Adv. Drug Deliv. Rev. 2019, 144, 16–34. [Google Scholar] [CrossRef]
- Proulx, S.T.; Luciani, P.; Dieterich, L.C.; Karaman, S.; Leroux, J.-C.; Detmar, M. Expansion of the lymphatic vasculature in cancer and inflammation: New opportunities for in vivo imaging and drug delivery. J. Control. Release 2013, 172, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; von der Weid, P.Y. Lymphatic system: An active pathway for immune protection. Semin. Cell Dev. Biol. 2015, 38, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Trevaskis, N.L.; Kaminskas, L.M.; Porter, C.J. From sewer to saviour—Targeting the lymphatic system to promote drug exposure and activity. Nat. Rev. Drug Discov. 2015, 14, 781–803. [Google Scholar] [CrossRef]
- Randolph, G.J.; Miller, N.E. Lymphatic transport of high-density lipoproteins and chylomicrons. J. Clin. Investig. 2014, 124, 929–935. [Google Scholar] [CrossRef] [Green Version]
- Miller, N.E.; Michel, C.C.; Nanjee, M.N.; Olszewski, W.L.; Miller, I.P.; Hazell, M.; Olivecrona, G.; Sutton, P.; Humphreys, S.M.; Frayn, K.N. Secretion of adipokines by human adipose tissue in vivo: Partitioning between capillary and lymphatic transport. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E659–E667. [Google Scholar] [CrossRef] [Green Version]
- Wiig, H.; Swartz, M.A. Interstitial Fluid and Lymph Formation and Transport: Physiological Regulation and Roles in Inflammation and Cancer. Physiol. Rev. 2012, 92, 1005–1060. [Google Scholar] [CrossRef]
- Roozendaal, R.; Mebius, R.E.; Kraal, G. The conduit system of the lymph node. Int. Immunol. 2008, 20, 1483–1487. [Google Scholar] [CrossRef] [Green Version]
- Gretz, J.E.; Anderson, A.O.; Shaw, S. Cords, channels, corridors and conduits: Critical architectural elements facilitating cell interactions in the lymph node cortex. Immunol. Rev. 1997, 156, 11–24. [Google Scholar] [CrossRef]
- Trevaskis, N.L.; Charman, W.N.; Porter, C.J.H. Lipid-based delivery systems and intestinal lymphatic drug transport: A mechanistic update. Adv. Drug Deliv. Rev. 2008, 60, 702–716. [Google Scholar] [CrossRef]
- Ryan, G.M.; Kaminskas, L.M.; Porter, C.J. Nano-chemotherapeutics: Maximising lymphatic drug exposure to improve the treatment of lymph-metastatic cancers. J. Control. Release 2014, 193, 241–256. [Google Scholar] [CrossRef]
- Das, R.P.; Gandhi, V.V.; Singh, B.G.; Kunwar, A. Passive and Active Drug Targeting: Role of Nanocarriers in Rational Design of Anticancer Formulations. Curr. Pharm. Des. 2019, 25, 3034–3056. [Google Scholar] [CrossRef]
- Alsaggar, M.; Liu, D. Organ-based drug delivery. J. Drug Target. 2018, 26, 385–397. [Google Scholar] [CrossRef]
- Rabanel, J.-M.; Aoun, V.; Elkin, I.; Mokhtar, M.; Hildgen, P. Drug-Loaded Nanocarriers: Passive Targeting and Crossing of Biological Barriers. Curr. Med. Chem. 2012, 19, 3070–3102. [Google Scholar] [CrossRef]
- Arslan, F.B.; Ozturk Atar, K.; Calis, S. Antibody-mediated drug delivery. Int. J. Pharm. 2021, 596, 120268. [Google Scholar] [CrossRef]
- Yousef, M.; Silva, D.; Bou Chacra, N.; Davies, N.; Löbenberg, R. The Lymphatic System: A Sometimes-Forgotten Compartment in Pharmaceutical Sciences. J. Pharm. Pharm. Sci. 2021, 24, 533–547. [Google Scholar] [CrossRef]
- Punjabi, M.S.; Naha, A.; Shetty, D.; Nayak, U.Y. Lymphatic Drug Transport and Associated Drug Delivery Technologies: A Comprehensive Review. Curr. Pharm. Des. 2021, 27, 1992–1998. [Google Scholar] [CrossRef]
- Xu, W.; Harris, N.R.; Caron, K.M. Lymphatic Vasculature: An Emerging Therapeutic Target and Drug Delivery Route. Annu. Rev. Med. 2021, 72, 167–182. [Google Scholar] [CrossRef]
- Jalkanen, S.; Salmi, M. Lymphatic endothelial cells of the lymph node. Nat. Rev. Immunol. 2020, 20, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Willard-Mack, C.L. Normal structure, function, and histology of lymph nodes. Toxicol. Pathol. 2006, 34, 409–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, T.; Harashima, H. Dawn of lipid nanoparticles in lymph node targeting: Potential in cancer immunotherapy. Adv. Drug Deliv. Rev. 2020, 167, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Baluk, P.; Fuxe, J.; Hashizume, H.; Romano, T.; Lashnits, E.; Butz, S.; Vestweber, D.; Corada, M.; Molendini, C.; Dejana, E.; et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J. Exp. Med. 2007, 204, 2349–2362. [Google Scholar] [CrossRef]
- Randolph, G.J.; Ivanov, S.; Zinselmeyer, B.H.; Scallan, J.P. The Lymphatic System: Integral Roles in Immunity. Annu. Rev. Immunol. 2017, 35, 31–52. [Google Scholar] [CrossRef] [Green Version]
- Sarris, M.; Sixt, M. Navigating in tissue mazes: Chemoattractant interpretation in complex environments. Curr. Opin. Cell Biol. 2015, 36, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Lämmermann, T.; Sixt, M. The microanatomy of T-cell responses. Immunol. Rev. 2008, 221, 21–43. [Google Scholar] [CrossRef]
- Gerner, M.Y.; Torabi-Parizi, P.; Germain, R.N. Strategically Localized Dendritic Cells Promote Rapid T Cell Responses to Lymph-Borne Particulate Antigens. Immunity 2015, 42, 172–185. [Google Scholar] [CrossRef] [Green Version]
- Moran, I.; Grootveld, A.K.; Nguyen, A.; Phan, T.G. Subcapsular Sinus Macrophages: The Seat of Innate and Adaptive Memory in Murine Lymph Nodes. Trends Immunol. 2019, 40, 35–48. [Google Scholar] [CrossRef]
- Ager, A. High Endothelial Venules and Other Blood Vessels: Critical Regulators of Lymphoid Organ Development and Function. Front. Immunol. 2017, 8, 45. [Google Scholar] [CrossRef]
- Ohtani, O.; Ohtani, Y. Structure and function of rat lymph nodes. Arch. Histol. Cytol. 2008, 71, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Thomas, S.N.; Rutkowski, J.M.; Pasquier, M.; Kuan, E.L.; Alitalo, K.; Randolph, G.J.; Swartz, M.A. Impaired Humoral Immunity and Tolerance in K14-VEGFR-3-Ig Mice That Lack Dermal Lymphatic Drainage. J. Immunol. 2012, 189, 2181–2190. [Google Scholar] [CrossRef] [Green Version]
- Lund, A.W.; Duraes, F.V.; Hirosue, S.; Raghavan, V.R.; Nembrini, C.; Thomas, S.N.; Issa, A.; Hugues, S.; Swartz, M.A. VEGF-C Promotes Immune Tolerance in B16 Melanomas and Cross-Presentation of Tumor Antigen by Lymph Node Lymphatics. Cell Rep. 2012, 1, 191–199. [Google Scholar] [CrossRef]
- Mebius, R.E.; Streeter, P.R.; Brevé, J.; Duijvestijn, A.M.; Kraal, G. The influence of afferent lymphatic vessel interruption on vascular addressin expression. J. Cell Biol. 1991, 115, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Rohner, N.A.; McClain, J.; Tuell, S.L.; Warner, A.; Smith, B.; Yun, Y.; Mohan, A.; Sushnitha, M.; Thomas, S.N. Lymph node biophysical remodeling is associated with melanoma lymphatic drainage. FASEB J. 2015, 29, 4512–4522. [Google Scholar] [CrossRef] [Green Version]
- Thomas, S.N.; Rohner, N.A.; Edwards, E.E. Implications of Lymphatic Transport to Lymph Nodes in Immunity and Immunotherapy. Annu. Rev. Biomed. Eng. 2016, 18, 207–233. [Google Scholar] [CrossRef] [Green Version]
- Yáñez, J.A.; Wang, S.W.J.; Knemeyer, I.W.; Wirth, M.A.; Alton, K.B. Intestinal lymphatic transport for drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 923–942. [Google Scholar] [CrossRef]
- Mortimer, P.S.; Rockson, S.G. New developments in clinical aspects of lymphatic disease. J. Clin. Investig. 2014, 124, 915–921. [Google Scholar] [CrossRef]
- Alitalo, K. The lymphatic vasculature in disease. Nat. Med. 2011, 17, 1371–1380. [Google Scholar] [CrossRef]
- Kesler, C.T.; Liao, S.; Munn, L.L.; Padera, T.P. Lymphatic vessels in health and disease. WIREs Syst. Biol. Med. 2013, 5, 111–124. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Oliver, G. Current views on the function of the lymphatic vasculature in health and disease. Genes Dev. 2010, 24, 2115–2126. [Google Scholar] [CrossRef] [Green Version]
- Swartz, M.A.; Lund, A.W. Lymphatic and interstitial flow in the tumour microenvironment: Linking mechanobiology with immunity. Nat. Rev. Cancer 2012, 12, 210–219. [Google Scholar] [CrossRef]
- Dieterich, L.C.; Seidel, C.D.; Detmar, M. Lymphatic vessels: New targets for the treatment of inflammatory diseases. Angiogenesis 2014, 17, 359–371. [Google Scholar] [CrossRef]
- Gasteiger, G.; Ataide, M.; Kastenmüller, W. Lymph node—An organ for T-cell activation and pathogen defense. Immunol. Rev. 2016, 271, 200–220. [Google Scholar] [CrossRef]
- Nakamura, T.; Kawai, M.; Sato, Y.; Maeki, M.; Tokeshi, M.; Harashima, H. The Effect of Size and Charge of Lipid Nanoparticles Prepared by Microfluidic Mixing on Their Lymph Node Transitivity and Distribution. Mol. Pharm. 2020, 17, 944–953. [Google Scholar] [CrossRef]
- Merad, M.; Sathe, P.; Helft, J.; Miller, J.; Mortha, A. The Dendritic Cell Lineage: Ontogeny and Function of Dendritic Cells and Their Subsets in the Steady State and the Inflamed Setting. Annu. Rev. Immunol. 2013, 31, 563–604. [Google Scholar] [CrossRef] [Green Version]
- Karaman, S.; Detmar, M. Mechanisms of lymphatic metastasis. J. Clin. Investig. 2014, 124, 922–928. [Google Scholar] [CrossRef] [Green Version]
- Hess, K.R.; Varadhachary, G.R.; Taylor, S.H.; Wei, W.; Raber, M.N.; Lenzi, R.; Abbruzzese, J.L. Metastatic patterns in adenocarcinoma. Cancer 2006, 106, 1624–1633. [Google Scholar] [CrossRef]
- Kawada, K.; Taketo, M.M. Significance and Mechanism of Lymph Node Metastasis in Cancer Progression. Cancer Res. 2011, 71, 1214–1218. [Google Scholar] [CrossRef] [Green Version]
- Kato, S.; Takeda, K.; Sukhbaatar, A.; Sakamoto, M.; Mori, S.; Shiga, K.; Kodama, T. Intranodal pressure of a metastatic lymph node reflects the response to lymphatic drug delivery system. Cancer Sci. 2020, 111, 4232–4241. [Google Scholar] [CrossRef]
- Medina, L.A.; Calixto, S.M.; Klipper, R.; Phillips, W.T.; Goins, B. Avidin/biotin-liposome system injected in the pleural space for drug delivery to mediastinal lymph nodes. J. Pharm. Sci. 2004, 93, 2595–2608. [Google Scholar] [CrossRef] [PubMed]
- Minchinton, A.I.; Tannock, I.F. Drug penetration in solid tumours. Nat. Rev. Cancer 2006, 6, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Shirai, Y.; Sakamoto, M.; Mori, S.; Kodama, T. Use of a Lymphatic Drug Delivery System and Sonoporation to Target Malignant Metastatic Breast Cancer Cells Proliferating in the Marginal Sinuses. Sci. Rep. 2019, 9, 13242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, Y.-C.; Xu, Z.; Guley, K.; Yuan, H.; Huang, L. Lipid–calcium phosphate nanoparticles for delivery to the lymphatic system and SPECT/CT imaging of lymph node metastases. Biomaterials 2014, 35, 4688–4698. [Google Scholar] [CrossRef] [Green Version]
- Chambers, A.F.; Groom, A.C.; MacDonald, I.C. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2002, 2, 563–572. [Google Scholar] [CrossRef]
- Pantel, K.; Brakenhoff, R.H. Dissecting the metastatic cascade. Nat. Rev. Cancer 2004, 4, 448–456. [Google Scholar] [CrossRef]
- Alvarado, R.; Yi, M.; Le-Petross, H.; Gilcrease, M.; Mittendorf, E.A.; Bedrosian, I.; Hwang, R.F.; Caudle, A.S.; Babiera, G.V.; Akins, J.S.; et al. The Role for Sentinel Lymph Node Dissection after Neoadjuvant Chemotherapy in Patients who Present with Node-Positive Breast Cancer. Ann. Surg. Oncol. 2012, 19, 3177–3184. [Google Scholar] [CrossRef]
- Shao, L.; Ouchi, T.; Sakamoto, M.; Mori, S.; Kodama, T. Activation of latent metastases in the lung after resection of a metastatic lymph node in a lymph node metastasis mouse model. Biochem. Biophys. Res. Commun. 2015, 460, 543–548. [Google Scholar] [CrossRef]
- Takeda, K.; Mori, S.; Kodama, T. Study of fluid dynamics reveals direct communications between lymphatic vessels and venous blood vessels at lymph nodes of mice. J. Immunol. Methods 2017, 445, 1–9. [Google Scholar] [CrossRef]
- Wada, N.; Duh, Q.Y.; Sugino, K.; Iwasaki, H.; Kameyama, K.; Mimura, T.; Ito, K.; Takami, H.; Takanashi, Y. Lymph node metastasis from 259 papillary thyroid microcarcinomas: Frequency, pattern of occurrence and recurrence, and optimal strategy for neck dissection. Ann. Surg. 2003, 237, 399–407. [Google Scholar] [CrossRef]
- Brown, M.; Assen, F.P.; Leithner, A.; Abe, J.; Schachner, H.; Asfour, G.; Bago-Horvath, Z.; Stein, J.V.; Uhrin, P.; Sixt, M.; et al. Lymph node blood vessels provide exit routes for metastatic tumor cell dissemination in mice. Science 2018, 359, 1408–1411. [Google Scholar] [CrossRef] [Green Version]
- Pereira, E.R.; Kedrin, D.; Seano, G.; Gautier, O.; Meijer, E.F.J.; Jones, D.; Chin, S.-M.; Kitahara, S.; Bouta, E.M.; Chang, J.; et al. Lymph node metastases can invade local blood vessels, exit the node, and colonize distant organs in mice. Science 2018, 359, 1403–1407. [Google Scholar] [CrossRef] [Green Version]
- Van Dongen, J.A.; Voogd, A.C.; Fentiman, I.S.; Legrand, C.; Sylvester, R.J.; Tong, D.; Van Der Schueren, E.; Helle, P.A.; Van Zijl, K.; Bartelink, H. Long-Term Results of a Randomized Trial Comparing Breast-Conserving Therapy with Mastectomy: European Organization for Research and Treatment of Cancer 10801 Trial. J. Natl. Cancer Inst. 2000, 92, 1143–1150. [Google Scholar] [CrossRef] [Green Version]
- Cai, S.; Yang, Q.; Bagby, T.R.; Forrest, M.L. Lymphatic drug delivery using engineered liposomes and solid lipid nanoparticles. Adv. Drug Deliv. Rev. 2011, 63, 901–908. [Google Scholar] [CrossRef] [Green Version]
- López-Suárez, A.; Bascuñana, A.; Elvira, J.; García-Del-Río, E.; Escribano, J.C.; Girón, J.A. Fatal mediastinal lymph node drainage into the airways of two patients with human immunodeficiency virus-related tuberculosis. Eur. J. Clin. Microbiol. Infect. Dis. 1998, 17, 670–671. [Google Scholar] [CrossRef]
- Geldmacher, H.; Taube, C.; Kroeger, C.; Magnussen, H.; Kirsten, D.K. Assessment of lymph node tuberculosis in northern Germany: A clinical review. Chest 2002, 121, 1177–1182. [Google Scholar] [CrossRef]
- Izbicki, J.R.; Passlick, B.; Hosch, S.B.; Kubuschock, B.; Schneider, C.; Busch, C.; Knoefel, W.T.; Thetter, O.; Pantel, K. Mode of spread in the early phase of lymphatic metastasis in non-small-cell lung cancer: Significance of nodal micrometastasis. J. Thorac. Cardiovasc. Surg. 1996, 112, 623–630. [Google Scholar] [CrossRef] [Green Version]
- Alitalo, A.; Detmar, M. Interaction of tumor cells and lymphatic vessels in cancer progression. Oncogene 2011, 31, 4499–4508. [Google Scholar] [CrossRef] [Green Version]
- Betticher, D.C.; Schmitz, S.-F.H.; Tötsch, M.; Hansen, E.; Joss, C.; Von Briel, C.; Schmid, R.A.; Pless, M.; Habicht, J.; Roth, A.D.; et al. Mediastinal Lymph Node Clearance After Docetaxel-Cisplatin Neoadjuvant Chemotherapy Is Prognostic of Survival in Patients with Stage IIIA pN2 Non–Small-Cell Lung Cancer: A Multicenter Phase II Trial. J. Clin. Oncol. 2003, 21, 1752–1759. [Google Scholar] [CrossRef]
- Rohner, N.A.; Thomas, S.N. Melanoma growth effects on molecular clearance from tumors and biodistribution into systemic tissues versus draining lymph nodes. J. Control. Release 2016, 223, 99–108. [Google Scholar] [CrossRef] [Green Version]
- Hoshida, T.; Isaka, N.; Hagendoorn, J.; di Tomaso, E.; Chen, Y.-L.; Pytowski, B.; Fukumura, D.; Padera, T.P.; Jain, R.K. Imaging Steps of Lymphatic Metastasis Reveals That Vascular Endothelial Growth Factor-C Increases Metastasis by Increasing Delivery of Cancer Cells to Lymph Nodes: Therapeutic Implications. Cancer Res. 2006, 66, 8065–8075. [Google Scholar] [CrossRef] [Green Version]
- Abe, N.; Ohtake, T.; Saito, K.; Kumamoto, K.; Sugino, T.; Takenoshita, S. Clinicopathological significance of lymphangiogenesis detected by immunohistochemistry using D2-40 monoclonal antibody in breast cancer. Fukushima J. Med Sci. 2016, 62, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Babar, I.A.; Cheng, C.J.; Booth, C.J.; Liang, X.; Weidhaas, J.B.; Saltzman, W.M.; Slack, F.J. Nanoparticle-based therapy in an in vivo microRNA-155 (miR-155)-dependent mouse model of lymphoma. Proc. Natl. Acad. Sci. USA 2012, 109, E1695–E1704. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo-Redondo, R.; Fryer, H.R.; Bedford, T.; Kim, E.Y.; Archer, J.; Kosakovsky Pond, S.L.K.; Chung, Y.S.; Penugonda, S.; Chipman, J.G.; Fletcher, C.V.; et al. Persistent HIV-1 replication maintains the tissue reservoir during therapy. Nature 2016, 530, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Kuerer, H.M.; Newman, L.A.; Buzdar, A.U.; Hunt, K.K.; Dhingra, K.; Buchholz, T.A.; Binkley, S.M.; Ames, F.C.; Feig, B.W.; Ross, M.I.; et al. Residual metastatic axillary lymph nodes following neoadjuvant chemotherapy predict disease-free survival in patients with locally advanced breast cancer. Am. J. Surg. 1998, 176, 502–509. [Google Scholar] [CrossRef]
- Liu, H.; Moynihan, K.D.; Zheng, Y.; Szeto, G.L.; Li, A.V.; Huang, B.; Van Egeren, D.S.; Park, C.; Irvine, D.J. Structure-based programming of lymph-node targeting in molecular vaccines. Nature 2014, 507, 519–522. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-W.; Epardaud, M.; Sun, J.; Becker, J.E.; Cheng, A.C.; Yonekura, A.-R.; Heath, J.K.; Turley, S.J. Peripheral antigen display by lymph node stroma promotes T cell tolerance to intestinal self. Nat. Immunol. 2007, 8, 181–190. [Google Scholar] [CrossRef]
- Komori, J.; Boone, L.; DeWard, A.; Hoppo, T.; Lagasse, E. The mouse lymph node as an ectopic transplantation site for multiple tissues. Nat. Biotechnol. 2012, 30, 976–983. [Google Scholar] [CrossRef] [Green Version]
- Schudel, A.; Chapman, A.P.; Yau, M.-K.; Higginson, C.J.; Francis, D.M.; Manspeaker, M.P.; Avecilla, A.R.C.; Rohner, N.A.; Finn, M.G.; Thomas, S.N. Programmable multistage drug delivery to lymph nodes. Nat. Nanotechnol. 2020, 15, 491–499. [Google Scholar] [CrossRef]
- Thomas, S.N.; Schudel, A. Overcoming transport barriers for interstitial-, lymphatic-, and lymph node-targeted drug delivery. Curr. Opin. Chem. Eng. 2015, 7, 65–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H. Nanoparticle uptake: The phagocyte problem. Nano Today 2015, 10, 487–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsehli, M. Polymeric nanocarriers as stimuli-responsive systems for targeted tumor (cancer) therapy: Recent advances in drug delivery. Saudi Pharm. J. 2020, 28, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Silva, J.M.; Videira, M.; Gaspar, R.; Préat, V.; Florindo, H.F. Immune system targeting by biodegradable nanoparticles for cancer vaccines. J. Control. Release 2013, 168, 179–199. [Google Scholar] [CrossRef]
- Silva, A.L.; Soema, P.C.; Slütter, B.; Ossendorp, F.; Jiskoot, W. PLGA particulate delivery systems for subunit vaccines: Linking particle properties to immunogenicity. Hum. Vaccines Immunother. 2016, 12, 1056–1069. [Google Scholar] [CrossRef]
- Zolnik, B.S.; González-Fernández, A.; Sadrieh, N.; Dobrovolskaia, M.A. Nanoparticles and the Immune System. Endocrinology 2010, 151, 458–465. [Google Scholar] [CrossRef]
- Lucarelli, M.; Gatti, A.M.; Savarino, G.; Quattroni, P.; Martinelli, L.; Monari, E.; Boraschi, D. Innate defence functions of macrophages can be biased by nano-sized ceramic and metallic particles. Eur. Cytokine Netw. 2004, 15, 339–346. [Google Scholar]
- Walkey, C.D.; Olsen, J.B.; Guo, H.; Emili, A.; Chan, W.C.W. Nanoparticle Size and Surface Chemistry Determine Serum Protein Adsorption and Macrophage Uptake. J. Am. Chem. Soc. 2012, 134, 2139–2147. [Google Scholar] [CrossRef]
- Shang, L.; Nienhaus, K.; Nienhaus, G.U. Engineered nanoparticles interacting with cells: Size matters. J. Nanobiotechnol. 2014, 12, 5. [Google Scholar] [CrossRef] [Green Version]
- Walkey, C.D.; Chan, W.C.W. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem. Soc. Rev. 2012, 41, 2780–2799. [Google Scholar] [CrossRef]
- Göppert, T.M.; Müller, R.H. Polysorbate-stabilized solid lipid nanoparticles as colloidal carriers for intravenous targeting of drugs to the brain: Comparison of plasma protein adsorption patterns. J. Drug Target. 2005, 13, 179–187. [Google Scholar] [CrossRef]
- Feliu, N.; Fadeel, B. Nanotoxicology: No small matter. Nanoscale 2010, 2, 2514–2520. [Google Scholar] [CrossRef]
- Owens, D.E., 3rd; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef]
- Borm, P.J.; Robbins, D.; Haubold, S.; Kuhlbusch, T.; Fissan, H.; Donaldson, K.; Schins, R.; Stone, V.; Kreyling, W.; Lademann, J.; et al. The Potential Risks of Nanomaterials: A Review Carried Out for ECETOC. Part. Fibre Toxicol. 2006, 3, 11. [Google Scholar] [CrossRef] [Green Version]
- Janko, C.; Friedrich, R.P.; Cicha, I.; Unterweger, H.; Lyer, S.; Alexiou, C. Modulation of immune responses by nanoparticles. Nanomedicine 2021, 16, 1925–1929. [Google Scholar] [CrossRef]
- Elamanchili, P.; Lutsiak, C.M.E.; Hamdy, S.; Diwan, M.; Samuel, J. “Pathogen-Mimicking” Nanoparticles for Vaccine Delivery to Dendritic Cells. J. Immunother. 2007, 30, 378–395. [Google Scholar] [CrossRef]
- Demento, S.L.; Siefert, A.L.; Bandyopadhyay, A.; Sharp, F.A.; Fahmy, T.M. Pathogen-associated molecular patterns on biomaterials: A paradigm for engineering new vaccines. Trends Biotechnol. 2011, 29, 294–306. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; McNeil, S.E. Immunological properties of engineered nanomaterials. Nat. Nanotechnol. 2007, 2, 469–478. [Google Scholar] [CrossRef]
- Huang, W.-C.; Deng, B.; Lin, C.; Carter, K.A.; Geng, J.; Razi, A.; He, X.; Chitgupi, U.; Federizon, J.; Sun, B.; et al. A malaria vaccine adjuvant based on recombinant antigen binding to liposomes. Nat. Nanotechnol. 2018, 13, 1174–1181. [Google Scholar] [CrossRef]
- Miao, L.; Li, L.; Huang, Y.; Delcassian, D.; Chahal, J.; Han, J.; Shi, Y.; Sadtler, K.; Gao, W.; Lin, J.; et al. Delivery of mRNA vaccines with heterocyclic lipids increases anti-tumor efficacy by STING-mediated immune cell activation. Nat. Biotechnol. 2019, 37, 1174–1185. [Google Scholar] [CrossRef]
- Kuai, R.; Ochyl, L.J.; Bahjat, K.S.; Schwendeman, A.; Moon, J.J. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat. Mater. 2017, 16, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Wang, H.; Wang, Z.; Cai, H.; Lu, Z.; Li, Y.; Du, M.; Huang, G.; Wang, C.; Chen, X.; et al. A STING-activating nanovaccine for cancer immunotherapy. Nat. Nanotechnol. 2017, 12, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Tran, K.T.M.; Gavitt, T.D.; Farrell, N.J.; Curry, E.J.; Mara, A.B.; Patel, A.; Brown, L.; Kilpatrick, S.; Piotrowska, R.; Mishra, N.; et al. Transdermal microneedles for the programmable burst release of multiple vaccine payloads. Nat. Biomed. Eng. 2021, 5, 998–1007. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.P.; Koutsonanos, D.G.; del Pilar Martin, M.; Lee, J.W.; Zarnitsyn, V.; Choi, S.-O.; Murthy, N.; Compans, R.W.; Skountzou, I.; Prausnitz, M.R. Dissolving polymer microneedle patches for influenza vaccination. Nat. Med. 2010, 16, 915–920. [Google Scholar] [CrossRef]
- Barbuto, J.A.M.; Ensina, L.F.; Neves, A.R.; Bergami-Santos, P.C.; Leite, K.; Marques, R.; Costa, F.; Martins, S.C.; Camara-Lopes, L.H.; Buzaid, A.C. Dendritic cell?tumor cell hybrid vaccination for metastatic cancer. Cancer Immunol. Immunother. 2004, 53, 1111–1118. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, Q.; Zhao, X.; Zhao, R.; Wang, Y.; Wang, Y.; Liu, J.; Shang, Y.; Zhao, S.; Wu, T.; et al. A DNA nanodevice-based vaccine for cancer immunotherapy. Nat. Mater. 2021, 20, 421–430. [Google Scholar] [CrossRef]
- Hong, X.; Zhong, X.; Du, G.; Hou, Y.; Zhang, Y.; Zhang, Z.; Gong, T.; Zhang, L.; Sun, X. The pore size of mesoporous silica nanoparticles regulates their antigen delivery efficiency. Sci. Adv. 2020, 6, eaaz4462. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, X.; Bian, Y.; Wang, S.; Chai, Q.; Guo, Z.; Wang, Z.; Zhu, P.; Peng, H.; Yan, X.; et al. Dual-targeting nanoparticle vaccine elicits a therapeutic antibody response against chronic hepatitis B. Nat. Nanotechnol. 2020, 15, 406–416. [Google Scholar] [CrossRef]
- Hassan, A.O.; Kafai, N.M.; Dmitriev, I.P.; Fox, J.M.; Smith, B.K.; Harvey, I.B.; Chen, R.E.; Winkler, E.S.; Wessel, A.W.; Case, J.B.; et al. A Single-Dose Intranasal ChAd Vaccine Protects Upper and Lower Respiratory Tracts against SARS-CoV-2. Cell 2020, 183, 169–184.e13. [Google Scholar] [CrossRef]
- Getts, D.R.; Shea, L.D.; Miller, S.D.; King, N.J.C. Harnessing nanoparticles for immune modulation. Trends Immunol. 2015, 36, 419–427. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi-Samani, S.; Taghipour, B. PLGA micro and nanoparticles in delivery of peptides and proteins; problems and approaches. Pharm. Dev. Technol. 2015, 20, 385–393. [Google Scholar] [CrossRef]
- Petros, R.A.; DeSimone, J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef]
- Champion, J.A.; Mitragotri, S. Role of target geometry in phagocytosis. Proc. Natl. Acad. Sci. USA 2006, 103, 4930–4934. [Google Scholar] [CrossRef] [Green Version]
- Gratton, S.E.; Ropp, P.A.; Pohlhaus, P.D.; Luft, J.C.; Madden, V.J.; Napier, M.E.; DeSimone, J.M. The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. USA 2008, 105, 11613–11618. [Google Scholar] [CrossRef] [Green Version]
- Champion, J.A.; Katare, Y.K.; Mitragotri, S. Particle shape: A new design parameter for micro- and nanoscale drug delivery carriers. J. Control. Release 2007, 121, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Geng, Y.; Dalhaimer, P.; Cai, S.; Tsai, R.; Tewari, M.; Minko, T.; Discher, D.E. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat. Nanotechnol. 2007, 2, 249–255. [Google Scholar] [CrossRef]
- Conner, S.D.; Schmid, S.L. Regulated portals of entry into the cell. Nature 2003, 422, 37–44. [Google Scholar] [CrossRef]
- Sinha, R.; Kim, G.J.; Nie, S.; Shin, D.M. Nanotechnology in cancer therapeutics: Bioconjugated nanoparticles for drug delivery. Mol. Cancer Ther. 2006, 5, 1909–1917. [Google Scholar] [CrossRef] [Green Version]
- Kaur, C.D.; Nahar, M.; Jain, N.K. Lymphatic targeting of zidovudine using surface-engineered liposomes. J. Drug Target. 2008, 16, 798–805. [Google Scholar] [CrossRef]
- Jewell, C.M.; López, S.C.B.; Irvine, D.J. In situ engineering of the lymph node microenvironment via intranodal injection of adjuvant-releasing polymer particles. Proc. Natl. Acad. Sci. USA 2011, 108, 15745–15750. [Google Scholar] [CrossRef] [Green Version]
- Mottas, I.; Bekdemir, A.; Cereghetti, A.; Spagnuolo, L.; Yang, Y.-S.S.; Müller, M.; Irvine, D.J.; Stellacci, F.; Bourquin, C. Amphiphilic nanoparticle delivery enhances the anticancer efficacy of a TLR7 ligand via local immune activation. Biomaterials 2019, 190–191, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Gulla, S.K.; Rao, B.R.; Moku, G.; Jinka, S.; Nimmu, N.V.; Khalid, S.; Patra, C.R.; Chaudhuri, A. In vivo targeting of DNA vaccines to dendritic cells using functionalized gold nanoparticles. Biomater. Sci. 2019, 7, 773–788. [Google Scholar] [CrossRef]
- Chu, Y.; Qian, L.; Ke, Y.; Feng, X.; Chen, X.; Liu, F.; Yu, L.; Zhang, L.; Tao, Y.; Xu, R.; et al. Lymph node-targeted neoantigen nanovaccines potentiate anti-tumor immune responses of post-surgical melanoma. J. Nanobiotechnol. 2022, 20, 1–15. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, H.; Jiang, H.; Yu, J.; Wang, Y.; Ke, H.; Gong, T.; Zhang, Z.; Sun, X. Tailoring polymeric hybrid micelles with lymph node targeting ability to improve the potency of cancer vaccines. Biomaterials 2017, 122, 105–113. [Google Scholar] [CrossRef]
- Wang, Y.; Miao, L.; Satterlee, A.; Huang, L. Delivery of oligonucleotides with lipid nanoparticles. Adv. Drug Deliv. Rev. 2015, 87, 68–80. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Yu, Y.; Ma, J.; Zhang, H.; Zhang, H.; Wang, X.; Wang, J.; Zhang, X.; Zhang, Q. LyP-1 Modification to Enhance Delivery of Artemisinin or Fluorescent Probe Loaded Polymeric Micelles to Highly Metastatic Tumor and Its Lymphatics. Mol. Pharm. 2012, 9, 2646–2657. [Google Scholar] [CrossRef]
- Luo, G.; Yu, X.; Jin, C.; Yang, F.; Fu, D.; Long, J.; Xu, J.; Zhan, C.; Lu, W. LyP-1-conjugated nanoparticles for targeting drug delivery to lymphatic metastatic tumors. Int. J. Pharm. 2010, 385, 150–156. [Google Scholar] [CrossRef]
- Ahmed, M.S.U.; Bin Salam, A.; Yates, C.; Willian, K.; Jaynes, J.; Turner, T.; Abdalla, M.O. Double-receptor-targeting multifunctional iron oxide nanoparticles drug delivery system for the treatment and imaging of prostate cancer. Int. J. Nanomed. 2017, 12, 6973–6984. [Google Scholar] [CrossRef] [Green Version]
- Li, A.W.; Sobral, M.C.; Badrinath, S.; Choi, Y.; Graveline, A.; Stafford, A.G.; Weaver, J.C.; Dellacherie, M.O.; Shih, T.-Y.; Ali, O.A.; et al. A facile approach to enhance antigen response for personalized cancer vaccination. Nat. Mater. 2018, 17, 528–534. [Google Scholar] [CrossRef]
- Zhuang, X.; Wu, T.; Zhao, Y.; Hu, X.; Bao, Y.; Guo, Y.; Song, Q.; Li, G.; Tan, S.; Zhang, Z. Lipid-enveloped zinc phosphate hybrid nanoparticles for codelivery of H-2K(b) and H-2D(b)-restricted antigenic peptides and monophosphoryl lipid A to induce antitumor immunity against melanoma. J. Control. Release 2016, 228, 26–37. [Google Scholar] [CrossRef]
- Song, H.; Huang, P.; Niu, J.; Shi, G.; Zhang, C.; Kong, D.; Wang, W. Injectable polypeptide hydrogel for dual-delivery of antigen and TLR3 agonist to modulate dendritic cells in vivo and enhance potent cytotoxic T-lymphocyte response against melanoma. Biomaterials 2018, 159, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, C.S.; Gordo, S.; Schubert, D.A.; Lewin, S.A.; Desai, R.M.; Dobbins, J.; Wucherpfennig, K.W.; Mooney, D.J. Multicomponent Injectable Hydrogels for Antigen-Specific Tolerogenic Immune Modulation. Adv. Healthc. Mater. 2017, 6, 1600773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, G.; Swanson, J.A.; Lee, K.-D. Drug delivery strategy utilizing conjugation via reversible disulfide linkages: Role and site of cellular reducing activities. Adv. Drug Deliv. Rev. 2003, 55, 199–215. [Google Scholar] [CrossRef]
- Daniels, T.R.; Delgado, T.; Helguera, G.; Penichet, M.L. The transferrin receptor part II: Targeted delivery of therapeutic agents into cancer cells. Clin. Immunol. 2006, 121, 159–176. [Google Scholar] [CrossRef]
- Kobayashi, H.; Watanabe, R.; Choyke, P.L. Improving Conventional Enhanced Permeability and Retention (EPR) Effects; What Is the Appropriate Target? Theranostics 2013, 4, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Coates, P.T.H.; Colvin, B.L.; Hackstein, H.; Thomson, A.W. Manipulation of dendritic cells as an approach to improved outcomes in transplantation. Expert Rev. Mol. Med. 2002, 4, 1–21. [Google Scholar] [CrossRef]
- Ehser, S.; Chuang, J.J.; Kleist, C.; Sandra-Petrescu, F.; Iancu, M.; Wang, D.; Opelz, G.; Terness, P. Suppressive dendritic cells as a tool for controlling allograft rejection in organ transplantation: Promises and difficulties. Hum. Immunol. 2008, 69, 165–173. [Google Scholar] [CrossRef]
- Schudel, A.; Francis, D.M.; Thomas, S.N. Material design for lymph node drug delivery. Nat. Rev. Mater. 2019, 4, 415–428. [Google Scholar] [CrossRef]
- Chen, Y.; De Koker, S.; De Geest, B.G. Engineering Strategies for Lymph Node Targeted Immune Activation. Acc. Chem. Res. 2020, 53, 2055–2067. [Google Scholar] [CrossRef]
- Girard, J.-P.; Moussion, C.; Forster, R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat. Rev. Immunol. 2012, 12, 762–773. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Q.; Sun, X. Lymph node targeting strategies to improve vaccination efficacy. J. Control. Release 2017, 267, 47–56. [Google Scholar] [CrossRef]
- Guo, P.; Huang, J.; Moses, M.A. Cancer Nanomedicines in an Evolving Oncology Landscape. Trends Pharmacol. Sci. 2020, 41, 730–742. [Google Scholar] [CrossRef]
- Bangham, A.D.; Horne, R.W. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J. Mol. Biol. 1964, 8, 660–668. [Google Scholar] [CrossRef]
- Zhu, F. Encapsulation and delivery of food ingredients using starch based systems. Food Chem. 2017, 229, 542–552. [Google Scholar] [CrossRef]
- Grimaldi, N.; Andrade, F.; Segovia, N.; Ferrer-Tasies, L.; Sala, S.; Veciana, J.; Ventosa, N. Lipid-based nanovesicles for nanomedicine. Chem. Soc. Rev. 2016, 45, 6520–6545. [Google Scholar] [CrossRef] [Green Version]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New Developments in Liposomal Drug Delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef]
- Bally, M.; Bailey, K.; Sugihara, K.; Grieshaber, D.; Vörös, J.; Städler, B. Liposome and Lipid Bilayer Arrays Towards Biosensing Applications. Small 2010, 6, 2481–2497. [Google Scholar] [CrossRef]
- Pohorille, A.; Deamer, D. Artificial cells: Prospects for biotechnology. Trends Biotechnol. 2002, 20, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Alexander-Bryant, A.A.; Berg-Foels, W.S.V.; Wen, X. Bioengineering Strategies for Designing Targeted Cancer Therapies. Adv. Cancer Res. 2013, 118, 1–59. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Jin, C.; Lv, W.; Ding, Q.; Han, X. Developing a highly stable PLGA-mPEG nanoparticle loaded with cisplatin for chemotherapy of ovarian cancer. PLoS ONE 2011, 6, e25433. [Google Scholar] [CrossRef]
- Lajunen, T.; Viitala, L.; Kontturi, L.-S.; Laaksonen, T.; Liang, H.; Vuorimaa-Laukkanen, E.; Viitala, T.; Le Guével, X.; Yliperttula, M.; Murtomäki, L.; et al. Light induced cytosolic drug delivery from liposomes with gold nanoparticles. J. Control. Release 2015, 203, 85–98. [Google Scholar] [CrossRef]
- Kim, S.K.; Huang, L. Nanoparticle delivery of a peptide targeting EGFR signaling. J. Control. Release 2012, 157, 279–286. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, H.; Akita, H.; Ishiba, R.; Tange, K.; Arai, M.; Kubo, K.; Harashima, H. Neutral biodegradable lipid-envelope-type nanoparticle using vitamin A-Scaffold for nuclear targeting of plasmid DNA. Biomaterials 2014, 35, 1755–1761. [Google Scholar] [CrossRef]
- Thi, E.P.; Mire, C.E.; Lee, A.C.H.; Geisbert, J.B.; Zhou, J.Z.; Agans, K.N.; Snead, N.M.; Deer, D.J.; Barnard, T.R.; Fenton, K.A.; et al. Lipid nanoparticle siRNA treatment of Ebola-virus-Makona-infected nonhuman primates. Nature 2015, 521, 362–365. [Google Scholar] [CrossRef] [Green Version]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Lee, R.J. The role of helper lipids in lipid nanoparticles (LNPs) designed for oligonucleotide delivery. Adv. Drug Deliv. Rev. 2016, 99, 129–137. [Google Scholar] [CrossRef]
- Maeki, M.; Kimura, N.; Sato, Y.; Harashima, H.; Tokeshi, M. Advances in microfluidics for lipid nanoparticles and extracellular vesicles and applications in drug delivery systems. Adv. Drug Deliv. Rev. 2018, 128, 84–100. [Google Scholar] [CrossRef]
- Roces, C.B.; Khadke, S.; Christensen, D.; Perrie, Y. Scale-Independent Microfluidic Production of Cationic Liposomal Adjuvants and Development of Enhanced Lymphatic Targeting Strategies. Mol. Pharm. 2019, 16, 4372–4386. [Google Scholar] [CrossRef] [Green Version]
- Milicic, A.; Kaur, R.; Reyes-Sandoval, A.; Tang, C.-K.; Honeycutt, J.; Perrie, Y.; Hill, A.V.S. Small Cationic DDA:TDB Liposomes as Protein Vaccine Adjuvants Obviate the Need for TLR Agonists in Inducing Cellular and Humoral Responses. PLoS ONE 2012, 7, e34255. [Google Scholar] [CrossRef] [Green Version]
- Forbes, N.; Hussain, M.T.; Briuglia, M.L.; Edwards, D.P.; ter Horst, J.H.; Szita, N.; Perrie, Y. Rapid and scale-independent microfluidic manufacture of liposomes entrapping protein incorporating in-line purification and at-line size monitoring. Int. J. Pharm. 2019, 556, 68–81. [Google Scholar] [CrossRef] [Green Version]
- Chiesa, E.; Dorati, R.; Modena, T.; Conti, B.; Genta, I. Multivariate analysis for the optimization of microfluidics-assisted nanoprecipitation method intended for the loading of small hydrophilic drugs into PLGA nanoparticles. Int. J. Pharm. 2018, 536, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Hussain, M.T.; Roces, C.B.; Anderluzzi, G.; Kastner, E.; Salmaso, S.; Kirby, D.J.; Perrie, Y. Microfluidics based manufacture of liposomes simultaneously entrapping hydrophilic and lipophilic drugs. Int. J. Pharm. 2016, 514, 160–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kastner, E.; Verma, V.; Lowry, D.; Perrie, Y. Microfluidic-controlled manufacture of liposomes for the solubilisation of a poorly water soluble drug. Int. J. Pharm. 2015, 485, 122–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeta, M.; Miura, N.; Tanaka, H.; Nakamura, T.; Kawanishi, R.; Nishikawa, Y.; Asano, K.; Tanaka, M.; Tamagawa, S.; Nakai, Y.; et al. Vitamin E Scaffolds of pH-Responsive Lipid Nanoparticles as DNA Vaccines in Cancer and Protozoan Infection. Mol. Pharm. 2020, 17, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Warashina, S.; Nakamura, T.; Sato, Y.; Fujiwara, Y.; Hyodo, M.; Hatakeyama, H.; Harashima, H. A lipid nanoparticle for the efficient delivery of siRNA to dendritic cells. J. Control. Release 2016, 225, 183–191. [Google Scholar] [CrossRef]
- Hanson, M.C.; Crespo, M.P.; Abraham, W.; Moynihan, K.D.; Szeto, G.L.; Chen, S.H.; Melo, M.B.; Mueller, S.; Irvine, D.J. Nanoparticulate STING agonists are potent lymph node–targeted vaccine adjuvants. J. Clin. Investig. 2015, 125, 2532–2546. [Google Scholar] [CrossRef] [Green Version]
- Sakurai, Y.; Suzuoki, M.; Gomi, M.; Tanaka, H.; Akita, H. Optimization of Sentinel Lymph Node Imaging Methodology Using Anionic Liposome and Hyaluronidase. Pharmaceutics 2021, 13, 1462. [Google Scholar] [CrossRef]
- Wan, D.; Que, H.; Chen, L.; Lan, T.; Hong, W.; He, C.; Yang, J.; Wei, Y.; Wei, X. Lymph-Node-Targeted Cholesterolized TLR7 Agonist Liposomes Provoke a Safe and Durable Antitumor Response. Nano Lett. 2021, 21, 7960–7969. [Google Scholar] [CrossRef]
- Oussoren, C.; Storm, G. Liposomes to target the lymphatics by subcutaneous administration. Adv. Drug Deliv. Rev. 2001, 50, 143–156. [Google Scholar] [CrossRef]
- Khadke, S.; Roces, C.B.; Cameron, A.; Devitt, A.; Perrie, Y. Formulation and manufacturing of lymphatic targeting liposomes using microfluidics. J. Control. Release 2019, 307, 211–220. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Li, S. Polymeric Micelles: Nanocarriers for Cancer-Targeted Drug Delivery. AAPS PharmSciTech 2014, 15, 862–871. [Google Scholar] [CrossRef] [Green Version]
- Yokoyama, M. Polymeric micelles as drug carriers: Their lights and shadows. J. Drug Target. 2014, 22, 576–583. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Gao, X.; Chen, Y.; Liu, T. Nanotechnology in cancer diagnosis: Progress, challenges and opportunities. J. Hematol. Oncol. 2019, 12, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Mao, S. Amphiphilic polymeric micelles as the nanocarrier for peroral delivery of poorly soluble anticancer drugs. Expert Opin. Drug Deliv. 2012, 9, 687–700. [Google Scholar] [CrossRef]
- Davis, M.E.; Chen, Z.G.; Shin, D.M. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008, 7, 771–782. [Google Scholar] [CrossRef]
- Li, X.; Dong, Q.; Yan, Z.; Lu, W.; Feng, L.; Xie, C.; Xie, Z.; Su, B.; Liu, M. MPEG-DSPE polymeric micelle for translymphatic chemotherapy of lymph node metastasis. Int. J. Pharm. 2015, 487, 8–16. [Google Scholar] [CrossRef]
- Widmer, J.; Thauvin, C.; Mottas, I.; Nguyen, V.N.; Delie, F.; Allémann, E.; Bourquin, C. Polymer-based nanoparticles loaded with a TLR7 ligand to target the lymph node for immunostimulation. Int. J. Pharm. 2018, 535, 444–451. [Google Scholar] [CrossRef] [Green Version]
- De Vrieze, J.; Louage, B.; Deswarte, K.; Zhong, Z.; De Coen, R.; Van Herck, S.; Nuhn, L.; Kaas Frich, C.; Zelikin, A.N.; Lienenklaus, S.; et al. Potent Lymphatic Translocation and Spatial Control Over Innate Immune Activation by Polymer-Lipid Amphiphile Conjugates of Small-Molecule TLR7/8 Agonists. Angew. Chem. Int. Ed. Engl. 2019, 58, 15390–15395. [Google Scholar] [CrossRef]
- Doddapaneni, B.S.; Kyryachenko, S.; Chagani, S.E.; Alany, R.G.; Rao, D.A.; Indra, A.K.; Alani, A.W.G. A three-drug nanoscale drug delivery system designed for preferential lymphatic uptake for the treatment of metastatic melanoma. J. Control. Release 2015, 220, 503–514. [Google Scholar] [CrossRef] [Green Version]
- Chida, T.; Miura, Y.; Cabral, H.; Nomoto, T.; Kataoka, K.; Nishiyama, N. Epirubicin-loaded polymeric micelles effectively treat axillary lymph nodes metastasis of breast cancer through selective accumulation and pH-triggered drug release. J. Control. Release 2018, 292, 130–140. [Google Scholar] [CrossRef]
- Eby, J.K.; Dane, K.Y.; O’Neil, C.P.; Hirosue, S.; Swartz, M.A.; Hubbell, J.A. Polymer micelles with pyridyl disulfide-coupled antigen travel through lymphatics and show enhanced cellular responses following immunization. Acta Biomater. 2012, 8, 3210–3217. [Google Scholar] [CrossRef]
- Wang, L.; He, Y.; He, T.; Liu, G.; Lin, C.; Li, K.; Lu, L.; Cai, K. Lymph node-targeted immune-activation mediated by imiquimod-loaded mesoporous polydopamine based-nanocarriers. Biomaterials 2020, 255, 120208. [Google Scholar] [CrossRef]
- Dane, K.Y.; Nembrini, C.; Tomei, A.A.; Eby, J.K.; O’Neil, C.P.; Velluto, D.; Swartz, M.A.; Inverardi, L.; Hubbell, J.A. Nano-sized drug-loaded micelles deliver payload to lymph node immune cells and prolong allograft survival. J. Control. Release 2011, 156, 154–160. [Google Scholar] [CrossRef]
- Thomas, S.N.; Vokali, E.; Lund, A.W.; Hubbell, J.A.; Swartz, M.A. Targeting the tumor-draining lymph node with adjuvanted nanoparticles reshapes the anti-tumor immune response. Biomaterials 2014, 35, 814–824. [Google Scholar] [CrossRef] [Green Version]
- Van Herck, S.; Deswarte, K.; Nuhn, L.; Zhong, Z.; Portela Catani, J.P.; Li, Y.; Sanders, N.N.; Lienenklaus, S.; De Koker, S.; Lambrecht, B.N.; et al. Lymph-Node-Targeted Immune Activation by Engineered Block Copolymer Amphiphiles-TLR7/8 Agonist Conjugates. J. Am. Chem Soc. 2018, 140, 14300–14307. [Google Scholar] [CrossRef]
- Reddy, S.T.; van der Vlies, A.J.; Simeoni, E.; Angeli, V.; Randolph, G.J.; O’Neil, C.P.; Lee, L.K.; Swartz, M.A.; Hubbell, J.A. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat. Biotechnol. 2007, 25, 1159–1164. [Google Scholar] [CrossRef]
- Rehor, A.; Hubbell, J.A.; Tirelli, N. Oxidation-Sensitive Polymeric Nanoparticles. Langmuir 2005, 21, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Cabral, H.; Makino, J.; Matsumoto, Y.; Mi, P.; Wu, H.; Nomoto, T.; Toh, K.; Yamada, N.; Higuchi, Y.; Konishi, S.; et al. Systemic Targeting of Lymph Node Metastasis through the Blood Vascular System by Using Size-Controlled Nanocarriers. ACS Nano 2015, 9, 4957–4967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Li, Y.; Wang, X.; Hou, Y.; Hong, X.; Gong, T.; Zhang, Z.; Sun, X. Rational design of Polymeric Hybrid Micelles to Overcome Lymphatic and Intracellular Delivery Barriers in Cancer Immunotherapy. Theranostics 2017, 7, 4383–4398. [Google Scholar] [CrossRef] [PubMed]
- Jeanbart, L.; Ballester, M.; de Titta, A.; Corthésy, P.; Romero, P.; Hubbell, J.A.; Swartz, M.A. Enhancing Efficacy of Anticancer Vaccines by Targeted Delivery to Tumor-Draining Lymph Nodes. Cancer Immunol. Res. 2014, 2, 436–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Riley, R.S.; Day, E.S. Gold nanoparticle-mediated photothermal therapy: Applications and opportunities for multimodal cancer treatment. WIREs Nanomed. Nanobiotechnol. 2017, 9, e1449. [Google Scholar] [CrossRef]
- Wang, J.; Potocny, A.M.; Rosenthal, J.; Day, E.S. Gold Nanoshell-Linear Tetrapyrrole Conjugates for Near Infrared-Activated Dual Photodynamic and Photothermal Therapies. ACS Omega 2020, 5, 926–940. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, L.; Huang, X.; Niu, G.; Chen, X. Differentiation of Reactive and Tumor Metastatic Lymph Nodes with Diffusion-weighted and SPIO-Enhanced MRI. Mol. Imaging Biol. 2013, 15, 40–47. [Google Scholar] [CrossRef]
- Liang, C.; Diao, S.; Wang, C.; Gong, H.; Liu, T.; Hong, G.; Shi, X.; Dai, H.; Liu, Z. Tumor Metastasis Inhibition by Imaging-Guided Photothermal Therapy with Single-Walled Carbon Nanotubes. Adv. Mater. 2014, 26, 5646–5652. [Google Scholar] [CrossRef]
- Oladipo, A.O.; Oluwafemi, O.S.; Songca, S.P.; Sukhbaatar, A.; Mori, S.; Okajima, J.; Komiya, A.; Maruyama, S.; Kodama, T. A novel treatment for metastatic lymph nodes using lymphatic delivery and photothermal therapy. Sci. Rep. 2017, 7, 45459. [Google Scholar] [CrossRef]
- Oberdörster, G. Significance of particle parameters in the evaluation of exposure-dose response relationships of inhaled particles. Inhal. Toxicol. 1996, 8, 73–89. [Google Scholar] [CrossRef]
- Mohammadpour, R.; Dobrovolskaia, M.A.; Cheney, D.L.; Greish, K.F.; Ghandehari, H. Subchronic and chronic toxicity evaluation of inorganic nanoparticles for delivery applications. Adv. Drug Deliv. Rev. 2019, 144, 112–132. [Google Scholar] [CrossRef]
- Gao, W.; Fang, R.H.; Thamphiwatana, S.; Luk, B.T.; Li, J.; Angsantikul, P.; Zhang, Q.; Hu, C.-M.J.; Zhang, L. Modulating Antibacterial Immunity via Bacterial Membrane-Coated Nanoparticles. Nano Lett. 2015, 15, 1403–1409. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.-M.J.; Fang, R.H.; Luk, B.T.; Zhang, L. Nanoparticle-detained toxins for safe and effective vaccination. Nat. Nanotechnol. 2013, 8, 933–938. [Google Scholar] [CrossRef] [Green Version]
- Dehaini, D.; Wei, X.; Fang, R.H.; Masson, S.; Angsantikul, P.; Luk, B.T.; Zhang, Y.; Ying, M.; Jiang, Y.; Kroll, A.V.; et al. Erythrocyte-Platelet Hybrid Membrane Coating for Enhanced Nanoparticle Functionalization. Adv. Mater. 2017, 29, 1606209. [Google Scholar] [CrossRef] [Green Version]
- Fang, R.H.; Hu, C.-M.J.; Luk, B.T.; Gao, W.; Copp, J.A.; Tai, Y.; O’Connor, D.E.; Zhang, L. Cancer Cell Membrane-Coated Nanoparticles for Anticancer Vaccination and Drug Delivery. Nano Lett. 2014, 14, 2181–2188. [Google Scholar] [CrossRef]
- Hu, C.-M.J.; Fang, R.H.; Wang, K.-C.; Luk, B.T.; Thamphiwatana, S.; Dehaini, D.; Nguyen, P.; Angsantikul, P.; Wen, C.H.; Kroll, A.V.; et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature 2015, 526, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.P.M.; Lin, A.Y.; Figueroa, E.R.; Foster, A.E.; Drezek, R.A. In vivo Gold Nanoparticle Delivery of Peptide Vaccine Induces Anti-Tumor Immune Response in Prophylactic and Therapeutic Tumor Models. Small 2015, 11, 1453–1459. [Google Scholar] [CrossRef] [Green Version]
- Frey, N.A.; Peng, S.; Cheng, K.; Sun, S. Magnetic nanoparticles: Synthesis, functionalization, and applications in bioimaging and magnetic energy storage. Chem. Soc. Rev. 2009, 38, 2532–2542. [Google Scholar] [CrossRef]
- Jun, Y.-W.; Choi, J.-S.; Cheon, J. Heterostructured magnetic nanoparticles: Their versatility and high performance capabilities. Chem. Commun. 2007, 12, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Ambashta, R.D.; Sillanpää, M. Water purification using magnetic assistance: A review. J. Hazard. Mater. 2010, 180, 38–49. [Google Scholar] [CrossRef]
- Vangijzegem, T.; Stanicki, D.; Laurent, S. Magnetic iron oxide nanoparticles for drug delivery: Applications and characteristics. Expert Opin. Drug Deliv. 2018, 16, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Estelrich, J.; Escribano, E.; Queralt, J.; Busquets, M.A. Iron Oxide Nanoparticles for Magnetically-Guided and Magnetically-Responsive Drug Delivery. Int. J. Mol. Sci. 2015, 16, 8070–8101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.-S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16, 023501. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Misra, R.; Sahoo, S.K. Nanoparticles: A boon to drug delivery, therapeutics, diagnostics and imaging. Nanomedicine 2012, 8, 147–166. [Google Scholar] [CrossRef]
- Zaloga, J.; Janko, C.; Nowak, J.; Matuszak, J.; Knaup, S.; Eberbeck, D.; Tietze, R.; Unterweger, H.; Friedrich, R.P.; Heimke-Brinck, R.; et al. Development of a lauric acid/albumin hybrid iron oxide nanoparticle system with improved biocompatibility. Int. J. Nanomed. 2014, 9, 4847–4866. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-M.; Jeong, H.-J.; Kim, E.-M.; Kim, D.W.; Lim, S.T.; Kim, H.T.; Park, I.-K.; Jeong, Y.Y.; Kim, J.W.; Sohn, M.-H. Superparamagnetic iron oxide nanoparticles as a dual imaging probe for targeting hepatocytes in vivo. Magn. Reson. Med. 2009, 62, 1440–1446. [Google Scholar] [CrossRef]
- Chen, T.-J.; Cheng, T.-H.; Chen, C.-Y.; Hsu, S.C.N.; Cheng, T.-L.; Liu, G.-C.; Wang, Y.-M. Targeted Herceptin-dextran iron oxide nanoparticles for noninvasive imaging of HER2/neu receptors using MRI. JBCI J. Biol. Inorgan. Chem. 2009, 14, 253–260. [Google Scholar] [CrossRef]
- Kjellman, P.; in ’t Zandt, R.; Fredriksson, S.; Strand, S.E. Optimizing retention of multimodal imaging nanostructures in sentinel lymph nodes by nanoscale size tailoring. Nanomedicine 2014, 10, 1089–1095. [Google Scholar] [CrossRef]
- Gautier, J.; Munnier, E.; Paillard, A.; Hervé, K.; Douziech-Eyrolles, L.; Soucé, M.; Dubois, P.; Chourpa, I. A pharmaceutical study of doxorubicin-loaded PEGylated nanoparticles for magnetic drug targeting. Int. J. Pharm. 2012, 423, 16–25. [Google Scholar] [CrossRef]
- Zou, Y.; Liu, P.; Liu, C.-H.; Zhi, X.-T. Doxorubicin-loaded mesoporous magnetic nanoparticles to induce apoptosis in breast cancer cells. Biomed. Pharmacother. 2015, 69, 355–360. [Google Scholar] [CrossRef]
- Quinto, C.A.; Mohindra, P.; Tong, S.; Bao, G. Multifunctional superparamagnetic iron oxide nanoparticles for combined chemotherapy and hyperthermia cancer treatment. Nanoscale 2015, 7, 12728–12736. [Google Scholar] [CrossRef] [Green Version]
- Cha, B.G.; Jeong, J.H.; Kim, J. Extra-Large Pore Mesoporous Silica Nanoparticles Enabling Co-Delivery of High Amounts of Protein Antigen and Toll-like Receptor 9 Agonist for Enhanced Cancer Vaccine Efficacy. ACS Central Sci. 2018, 4, 484–492. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Yang, Y.; Gu, Z.; Zhang, J.; Song, H.; Xiang, G.; Yu, C. Glutathione-depletion mesoporous organosilica nanoparticles as a self-adjuvant and Co-delivery platform for enhanced cancer immunotherapy. Biomaterials 2018, 175, 82–92. [Google Scholar] [CrossRef]
- Ajima, K.; Murakami, T.; Mizoguchi, Y.; Tsuchida, K.; Ichihashi, T.; Iijima, S.; Yudasaka, M. Enhancement of In Vivo Anticancer Effects of Cisplatin by Incorporation Inside Single-Wall Carbon Nanohorns. ACS Nano 2008, 2, 2057–2064. [Google Scholar] [CrossRef]
- Ali-Boucetta, H.; Al-Jamal, K.T.; McCarthy, D.; Prato, M.; Bianco, A.; Kostarelos, K. Multiwalled carbon nanotube–doxorubicin supramolecular complexes for cancer therapeutics. Chem. Commun. 2008, 4, 459–461. [Google Scholar] [CrossRef]
- Hampel, S.; Kunze, D.; Haase, D.; Krämer, K.; Rauschenbach, M.; Ritschel, M.; Leonhardt, A.; Thomas, J.; Oswald, S.; Hoffmann, V.; et al. Carbon nanotubes filled with a chemotherapeutic agent: A nanocarrier mediates inhibition of tumor cell growth. Nanomedicine 2008, 3, 175–182. [Google Scholar] [CrossRef]
- Xu, C.; Hong, H.; Lee, Y.; Park, K.S.; Sun, M.; Wang, T.; Aikins, M.E.; Xu, Y.; Moon, J.J. Efficient Lymph Node-Targeted Delivery of Personalized Cancer Vaccines with Reactive Oxygen Species-Inducing Reduced Graphene Oxide Nanosheets. ACS Nano 2020, 14, 13268–13278. [Google Scholar] [CrossRef]
- Yang, F.; Jin, C.; Yang, D.; Jiang, Y.; Li, J.; Di, Y.; Hu, J.; Wang, C.; Ni, Q.; Fu, D. Magnetic functionalised carbon nanotubes as drug vehicles for cancer lymph node metastasis treatment. Eur. J. Cancer 2011, 47, 1873–1882. [Google Scholar] [CrossRef]
- Liu, D.; Poon, C.; Lu, K.; He, C.; Lin, W. Self-assembled nanoscale coordination polymers with trigger release properties for effective anticancer therapy. Nat. Commun. 2014, 5, 4182. [Google Scholar] [CrossRef]
- He, C.; Duan, X.; Guo, N.; Chan, C.; Poon, C.; Weichselbaum, R.R.; Lin, W. Core-shell nanoscale coordination polymers combine chemotherapy and photodynamic therapy to potentiate checkpoint blockade cancer immunotherapy. Nat. Commun. 2016, 7, 12499. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Li, Y.; Jiao, J.; Hu, H.-M. Alpha-alumina nanoparticles induce efficient autophagy-dependent cross-presentation and potent antitumour response. Nat. Nanotechnol. 2011, 6, 645–650. [Google Scholar] [CrossRef]
- Bocanegra Gondan, A.I.; Ruiz-de-Angulo, A.; Zabaleta, A.; Gomez Blanco, N.; Cobaleda-Siles, B.M.; Garcia-Granda, M.J.; Padro, D.; Llop, J.; Arnaiz, B.; Gato, M.; et al. Effective cancer immunotherapy in mice by polyIC-imiquimod complexes and engineered magnetic nanoparticles. Biomaterials 2018, 170, 95–115. [Google Scholar] [CrossRef] [Green Version]
- Das, V.; Bruzzese, F.; Konečný, P.; Iannelli, F.; Budillon, A.; Hajdúch, M. Pathophysiologically relevant in vitro tumor models for drug screening. Drug Discov. Today 2015, 20, 848–855. [Google Scholar] [CrossRef]
- Norouzi, M.; Shabani, I.; Ahvaz, H.H.; Soleimani, M. PLGA/gelatin hybrid nanofibrous scaffolds encapsulating EGF for skin regeneration. J. Biomed. Mater. Res. Part A 2015, 103, 2225–2235. [Google Scholar] [CrossRef] [PubMed]
- Mirdailami, O.; Soleimani, M.; Dinarvand, R.; Khoshayand, M.R.; Norouzi, M.; Hajarizadeh, A.; Dodel, M.; Atyabi, F. Controlled release of rhEGF and rhbFGF from electrospun scaffolds for skin regeneration. J. Biomed. Mater. Res. Part A 2015, 103, 3374–3385. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, M.; Boroujeni, S.M.; Omidvarkordshouli, N.; Soleimani, M. Advances in Skin Regeneration: Application of Electrospun Scaffolds. Adv. Healthc. Mater. 2015, 4, 1114–1133. [Google Scholar] [CrossRef] [PubMed]
- Dimatteo, R.; Darling, N.J.; Segura, T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Deliv. Rev. 2018, 127, 167–184. [Google Scholar] [CrossRef]
- Li, Y.; Rodrigues, J.M.; Tomás, H. Injectable and biodegradable hydrogels: Gelation, biodegradation and biomedical applications. Chem. Soc. Rev. 2012, 41, 2193–2221. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Lee, D.S. Injectable Biodegradable Hydrogels. Macromol. Biosci. 2010, 10, 563–579. [Google Scholar] [CrossRef]
- Xiong, L.; Luo, Q.; Wang, Y.; Li, X.; Shen, Z.; Zhu, W. An injectable drug-loaded hydrogel based on a supramolecular polymeric prodrug. Chem. Commun. 2015, 51, 14644–14647. [Google Scholar] [CrossRef]
- Wu, X.; He, C.; Wu, Y.; Chen, X. Synergistic therapeutic effects of Schiff’s base cross-linked injectable hydrogels for local co-delivery of metformin and 5-fluorouracil in a mouse colon carcinoma model. Biomaterials 2016, 75, 148–162. [Google Scholar] [CrossRef]
- Norouzi, M.; Nazari, B.; Miller, D.W. Injectable hydrogel-based drug delivery systems for local cancer therapy. Drug Discov. Today 2016, 21, 1835–1849. [Google Scholar] [CrossRef]
- Muraoka, D.; Harada, N.; Hayashi, T.; Tahara, Y.; Momose, F.; Sawada, S.-I.; Mukai, S.-A.; Akiyoshi, K.; Shiku, H. Nanogel-Based Immunologically Stealth Vaccine Targets Macrophages in the Medulla of Lymph Node and Induces Potent Antitumor Immunity. ACS Nano 2014, 8, 9209–9218. [Google Scholar] [CrossRef]
- Abellan-Pose, R.; Teijeiro-Valiño, C.; Santander-Ortega, M.J.; Borrajo, E.; Vidal, A.; Garcia-Fuentes, M.; Csaba, N.; Alonso, M.J. Polyaminoacid nanocapsules for drug delivery to the lymphatic system: Effect of the particle size. Int. J. Pharm. 2016, 509, 107–117. [Google Scholar] [CrossRef]
- Wauthoz, N.; Bastiat, G.; Moysan, E.; Cieślak, A.; Kondo, K.; Zandecki, M.; Moal, V.; Rousselet, M.-C.; Hureaux, J.; Benoit, J.-P. Safe lipid nanocapsule-based gel technology to target lymph nodes and combat mediastinal metastases from an orthotopic non-small-cell lung cancer model in SCID-CB17 mice. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1237–1245. [Google Scholar] [CrossRef]
- Nuhn, L.; Vanparijs, N.; De Beuckelaer, A.; Lybaert, L.; Verstraete, G.; Deswarte, K.; Lienenklaus, S.; Shukla, N.M.; Salyer, A.C.; Lambrecht, B.N.; et al. pH-degradable imidazoquinoline-ligated nanogels for lymph node-focused immune activation. Proc. Natl. Acad. Sci. USA 2016, 113, 8098–8103. [Google Scholar] [CrossRef] [Green Version]
- De Koker, S.; Cui, J.; Vanparijs, N.; Albertazzi, L.; Grooten, J.; Caruso, F.; De Geest, B.G. Engineering Polymer Hydrogel Nanoparticles for Lymph Node-Targeted Delivery. Angew. Chem. Int. Ed. Engl. 2016, 55, 1334–1339. [Google Scholar] [CrossRef] [Green Version]
- Tőke, E.R.; Lőrincz, O.; Csiszovszki, Z.; Somogyi, E.; Felföldi, G.; Molnár, L.; Szipőcs, R.; Kolonics, A.; Malissen, B.; Lori, F.; et al. Exploitation of Langerhans cells for in vivo DNA vaccine delivery into the lymph nodes. Gene Ther. 2014, 21, 566–574. [Google Scholar] [CrossRef]
- Vicente, S.; Goins, B.A.; Sanchez, A.; Alonso, M.J.; Phillips, W.T. Biodistribution and lymph node retention of polysaccharide-based immunostimulating nanocapsules. Vaccine 2014, 32, 1685–1692. [Google Scholar] [CrossRef]
- Li, A.V.; Moon, J.J.; Abraham, W.; Suh, H.; Elkhader, J.; Seidman, M.A.; Yen, M.; Im, E.-J.; Foley, M.H.; Barouch, D.H.; et al. Generation of Effector Memory T Cell–Based Mucosal and Systemic Immunity with Pulmonary Nanoparticle Vaccination. Sci. Transl. Med. 2013, 5, 204ra130. [Google Scholar] [CrossRef] [Green Version]
- Attili-Qadri, S.; Karra, N.; Nemirovski, A.; Schwob, O.; Talmon, Y.; Nassar, T.; Benita, S. Oral delivery system prolongs blood circulation of docetaxel nanocapsules via lymphatic absorption. Proc. Natl. Acad. Sci. USA 2013, 110, 17498–17503. [Google Scholar] [CrossRef] [Green Version]
- Vlassov, A.V.; Magdaleno, S.; Setterquist, R.; Conrad, R. Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta BBA Gen. Subj. 2012, 1820, 940–948. [Google Scholar] [CrossRef]
- El Andaloussi, S.; Mäger, I.; Breakefield, X.O.; Wood, M.J.A. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 2013, 12, 347–357. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Gudbergsson, J.M.; Skov, M.N.; Pilgaard, L.; Moos, T.; Duroux, M. A comprehensive overview of exosomes as drug delivery vehicles—Endogenous nanocarriers for targeted cancer therapy. Biochim. Biophys. Acta 2014, 1846, 75–87. [Google Scholar] [CrossRef]
- Munoz, J.L.; Bliss, S.A.; Greco, S.J.; Ramkissoon, S.H.; Ligon, K.L.; Rameshwar, P. Delivery of Functional Anti-miR-9 by Mesenchymal Stem Cell-derived Exosomes to Glioblastoma Multiforme Cells Conferred Chemosensitivity. Mol. Ther. Nucleic Acids 2013, 2, e126. [Google Scholar] [CrossRef]
- Ohno, S.-I.; Takanashi, M.; Sudo, K.; Ueda, S.; Ishikawa, A.; Matsuyama, N.; Fujita, K.; Mizutani, T.; Ohgi, T.; Ochiya, T.; et al. Systemically Injected Exosomes Targeted to EGFR Deliver Antitumor MicroRNA to Breast Cancer Cells. Mol. Ther. 2013, 21, 185–191. [Google Scholar] [CrossRef] [Green Version]
- Xin, H.; Li, Y.; Buller, B.; Katakowski, M.; Zhang, Y.; Wang, X.; Shang, X.; Zhang, Z.G.; Chopp, M. Exosome-Mediated Transfer of miR-133b from Multipotent Mesenchymal Stromal Cells to Neural Cells Contributes to Neurite Outgrowth. Stem Cells 2012, 30, 1556–1564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Q.; Ramakrishnaiah, V.; Henry, S.; Fouraschen, S.; de Ruiter, P.E.; Kwekkeboom, J.; Tilanus, H.W.; Janssen, H.L.A.; van der Laan, L.J.W. Hepatic cell-to-cell transmission of small silencing RNA can extend the therapeutic reach of RNA interference (RNAi). Gut 2012, 61, 1330–1339. [Google Scholar] [CrossRef]
- Kosaka, N.; Iguchi, H.; Yoshioka, Y.; Hagiwara, K.; Takeshita, F.; Ochiya, T. Competitive Interactions of Cancer Cells and Normal Cells via Secretory MicroRNAs. J. Biol. Chem. 2012, 287, 1397–1405. [Google Scholar] [CrossRef] [Green Version]
- Katakowski, M.; Buller, B.; Zheng, X.; Lu, Y.; Rogers, T.; Osobamiro, O.; Shu, W.; Jiang, F.; Chopp, M. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 2013, 335, 201–204. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liu, D.; Chen, X.; Li, J.; Li, L.; Bian, Z.; Sun, F.; Lu, J.; Yin, Y.; Cai, X.; et al. Secreted Monocytic miR-150 Enhances Targeted Endothelial Cell Migration. Mol. Cell 2010, 39, 133–144. [Google Scholar] [CrossRef] [Green Version]
- Bryniarski, K.; Ptak, W.; Jayakumar, A.; Püllmann, K.; Caplan, M.J.; Chairoungdua, A.; Lu, J.; Adams, B.D.; Sikora, E.; Nazimek, K.; et al. Antigen-specific, antibody-coated, exosome-like nanovesicles deliver suppressor T-cell microRNA-150 to effector T cells to inhibit contact sensitivity. J. Allergy Clin. Immunol. 2013, 132, 170–181.e9. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Charrier, A.; Zhou, Y.; Chen, R.; Yu, B.; Agarwal, K.; Tsukamoto, H.; Lee, L.J.; Paulaitis, M.E.; Brigstock, D.R. Epigenetic regulation of connective tissue growth factor by MicroRNA-214 delivery in exosomes from mouse or human hepatic stellate cells. Hepatology 2014, 59, 1118–1129. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Wahlgren, J.; Karlson, T.D.L.; Brisslert, M.; Vaziri Sani, F.; Telemo, E.; Sunnerhagen, P.; Valadi, H. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res. 2012, 40, e130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shtam, T.A.; Kovalev, R.A.; Varfolomeeva, E.Y.; Makarov, E.M.; Kil, Y.V.; Filatov, M.V. Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Commun. Signal. 2013, 11, 88. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Zhuang, X.; Xiang, X.; Liu, Y.; Zhang, S.; Liu, C.; Barnes, S.; Grizzle, W.; Miller, D.; Zhang, H.-G. A Novel Nanoparticle Drug Delivery System: The Anti-inflammatory Activity of Curcumin Is Enhanced When Encapsulated in Exosomes. Mol. Ther. 2010, 18, 1606–1614. [Google Scholar] [CrossRef]
- Zhuang, X.; Xiang, X.; Grizzle, W.; Sun, D.; Zhang, S.; Axtell, R.C.; Ju, S.; Mu, J.; Zhang, L.; Steinman, L.; et al. Treatment of Brain Inflammatory Diseases by Delivering Exosome Encapsulated Anti-inflammatory Drugs From the Nasal Region to the Brain. Mol. Ther. 2011, 19, 1769–1779. [Google Scholar] [CrossRef]
- Maguire, C.A.; Balaj, L.; Sivaraman, S.; Crommentuijn, M.H.; Ericsson, M.; Mincheva-Nilsson, L.; Baranov, V.; Gianni, D.; Tannous, B.A.; Sena-Esteves, M.; et al. Microvesicle-associated AAV Vector as a Novel Gene Delivery System. Mol. Ther. 2012, 20, 960–971. [Google Scholar] [CrossRef] [Green Version]
- Mizrak, A.; Bolukbasi, M.F.; Ozdener, G.B.; Brenner, G.J.; Madlener, S.; Erkan, E.P.; Ströbel, T.; Breakefield, X.O.; Saydam, O. Genetically Engineered Microvesicles Carrying Suicide mRNA/Protein Inhibit Schwannoma Tumor Growth. Mol. Ther. 2013, 21, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Hood, J.L.; Scott, M.J.; Wickline, S.A. Maximizing exosome colloidal stability following electroporation. Anal. Biochem. 2014, 448, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.C.; Kim, O.Y.; Yoon, C.M.; Choi, D.-S.; Roh, T.-Y.; Park, J.; Nilsson, J.; Lötvall, J.; Kim, Y.-K.; Gho, Y.S. Bioinspired Exosome-Mimetic Nanovesicles for Targeted Delivery of Chemotherapeutics to Malignant Tumors. ACS Nano 2013, 7, 7698–7710. [Google Scholar] [CrossRef]
- Tian, Y.; Li, S.; Song, J.; Ji, T.; Zhu, M.; Anderson, G.J.; Wei, J.; Nie, G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 2014, 35, 2383–2390. [Google Scholar] [CrossRef]
- Sato, Y.; Hashiba, K.; Sasaki, K.; Maeki, M.; Tokeshi, M.; Harashima, H. Understanding structure-activity relationships of pH-sensitive cationic lipids facilitates the rational identification of promising lipid nanoparticles for delivering siRNAs in vivo. J. Control. Release 2019, 295, 140–152. [Google Scholar] [CrossRef]
- Lori, F.; Calarota, S.; Lisziewicz, J. Nanochemistry-based immunotherapy for HIV-1. Curr. Med. Chem. 2007, 14, 1911–1919. [Google Scholar] [CrossRef]
- Leal, L.; Guardo, A.C.; Morón-López, S.; Salgado, M.; Mothe, B.; Heirman, C.; Pannus, P.; Vanham, G.; van den Ham, H.J.; Gruters, R.; et al. Phase I clinical trial of an intranodally administered mRNA-based therapeutic vaccine against HIV-1 infection. Aids 2018, 32, 2533–2545. [Google Scholar] [CrossRef] [Green Version]
- Gong, N.; Sheppard, N.C.; Billingsley, M.M.; June, C.H.; Mitchell, M.J. Nanomaterials for T-cell cancer immunotherapy. Nat. Nanotechnol. 2021, 16, 25–36. [Google Scholar] [CrossRef]
- Smith, T.T.; Stephan, S.B.; Moffett, H.F.; McKnight, L.E.; Ji, W.; Reiman, D.; Bonagofski, E.; Wohlfahrt, M.E.; Pillai, S.P.S.; Stephan, M.T. In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nat. Nanotechnol. 2017, 12, 813–820. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Stephan, M.T.; Gai, S.A.; Abraham, W.; Shearer, A.; Irvine, D.J. In vivo targeting of adoptively transferred T-cells with antibody- and cytokine-conjugated liposomes. J. Control. Release 2013, 172, 426–435. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Tang, L.; Mabardi, L.; Kumari, S.; Irvine, D.J. Enhancing Adoptive Cell Therapy of Cancer through Targeted Delivery of Small-Molecule Immunomodulators to Internalizing or Noninternalizing Receptors. ACS Nano 2017, 11, 3089–3100. [Google Scholar] [CrossRef] [Green Version]
- Schmid, D.; Park, C.G.; Hartl, C.A.; Subedi, N.; Cartwright, A.N.; Puerto, R.B.; Zheng, Y.; Maiarana, J.; Freeman, G.J.; Wucherpfennig, K.W.; et al. T cell-targeting nanoparticles focus delivery of immunotherapy to improve antitumor immunity. Nat. Commun. 2017, 8, 1747. [Google Scholar] [CrossRef]
- Ou, W.; Thapa, R.K.; Jiang, L.; Soe, Z.C.; Gautam, M.; Chang, J.-H.; Jeong, J.-H.; Ku, S.K.; Choi, H.-G.; Yong, C.S.; et al. Regulatory T cell-targeted hybrid nanoparticles combined with immuno-checkpoint blockage for cancer immunotherapy. J. Control. Release 2018, 281, 84–96. [Google Scholar] [CrossRef]
- Kosmides, A.K.; Sidhom, J.-W.; Fraser, A.; Bessell, C.A.; Schneck, J.P. Dual Targeting Nanoparticle Stimulates the Immune System To Inhibit Tumor Growth. ACS Nano 2017, 11, 5417–5429. [Google Scholar] [CrossRef]
- Park, J.; Wrzesinski, S.H.; Stern, E.; Look, M.; Criscione, J.M.; Ragheb, R.; Jay, S.M.; Demento, S.L.; Agawu, A.; Limon, P.L.; et al. Combination delivery of TGF-β inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nat. Mater. 2012, 11, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Stephan, S.B.; Taber, A.M.; Jileaeva, I.; Pegues, E.P.; Sentman, C.L.; Stephan, M.T. Biopolymer implants enhance the efficacy of adoptive T-cell therapy. Nat. Biotechnol. 2015, 33, 97–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coon, M.E.; Stephan, S.B.; Gupta, V.; Kealey, C.P.; Stephan, M.T. Nitinol thin films functionalized with CAR-T cells for the treatment of solid tumours. Nat. Biomed. Eng. 2020, 4, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Unsoy, G.; Gunduz, U. Smart Drug Delivery Systems in Cancer Therapy. Curr. Drug Targets 2018, 19, 202–212. [Google Scholar] [CrossRef]
- Park, K. Controlled drug delivery systems: Past forward and future back. J. Control. Release 2014, 190, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Navya, P.N.; Kaphle, A.; Srinivas, S.P.; Bhargava, S.K.; Rotello, V.M.; Daima, H.K. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019, 6, 1–30. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, S.M.; Veeranarayanan, S.; Maekawa, T.; Kumar, S.D. External stimulus responsive inorganic nanomaterials for cancer theranostics. Adv. Drug Deliv. Rev. 2019, 138, 18–40. [Google Scholar] [CrossRef]
- Ding, C.; Tong, L.; Feng, J.; Fu, J. Recent Advances in Stimuli-Responsive Release Function Drug Delivery Systems for Tumor Treatment. Molecules 2016, 21, 1715. [Google Scholar] [CrossRef]
- Lafuente-Gómez, N.; Latorre, A.; Milán-Rois, P.; Rodriguez Diaz, C.; Somoza, Á. Stimuli-responsive nanomaterials for cancer treatment: Boundaries, opportunities and applications. Chem. Commun. 2021, 57, 13662–13677. [Google Scholar] [CrossRef]
- Kanamala, M.; Wilson, W.R.; Yang, M.; Palmer, B.D.; Wu, Z. Mechanisms and biomaterials in pH-responsive tumour targeted drug delivery: A review. Biomaterials 2016, 85, 152–167. [Google Scholar] [CrossRef]
- Li, X.; Yu, X.; Dai, D.; Song, X.; Xu, W. The altered glucose metabolism in tumor and a tumor acidic microenvironment associated with extracellular matrix metalloproteinase inducer and monocarboxylate transporters. Oncotarget 2016, 7, 23141–23155. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Kuang, Y.; Lv, R.; Yang, P.; Li, C.; Bi, H.; Liu, B.; Yang, D.; Dai, Y.; Gai, S.; et al. Charge convertibility and near infrared photon co-enhanced cisplatin chemotherapy based on upconversion nanoplatform. Biomaterials 2017, 130, 42–55. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, F.; Wang, Y.; Wang, J.; Yu, Y.; Guo, S.; Chen, R.; Zhou, D. pH and near-infrared light dual-stimuli responsive drug delivery using DNA-conjugated gold nanorods for effective treatment of multidrug resistant cancer cells. J. Control. Release 2016, 232, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Shen, T.; Zhou, S.; Wang, W.; Lin, S.; Zhu, G. pH-Responsive STING-Activating DNA Nanovaccines for Cancer Immunotherapy. Adv. Ther. 2020, 3, 2000083. [Google Scholar] [CrossRef]
- Li, H.J.; Du, J.Z.; Liu, J.; Du, X.J.; Shen, S.; Zhu, Y.H.; Wang, X.; Ye, X.; Nie, S.; Wang, J. Smart Superstructures with Ultrahigh pH-Sensitivity for Targeting Acidic Tumor Microenvironment: Instantaneous Size Switching and Improved Tumor Penetration. ACS Nano 2016, 10, 6753–6761. [Google Scholar] [CrossRef]
- Torchilin, V.P. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat. Rev. Drug Discov. 2014, 13, 813–827. [Google Scholar] [CrossRef] [Green Version]
- Wolski, P.; Nieszporek, K.; Panczyk, T. Cytosine-Rich DNA Fragments Covalently Bound to Carbon Nanotube as Factors Triggering Doxorubicin Release at Acidic pH. A Molecular Dynamics Study. Int. J. Mol. Sci. 2021, 22, 68466. [Google Scholar] [CrossRef]
- Wu, W.; Luo, L.; Wang, Y.; Wu, Q.; Dai, H.B.; Li, J.S.; Durkan, C.; Wang, N.; Wang, G.X. Endogenous pH-responsive nanoparticles with programmable size changes for targeted tumor therapy and imaging applications. Theranostics 2018, 8, 3038–3058. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, T.; Li, Y.; Huang, G.; White, M.A.; Gao, J. Investigation of endosome and lysosome biology by ultra pH-sensitive nanoprobes. Adv. Drug Deliv. Rev. 2017, 113, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Tong, L.; Chuang, C.-C.; Wu, S.; Zuo, L. Reactive oxygen species in redox cancer therapy. Cancer Lett. 2015, 367, 18–25. [Google Scholar] [CrossRef]
- Chibh, S.; Kour, A.; Yadav, N.; Kumar, P.; Yadav, P.; Chauhan, V.S.; Panda, J.J. Redox-Responsive Dipeptide Nanostructures toward Targeted Cancer Therapy. ACS Omega 2020, 5, 3365–3375. [Google Scholar] [CrossRef]
- Wang, J.; Wang, F.; Li, F.; Zhang, W.; Shen, Y.; Zhou, D.; Guo, S. A multifunctional poly(curcumin) nanomedicine for dual-modal targeted delivery, intracellular responsive release, dual-drug treatment and imaging of multidrug resistant cancer cells. J. Mater. Chem. B 2016, 4, 2954–2962. [Google Scholar] [CrossRef] [Green Version]
- García-Soriano, D.; Amaro, R.; Lafuente-Gomez, N.L.; Milán-Rois, P.M.; Somoza, Á.; Navío, C.; Herranz, F.; Gutiérrez, L.; Salas, G. The influence of cation incorporation and leaching in the properties of Mn-doped nanoparticles for biomedical applications. J. Colloid Interface Sci. 2020, 578, 510–521. [Google Scholar] [CrossRef]
- Xu, C.; Sun, S. New forms of superparamagnetic nanoparticles for biomedical applications. Adv. Drug Deliv. Rev. 2013, 65, 732–743. [Google Scholar] [CrossRef]
- Garanina, A.S.; Naumenko, V.A.; Nikitin, A.A.; Myrovali, E.; Petukhova, A.Y.; Klimyuk, S.V.; Nalench, Y.A.; Ilyasov, A.R.; Vodopyanov, S.S.; Erofeev, A.S.; et al. Temperature-controlled magnetic nanoparticles hyperthermia inhibits primary tumor growth and metastases dissemination. Nanomed. Nanotechnol. Biol. Med. 2020, 25, 102171. [Google Scholar] [CrossRef]
- Jhaveri, A.; Deshpande, P.; Torchilin, V. Stimuli-sensitive nanopreparations for combination cancer therapy. J. Control. Release 2014, 190, 352–370. [Google Scholar] [CrossRef]
- Yang, X.; Liu, X.; Liu, Z.; Pu, F.; Ren, J.; Qu, X. Near-infrared light-triggered, targeted drug delivery to cancer cells by aptamer gated nanovehicles. Adv. Mater. 2012, 24, 2890–2895. [Google Scholar] [CrossRef]
- Lino, M.M.; Ferreira, L. Light-triggerable formulations for the intracellular controlled release of biomolecules. Drug Discov. Today 2018, 23, 1062–1070. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Y.; Liu, M.; Chen, Z.; Lin, Y.; Li, W.; Cao, F.; Liu, Z.; Ren, J.; Qu, X. A NIR-controlled cage mimicking system for hydrophobic drug mediated cancer therapy. Biomaterials 2017, 139, 151–162. [Google Scholar] [CrossRef]
- Xiang, J.; Tong, X.; Shi, F.; Yan, Q.; Yu, B.; Zhao, Y. Near-infrared light-triggered drug release from UV-responsive diblock copolymer-coated upconversion nanoparticles with high monodispersity. J. Mater. Chem. B 2018, 6, 3531–3540. [Google Scholar] [CrossRef]
- Men, Y.; Brevé, T.G.; Liu, H.; Denkova, A.G.; Eelkema, R. Photo cleavable thioacetal block copolymers for controlled release. Polym. Chem. 2021, 12, 3612–3618. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Chen, D.; Huang, S.; Deng, D.; Tang, L.; Gu, Y. Multifunctional near-infrared light-triggered biodegradable micelles for chemo- and photo-thermal combination therapy. Oncotarget 2016, 7, 82170–82184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paris, J.L.; Cabañas, M.V.; Manzano, M.; Vallet-Regí, M. Polymer-Grafted Mesoporous Silica Nanoparticles as Ultrasound-Responsive Drug Carriers. ACS Nano 2015, 9, 11023–11033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Al-Jawadi, S.; Thakur, S.S. Ultrasound-responsive lipid microbubbles for drug delivery: A review of preparation techniques to optimise formulation size, stability and drug loading. Int. J. Pharm. 2020, 585, 119559. [Google Scholar] [CrossRef]
- Chandan, R.; Mehta, S.M.; Banerjee, R. Ultrasound-Responsive Carriers for Therapeutic Applications. ACS Biomater. Sci. Eng. 2020, 6, 4731–4747. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.H.; Kim, S.E.; Kang, S.S.; Tae, G. Nanosized Ultrasound Enhanced-Contrast Agent for in Vivo Tumor Imaging via Intravenous Injection. ACS Appl. Mater. Interfaces 2016, 8, 8409–8418. [Google Scholar] [CrossRef]
- Zhou, Y.; Cunitz, B.W.; Dunmire, B.; Wang, Y.; Karl, S.G.; Warren, C.; Mitchell, S.; Hwang, J.H. Characterization and Ex Vivo evaluation of an extracorporeal high-intensity focused ultrasound (HIFU) system. J. Appl. Clin. Med Phys. 2021, 22, 345–359. [Google Scholar] [CrossRef]
- Kooiman, K.; Vos, H.J.; Versluis, M.; de Jong, N. Acoustic behavior of microbubbles and implications for drug delivery. Adv. Drug Deliv. Rev. 2014, 72, 28–48. [Google Scholar] [CrossRef]
- Ranjan, A.; Jacobs, G.C.; Woods, D.L.; Negussie, A.H.; Partanen, A.; Yarmolenko, P.S.; Gacchina, C.E.; Sharma, K.V.; Frenkel, V.; Wood, B.J.; et al. Image-guided drug delivery with magnetic resonance guided high intensity focused ultrasound and temperature sensitive liposomes in a rabbit Vx2 tumor model. J. Control. Release 2012, 158, 487–494. [Google Scholar] [CrossRef] [Green Version]
| Vaccine Delivery Systems | Disease | Antigen Type | Administration Routes | Delivery Efficiency | Ref. |
|---|---|---|---|---|---|
| Liposome | Malaria | Recombinant Pfs25 | Intramuscularly | Enhanced several-fold | [99] |
| Liposome | Tumor | mRNA | Subcutaneously | Induced protein expression | [100] |
| Lipoprotein | Tumor | Antigens and CpG | Subcutaneously | Increased LN accumulation | [101] |
| Polymer | Tumor | OVA | Subcutaneously | Efficient LN accumulation | [102] |
| Polymer | Pneumonia | Prevnar-13 | Microneedle insertion | Controlled antigen release | [103] |
| Polymer | Influenza | Inactivated influenza virus | Microneedle insertion | Efficient LN immune activation | [104] |
| Cell | Tumor | Hybrid cells | Intradermal Immunization | Immune function recovery | [105] |
| DNA nanodevice | Tumor | Tumor antigen peptide/CpG loop/dsRNA | Subcutaneously | Enhanced antigen-fluorescence signals in LN | [106] |
| Inorganic materials | Tumor | OVA | Subcutaneously | Much greater extent in LN | [107] |
| Peptide/protein | Chronic hepatitis B | preS1 | Subcutaneously | Mainly captured by SIGNR1+ DCs and macrophages in LN | [108] |
| Virus | SARS-CoV-2 | Prefusion stabilized spike protein | Intramuscularly/ Intranasally | Protecting upper and lower respiratory tracts | [109] |
| Research Group | Ligand | Target | Ref. |
|---|---|---|---|
| Kaur et al. | Mannose | HIV | [119] |
| Jewell et al. | Poly (inosinic acid: cytidine) (PolyIC) | Therapeutic or prophylactic vaccine | [120] |
| Mottas et al. | TLR7 ligands | Tumor | [121] |
| Gulla et al. | Thiol ligands containing shikimoyl and guanidine groups | Melanoma | [122] |
| Liu et al. | polyethylene glycol phospholipid derivatives, anti-PD1 antibody and Treg inhibitory peptide P60 | Tumor | [123] |
| Zeng et al. | Trp2 and TLR-9 | Melanoma | [124] |
| Li et al. | Trp2 and CpG oligonucleotides | Tumor | [125] |
| Wang et al. | LYP-1 | Tumor | [126] |
| Luo et al. | LYP-1 | Tumor | [127] |
| Ahmed et al. | LHRHR and uPAR | Tumor | [128] |
| Mooney et al. | E7 peptide | Tumor | [129] |
| Zhuang et al. | Trp2180-188 and Hgp10025-33 | Tumor | [130] |
| Song et al. | polypeptide hydrogel | Melanoma | [131] |
| Verbeke et al. | BDC peptides | Diabetes | [132] |
| Type | Advantage/Benefit | Deficiency/Limitation |
|---|---|---|
| Liposome | Good controllability of organizational distribution Long term effect Low toxicity Multiple ways of drug administration Slow-release drug delivery Good modifiability | Difficult for industrialized production Low encapsulation efficiency of water-soluble drugs Poor stability, easy hydrolyzed, and oxidized |
| Micelle | Improve the water solubility of drugs Highly stable structure Low toxicity Highly functional structure | Instability in the blood circulatory system |
| Inorganic nanoparticle | Designed in a variety of sizes, structures and geometric shapes Good biocompatibility and stability Have a high specific surface area Different drug loading scales | Low solubility Low clearance rate in vivo Possible long-term potential toxicity Induced cytotoxicity |
| Hydrogel | Prevent protein denaturation Low toxicity Long term effect | Slow response rate Poor mechanical strength |
| Nanocapsule | Better biodistribution Better bioavailability Protect from protease and nuclease degradation | Low entrapment efficiency Low drug loading |
| Type | Research Group | Therapeutic Cargo | Ref. |
|---|---|---|---|
| Interfering RNAs | Munoz et al. | Cy5-anti-miR-9 | [252] |
| Ohno et al. | Let-7a | [253] | |
| Xin et al. | miR-133b | [254] | |
| Pan et al. | miR-122 | [255] | |
| Kosaka et al. | miR-143 | [256] | |
| Katakowski et al. | miR-146b | [257] | |
| Zhang et al. | miR-150 | [258] | |
| Bryniarski et al. | miR-150 | [259] | |
| Chen et al. | miR-214 | [260] | |
| Pan et al. | shNS5b, shCD81 | [255] | |
| Alvarez-Erviti et al. | GAPDH siRNA and BACE1 siRNA | [261] | |
| Wahlgren et al. | MAPK1 siRNA | [262] | |
| Shtam et al. | siRNA against RAD51 and RAD52 | [263] | |
| Other types of therapeutic cargo | Sun et al. | Curcumin | [264] |
| Zhuang et al. | Curcumin and JSI-124 | [265] | |
| Maguire et al. | Adeno-associated viral vector | [266] | |
| Mizrak et al. | Cytosine deaminase (CD) fused with uracil phosphoribosyltransferase (UPRT) and EGFP | [267] | |
| Other types of therapeutic cargo | Hood et al. | Superparamagnetic iron oxide nanoparticles (SPION5) | [268] |
| Jang et al. | Doxorubicin | [269] | |
| Tian et al. | Doxorubicin | [270] |
| Nanomaterials | Cargo Molecules | Disease | Ref. |
|---|---|---|---|
| Poly(beta-amino ester)-based nanomaterial | Plasmids encoding a 194-1BBz CAR and a piggyBac transposase | N/A | [275] |
| Liposome | IL-2–Fc fusion protein | Mouse melanoma | [276] |
| Liposome | TGF-β inhibitor (SB525334) | Mouse melanoma | [277] |
| PLGA–PEG nanomaterial | TGF-β receptor inhibitor (SD-208) | Mouse colon cancer | [278] |
| T-cell (Treg)-targeted hybrid nanomaterial | STAT3/STAT5 pathway inhibitor (imatinib) | Mouse melanoma | [279] |
| Iron nanomaterial | Anti-CD137 and anti-PD-L1 | Mouse melanoma | [280] |
| Liposome-coated polymeric gel | Mouse IL-2 and a TGF-β inhibitor (SB505124) | Mouse melanoma | [281] |
| Macroporous alginate scaffolds | IL-15 superagonists, antibodies for CD3, CD28, and CD137 | Mouse breast cancer, mouse ovarian cancer | [282] |
| Nickel–titanium alloys | Antibodies for CD3, CD28, and CD137 | Mouse model of human pancreatic cancer expressing receptor tyrosine kinase-like orphan receptor (ROR1) | [283] |
| Type | Drug | Date of Approval | Application | Company |
|---|---|---|---|---|
| Liposome | Onpattro | 2018 | Transthyretin-mediated amyloidosis | Alnylam Pharmaceuticals |
| Vyxeos | 2017 | Acute myeloid leukaemia | Jazz Pharmaceuticals | |
| Onivyde | 2015 | Metastatic pancreatic cancer | Ipsen | |
| Marqibo | 2012 | Acute lymphoblastic leukaemia | Acrotech Biopharma | |
| Visudyne | 2000 | Wet age-related macular degeneration, myopia, and ocular histoplasmosis | Bausch and Lomb | |
| AmBisome | 1997 | Fungal/protozoal infections | Gilead Sciences | |
| DaunoXome | 1996 | Kaposi’s sarcoma | Galen | |
| Doxil | 1995 | Kaposi’s sarcoma, ovarian cancer, and multiple myeloma | Janssen | |
| Polymer-based | ADYNOVATE | 2015 | Hemophilia | Takeda |
| Plegridy | 2014 | Multiple sclerosis | Biogen | |
| Cimiza | 2008 | Crohn’s disease, rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis | UCB | |
| Abraxane | 2005 | Lung cancer, metastatic breast cancer, and metastatic pancreatic cancer | Celgene | |
| Neulasta | 2002 | Neutropenia, chemotherapy induced | Amgen | |
| Eligard | 2002 | Prostate cancer | Tolmar | |
| PegIntron | 2001 | Hepatitis C infection | Merck | |
| Copaxone | 1996 | Multiple sclerosis | Teva | |
| Oncaspar | 1994 | Acute lymphoblastic leukaemia | Servier Pharmaceuticals | |
| Inorganic | Injectafer | 2013 | Iron-deficient anaemia | American Regent |
| Feraheme | 2009 | Iron deficiency in chronic kidney disease | AMAG | |
| Venofer | 2000 | Iron deficiency in chronic kidney disease | American Regent | |
| Ferrlecit | 1999 | Iron deficiency in chronic kidney disease | Sanofi | |
| DexFerrum | 1996 | Iron-deficient anaemia | American Regent | |
| INFeD | 1992 | Iron-deficient anaemia | Allergan |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Z.; Que, H.; Chen, L.; Sun, Q.; Wei, X. Nanomaterial-Based Drug Delivery System Targeting Lymph Nodes. Pharmaceutics 2022, 14, 1372. https://doi.org/10.3390/pharmaceutics14071372
Cheng Z, Que H, Chen L, Sun Q, Wei X. Nanomaterial-Based Drug Delivery System Targeting Lymph Nodes. Pharmaceutics. 2022; 14(7):1372. https://doi.org/10.3390/pharmaceutics14071372
Chicago/Turabian StyleCheng, Zesheng, Haiying Que, Li Chen, Qiu Sun, and Xiawei Wei. 2022. "Nanomaterial-Based Drug Delivery System Targeting Lymph Nodes" Pharmaceutics 14, no. 7: 1372. https://doi.org/10.3390/pharmaceutics14071372
APA StyleCheng, Z., Que, H., Chen, L., Sun, Q., & Wei, X. (2022). Nanomaterial-Based Drug Delivery System Targeting Lymph Nodes. Pharmaceutics, 14(7), 1372. https://doi.org/10.3390/pharmaceutics14071372






