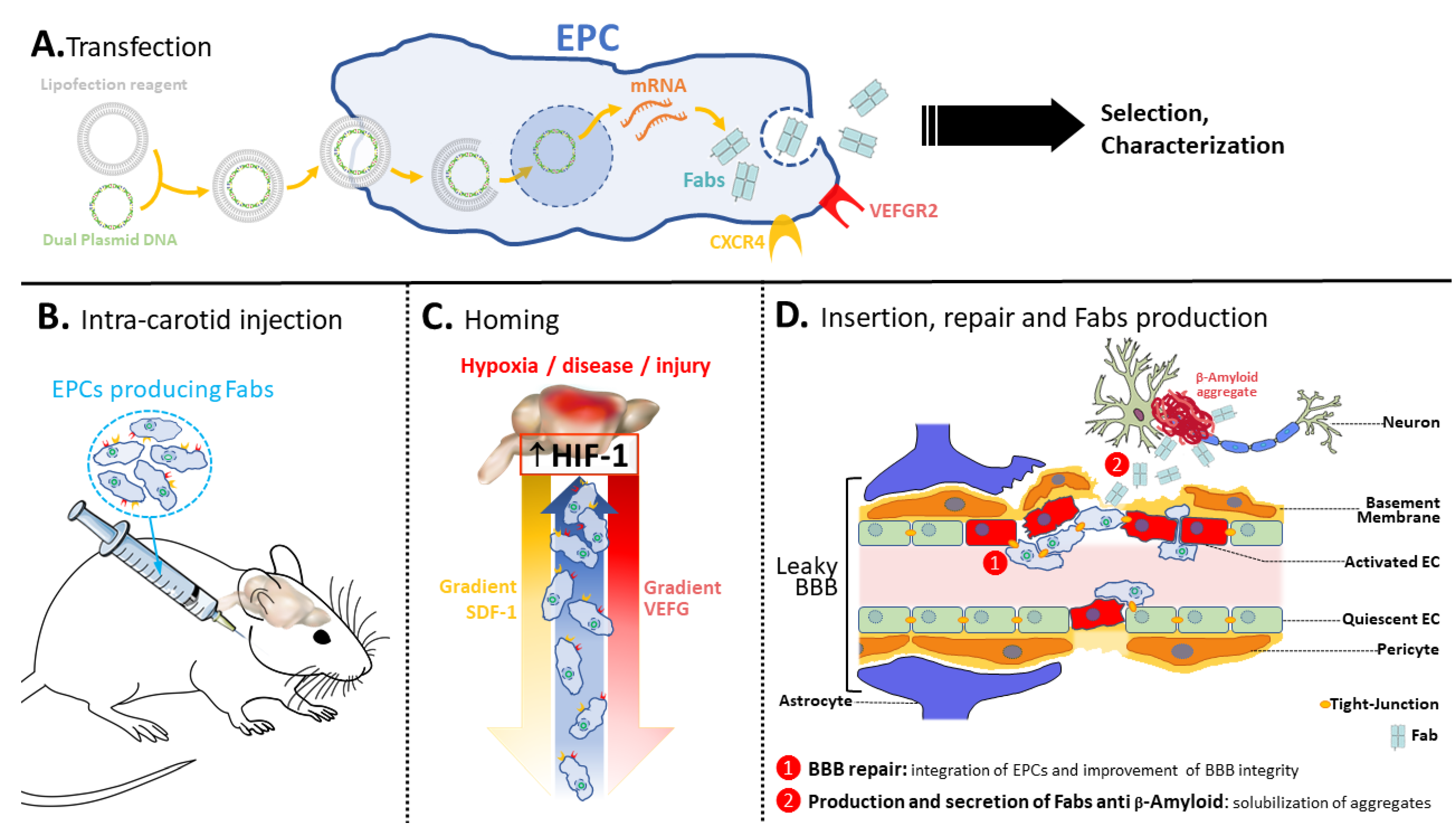
“Endothelial Antibody Factory” at the Blood Brain Barrier: Novel Approach to Therapy of Neurodegenerative Diseases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Tetrapalmitoyl-Tris-Lysine-β-Amyloid-Peptide and TDP-43-Peptide
2.2. Preparation of the Antigens
2.3. Immunizations, Antibody Fragments (Fabs) Cloning and Production
2.4. Solubilization of Anti-β-Amyloid and Anti-TDP-43 Aggregates by Antibodies and Antibody Fragments
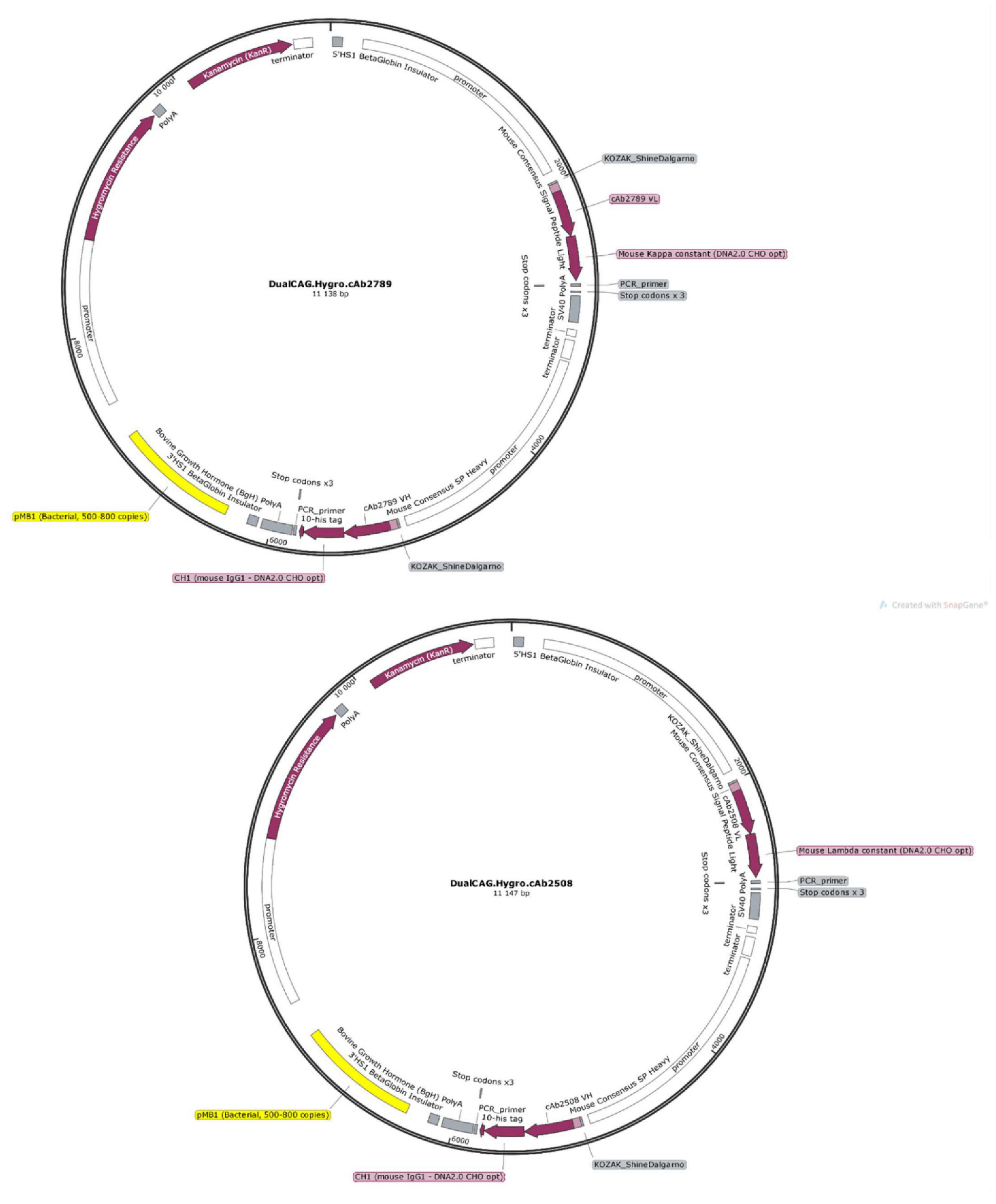
2.5. Plasmids
2.6. Cell Line
2.7. Cell Transduction with tdTomato Expression Lentiviral Vectors
2.8. Cell Transfection and Cloning
2.9. Western Blot His-Tagged Fab
2.10. Assessment of Surface Markers Using Flow Cytometry
2.11. Hypoxia Sensitivity of Murine Brain-Derived Endothelial Cells (MBrMECs) Recognition by MAgEC 10.5 RT Cell Line by Adhesion Experiment
2.12. Endothelial Progenitor Cells Recruitment Assays by BBB Brain Spheroid
2.13. Animal Experiments and Immunofluorescence
2.14. Statistics
3. Results
3.1. New Antibodies and Antibody Fragments Directed against β-Amyloid and TDP-43 Proteins
3.2. Construction of Expression Vectors Encoding Anti-β-Amyloid and TDP-43 Fabs
3.3. Preliminary Study with Stable Expression of s-EGFP and Transient Expression of Fab
3.4. Creation and Characterization of the MAgEC 10.5 RT Anti-β-Amyloid and MAgEC 10.5 RT Anti-TDP-43 Cells: Solubilizing Fab Production and Cell Markers
3.5. Endothelial Progenitor Cells Recruitment Assays by BBB Brain Spheroid
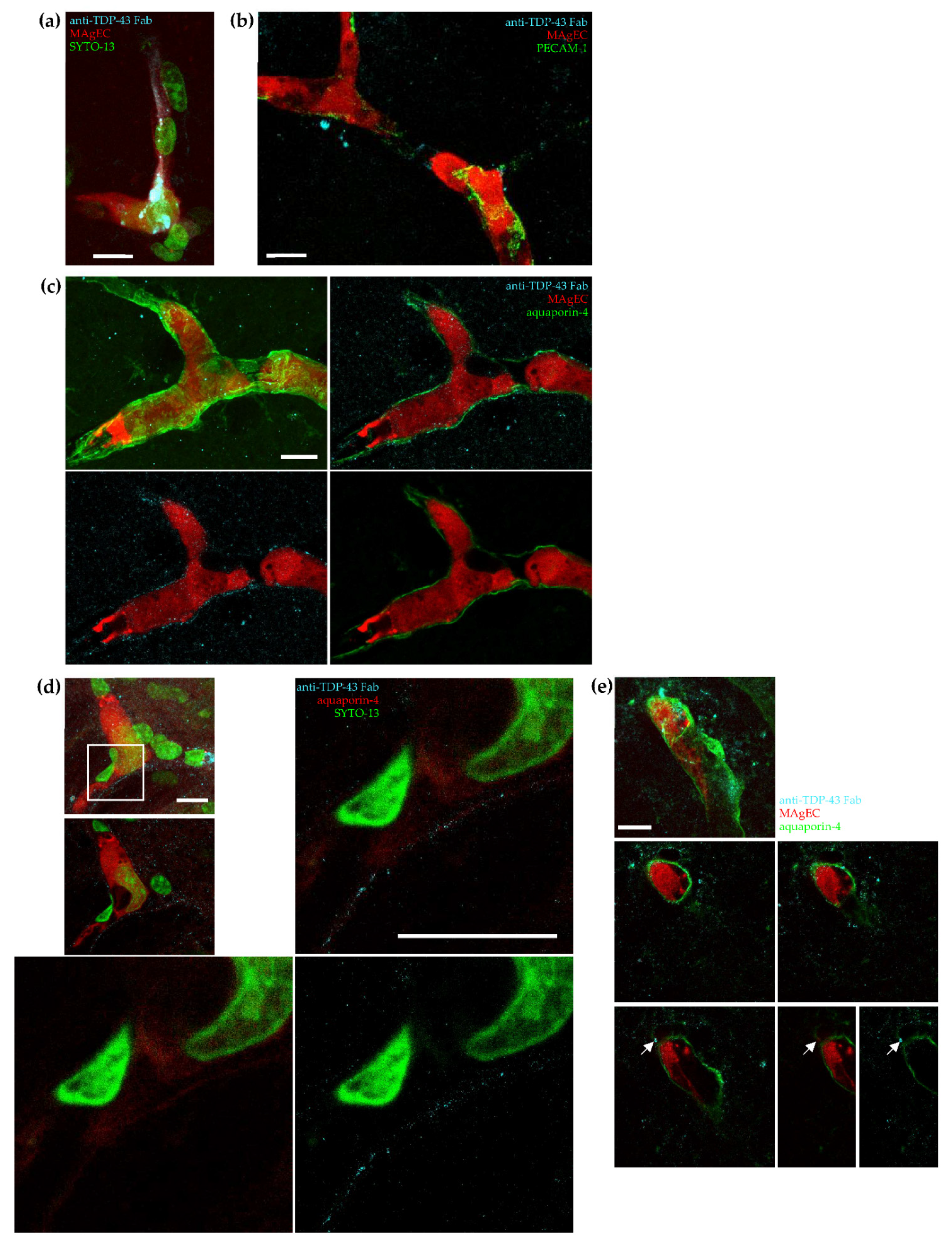
3.6. Homing, Adhesion and Integration of the Injected Cells into the Brain Vasculature
3.7. Exogenic TDP-43 Antibody Secreted by Therapeutic MAgEC Cells Observed in the Brain Vasculature and Brain Parenchyma
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hickman, D.T.; López-Deber, M.P.; Ndao, D.M.; Silva, A.B.; Nand, D.; Pihlgren, M.; Giriens, V.; Madani, R.; St-Pierre, A.; Karastaneva, H.; et al. Sequence-independent Control of Peptide Conformation in Liposomal Vaccines for Targeting Protein Misfolding Diseases. J. Biol. Chem. 2011, 286, 13966–13976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polymenidou, M.; Cleveland, D.W. The seeds of neurodegeneration: Prion-like spreading in ALS. Cell 2011, 147, 498–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polymenidou, M.; Cleveland, D.W. Prion-like spread of protein aggregates in neurodegeneration. J. Exp. Med. 2012, 209, 889–893. [Google Scholar] [CrossRef] [Green Version]
- Hergesheimer, R.C.; Chami, A.A.; De Assis, D.R.; Vourc’h, P.; Andres, C.R.; Corcia, P.; Lanznaster, D.; Blasco, H. The debated toxic role of aggregated TDP-43 in amyotrophic lateral sclerosis: A resolution in sight? Brain 2019, 142, 1176–1194. [Google Scholar] [CrossRef]
- Muhs, A.; Hickman, D.T.; Pihlgren, M.; Chuard, N.; Giriens, V.; Meerschman, C.; Van der Auwera, I.; Van Leuven, F.; Sugawara, M.; Weingertner, M.-C.; et al. Liposomal vaccines with conformation-specific amyloid peptide antigens define immune response and efficacy in APP transgenic mice. Proc. Natl. Acad. Sci. USA 2007, 104, 9810–9815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwong, L.K.; Irwin, D.J.; Walker, A.K.; Xu, Y.; Riddle, D.M.; Trojanowski, J.Q.; Lee, V.M.Y. Novel monoclonal antibodies to normal and pathologically altered human TDP-43 proteins. Acta Neuropathol. Commun. 2014, 2, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K. Conformation-specific antibodies to target amyloid β oligomers and their application to immunotherapy for Alzheimer’s disease. Biosci. Biotechnol. Biochem. 2014, 78, 1293–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thom, G.; Hatcher, J.; Hearn, A.; Paterson, J.; Rodrigo, N.; Beljean, A.; Gurrell, I.; Webstera, C. Isolation of blood-brain barrier-crossing antibodies from a phage display library by competitive elution and their ability to penetrate the central nervous system. MAbs 2018, 10, 304–314. [Google Scholar] [CrossRef] [Green Version]
- YuZuchero, Y.J.; Chen, X.; Bien-Ly, N.; Bumbaca, D.; Tong, R.K.; Gao, X.; Zhang, S.; Hoyte, K.; Luk, W.; Huntley, M.A.; et al. Discovery of Novel Blood Brain Barrier targets to enhance brain uptake of therapeutic antibodies. Neuron 2016, 89, 70–82. [Google Scholar] [CrossRef] [Green Version]
- Pardrigde, W.M. Blood Brain Barrier delivery. Drug Discov. Today 2007, 12, 54–61. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood Brain Barrier: From physiology to disease and back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef] [PubMed]
- Garbuzova-Davis, S.; Kurien, C.; Haller, E.; Eve, D.J.; Navarro, S.; Steiner, G.; Mahendrasah, A.; Hailu, S.; Khatib, M.; Boccio, K.J.; et al. Human bone marrow endothelial progenitor cell transplantation into symptomatic ALS mice delays disease progression and increases motor neuron survival by repairing blood-spinal cord barrier. Sci. Rep. 2019, 9, 5280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalka, C.; Masuda, H.; Takahashi, T.; Kalka-Moll, W.M.; Silver, M.; Kearney, M.; Li, T.; Isner, J.M.; Asahara, T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neurovascularization. Proc. Natl. Acad. Sci. USA 2000, 97, 3422–3427. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Hong, Y.; Zhu, J.; Cheng, X.; Liu, Y. Endothelial progenitor cells improve functional recovery in focal cerebral ischemia of rat by promoting angiogenesis vis VEGF. J. Clin. Neurosci. 2018, 55, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Collet, G.; Grillon, C.; Nadim, M.; Kieda, C. Trojan horse at cellular level for tumor gene therapies. Gene 2013, 525, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.P.; He, X.P.; Xu, H.T.; Zou, Z.; Shi, X.Y. Autologous transplantation of peripheral blood-derived circulating endothelial progenitor cells attenuates endotoxin-induced acute lung injury in rabbits by direct endothelial repair and indirect immunomodulation. Anesthesiology 2012, 116, 1278–1287. [Google Scholar] [CrossRef] [Green Version]
- Kado, M.; Tanaka, R.; Arita, K.; Okada, K.; Ito-Hirano, R.; Fujimura, S.; Mizuno, H. Human peripheral blood mononuclear cells enriched in endothelial progenitor cells via quality and quantity-controlled culture accelerate vascularization and wound healing in porcine wound model. Cell Transpl. 2018, 27, 1068–1079. [Google Scholar] [CrossRef] [Green Version]
- Suh, W.; Kim, K.L.; Kim, J.M.; Shin, I.S.; Lee, Y.S.; Lee, J.Y.; Jang, H.S.; Lee, J.S.; Byun, J.; Choi, J.H.; et al. Transplantation of endothelial progenitor cells accelerates dermal wound healing with increased recruitment of monocytes/macrophages and neovascularization. Stem Cells 2005, 23, 1571–1578. [Google Scholar] [CrossRef] [Green Version]
- Jung, K.H.; Roh, J.K. Circulating endothelial progenitor cells in cerebrovascular disease. J. Clin. Neurol. 2008, 4, 139–147. [Google Scholar] [CrossRef] [Green Version]
- Collet, G.; Szade, K.; Nowak, W.; Klimkiewicz, K.; El Hafny-Rahbi, B.; Szczepanek, K.; Sugiyama, D.; Weglarczyk, K.; Foucault-Collet, A.; Guichard, A.; et al. Endothelial precursor cell-based therapy to target the pathological angiogenesis and compensate tumor hypoxia. Cancer Lett. 2016, 370, 345–357. [Google Scholar] [CrossRef]
- Fries, L.F.; Gordon, D.M.; Richards, R.L.; Egan, J.E.; Hollingdale, M.R.; Gross, M.; Silverman, C.; Alving, C.R. Liposomal malaria vaccine in humans: A safe and potent adjuvant strategy. Proc. Natl. Acad. Sci. USA 1992, 89, 358–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heppner, D.G.; Gordon, D.M.; Gross, M.; Wellde, B.; Leitner, W.; Krzych, U.; Schneider, I.; Wirtz, R.A.; Richards, R.L.; Trofa, A.; et al. Safety, immunogenicity, and efficacy of Plasmodium falciparum repeatless circumsporozoite protein vaccine encapsulated in liposomes. J. Infect. Dis. 1996, 174, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Nicolau, C.; Greferath, R.; Balaban, T.S.; Lazarte, J.E.; Hopkins, R.J. A liposome-based therapeutic vaccine against beta-amyloid plaques on the pancreas of transgenic NORBA mice. Proc. Natl. Acad. Sci. USA 2002, 99, 2332–2337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossowska, J.; Anger, N.; Szczygieł, A.; Mierzejewska, J.; Pajtasz-Piasecka, E. Intratumoral Lentivector-Mediated TGF-Β1 Gene Downregulation as a Potent Strategy for Enhancing the Antitumor Effect of Therapy Composed of Cyclophosphamide and Dendritic Cells. Front. Immunol. 2017, 8, 713. [Google Scholar] [CrossRef] [PubMed]
- Paprocka, M.; Duśa, D.; Mitterrand, M.; Lamerant-Fayel, N.; Kieda, C. Flow cytometric assay for quantitative and qualitative evaluation of adhesive interactions of tumor cells with endothelial cells. Microvasc. Res. 2008, 76, 134–138. [Google Scholar] [CrossRef]
- Klimkiewicz, K.; Weglarczyk, K.; Collet, G.; Paprocka, M.; Guichard, A.; Sarna, M.; Jozkowicz, A.; Dulak, J.; Sarna, T.; Grillon, C.; et al. A 3d model of tumour angiogenic microenvironment to monitor hypoxia effects on cell interactions and cancer stem cell selection. Cancer Lett. 2017, 396, 10–20. [Google Scholar] [CrossRef]
- Cho, C.F.; Wolfe, J.M.; Fadzen, C.M.; Calligaris, D.; Hornburg, K.; Chiocca, E.A.; Agar, N.Y.R.; Pentelute, B.L.; Lawler, S.E. Blood-brain-barrier spheroids as an in vitro screening platform for brain-penetrating agents. Nat. Commun. 2017, 8, 15623. [Google Scholar] [CrossRef]
- Heller, L.; Thinard, R.; Chevalier, M.; Arpag, S.; Jing, Y.; Greferath, R.; Heller, R.; Nicolau, C. Secretion of proteins and antibody fragments from transiently transfected endothelial progenitor cells. J. Cell. Mol. Med. 2020, 24, 8772–8778. [Google Scholar] [CrossRef]
- Garbuzova-Davis, S.; Saporta, S.; Sanberg, P.R. Implication of Blood Brain Barrier disruption in ALS. Amyotroph. Lateral Scler. 2008, 9, 375–376. [Google Scholar] [CrossRef]
- Wang, J.; Ohno-Matsui, K.; Nakahama, K.; Okamoto, A.; Yoshida, T.; Shimada, N.; Mochizuki, M.; Morita, I. Amyloid beta enhances migration of endothelial progenitor cells by upregulating CX3CR1 in response to Fractalkine, which may be associated with development of choroidal neovascularization. Arter. Thromb. Vasc. Biol. 2011, 31, e11–e18. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Liu, Q.; Fang, Z.; Hu, X.; Huang, F.; Tang, L.; Zhou, S. Hypoxia induces the proliferation of endothelial progenitor cells via upregulation of Apelin/APLNR/MAPK signaling. Mol. Med. Rep. 2016, 13, 1801–1806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joyce, P.I.; Mcgoldrick, P.; Saccon, R.A.; Weber, W.; Fratta, P.; West, S.J.; Zhu, N.; Carter, S.; Phatak, V.; Stewart, M.; et al. A novel SOD1-ALS mutation separates central and peripheral effects of mutant sod1 toxicity. Hum. Mol. Genet. 2015, 24, 1883–1897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sen, S.; McDonald, S.P.; Coates, P.T.H.; Bonder, C.S. Endothelial progenitor cells: Novel biomarker and promising cell therapy for cardiovascular disease. Clin. Sci. 2011, 120, 263–283. [Google Scholar] [CrossRef] [Green Version]
- Chopra, H.; Hung, M.K.; Kwong, D.L.; Zhang, C.F.; Pow, E.H.N. Insights into Endothelial Progenitor Cells: Origin, Classification, Potentials, and Prospects. Stem Cells Int. 2018, 18, 9847015. [Google Scholar] [CrossRef] [PubMed]




| Primary Antibodies | Catalog Number |
|---|---|
| Anti-Histag Chimeric | Merck, SAB5600096 |
| Anti-Mouse IgG (Fab specific) | ThermoFisher, 31413 |
| Secondary antibodies | |
| Anti-Histag Chimeric secondary | Merck, A1293 |
| Rabbit anti-Goat IgG (H + L) secondary | ThermoFisher, 81-1620 |
| Antibody | Catalog Number | Details |
|---|---|---|
| CD34 | 152207 (Biolegend) | Rat IgG2a Brilliant Violet 421™ |
| CD45 | 103111(Biolegend) | Rat IgG2b APC |
| UEA-1 | DL-1069 (Vector Labratories) | DyLight649 labeling |
| Isotype Control Antibody | 400511 (Biolegend) | Rat IgG2a APC |
| Isotype Control Antibody | 400611(Biolegend) | Rat IgG2b APC |
| Clone Name | Primary Heavy Chain Protein Sequence | |||
|---|---|---|---|---|
| HC Type | Sig. Pep. | VH | Constant | |
| anti-TDP-43 antibody (cAb2508) | Mouse IgG2b | MEWIWIFLFILSGTAGVQS | QVQLQQSGAELARPGASVKLSCKASGYTFTSYGISWVRQRTGQGLEWIGEIYPRRGNTYYNEKFKGKATLTAYKSSGTAYMELRSLTSEDSAVFFCARGGIYYGNLFDYWGQGTTLTVSS | AKTTPPSVYPLAPGCGDTTGSSVTLGCLVKGYFPESVTVTWNSGSLSSSVHTFPALLQSGLYTMSSSVTVPSSTWPSQTVTCSVAHPASSTTVDKKLEPSGPISTINPCPPCKECHKCPAPNLEGGPSVFIFPPNIKDVLMISLTPKVTCVVVDVSEDDPDVRISWFVNNVEVHTAQTQTHREDYNSTIRVVSALPIQHQDWMSGKEFKCKVNNKDLPSPIERTISKIKGLVRAPQVYILPPPAEQLSRKDVSLTCLVVGFNPGDISVEWTSNGHTEENYKDTAPVLDSDGSYFIYSKLDIKTSKWEKTDSFSCNVRHEGLKNYYLKKTISRSPGK |
| Primary light chain protein sequence | ||||
| LC type | Sig. pep. | VL | Constant | |
| Mouse lambda | MAWISLILSLLALSSGAIS | QAVVTQESALTTSPGETVTLTCRSSTGAVTTSNYANWVQEKPDHLFTGLIGGTNNRAPGVPARFSGSLIGDKAALTITGAQTEDEAIYFCALWFSNHWVFGGGTKLTVLG | QPKSSPSVTLFPPSSEELETNKATLVCTITDFYPGVVTVDWKVDGTPVTQGMETTQPSKQSNNKYMASSYLTLTARAWERHSSYSCQVTHEGHTVEKSLSRADCS | |
| Primary heavy chain protein sequence | ||||
| HC type | Sig. pep. | VH | Constant | |
| anti-β-Amyloid antibody (cAb2789) | Mouse IgM | MEWPLIFLFLLSGTAGVQS | QVQLQQSGAELVKPGASVKISCKASGYAFSNYWMNWVKQRPGKGLEWIGQIYPGDGDTNYNGKFKGKATLTADKSSSTAYMQLSSLTSEDSAVYFCARGDYWGQGTTLTVSS | ESQSFPNVFPLVSCESPLSDKNLVAMGCLARDFLPSTISFTWNYQNNTEVIQGIRTFPTLRTGGKYLATSQVLLSPKSILEGSDEYLVCKIHYGGKNKDLHVPIPAVAEMNPNVNVFVPPRDGFSGPAPRKSKLICEATNFTPKPITVSWLKDGKLVESGFTTDPVTIENKGSTPQTYKVISTLTISEIDWLNLNVYTCRVDHRGLTFLKNVSSTCAASPSTDILTFTIPPSFADIFLSKSANLTCLVSNLATYETLNISWASQSGEPLETKIKIMESHPNGTFSAKGVASVCVEDWNNRKEFVCTVTHRDLPSPQKKFISKPNEVHKHPPAVYLLPPAREQLNLRESATVTCLVKGFSPADISVQWLQRGQLLPQEKYVTSAPMPEPGAPGFYFTHSILTVTEEEWNSGETYTCVVGHEALPHLVTERTVDKSTGKPTLYNVSLIMSDTGGTCY |
| Primary light chain protein sequence | ||||
| LC type | Sig. pep. | VL | Constant | |
| Mouse kappa | MESQTQVLMFLLLWVSGACA | DIVMTQSPSSLAMSVGQKVTMSCKSSQSLLNSSNQKNYLAWYQQKPGQSPKLLVYFASTRESGVPDRFIGSGSGTDFTLTISSVQAEDLADYFCQQHYNTPLTFGAGTKLELK | RADAAPTVSIFPPSSEQLTSGGASVVCFLNNFYPKDINVKWKIDGSERQNGVLNSWTDQDSKDSTYSMSSTLTLTKDEYERHNSYTCEATHKTSTSPIVKSFNRNEC | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thinard, R.; Farkas, A.E.; Halasa, M.; Chevalier, M.; Brodaczewska, K.; Majewska, A.; Zdanowski, R.; Paprocka, M.; Rossowska, J.; Duc, L.T.; et al. “Endothelial Antibody Factory” at the Blood Brain Barrier: Novel Approach to Therapy of Neurodegenerative Diseases. Pharmaceutics 2022, 14, 1418. https://doi.org/10.3390/pharmaceutics14071418
Thinard R, Farkas AE, Halasa M, Chevalier M, Brodaczewska K, Majewska A, Zdanowski R, Paprocka M, Rossowska J, Duc LT, et al. “Endothelial Antibody Factory” at the Blood Brain Barrier: Novel Approach to Therapy of Neurodegenerative Diseases. Pharmaceutics. 2022; 14(7):1418. https://doi.org/10.3390/pharmaceutics14071418
Chicago/Turabian StyleThinard, Reynald, Attila E. Farkas, Marta Halasa, Melanie Chevalier, Klaudia Brodaczewska, Aleksandra Majewska, Robert Zdanowski, Maria Paprocka, Joanna Rossowska, Lam Tri Duc, and et al. 2022. "“Endothelial Antibody Factory” at the Blood Brain Barrier: Novel Approach to Therapy of Neurodegenerative Diseases" Pharmaceutics 14, no. 7: 1418. https://doi.org/10.3390/pharmaceutics14071418
APA StyleThinard, R., Farkas, A. E., Halasa, M., Chevalier, M., Brodaczewska, K., Majewska, A., Zdanowski, R., Paprocka, M., Rossowska, J., Duc, L. T., Greferath, R., Krizbai, I., Van Leuven, F., Kieda, C., & Nicolau, C. (2022). “Endothelial Antibody Factory” at the Blood Brain Barrier: Novel Approach to Therapy of Neurodegenerative Diseases. Pharmaceutics, 14(7), 1418. https://doi.org/10.3390/pharmaceutics14071418







