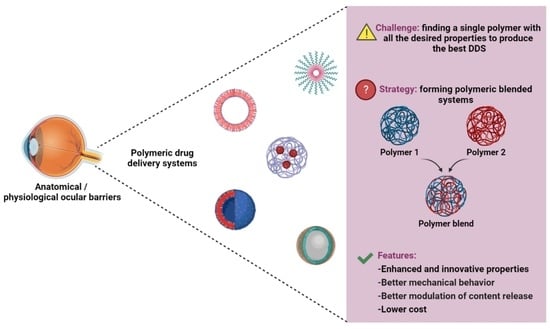
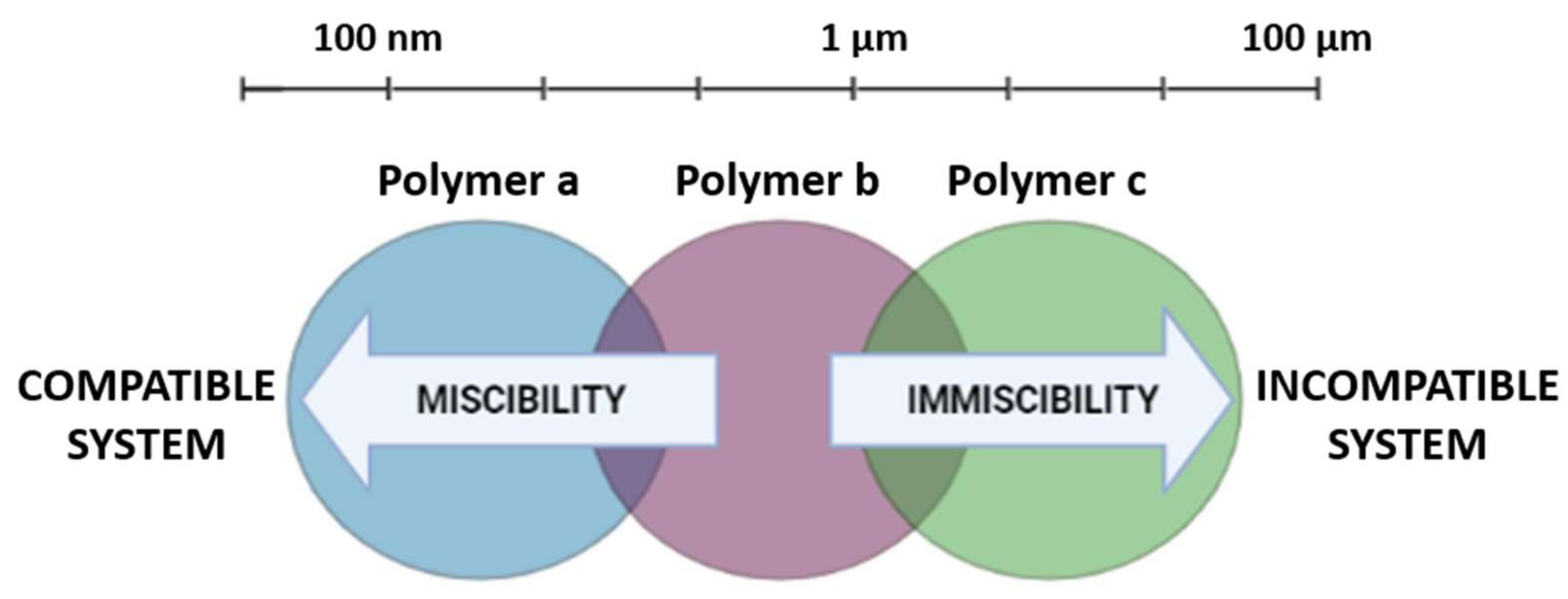
The Use of Polymer Blends in the Treatment of Ocular Diseases
Abstract
:1. Introduction
2. Methods
3. Results
3.1. Common Polymers in Ocular Use
3.1.1. Synthetic Polymers
3.1.2. Natural Polymers
3.2. DDS Made from Polymer Blends
3.2.1. Hydrogels
3.2.2. Nano and Microparticulated Systems
3.2.3. Polymeric Micelles
3.2.4. Ocular Inserts
3.3. Application of Polymer Blends in Various Ocular Conditions
3.3.1. Glaucoma
3.3.2. Dry Eye Syndrome
3.3.3. Infectious Keratitis
3.4. Application of Polymer Blends for Specific Ocular Tissues
3.4.1. The Retina
3.4.2. The Cornea
4. Final Considerations
5. Conclusions and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Huang, D.; Chen, Y.; Rupenthal, I.D. Overcoming Ocular Drug Delivery Barriers through the Use of Physical Forces. Adv. Drug Deliv. Rev. 2017, 126, 96–112. [Google Scholar] [CrossRef] [PubMed]
- Lalu, L.; Tambe, V.; Pradhan, D.; Nayak, K.; Bagchi, S.; Maheshwari, R.; Kalia, K.; Tekade, R.K. Novel Nanosystems for the Treatment of Ocular Inflammation: Current Paradigms and Future Research Directions. J. Control. Release 2017, 268, 19–39. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.; Wang, P.; Lin, I.; Huang, H.; Liu, G. Ocular Drug Delivery: Role of Degradable Polymeric Nanocarriers for Ophthalmic Application. Mol. Sci. 2018, 19, 2830. [Google Scholar] [CrossRef] [Green Version]
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular Drug Delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Molokhia, S.A.; Thomas, S.C.; Garff, K.J.; Mandell, K.J.; Wirostko, B.M. Anterior Eye Segment Drug Delivery Systems: Current Treatments and Future Challenges. J. Ocul. Pharmacol. Ther. 2013, 29, 92–105. [Google Scholar] [CrossRef]
- Patel, A. Ocular Drug Delivery Systems: An Overview. World J. Pharmacol. 2013, 2, 47. [Google Scholar] [CrossRef]
- Gote, V. Ocular Drug Delivery: Present Innovations and Future Challenges. Ocular Drug Delivery: Past Present and Future. Pharmacol. Exp. Ther. 2019, 370, 602–624. [Google Scholar] [CrossRef]
- Fialho, S.L.; Rego, M.G.B.; Cardillo, J.A.; Siqueira, R.C.; Jorge, R.; da Silva Cunha Júnior, A. Implantes Biodegradáveis Destinados à Administração Intra-Ocular. Arq. Bras. Oftalmol. 2003, 66, 891–896. [Google Scholar] [CrossRef] [Green Version]
- Nayak, K.; Misra, M. A Review on Recent Drug Delivery Systems for Posterior Segment of Eye. Biomed. Pharmacother. 2018, 107, 1564–1582. [Google Scholar] [CrossRef]
- Cabrera, F.J.; Wang, D.C.; Reddy, K.; Acharya, G.; Shin, C.S. Challenges and Opportunities for Drug Delivery to the Posterior of the Eye. Drug Discov. Today 2019, 24, 1679–1684. [Google Scholar] [CrossRef]
- Ustundag Okur, N.; Çaglar, E.S.; Siafaka, P.I. Novel Ocular Drug Delivery Systems: An Update on Microemulsions. J. Ocul. Pharmacol. Ther. 2020, 36, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.B.; Ichhpujani, P.; Thakur, S.; Jindal, S. Promising Therapeutic Drug Delivery Systems for Glaucoma: A Comprehensive Review. Ther. Adv. Ophthalmol. 2020, 12, 12515841420905740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varela-Fernández, R.; Díaz-Tomé, V.; Luaces-Rodríguez, A.; Conde-Penedo, A.; García-Otero, X.; Luzardo-Álvarez, A.; Fernández-Ferreiro, A.; Otero-Espinar, F.J. Drug Delivery to the Posterior Segment of the Eye: Biopharmaceutic and Pharmacokinetic Considerations. Pharmaceutics 2020, 12, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imperiale, J.C.; Acosta, G.B.; Sosnik, A. Polymer-Based Carriers for Ophthalmic Drug Delivery. J. Control. Release 2018, 285, 106–141. [Google Scholar] [CrossRef]
- Behar-cohen, F. Expert Opinion on Drug Delivery Recent Advances in Slow and Sustained Drug Release for Retina Drug Delivery Recent Advances in Slow and Sustained Drug Release for Retina Drug Delivery. Expert Opin. Drug Deliv. 2019, 16, 679–686. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Morreale, M.; Botta, L.; Mistretta, M.C.; Ceraulo, M.; Scaffaro, R. Degradation of Polymer Blends: A Brief Review. Polym. Degrad. Stab. 2017, 145, 79–92. [Google Scholar] [CrossRef]
- Alam, T.M.; Otaigbe, J.U.; Rhoades, D.; Holland, G.P.; Cherry, B.R.; Kotula, P.G. Nanostructured Polymer Blends: Synthesis and Structure. Polymer 2005, 46, 12468–12479. [Google Scholar] [CrossRef]
- He, Y.; Zhu, B.; Inoue, Y. Hydrogen Bonds in Polymer Blends. Prog. Polym. Sci. 2004, 29, 1021–1051. [Google Scholar] [CrossRef]
- Soares, D.C.F.; Arribada, R.G.; Branco de Barros, A.L.; Tebaldi, M.L. Polymeric Nanoblends Compatibilization: A Strategic Design to Enhance the Effectiveness of Nanocarriers for Biomedical Applications. Int. J. Polym. Mater. Polym. Biomater. 2019, 69, 567–579. [Google Scholar] [CrossRef]
- Terence, M.C.; Faldini, S.B.; de Miranda, L.F.; Munhoz, A.H.; de Castro, P.J. Preparation and Characterization of a Polymeric Blend of PVP/PVAL for Use in Drug Delivery System. J Biomed. Nanotechnol. 2011, 7, 446–449. [Google Scholar] [CrossRef]
- Changez, M.; Burugapalli, K.; Koul, V.; Choudhary, V. The Effect of Composition of Poly(Acrylic Acid)-Gelatin Hydrogel on Gentamicin Sulphate Release: In Vitro. Biomaterials 2003, 24, 527–536. [Google Scholar] [CrossRef]
- Tucker, C.L., III; Moldenaers, P. Microstructural evolution in polymer blends. Annu. Rev. Fluid Mech. 2002, 34, 177–210. [Google Scholar] [CrossRef]
- Sundararaj, U.; Macosko, C.W. Evidence for Inversion of Phase Continuity During Morphology Development in Polymer Blending. Polym. Eng. Sci. 1996, 36, 1769–1781. [Google Scholar] [CrossRef]
- Koning, C.; Van Duin, M.; Pagnoulle, C.; Jerome, R. Strategies for Compatibilization of Polymer Blends. Polym. Sci. 1998, 23, 707–757. [Google Scholar] [CrossRef]
- Imre, B.; Pukánszky, B. Compatibilization in Bio-Based and Biodegradable Polymer Blends. Eur. Polym. J. 2013, 49, 1215–1233. [Google Scholar] [CrossRef] [Green Version]
- Hamad, K.; Kaseem, M.; Ayyoob, M.; Joo, J.; Deri, F. Progress in Polymer Science Polylactic Acid Blends: The Future of Green, Light and Tough. Prog. Polym. Sci. 2018, 85, 83–127. [Google Scholar] [CrossRef]
- Nofar, M.; Sacligil, D.; Carreau, P.J.; Kamal, M.R.; Heuzey, M. Poly (Lactic Acid) Blends: Processing, Properties and Applications. Int. J. Biol. Macromol. 2019, 125, 307–360. [Google Scholar] [CrossRef]
- Huang, L.; Wu, C.; Hua, C.; Huang, T. Multiscale Simulations of Coupled Composition-Stress-Morphology of Binary Polymer Blend. Polymer 2020, 193, 122366. [Google Scholar] [CrossRef]
- Charfeddine, I.; Majest, J.C.; Carrot, C.; Lhost, O. A Model for the Prediction of the Morphology of Immiscible Blends of Polymers. Polymer 2020, 193, 1–11. [Google Scholar] [CrossRef]
- Walther, A.; Matussek, K.; Mu, A.H.E. Engineering Nanostructured Polymer Blends with Controlled Nanoparticle Location Using Janus Particles. ACS Nano 2008, 2, 1167–1178. [Google Scholar] [CrossRef]
- Macosko, C.W.; Jeon, H.K.; Hoye, T.R. Reactions at Polymer—Polymer Interfaces for Blend Compatibilization. Prog. Polym. Sci. 2005, 30, 939–947. [Google Scholar] [CrossRef]
- Grimaudo, M.A.; Amato, G.; Carbone, C.; Diaz-Rodriguez, P.; Musumeci, T.; Concheiro, A.; Alvarez-Lorenzo, C.; Puglisi, G. Micelle-Nanogel Platform for Ferulic Acid Ocular Delivery. Int. J. Pharm. 2020, 576, 118986. [Google Scholar] [CrossRef] [PubMed]
- Terreni, E.; Burgalassi, S.; Chetoni, P.; Tampucci, S.; Zucchetti, E.; Fais, R.; Ghelardi, E.; Lupetti, A.; Monti, D. Development and Characterization of a Novel Peptide-Loaded Antimicrobial Ocular Insert. Biomolecules 2020, 10, 664. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Ding, Z.; Luo, L.; Chen, B.; Schneider, J.; Yang, J.; Eberhart, C.G.; Stark, W.J.; Xu, Q. Shear-Thinning Viscous Materials for Subconjunctival Injection of Microparticles. AAPS PharmSciTech 2020, 22, 8. [Google Scholar] [CrossRef]
- Shahab, M.S.; Rizwanullah, M.; Alshehri, S.; Imam, S.S. Optimization to Development of Chitosan Decorated Polycaprolactone Nanoparticles for Improved Ocular Delivery of Dorzolamide: In Vitro, Ex Vivo and Toxicity Assessments. Int. J. Biol. Macromol. 2020, 163, 2392–2404. [Google Scholar] [CrossRef]
- Sun, F.; Zheng, Z.; Lan, J.; Li, X.; Li, M.; Song, K.; Wu, X. New Micelle Myricetin Formulation for Ocular Delivery: Improved Stability, Solubility, and Ocular Anti-Inflammatory Treatment. Drug Deliv. 2019, 26, 575–585. [Google Scholar] [CrossRef] [Green Version]
- Roy, G.; Galigama, R.D.; Thorat, V.S.; Mallela, L.S.; Roy, S.; Garg, P.; Venuganti, V.V.K. Amphotericin B containing microneedle ocular patch for effective treatment of fungal keratitis. Int. J. Pharm. 2019, 572, 118808. [Google Scholar] [CrossRef]
- Srinivasarao, D.A.; Reddy, S.S.; Reddy, G.B.; Katti, D.S. Spatio-Temporal Control on the Delivery of Triamcinolone Acetonide Using Polymeric Nanoparticles Reduces Steroid Induced Cataract. Int. J. Pharm. 2019, 568, 118474. [Google Scholar] [CrossRef]
- Tighsazzadeh, M.; Mitchell, J.C.; Boateng, J.S. Development and Evaluation of Performance Characteristics of Timolol-Loaded Composite Ocular Films as Potential Delivery Platforms for Treatment of Glaucoma. Int. J. Pharm. 2019, 566, 111–125. [Google Scholar] [CrossRef]
- Liu, D.; Wu, Q.; Chen, W.; Lin, H.; Zhu, Y.; Liu, Y.; Liang, H.; Zhu, F.M. A Novel FK506 Loaded Nanomicelles Consisting of Amino-Terminated Poly(Ethylene Glycol)-Block-Poly(D,L)-Lactic Acid and Hydroxypropyl Methylcellulose for Ocular Drug Delivery. Int. J. Pharm. 2019, 562, 1–10. [Google Scholar] [CrossRef]
- Eid, H.M.; Elkomy, M.H.; El Menshawe, S.F.; Salem, H.F. Development, Optimization, and In Vitro/In Vivo Characterization of Enhanced Lipid Nanoparticles for Ocular Delivery of Ofloxacin: The Influence of Pegylation and Chitosan Coating. AAPS PharmSciTech 2019, 20, 103527. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Peng, F.; Zheng, Q.; Zeng, L.; Chen, H.; Li, X.; Huang, J. Micelle-Solubilized Axitinib for Ocular Administration in Anti-Neovascularization. Int. J. Pharm. 2019, 560, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Kouchak, M.; Mahmoodzadeh, M.; Farrahi, F. Designing of a PH-Triggered Carbopol®/HPMC In Situ Gel for Ocular Delivery of Dorzolamide HCl: In Vitro, In Vivo, and Ex Vivo Evaluation. AAPS PharmSciTech 2019, 20, 210. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Jaiswal, C.P.; Mirza, M.A.; Anwer, M.K.; Iqbal, Z. Preparation of Levofloxacin Loaded in Situ Gel for Sustained Ocular Delivery: In Vitro and Ex Vivo Evaluations. Drug Dev. Ind. Pharm. 2019, 46, 50–56. [Google Scholar] [CrossRef]
- Devi, S.; Saini, V.; Kumar, M.; Bhatt, S.; Gupta, S.; Deep, A. A Novel Approach of Drug Localization through Development of Polymeric Micellar System Containing Azelastine HCl for Ocular Delivery. Pharm. Nanotechnol. 2019, 7, 314–327. [Google Scholar] [CrossRef]
- El-Nabarawi, M.A.; Abd El Rehem, R.T.; Teaima, M.; Abary, M.; El-Mofty, H.M.; Khafagy, M.M.; Lotfy, N.M.; Salah, M. Natamycin Niosomes as a Promising Ocular Nanosized Delivery System with Ketorolac Tromethamine for Dual Effects for Treatment of Candida Rabbit Keratitis; in Vitro/in Vivo and Histopathological Studies. Drug Dev. Ind. Pharm. 2019, 45, 922–936. [Google Scholar] [CrossRef]
- Di Prima, G.; Licciardi, M.; Carfì Pavia, F.; Lo Monte, A.I.; Cavallaro, G.; Giammona, G. Microfibrillar Polymeric Ocular Inserts for Triamcinolone Acetonide Delivery. Int. J. Pharm. 2019, 567, 118459. [Google Scholar] [CrossRef]
- Mandal, A.; Patel, P.; Pal, D.; Mitra, A.K. Multi-Layered Nanomicelles as Self-Assembled Nanocarrier Systems for Ocular Peptide Delivery. AAPS PharmSciTech 2019, 20, 66. [Google Scholar] [CrossRef]
- Mehta, P.; Al-Kinani, A.A.; Arshad, M.S.; Singh, N.; van der Merwe, S.M.; Chang, M.W.; Alany, R.G.; Ahmad, Z. Engineering and Development of Chitosan-Based Nanocoatings for Ocular Contact Lenses. J. Pharm. Sci. 2019, 108, 1540–1551. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Li, Z.; Liang, Z.; Han, L.; Feng, H.; He, S.; Zhang, J. Fabrication of a Drug Delivery System That Enhances Antifungal Drug Corneal Penetration. Drug Deliv. 2018, 25, 938–949. [Google Scholar] [CrossRef]
- Zhu, Q.; Cheng, H.; Huo, Y.; Mao, S. Sustained Ophthalmic Delivery of Highly Soluble Drug Using PH-Triggered Inner Layer-Embedded Contact Lens. Int. J. Pharm. 2018, 544, 100–111. [Google Scholar] [CrossRef]
- Zhu, Q.; Liu, C.; Sun, Z.; Zhang, X.; Liang, N.; Mao, S. Inner Layer-Embedded Contact Lenses for PH-Triggered Controlled Ocular Drug Delivery. Eur. J. Pharm. Biopharm. 2018, 128, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Kabiri, M.; Kamal, S.H.; Pawar, S.V.; Roy, P.R.; Derakhshandeh, M.; Kumar, U.; Hatzikiriakos, S.G.; Hossain, S.; Yadav, V.G. A Stimulus-Responsive, in Situ-Forming, Nanopartcile-Laden Hydrogel for Ocular Drug Delivery. Drug Deliv. Transl. Res. 2018, 8, 484–495. [Google Scholar] [CrossRef] [Green Version]
- El-Feky, G.S.; Zayed, G.M.; Elshaier, Y.A.M.M.; Alsharif, F.M. Chitosan-Gelatin Hydrogel Crosslinked with Oxidized Sucrose for the Ocular Delivery of Timolol Maleate. J. Pharm. Sci. 2018, 107, 3098–3104. [Google Scholar] [CrossRef]
- Silva, D.; de Sousa, H.C.; Gil, M.H.; Santos, L.F.; Moutinho, G.M.; Serro, A.P.; Saramago, B. Antibacterial Layer-by-Layer Coatings to Control Drug Release from Soft Contact Lenses Material. Int. J. Pharm. 2018, 553, 186–200. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chen, D.; Li, Y.; Yang, W.; Tu, J.; Shen, Y. Improving the Topical Ocular Pharmacokinetics of Lyophilized Cyclosporine A-Loaded Micelles: Formulation, in Vitro and in Vivo Studies. Drug Deliv. 2018, 25, 888–899. [Google Scholar] [CrossRef] [Green Version]
- Lancina, M.G.; Wang, J.; Williamson, G.S.; Yang, H. DenTimol as A Dendrimeric Timolol Analogue for Glaucoma Therapy: Synthesis and Preliminary Efficacy and Safety Assessment. Mol. Pharm. 2018, 15, 2883–2889. [Google Scholar] [CrossRef] [PubMed]
- Kasper, M.; Gabriel, D.; Möller, M.; Bauer, D.; Wildschütz, L.; Courthion, H.; Rodriguez-Aller, M.; Busch, M.; Böhm, M.R.R.; Loser, K.; et al. Cyclosporine A-Loaded Nanocarriers for Topical Treatment of Murine Experimental Autoimmune Uveoretinitis. Mol. Pharm. 2018, 15, 2539–2547. [Google Scholar] [CrossRef]
- Bhamra, T.S.; Tighe, B.J.; Li, J. High Modulus Hydrogels for Ophthalmic and Related Biomedical Applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 107, 1645–1653. [Google Scholar] [CrossRef]
- Khan, S.; Warade, S.; Singhavi, D.J. Improvement in Ocular Bioavailability and Prolonged Delivery of Tobramycin Sulfate Following Topical Ophthalmic Administration of Drug-Loaded Mucoadhesive Microparticles Incorporated in Thermosensitive in Situ Gel. J. Ocul. Pharmacol. Ther. 2017, 34, 287–297. [Google Scholar] [CrossRef]
- Calles, J.A.; Mora, M.J.; Onnainty, R.; Tartara, L.I.; Granero, G.E.; Longhi, M.R.; Diebold, Y.; Vallés, E.M.; Palma, S.D. Cross-Linked Hyaluronan Films Loaded with Acetazolamide-Cyclodextrin-Triethanolamine Complexes for Glaucoma Treatment. Ther. Deliv. 2018, 9, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Sebastián-Morelló, M.; Calatayud-Pascual, M.A.; Rodilla, V.; Balaguer-Fernández, C.; López-Castellano, A. Ex Vivo Rabbit Cornea Diffusion Studies with a Soluble Insert of Moxifloxacin. Drug Deliv. Transl. Res. 2017, 8, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.M.; Calado, R.; Marto, J.; Bettencourt, A.; Almeida, A.J.; Gonçalves, L.M.D. Chitosan Nanoparticles as a Mucoadhesive Drug Delivery System for Ocular Administration. Mar. Drugs 2017, 15, 370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatnagar, S.; Saju, A.; Cheerla, K.D.; Gade, S.K.; Garg, P.; Venuganti, V.V.K. Corneal Delivery of Besifloxacin Using Rapidly Dissolving Polymeric Microneedles. Drug Deliv. Transl. Res. 2017, 8, 473–483. [Google Scholar] [CrossRef]
- Li, M.; Xin, M.; Guo, C.; Lin, G.; Wu, X. New Nanomicelle Curcumin Formulation for Ocular Delivery: Improved Stability, Solubility, and Ocular Anti-Inflammatory Treatment. Drug Dev. Ind. Pharm. 2017, 43, 1846–1857. [Google Scholar] [CrossRef]
- Pandian, S.; Jeevanesan, V.; Ponnusamy, C.; Natesan, S. RES-Loaded Pegylated CS NPs: For Efficient Ocular Delivery. IET Nanobiotechnol. 2017, 11, 32–39. [Google Scholar] [CrossRef]
- Luo, L.J.; Lai, J.Y. The Role of Alkyl Chain Length of Monothiol-Terminated Alkyl Carboxylic Acid in the Synthesis, Characterization, and Application of Gelatin-g-Poly(N-Isopropylacrylamide) Carriers for Antiglaucoma Drug Delivery. Acta Biomater. 2017, 49, 344–357. [Google Scholar] [CrossRef]
- Luo, L.J.; Lai, J.Y. Epigallocatechin Gallate-Loaded Gelatin-g-Poly(N-Isopropylacrylamide) as a New Ophthalmic Pharmaceutical Formulation for Topical Use in the Treatment of Dry Eye Syndrome. Sci. Rep. 2017, 7, 9380. [Google Scholar] [CrossRef] [Green Version]
- Fu, T.; Yi, J.; Lv, S.; Zhang, B. Ocular Amphotericin B Delivery by Chitosan-Modified Nanostructured Lipid Carriers for Fungal Keratitis-Targeted Therapy. J. Liposome Res. 2016, 27, 228–233. [Google Scholar] [CrossRef]
- Quinteros, D.A.; Ferreira, L.M.; Schaffazick, S.R.; Palma, S.D.; Allemandi, D.A.; Cruz, L. Novel Polymeric Nanoparticles Intended for Ophthalmic Administration of Acetazolamide. J. Pharm. Sci. 2016, 105, 3183–3190. [Google Scholar] [CrossRef]
- Chou, S.F.; Luo, L.J.; Lai, J.Y.; Ma, D.H.K. On the Importance of Bloom Number of Gelatin to the Development of Biodegradable in Situ Gelling Copolymers for Intracameral Drug Delivery. Int. J. Pharm. 2016, 511, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.; Marto, J.; Braz, B.S.; Delgado, E.; Almeida, A.J.; Gonçalves, L. New Nanoparticles for Topical Ocular Delivery of Erythropoietin. Int. J. Pharm. 2020, 576, 119020. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Luo, R.; Zhang, H.; Dai, M.; Bai, L.; Fei, Q.; Lei, F.; He, N. Design, Characterization, and Application of a PH-Triggered In Situ Gel for Ocular Delivery of Vinpocetine. AAPS PharmSciTech 2020, 21, 253. [Google Scholar] [CrossRef]
- Xu, T.; Xu, X.; Gu, Y.; Fang, L.; Cao, F. Functional Intercalated Nanocomposites with Chitosan-Glutathione-Glycylsarcosine and Layered Double Hydroxides for Topical Ocular Drug Delivery. Int. J. Nanomed. 2018, 13, 917–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cauldbeck, H.; Le Hellaye, M.; Long, M.; Kennedy, S.M.; Williams, R.L.; Kearns, V.R.; Rannard, S.P. Controlling Drug Release from Non-Aqueous Environments: Moderating Delivery from Ocular Silicone Oil Drug Reservoirs to Combat Proliferative Vitreoretinopathy. J. Control. Release 2016, 244, 41–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowhan, A.; Giri, T.K. Polysaccharide as Renewable Responsive Biopolymer for in Situ Gel in the Delivery of Drug through Ocular Route. Int. J. Biol. Macromol. 2020, 150, 559–572. [Google Scholar] [CrossRef]
- Qamar, Z.; Qizilbash, F.F.; Iqubal, M.K.; Ali, A.; Narang, J.K.; Ali, J.; Baboota, S. Nano-Based Drug Delivery System: Recent Strategies for the Treatment of Ocular Disease and Future Perspective. Recent Pat. Drug Deliv. Formul. 2019, 13, 246–254. [Google Scholar] [CrossRef]
- Holgado, M.A.; Anguiano-Domínguez, A.; Martín-Banderas, L. Contact Lenses as Drug-Delivery Systems: A Promising Therapeutic Tool. Arch. Soc. Esp. Oftalmol. 2020, 95, 24–33. [Google Scholar] [CrossRef]
- Grimaudo, M.A.; Pescina, S.; Padula, C.; Santi, P.; Concheiro, A.; Alvarez-Lorenzo, C.; Nicoli, S. Topical Application of Polymeric Nanomicelles in Ophthalmology: A Review on Research Efforts for the Noninvasive Delivery of Ocular Therapeutics. Expert Opin. Drug Deliv. 2019, 16, 397–413. [Google Scholar] [CrossRef]
- Mutlu, Z.; Shams Es-haghi, S.; Cakmak, M. Recent Trends in Advanced Contact Lenses. Adv. Healthc. Mater. 2019, 8, 1801390. [Google Scholar] [CrossRef]
- Pontillo, A.R.N.; Detsi, A. Nanoparticles for Ocular Drug Delivery: Modified and Non-Modified Chitosan as a Promising Biocompatible Carrier. Nanomedicine 2019, 14, 1889–1909. [Google Scholar] [CrossRef] [PubMed]
- Göttel, B.; de Souza e Silva, J.M.; Santos de Oliveira, C.; Syrowatka, F.; Fiorentzis, M.; Viestenz, A.; Viestenz, A.; Mäder, K. Electrospun Nanofibers—A Promising Solid in-Situ Gelling Alternative for Ocular Drug Delivery. Eur. J. Pharm. Biopharm. 2020, 146, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Von Deylen, D.; Dreher, C.; Seidelmann, O.; Reichl, S. New Classes of Polycationic Compounds as Preservatives for Ophthalmic Formulations. Pharm. Res. 2019, 36, 11. [Google Scholar] [CrossRef] [PubMed]
- McAvoy, K.; Jones, D.; Thakur, R.R.S. Synthesis and Characterisation of Photocrosslinked Poly(Ethylene Glycol) Diacrylate Implants for Sustained Ocular Drug Delivery. Pharm. Res. 2018, 35, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuwahatu, C.A.; Yeung, C.C.; Lam, Y.W.; Roy, V.A.L. The Molecularly Imprinted Polymer Essentials: Curation of Anticancer, Ophthalmic, and Projected Gene Therapy Drug Delivery Systems. J. Control. Release 2018, 287, 24–34. [Google Scholar] [CrossRef]
- Kutlehria, S.; Vhora, I.; Bagde, A.; Chowdhury, N.; Behl, G.; Patel, K.; Singh, M. Tacrolimus Loaded PEG-Cholecalciferol Based Micelles for Treatment of Ocular Inflammation. Pharm. Res. 2018, 35, 117. [Google Scholar] [CrossRef]
- Wen, Y.; Ban, J.; Mo, Z.; Zhang, Y.; An, P.; Liu, L.; Xie, Q.; Du, Y.; Xie, B.; ZHan, X.; et al. A Potential Nanoparticle-Loaded in Situ Gel for Enhanced and Sustained Ophthalmic Delivery of Dexamethasone. Nanotechnology 2018, 29, 425101. [Google Scholar] [CrossRef]
- Ngatuni, M.J.; Trinh, H.M.; Pal, D.; Mitra, A.K. Novel Random Triblock Copolymers for Sustained Delivery of Macromolecules for the Treatment of Ocular Diseases. AAPS PharmSciTech 2018, 19, 3871–3885. [Google Scholar] [CrossRef]
- Bongiovì, F.; Fiorica, C.; Palumbo, F.S.; Di Prima, G.; Giammona, G.; Pitarresi, G. Imatinib-Loaded Micelles of Hyaluronic Acid Derivatives for Potential Treatment of Neovascular Ocular Diseases. Mol. Pharm. 2018, 15, 5031–5045. [Google Scholar] [CrossRef]
- Egbu, R.; Brocchini, S.; Khaw, P.T.; Awwad, S. Antibody Loaded Collapsible Hyaluronic Acid Hydrogels for Intraocular Delivery. Eur. J. Pharm. Biopharm. 2018, 124, 95–103. [Google Scholar] [CrossRef]
- Silva-Abreu, M.; Calpena, A.C.; Espina, M.; Silva, A.M.; Gimeno, A.; Egea, M.A.; García, M.L. Optimization, Biopharmaceutical Profile and Therapeutic Efficacy of Pioglitazone-Loaded PLGA-PEG Nanospheres as a Novel Strategy for Ocular Inflammatory Disorders. Pharm. Res. 2018, 35, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krtalić, I.; Radošević, S.; Hafner, A.; Grassi, M.; Nenadić, M.; Cetina-Čižmek, B.; Filipović-Grčić, J.; Pepić, I.; Lovrić, J. D-Optimal Design in the Development of Rheologically Improved In Situ Forming Ophthalmic Gel. J. Pharm. Sci. 2018, 107, 1562–1571. [Google Scholar] [CrossRef] [PubMed]
- Orasugh, J.T.; Sarkar, G.; Saha, N.R.; Das, B.; Bhattacharyya, A.; Das, S.; Mishra, R.; Roy, I.; Chattoapadhyay, A.; Ghosh, S.K.; et al. Effect of Cellulose Nanocrystals on the Performance of Drug Loaded in Situ Gelling Thermo-Responsive Ophthalmic Formulations. Int. J. Biol. Macromol. 2018, 124, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Lian, Y.; Fang, Q.; Liu, L.; Zhang, J.; Li, J. Hyaluronic-Acid-Modified Lipid-Polymer Hybrid Nanoparticles as an Efficient Ocular Delivery Platform for Moxifloxacin Hydrochloride. Int. J. Biol. Macromol. 2018, 116, 1026–1036. [Google Scholar] [CrossRef]
- Mandal, A.; Bisht, R.; Rupenthal, I.D.; Mitra, A.K. Polymeric Micelles for Ocular Drug Delivery: From Structural Frameworks to Recent Preclinical Studies. J. Control. Release 2017, 248, 96–116. [Google Scholar] [CrossRef] [Green Version]
- Mehta, P.; Haj-Ahmad, R.; Al-kinani, A.; Arshad, M.S.; Chang, M.W.; Alany, R.G.; Ahmad, Z. Approaches in Topical Ocular Drug Delvery and Developments in the Use of Contact Lenses as Drug-Delivery Devices. Ther. Deliv. 2017, 8, 117–138. [Google Scholar] [CrossRef] [Green Version]
- Chu, Y.; Chen, N.; Yu, H.; Mu, H.; He, B.; Hua, H.; Wang, A.; Sun, K. Topical Ocular Delivery to Laser-Induced Choroidal Neovascularization by Dual Internalizing RGD and TAT Peptide-Modified Nanoparticles. Int. J. Nanomed. 2017, 12, 1353–1368. [Google Scholar] [CrossRef] [Green Version]
- Yousry, C.; Elkheshen, S.A.; El-laithy, H.M.; Essam, T.; Fahmy, R.H. Studying the Influence of Formulation and Process Variables on Vancomycin-Loaded Polymeric Nanoparticles as Potential Carrier for Enhanced Ophthalmic Delivery; Elsevier B.V.: Amsterdam, The Netherlands, 2017; Volume 100. [Google Scholar] [CrossRef]
- Dewan, M.; Sarkar, G.; Bhowmik, M.; Das, B.; Chattoapadhyay, A.K.; Rana, D.; Chattopadhyay, D. Effect of Gellan Gum on the Thermogelation Property and Drug Release Profile of Poloxamer 407 Based Ophthalmic Formulation. Int. J. Biol. Macromol. 2017, 102, 258–265. [Google Scholar] [CrossRef]
- Yacasi, G.R.R.; Calpena Campmany, A.C.; Egea Gras, M.A.; Espina García, M.; García López, M.L. Freeze Drying Optimization of Polymeric Nanoparticles for Ocular Flurbiprofen Delivery: Effect of Protectant Agents and Critical Process Parameters on Long-Term Stability. Drug Dev. Ind. Pharm. 2017, 43, 637–651. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Soni, T.G.; Shah, D.O. A Review on Therapeutic Contact Lenses for Ocular Drug Delivery. Drug Deliv. 2016, 23, 3017–3026. [Google Scholar] [CrossRef]
- Rodríguez Villanueva, J.; Navarro, M.G.; Rodríguez Villanueva, L. Dendrimers as a Promising Tool in Ocular Therapeutics: Latest Advances and Perspectives. Int. J. Pharm. 2016, 511, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Despanie, J.; Dhandhukia, J.P.; Hamm-Alvarez, S.F.; MacKay, J.A. Elastin-like Polypeptides: Therapeutic Applications for an Emerging Class of Nanomedicines. J. Control. Release 2016, 240, 93–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, N.; Thakkar, V.; Metalia, V.; Baldaniya, L.; Gandhi, T.; Gohel, M. Formulation and Development of Ophthalmic in Situ Gel for the Treatment Ocular Inflammation and Infection Using Application of Quality by Design Concept. Drug Dev. Ind. Pharm. 2016, 42, 1406–1423. [Google Scholar] [CrossRef] [PubMed]
- Al Khateb, K.; Ozhmukhametova, E.K.; Mussin, M.N.; Seilkhanov, S.K.; Rakhypbekov, T.K.; Lau, W.M.; Khutoryanskiy, V.V. In Situ Gelling Systems Based on Pluronic F127/Pluronic F68 Formulations for Ocular Drug Delivery. Int. J. Pharm. 2016, 502, 70–79. [Google Scholar] [CrossRef]
- Sharma, M.; Bhowmick, R.; Gappa-Fahlenkamp, H. Drug-Loaded Nanoparticles Embedded in a Biomembrane Provide a Dual-Release Mechanism for Drug Delivery to the Eye. J. Ocul. Pharmacol. Ther. 2016, 32, 565–573. [Google Scholar] [CrossRef]
- Zhang, Z.; He, Z.; Liang, R.; Ma, Y.; Huang, W.; Jiang, R.; Shi, S.; Chen, H.; Li, X. Fabrication of a Micellar Supramolecular Hydrogel for Ocular Drug Delivery. Biomacromolecules 2016, 17, 798–807. [Google Scholar] [CrossRef]
- Kumari, A.; Jain, A.; Hurkat, P.; Verma, A.; Jain, S.K. Microsponges: A Pioneering Tool for Biomedical Applications. Crit. Rev. Ther. Drug Carr. Syst. 2016, 33, 77–105. [Google Scholar] [CrossRef]
- Mitra, R.N.; Zheng, M.; Han, Z. Nanoparticle-Motivated Gene Delivery for Ophthalmic Application. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 160–174. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, D.N.; Bhatia, A.; Kaur, R.; Sharma, R.; Kaur, G.; Dhawan, S. PLGA: A Unique Polymer for Drug Delivery. Ther. Deliv. 2015, 6, 41–58. [Google Scholar] [CrossRef]
- Kamali, H.; Khodaverdi, E.; Hadizadeh, F. In-Vitro, Ex-Vivo, and In-Vivo Release Evaluation of In Situ Forming Buprenorphine Implants Using Mixture of PLGA Copolymers and Additives. Int. J. Polym. Mater. Polym. Biomater. 2018, 68, 965–977. [Google Scholar] [CrossRef]
- Mir, M.; Ahmed, N.; ur Rehman, A. Recent Applications of PLGA Based Nanostructures in Drug Delivery. Colloids Surf. B Biointerfaces 2017, 159, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Basar, A.O.; Sadhu, V.; Turkoglu Sasmazel, H. Preparation of Electrospun PCL-Based Scaffolds by Mono/Multi-Functionalized GO. Biomed. Mater. 2019, 14, 045012. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.; Asawa, S.; Birru, B.; Baadhe, R.; Rao, S. PCL-Based Composite Scaffold Matrices for Tissue Engineering Applications. Mol. Biotechnol. 2018, 60, 506–532. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Feng, B.; Zhang, W.; Yan, C.; Yao, Q.; Shao, C.; Yu, F.; Li, F.; Fu, Y. Electrospun Gelatin/PCL and Collagen/PCL Scaffolds for Modulating Responses of Bone Marrow Endothelial Progenitor Cells. Exp. Ther. Med. 2019, 17, 3717–3726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arrieta, M.P.; Gil, A.L.; Yusef, M.; Kenny, J.M.; Peponi, L. Electrospinning of PCL-Based Blends: Processing Optimization for Their Scalable Production. Materials 2020, 13, 3853. [Google Scholar] [CrossRef]
- Rahme, K.; Dagher, N. Chemistry Routes for Copolymer Synthesis Containing Peg for Targeting, Imaging, and Drug Delivery Purposes. Pharmaceutics 2019, 11, 327. [Google Scholar] [CrossRef] [Green Version]
- Sellaturay, P.; Nasser, S.; Ewan, P. Polyethylene Glycol–Induced Systemic Allergic Reactions (Anaphylaxis). J. Allergy Clin. Immunol. Pract. 2021, 9, 670–675. [Google Scholar] [CrossRef]
- D’souza, A.A.; Shegokar, R. Polyethylene Glycol (PEG): A Versatile Polymer for Pharmaceutical Applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef]
- Mašková, E.; Kubová, K.; Raimi-Abraham, B.T.; Vllasaliu, D.; Vohlídalová, E.; Turánek, J.; Mašek, J. Hypromellose—A Traditional Pharmaceutical Excipient with Modern Applications in Oral and Oromucosal Drug Delivery. J. Contro. Release 2020, 324, 695–727. [Google Scholar] [CrossRef]
- Esteban-Pérez, S.; Andrés-Guerrero, V.; López-Cano, J.J.; Molina-Martínez, I.; Herrero-Vanrell, R.; Bravo-Osuna, I. Gelatin Nanoparticles-HPMC Hybrid System for Effective Ocular Topical Administration of Antihypertensive Agents. Pharmaceutics 2020, 12, 306. [Google Scholar] [CrossRef] [Green Version]
- Nair, A.R.; Lakshman, Y.D.; Anand, V.S.K.; Sree, K.S.N.; Bhat, K.; Dengale, S.J. Overview of Extensively Employed Polymeric Carriers in Solid Dispersion Technology. AAPS PharmSciTech 2020, 21, 309. [Google Scholar] [CrossRef] [PubMed]
- Migliozzi, S.; Meridiano, G.; Angeli, P.; Mazzei, L. Investigation of the Swollen State of Carbopol Molecules in Non-Aqueous Solvents through Rheological Characterization. Soft Matter 2020, 16, 9799–9815. [Google Scholar] [CrossRef] [PubMed]
- Graziano, R.; Preziosi, V.; Uva, D.; Tomaiuolo, G.; Mohebbi, B.; Claussen, J.; Guido, S. The Microstructure of Carbopol in Water under Static and Flow Conditions and Its Effect on the Yield Stress. J. Colloid Interface Sci. 2021, 582, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Thakral, S.; Thakral, N.K.; Majumdar, D.K. Eudragit®: A Technology Evaluation. Expert Opin. Drug Deliv. 2013, 10, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Kurakula, M.; Rao, G.S.N.K. Pharmaceutical Assessment of Polyvinylpyrrolidone (PVP): As Excipient from Conventional to Controlled Delivery Systems with a Spotlight on COVID-19 Inhibition. J. Drug Deliv. Sci. Technol. 2020, 60, 102046. [Google Scholar] [CrossRef]
- Charron, P.N.; Braddish, T.A.; Oldinski, R.A. PVA-Gelatin Hydrogels Formed Using Combined Theta-Gel and Cryo-Gel Fabrication Techniques. J. Mech. Behav. Biomed. Mater. 2019, 92, 90–96. [Google Scholar] [CrossRef]
- Kuźmińska, A.; Butruk-Raszeja, B.A.; Stefanowska, A.; Ciach, T. Polyvinylpyrrolidone (PVP) Hydrogel Coating for Cylindrical Polyurethane Scaffolds. Colloids Surf. B Biointerfaces 2020, 192, 4–9. [Google Scholar] [CrossRef]
- Vecchi, C.F.; Cesar, G.B.; de Souza, P.R.; Caetano, W.; Bruschi, M.L. Mucoadhesive Polymeric Films Comprising Polyvinyl Alcohol, Polyvinylpyrrolidone, and Poloxamer 407 for Pharmaceutical Applications. Pharm. Dev. Technol. 2021, 26, 138–149. [Google Scholar] [CrossRef]
- Teodorescu, M.; Bercea, M.; Morariu, S. Biomaterials of PVA and PVP in Medical and Pharmaceutical Applications: Perspectives and Challenges. Biotechnol. Adv. 2019, 37, 109–131. [Google Scholar] [CrossRef]
- Russo, E.; Villa, C. Poloxamer Hydrogels for Biomedical Applications. Pharmaceutics 2019, 11, 671. [Google Scholar] [CrossRef] [Green Version]
- Zarrintaj, P.; Ramsey, J.D.; Samadi, A.; Atoufi, Z.; Yazdi, M.K.; Ganjali, M.R.; Amirabad, L.M.; Zangene, E.; Farokhi, M.; Formela, K.; et al. Poloxamer: A Versatile Tri-Block Copolymer for Biomedical Applications. Acta Biomater. 2020, 110, 37–67. [Google Scholar] [CrossRef] [PubMed]
- Vasvani, S.; Kulkarni, P.; Rawtani, D. Hyaluronic Acid: A Review on Its Biology, Aspects of Drug Delivery, Route of Administrations and a Special Emphasis on Its Approved Marketed Products and Recent Clinical Studies. Int. J. Biol. Macromol. 2020, 151, 1012–1029. [Google Scholar] [CrossRef] [PubMed]
- Nien, H.K.; Yap, W.H.; Lim, C.L.H.; Goh, B.H.; Lai, Z.W. Hyaluronic Acid-Mediated Drug Delivery System Targeting for Inflammatory Skin Diseases: A Mini Review. Front. Pharmacol. 2020, 11, 1105. [Google Scholar] [CrossRef]
- Passi, A.; Vigetti, D. Hyaluronan as Tunable Drug Delivery System. Adv. Drug Deliv. Rev. 2019, 146, 83–96. [Google Scholar] [CrossRef]
- Madkhali, O.; Mekhail, G.; Wettig, S.D. Modified Gelatin Nanoparticles for Gene Delivery. Int. J. Pharm. 2019, 554, 224–234. [Google Scholar] [CrossRef]
- Calixto, S.; Ganzherli, N.; Gulyaev, S.; Figueroa-Gerstenmaier, S. Gelatin as a Photosensitive Material. Molecules 2018, 23, 2064. [Google Scholar] [CrossRef] [Green Version]
- Hathout, R.M.; Metwally, A.A. Gelatin Nanoparticles. Methods Mol. Biol. 2019, 2000, 71–78. [Google Scholar] [CrossRef]
- Afinjuomo, F.; Abdella, S.; Youssef, S.H.; Song, Y.; Garg, S. Inulin and Its Application in Drug Delivery. Pharmaceuticals 2021, 14, 855. [Google Scholar] [CrossRef]
- Imran, S.; Gillis, R.B.; Kok, M.S.; Harding, S.E.; Adams, G.G. Application and Use of Inulin as a Tool for Therapeutic Drug Delivery. Biotechnol. Genet. Eng. Rev. 2012, 28, 33–46. [Google Scholar] [CrossRef] [Green Version]
- Di Prima, G.; Licciardi, M.; Bongiovì, F.; Pitarresi, G.; Giammona, G. Inulin-Based Polymeric Micelles Functionalized with Ocular Permeation Enhancers: Improvement of Dexamethasone Permeation/Penetration through Bovine Corneas. Pharmaceutics 2021, 13, 1431. [Google Scholar] [CrossRef]
- Di Prima, G.; Bongiovì, F.; Palumbo, F.S.; Pitarresi, G.; Licciardi, M.; Giammona, G. Mucoadhesive PEGylated Inulin-Based Self-Assembling Nanoparticles: In Vitro and Ex Vivo Transcorneal Permeation Enhancement of Corticosteroids. J. Drug Deliv. Sci. Technol. 2019, 49, 195–208. [Google Scholar] [CrossRef]
- Sardo, C.; Mencherini, T.; Tommasino, C.; Esposito, T.; Russo, P.; Del Gaudio, P.; Aquino, R.P. Inulin-g-Poly-D,L-Lactide, a Sustainable Amphiphilic Copolymer for Nano-Therapeutics. Drug Deliv. Transl. Res. 2022, 12, 1974–1990. [Google Scholar] [CrossRef] [PubMed]
- Bale, S.; Khurana, A.; Reddy, A.S.S.; Singh, M.; Godugu, C. Overview on Therapeutic Applications of Microparticulate Drug Delivery Systems. Crit. Rev. Ther. Drug Carr. Syst. 2016, 33, 309–361. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.Y.; Al-Salami, H.; Dass, C.R. Microparticles, Microcapsules and Microspheres: A Review of Recent Developments and Prospects for Oral Delivery of Insulin. Int. J. Pharm. 2018, 537, 223–244. [Google Scholar] [CrossRef]
- Mauro, N.; Di Prima, G.; Varvarà, P.; Licciardi, M.; Cavallaro, G.; Giammona, G. Core-Shell Arginine-Containing Chitosan Microparticles for Enhanced Transcorneal Permeation of Drugs. J. Pharm. Sci. 2019, 108, 960–969. [Google Scholar] [CrossRef]
- Mandal, A.; Cholkar, K.; Khurana, V.; Shah, A.; Agrahari, V.; Bisht, R.; Pal, D.; Mitra, A.K. Topical Formulation of Self-Assembled Antiviral Prodrug Nanomicelles for Targeted Retinal Delivery. Mol. Pharm. 2017, 14, 2056–2069. [Google Scholar] [CrossRef]
- Rassu, G.; Pavan, B.; Mandracchia, D.; Tripodo, G.; Botti, G.; Dalpiaz, A.; Gavini, E.; Giunchedi, P. Polymeric Nanomicelles Based on Inulin D α-Tocopherol Succinate for the Treatment of Diabetic Retinopathy. J. Drug Deliv. Sci. Technol. 2021, 61, 102286. [Google Scholar] [CrossRef]
- Weiss, S.L.; Kramer, W.G. Ocular Distribution of Cyclosporine Following Topical Administration of OTX-101 in New Zealand White Rabbits. J. Ocul. Pharmacol. Ther. 2019, 35, 395–402. [Google Scholar] [CrossRef]
- Mandal, A.; Gote, V.; Pal, D.; Ogundele, A.; Mitra, A.K. Ocular Pharmacokinetics of a Topical Ophthalmic Nanomicellar Solution of Cyclosporine (Cequa®) for Dry Eye Disease. Pharm. Res. 2019, 36, 36. [Google Scholar] [CrossRef]
- Goldberg, D.F.; Malhotra, R.P.; Schechter, B.A.; Justice, A.; Weiss, S.L.; Sheppard, J.D. A Phase 3, Randomized, Double-Masked Study of OTX-101 Ophthalmic Solution 0.09% in the Treatment of Dry Eye Disease. Ophthalmology 2019, 126, 1230–1237. [Google Scholar] [CrossRef] [Green Version]
- Aburahma, M.H.; Mahmoud, A.A. Biodegradable Ocular Inserts for Sustained Delivery of Brimonidine Tartarate: Preparation and in Vitro/in Vivo Evaluation. AAPS PharmSciTech 2011, 12, 1335–1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammad, D.A.; Sweet, B.V.; Elner, S.G. Retisert: Is the New Advance in Treatment of Uveitis a Good One? Ann. Pharmacother. 2007, 41, 449–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habib, M.S. ILUVIEN ® Technology in the Treatment of Center-Involving Diabetic Macular Edema: A Review of the Literature. Ther. Deliv. 2018, 9, 547–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirley, M. Bimatoprost Implant: First Approval. Drugs Aging 2020, 37, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Carvalho, E.; Banthia, A.K.; Banerjee, R. Development of Polyvinyl Alcohol—Gelatin Membranes for Antibiotic Delivery in the Eye. Drug Dev. 2011, 37, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Ng, X.W.; Liu, K.L.; Veluchamy, A.B.; Lwin, N.C.; Wong, T.T.; Venkatraman, S.S. A Biodegradable Ocular Implant for Long-Term Suppression of Intraocular Pressure. Drug Deliv. Transl. Res. 2015, 5, 469–479. [Google Scholar] [CrossRef] [Green Version]
- Natu, M.V.; Gaspar, M.N.; Fontes Ribeiro, C.A.; Cabrita, A.M.; De Sousa, H.C.; Gil, M.H. In Vitro and in Vivo Evaluation of an Intraocular Implant for Glaucoma Treatment. Int. J. Pharm. 2011, 415, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Wischke, C.; Neffe, A.T.; Duc, B.; Kreiner, C.F.; Sternberg, K.; Stachs, O.; Guthoff, R.F.; Lendlein, A. A Multifunctional Bilayered Microstent as Glaucoma Drainage Device. J. Control. Release 2013, 172, 1002–1010. [Google Scholar] [CrossRef]
- Austin, A.; Lietman, T.; Rose-Nussbaumer, J. Update on the Management of Infectious Keratitis. Ophthalmology 2017, 124, 1678–1689. [Google Scholar] [CrossRef]
- Lakhundi, S.; Siddiqui, R.; Khan, N.A. Pathogenesis of Microbial Keratitis. Microb. Pathog. 2017, 104, 97–109. [Google Scholar] [CrossRef] [Green Version]
- Hunt, N.C.; Hallam, D.; Chichagova, V.; Steel, D.H.; Lako, M. The Application of Biomaterials to Tissue Engineering Neural Retina and Retinal Pigment Epithelium. Adv. Healthc. Mater. 2018, 7, 1800226. [Google Scholar] [CrossRef] [PubMed]
- Thomson, H.A.; Treharne, A.J.; Backholer, L.S.; Cuda, F.; Grossel, M.C.; Lotery, A.J. Biodegradable Poly (a-Hydroxy Ester) Blended Microspheres as Suitable Carriers for Retinal Pigment Epithelium Cell Transplantation. Biomed. Mater. Res. 2010, 95, 1233–1243. [Google Scholar] [CrossRef] [PubMed]
- Thomson, H.A.J.; Treharne, A.J.; Walker, P.; Grossel, M.C.; Lotery, A.J. Optimisation of Polymer Scaffolds for Retinal Pigment Epithelium (RPE) Cell Transplantation. Br. J. Ophtalmol. 2011, 95, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Cauldbeck, H.; Le Hellaye, M.; Mcdonald, T.O.; Long, M.; Williams, R.L.; Rannard, S.P.; Kearns, V.R. Modulated Release from Implantable Ocular Silicone Oil Tamponade Drug Reservoirs. Polym. Chem. 2018, 56, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhao, Z.; Zhao, Y.; Yang, Y. Fabrication and Evaluation of Chitosan-Gelatin Based Buckling Implant for Retinal Detachment Surgery. J. Mater. Sci. Mater. Med. 2010, 21, 2887–2895. [Google Scholar] [CrossRef]
- Wong, C.W.; Wong, T.T. Posterior Segment Drug Delivery for the Treatment of Exudative Age-Related Macular Degeneration and Diabetic Macular Oedema. Br. J. Ophthalmol. 2019, 103, 1356–1360. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, X.; Gu, Y.; Cheng, Y.; Cao, F. Recent Advance of Nanoparticle-Based Topical Drug Delivery to the Posterior Segment of the Eye. Expert Opin. Drug Deliv. 2018, 15, 687–701. [Google Scholar] [CrossRef]
- Nayak, K.; Misra, M. PEGylated Microemulsion for Dexamethasone Delivery to Posterior Segment of Eye. J. Biomater. Sci. Polym. Ed. 2020, 31, 1071–1090. [Google Scholar] [CrossRef]
- Tahara, K.; Karasawa, K.; Onodera, R.; Takeuchi, H. Feasibility of Drug Delivery to the Eye’s Posterior Segment by Topical Instillation of PLGA Nanoparticles. Asian J. Pharm. Sci. 2017, 12, 394–399. [Google Scholar] [CrossRef]
- Lechner, J.; O’Leary, O.E.; Stitt, A.W. The Pathology Associated with Diabetic Retinopathy. Vis. Res. 2017, 139, 7–14. [Google Scholar] [CrossRef]
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic Retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef]
- Radwan, S.E.S.; El-Kamel, A.; Zaki, E.I.; Burgalassi, S.; Zucchetti, E.; El-Moslemany, R.M. Hyaluronic-Coated Albumin Nanoparticles for the Non-Invasive Delivery of Apatinib in Diabetic Retinopathy. Int. J. Nanomed. 2021, 16, 4481–4494. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Ma, W.; Shi, L.; Chen, X.; Wu, R.; Zhang, Y.; Chen, H.; Chen, H. Poly(Lactic-Co-Glycolic Acid) Nanoparticle-Mediated Interleukin-12 Delivery for the Treatment of Diabetic Retinopathy. Int. J. Nanomed. 2019, 14, 6357–6369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahaling, B.; Srinivasarao, D.A.; Raghu, G.; Kasam, R.K.; Bhanuprakash Reddy, G.; Katti, D.S. A Non-Invasive Nanoparticle Mediated Delivery of Triamcinolone Acetonide Ameliorates Diabetic Retinopathy in Rats. Nanoscale 2018, 10, 16485–16498. [Google Scholar] [CrossRef] [PubMed]
- Young, T.H.; Wang, I.J.; Hu, F.R.; Wang, T.J. Fabrication of a Bioengineered Corneal Endothelial Cell Sheet Using Chitosan/Polycaprolactone Blend Membranes. Colloids Surf. B Biointerfaces 2014, 116, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Young, T.; Wang, T. Investigating the Effect of Chitosan / Polycaprolactone Blends in Differentiation of Corneal Endothelial Cells and Extracellular Matrix Compositions. Exp. Eye Res. 2019, 185, 107679. [Google Scholar] [CrossRef]

| Author | Target | Drugs | Polymers | Technology | Administration |
|---|---|---|---|---|---|
| Anterior Ocular Disorders | |||||
| [32] | Corneal wound healing | Ferulic acid | Pluronic® F68 and hyaluronan | Nanocomposite (micelle-nanogel) | Topical (ocular) |
| [33] | Infectious ocular keratitis | hLF 1-11 (synthetic antimicrobial peptide derived from human lactoferrin) | Hydroxypropylmethylcellulose (HPMC) and HA | Freeze-dried ocular insert | Topical (ocular)—no in vivo tests |
| [34] | Subconjunctival retention | Sunitinib malate | Methylcellulose (MC), HA and PLGA | Microparticles | Subconjunctival injection |
| [35] | Ocular hypertension | Dorzolamide HCl | Chitosan, PCL, and PVA | Polymeric nanoparticles | Topical (ocular) |
| [36] | Cornal permeability | Myricetin | Polyvinyl caprolactam, polyvinyl acetate, and polyethylene glycol graft copolymer | Polymeric micelles | Topical (ocular) |
| [37] | Fungal keratitis | Amphotericin B | PVA and PVP | Microneedle ocular patch (polymer composite) | Micromolding technique to mimic contact lenses |
| [38] | Steroid-induced cataract | Triamcinolone acetonide | PLC and Pluronic® F68 | Polymeric core-shell nanoparticles | Topical (eye drops) |
| [39] | Ocular hypertension | Timolol maleate | HPMC and HA | Composite ocular films | Topical (eye drops) |
| [40] | Increase hydrophobic drugs penetration | Tacrolimus | Amino-terminated poly(ethylene glycol-block-poly(D,L)-lactic acid) (NH2-PEG-b-PLA) and HPMC | Nanomicelles | Topical (eye drops) |
| [41] | Bacterial Keratitis | Ofloxacin | Chitosan and PEG | Enhanced lipid nanoparticles | Topical (eye drops) |
| [42] | Corneal neovascularization | Axitinib | MPEG and PCL | Polymeric micelles | Topical (ocular) |
| [43] | Ocular hypertension | Dorzolamide HCl | Carbopol and HPCM | pH-triggered in situ gel (ISG) | Topical (ocular) |
| [44] | Dry eye disorders and corneal ulcer | Levofloxacin | HPMC and sodium alginate | pH-triggered in situ gel (ISG) | Topical (ocular) |
| [45] | Ocular drug delivery | Azelastine HCl | Pluronic® F127 and carbopol | Polymeric micellar gel | Topical (eye drops) |
| [46] | Ocular drug delivery | Levofloxacin | Eudragit® RS and carbopol | Mucoadhesive minitablets | Topical (ocular) |
| [47] | Ocular drug delivery | Triamcinolone acetonide | α,β-poly(N-2-hydroxyethyl)-D,L-aspartamide (PHEA), and poly-butylene succinate (PBS) | Microfibrillar polymeric ocular inserts | Topical (ocular) |
| [48] | Ocular drug delivery | Small peptides | Polyoxyethylene hydrogenated castor oil 40 (HCO-40) and octoxynol 40 (OC-40) | Self-assembling multi-layered nanomicelles | Topical (ocular) |
| [49] | Glaucoma | Timolol maleate | Chitosan, PVP, and poly (N-isopropylacrylamide) | Ocular contact lenses | Topical (ocular) |
| [50] | Fungal keratitis | Econazole | Carboxymethyl-α-cyclodextrin and chitosan | Eye drops | Topical (ocular) |
| [51] | Anterior segment of the eye | Betaxol hydrochloride | Cellulose acetate and Eudragit S100 | Inner layer-embedded contact lenses | Topical (ocular) |
| [52] | Anterior segment of the eye | Diclofenac sodium | Ethyl cellulose and Eudragit S100 | Inner layer-embedded contact lenses | Topical (ocular) |
| [53] | Controlled release of poorly bioavailable drugs into the aqueous humor | Cannabigerolic acid | Hydrogel: Methylcellulose (MC) and HAnanoparticles: poly(ethylene oxide) and PLA | In-situ forming nanoparticle-laden hydrogel | Topical (ocular) |
| [54] | Ocular hypertension | Timolol maleate | Chitosan and gelatin | Hydrogel | Topical (ocular) |
| [55] | Bacterial growth | Moxifloxacin hydrochloride, chlorhexidine diacetate monohydrate, and diclofenac sodium salt | Sodium alginate, HA, chitosan, and polylysine hydrobromide | Layer-by-layer coatings on contact lenses (hydrogel) | Topical (ocular) |
| [56] | Dry eye syndrome | Cyclosporine A | PEG and PLA | Polymeric micelles | Topical (ocular) |
| [57] | Glaucoma | Timolol (precursor) | PEG and polyamidoamine (PAMAM) | Polymeric dendrimer | Topical (ocular) |
| [58] | Autoimmune uveitis | Cyclosporine A | Methoxy-poly(ethylene-glycol)-hexyl substituted poly-(lactic acid) (mPEGhexPLA) | Nanocarriers | Topical (ocular) |
| [59] | Keratoprosthesis, orthokeratology, and mini-scleral lens | - | Ester-based polyurethane (EBPU), N,N-dimethylacrylamide (NNDMA), N-vinyl pyrrolidone (NVP), and acryloylmorpholine (AMO) | High modulus hydrogels | Topical (ocular) |
| [60] | Hyperacute bacterial conjunctivitis and endophthalmitis | Tobramycin sulfate | Chitosan HCl and Poloxamer 407 | Mucoadhesive microparticles incorporated in thermosensitive in situ gel | Topical (ocular) |
| [61] | Glaucoma | Acetazolamide | HA and PEG | Polymeric films | Topical (ocular) |
| [62] | Corneal keratitis or bacterial endophthalmitis | Moxifloxacin | HPMC, PVP-K30, and PEG | Ocular inserts | Topical (ocular) |
| [63] | Bacterial keratitis | Ceftazidime | Chitosan, HPMC, and HA | Mucoadhesive nanoparticles | Topical (eye drops) |
| [64] | Corneal delivery | Besifloxacin | PVA and PVP | Polymeric microneedles | Topical (ocular) |
| [65] | Anterior segment of the eye | Curcumin | PVCL, PVA, and PEG | Polymeric nanomicelles | Topical (ocular) |
| [66] | Glaucoma | Resveratrol | PEG and chitosan | Polymeric nanoparticles | Topical (ocular) |
| [67] | Glaucoma | - | Poly(N-isopropylacrylamide) and gelatin | Hydrogel | Intracameral injection |
| [68] | Dry eye syndrome | Epigallocatechin gallate | Gelatin-gpoly(N-isopropylacrylamide) | In situ gelling carriers | Topical (ocular) |
| [69] | Fungal keratitis | Amphotericin B | Chitosan and Poloxamer® 188 | Nanostructured lipid carriers | Topical (ocular) |
| [70] | Glaucoma | Acetazolamide | Ethyl cellulose and Eudragit RS100 | Polymeric nanocapsules | Topical (ocular) |
| [71] | Glaucoma | Pilocarpine hydrochloride | Gelatin-gpoly(N-isopropylacrylamide) | In situ forming hydrogel | Intracameral injection |
| Posterior ocular disorders | |||||
| [72] | Retinal diseases | Erythropoietin | Chitosan and hyaluronic acid (HA) | Nanoparticles | Topical (ocular) |
| [73] | Systemic absorption and brain-targeting effect | Vinpocetine | Carbopol and HPCM | pH-triggered in situ gel (ISG) | Topical (ocular) |
| [74] | Treatment of mid-posterior diseases. | Glycylsarcosine | Chitosan-glutathione | Functional intercalated nanocomposites | Topical (ocular) |
| [75] | Proliferative vitreoretinopathy | Ibuprofen and all-trans retinoic acid | Dimethylsiloxiane, PEG, and silicone | Polymer grafts | - |
| General ocular disorders | |||||
| [76] | Delivery to ocular tissues | - | Polysaccharides | In situ forming gel | Topical (ocular) |
| [77] | Ocular drug delivery | - | - | Liposomes, SLN, NLC, niosomes... | Ocular |
| [78] | Ocular drug delivery | - | - | Contact lenses | Topical (ocular) |
| [79] | Ocular drug delivery | - | - | Polymeric nanomicelles | Topical (ocular) |
| [80] | Ocular drug delivery | - | - | Contact lenses | Topical (ocular) |
| [81] | Improve drug delivery and encapsulation in nanocarriers | - | Chitosan, PLGA, alginate | Nanoparticles | Topical (ocular) |
| [82] | Improve drug delivery and residence time | - | Gellan gum and Pullulan | Electrospun nanofibers | Topical (ocular) |
| [83] | Bacteria and fungi ocular infections | - | Two antibacterial synthetic polymers with dipyridine motif | ? | Topical (ocular) |
| [3] | Ocular drug/gene delivery | - | PLGA, chitosan and gelatin | Nanocarriers | Ocular |
| [14] | Ocular drug delivery | - | - | Micro and nanoparticles (gels) | Topical (ocular) |
| [84] | Drug delivery | Triamcinolone acetonide and ovoalbumin | PEG and diacrylate (PEGDA) | Implants | - |
| [85] | Oncology and ophthalmology | - | - | Molecularly imprinted polymers (MIP) | - |
| [86] | Ocular inflammation | Tacrolimus | PEG2000 and derivatives | Polymeric micelles | Topical (ocular) |
| [87] | Ocular drug delivery | Dexamethasone sodium phosphate | Poloxamer 188 and Poloxamer 407 | In situ gel loaded with nanoparticles | Topical (ocular) |
| [88] | Sustained delivery of macromolecules | Insulin, catalase, octreotide, IgG, IgG Fab, Lysozyme, BSA | P(CL-co-GA)-PEG-P(GA-co-CL) | Polymeric nanoparticles | Ocular |
| [89] | Neovascular ocular diseases | Imatinib | HA and PEG | Polymeric micelles | Topical (ocular) |
| [90] | Ocular drug delivery | Infliximab | HA, N-isopropylacylamide (pNIPAAM) and PEG | Collapsible hyaluronic acid hydrogels | Intraocular injection |
| [91] | Ocular inflammatory disorders | Pioglitazone | PLGA and PEG | Polymeric nanospheres | Topical (ocular) |
| [92] | Ocular drug delivery | Timolol maleate, dexamethasone, and dorzolamide hydrochloride | Poloxamer 407, Poloxamer 188, and chitosan | In situ forming ophthalmic gel | Topical (eye drops) |
| [93] | Ocular drug delivery | Pilocarpine hydrochloride | Cellulose and Poloxamer 407 | In situ gelling thermo-responsive hydrogels | Topical (ocular) |
| [94] | Intraocular inflammation | Moxifloxacin hydrochloride | Chitosan and HA | Lipid-polymer hybrid nanoparticles | Topical (ocular) |
| [95] | Ocular drug delivery | - | - | Polymeric micelles | Ocular |
| [96] | Ocular drug delivery | - | - | Lens-based and conventional drug delivery | Topical (ocular) |
| [97] | Choroidal neovascularization | Cell-penetrating peptides | PEG and PLGA | Polymeric nanoparticles | Topical (ocular) |
| [98] | Ocular drug delivery | Vancomycin | Eudragit® RS100 and carbopol | Polymeric nanoparticles | Topical (ocular) |
| [99] | Ocular drug delivery | Pilocarpine hydrochloride | Poloxamer 407 and gellan gum | In situ gelling systems | Topical (ocular) |
| [100] | Ocular drug delivery | Flurbiprofen | PCL and poloxamer 188 | Freeze-dried polymeric nanoparticles | Topical (eye drops) |
| [101] | Delivery to ocular tissues | - | - | Contact lenses | Topical (ocular) |
| [102] | Ocular drug delivery | - | - | Dendrimers | - |
| [103] | Ocular drug delivery | - | Elastin-like polypeptides | - | - |
| [104] | Ocular inflammation and infection | Dexamethasone sodium phosphate and Tobramycin sulfate | Poloxamer 407 and HPMC K4M | Thermoresponsive ophthalmic in situ gel | Topical (ocular) |
| [105] | Ocular drug delivery | Fluorescein sodium and ofloxacin | Pluronic® F127 and Pluronic® F68 | In situ gelling system | Topical (ocular) |
| [106] | Ocular drug delivery | Lidocaine | PLGA and collagen | Polymeric nanoparticles | Topical (ocular) |
| [107] | Ocular drug delivery | Diclofenac sodium | Methoxy PEG-PCL copolymers and α-cyclodextrin | Micellar supramolecular hydrogel | Topical (ocular) |
| [108] | Ocular drug delivery | - | - | Polymeric microsponges | Topical (ocular) |
| [109] | Ophthalmic gene therapy | - | - | - | - |
| Disease/Tissue | DDS (Technology) | Most Used Polymers |
|---|---|---|
| Posterior segment of the eye | Nanoparticles/nanocomposites/polymer grafts | Chitosan/HA/PEG |
| Anterior segment of the eye | Micelles/contact lenses | PCL/PEG/PVA/PVP/EC/Eudragit® |
| Keratitis | Inserts/composites/nanoparticles | HPMC/HA/PVA/PVP/chitosan/PLGA |
| Dry eye | In situ hydrogel/micelles | HPMC/PEG/gelatin |
| Glaucoma | Nanoparticles/in situ hydrogel | Chitosan/HPMC/HA/PEG/gelatin |
| DDS | In situ hydrogel/nanoparticles | Poloxamers/PLGA/PEG/carbopol |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arribada, R.G.; Behar-Cohen, F.; de Barros, A.L.B.; Silva-Cunha, A. The Use of Polymer Blends in the Treatment of Ocular Diseases. Pharmaceutics 2022, 14, 1431. https://doi.org/10.3390/pharmaceutics14071431
Arribada RG, Behar-Cohen F, de Barros ALB, Silva-Cunha A. The Use of Polymer Blends in the Treatment of Ocular Diseases. Pharmaceutics. 2022; 14(7):1431. https://doi.org/10.3390/pharmaceutics14071431
Chicago/Turabian StyleArribada, Raquel Gregorio, Francine Behar-Cohen, Andre Luis Branco de Barros, and Armando Silva-Cunha. 2022. "The Use of Polymer Blends in the Treatment of Ocular Diseases" Pharmaceutics 14, no. 7: 1431. https://doi.org/10.3390/pharmaceutics14071431
APA StyleArribada, R. G., Behar-Cohen, F., de Barros, A. L. B., & Silva-Cunha, A. (2022). The Use of Polymer Blends in the Treatment of Ocular Diseases. Pharmaceutics, 14(7), 1431. https://doi.org/10.3390/pharmaceutics14071431