Co-Delivery of siRNA and Chemotherapeutic Drug Using 2C5 Antibody-Targeted Dendrimer-Based Mixed Micelles for Multidrug Resistant Cancers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Dox-Loaded MDM and 2C5-Modified Micelle
2.2.2. Particle Size, Polydispersity, Zeta Potential Measurement and Drug Concentration Analysis
2.2.3. Surface Morphology
2.2.4. Homolysis of Dendrimer-Based Nanoparticles
2.2.5. Two-Dimensional Cell Culture Preparation
2.2.6. Cellular Uptake of MDM Nanoparticles in Monolayer
2.2.7. P-gp Downregulation in Monolayers Using Western Blot
2.2.8. Cellular Association by Fluorescence Imaging
2.2.9. Cytotoxicity of Nanoparticles in a Monolayer Model
2.2.10. Statistical Analysis
3. Results
3.1. Stability of MDM
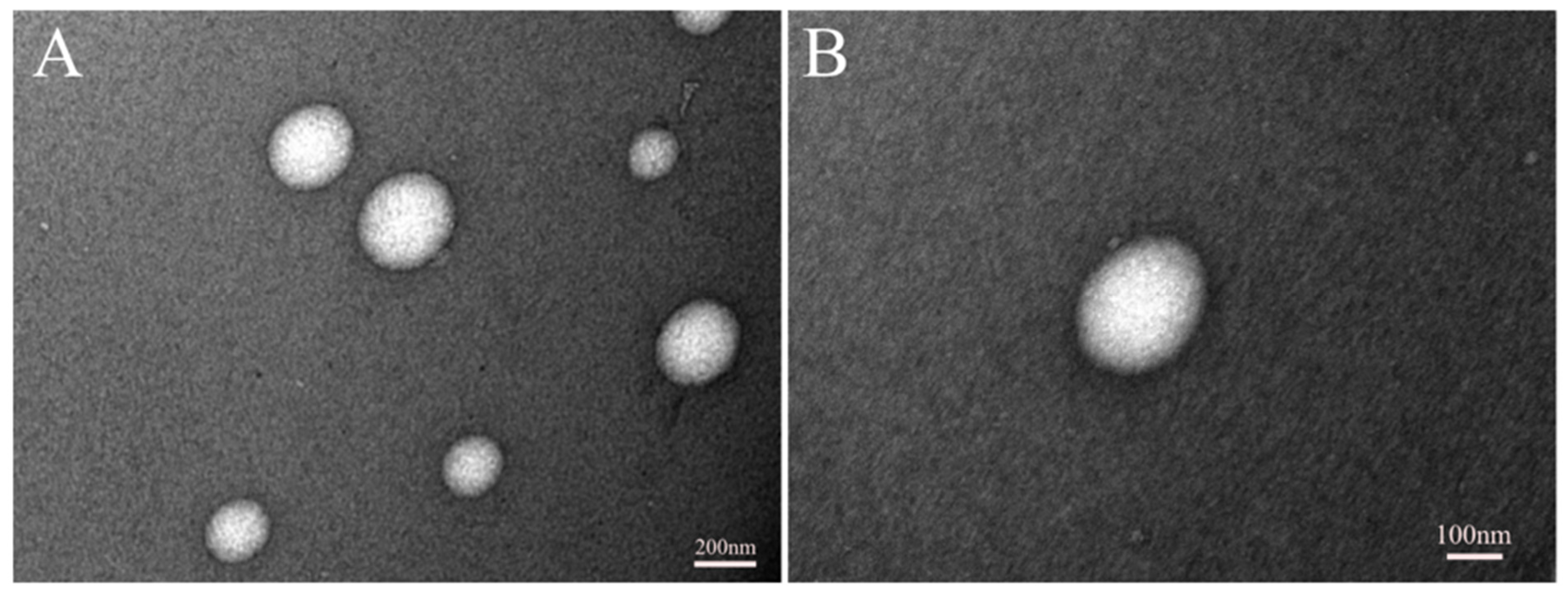
3.2. Imaging of Micelles Using TEM
3.3. Hemolysi
3.4. Cellular Association of 2C5-Modified MDM with Cancer Cells
3.5. Cellular Uptake by FACS
3.6. Downregulation of Membrane Bound P-gp
3.7. Cytotoxicity in Monolayer Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef]
- Szakács, G.; Paterson, J.K.; Ludwig, J.A.; Booth-Genthe, C.; Gottesman, M.M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006, 5, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.R.; Pattni, B.S.; Abouzeid, A.H.; Torchilin, V.P. Nanopreparations to overcome multidrug resistance in cancer. Adv. Drug Deliv. Rev. 2013, 65, 1748–1762. [Google Scholar] [CrossRef] [Green Version]
- Doyle, L.A.; Yang, W.; Abruzzo, L.V.; Krogmann, T.; Gao, Y.; Rishi, A.K.; Ross, D.D. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc. Natl. Acad. Sci. USA 1998, 95, 15665–15670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allikmets, R.; Schriml, L.M.; Hutchinson, A.; Romano-Spica, V.; Dean, M. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res. 1998, 58, 5337–5339. [Google Scholar] [PubMed]
- Miyake, K.; Mickley, L.; Litman, T.; Zhan, Z.; Robey, R.; Cristensen, B.; Brangi, M.; Greenberger, L.; Dean, M.; Fojo, T.; et al. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: Demonstration of homology to ABC transport genes. Cancer Res. 1999, 59, 8–13. [Google Scholar]
- Lage, H. An overview of cancer multidrug resistance: A still unsolved problem. Cell Mol. Life Sci. 2008, 65, 3145–3167. [Google Scholar] [CrossRef]
- Cordon-Cardo, C.; O’Brien, J.P.; Boccia, J.; Casals, D.; Bertino, J.R.; Melamed, M.R. Expression of the multidrug resistance gene product (P-glycoprotein) in human normal and tumor tissues. J. Histochem. Cytochem. 1990, 38, 1277–1287. [Google Scholar] [CrossRef]
- Thiebaut, F.; Tsuruo, T.; Hamada, H.; Gottesman, M.M.; Pastan, I.; Willingham, M.C. Immunohistochemical localization in normal tissues of different epitopes in the multidrug transport protein P170: Evidence for localization in brain capillaries and crossreactivity of one antibody with a muscle protein. J. Histochem. Cytochem. 1989, 37, 159–164. [Google Scholar] [CrossRef] [Green Version]
- Hilgendorf, C.; Ahlin, G.; Seithel, A.; Artursson, P.; Ungell, A.L.; Karlsson, J. Expression of thirty-six drug transporter genes in human intestine, liver, kidney, and organotypic cell lines. Drug Metab. Dispos. 2007, 35, 1333–1340. [Google Scholar] [CrossRef] [Green Version]
- Szakács, G.; Annereau, J.P.; Lababidi, S.; Shankavaram, U.; Arciello, A.; Bussey, K.J.; Reinhold, W.; Guo, Y.; Kruh, G.D.; Reimers, M.; et al. Predicting drug sensitivity and resistance: Profiling ABC transporter genes in cancer cells. Cancer Cell 2004, 6, 129–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.; Fisher, M.H.; Xu, D.; Miyamoto, Y.J.; Marchand, A.; Van Aerschot, A.; Herdewijn, P.; Juliano, R.L. Inhibition of MDR1 gene expression by chimeric HNA antisense oligonucleotides. Nucleic Acids Res. 2004, 32, 4411–4419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finch, A.; Pillans, P. P-glycoprotein and its role in drug-drug interactions. Aust. Prescr. 2014, 37, 137–139. [Google Scholar] [CrossRef]
- Wu, H.; Hait, W.N.; Yang, J.M. Small interfering RNA-induced suppression of MDR1 (P-glycoprotein) restores sensitivity to multidrug-resistant cancer cells. Cancer Res. 2003, 63, 1515–1519. [Google Scholar] [PubMed]
- Gavrilov, K.; Saltzman, W.M. Therapeutic siRNA: Principles, challenges, and strategies. Yale J. Biol. Med. 2012, 85, 187–200. [Google Scholar]
- Sarisozen, C.; Pan, J.; Dutta, I.; Torchilin, V.P. Polymers in the co-delivery of siRNA and anticancer drugs to treat multidrug-resistant tumors. J. Pharm. Investig. 2017, 47, 37–49. [Google Scholar] [CrossRef]
- Pan, J.; Mendes, L.P.; Yao, M.; Filipczak, N.; Garai, S.; Thakur, G.A.; Sarisozen, C.; Torchilin, V.P. Polyamidoamine dendrimers-based nanomedicine for combination therapy with siRNA and chemotherapeutics to overcome multidrug resistance. Eur. J. Pharm. Biopharm. 2019, 136, 18–28. [Google Scholar] [CrossRef]
- Gao, J.; Dutta, K.; Zhuang, J.; Thayumanavan, S. Cellular-and Subcellular-Targeted Delivery Using a Simple All-in-One Polymeric Nanoassembly. Angew. Chem. Int. Ed. 2020, 59, 23466–23470. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H.A.; Alrokayan, S.A.; Kumar, S. Targeted anticancer therapy: Overexpressed receptors and nanotechnology. Clin. Chim. Acta 2014, 436, 78–92. [Google Scholar]
- Pan, J.; Attia, S.A.; Subhan, M.A.; Filipczak, N.; Mendes, L.P.; Li, X.; Kishan Yalamarty, S.S.; Torchilin, V.P. Monoclonal Antibody 2C5-Modified Mixed Dendrimer Micelles for Tumor-Targeted Codelivery of Chemotherapeutics and siRNA. Mol. Pharm. 2020, 17, 1638–1647. [Google Scholar] [CrossRef]
- Iakoubov, L.; Rokhlin, O.; Torchilin, V. Anti-nuclear autoantibodies of the aged reactive against the surface of tumor but not normal cells. Immunol. Lett. 1995, 47, 147–149. [Google Scholar] [CrossRef]
- Iakoubov, L.Z.; Torchilin, V.P. Nucleosome-releasing treatment makes surviving tumor cells better targets for nucleosome-specific anticancer antibodies. Cancer Detect. Prev. 1998, 22, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Elbayoumi, T.A.; Torchilin, V.P. Tumor-specific antibody-mediated targeted delivery of Doxil reduces the manifestation of auricular erythema side effect in mice. Int. J. Pharm. 2008, 357, 272–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudimack, J.; Lee, R.J. Targeted drug delivery via the folate receptor. Adv. Drug Deliv. Rev. 2000, 41, 147–162. [Google Scholar] [CrossRef]
- Torchilin, V.P.; Lukyanov, A.N. Peptide and protein drug delivery to and into tumors: Challenges and solutions. Drug Discov. Today 2003, 8, 259–266. [Google Scholar] [CrossRef]
- Navarro, G.; Sawant, R.R.; Essex, S.; Tros de Ilarduya, C.; Torchilin, V.P. Phospholipid–polyethylenimine conjugate-based micelle-like nanoparticles for siRNA delivery. Drug Deliv. Transl. Res. 2011, 1, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Sarisozen, C.; Abouzeid, A.H.; Torchilin, V.P. The effect of co-delivery of paclitaxel and curcumin by transferrin-targeted PEG-PE-based mixed micelles on resistant ovarian cancer in 3-D spheroids and in vivo tumors. Eur. J. Pharm. Biopharm. 2014, 88, 539–550. [Google Scholar] [CrossRef] [Green Version]
- Biswas, S.; Deshpande, P.P.; Navarro, G.; Dodwadkar, N.S.; Torchilin, V.P. Lipid modified triblock PAMAM-based nanocarriers for siRNA drug co-delivery. Biomaterials 2013, 34, 1289–1301. [Google Scholar]
- Amin, K.; Dannenfelser, R.M. In vitro hemolysis: Guidance for the pharmaceutical scientist. J. Pharm. Sci. 2006, 95, 1173–1176. [Google Scholar]
- Han, J.; Lim, W.; You, D.; Jeong, Y.; Kim, S.; Lee, J.E.; Shin, T.H.; Lee, G.; Park, S. Chemoresistance in the human triple-negative breast cancer cell line MDA-MB-231 induced by doxorubicin gradient is associated with epigenetic alterations in histone deacetylase. J. Oncol. 2019, 2019, 1345026. [Google Scholar] [CrossRef]
- Zou, W.; Sarisozen, C.; Torchilin, V.P. The reversal of multidrug resistance in ovarian carcinoma cells by co-application of tariquidar and paclitaxel in transferrin-targeted polymeric micelles. J. Drug Target. 2017, 25, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Riehle, R.; Pattni, B.; Jhaveri, A.; Kulkarni, A.; Thakur, G.; Degterev, A.; Torchilin, V. Combination Nanopreparations of a Novel Proapoptotic Drug–NCL-240, TRAIL and siRNA. Pharm. Res. 2016, 33, 1587–1601. [Google Scholar] [CrossRef] [PubMed]
- Brownlie, A.; Uchegbu, I.; Schätzlein, A. PEI-based vesicle-polymer hybrid gene delivery system with improved biocompatibility. Int. J. Pharm. 2004, 274, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Chouly, C.; Bordenave, L.; Bareille, R.; Guerin, V.; Baquey, A.; Pouliquen, D.; Baquey, C.; Jallet, P. In vitro study of the hemocompatibility of superparamagnetic contrast agent for magnetic resonance imaging. Clin. Mater. 1994, 15, 293–301. [Google Scholar] [CrossRef]
- Vittaz, M.; Bazile, D.; Spenlehauer, G.; Verrecchia, T.; Veillard, M.; Puisieux, F.; Labarre, D. Effect of PEO surface density on long-circulating PLA-PEO nanoparticles which are very low complement activators. Biomaterials 1996, 17, 1575–1581. [Google Scholar] [CrossRef]
- Krzyzaniak, J.F.; Núñez, F.A.A.; Raymond, D.M.; Yalkowsky, S.H. Lysis of human red blood cells. 4. Comparison of in vitro and in vivo hemolysis data. J. Pharm. Sci. 1997, 86, 1215–1217. [Google Scholar] [CrossRef]
- Torchilin, V.P.; Rammohan, R.; Weissig, V.; Levchenko, T.S. TAT peptide on the surface of liposomes affords their efficient intracellular delivery even at low temperature and in the presence of metabolic inhibitors. Proc. Natl. Acad. Sci. USA 2001, 98, 8786–8791. [Google Scholar] [CrossRef] [Green Version]







| Day 0 | Day 5 | Day 15 | Day 20 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Size (nm) | PdI | Zeta Potential (mV) | Size (nm) | PdI | Zeta Potential (mV) | Size (nm) | PdI | Zeta Potential (mV) | Size (nm) | PdI | Zeta Potential (mV) | |
| 2C5-modified MDM with siRNA and DOX (2C5-MDM-D-R) | 165.4 ± 70.1 | 0.175 | 2.04 ± 5.96 | 149.8 ± 71.1 | 0.172 | 2.12 ± 7.06 | 160.5 ± 60.8 | 0.120 | 2.62 ± 6.04 | 148.4 ± 78.8 | 0.181 | 1.17 ± 5.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yalamarty, S.S.K.; Filipczak, N.; Li, X.; Pathrikar, T.V.; Cotter, C.; Torchilin, V.P. Co-Delivery of siRNA and Chemotherapeutic Drug Using 2C5 Antibody-Targeted Dendrimer-Based Mixed Micelles for Multidrug Resistant Cancers. Pharmaceutics 2022, 14, 1470. https://doi.org/10.3390/pharmaceutics14071470
Yalamarty SSK, Filipczak N, Li X, Pathrikar TV, Cotter C, Torchilin VP. Co-Delivery of siRNA and Chemotherapeutic Drug Using 2C5 Antibody-Targeted Dendrimer-Based Mixed Micelles for Multidrug Resistant Cancers. Pharmaceutics. 2022; 14(7):1470. https://doi.org/10.3390/pharmaceutics14071470
Chicago/Turabian StyleYalamarty, Satya Siva Kishan, Nina Filipczak, Xiang Li, Tanvi Vinod Pathrikar, Colin Cotter, and Vladimir P. Torchilin. 2022. "Co-Delivery of siRNA and Chemotherapeutic Drug Using 2C5 Antibody-Targeted Dendrimer-Based Mixed Micelles for Multidrug Resistant Cancers" Pharmaceutics 14, no. 7: 1470. https://doi.org/10.3390/pharmaceutics14071470
APA StyleYalamarty, S. S. K., Filipczak, N., Li, X., Pathrikar, T. V., Cotter, C., & Torchilin, V. P. (2022). Co-Delivery of siRNA and Chemotherapeutic Drug Using 2C5 Antibody-Targeted Dendrimer-Based Mixed Micelles for Multidrug Resistant Cancers. Pharmaceutics, 14(7), 1470. https://doi.org/10.3390/pharmaceutics14071470







