Another Move towards Bicalutamide Dissolution and Permeability Improvement with Acetylated β-Cyclodextrin Solid Dispersion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. UV-Spectroscopy
2.2.2. Determination of BCL Phase Solubility in Me- and Ac-β-CD Solutions
2.2.3. Determination of the Complex Stoichiometry with Job’s Plot
2.2.4. Preparation of the Solid Samples by Mechanical Grinding Procedure
2.2.5. Differential Scanning Calorimetry
2.2.6. PXRD Analysis
2.2.7. IR-Spectroscopy
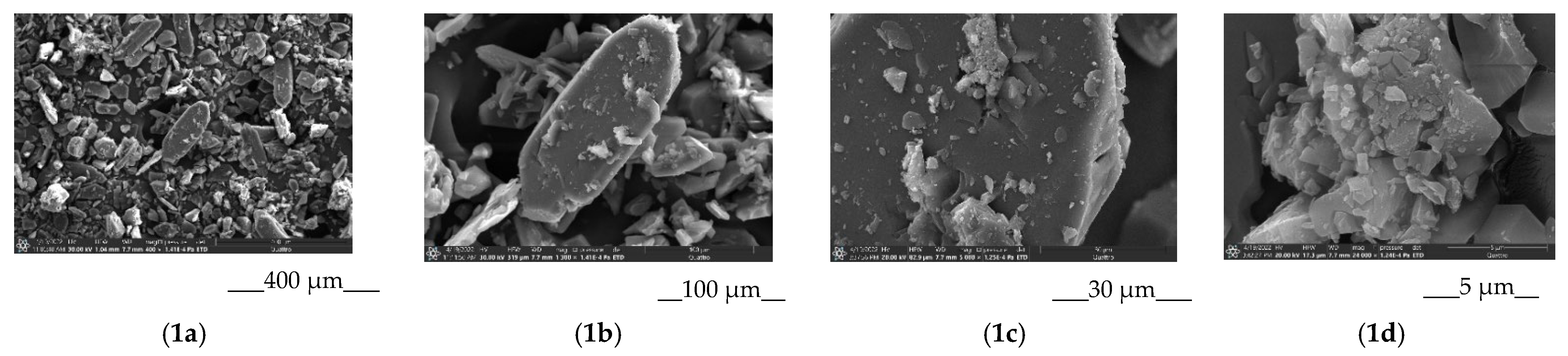
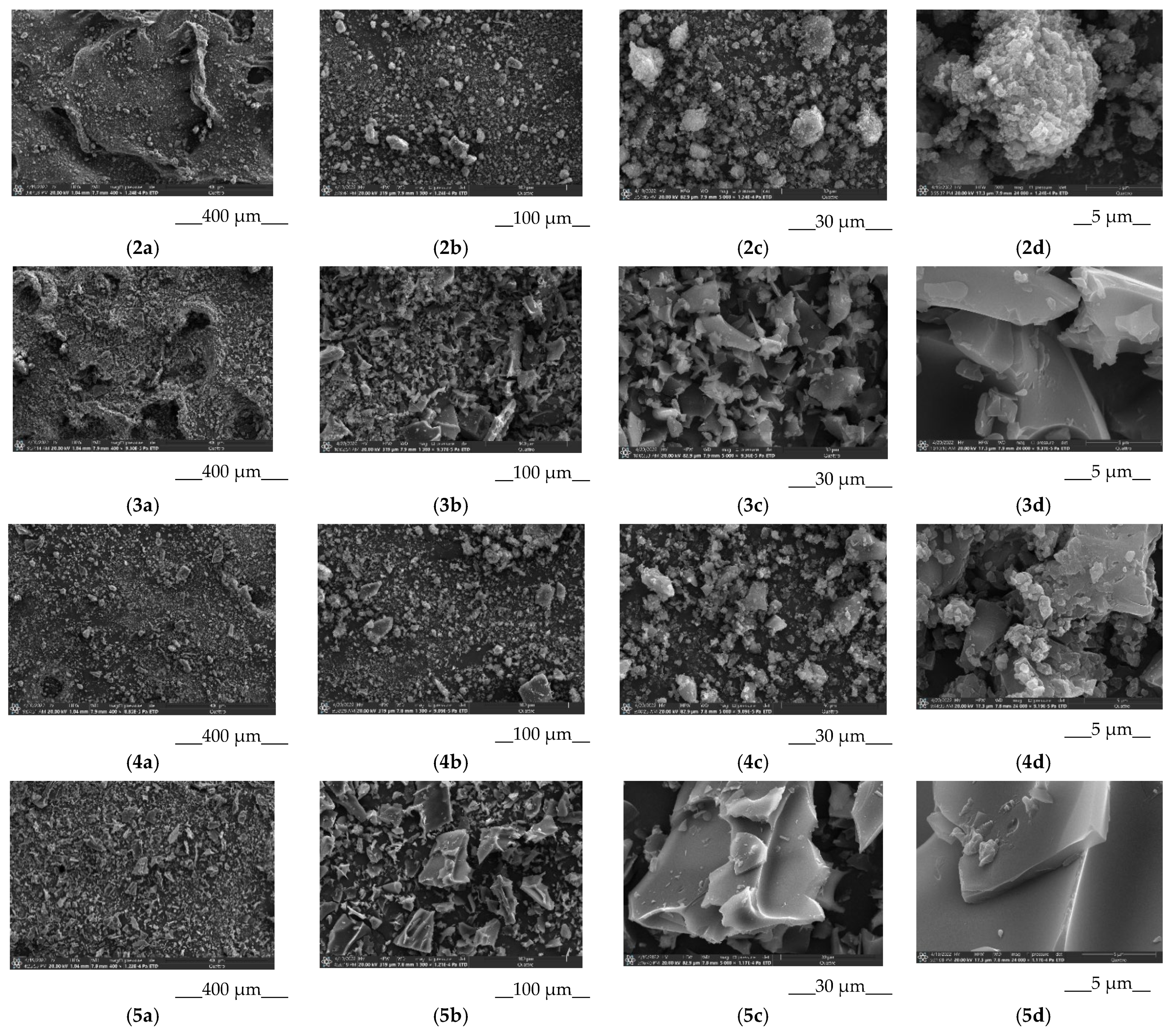
2.2.8. Scanning Electron Microscopy
2.2.9. Dissolution/Permeation Setup in a Side-by-Side Cell
2.2.10. Apparent Solubility Determination
2.2.11. In Vitro Permeation Experiments for the Apparent Permeability Coefficient Calculation
2.2.12. Calculation of the Complexation Thermodynamic Functions
3. Results
3.1. BCL Complex Formation with Me-β-CD and Ac-β-CD in Solution
3.2. Solid BCL/Ac-β-CD Complex
3.2.1. Characterization of the Solid BCL/Ac-β-CD Complex
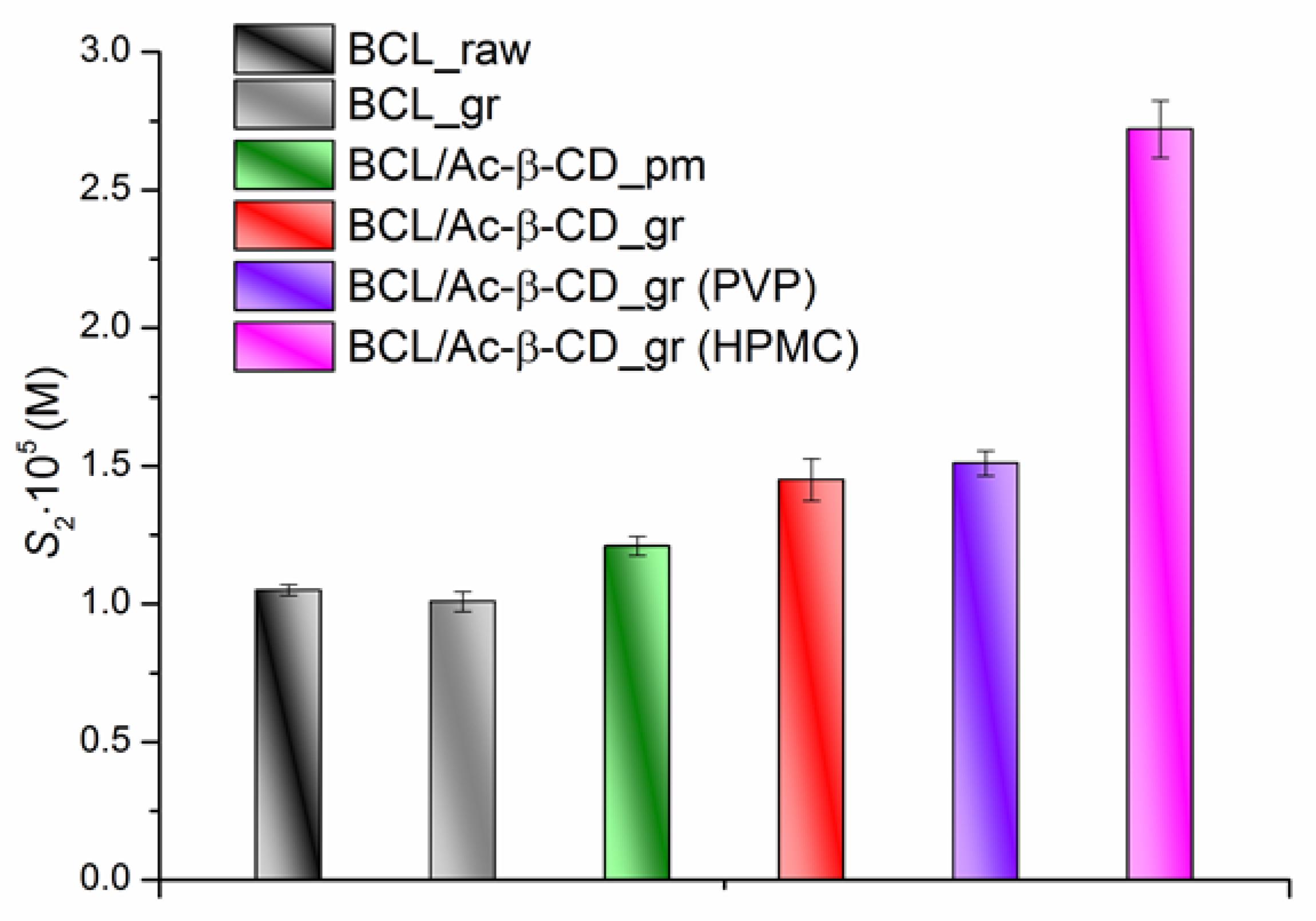
3.2.2. Equillibrium Solubility of the BCL/Ac-β-CD as Solid Samples
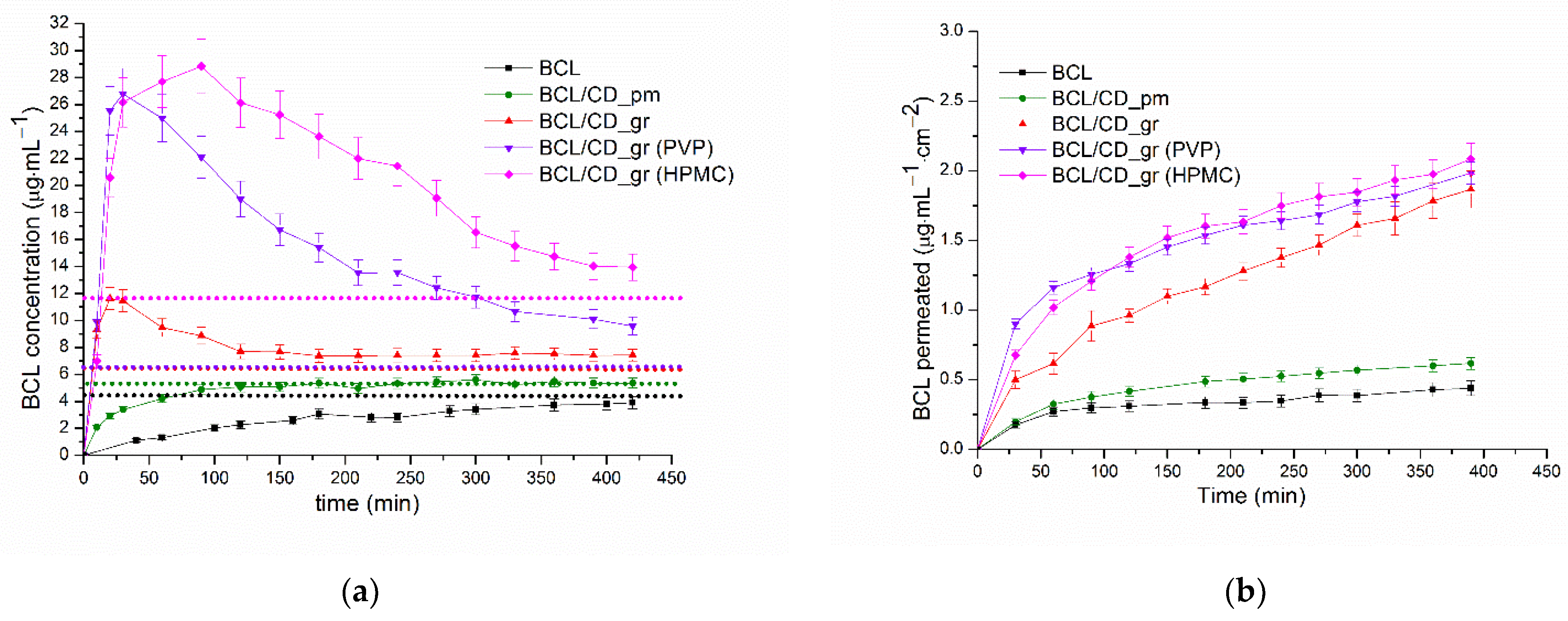
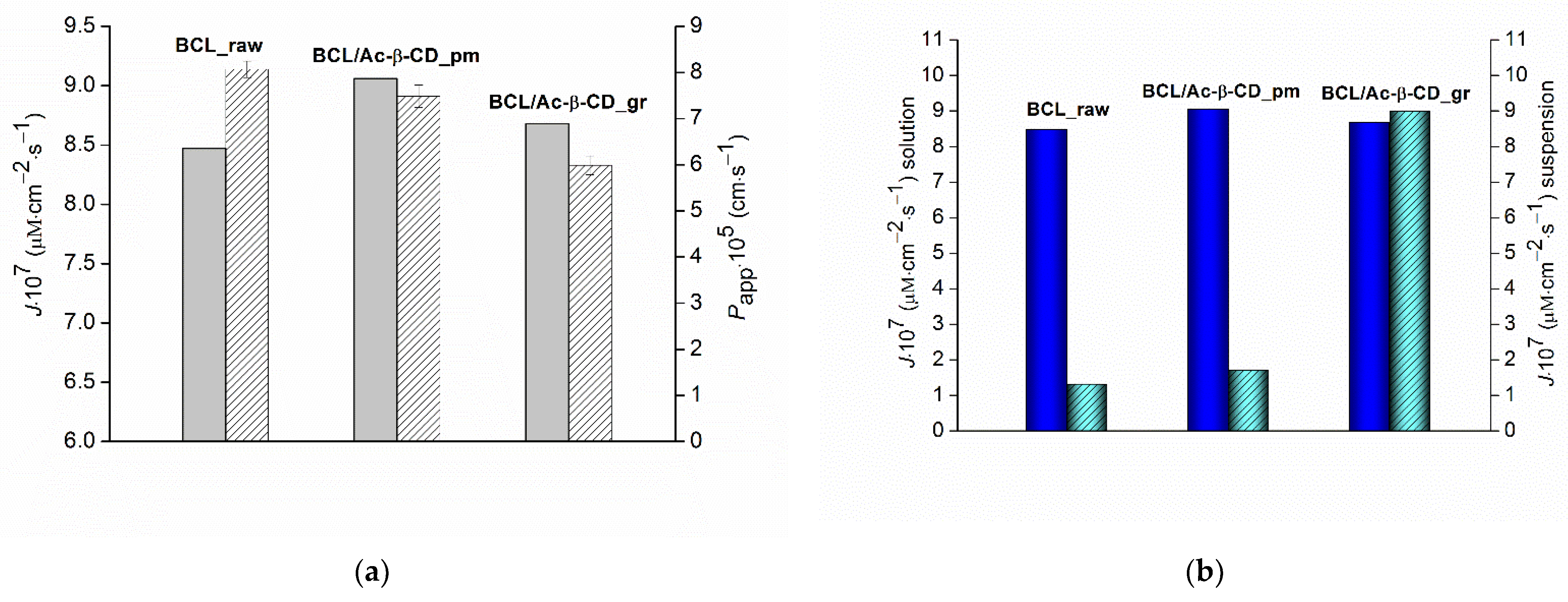
3.2.3. Non-Sink Dissolution/Permeation Behavior of the BCL Solid Samples
3.2.4. Permeability Experiments in Sink Conditions, Determination of the Steady State BCL Permeability Coefficients
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Denis, L.; Mahler, C. Pharmacodynamics and pharmacokinetics of bicalutamide: Defining an active dosing regimen. Urology 1996, 47, 26–28. [Google Scholar] [CrossRef]
- Fischer, J.; Ganellin, C.R. Analogue-Based Drug Discovery; John Wiley & Sons: Hoboken, NJ, USA, 2006; p. 515. ISBN 978-352-760-749-5. [Google Scholar]
- Boers, J.; Venema, C.M.; de Vries, E.F.J.; Hospers, G.A.P.; Boersma, H.H.; Rikhof, B.; Dorbritz, C.; Glaudemans, A.W.J.M.; Schröder, C.P. Serial [18F]-FDHT-PET to predict bicalutamide efficacy in patients with androgen receptor positive metastatic breast cancer. Eur. J. Cancer 2021, 144, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.L.; Pore, Y.V.; Kuchekar, B.S.; Late, S.G. Solid-state characterization and dissolution properties of bicalutamide- β-cyclodextrin inclusion complex. Pharmazie 2008, 63, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Mendyk, A.; Pacławski, A.; Szafraniec-Szczęsny, J.; Antosik, A.; Jamróz, W.; Paluch, M.; Jachowicz, R. Data-driven modeling of the bicalutamide dissolution from powder systems. AAPS PharmSciTech 2020, 21, 110–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cockshot, I.D. Bicalutamide clinical pharmacokinetics and metabolism. Clin. Pharmacokinet. 2004, 43, 855–878. [Google Scholar]
- Perlovich, G.L.; Blokhina, S.V.; Manin, N.G.; Volkova, T.V.; Tkachev, V.V. Polymorphism and solvatomorphism of bicalutamide. Thermophysical study and solubility. J. Therm. Anal. Calorim. 2013, 111, 655–662. [Google Scholar] [CrossRef]
- Volkova, T.V.; Simonova, O.R.; Perlovich, G.L. Physicochemical profile of antiandrogen drug bicalutamide: Solubility, distribution, permeability. Pharmaceutics 2022, 14, 674. [Google Scholar] [CrossRef]
- Surov, A.O.; Solanko, K.; Bond, A.D.; Bauer-Brandl, A.; Perlovich, G.L. Cocrystals of the antiandrogenic drug bicalutamide: Screening, crystal structures, formation thermodynamics and lattice energies. CrystEngComm 2016, 18, 4818–4829. [Google Scholar] [CrossRef]
- Palcsó, B.; Zelkó, R. Different types, applications and limits of enabling excipients of pharmaceutical dosage forms. Drug Discov. Today Technol. 2018, 27, 21–39. [Google Scholar] [CrossRef]
- Salts of therapeutic agents: Chemical, physicochemical, and biological considerations. Molecules 2018, 23, 1719. [CrossRef] [Green Version]
- Belsarkar, A.S.; Shinde, A.D.; Sonawane, S.P. An overview: Polymorphism and its applications in drug development process. Indo Am. J. Pharm. Sci. 2022, 9, 196–203. [Google Scholar]
- Garg, U.; Azim, Y. Challenges and opportunities of pharmaceutical cocrystals: A focused review on non-steroidal anti-inflammatory drugs. RSC Med. Chem. 2021, 12, 705–721. [Google Scholar] [CrossRef] [PubMed]
- Poulson, B.G.; Alsulami, Q.A.; Sharfalddin, A.; El Agammy, E.F.; Mouffouk, F.; Emwas, A.-H.; Jaremko, L.; Jaremko, M. Cyclodextrins: Structural, chemical, and physical properties, and applications. Polysaccharides 2022, 3, 1–31. [Google Scholar] [CrossRef]
- Loftsson, T.; Brewster, M.E. Cyclodextrins as pharmaceutical excipients. Pharm. Technol. Eur. 1997, 9, 26–33. [Google Scholar]
- Sogali, B.S.; Vikram, B.N.; Ramana Murthy, K.V. Comparative studies with different cyclodextrin derivatives in improving the solubility and dissolution of saquinavir. Asian J. Pharm. Clin. Res. 2018, 11, 509–516. [Google Scholar] [CrossRef]
- Trollope, L.; Cruickshank, D.L.; Noonan, T.; Bourne, S.A.; Sorrenti, M.; Catenacci, L.; Caira, M.R. Inclusion of trans-resveratrol in methylated cyclodextrins: Synthesis and solid-state structures. Beilstein J. Org. Chem. 2014, 10, 3136–3151. [Google Scholar] [CrossRef]
- Hirayama, F.; Mieda, S.; Miyamoto, Y.; Arima, H.; Uekama, K. Heptakis(2,6-di-O-methyl-3-O-acetyl)-β-CyD: A Water-soluble cyclodextrin derivative with low hemolytic activity. J. Pharm. Sci. 1999, 88, 970–975. [Google Scholar] [CrossRef]
- Lai, L.F.; Guo, H.X. Preparation of new 5-fluorouracil-loaded zein nanoparticles for liver targeting. Int. J. Pharm. 2011, 404, 317–323. [Google Scholar] [CrossRef]
- Le, Y.; Ji, H.; Chen, J.-F.; Shen, Z.; Yun, J.; Pu, M. Nanosized bicalutamide and its molecular structure in solvents. Int. J. Pharm. 2009, 370, 175–180. [Google Scholar] [CrossRef]
- Li, C.; Li, C.; Le, Y.; Chen, J.-F. Formation of bicalutamide nanodispersion for dissolution rate enhancement. Int. J. Pharm. 2011, 404, 257–263. [Google Scholar] [CrossRef]
- Szafraniec, J.; Antosik, A.; Knapik-Kowalczuk, J.; Kurek, M.; Syrek, K.; Chmiel, K.; Paluch, M.; Jachowicz, R. Planetary ball milling and supercritical fluid technology as a way to enhance dissolution of bicalutamide. Int. J. Pharm. 2017, 533, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Andrews, G.P.; AbuDiak, O.A.; Jones, D.S. Physicochemical characterization of hot melt extruded bicalutamide–polyvinylpyrrolidone solid dispersions. J. Pharm. Sci. 2010, 99, 1322–1335. [Google Scholar] [CrossRef] [PubMed]
- Szafraniec, J.; Antosik, A.; Knapik-Kowalczuk, J.; Gawlak, K.; Kurek, M.; Szlęk, J.; Jamróz, W.; Paluch, M.; Jachowicz, R. Molecular disorder of bicalutamide—amorphous solid dispersions obtained by solvent methods. Pharmaceutics 2018, 10, 194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AbuDiak, O.A.; Jones, D.S.; Andrews, G.P. Understanding the performance of melt-extruded poly(ethylene oxide)–bicalutamide solid dispersions: Characterisation of microstructural properties using thermal, spectroscopic and drug release methods. J. Pharm. Sci. 2012, 101, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Narkhede, K.B.; Laware, R.B.; Sharma, Y.P.; Rawat, S.S. Enhancement of solubility of bicalutamide drug using solid dispersion technique. Pharma Sci. Monit. Int. J. Pharm. Sci. 2012, 3, 2739–2748. [Google Scholar]
- Sancheti, P.P.; Vyas, V.M.; Shah, M.; Karekar, P.; Pore, Y.V. Development and characterization of bicalutamide-poloxamer F68 solid dispersion systems. Pharmazie 2008, 63, 571–575. [Google Scholar] [CrossRef]
- Szafraniec, J.; Antosik, A.; Knapik-Kowalczuk, J.; Chmiel, K.; Kurek, M.; Gawlak, K.; Odrobińska, J.; Paluch, M.; Jachowicz, R. The self-assembly phenomenon of poloxamers and its effect on the dissolution of a poorly soluble drug from solid dispersions obtained by solvent methods. Pharmaceutics 2019, 11, 130. [Google Scholar] [CrossRef] [Green Version]
- Srikanth, M.V.; Murali Mohan Babu, G.V.; Sreenivasa Rao, N.; Sunil, S.A.; Balaji, S.; Ramanamurthy, K.V. Dissolution rate enhancement of poorly soluble bicalutamide using β-cyclodextrin inclusion complexation. Int. J. Pharm. Pharm. Sci. 2010, 2, 191–198. [Google Scholar] [CrossRef]
- Patil, A.; Pore, Y.; Kuchekar, B. Effect of l-arginin on bicalutamide complexation with hydroxypropyl-β-cyclodextrin. Dig. J. Nanomater. Biostruct. 2008, 3, 89–98. [Google Scholar]
- Anton Smith, A.; Kannan, K.; Manavalan, R.; Rajendiran, N. Spectral characteristics of bicalutamide drug in different solvents and β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2007, 58, 161–167. [Google Scholar] [CrossRef]
- Kerns, E.H.; Di, L. Druglike Properties: Concepts, Structure Design and Methods; Academic Press: Cambridge, MA, USA, 2008. [Google Scholar]
- Sigurdsson, H.H.; Magnusdottir, A.; Másson, M.; Loftsson, T. The effects of cyclodextrins on hydrocortisone permeability through semi-permeable membranes. J. Incl. Phenom. Macrocycl. Chem. 2002, 44, 163–167. [Google Scholar] [CrossRef]
- Masson, M.; Loftsson, T.; Masson, G.; Stefansson, E. Cyclodextrins as permeation enhancers: Some theoretical evaluations and in vitro testing. J. Control. Release 1999, 59, 107–118. [Google Scholar] [CrossRef]
- Buch, P.; Langguth, P.; Kataoka, M.; Yamashita, S. IVIV Rinoral absorption for enofibrate immediate release tablets using dissolution and dissolution permeation methods. Pharmazie 2010, 65, 723–728. [Google Scholar] [CrossRef]
- Sironi, D.; Rosenberg, J.; Bauer-Brandl, A.; Brandl, M. Dynamic dissolution-/permeation-testing of nano- and microparticle formulations of fenofibrate. Eur. J. Pharm. Sci. 2017, 96, 20–27. [Google Scholar] [CrossRef]
- Kaur, M.; Yardley, V.; Wang, K.; Masania, J.; Botana, A.; Arroo, R.R.J.; Li, M. Artemisinin cocrystals for bioavailability enhancement. Part 1: Formulation design and role of the polymeric excipient. Mol. Pharm. 2021, 18, 4256–4271. [Google Scholar] [CrossRef]
- Buckley, S.T.; Frank, K.J.; Fricker, G.; Brandl, M. Biopharmaceutical classification of poorly soluble drugs with respect to “enabling formulations”. Eur. J. Pharm. Sci. 2013, 50, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Sironi, D.; Christensen, M.; Rosenberg, J.; Bauer-Brandl, A.; Brandl, M. Evaluation of a dynamic dissolution/permeation model: Mutual influence of dissolution and barrier-flux under non-steady state conditions. Int. J. Pharm. 2017, 522, 50–57. [Google Scholar] [CrossRef]
- Xu, S.; Dai, W.-G. Drug precipitation inhibitors in supersaturable formulations. Int. J. Pharm. 2013, 453, 36–43. [Google Scholar] [CrossRef]
- Vandecruys, R.; Peeters, J.; Verreck, G.; Brewster, M.E. Use of as creening method to determine excipients which optimize the extent and stability of supersaturated drug solutions and application of this system to solid formulation design. Int. J. Pharm. 2007, 342, 168–175. [Google Scholar] [CrossRef]
- Higuchi, T.; Connors, K. Phase-solubility techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–123. [Google Scholar]
- Job, P. Formation and stability of inorganic complexes in solution. Annal. Chim. Phys. 1928, 9, 113–203. [Google Scholar]
- Loftsson, T.; Masson, M.; Sigurdsson, H. Cyclodextrins and drug permeability through semi-permeablecellophane membranes. Int. J. Pharm. 2002, 232, 35–43. [Google Scholar] [CrossRef]
- Friedel, R.A.; Orchin, M. Ultraviolet Spectra of Aromatic Compounds; Wiley: New York, NY, USA, 1951. [Google Scholar]
- Loftsson, T.; Brewster, M.E. Pharmaceutical applications of cyclodextrins: Effects on drug permeation through biological membranes. J. Pharm. Pharmacol. 2011, 63, 1119–1135. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J. Cyclodextrin Technology; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1988; 450p. [Google Scholar]
- Loftsson, T.; O’Fee, R. Cyclodextrins in Solid Dosage Forms; A report, business briefing; Pharmatech: Denver, CO, USA, 2003; pp. 176–180. [Google Scholar]
- Van der Jagt, D.L.; Killian, F.L.; Bender, M.L. Cycloamyloses as enzyme models. Effects of inclusion complex formation on intramolecular participation. J. Am. Chem. Soc. 1970, 92, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Rekharsky, M.V.; Inoue, Y. Complexation and chiral recognition thermodynamics of 6-Amino-6-deoxy-β-cyclodextrin with anionic, cationic, and neutral chiral guests: Counterbalance between van der Waals and Coulombic interactions. J. Am. Chem. Soc. 2002, 124, 813–826. [Google Scholar] [CrossRef]
- Carrier, R.L.; Miller, L.A.; Ahmed, I. The utility of cyclodextrins for enhancing oral bioavailability. J. Control. Release 2007, 123, 78–99. [Google Scholar] [CrossRef]
- Miller, L.A.; Carrier, R.L.; Ahmed, I. Practical considerations in development of solid dosage forms that contain cyclodextrin. J. Pharm. Sci. 2007, 96, 1691–1707. [Google Scholar] [CrossRef]
- Rawlinson, C.F.; Williams, A.C.; Timmins, P.; Grimsey, I. Polymer-mediated disruption of drug crystallinity. Int. J. Pharm. 2007, 336, 42–48. [Google Scholar] [CrossRef]
- Liu, Z.-T.; Shen, L.-H.; Liu, Z.-W.; Lu, J. Acetylation of β-cyclodextrin in ionic liquid green solvent. J. Mater. Sci. 2009, 44, 1813–1820. [Google Scholar] [CrossRef]
- Ford, J. Current status of solid dispersions. J. Pharm. Acta Helv. 1986, 61, 69–88. [Google Scholar]
- Ogawa, N.; Takahashi, C.; Yamamoto, H. Physicochemical characterization of cyclodextrin-drug interactions in the solid state and the effect of water on these interactions. J. Pharm. Sci. 2015, 104, 942–954. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Fridriksdottir, H.; Gudmundsdottir, T.K. The effect of water soluble polymers on aqueous solubility of drugs. Int. J. Pharm. 1996, 127, 293–296. [Google Scholar] [CrossRef]
- Hirayama, F.; Uekama, K. Cyclodextrin-based controlled drug release system. Adv. Drug Deliv. Rev. 1999, 36, 125–141. [Google Scholar] [CrossRef]
- Brouwers, J.; Brewster, M.; Augustijns, P. Supersaturating drug delivery systems: The answer to solubility-limited oral bioavailability? J. Pharm. Sci. 2009, 98, 2549–2572. [Google Scholar] [CrossRef] [PubMed]
- Sugano, K.; Okazaki, A.; Sugimoto, S.; Tavornvipas, S.; Omura, A.; Mano, T. Solubility and dissolution profile assessment in drug discovery. Drug Metab. Pharmacokinet. 2007, 22, 225–254. [Google Scholar] [CrossRef]
- Raina, S.A.; Zhang, G.G.Z.; Alonzo, D.E.; Wu, J.; Zhu, D.; Catron, N.D.; Gao, Y.; Taylor, L.S. Enhancements and limits in drug membrane transport using supersaturated solutions of poorly water soluble drugs. J. Pharm. Sci. 2014, 103, 2736–2748. [Google Scholar] [CrossRef]
- Enxian, L.; Shoufeng, L.; Zhongqin, W. Biorelevant test for supersaturable formulation. Asian J. Pharm. Sci. 2017, 12, 9–20. [Google Scholar] [CrossRef]
- Lindfors, L.; Forssén, S.; Westergren, J.; Olsson, U. Nucleation and crystal growth in supersaturated solutions of a model drug. J. Colloid Interface Sci. 2008, 325, 404–413. [Google Scholar] [CrossRef]
- Salas-Zúñiga, R.; Rodríguez-Ruiz, C.; Höpf, H.; Morales-Rojas, H.; Sánchez-Guadarrama, O.; Rodríguez-Cuamatzi, P.; Herrera-Ruiz, D. Dissolution advantage of nitazoxanide cocrystals in the presence of cellulosic polymers. Pharmaceutics 2020, 12, 23. [Google Scholar] [CrossRef] [Green Version]
- Brewster, M.E.; Vandecruys, R.; Peeters, J.; Neeskens, P.; Verreck, G.; Loftsson, T. Comparative interaction of 2-hydroxypropyl-β-cyclodextrin and sulfobutylether-β-cyclodextrin with itraconazole: Phase-solubility behavior and stabilization of supersaturated drug solutions. Eur. J. Pharm. Sci. 2008, 34, 94–103. [Google Scholar] [CrossRef]
- Bevernage, J.; Forier, T.; Brouwers, J.; Tack, J.; Annaert, P.; Augustijns, P. Excipient-mediated supersaturation stabilization in human intestinal fluids. Mol. Pharm. 2011, 8, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Alonzo, D.E.; Raina, S.; Zhou, D.; Gao, Y.; Zhang, G.G.; Taylor, L.S. Characterizing the impact of hydroxypropylmethyl cellulose on the growth and nucleation kinetics of felodipine from supersaturated solutions. Cryst. Growth Des. 2012, 12, 1538–1547. [Google Scholar] [CrossRef]
- Ilevbare, G.A.; Liu, H.; Edgar, K.J.; Taylor, L.S. Impact of polymers on crystal growth rate of structurally diverse compounds from aqueous solution. Mol. Pharm. 2013, 10, 2381–2393. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.M.; Beig, A.; Carr, R.A.; Spence, J.K.; Dahan, A. A win–win solution in oral delivery of lipophilic drugs: Supersaturation via amorphous solid dspersions increases apparent solubility without sacrifice of intestinal membrane permeability. Mol. Pharm. 2012, 9, 2009–2016. [Google Scholar] [CrossRef] [PubMed]

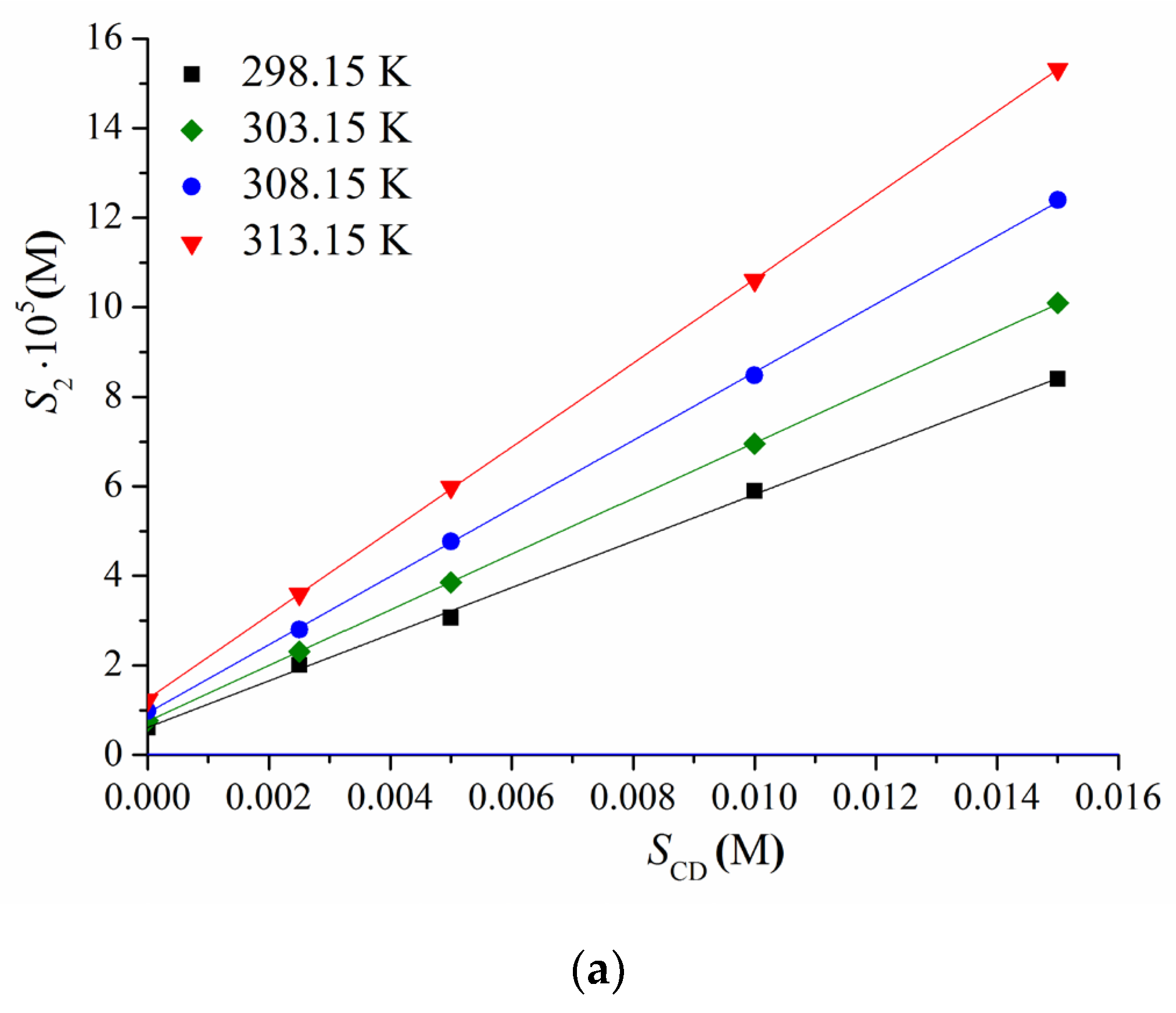

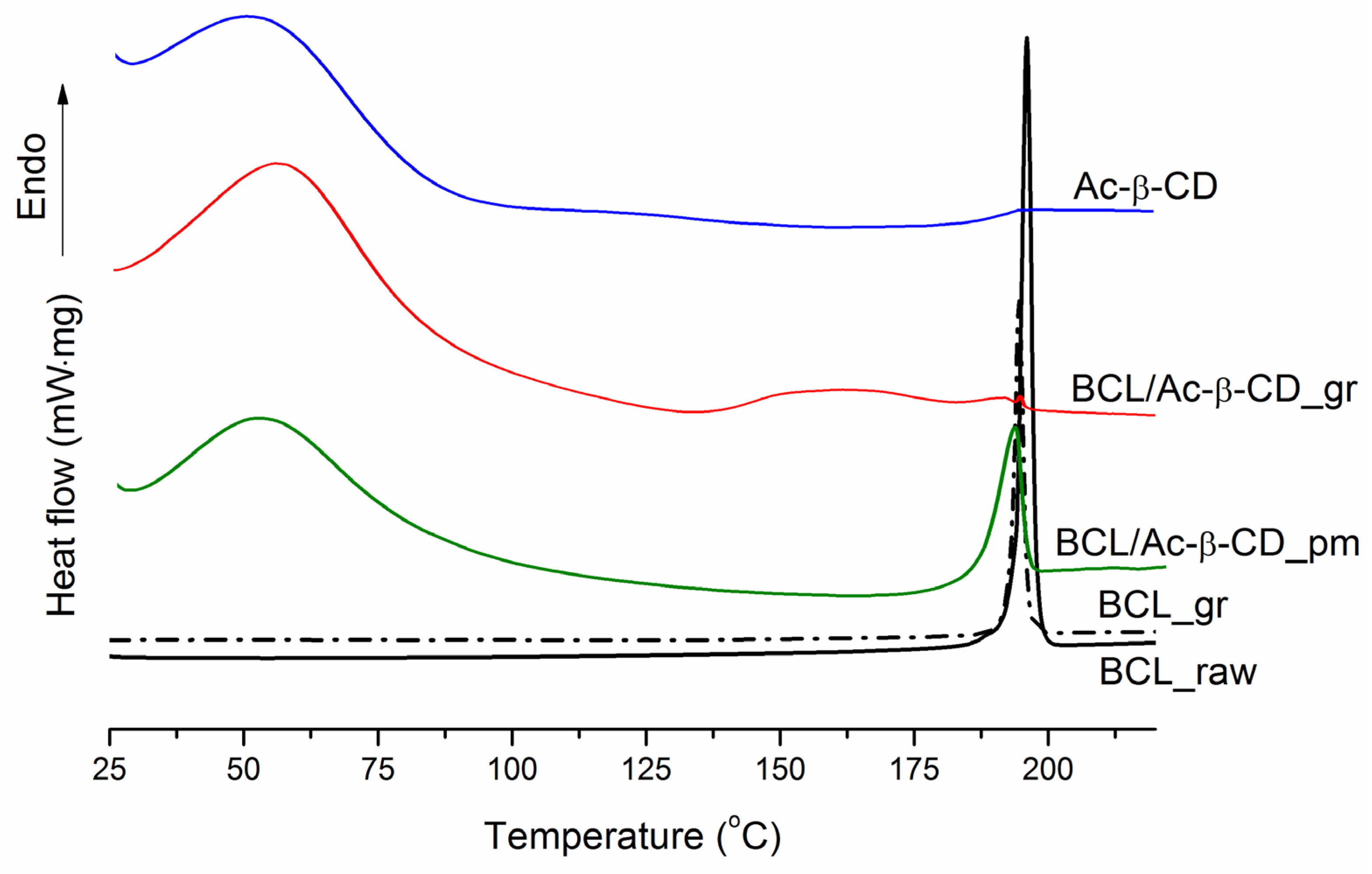
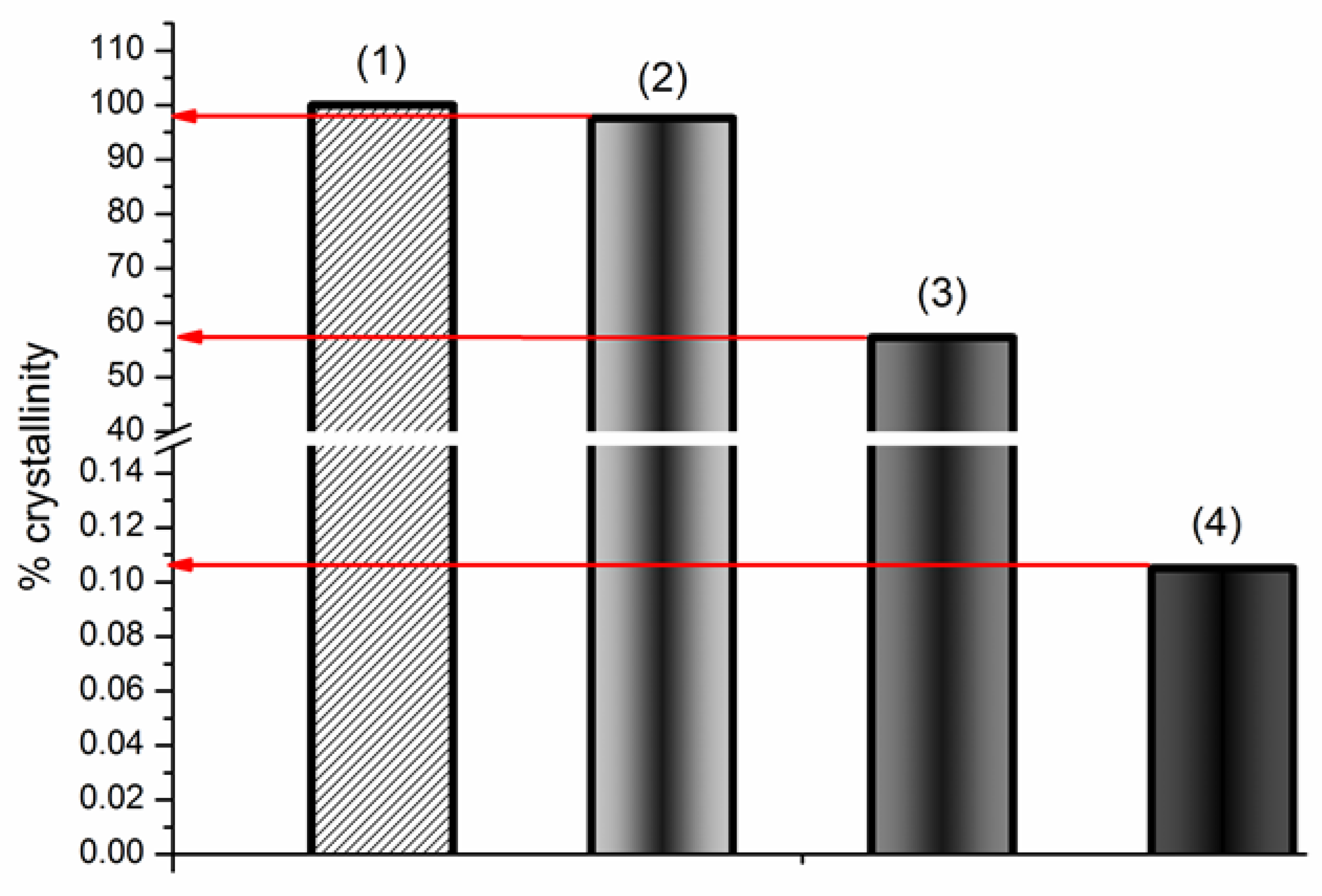
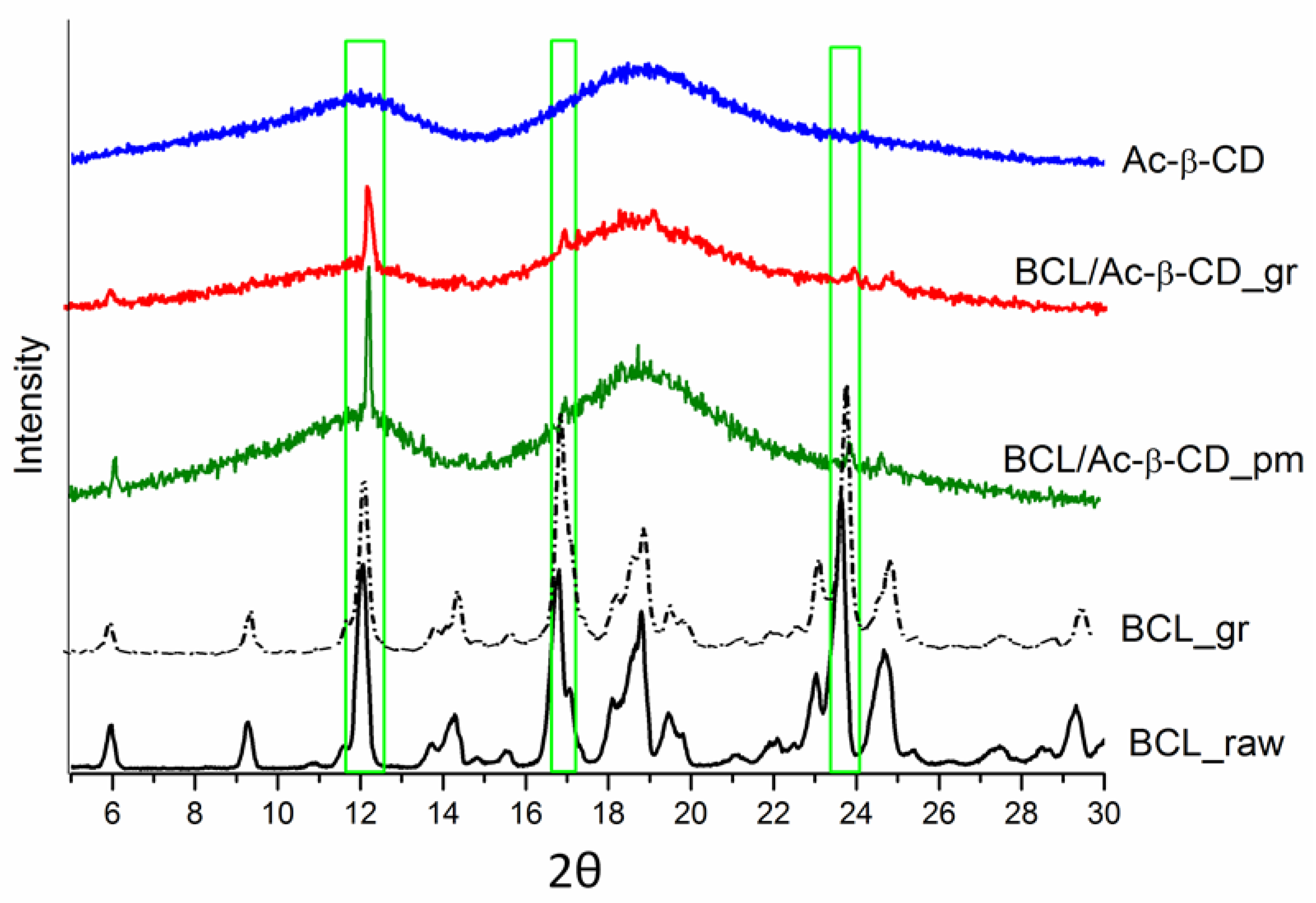

| T (K) | ||
|---|---|---|
| Me-β-Cyclodextrin | Ac-β-Cyclodextrin | |
| 298.15 | 849.2 ± 40.3 | 1919.3 ± 61.8 |
| 303.15 | 812.8 ± 37.5 | 1613.3 ± 35.3 |
| 308.15 | 782.5 ± 34.2 | 1332.5 ± 43.2 |
| 315.15 | 769.8 ± 33.6 | 1187.0 ± 28.7 |
| System | KC (M−1) | |||
|---|---|---|---|---|
| BCL/Me-β-CD a | 849.2 ± 40.3 | −16.7 ± 0.3 | −5.2 ± 0.6 | 11.5 ± 1.6 |
| BCL/Ac-β-CD b | 1919.3 ± 61.8 | −18.7 ± 0.4 | −25.4 ± 1.6 | −6.7 ± 0.6 |
| System | 1 timeCmax (min) | 2C20 (μg·mL−1) | 2C60 (μg·mL−1) | 2C120 (μg·mL−1) | 3 (μg·min) | 4 EGF |
|---|---|---|---|---|---|---|
| BCL_raw | 0.56 | 1.30 | 2.27 | 1113.99 | ||
| BCL/Ac-β-CD_pm | 2.92 | 4.20 | 5.07 | 2058.18 | ||
| BCL/Ac-β-CD_gr | 20 | 11.63 | 9.46 | 7.70 | 3346.34 | |
| BCL/Ac-β-CD_gr (PVP) | 30 | 25.54 | 24.98 | 19.00 | 6451.43 | 1.93 |
| BCL/Ac-β-CD_gr (HPMC) | 90 | 20.57 | 27.70 | 26.13 | 8653.46 | 2.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volkova, T.V.; Simonova, O.R.; Perlovich, G.L. Another Move towards Bicalutamide Dissolution and Permeability Improvement with Acetylated β-Cyclodextrin Solid Dispersion. Pharmaceutics 2022, 14, 1472. https://doi.org/10.3390/pharmaceutics14071472
Volkova TV, Simonova OR, Perlovich GL. Another Move towards Bicalutamide Dissolution and Permeability Improvement with Acetylated β-Cyclodextrin Solid Dispersion. Pharmaceutics. 2022; 14(7):1472. https://doi.org/10.3390/pharmaceutics14071472
Chicago/Turabian StyleVolkova, Tatyana V., Olga R. Simonova, and German L. Perlovich. 2022. "Another Move towards Bicalutamide Dissolution and Permeability Improvement with Acetylated β-Cyclodextrin Solid Dispersion" Pharmaceutics 14, no. 7: 1472. https://doi.org/10.3390/pharmaceutics14071472






