The Ca2+ Channel Blocker Verapamil Inhibits the In Vitro Activation and Function of T Lymphocytes: A 2022 Reappraisal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Blood Samples and T Cell Purification
2.2. T Cell Stimulation Assays
2.3. Cytokine Production Profile
2.4. Flow Cytometry
2.5. Statistical Analysis
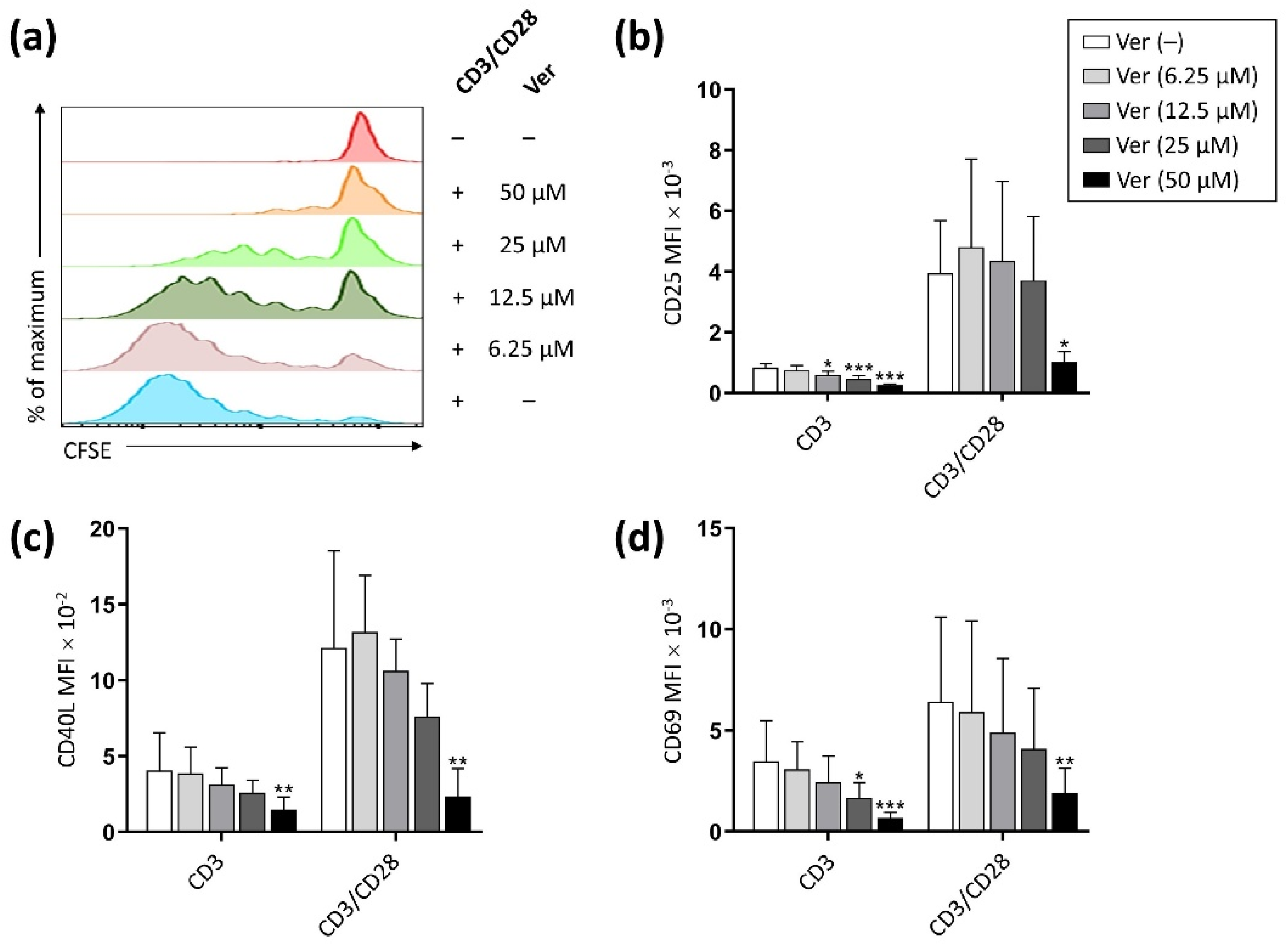
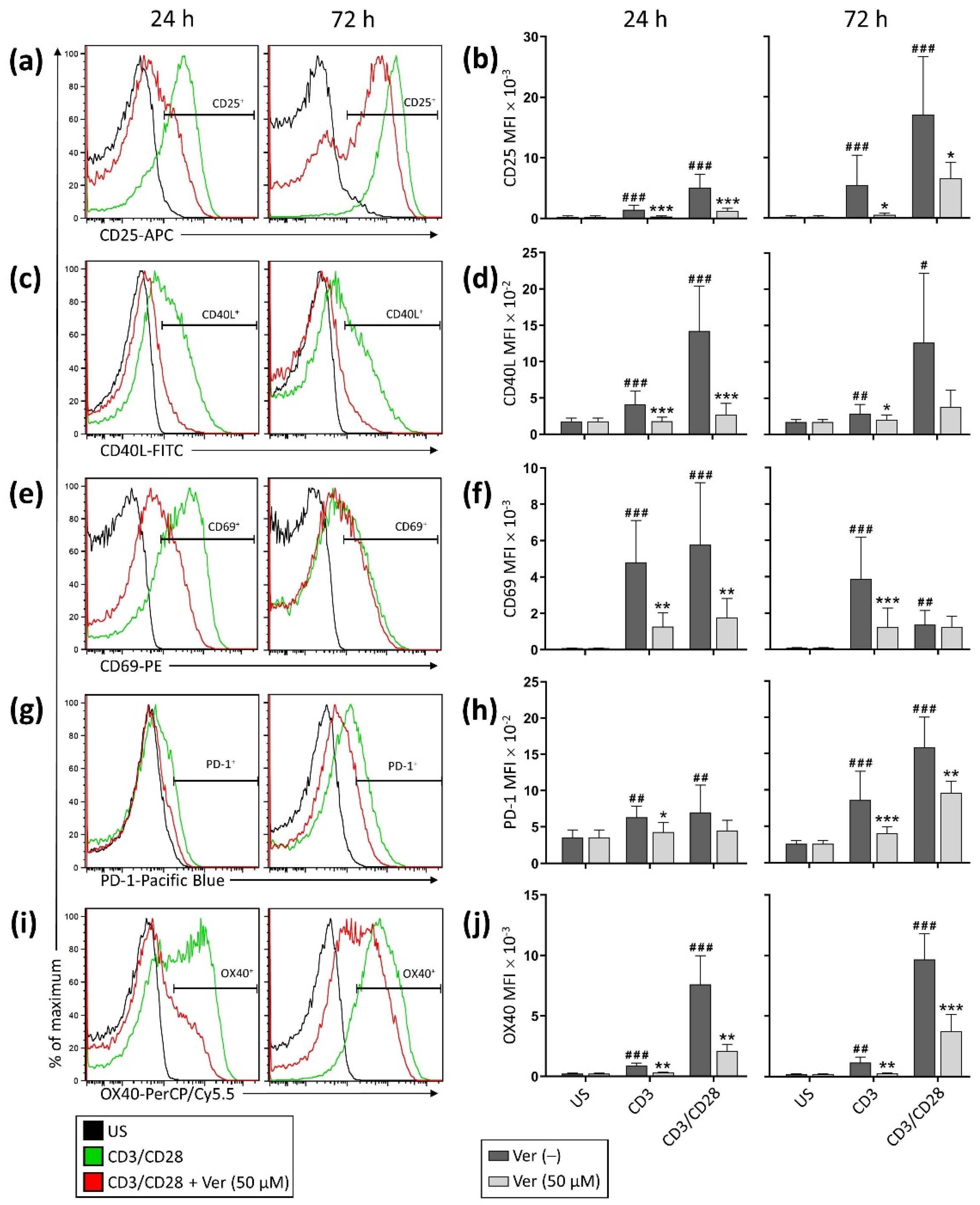
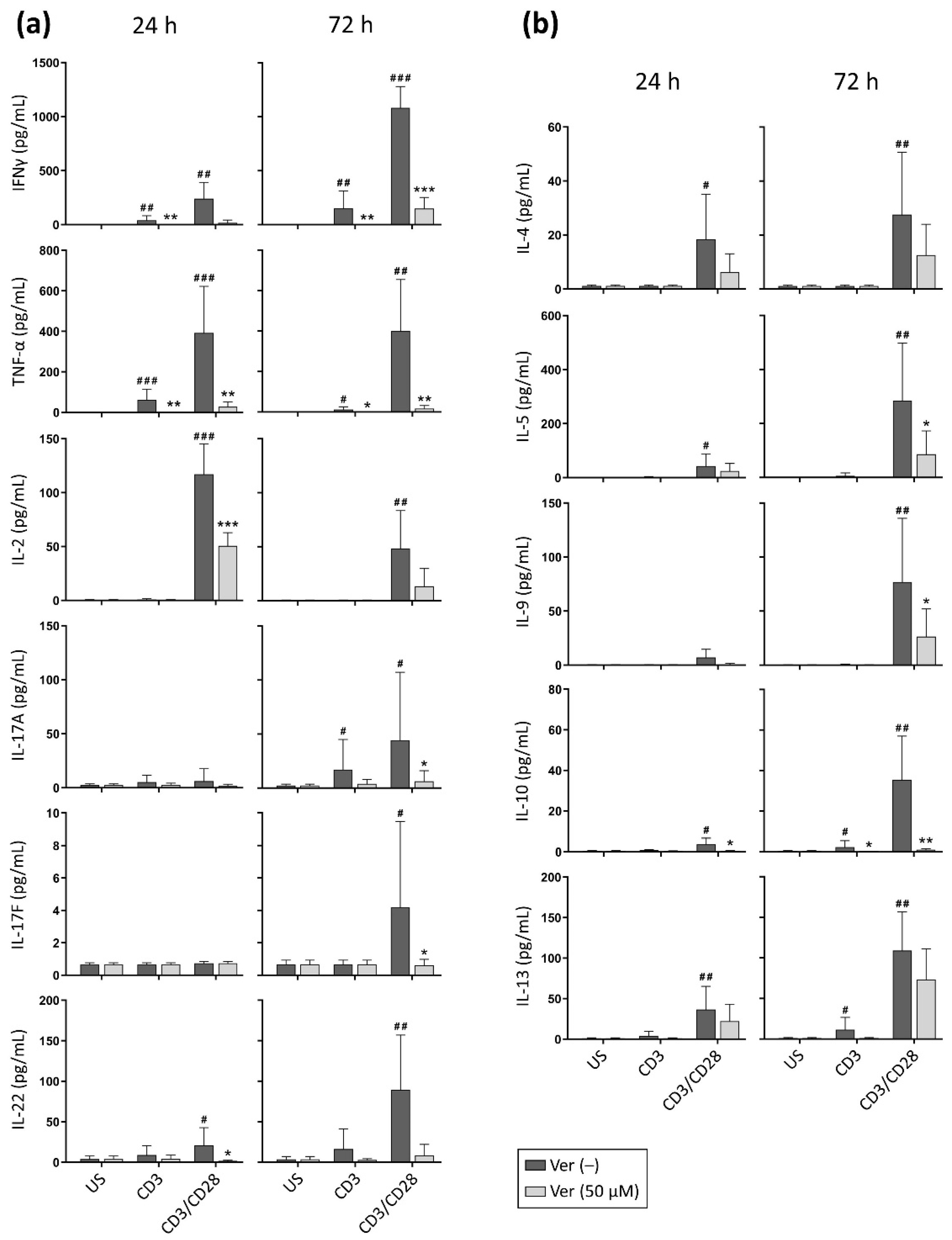
3. Results
3.1. Verapamil Inhibits T Cell Activation and Proliferation
3.2. Verapamil Impairs the Production of Cytokines
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feske, S.; Wulff, H.; Skolnik, E.Y. Ion Channels in Innate and Adaptive Immunity. Annu. Rev. Immunol. 2015, 33, 291–353. [Google Scholar] [CrossRef] [Green Version]
- Feske, S. Calcium Signalling in Lymphocyte Activation and Disease. Nat. Rev. Immunol. 2007, 7, 690–702. [Google Scholar] [CrossRef]
- Brini, M.; Calì, T.; Ottolini, D.; Carafoli, E. Neuronal Calcium Signaling: Function and Dysfunction. Cell. Mol. Life Sci. 2014, 71, 2787–2814. [Google Scholar] [CrossRef]
- Elliott, W.J.; Ram, C.V.S. Calcium Channel Blockers. J. Clin. Hypertens. 2011, 13, 687–689. [Google Scholar] [CrossRef]
- Abernethy, D.R.; Schwartz, J.B. Calcium-Antagonist Drugs. N. Engl. J. Med. 1999, 341, 1447–1457. [Google Scholar] [CrossRef]
- Derenne, F.; Vanhaeverbeek, M.; Brohée, D. Nifedipine-Induced Hyporeactivity in Delayed Hypersensitivity Skin Tests. Int. J. Immunopharmacol. 1987, 9, 741–744. [Google Scholar] [CrossRef]
- Tietz, N.W.; Thompson, J. Possible Concentration-Dependent Suppression of Immune Response by Verapamil. Arch. Fam. Med. 1995, 4, 368–369. [Google Scholar] [CrossRef]
- McFadden, J.; Bacon, K.; Camp, R. Topically Applied Verapamil Hydrochloride Inhibits Tuberculin-Induced Delayed-Type Hypersensitivity Reactions in Human Skin. J. Investig. Dermatol. 1992, 99, 784–786. [Google Scholar] [CrossRef] [Green Version]
- Moslen, M.T.; Balakumaran, A. Calcium Channel Blockers and Cancer: Due to Loss of Immune Surveillance? Am. J. Hypertens. 1996, 9, 1050–1051. [Google Scholar] [CrossRef] [Green Version]
- Suthanthiran, M.; Haschemeyer, R.H.; Riggio, R.R.; Adubor, C.; Friedman, G.S.; Cheigh, J.S.; Wang, J.C.; Fotino, M.; Stubenbord, W.T.; Saal, S.D. Excellent Outcome with a Calcium Channel Blocker-Supplemented Immunosuppressive Regimen in Cadaveric Renal Transplantation. A Potential Strategy to Avoid Antibody Induction Protocols. Transplantation 1993, 55, 1008–1013. [Google Scholar] [CrossRef]
- Dawidson, I.; Rooth, P.; Lu, C.; Sagalowsky, A.; Diller, K.; Palmer, B.; Peters, P.; Risser, R.; Sandor, Z.; Seney, F. Verapamil Improves the Outcome after Cadaver Renal Transplantation. J. Am. Soc. Nephrol. 1991, 2, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Chitwood, K.K.; Heim-Duthoy, K.L. Immunosuppressive Properties of Calcium Channel Blockers. Pharmacotherapy 1993, 13, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.F.; Dawidson, I.; Sagalowsky, A.; Sandor, Z.; Lu, C.Y. Improved Outcome of Cadaveric Renal Transplantation Due to Calcium Channel Blockers. Transplantation 1991, 52, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Pahor, M.; Guralnik, J.M.; Salive, M.E.; Corti, M.C.; Carbonin, P.; Havlik, R.J. Do Calcium Channel Blockers Increase the Risk of Cancer? Am. J. Hypertens. 1996, 9, 695–699. [Google Scholar] [CrossRef] [Green Version]
- Fenninger, F.; Jefferies, W.A. What’s Bred in the Bone: Calcium Channels in Lymphocytes. J. Immunol. 2019, 202, 1021–1030. [Google Scholar] [CrossRef] [Green Version]
- Trebak, M.; Kinet, J.-P. Calcium Signalling in T Cells. Nat. Rev. Immunol. 2019, 19, 154–169. [Google Scholar] [CrossRef]
- Chandy, K.G.; Wulff, H.; Beeton, C.; Pennington, M.; Gutman, G.A.; Cahalan, M.D. K+ Channels as Targets for Specific Immunomodulation. Trends Pharmacol. Sci. 2004, 25, 280–289. [Google Scholar] [CrossRef] [Green Version]
- Beeton, C.; Barbaria, J.; Giraud, P.; Devaux, J.; Benoliel, A.-M.; Gola, M.; Sabatier, J.M.; Bernard, D.; Crest, M.; Beraud, E. Selective Blocking of Voltage-Gated K+ Channels Improves Experimental Autoimmune Encephalomyelitis and Inhibits T Cell Activation. J. Immunol. 2001, 166, 936–944. [Google Scholar] [CrossRef] [Green Version]
- Riha, P.; Rudd, C.E. CD28 Co-Signaling in the Adaptive Immune Response. Self Nonself 2010, 1, 231–240. [Google Scholar] [CrossRef] [Green Version]
- Cantrell, D. Signaling in Lymphocyte Activation. Cold Spring Harb. Perspect. Biol. 2015, 7, a018788. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Kurlander, R.J. Comparison of Anti-CD3 and Anti-CD28-Coated Beads with Soluble Anti-CD3 for Expanding Human T Cells: Differing Impact on CD8 T Cell Phenotype and Responsiveness to Restimulation. J. Transl. Med. 2010, 8, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abate, G.; Ruminiski, P.G.; Kumar, M.; Singh, K.; Hamzabegovic, F.; Hoft, D.F.; Eickhoff, C.S.; Selimovic, A.; Campbell, M.; Chibale, K. New Verapamil Analogs Inhibit Intracellular Mycobacteria without Affecting the Functions of Mycobacterium-Specific T Cells. Antimicrob. Agents Chemother. 2015, 60, 1216–1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srikanth, S.; Gwack, Y. Orai1-NFAT Signalling Pathway Triggered by T Cell Receptor Stimulation. Mol. Cells 2013, 35, 182–194. [Google Scholar] [CrossRef] [Green Version]
- Luik, R.M.; Lewis, R.S. New Insights into the Molecular Mechanisms of Store-Operated Ca2+ Signaling in T Cells. Trends Mol. Med. 2007, 13, 103–107. [Google Scholar] [CrossRef]
- Vaeth, M.; Kahlfuss, S.; Feske, S. CRAC Channels and Calcium Signaling in T Cell-Mediated Immunity. Trends Immunol. 2020, 41, 878–901. [Google Scholar] [CrossRef]
- Jash, A.; Sahoo, A.; Kim, G.-C.; Chae, C.-S.; Hwang, J.-S.; Kim, J.-E.; Im, S.-H. Nuclear Factor of Activated T Cells 1 (NFAT1)-Induced Permissive Chromatin Modification Facilitates Nuclear Factor-ΚB (NF-ΚB)-Mediated Interleukin-9 (IL-9) Transactivation. J. Biol. Chem. 2012, 287, 15445–15457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karnell, J.L.; Rieder, S.A.; Ettinger, R.; Kolbeck, R. Targeting the CD40-CD40L Pathway in Autoimmune Diseases: Humoral Immunity and Beyond. Adv. Drug Deliv. Rev. 2019, 141, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Cibrián, D.; Sánchez-Madrid, F. CD69: From Activation Marker to Metabolic Gatekeeper. Eur. J. Immunol. 2017, 47, 946–953. [Google Scholar] [CrossRef]
- Létourneau, S.; Krieg, C.; Pantaleo, G.; Boyman, O. IL-2- and CD25-Dependent Immunoregulatory Mechanisms in the Homeostasis of T-Cell Subsets. J. Allergy Clin. Immunol. 2009, 123, 758–762. [Google Scholar] [CrossRef]
- Webb, G.J.; Hirschfield, G.M.; Lane, P.J.L. OX40, OX40L and Autoimmunity: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2016, 50, 312–332. [Google Scholar] [CrossRef]
- Sharpe, A.H.; Pauken, K.E. The Diverse Functions of the PD1 Inhibitory Pathway. Nat. Rev. Immunol. 2018, 18, 153–167. [Google Scholar] [CrossRef] [PubMed]
- González-Amaro, R.; Cortés, J.R.; Sánchez-Madrid, F.; Martín, P. Is CD69 an Effective Brake to Control Inflammatory Diseases? Trends Mol. Med. 2013, 19, 625–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weir, M.R.; Peppler, R.; Gomolka, D.; Handwerger, B.S. Additive Inhibition of Afferent and Efferent Immunological Responses of Human Peripheral Blood Mononuclear Cells by Verapamil and Cyclosporine. Transplantation 1991, 51, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Walz, G.; Zanker, B.; Wieder, K.; Hadro, E.; Moscovitch-Lopatin, M.; Smith, B.R.; Strom, T.B. Similar Effects of Cyclosporine and Verapamil on Lymphokine, Interleukin 2 Receptor, and Proto-Oncogene Expression. Transplantation 1989, 47, 331–334. [Google Scholar] [CrossRef]
- Matsumori, A.; Nishio, R.; Nose, Y. Calcium Channel Blockers Differentially Modulate Cytokine Production by Peripheral Blood Mononuclear Cells. Circ. J. 2010, 74, 567–571. [Google Scholar] [CrossRef] [Green Version]
- Weir, M.R. Therapeutic Benefits of Calcium Channel Blockers in Cyclosporine-Treated Organ Transplant Recipients: Blood Pressure Control and Immunosuppression. Am. J. Med. 1991, 90, 32S–36S. [Google Scholar] [CrossRef]
- Bruserud, O. In Vitro Effects of R-Verapamil on the Cytokine Environment and T-Lymphocyte Proliferation When Human T-Lymphocyte Activation Takes Place in the Presence of Acute Myelogenous Leukemia Blasts. Cancer Chemother. Pharmacol. 1996, 39, 71–78. [Google Scholar] [CrossRef]
- Walz, G.; Zanker, B.; Barth, C.; Wieder, K.J.; Clark, S.C.; Strom, T.B. Transcriptional Modulation of Human IL-6 Gene Expression by Verapamil. J. Immunol. 1990, 144, 4242–4248. [Google Scholar]
- Weir, M.R.; Peppler, R.; Gomolka, D.; Handwerger, B.S. Evidence That the Antiproliferative Effect of Verapamil on Afferent and Efferent Immune Responses Is Independent of Calcium Channel Inhibition. Transplantation 1992, 54, 681–685. [Google Scholar] [CrossRef]
- Zanker, B.; Marx, S.; Strom, T.B.; Köhler, H. The Immunosuppressive Effects of Verapamil upon Mitogen Activated and Allo-Antigen Inducible Human Cytotoxic T-Lymphocytes. Int. J. Immunopharmacol. 1994, 16, 507–517. [Google Scholar] [CrossRef]
- Bruserud, O. Effect of Verapamil on T-Lymphocyte Activation in Vitro. Scand. J. Immunol. 1985, 21, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Drach, J.; Gsur, A.; Hamilton, G.; Zhao, S.; Angerler, J.; Fiegl, M.; Zojer, N.; Raderer, M.; Haberl, I.; Andreeff, M.; et al. Involvement of P-Glycoprotein in the Transmembrane Transport of Interleukin-2 (IL-2), IL-4, and Interferon-Gamma in Normal Human T Lymphocytes. Blood 1996, 88, 1747–1754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kooijman, R.; Devos, S.; Hooghe-Peters, E. Inhibition of in Vitro Cytokine Production by Human Peripheral Blood Mononuclear Cells Treated with Xenobiotics: Implications for the Prediction of General Toxicity and Immunotoxicity. Toxicol. Vitr. 2010, 24, 1782–1789. [Google Scholar] [CrossRef] [PubMed]
- Kennes, B.; Hubert, C.; Brohee, D.; Neve, P. Early Biochemical Events Associated with Lymphocyte Activation in Ageing. I. Evidence That Ca2+ Dependent Processes Induced by PHA Are Impaired. Immunology 1981, 42, 119–126. [Google Scholar]
- Bruserud, O.; Hamann, W.; Patel, S.; Pawelec, G. CD4+ TCR Alpha Beta+ T-Cell Clones Derived Shortly after Allogeneic Bone Marrow Transplantation: Theophyllamine and Verapamil Inhibit Proliferation of Functionally Heterogeneous T-Cells. Int. J. Immunopharmacol. 1992, 14, 783–789. [Google Scholar] [CrossRef]
- Witkowski, J.M.; Siebert, J.; Lukaszuk, K.; Trawicka, L. Comparison of Effect of a Panel of Membrane Channel Blockers on the Proliferative, Cytotoxic and Cytoadherence Abilities of Human Peripheral Blood Lymphocytes. Immunopharmacology 1993, 26, 53–63. [Google Scholar] [CrossRef]
- Marx, M.; Weber, M.; Merkel, F.; Meyer zum Büschenfelde, K.H.; Köhler, H. Additive Effects of Calcium Antagonists on Cyclosporin A-Induced Inhibition of T-Cell Proliferation. Nephrol. Dial. Transplant. 1990, 5, 1038–1044. [Google Scholar] [CrossRef]
- Nakabayashi, H.; Komada, H.; Yoshida, T.; Takanari, H.; Izutsu, K. Lymphocyte Calmodulin and Its Participation in the Stimulation of T Lymphocytes by Mitogenic Lectins. Biol. Cell 1992, 75, 55–59. [Google Scholar] [CrossRef]
- Birx, D.L.; Berger, M.; Fleisher, T.A. The Interference of T Cell Activation by Calcium Channel Blocking Agents. J. Immunol. 1984, 133, 2904–2909. [Google Scholar]
- Weir, M.R.; Peppler, R.; Gomolka, D.; Handwerger, B.S. Calcium Channel Blockers Inhibit Cellular Uptake of Thymidine, Uridine and Leucine: The Incorporation of These Molecules into DNA, RNA and Protein in the Presence of Calcium Channel Blockers Is Not a Valid Measure of Lymphocyte Activation. Immunopharmacology 1993, 25, 75–82. [Google Scholar] [CrossRef]
- Maisel, A.S.; Murray, D.; Polizzi, S.; Motulsky, H.J.; Brodde, O.E.; van Tits, L.J.; Michel, M.C. Does Verapamil Act as an Immunomodulatory Drug in Vivo? Immunopharmacology 1991, 22, 85–91. [Google Scholar] [CrossRef]
- Bacon, K.B.; Westwick, J.; Camp, R.D.R. Potent and Specific Inhibition of IL-8-, IL-1α- and IL-1β-Induced in Vitro Human Lymphocyte Migration by Calcium Channel Antagonists. Biochem. Biophys. Res. Commun. 1989, 165, 349–354. [Google Scholar] [CrossRef]
- Blaheta, R.A.; Hailer, N.P.; Brude, N.; Wittig, B.; Leckel, K.; Oppermann, E.; Bachmann, M.; Harder, S.; Cinatl, J.; Scholz, M.; et al. In Vitro Analysis of Verapamil-Induced Immunosuppression: Potent Inhibition of T Cell Motility and Lymphocytic Transmigration through Allogeneic Endothelial Cells. Transplantation 2000, 69, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Suwa, H.; Miyasaka, M.; Kumada, K. Selective Inhibition of Vascular Cell Adhesion Molecule-1 Expression by Verapamil in Human Vascular Endothelial Cells. Transplantation 1997, 63, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Chandy, K.G.; DeCoursey, T.E.; Cahalan, M.D.; McLaughlin, C.; Gupta, S. Voltage-Gated Potassium Channels Are Required for Human T Lymphocyte Activation. J. Exp. Med. 1984, 160, 369–385. [Google Scholar] [CrossRef] [Green Version]
- DeCoursey, T.E.; Chandy, K.G.; Gupta, S.; Cahalan, M.D. Voltage-Dependent Ion Channels in T-Lymphocytes. J. Neuroimmunol. 1985, 10, 71–95. [Google Scholar] [CrossRef]
- Rauer, H.; Grissmer, S. Evidence for an Internal Phenylalkylamine Action on the Voltage-Gated Potassium Channel Kv1.3. Mol. Pharmacol. 1996, 50, 1625–1634. [Google Scholar]
- Johnson, B.D.; Hockerman, G.H.; Scheuer, T.; Catterall, W.A. Distinct Effects of Mutations in Transmembrane Segment IVS6 on Block of L-Type Calcium Channels by Structurally Similar Phenylalkylamines. Mol. Pharmacol. 1996, 50, 1388–1400. [Google Scholar]
- Freeze, B.S.; McNulty, M.M.; Hanck, D.A. State-Dependent Verapamil Block of the Cloned Human Ca(v)3.1 T-Type Ca(2+) Channel. Mol. Pharmacol. 2006, 70, 718–726. [Google Scholar] [CrossRef] [Green Version]
- Bergson, P.; Lipkind, G.; Lee, S.P.; Duban, M.-E.; Hanck, D.A. Verapamil Block of T-Type Calcium Channels. Mol. Pharmacol. 2011, 79, 411–419. [Google Scholar] [CrossRef]
- Hamann, S.R.; Todd, G.D.; McAllister, R.G. The Pharmacology of Verapamil. V. Tissue Distribution of Verapamil and Norverapamil in Rat and Dog. Pharmacology 1983, 27, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hamann, S.R.; Blouin, R.A.; McAllister, R.G. Clinical Pharmacokinetics of Verapamil. Clin. Pharmacokinet. 1984, 9, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.; Dana, R.; Gold, B.; Alkan, M.; Schlaeffer, F. Influence of Calcium Channel Blockers on Polymorphonuclear and Monocyte Bactericidal and Fungicidal Activity. Isr. J. Med. Sci. 1991, 27, 301–306. [Google Scholar]
- Kazanjian, P.H.; Pennington, J.E. Influence of Drugs That Block Calcium Channels on the Microbicidal Function of Human Neutrophils. J. Infect. Dis. 1985, 151, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Marino, F.; Cattaneo, S.; Cosentino, M.; Rasini, E.; Di Grazia, L.; Fietta, A.M.; Lecchini, S.; Frigo, G. Diazepam Stimulates Migration and Phagocytosis of Human Neutrophils: Possible Contribution of Peripheral-Type Benzodiazepine Receptors and Intracellular Calcium. Pharmacology 2001, 63, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, T.; Yano, E.; Evans, P.H. Cellular Mechanisms of Reactive Oxygen Metabolite Generation from Human Polymorphonuclear Leukocytes Induced by Crocidolite Asbestos. Environ. Res. 1997, 75, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Huwyler, T.; Hirt, A.; Felix, D.; Morell, A. Effect of Cations and Cation Channel Blockers on Human Natural Killer Cells. Int. J. Immunopharmacol. 1985, 7, 573–576. [Google Scholar] [CrossRef]
- Markham, P.N.; Ellis, T.M.; Tambur, A.R.; Gebel, H.M. Differential Sensitivity of Resting and IL-2 Activated NK Cells to R-Verapamil. Transplantation 1996, 62, 1883–1888. [Google Scholar] [CrossRef]
- Degiannis, D.; Hornung, N.; Luke-Gustites, D.; Raskova, J.; Raska, K. IL-4 Receptor Expression by SAC-Activated B-Lymphocytes: Its Role in B-Cell Proliferation and the Effect of Cyclosporine (CsA), Prednisolone and Verapamil. Int. J. Immunopharmacol. 1993, 15, 829–832. [Google Scholar] [CrossRef]
- Brent, L.H.; Butler, J.L.; Woods, W.T.; Bubien, J.K. Transmembrane Ion Conductance in Human B Lymphocyte Activation. J. Immunol. 1990, 145, 2381–2389. [Google Scholar]
- Dugas, B.; Vazquez, A.; Delfraissy, J.-F.; Gérard, J.-P.; Rannou, M.-T.; Galanaud, P. Human B Cell Activation: Selective Sensitivity of the Early Stages to Calcium Channel-Blocking Drugs. Eur. J. Immunol. 1986, 16, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Blaheta, R.A.; Nelson, K.; Oppermann, E.; Leckel, K.; Harder, S.; Cinatl, J.; Weber, S.; Shipkova, M.; Encke, A.; Markus, B.H. Mycophenolate Mofetil Decreases Endothelial Prostaglandin E2 in Response to Allogeneic T Cells or Cytokines. Transplantation 2000, 69, 1977–1981. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veytia-Bucheli, J.I.; Alvarado-Velázquez, D.A.; Possani, L.D.; González-Amaro, R.; Rosenstein, Y. The Ca2+ Channel Blocker Verapamil Inhibits the In Vitro Activation and Function of T Lymphocytes: A 2022 Reappraisal. Pharmaceutics 2022, 14, 1478. https://doi.org/10.3390/pharmaceutics14071478
Veytia-Bucheli JI, Alvarado-Velázquez DA, Possani LD, González-Amaro R, Rosenstein Y. The Ca2+ Channel Blocker Verapamil Inhibits the In Vitro Activation and Function of T Lymphocytes: A 2022 Reappraisal. Pharmaceutics. 2022; 14(7):1478. https://doi.org/10.3390/pharmaceutics14071478
Chicago/Turabian StyleVeytia-Bucheli, José Ignacio, Den Alejandro Alvarado-Velázquez, Lourival Domingos Possani, Roberto González-Amaro, and Yvonne Rosenstein. 2022. "The Ca2+ Channel Blocker Verapamil Inhibits the In Vitro Activation and Function of T Lymphocytes: A 2022 Reappraisal" Pharmaceutics 14, no. 7: 1478. https://doi.org/10.3390/pharmaceutics14071478





