The Application of Hollow Fiber Cartridge in Biomedicine
Abstract
1. Introduction
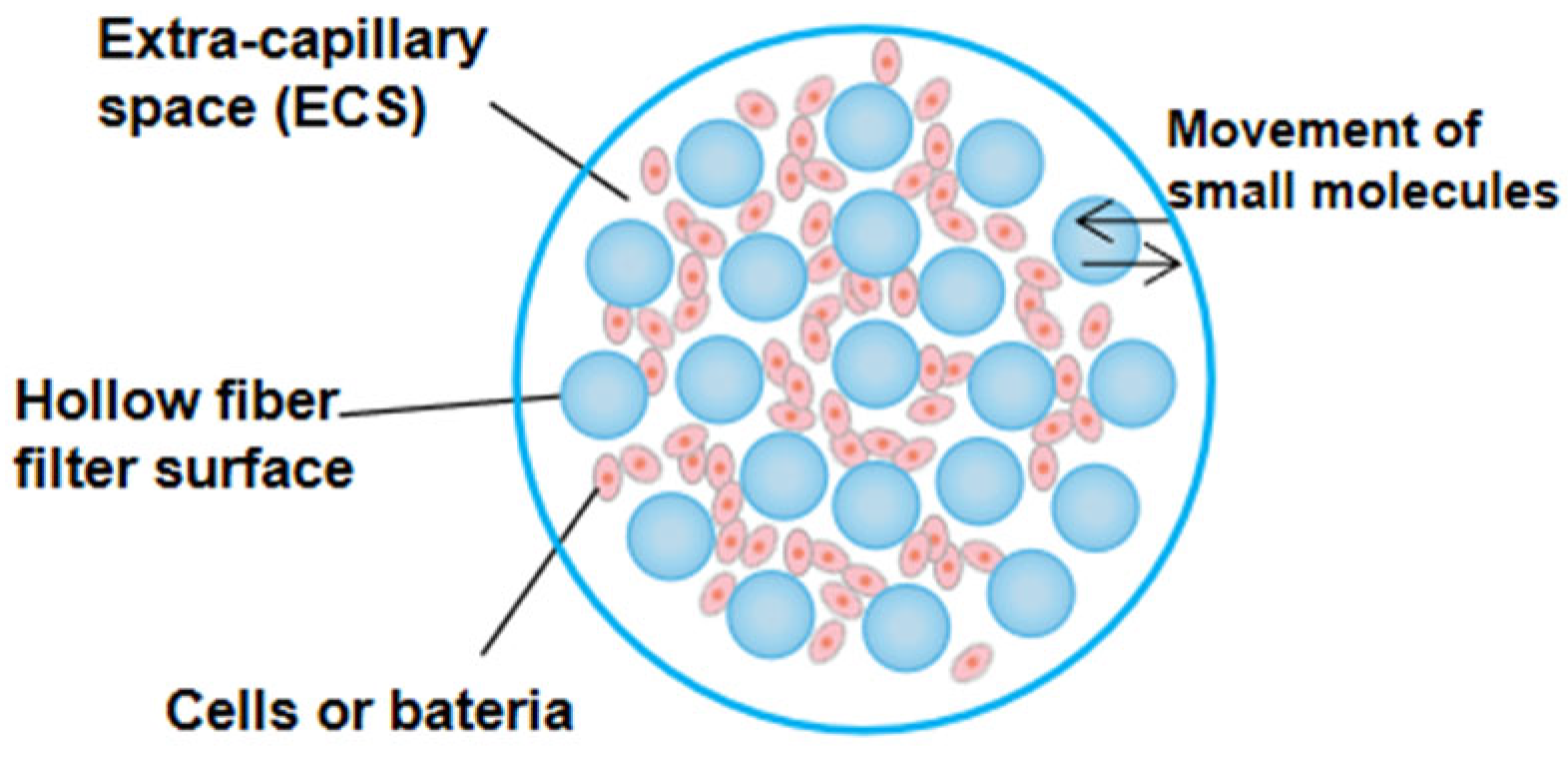
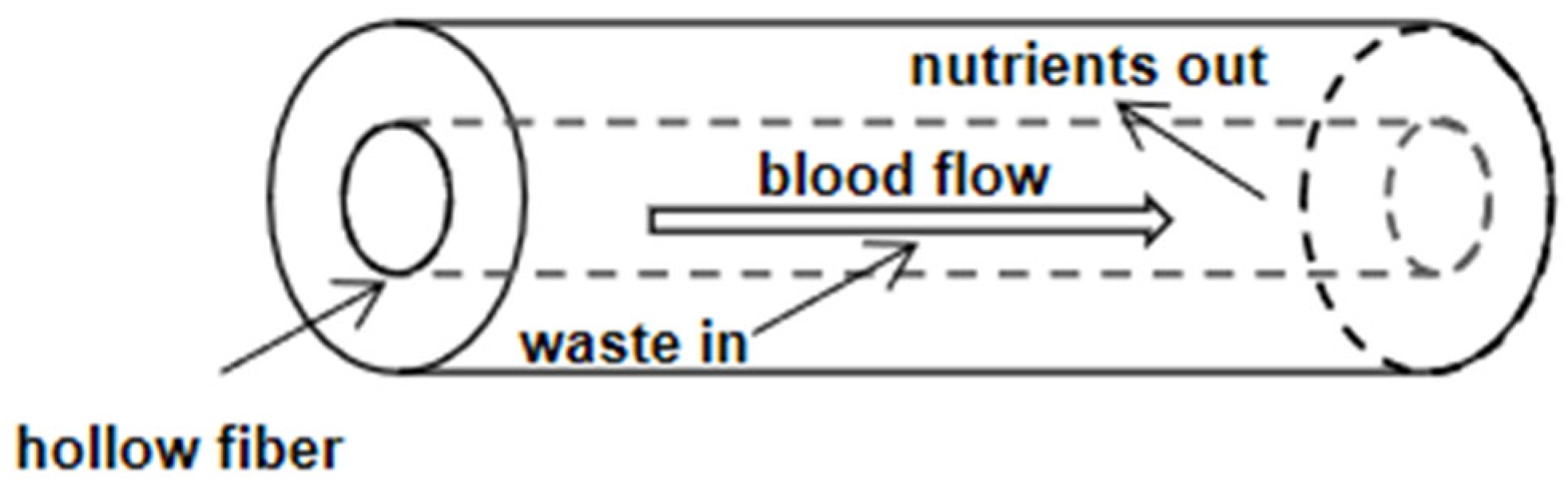
2. Basic Structure of Hollow Fiber Cartridge
3. Construction and Application of Hollow Fiber Infection Model
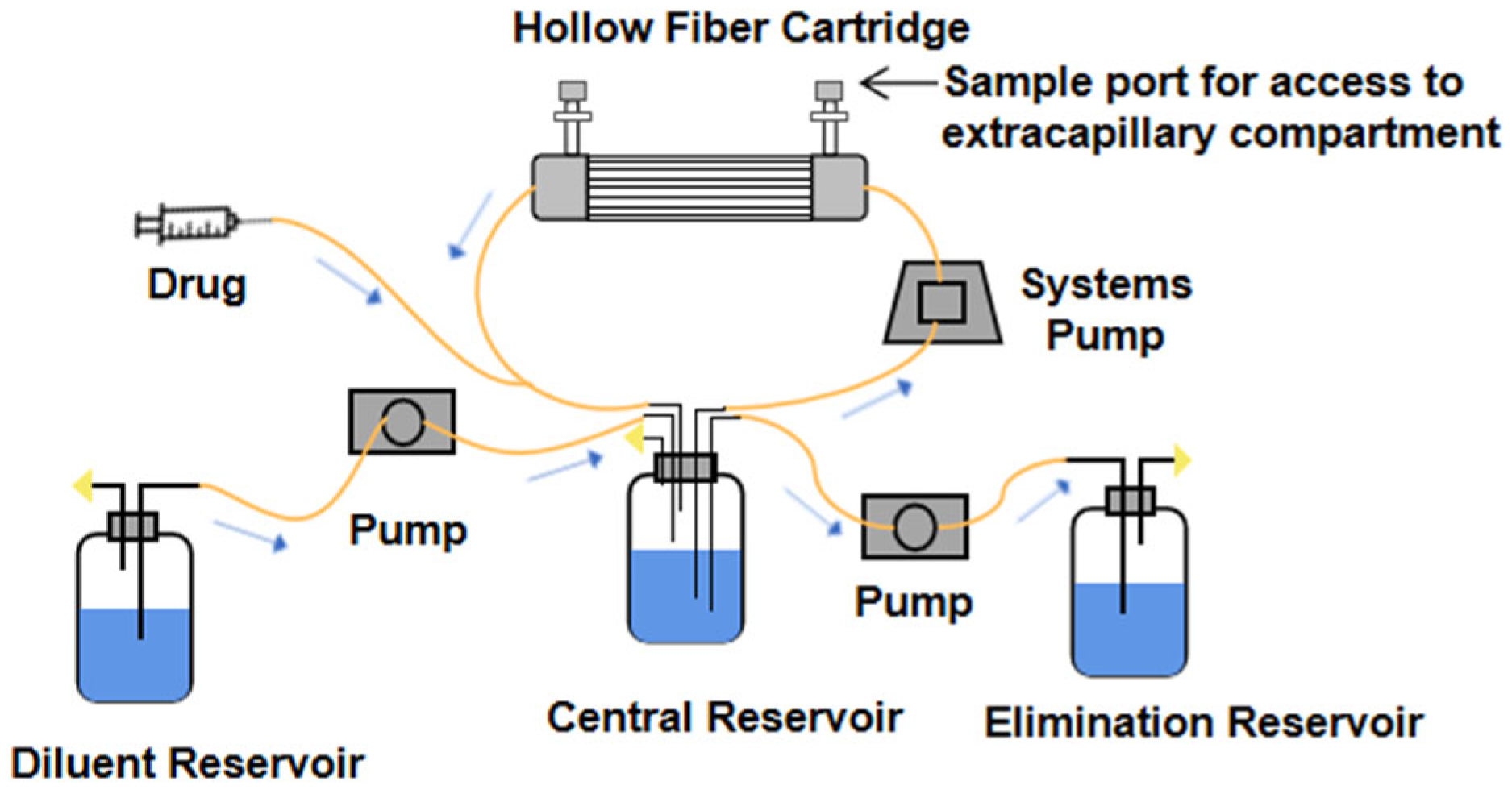
3.1. Design Principle of Hollow Fiber Infection Model
3.2. Characteristics of Hollow Fiber Infection Model
3.3. Application of HFIM in the Medicine
3.3.1. Evaluation of Drug Effects
3.3.2. Study of Drug Resistance
3.3.3. Evaluation of Combination Regimen
| Bacteria | Combination Regimen | Effect | Reference |
|---|---|---|---|
| M. tuberculosis | rifampicin(100 mg/d) + moxifloxacin(100 mg/d) | reduce drug exposure for drug resistance inhibition | [57] |
| ceftriaxone(100 mg/kg) + avibactam(15 mg/mL) | shortening the treatment time of children with disseminated tuberculosis | [49] | |
| A. baumannii | ampicillin-sulbactam(8/4 g/8 h) + meropenem(2 g/8 h)+polymyxin B(1.43 mg/kg/12 h) | rapid (96 h) eradication of A. baumannii | [58] |
| K. pneumoniae | amikacin(300 mg/L) + fosfomycin(1200 mg/mL) | synergetic sterilization and resistance inhibition | [46] |
| P.aeruginosa | intravenous imipenem(500 mg/6 h) + REL(250 gm/6 h) | the best therapeutic effect | [52] |
| meropenem(1 g/8 h,0.5 h infusion) + tobramycin(10 mg/kg/24 h) | synergetic sterilization and resistance inhibition | [59] | |
| piperacillin(4 g/4 h 0.5 h infusion) + tobramycin(5 mg/kg/24 h, 7 mg/kg/q24 h, 10 mg/kg/48 h, 0.5 h infusion) | synergetic sterilization and resistance inhibition | [56] | |
| E.coli | polymyxin B(30,000 U/kg/day) + tigecycline(100 mg/12 h) | synergetic sterilization and resistance inhibition | [60] |
| ceftazidime/avibactam + (2/0.5 g/8 h)aztreonam(2 g/6 h) | synergetic sterilization and resistance inhibition | [53] |
4. Construction and Application of Hollow Fiber Bioreactor
4.1. Characteristics of Hollow Fiber Bioreactor
4.2. Application of Hollow Fiber Bioreactor in the Medicine
4.2.1. Bioartificial Liver Bioreactor
4.2.2. Other Applications
5. Application of Hollow Fiber Dialyzer
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, T.R.; Hadidi, M.; Motevalian, S.P.; Sunohara, T.; Zydney, A.L. Transport Characteristics of Asymmetric Cellulose Triacetate Hemodialysis Membranes. Blood Purif. 2018, 45, 46–52. [Google Scholar] [CrossRef]
- Cadwell, J.J.S. The hollow fiber infection model: Principles and practice. Arch. Clin. Microbiol. 2015, 1, 101. [Google Scholar]
- Romero, K.; Clay, R.; Hanna, D. Strategic Regulatory Evaluation and Endorsement of the Hollow Fiber Tuberculosis System as a Novel Drug Development Tool. Clin. Infect. Dis. 2015, 61 (Suppl. S1), S5–S9. [Google Scholar] [CrossRef][Green Version]
- Cadwell, J.J.S.; Whitford, W.G. Three-dimensional cell-based assays in hollow fibre bioreactors. In Technology Platforms for 3D Cell Culture; Przyborski, S., Ed.; John Wiley and Sons: Hoboken, NJ, USA, 2017; pp. 327–350. [Google Scholar]
- High, K.M.; Snider, M.T.; Panol, G.R.; Richard, R.B.; Gray, D.N. Polysulfone coating for hollow fiber artificial lungs operated at hypobaric and hyperbaric pressures. ASAIO J. 1996, 42, M442–M445. [Google Scholar] [CrossRef]
- Palakkan, A.A.; Raj, D.K.; Rojan, J.; Raj, R.G.S.; Anil Kumar, P.R.; Muraleedharan, C.V.; Kumary, T.V. Evaluation of polypropylene hollow-fiber prototype bioreactor for bioartificialliver. Tissue Eng. Part A 2013, 19, 1056–1066. [Google Scholar] [CrossRef]
- Mohebbi-Kalhori, D.; Behzadmehr, A.; Doillon, C.J.; Hadjizadeh, A. Computational modeling of adherent cell growth in a hollow-fiber membrane bioreactor for large-scale 3-D bone tissue engineering. J. Artif. Organs 2012, 15, 250–265. [Google Scholar] [CrossRef]
- Grasso, S.; Meinardi, G.; de Carneri, I.; Tamassia, V. New in vitro model to study the effect of antibiotic concentration and rate of elimination on antibacterial activity. Antimicrob. Agents Chemother. 1978, 13, 570–576. [Google Scholar] [CrossRef]
- Wang, L.; Wismer, M.K.; Racine, F.; Conway, D.; Giacobbe, R.A.; Berejnaia, O.; Kath, G.S. Development of an integrated semi-automated system for in vitro pharmacodynamic modelling. J. Antimicrob. Chemother. 2008, 62, 1070–1077. [Google Scholar] [CrossRef][Green Version]
- Tomita, T.; Ohara-Nemoto, Y.; Moriyama, H.; Ozawa, A.; Takeda, Y.; Kikuchi, K. A novel in vitro pharmacokinetic/pharmacodynamic model based on two-compartment open model used to simulate serum drug concentration-time profiles. Microbiol. Immunol. 2007, 51, 567–575. [Google Scholar] [CrossRef]
- Zinner, S.H.; Husson, M.; Klastersky, J. An artificial capillary in vitro kinetic model of antibiotic bactericidal activity. J. Infect. Dis. 1981, 144, 583–587. [Google Scholar] [CrossRef]
- Bilello, J.A.; Bauer, G.; Dudley, M.N.; Cole, G.A.; Drusano, G.L. Effect of 2′,3′-didehydro-3′-deoxythymidine in an in vitro hollow-fiber pharmacodynamic model system correlates with results of dose-ranging clinical studies. Antimicrob. Agents Chemother. 1994, 38, 1386–1391. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cavaleri, M.; Manolis, E. Hollow Fiber System Model for Tuberculosis: The European Medicines Agency Experience. Clin. Infect. Dis. 2015, 61 (Suppl. S1), S1–S4. [Google Scholar] [CrossRef] [PubMed]
- Blaser, J.; Stone, B.B.; Zinner, S.H. Two compartment kinetic model with multiple artificial capillary units. Antimicrob. Chemother. 1985, 15 (Suppl. A), 131–137. [Google Scholar] [CrossRef]
- Blaser, J. In-vitro model for simultaneous simulation of the serum kinetics of two drugs with different half-lives. Antimicrob. Chemother. 1985, 15 (Suppl. A), 125–130. [Google Scholar] [CrossRef]
- Zhao, L.; Li, X.; He, X.; Jian, L. Levofloxacin-ceftazidime administration regimens combat Pseudomonas aeruginosa in the hollow-fiber infection model simulating abnormal renal function in critically ill patients. BMC Pharmacol. Toxicol. 2020, 21, 20. [Google Scholar] [CrossRef] [PubMed]
- Bonapace, C.R.; Friedrich, L.V.; Bosso, J.A.; White, R.L. Determination of Antibiotic Effect in an in vitro Pharmacodynamic Model: Comparison with an Established Animal Model of Infection. Antimicrob. Agents Chemother. 2002, 46, 3574–3579. [Google Scholar] [CrossRef]
- Andes, D.; Craig, W.A. Pharmacodynamics of the new fluoroquinolone gatifloxacin in murine thigh and lung infection models. Antimicrob. Agents Chemother. 2002, 46, 1665–1670. [Google Scholar] [CrossRef]
- Booker, B.M.; Smith, P.F.; Forrest, A.; Bullock, J.; Kelchlin, P.; Bhavnani, S.M.; Jones, R.N.; Ambrose, P.G. Application of an in vitro Infection Model and Simulation for Reevaluation of Fluoroquinolone Breakpoints for Salmonella enterica Serotype Typhi. Antimicrob. Agents Chemother. 2005, 49, 1775–1781. [Google Scholar] [CrossRef]
- Liang, W.; Chen, Y.C.; Cao, Y.R.; Liu, X.F.; Huang, J.; Hu, J.L.; Zhao, M.; Guo, Q.L.; Zhang, S.J.; Wu, X.J.; et al. Pharmacokinetics and Pharmacodynamics of Nemonoxacin against Streptococcus pneumoniae in an in vitro Infection Model. Antimicrob. Agents Chemother. 2013, 57, 2942–2947. [Google Scholar] [CrossRef][Green Version]
- Hu, J.; Zhang, J.; Chen, Y.; Liang, W.; Wu, S. In vitro Anaerobic Pharmacokinetic/Pharmacodynamic Model to Simulate the Bactericidal Activity of Levornidazole Against Bacteroides fragilis. Clin. Ther. 2017, 39, 828–836. [Google Scholar] [CrossRef]
- Meletiadis, J.; Al-Saigh, R.; Velegraki, A.; Walsh, T.J.; Roilides, E.; Zerva, L. Pharmacodynamic Effects of Simulated Standard Doses of Antifungal Drugs against Aspergillus Species in a New in vitro Pharmacokinetic/Pharmacodynamic Model. Antimicrob. Agents Chemother. 2012, 56, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Ayestarán, A.; López, R.M.; Montoro, J.B.; Estíbalez, A.; Pou, L.; Julià, A.; López, A.; Pascual, B. Pharmacokinetics of conventional formulation versus fat emulsion formulation of amphotericin B in a group of patients with neutropenia. Antimicrob. Agents Chemother. 1996, 40, 609–612. [Google Scholar] [CrossRef] [PubMed]
- Purkins, L.; Wood, N.; Greenhalgh, K.; Eve, M.D.; Oliver, S.D.; Nichols, D. The pharmacokinetics and safety of intravenous voriconazole-a novel wide-spectrum antifungal agent. Br. J. Clin. Pharmacol. 2003, 56 (Suppl. S1), 2–9. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.J.; Adamson, P.C.; Seibel, N.L.; Flynn, P.M.; Neely, M.N.; Schwartz, C.; Shad, A.; Kaplan, S.L.; Roden, M.M.; Stone, J.A.; et al. Pharmacokinetics, safety, and tolerability of caspofungin in children and adolescents. Antimicrob. Agents Chemother. 2005, 49, 4536–4545. [Google Scholar] [CrossRef] [PubMed]
- Gloede, J.; Scheerans, C.; Derendorf, H.; Kloft, C. In vitro pharmacodynamic models to determine the effect of antibacterial drugs. J. Antimicrob. Chemother. 2010, 65, 186–201. [Google Scholar] [CrossRef]
- Huang, Z.; Wu, Y.; Zhou, Z.; Xia, X.; Gu, X.; Cai, Q.; Shen, X.; Yang, H.; Ding, H. Pharmacokinetic and Pharmacodynamic Integration and Resistance Analysis of Tilmicosin Against Mycoplasma gallisepticum in an in vitro Dynamic Model. Front. Pharmacol. 2019, 10, 670. [Google Scholar] [CrossRef]
- Wang, S.; Liu, H.; Mao, J.; Peng, Y.; Yan, Y.; Li, Y.; Zhang, N.; Jiang, L.; Liu, Y.; Li, J.; et al. Pharmacodynamics of Linezolid Plus Fosfomycin Against Vancomycin-Resistant Enterococcus faecium in a Hollow Fiber Infection Model. Front. Microbiol. 2021, 12, 779885. [Google Scholar] [CrossRef]
- Nielsen, E.I.; Khan, D.D.; Cao, S.; Lustig, U.; Hughes, D.; Andersson, D.I.; Friberg, L.E. Can a pharmacokinetic/pharmacodynamic (PKPD) model be predictive across bacterial densities and strains? External evaluation of a PKPD model describing longitudinal in vitro data. J. Antimicrob. Chemother. 2017, 72, 3108–3116. [Google Scholar] [CrossRef]
- Bradford, P.A.; Miller, A.A.; O’Donnell, J.; Mueller, J.P. Zoliflodacin: An Oral Spiropyrimidinetrione Antibiotic for the Treatment of Neisseria gonorrheae, Including Multi-Drug-Resistant Isolates. ACS Infect. Dis. 2020, 6, 1332–1345. [Google Scholar] [CrossRef]
- Singh, R.; Almutairi, M.; Alm, R.A.; Lahiri, S.D.; San Martin, M.; Chen, A.; Ambler, J.E. Ceftaroline efficacy against high-MIC clinical Staphylococcus aureus isolates in an in vitro hollow-fibre infection model. J. Antimicrob. Chemother. 2017, 72, 2796–2803. [Google Scholar] [CrossRef]
- Drusano, G.L.; Myrick, J.; Maynard, M.; Nole, J.; Duncanson, B.; Brown, D.; Schmidt, S.; Neely, M.; Scanga, C.A.; Peloquin, C.; et al. Linezolid Kills Acid-Phase and Nonreplicative-Persister-Phase Mycobacterium tuberculosis in a Hollow-Fiber Infection Model. Antimicrob. Agents Chemother. 2018, 62, e00221-18. [Google Scholar] [CrossRef] [PubMed]
- Marum, D.; Manning, L.; Raby, E. Revisiting the inoculum effect for Streptococcus pyogenes with a hollow fibre infection model. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 2137–2144. [Google Scholar] [CrossRef] [PubMed]
- Boorgula, G.D.; Jakkula, L.; Gumbo, T.; Jung, B.; Srivastava, S. Comparison of Rifamycins for Efficacy Against Mycobacterium avium Complex and Resistance Emergence in the Hollow Fiber Model System. Front. Pharmacol. 2021, 12, 645264. [Google Scholar] [CrossRef] [PubMed]
- VanScoy, B.D.; Lakota, E.A.; Conde, H.; Fikes, S.; Bhavnani, S.M.; Elefante, P.B.; Scangarella-Oman, N.E.; Ambrose, P.G. Gepotidacin Pharmacokinetics-Pharmacodynamics against Escherichia coli in the One-Compartment and Hollow-Fiber In Vitro Infection Model Systems. Antimicrob. Agents Chemother. 2021, 65, e0012221. [Google Scholar] [CrossRef]
- Jacobsson, S.; Golparian, D.; Oxelbark, J.; Alirol, E.; Franceschi, F.; Gustafsson, T.N.; Brown, D.; Louie, A.; Drusano, G.; Unemo, M. Pharmacodynamic Evaluation of Dosing, Bacterial Kill, and Resistance Suppression for Zoliflodacin Against Neisseria gonorrhoeae in a Dynamic Hollow Fiber Infection Model. Front. Pharmacol. 2021, 12, 682135. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Lipman, J.; Mouton, J.W.; Hope, W.W.; Roberts, J.A. Applying pharmacokinetic/pharmacodynamic principles in critically ill patients: Optimizing efficacy and reducing resistance development. Semin. Respir. Crit. Care Med. 2015, 36, 136–153. [Google Scholar] [CrossRef]
- Alieva, K.N.; Strukova, E.N.; Golikova, M.V.; Portnoy, Y.A.; Zinner, S.H.; Firsov, A.A. Time inside the mutant selection window as a predictor of staphylococcal resistance to linezolid. J. Antibiot. 2018, 71, 514–521. [Google Scholar] [CrossRef]
- Firsov, A.A.; Smirnova, M.V.; Lubenko, I.Y.; Vostrov, S.N.; Portnoy, Y.A.; Zinner, S.H. Testing the mutant selection window hypothesis with Staphylococcus aureus exposed to daptomycin and vancomycin in an in vitro dynamic model. J. Antimicrob. Chemother. 2006, 58, 1185–1192. [Google Scholar] [CrossRef]
- Firsov, A.A.; Alieva, K.N.; Strukova, E.N.; Golikova, M.V.; Portnoy, Y.A.; Dovzhenko, S.A.; Kobrin, M.B.; Romanov, A.V.; Edelstein, M.V.; Zinner, S.H. Testing the mutant selection window hypothesis with Staphylococcus aureus exposed to linezolid in an in vitro dynamic model. J. Antimicrob. Chemother. 2017, 72, 3100–3107. [Google Scholar] [CrossRef]
- Deshpande, D.; Pasipanodya, J.G.; Mpagama, S.G.; Bendet, P.; Srivastava, S.; Koeuth, T.; Lee, P.S.; Bhavnani, S.M.; Ambrose, P.G.; Thwaites, G.; et al. Levofloxacin Pharmacokinetics/Pharmacodynamics, Dosing, Susceptibility Breakpoints, and Artificial Intelligence in the Treatment of Multidrug-resistant Tuberculosis. Clin. Infect. Dis. 2018, 67 (Suppl. S3), S293–S302. [Google Scholar] [CrossRef]
- Drusano, G.L.; Rogers, S.; Brown, D.; Peloquin, C.A.; Neely, M.; Yamada, W.; Kim, S.; Almoslem, M.; Schmidt, S.; Louie, A. Dose Fractionation of Moxifloxacin for Treatment of Tuberculosis: Impact of Dosing Interval and Elimination Half-Life on Microbial Kill and Resistance Suppression. Antimicrob. Agents Chemother. 2021, 65, e02533-20. [Google Scholar] [CrossRef] [PubMed]
- VanScoy, B.; McCauley, J.; Bhavnani, S.M.; Ellis-Grosse, E.J.; Ambrose, P.G. Relationship between Fosfomycin Exposure and Amplification of Escherichia coli Subpopulations with Reduced Susceptibility in a Hollow-Fiber Infection Model. Antimicrob. Agents Chemother. 2016, 60, 5141–5145. [Google Scholar] [CrossRef] [PubMed]
- Garimella, N.; Zere, T.; Hartman, N.; Gandhi, A.; Bekele, A.; Li, X.; Stone, H.; Sacks, L.; Weaver, J.L. Effect of drug combinations on the kinetics of antibiotic resistance emergence in Escherichia coli CFT073 using an in vitro hollow-fibre infection model. Antimicrob. Agents Chemother. 2020, 55, 105861. [Google Scholar] [CrossRef] [PubMed]
- Bilal, H.; Peleg, A.Y.; McIntosh, M.P.; Styles, I.K.; Hirsch, E.B.; Landersdorfer, C.B.; Bergen, P.J. Elucidation of the pharmacokinetic/pharmacodynamic determinants of fosfomycin activity against Pseudomonas aeruginosa using a dynamic in vitro model. J. Antimicrob. Chemother. 2018, 73, 1570–1578. [Google Scholar] [CrossRef]
- Heffernan, A.J.; Sime, F.B.; Sarovich, D.S.; Neely, M.; Guerra-Valero, Y.; Naicker, S.; Cottrell, K.; Harris, P.; Andrews, K.T.; Ellwood, D.; et al. Pharmacodynamic Evaluation of Plasma and Epithelial Lining Fluid Exposures of Amikacin against Pseudomonas aeruginosa in a Dynamic In Vitro Hollow-Fiber Infection Model. Antimicrob. Agents Chemother. 2020, 64, e00879-20. [Google Scholar] [CrossRef]
- Alm, R.A.; Lahiri, S.D.; Kutschke, A.; Otterson, L.G.; McLaughlin, R.E.; Whiteaker, J.D.; Lewis, L.A.; Su, X.; Huband, M.D.; Gardner, H.; et al. Characterization of the novel DNA gyrase inhibitor AZD0914: Low resistance potential and lack of cross-resistance in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 2015, 59, 1478–1486. [Google Scholar] [CrossRef]
- Hope, W.; Stone, N.R.H.; Johnson, A.; McEntee, L.; Farrington, N.; Santoro-Castelazo, A.; Liu, X.; Lucaci, A.; Hughes, M.; Oliver, J.D.; et al. Fluconazole Monotherapy Is a Suboptimal Option for Initial Treatment of Cryptococcal Meningitis Because of Emergence of Resistance. mBio 2019, 10, e02575-19. [Google Scholar] [CrossRef]
- Nielsen, E.I.; Friberg, L.E. Pharmacokinetic-pharmacodynamic modeling of antibacterial drugs. Pharmacol. Rev. 2013, 65, 1053–1090. [Google Scholar] [CrossRef]
- Bhagunde, P.; Zhang, Z.; Racine, F.; Carr, D.; Wu, J.; Young, K.; Rizk, M.L. A translational pharmacokinetic/pharmacodynamic model to characterize bacterial kill in the presence of imipenem-relebactam. Int J Infect Dis. 2019, 89, 55–61. [Google Scholar] [CrossRef]
- Wu, J.; Racine, F.; Wismer, M.K.; Young, K.; Carr, D.M.; Xiao, J.C.; Katwaru, R.; Si, Q.; Harradine, P.; Motyl, M.; et al. Exploring the Pharmacokinetic/Pharmacodynamic Relationship of Relebactam (MK-7655) in Combination with Imipenem in a Hollow-Fiber Infection Model. Antimicrob. Agents Chemother. 2018, 62, e02323-17. [Google Scholar] [CrossRef]
- Srivastava, S.; van Zyl, J.; Cirrincione, K.; Martin, K.; Thomas, T.; Deshpande, D.; Alffenaar, J.W.; Seddon, J.A.; Gumbo, T. Evaluation of Ceftriaxone Plus Avibactam in an Intracellular Hollow Fiber Model of Tuberculosis: Implications for the Treatment of Disseminated and Meningeal Tuberculosis in Children. Pediatr. Infect. Dis. J. 2020, 39, 1092–1100. [Google Scholar] [CrossRef] [PubMed]
- Lodise, T.P.; Smith, N.M.; O’Donnell, N.; Eakin, A.E.; Holden, P.; Boissonneault, K.R.; Zhou, J.; Tao, X.; Bulitta, J.B.; Fowler, V.G.; et al. Determining the optimal dosing of a novel combination regimen of ceftazidime/avibactam with aztreonam against NDM-1-producing Enterobacteriaceae using a hollow-fibre infection model. J. Antimicrob. Chemother. 2020, 75, 2622–2632. [Google Scholar] [CrossRef]
- Tam, V.H.; Abodakpi, H.; Wang, W.; Ledesma, K.R.; Merlau, P.R.; Chan, K.; Altman, R.; Tran, T.T.; Nikolaou, M.; Sofjan, A.K. Optimizing pharmacokinetics/pharmacodynamics of β-lactam/β-lactamase inhibitor combinations against high inocula of ESBL-producing bacteria. J. Antimicrob. Chemother. 2021, 76, 179–183. [Google Scholar] [CrossRef]
- Roberts, J.A.; Abdul-Aziz, M.H.; Lipman, J.; Mouton, J.W.; Vinks, A.A.; Felton, T.W.; Hope, W.W.; Farkas, A.; Neely, M.N.; Schentag, J.J.; et al. International Society of Anti-Infective Pharmacology and the Pharmacokinetics and Pharmacodynamics Study Group of the European Society of Clinical Microbiology and Infectious Diseases. Individualised antibiotic dosing for patients who are critically ill: Challenges and potential solutions. Lancet Infect. Dis. 2014, 14, 498–509. [Google Scholar] [PubMed]
- Yadav, R.; Rogers, K.E.; Bergen, P.J.; Bulitta, J.B.; Kirkpatrick, C.M.J.; Wallis, S.C.; Paterson, D.L.; Nation, R.L.; Lipman, J.; Roberts, J.A.; et al. Optimization and Evaluation of Piperacillin-Tobramycin Combination Dosage Regimens against Pseudomonas aeruginosa for Patients with Altered Pharmacokinetics via the Hollow-Fiber Infection Model and Mechanism-Based Modeling. Antimicrob. Agents Chemother. 2018, 62, e00078-18. [Google Scholar] [CrossRef] [PubMed]
- Drusano, G.L.; Sgambati, N.; Eichas, A.; Brown, D.L.; Kulawy, R.; Louie, A.; Low, D.E. The Combination of Rifampin plus Moxifloxacin Is Synergistic for Suppression of Resistance but Antagonistic for Cell Kill of Mycobacterium tuberculosis as Determined in a Hollow-Fiber Infection Model. mBio 2010, 1, e00139-10. [Google Scholar] [CrossRef]
- Lenhard, J.R.; Smith, N.M.; Bulman, Z.P.; Tao, X.; Thamlikitkul, V.; Shin, B.S.; Nation, R.L.; Li, J.; Bulitta, J.B.; Tsuji, B.T. High-Dose Ampicillin-Sulbactam Combinations Combat Polymyxin-Resistant Acinetobacter baumannii in a Hollow-Fiber Infection Model. Antimicrob. Agents Chemother. 2017, 61, e01268-16. [Google Scholar] [CrossRef]
- Landersdorfer, C.B.; Rees, V.E.; Yadav, R.; Rogers, K.E.; Kim, T.H.; Bergen, P.J.; Cheah, S.E.; Boyce, J.D.; Peleg, A.Y.; Oliver, A.; et al. Optimization of a Meropenem-Tobramycin Combination Dosage Regimen against Hypermutable and Nonhypermutable Pseudomonas aeruginosa via Mechanism-Based Modeling and the Hollow-Fiber Infection Model. Antimicrob. Agents Chemother. 2018, 62, e02055-17. [Google Scholar] [CrossRef]
- Cai, Y.; Lim, T.P.; Teo, J.Q.; Sasikala, S.; Chan, E.C.; Hong. Y., J.; Lee, W.; Tan, T.Y.; Tan, T.T.; Koh, T.H.; et al. Evaluating Polymyxin B-Based Combinations against Carbapenem-Resistant Escherichia coli in Time-Kill Studies and in a Hollow-Fiber Infection Model. Antimicrob. Agents Chemother. 2016, 61, e01509-16. [Google Scholar] [CrossRef]
- Knazek, R.A.; Gullino, P.M.; Kohler, P.O.; Dedrick, R.L. Cell culture on artificial capillaries: An approach to tissue growth in vitro. Sciences 1972, 178, 65–66. [Google Scholar] [CrossRef]
- Vermasvuori, R.; Hurme, M. Economic comparison of diagnostic antibody production in perfusion stirred tank and in hollow fiber bioreactor processes. Biotechnol. Prog. 2011, 27, 1588–1598. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Zhu, X.J.; Yuan, T.J.; Wang, Z.Y.; Bian, Z.Q.; Jing, H.S.; Shi, X.; Chen, C.Y.; Fu, G.B.; Huang, W.J.; et al. An extracorporeal bioartificial liver embedded with 3D-layered human liver progenitor-like cells relieves acute liver failure in pigs. Sci. Transl. Med. 2020, 12, eaba5146. [Google Scholar] [CrossRef] [PubMed]
- Riordan, S.; Williams, R. Bioartificial liver support: Developments in hepatocyte culture and bioreactor design. Br. Med. Bull. 1997, 53, 730–744. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.M.M.; Salerno, S.; Piscioneri, A.; Khakpour, S.; Giorno, L.; De Bartolo, L. Human liver microtissue spheroids in hollow fiber membrane bioreactor. Colloids Surf. B Biointerfaces 2017, 160, 272–280. [Google Scholar] [CrossRef]
- Sakiyama, R.; Hamada, H.; Blau, B.; Freyer, N.; Zeilinger, K.; Schubert, F.; Miki, T. Evaluation of the mass transfer rate using computer simulation in a three-dimensional interwoven hollow fiber-type bioartificial liver. Biotechnol. Lett. 2018, 40, 1567–1578. [Google Scholar] [CrossRef] [PubMed]
- Valdés, R.; Ibarra, N.; González, M.; Alvarez, T.; García, J.; Llambias, R.; Pérez, C.A.; Quintero, O.; Fischer, R. CB.Hep-1 hybridoma growth and antibody production using protein-free medium in a hollow fiber bioreactor. Cytotechnology 2001, 35, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Legazpi, L. Diffusion and Inhibition Processes in a Hollow-fiber Membrane Bioreactor for Hybridoma Culture. Development of a Mathematical Model. Chem. Biochem. Eng. Q. 2016, 30, 213–225. [Google Scholar] [CrossRef]
- Legazpi, L.; Díaz, J.; Laca, A.; Díaz, M. Kinetic analysis of hybridoma cell culture in a protein-free medium: Substrate and agitation effects. Biochem. Eng. J. 2005, 26, 122. [Google Scholar] [CrossRef]
- Eghbali, H.; Nava, M.M.; Mohebbi-Kalhori, D.; Raimondi, M.T. Hollow fiber bioreactor technology for tissue engineering applications. Int. J. Artif. Organs 2016, 39, 1–15. [Google Scholar] [CrossRef]
- Vymetalova, L.; Kucirkova, T.; Knopfova, L.; Pospisilova, V.; Kasko, T.; Lejdarova, H.; Makaturova, E.; Kuglik, P.; Oralova, V.; Matalova, E.; et al. Large-Scale Automated Hollow-Fiber Bioreactor Expansion of Umbilical Cord-Derived Human Mesenchymal Stromal Cells for Neurological Disorders. Neurochem. Res. 2020, 45, 204–214. [Google Scholar] [CrossRef]
- Uslu, U.; Erdmann, M.; Wiesinger, M.; Schuler, G.; Schuler-Thurner, B. Automated Good Manufacturing Practice-compliant generation of human monocyte-derived dendritic cells from a complete apheresis product using a hollow-fiber bioreactor system overcomes a major hurdle in the manufacture of dendritic cells for cancer vaccines. Cytotherapy 2019, 21, 1166–1178. [Google Scholar] [CrossRef] [PubMed]
- Weeraphan, C.; Diskul-Na-Ayudthaya, P.; Chiablaem, K.; Khongmanee, A.; Chokchaichamnankit, D.; Subhasitanont, P.; Svasti, J.; Srisomsap, C. Effective enrichment of cholangiocarcinoma secretomes using the hollow fiber bioreactor culture system. Talanta 2012, 99, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.C.; Bayik, D.; Srivatsan, A.; Bergamaschi, C.; Valentin, A.; Niu, G.; Bear, J.; Monninger, M.; Sun, M.; Morales-Kastresana, A.; et al. Efficient production and enhanced tumor delivery of engineered extracellular vesicles. Biomaterials 2016, 105, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Zhang, G.; Shen, C.; Yin, J.; Meng, Q. Hollow fiber culture accelerates differentiation of Caco-2 cells. Appl. Microbiol. Biotechnol. 2013, 97, 6943–6955. [Google Scholar] [CrossRef]
- Clark, W.R.; Rocha, E.; Ronco, C. Solute removal by hollow-fiber dialyzers. Contrib. Nephrol. 2007, 158, 20–33. [Google Scholar] [PubMed]
- Hirano, A.; Yamamoto, K.; Matsuda, M.; Ogawa, T.; Yakushiji, T.; Miyasaka, T.; Sakai, K. Evaluation of dialyzer jacket structure and hollow-fiber dialysis membranes to achieve high dialysis performance. Ther. Apher. Dial. 2011, 15, 66–74. [Google Scholar] [CrossRef]
- Lin, C.-C.; Yang, M.-C. Urea permeation and hydrolysis through hollow fiber dialyzer immobilized with urease: Storage and operation properties. Biomaterials 2003, 24, 1989–1994. [Google Scholar] [CrossRef]
- Modi, A.; Verma, S.K.; Bellare, J. Extracellular matrix-coated polyethersulfone-TPGS hollow fiber membranes showing improved biocompatibility and uremic toxins removal for bioartificial kidney application. Colloids Surf. B Biointerfaces 2018, 167, 457–467. [Google Scholar] [CrossRef]
- Yehl, C.J.; Jabra, M.G.; Zydney, A.L. Hollow fiber countercurrent dialysis for continuous buffer exchange of high-value biotherapeutics. Biotechnol. Prog. 2019, 35, e2763. [Google Scholar] [CrossRef]
- Savarimuthu, S.; BinSaeid, J.; Harky, A. The role of ECMO in COVID-19: Can it provide rescue therapy in those who are critically ill? J. Card. Surg. 2020, 35, 1298–1301. [Google Scholar] [CrossRef]
- Taşkan, B.; Casey, E.; Hasar, H. Simultaneous oxidation of ammonium and tetracycline in a membrane aerated biofilm reactor. Sci. Total Environ. 2019, 682, 553–560. [Google Scholar] [CrossRef] [PubMed]
| Drug (Simulated Dose [mg/kg]) and Pharmacokinetic Parameter | Avg Value in Human Plasma a | Mean Value In Vitro Model b ± SEM |
|---|---|---|
| Amphotericin B | ||
| Cmax (mg/L) | 2.83 | 2.6 ± 0.1 |
| t1/2 (h) | 19.65 | 11 ± 1.5 |
| AUC0–24 (mg·h/L) | 28.98 | 34.52 |
| Voriconazole | ||
| Cmax (mg/L) | 3.62 | 3.7 ± 0.17 |
| t1/2 (h) | 6.5 | 5.9 ± 0.6 |
| AUC0–24 (mg·h/L) | 22.7 | 30.37 |
| Caspofungin | ||
| Cmax (mg/L) | 10 | 9.3 ± 0.25 |
| t1/2 (h) | 12.2 | 14 ± 1.25 |
| AUC0–24 (mg·h/L) | 97.20 | 120.31 |
| i-MAb Bag a (500 mL) | T150-Flask a | HFBR b | |
|---|---|---|---|
| MAb maximum concentration (mg mL−1) | 0.8–0.74 | 0.030 | 0.220 |
| Time to achieve the maximum MAb concentration (h) | 720 | 50 | 4 |
| Productivity (mg mL–1 h–1) | 0.0001 | 0.0006 | 0.0021 |
| Medium yield (MAb obtained/culture medium consumed) (mg mL–1) | 0.074 | 0.030 | 0.009 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Y.; Mi, K.; Sun, L.; Zhou, K.; Wang, L.; Zhang, L.; Liu, Z.; Huang, L. The Application of Hollow Fiber Cartridge in Biomedicine. Pharmaceutics 2022, 14, 1485. https://doi.org/10.3390/pharmaceutics14071485
Hou Y, Mi K, Sun L, Zhou K, Wang L, Zhang L, Liu Z, Huang L. The Application of Hollow Fiber Cartridge in Biomedicine. Pharmaceutics. 2022; 14(7):1485. https://doi.org/10.3390/pharmaceutics14071485
Chicago/Turabian StyleHou, Yixuan, Kun Mi, Lei Sun, Kaixiang Zhou, Lei Wang, Lan Zhang, Zhenli Liu, and Lingli Huang. 2022. "The Application of Hollow Fiber Cartridge in Biomedicine" Pharmaceutics 14, no. 7: 1485. https://doi.org/10.3390/pharmaceutics14071485
APA StyleHou, Y., Mi, K., Sun, L., Zhou, K., Wang, L., Zhang, L., Liu, Z., & Huang, L. (2022). The Application of Hollow Fiber Cartridge in Biomedicine. Pharmaceutics, 14(7), 1485. https://doi.org/10.3390/pharmaceutics14071485






