Reducing the Kidney Uptake of High Contrast CXCR4 PET Imaging Agents via Linker Modifications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemistry
2.2. Chemical Synthesis
2.3. Radiochemistry
2.4. Cell Culture
2.5. In Vitro Competitive Binding Assay
2.6. LogD7.4 Measurement
2.7. Animal Models
2.8. PET/CT Imaging
2.9. Biodistribution Studies
2.10. Statistical Analysis
3. Results
3.1. Synthesis of the Derivatives
3.2. In Vitro Characterization and Radiolabeling of BL02 Derivatives
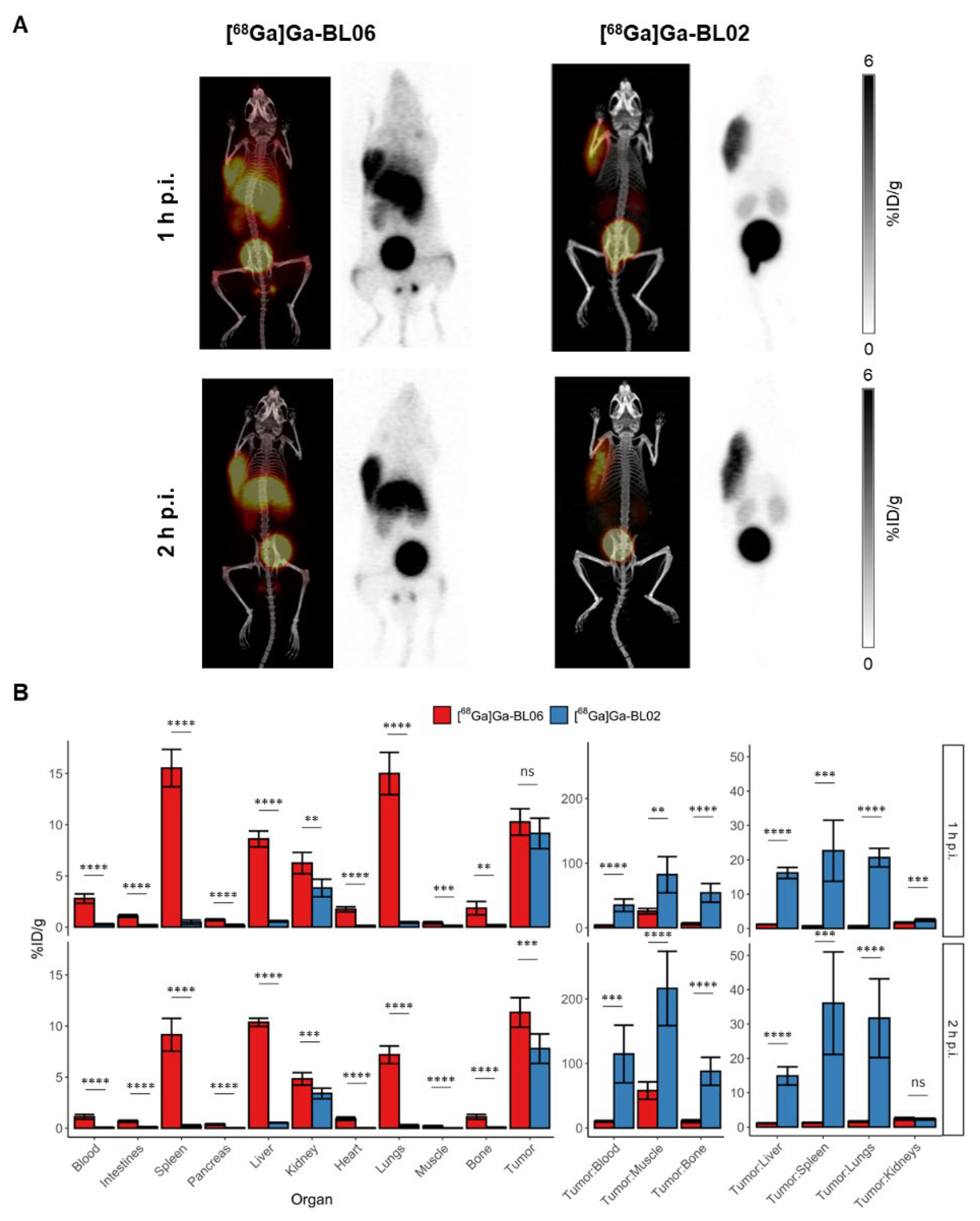
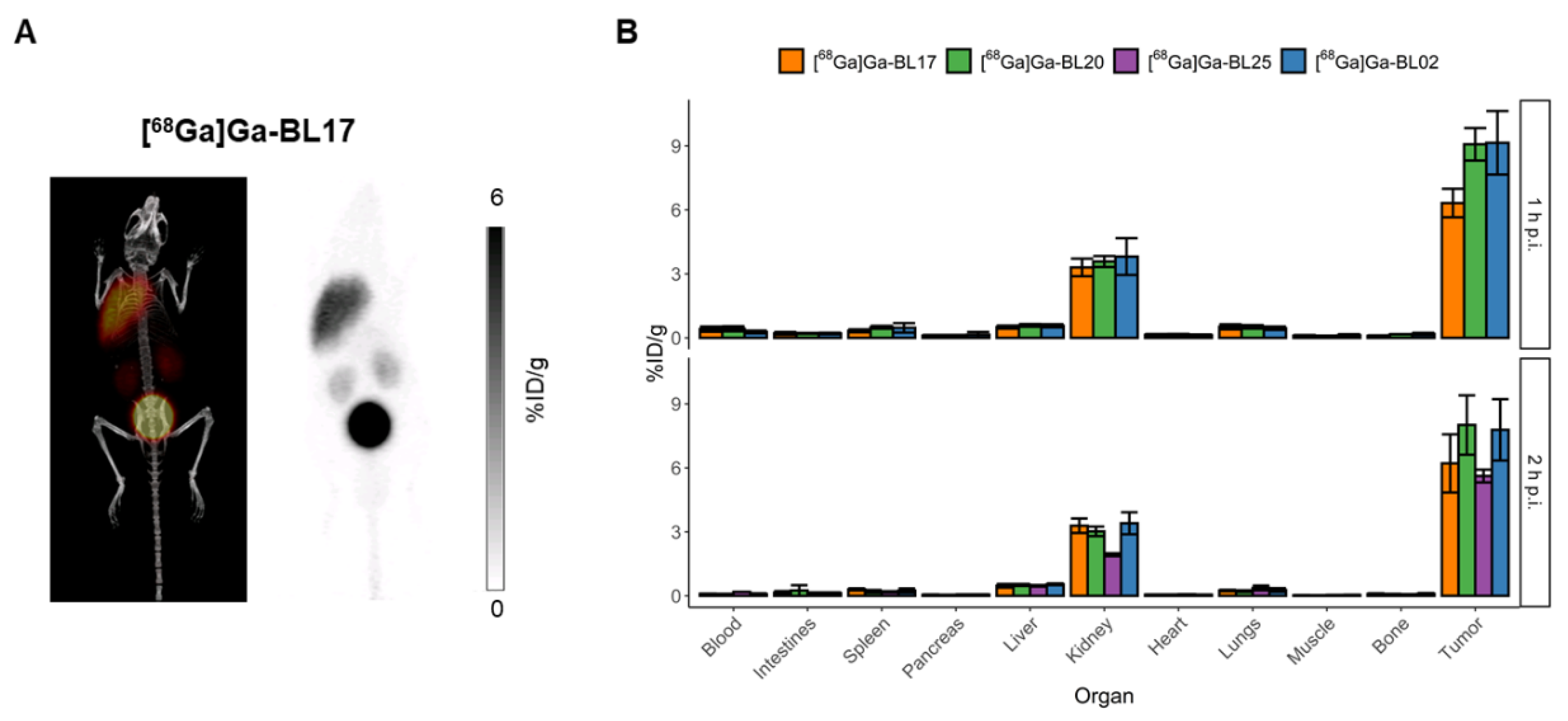
3.3. Biodistribution and PET Imaging Studies
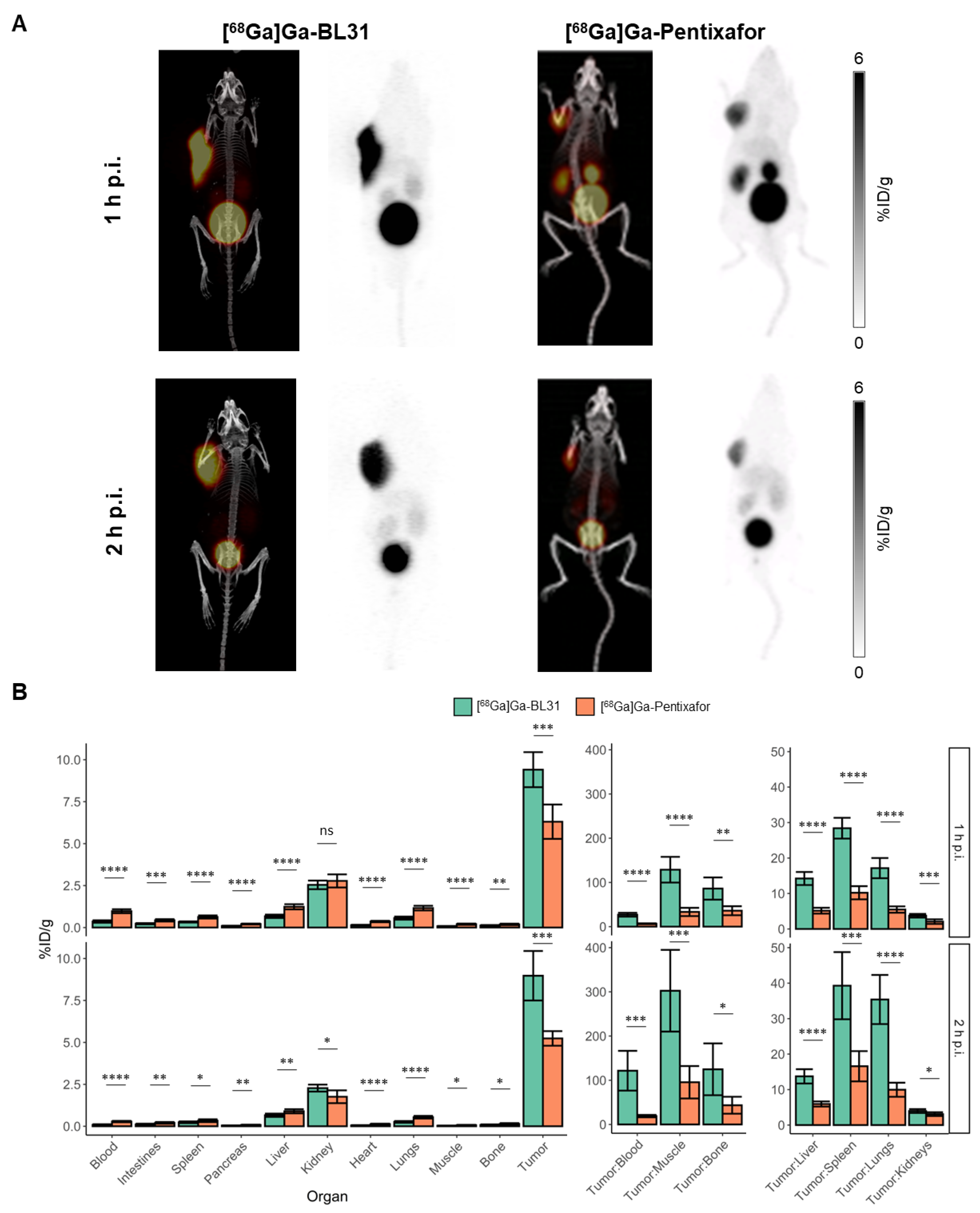
3.4. Comparison of [68Ga]Ga-BL31 with [68Ga]Ga-Pentixafor
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Andricopulo, A.D.; Montanari, C.A. Structure-Activity Relationships for the Design of Small-Molecule Inhibitors. Mini-Rev. Med. Chem. 2005, 5, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Winkler, D.A. The Role of Quantitative Structure—Activity Relationships (QSAR) in Biomolecular Discovery. Brief. Bioinform. 2002, 3, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Guha, R. On Exploring Structure-Activity Relationships. Methods Mol. Biol. 2013, 993, 81–94. [Google Scholar] [CrossRef] [Green Version]
- Bohacek, R.S.; McMartin, C.; Guida, W.C. The Art and Practice of Structure-Based Drug Design: A Molecular Modeling Perspective. Med. Res. Rev. 1996, 16, 3–50. [Google Scholar] [CrossRef]
- Derendorf, H.; Lesko, L.J.; Chaikin, P.; Colburn, W.A.; Lee, P.; Miller, R.; Powell, R.; Rhodes, G.; Stanski, D.; Venitz, J. Pharmacokinetic/Pharmacodynamic Modeling in Drug Research and Development. J. Clin. Pharm. 2000, 40, 1399–1418. [Google Scholar]
- Gombar, V.K.; Hall, S.D. Quantitative Structure-Activity Relationship Models of Clinical Pharmacokinetics: Clearance and Volume of Distribution. J. Chem. Inf. Model. 2013, 53, 948–957. [Google Scholar] [CrossRef]
- Van de Waterbeemd, H.; Smith, D.A.; Beaumont, K.; Walker, D.K. Property-Based Design: Optimization of Drug Absorption and Pharmacokinetics. J. Med. Chem. 2001, 44, 1313–1333. [Google Scholar] [CrossRef]
- Kim, D.; Wang, L.; Beconi, M.; Eiermann, G.J.; Fisher, M.H.; He, H.; Hickey, G.J.; Kowalchick, J.E.; Leiting, B.; Lyons, K.; et al. (2R)-4-Oxo-4-[3-(Trifluoromethyl)-5,6-Dihydro[1,2,4]Triazolo[4,3-a]Pyrazin-7(8H)-Yl]-1-(2,4,5-Trifluorophenyl)Butan-2-Amine: A Potent, Orally Active Dipeptidyl Peptidase IV Inhibitor for the Treatment of Type 2 Diabetes. J. Med. Chem. 2005, 48, 141–151. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, X.; Zhang, X.; Tian, G.; Yang, R.; Wu, J.; Zou, X.; Liu, Z.; Yang, X.; Wu, C.; et al. Pharmacokinetics-Driven Optimization of 4(3 H)-Pyrimidinones as Phosphodiesterase Type 5 Inhibitors Leading to TPN171, a Clinical Candidate for the Treatment of Pulmonary Arterial Hypertension. J. Med. Chem. 2019, 62, 4979–4990. [Google Scholar] [CrossRef]
- Lau, J.; Rousseau, E.; Kwon, D.; Lin, K.-S.; Bénard, F.; Chen, X. Insight into the Development of PET Radiopharmaceuticals for Oncology. Cancers 2020, 12, 1312. [Google Scholar] [CrossRef]
- Baranski, A.-C.; Schäfer, M.; Bauder-Wüst, U.; Wacker, A.; Schmidt, J.; Liolios, C.; Mier, W.; Haberkorn, U.; Eisenhut, M.; Kopka, K.; et al. Improving the Imaging Contrast of 68Ga-PSMA-11 by Targeted Linker Design: Charged Spacer Moieties Enhance the Pharmacokinetic Properties. Bioconjug Chem. 2017, 28, 2485–2492. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.-T.; Pan, J.; Zhang, Z.; Lau, J.; Merkens, H.; Zhang, C.; Colpo, N.; Lin, K.-S.; Bénard, F. Effects of Linker Modification on Tumor-to-Kidney Contrast of 68Ga-Labeled PSMA-Targeted Imaging Probes. Mol. Pharm. 2018, 15, 3502–3511. [Google Scholar] [CrossRef]
- Mitran, B.; Varasteh, Z.; Selvaraju, R.K.; Lindeberg, G.; Sörensen, J.; Larhed, M.; Tolmachev, V.; Rosenström, U.; Orlova, A. Selection of Optimal Chelator Improves the Contrast of GRPR Imaging Using Bombesin Analogue RM26. Int. J. Oncol. 2016, 48, 2124–2134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Witting, E.; Garousi, J.; Lindbo, S.; Vorobyeva, A.; Altai, M.; Oroujeni, M.; Mitran, B.; Orlova, A.; Hober, S.; Tolmachev, V. Selection of the Optimal Macrocyclic Chelators for Labeling with 111In and 68Ga Improves Contrast of HER2 Imaging Using Engineered Scaffold Protein ADAPT6. Eur. J. Pharm. Biopharm. 2019, 140, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Domanska, U.M.; Kruizinga, R.C.; Nagengast, W.B.; Timmer-Bosscha, H.; Huls, G.; de Vries, E.G.E.; Walenkamp, A.M.E. A Review on CXCR4/CXCL12 Axis in Oncology: No Place to Hide. Eur. J. Cancer 2013, 49, 219–230. [Google Scholar] [CrossRef]
- Zhao, H.; Guo, L.; Zhao, H.; Zhao, J.; Weng, H.; Zhao, B. CXCR4 Over-Expression and Survival in Cancer: A System Review and Meta-Analysis. Oncotarget 2015, 6, 5022–5040. [Google Scholar] [CrossRef] [Green Version]
- Balkwill, F. Cancer and the Chemokine Network. Nat. Rev. Cancer 2004, 4, 540–550. [Google Scholar] [CrossRef]
- Zeng, Z.; Xi Shi, Y.; Samudio, I.J.; Wang, R.-Y.; Ling, X.; Frolova, O.; Levis, M.; Rubin, J.B.; Negrin, R.R.; Estey, E.H.; et al. Targeting the Leukemia Microenvironment by CXCR4 Inhibition Overcomes Resistance to Kinase Inhibitors and Chemotherapy in AML. Blood 2009, 113, 6215–6224. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Teo, A.E.; McCarty, N. ROS-Induced CXCR4 Signaling Regulates Mantle Cell Lymphoma (MCL) Cell Survival and Drug Resistance in the Bone Marrow Microenvironment via Autophagy. Clin. Cancer Res. 2016, 22, 187–199. [Google Scholar] [CrossRef] [Green Version]
- Darash-Yahana, M.; Pikarsky, E.; Abramovitch, R.; Zeira, E.; Pal, B.; Karplus, R.; Beider, K.; Avniel, S.; Kasem, S.; Galun, E.; et al. Role of High Expression Levels of CXCR4 in Tumor Growth, Vascularization, and Metastasis. FASEB J. 2004, 18, 1240–1242. [Google Scholar] [CrossRef]
- Yan, L.; Cai, Q.; Xu, Y. The Ubiquitin-CXCR4 Axis Plays an Important Role in Acute Lung Infection-Enhanced Lung Tumor Metastasis. Clin. Cancer Res. 2013, 19, 4706–4716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrmann, K.; Lapa, C.; Wester, H.-J.; Schottelius, M.; Schiepers, C.; Eberlein, U.; Bluemel, C.; Keller, U.; Knop, S.; Kropf, S.; et al. Biodistribution and Radiation Dosimetry for the Chemokine Receptor CXCR4-Targeting Probe 68Ga-Pentixafor. J. Nucl. Med. 2015, 56, 410–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schottelius, M.; Osl, T.; Poschenrieder, A.; Hoffmann, F.; Beykan, S.; Schirbel, A.; Buck, A.K.; Kropf, S.; Schwaiger, M.; Keller, U.; et al. [177Lu] Pentixather: Comprehensive Preclinical Characterization of a First CXCR4-Directed Endoradiotherapeutic Agent. Theranostics 2017, 7, 2350–2362. [Google Scholar] [CrossRef]
- Kwon, D.; Takata, K.; Zhang, Z.; Chong, L.; Fraser, B.; Zeisler, J.; Miyata-Takata, T.; Merkens, H.; Rousseau, J.; Aoki, T.; et al. Targeting Refractory Mantle Cell Lymphoma for Imaging and Therapy Using C-X-C Chemokine Receptor Type 4 Radioligands. Clin. Cancer Res. 2022, 28, 1628–1639. [Google Scholar] [CrossRef] [PubMed]
- Melis, M.; Krenning, E.P.; Bernard, B.F.; Barone, R.; Visser, T.J.; de Jong, M. Localisation and Mechanism of Renal Retention of Radiolabelled Somatostatin Analogues. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Barone, R.; van der Smissen, P.; Devuyst, O.; Beaujean, V.; Pauwels, S.; Courtoy, P.J.; Jamar, F. Endocytosis of the Somatostatin Analogue, Octreotide, by the Proximal Tubule-Derived Opossum Kidney (OK) Cell Line. Kidney Int. 2005, 67, 969–976. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, K.; Ishizaki, A.; Takai, K.; Kitamura, Y.; Makino, A.; Kozaka, T.; Kiyono, Y.; Shiba, K.; Odani, A. Evaluation of Ga-DOTA-(D-Asp)n as Bone Imaging Agents: D-Aspartic Acid Peptides as Carriers to Bone. Sci. Rep. 2017, 7, 13971. [Google Scholar] [CrossRef] [Green Version]
- Kwon, D.; Lozada, J.; Zhang, Z.; Zeisler, J.; Poon, R.; Zhang, C.; Roxin, Á.; Lin, K.-S.; Perrin, D.; Benard, F. High-Contrast CXCR4-Targeted 18 F-PET Imaging Using a Potent and Selective Antagonist. Mol. Pharm. 2021, 18, 187–197. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Z.; Lin, K.-S.; Pan, J.; Dude, I.; Hundal-Jabal, N.; Colpo, N.; Bénard, F. Preclinical Melanoma Imaging with 68 Ga-Labeled α-Melanocyte-Stimulating Hormone Derivatives Using PET. Theranostics 2017, 7, 805–813. [Google Scholar] [CrossRef] [Green Version]
- Motulsky, H.J.; Brown, R.E. Detecting Outliers When Fitting Data with Nonlinear Regression—A New Method Based on Robust Nonlinear Regression and the False Discovery Rate. BMC Bioinform. 2006, 7, 123. [Google Scholar] [CrossRef] [Green Version]
- Weineisen, M.; Simecek, J.; Schottelius, M.; Schwaiger, M.; Wester, H.-J. Synthesis and Preclinical Evaluation of DOTAGA-Conjugated PSMA Ligands for Functional Imaging and Endoradiotherapy of Prostate Cancer. EJNMMI Res. 2014, 4, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michels, T.; Dölling, R.; Haberkorn, U.; Mier, W. Acid-Mediated Prevention of Aspartimide Formation in Solid Phase Peptide Synthesis. Org. Lett. 2012, 14, 5218–5221. [Google Scholar] [CrossRef] [PubMed]
- Behrendt, R.; Huber, S.; Martí, R.; White, P. New T-Butyl Based Aspartate Protecting Groups Preventing Aspartimide Formation in Fmoc SPPS. J. Pept. Sci. 2015, 21, 680–687. [Google Scholar] [CrossRef]
- Geenen, L.; Nonnekens, J.; Konijnenberg, M.; Baatout, S.; de Jong, M.; Aerts, A. Overcoming Nephrotoxicity in Peptide Receptor Radionuclide Therapy Using [177Lu]Lu-DOTA-TATE for the Treatment of Neuroendocrine Tumours. Nucl. Med. Biol. 2021, 102–103, 1–11. [Google Scholar] [CrossRef]
- Buitinga, M.; Jansen, T.; van der Kroon, I.; Woliner-van der Weg, W.; Boss, M.; Janssen, M.; Aarntzen, E.; Béhé, M.; Wild, D.; Visser, E.; et al. Succinylated Gelatin Improves the Theranostic Potential of Radiolabeled Exendin-4 in Insulinoma Patients. J. Nucl. Med. 2019, 60, 812–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurth, J.; Krause, B.J.; Schwarzenböck, S.M.; Bergner, C.; Hakenberg, O.W.; Heuschkel, M. First-in-Human Dosimetry of Gastrin-Releasing Peptide Receptor Antagonist [177Lu]Lu-RM2: A Radiopharmaceutical for the Treatment of Metastatic Castration-Resistant Prostate Cancer. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 123–135. [Google Scholar] [CrossRef]
- Felber, V.B.; Wester, H.-J. Small Peptide-Based GLP-1R Ligands: An Approach to Reduce the Kidney Uptake of Radiolabeled GLP-1R-Targeting Agents? EJNMMI Radiopharm. Chem. 2021, 6, 29. [Google Scholar] [CrossRef]
- Melis, M.; Krenning, E.P.; Bernard, B.F.; de Visser, M.; Rolleman, E.; de Jong, M. Renal Uptake and Retention of Radiolabeled Somatostatin, Bombesin, Neurotensin, Minigastrin and CCK Analogues: Species and Gender Differences. Nucl. Med. Biol. 2007, 34, 633–641. [Google Scholar] [CrossRef]
- Atkins, M.B.; Tannir, N.M. Current and Emerging Therapies for First-Line Treatment of Metastatic Clear Cell Renal Cell Carcinoma. Cancer Treat. Rev. 2018, 70, 127–137. [Google Scholar] [CrossRef] [Green Version]
- Béhé, M.; Kluge, G.; Becker, W.; Gotthardt, M.; Behr, T.M. Use of Polyglutamic Acids to Reduce Uptake of Radiometal-Labeled Minigastrin in the Kidneys. J. Nucl. Med. 2005, 46, 1012–1015. [Google Scholar]
- Nanda, P.K.; Lane, S.R.; Retzloff, L.B.; Pandey, U.S.; Smith, C.J. Radiolabeled Regulatory Peptides for Imaging and Therapy. In Current Opinion in Endocrinology, Diabetes and Obesity; NIH: Bethesda, MD, USA, 2010; pp. 69–76. [Google Scholar] [CrossRef] [Green Version]
- Vaidyanathan, G.; Kang, C.M.; McDougald, D.; Minn, I.; Brummet, M.; Pomper, M.G.; Zalutsky, M.R. Brush Border Enzyme-Cleavable Linkers: Evaluation for Reducing Renal Uptake of Radiolabeled Prostate-Specific Membrane Antigen Inhibitors. Nucl. Med. Biol. 2018, 62–63, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Liu, Z.; Shi, J.; Yu, Z.; Yang, Z.; Zhao, H.; He, Z.; Liu, S.; Wang, F. Linker Effects on Biological Properties of 111 In-Labeled DTPA Conjugates of a Cyclic RGDfK Dimer. Bioconjugate Chem. 2008, 19, 201–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagai, J.; Takano, M. Molecular-Targeted Approaches to Reduce Renal Accumulation of Nephrotoxic Drugs. Expert Opin. Drug Metab. Toxicol. 2010, 6, 1125–1138. [Google Scholar] [CrossRef]
- De Jong, M.; Barone, R.; Krenning, E.; Bernard, B.; Melis, M.; Visser, T.; Gekle, M.; Willnow, T.E.; Walrand, S.; Jamar, F.; et al. Megalin Is Essential for Renal Proximal Tubule Reabsorption of (111)In-DTPA-Octreotide. J. Nucl. Med. 2005, 46, 1696–1700. [Google Scholar] [PubMed]
- Schottelius, M.; Herrmann, K.; Lapa, C. In Vivo Targeting of CXCR4—New Horizons. Cancers 2021, 13, 5920. [Google Scholar] [CrossRef]
- Lau, J.; Kwon, D.; Rousseau, E.; Zhang, Z.; Zeisler, J.; Uribe, C.F.; Kuo, H.T.; Zhang, C.; Lin, K.S.; Bénard, F. Ga/[177Lu]Lu-BL01, a Novel Theranostic Pair for Targeting C-X-C Chemokine Receptor 4. Mol. Pharm. 2019, 16, 4688–4695. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.B.; van Horn, R.D.; Yin, T.; Brown, R.M.; Roell, W.C.; Obungu, V.H.; Ruegg, C.; Wroblewski, V.J.; Raddad, E.; Stille, J.R. Distinct Mobilization of Leukocytes and Hematopoietic Stem Cells by CXCR4 Peptide Antagonist LY2510924 and Monoclonal Antibody LY2624587. Oncotarget 2017, 8, 94619–94634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westerlund, K.; Honarvar, H.; Norrström, E.; Strand, J.; Mitran, B.; Orlova, A.; Eriksson Karlström, A.; Tolmachev, V. Increasing the Net Negative Charge by Replacement of DOTA Chelator with DOTAGA Improves the Biodistribution of Radiolabeled Second-Generation Synthetic Affibody Molecules. Mol. Pharm. 2016, 13, 1668–1678. [Google Scholar] [CrossRef]
- Yao, J.-F.; Yang, H.; Zhao, Y.-Z.; Xue, M. Metabolism of Peptide Drugs and Strategies to Improve Their Metabolic Stability. Curr. Drug Metab. 2018, 19, 892–901. [Google Scholar] [CrossRef]
- Ballatore, C.; Huryn, D.M.; Smith, A.B. Carboxylic Acid (Bio)Isosteres in Drug Design. ChemMedChem 2013, 8, 385–395. [Google Scholar] [CrossRef] [Green Version]
- Etoga, J.-L.G.; Ahmed, S.K.; Patel, S.; Bridges, R.J.; Thompson, C.M. Conformationally-Restricted Amino Acid Analogues Bearing a Distal Sulfonic Acid Show Selective Inhibition of System over the Vesicular Glutamate Transporter. Bioorganic Med. Chem. Lett. 2010, 20, 2680–2683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, G.A.; Kilpatrick, I.C. The Neurotransmitter Candidature of Sulphur-Containing Excitatory Amino Acids in the Mammalian Central Nervous System. Pharmacol. Ther. 1996, 72, 25–36. [Google Scholar] [CrossRef]
- Curtis, D.R.; Watkins, J.C. Acidic Amino Acids with Strong Excitatory Actions on Mammalian Neurones. J. Physiol. 1963, 166, 1–14. [Google Scholar] [CrossRef] [PubMed]


| Radiotracer | IC50 (nM) | Peptide Net Charge | LogD7.4 |
|---|---|---|---|
| [68Ga]BL02 | 27.9 ± 12.5 † | 3.0 | −4.20 ± 0.44 |
| [68Ga]BL06 | 26.3 ± 27.6 † | 4.0 | N.D. |
| [68Ga]BL17 | 13.0 ± 8.6 † | 3.0 | N.D. |
| [68Ga]BL20 | N.D. | 3.0 | N.D. |
| [68Ga]BL25 | 21.3 ± 0.1 | 3.0 | N.D. |
| [68Ga]BL30 | 22.7 ± 1.2 | 2.0 | N.D. |
| [68Ga]BL31 | 16.2 ± 4.2 | 3.0 | −4.17 ± 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, D.; Zhang, Z.; Zeisler, J.; Kuo, H.-T.; Lin, K.-S.; Benard, F. Reducing the Kidney Uptake of High Contrast CXCR4 PET Imaging Agents via Linker Modifications. Pharmaceutics 2022, 14, 1502. https://doi.org/10.3390/pharmaceutics14071502
Kwon D, Zhang Z, Zeisler J, Kuo H-T, Lin K-S, Benard F. Reducing the Kidney Uptake of High Contrast CXCR4 PET Imaging Agents via Linker Modifications. Pharmaceutics. 2022; 14(7):1502. https://doi.org/10.3390/pharmaceutics14071502
Chicago/Turabian StyleKwon, Daniel, Zhengxing Zhang, Jutta Zeisler, Hsiou-Ting Kuo, Kuo-Shyan Lin, and Francois Benard. 2022. "Reducing the Kidney Uptake of High Contrast CXCR4 PET Imaging Agents via Linker Modifications" Pharmaceutics 14, no. 7: 1502. https://doi.org/10.3390/pharmaceutics14071502
APA StyleKwon, D., Zhang, Z., Zeisler, J., Kuo, H.-T., Lin, K.-S., & Benard, F. (2022). Reducing the Kidney Uptake of High Contrast CXCR4 PET Imaging Agents via Linker Modifications. Pharmaceutics, 14(7), 1502. https://doi.org/10.3390/pharmaceutics14071502






