Nanoparticles in Endodontics Disinfection: State of the Art
Abstract
:1. Introduction
2. The Oral Cavity and the Dentin-Pulp Complex
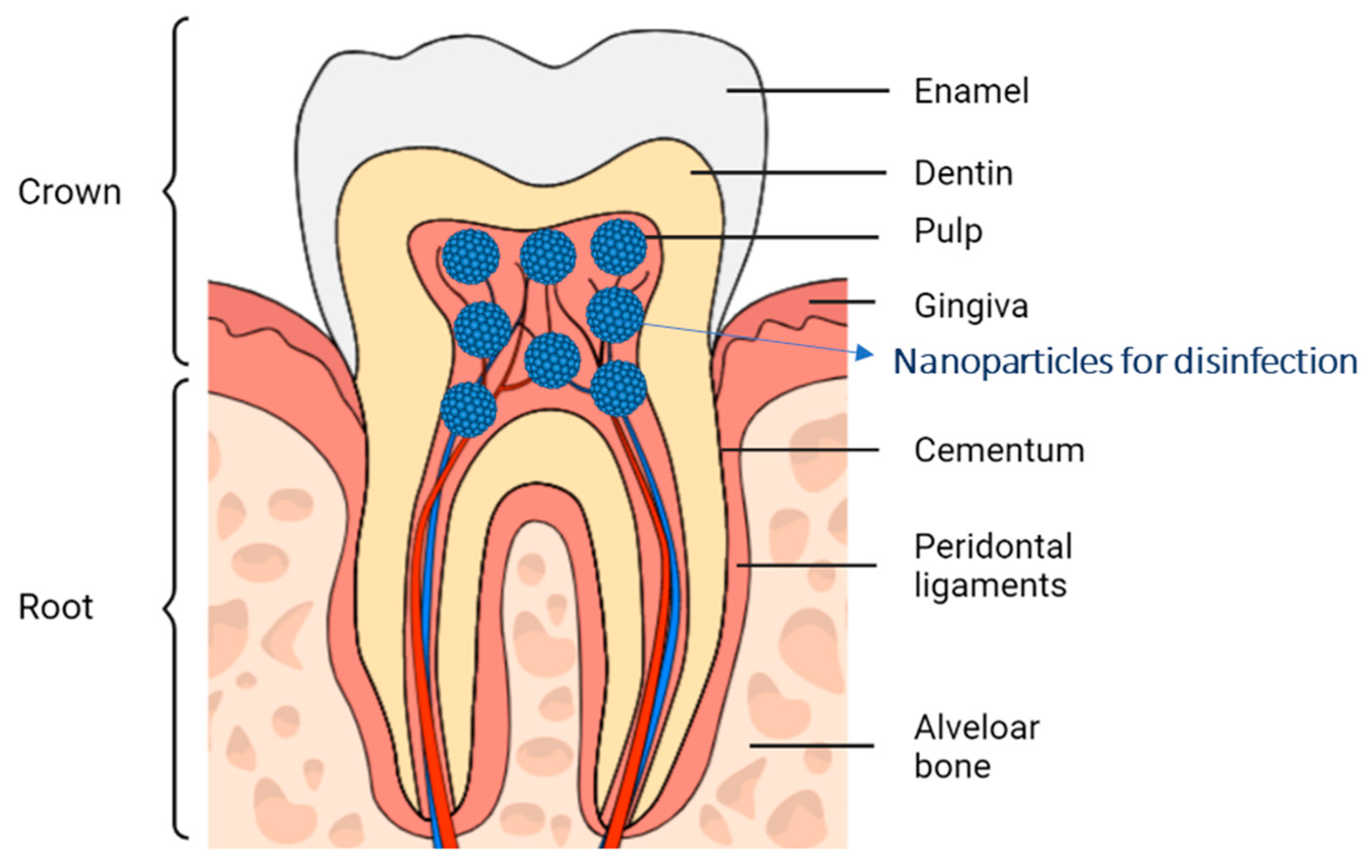
2.1. Anatomical Structures of the Dentin-Pulp Complex
2.2. Bacterial Infections of the Pulp and Dentinal-Pulp Complex
2.3. Current Bacterial Disinfection Techniques
3. Nanoparticles in the Medical Field
3.1. Nanoparticles in Endodontics
3.2. Biodegradable Nanoparticles
3.3. Inorganic Nanoparticles
3.4. Metal Nanoparticles
3.5. Mesoporous Calcium Silicate
3.6. Nanoparticles Functionalization
4. Biodegradable Nanoparticle in Endodontics Disinfection
4.1. Dentinal Biofilms
4.2. Biodegradable Nanoparticles in Endodontics
5. Conclusions
6. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Couvreur, P. Nanoparticles in Drug Delivery: Past, Present and Future. Adv. Drug Deliv. Rev. 2013, 65, 21–23. [Google Scholar] [CrossRef]
- Yohan, D.; Chithrani, B.D. Applications of Nanoparticles in Nanomedicine. J. Biomed. Nanotechnol. 2014, 10, 2371–2392. [Google Scholar] [CrossRef]
- Wolfram, J.; Zhu, M.; Yang, Y.; Shen, J.; Gentile, E.; Paolino, D.; Fresta, M.; Nie, G.; Chen, C.; Shen, H.; et al. Safety of Nanoparticles in Medicine HHS Public Access This Mini-Review Addresses the Safety Considerations for Nanoparticles in Medicine. Curr. Drug Targets 2015, 16, 1671–1681. [Google Scholar] [CrossRef] [Green Version]
- De Jong, W.H.; Borm, P.J.A. Drug Delivery and Nanoparticles: Applications and Hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef] [Green Version]
- Baetke, S.C.; Lammers, T.; Kiessling, F. Applications of Nanoparticles for Diagnosis and Therapy of Cancer. Br. J. Radiol. 2015, 88, 20150207. [Google Scholar] [CrossRef] [PubMed]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A New Classification Scheme for Periodontal and Peri-Implant Diseases and Conditions—Introduction and Key Changes from the 1999 Classification. J. Periodontol. 2018, 89, S1–S8. [Google Scholar] [CrossRef] [PubMed]
- Laudenbach, J.M.; Simon, Z. Common Dental and Periodontal Diseases: Evaluation and Management. Med. Clin. N. Am. 2014, 98, 1239–1260. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Meng, M.; Law, C.S.; Rao, Y.; Zhou, X. Common Dental Diseases in Children and Malocclusion. Int. J. Oral Sci. 2018, 10, 7. [Google Scholar] [CrossRef]
- Farges, J.C.; Alliot-Licht, B.; Renard, E.; Ducret, M.; Gaudin, A.; Smith, A.J.; Cooper, P.R. Dental Pulp Defence and Repair Mechanisms in Dental Caries. Mediat. Inflamm. 2015, 2015, 230251. [Google Scholar] [CrossRef] [Green Version]
- Listl, S.; Galloway, J.; Mossey, P.A.; Marcenes, W. Global Economic Impact of Dental Diseases. J. Dent. Res. 2015, 94, 1355–1361. [Google Scholar] [CrossRef]
- Raura, N.; Garg, A.; Arora, A.; Roma, M. Nanoparticle Technology and Its Implications in Endodontics: A Review. Biomater. Res. 2020, 24, 21. [Google Scholar] [CrossRef]
- Wong, J.; Zou, T.; Lee, A.H.C.; Zhang, C. The Potential Translational Applications of Nanoparticles in Endodontics. Int. J. Nanomed. 2021, 16, 2087–2106. [Google Scholar] [CrossRef]
- Shrestha, A.; Kishen, A. Antibacterial Nanoparticles in Endodontics: A Review. J. Endod. 2016, 42, 1417–1426. [Google Scholar] [CrossRef]
- Noronha, V.T.; Paula, A.J.; Durán, G.; Galembeck, A.; Cogo-Müller, K.; Franz-Montan, M.; Durán, N. Silver Nanoparticles in Dentistry. Dent. Mater. 2017, 33, 1110–1126. [Google Scholar] [CrossRef]
- Song, W.; Ge, S. Application of Antimicrobial Nanoparticles in Dentistry. Molecules 2019, 24, 1033. [Google Scholar] [CrossRef] [Green Version]
- Kirsch, C. Oral Cavity Cancer. Top. Magn. Reson. Imaging 2007, 18, 269–280. [Google Scholar] [CrossRef]
- Montero, P.H.; Patel, S.G. Cancer of the Oral Cavity. Surg. Oncol. Clin. N. Am. 2015, 24, 491–508. [Google Scholar] [CrossRef] [Green Version]
- Wanasathop, A.; Li, S.K. Iontophoretic Drug Delivery in the Oral Cavity. Pharmaceutics 2018, 10, 121. [Google Scholar] [CrossRef] [Green Version]
- Madani, M.; Berardi, T.; Stoopler, E.T. Anatomic and Examination Considerations of the Oral Cavity. Med. Clin. N. Am. 2014, 98, 1225–1238. [Google Scholar] [CrossRef]
- Galler, K.M.; Weber, M.; Korkmaz, Y.; Widbiller, M.; Feuerer, M. Inflammatory Response Mechanisms of the Dentine–Pulp Complex and the Periapical Tissues. Int. J. Mol. Sci. 2021, 22, 1480. [Google Scholar] [CrossRef]
- Amarie, S.; Zaslansky, P.; Kajihara, Y.; Griesshaber, E.; Schmahl, W.W.; Keilmann, F. Nano-FTIR Chemical Mapping of Minerals in Biological Materials. Beilstein J. Nanotechnol. 2012, 3, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Arola, D.D.; Gao, S.; Zhang, H.; Masri, R. The Tooth: Its Structure and Properties. Dent. Clin. N. Am. 2017, 61, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Akintoye, S.O.; Mupparapu, M. Clinical Evaluation and Anatomic Variation of the Oral Cavity. Dermatol. Clin. 2020, 38, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Nanci, A. Ten Cate’s Oral Histology: Development, Structure, and Function; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780323485180. [Google Scholar]
- Elmsmari, F.; González Sánchez, J.A.; Duran-Sindreu, F.; Belkadi, R.; Espina, M.; García, M.L.; Sánchez-López, E. Calcium Hydroxide-Loaded PLGA Biodegradable Nanoparticles as an Intracanal Medicament. Int. Endod. J. 2021, 54, 2086–2098. [Google Scholar] [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The Oral Microbiota: Dynamic Communities and Host Interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef]
- Lakhani, C.M. Tissue-specific immunity at the oral mucosal barrier, HHS Public Access. Physiol. Behav. 2019, 176, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.; Abbott, P.V. An Overview of the Dental Pulp: Its Functions and Responses to Injury. Aust. Dent. J. 2007, 52, S4–S6. [Google Scholar] [CrossRef]
- Walsh, L.J. Serious Complications of Endodontic Infections: Some Cautionary Tales. Aust. Dent. J. 1997, 42, 156–159. [Google Scholar] [CrossRef]
- Mejàre, I.A.; Axelsson, S.; Davidson, T.; Frisk, F.; Hakeberg, M.; Kvist, T.; Norlund, A.; Petersson, A.; Portenier, I.; Sandberg, H.; et al. Diagnosis of the Condition of the Dental Pulp: A Systematic Review. Int. Endod. J. 2012, 45, 597–613. [Google Scholar] [CrossRef]
- Pereira, T.C.; Dijkstra, R.J.B.; Petridis, X.; Sharma, P.K.; van de Meer, W.J.; van der Sluis, L.W.M.; de Andrade, F.B. Chemical and Mechanical Influence of Root Canal Irrigation on Biofilm Removal from Lateral Morphological Features of Simulated Root Canals, Dentine Discs and Dentinal Tubules. Int. Endod. J. 2021, 54, 112–129. [Google Scholar] [CrossRef]
- Haapasalo, M.; Shen, Y.; Wang, Z.; Gao, Y. Irrigation in Endodontics. Br. Dent. J. 2014, 216, 299–303. [Google Scholar] [CrossRef]
- Steve, S.; Marshall, J.; Rosen, S. The Solvent Action of Sodium Hypochlorite on Pulp Tissue of Extracted Teeth. Oral Surg. Oral Med. Oral Pathol. 1971, 31, 96–103. [Google Scholar]
- Haapasalo, H.K.; Sirén, E.K.; Waltimo, T.M.T.; Ørstavik, D.; Haapasalo, M.P.P. Inactivation of Local Root Canal Medicaments by Dentine: An in Vitro Study. Int. Endod. J. 2000, 33, 126–131. [Google Scholar] [CrossRef]
- Athanassiadis, B.; Abbott, P.V.; Walsh, L.J. The Use of Calcium Hydroxide, Antibiotics and Biocides as Antimicrobial Medicaments in Endodontics. Aust. Dent. J. 2007, 52, S64–S82. [Google Scholar] [CrossRef]
- Giardino, L.; Ambu, E.; Savoldi, E.; Rimondini, R.; Cassanelli, C.; Debbia, E.A. Comparative Evaluation of Antimicrobial Efficacy of Sodium Hypochlorite, MTAD, and Tetraclean Against Enterococcus Faecalis Biofilm. J. Endod. 2007, 33, 852–855. [Google Scholar] [CrossRef]
- Ng, Y.L.; Mann, V.; Gulabivala, K. A Prospective Study of the Factors Affecting Outcomes of Nonsurgical Root Canal Treatment: Part 1: Periapical Health. Int. Endod. J. 2011, 44, 583–609. [Google Scholar] [CrossRef]
- Vasudeva, A.; Sinha, D.J.; Tyagi, S.P.; Singh, N.N.; Garg, P.; Upadhyay, D. Disinfection of Dentinal Tubules with 2% Chlorhexidine Gel, Calcium Hydroxide and Herbal Intracanal Medicaments against Enterococcus Faecalis: An in-Vitro Study. Singap. Dent. J. 2017, 38, 39–44. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Dummer, P.M.H. Properties and Applications of Calcium Hydroxide in Endodontics and Dental Traumatology. Int. Endod. J. 2011, 44, 697–730. [Google Scholar] [CrossRef]
- Momenijavid, M.; Salimizand, H.; Korani, A.; Dianat, O.; Nouri, B.; Ramazanzadeh, R.; Ahmadi, A.; Rostamipour, J.; Khosravi, M.R. Effect of Calcium Hydroxide on Morphology and Physicochemical Properties of Enterococcus Faecalis Biofilm. Sci. Rep. 2022, 12, 7595. [Google Scholar] [CrossRef]
- Mizuno, M.; Banzai, Y. Calcium Ion Release from Calcium Hydroxide Stimulated Fibronectin Gene Expression in Dental Pulp Cells and the Differentiation of Dental Pulp Cells to Mineralized Tissue Forming Cells by Fibronectin. Int. Endod. J. 2008, 41, 933–938. [Google Scholar] [CrossRef]
- Al-Hiyasat, A.S.; El-Farraj, H.S.; Alebrahim, M.A. The Effect of Calcium Hydroxide on Dentine Composition and Root Fracture Resistance of Human Teeth: An in Vitro Study. Eur. J. Oral Sci. 2021, 129, e12798. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.P.M.; Chen, A.L.; Foster, A.; Drezek, R. In Vivo Biodistribution of Nanoparticles. Nanomedicine 2011, 6, 815–835. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, A.P.R.; Moreira, F.C.L.; Alves, D.R.S.; Estrela, C.R.A.; Estrela, C.; Carrião, M.S.; Bakuzis, A.F.; Lopes, L.G. Silver Nanoparticles in Resin Luting Cements: Antibacterial and Physiochemical Properties. J. Clin. Exp. Dent. 2016, 18, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Ettcheto, M.; Espina, M.; Auladell, C.; Folch, J.; Kuhne, B.A.; Barenys, M.; Sanchez-Lopez, E.; Souto, E.B.; Garcia, M.L.; et al. Epigallocatechin-3-gallate PEGylated poly(lactic-co-glycolic) acid nanoparticles mitigate striatal pathology and motor deficits in 3-nitropropionic acid intoxicated mice. Nanomedicine 2021, 16, 19–35. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Boisseau, P.; Loubaton, B. Nanomedicine, Nanotechnology in Medicine. Comptes Rendus Phys. 2011, 12, 620–636. [Google Scholar] [CrossRef] [Green Version]
- Aeran, H.; Kumar, V.; Uniyal, S.; Tanwer, P. Nanodentistry: Is Just a Fiction or Future. J. Oral Biol. Craniofacial Res. 2015, 5, 207–211. [Google Scholar] [CrossRef] [Green Version]
- Priyadarsini, S.; Mukherjee, S.; Mishra, M. Nanoparticles Used in Dentistry: A Review. J. Oral Biol. Craniofacial Res. 2018, 8, 58–67. [Google Scholar] [CrossRef] [Green Version]
- Moraes, G.; Zambom, C.; Siqueira, W.L. Nanoparticles in Dentistry: A Comprehensive Review. Pharmaceuticals 2021, 14, 752. [Google Scholar] [CrossRef]
- LaVan, D.A.; McGuire, T.; Langer, R. Small-Scale Systems for in Vivo Drug Delivery. Nat. Biotechnol. 2003, 21, 1184–1191. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable Polymeric Nanoparticles Based Drug Delivery Systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef]
- Folle, C.; Marqués, A.M.; Díaz-Garrido, N.; Espina, M.; Sánchez-López, E.; Badia, J.; Baldoma, L.; Calpena, A.C.; García, M.L. Thymol-Loaded PLGA Nanoparticles: An Efficient Approach for Acne Treatment. J. Nanobiotechnol. 2021, 19, 359. [Google Scholar] [CrossRef]
- Esteruelas, G.; Halbaut, L.; García-Torra, V.; Espina, M.; Cano, A.; Ettcheto, M.; Camins, A.; Souto, E.B.; García, M.L.; Sánchez-López, E. Development and Optimization of Riluzole-Loaded Biodegradable Nanoparticles Incorporated in a Mucoadhesive in Situ Gel for the Posterior Eye Segment. Int. J. Pharm. 2021, 612, 121379. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Ettcheto, M.; Egea, M.A.; Espina, M.; Cano, A.; Calpena, A.C.; Camins, A.; Carmona, N.; Silva, A.M.; Souto, E.B.; et al. Memantine Loaded PLGA PEGylated Nanoparticles for Alzheimer’s Disease: In Vitro and in Vivo Characterization. J. Nanobiotechnol. 2018, 16, 32. [Google Scholar] [CrossRef]
- Anderson, J.M.; Shive, M.S. Biodegradation and Biocompatibility of PLA and PLGA Microspheres. Adv. Drug Deliv. Rev. 2012, 64, 72–82. [Google Scholar] [CrossRef]
- Zhao, J.; Weng, G.; Li, J.; Zhu, J.; Zhao, J. Polyester-Based Nanoparticles for Nucleic Acid Delivery. Mater. Sci. Eng. C 2018, 92, 983–994. [Google Scholar] [CrossRef]
- Hans, M.; Lowman, A. A Dual-Responsive Mesoporous Silica Nanoparticle for Tumor-Triggered Targeting Drug Deliverys for Drug Delivery and Targeting. Curr. Opin. Solid State Mater. Sci. 2002, 6, 319–327. [Google Scholar] [CrossRef]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in Cancer Therapy: Challenges, Opportunities, and Clinical Applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef]
- Rezvantalab, S.; Drude, N.I.; Moraveji, M.K.; Güvener, N.; Koons, E.K.; Shi, Y.; Lammers, T.; Kiessling, F. PLGA-Based Nanoparticles in Cancer Treatment. Front. Pharmacol. 2018, 9, 1260. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-López, E.; Egea, M.A.; Davis, B.M.; Guo, L.; Espina, M.; Silva, A.M.; Calpena, A.C.; Souto, E.M.B.; Ravindran, N.; Ettcheto, M.; et al. Memantine-Loaded PEGylated Biodegradable Nanoparticles for the Treatment of Glaucoma. Small 2018, 14, 1701808. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Egea, M.A.; Cano, A.; Espina, M.; Calpena, A.C.; Ettcheto, M.; Camins, A.; Souto, E.B.; Silva, A.M.; García, M.L. PEGylated PLGA Nanospheres Optimized by Design of Experiments for Ocular Administration of Dexibuprofen– in Vitro, Ex Vivo and in Vivo Characterization. Colloids Surf. B Biointerfaces 2016, 145, 241–250. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-López, E.; Esteruelas, G.; Ortiz, A.; Espina, M.; Prat, J.; Muñoz, M.; Cano, A.; Calpena, A.C.; Ettcheto, M.; Camins, A.; et al. Dexibuprofen Biodegradable Nanoparticles: One Step Closer towards a Better Ocular Interaction Study. Nanomaterials 2020, 10, 720. [Google Scholar] [CrossRef] [Green Version]
- Cano, A.; Ettcheto, M.; Chang, J.H.; Barroso, E.; Espina, M.; Kühne, B.A.; Barenys, M.; Auladell, C.; Folch, J.; Souto, E.B.; et al. Dual-Drug Loaded Nanoparticles of Epigallocatechin-3-Gallate (EGCG)/Ascorbic Acid Enhance Therapeutic Efficacy of EGCG in a APPswe/PS1dE9 Alzheimer’s Disease Mice Model. J. Control. Release 2019, 301, 62–75. [Google Scholar] [CrossRef]
- Wong, K.H.; Lu, A.; Chen, X.; Yang, Z. Natural Ingredient-Based Polymeric Nanoparticles for Cancer Treatment. Molecules 2020, 25, 3620. [Google Scholar] [CrossRef]
- Misra, S.; Heldin, P.; Hascall, V.C.; Karamanos, N.K.; Skandalis, S.S.; Markwald, R.R.; Ghatak, S. Hyaluronan-CD44 Interactions as Potential Targets for Cancer Therapy. FEBS J. 2011, 278, 1429–1443. [Google Scholar] [CrossRef] [Green Version]
- Sekar, V.; Rajendran, K.; Vallinayagam, S.; Deepak, V.; Mahadevan, S. Synthesis and Characterization of Chitosan Ascorbate Nanoparticles for Therapeutic Inhibition for Cervical Cancer and Their in Silico Modeling. J. Ind. Eng. Chem. 2018, 62, 239–249. [Google Scholar] [CrossRef]
- Motevalli, S.M.; Eltahan, A.S.; Liu, L.; Magrini, A.; Rosato, N.; Guo, W.; Bottini, M.; Liang, X.-J. Co-Encapsulation of Curcumin and Doxorubicin in Albumin Nanoparticles Blocks the Adaptive Treatment Tolerance of Cancer Cells. Biophys. Rep. 2019, 5, 19–30. [Google Scholar] [CrossRef] [Green Version]
- Koo, H.; Min, K.H.; Lee, S.C.; Park, J.H.; Park, K.; Jeong, S.Y.; Choi, K.; Kwon, I.C.; Kim, K. Enhanced Drug-Loading and Therapeutic Efficacy of Hydrotropic Oligomer-Conjugated Glycol Chitosan Nanoparticles for Tumor-Targeted Paclitaxel Delivery. J. Control. Release 2013, 172, 823–831. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, Y.S.; Park, K.; Lee, S.; Nam, H.Y.; Min, K.H.; Jo, H.G.; Park, J.H.; Choi, K.; Jeong, S.Y.; et al. Antitumor Efficacy of Cisplatin-Loaded Glycol Chitosan Nanoparticles in Tumor-Bearing Mice. J. Control. Release 2008, 127, 41–49. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parra-Nieto, J.; del Cid, M.A.G.; de Cárcer, I.A.; Baeza, A. Inorganic Porous Nanoparticles for Drug Delivery in Antitumoral Therapy. Proteomics 2020, 16, 2000150. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Ge, J.; Miao, Q.; Zhu, R.; Wen, L.; Zeng, J.; Gao, M. Biodegradable Inorganic Nanoparticles for Cancer Theranostics: Insights into the Degradation Behavior. Bioconjug. Chem. 2020, 31, 315–331. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Beack, S.; Yoo, J.; Kim, S.K.; Lee, C.; Kwon, W.; Hahn, S.K.; Kim, C. In Vivo Photoacoustic Imaging of Livers Using Biodegradable Hyaluronic Acid-Conjugated Silica Nanoparticles. Adv. Funct. Mater. 2018, 28, 1800941. [Google Scholar] [CrossRef]
- Park, Y.; Yoo, J.; Kang, M.H.; Kwon, W.; Joo, J. Photoluminescent and Biodegradable Porous Silicon Nanoparticles for Biomedical Imaging. J. Mater. Chem. B 2019, 7, 6271–6292. [Google Scholar] [CrossRef]
- Zhang, S.; Chu, Z.; Yin, C.; Zhang, C.; Lin, G.; Li, Q. Controllable Drug Release and Simultaneously Carrier Decomposition of SiO2-Drug Composite Nanoparticles. J. Am. Chem. Soc. 2013, 135, 5709–5716. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, S.; Ma, J.; Fan, L.; Yin, C.; Lin, G.; Li, Q. Double Loaded Self-Decomposable SiO2 Nanoparticles for Sustained Drug Release. Nanoscale 2015, 7, 16389–16398. [Google Scholar] [CrossRef] [Green Version]
- Girirajan, S.; Campbell, C.; Eichler, E. Radiolabeled Inorganic Nanoparticles for Positron Emission Tomography Imaging of Cancer: An Overview. Physiol. Behav. 2018, 176, 139–148. [Google Scholar] [CrossRef]
- Phillips, E.; Penate-Medina, O.; Zanzonico, P.B.; Carvajal, R.D.; Mohan, P.; Ye, Y.; Humm, J.; Gönen, M.; Kalaigian, H.; Schöder, H.; et al. Clinical Translation of an Ultrasmall Inorganic Optical-PET Imaging Nanoparticle Probe. Physiol. Behav. 2015, 176, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Dizaj, S.M.; Barzegar-Jalali, M.; Hossein Zarrintan, M.; Adibkia, K.; Lotfipour, F. Calcium Carbonate Nanoparticles; Potential in Bone and Tooth Disorders. Pharm. Sci. 2015, 20, 175–182. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [Green Version]
- Aguado, B.A.; Grim, J.C.; Rosales, A.M.; Watson-Capps, J.J.; Anseth, K.S. Engineering Precision Biomaterials for Personalized Medicine. Sci. Transl. Med. 2018, 10, eaam8645. [Google Scholar] [CrossRef]
- Yang, W.; Liang, H.; Ma, S.; Wang, D.; Huang, J. Gold Nanoparticle Based Photothermal Therapy: Development and Application for Effective Cancer Treatment. Sustain. Mater. Technol. 2019, 22, e00109. [Google Scholar] [CrossRef]
- Rastinehad, A.R.; Anastos, H.; Wajswol, E.; Winoker, J.S.; Sfakianos, J.P.; Doppalapudi, S.K.; Carrick, M.R.; Knauer, C.J.; Taouli, B.; Lewis, S.C.; et al. Gold Nanoshell-Localized Photothermal Ablation of Prostate Tumors in a Clinical Pilot Device Study. Proc. Natl. Acad. Sci. USA 2019, 116, 18590–18596. [Google Scholar] [CrossRef] [Green Version]
- Briley-Saebo, K.C.; Mani, V.; Hyafil, F.; Cornily, J.C.; Fayad, Z.A. Fractionated Feridex and Positive Contrast: In Vivo MR Imaging of Atherosclerosis. Magn. Reson. Med. 2008, 59, 721–730. [Google Scholar] [CrossRef]
- Aulic, S.; Marson, D.; Laurini, E.; Fermeglia, M.; Pricl, S. Breast Cancer Nanomedicine Market Update and Other Industrial Perspectives of Nanomedicine. In Nanomedicines for Breast Cancer Theranostics; Elsevier: Amsterdam, The Netherlands, 2020; pp. 371–404. [Google Scholar]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic Photothermal Therapy (PPTT) Using Gold Nanoparticles. Lasers Med. Sci. 2008, 23, 217–228. [Google Scholar] [CrossRef]
- Aderibigbe, B.A. Metal-Based Nanoparticles for the Treatment of Infectious Diseases. Molecules 2017, 22, 1370. [Google Scholar] [CrossRef]
- Kirmanidou, Y.; Sidira, M.; Drosou, M.E.; Bennani, V.; Bakopoulou, A.; Tsouknidas, A.; Michailidis, N.; Michalakis, K. New Ti-Alloys and Surface Modifications to Improve the Mechanical Properties and the Biological Response to Orthopedic and Dental Implants: A Review. Biomed Res. Int. 2016, 2016, 2908570. [Google Scholar] [CrossRef] [Green Version]
- Vi, T.T.T.; Kumar, S.R.; Huang, Y.T.; Chen, D.W.; Liu, Y.K.; Lue, S.J. Size-Dependent Antibacterial Activity of Silver Nanoparticle-Loaded Graphene Oxide Nanosheets. Nanomaterials 2020, 10, 1270. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.Y.; Huang, J.; Chen, C.Y.; Wang, Z.X.; Xie, H. Silver Nanoparticles: Synthesis, Medical Applications and Biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef]
- Salomoni, R.; Léo, P.; Rodrigues, M.F.A. Antibacterial Activity of Silver Nanoparticles (AgNPs) in Staphylococcus Aureus and Cytotoxicity Effect in Mammalian Cells. Battle Against Microb. Pathog. Basic Sci. Technol. Adv. Educ. Programs 2015, 17, 851–857. [Google Scholar]
- Yilma, A.N.; Singh, S.R.; Dixit, S.; Dennis, V.A. Anti-Inflammatory Effects of Silver-Polyvinyl Pyrrolidone (Ag-PVP) Nanoparticles in Mouse Macrophages Infected with Live Chlamydia Trachomatis. Int. J. Nanomed. 2013, 8, 2421. [Google Scholar] [CrossRef] [Green Version]
- Thirumurugan, G.; Seshagiri Rao, J.V.L.N.; Dhanaraju, M.D. Elucidating Pharmacodynamic Interaction of Silver Nanoparticle—Topical Deliverable Antibiotics. Sci. Rep. 2016, 6, 29982. [Google Scholar] [CrossRef] [Green Version]
- Bayda, S.; Hadla, M.; Palazzolo, S.; Riello, P.; Corona, G.; Toffoli, G.; Rizzolio, F. Inorganic Nanoparticles for Cancer Therapy: A Transition from Lab to Clinic. Curr. Med. Chem. 2017, 25, 4269–4303. [Google Scholar] [CrossRef]
- Guo, D.; Zhu, L.; Huang, Z.; Zhou, H.; Ge, Y.; Ma, W.; Wu, J.; Zhang, X.; Zhou, X.; Zhang, Y.; et al. Anti-Leukemia Activity of PVP-Coated Silver Nanoparticles via Generation of Reactive Oxygen Species and Release of Silver Ions. Biomaterials 2013, 34, 7884–7894. [Google Scholar] [CrossRef]
- Huang, C.Y.; Huang, T.H.; Kao, C.T.; Wu, Y.H.; Chen, W.C.; Shie, M.Y. Mesoporous Calcium Silicate Nanoparticles with Drug Delivery and Odontogenesis Properties. J. Endod. 2017, 43, 69–76. [Google Scholar] [CrossRef]
- Gheibi Hayat, S.M.; Bianconi, V.; Pirro, M.; Sahebkar, A. Stealth Functionalization of Biomaterials and Nanoparticles by CD47 Mimicry. Int. J. Pharm. 2019, 569, 118628. [Google Scholar] [CrossRef]
- Veerapandian, M.; Yun, K. Functionalization of Biomolecules on Nanoparticles: Specialized for Antibacterial Applications. Appl. Microbiol. Biotechnol. 2011, 90, 1655–1667. [Google Scholar] [CrossRef]
- Folle, C.; Díaz-Garrido, N.; Sánchez-López, E.; Marqués, A.M.; Badia, J.; Baldomà, L.; Espina, M.; Calpena, A.C.; García, M.L. Surface-Modified Multifunctional Thymol-Loaded Biodegradable Nanoparticles for Topical Acne Treatment. Pharmaceutics 2021, 13, 1501. [Google Scholar] [CrossRef]
- Araujo, J.; Vega, E.; Lopes, C.; Egea, M.A.; Garcia, M.L.; Souto, E.B. Effect of polymer viscosity on physicochemical properties and ocular tolerance of FB-loaded PLGA nanospheres. Colloids Surf B Biointerfaces 2009, 72, 48–56. [Google Scholar] [CrossRef]
- Buzyurova, D.N.; Pashirova, T.N.; Zueva, I.V.; Burilova, E.A.; Shaihutdinova, Z.M.; Rizvanov, I.K.; Babaev, V.M.; Petrov, K.A.; Souto, E.B. Surface modification of pralidoxime chloride-loaded solid lipid nanoparticles for enhanced brain reactivation of organophosphorus-inhibited AChE: Pharmacokinetics in rat. Toxicology 2020, 444, 152578. [Google Scholar] [CrossRef] [PubMed]
- Marega, R.; Karmani, L.; Flamant, L.; Nageswaran, P.G.; Valembois, V.; Masereel, B.; Feron, O.; Borght, T.V.; Lucas, S.; Michiels, C.; et al. Antibody-Functionalized Polymer-Coated Gold Nanoparticles Targeting Cancer Cells: An in Vitro and in Vivo Study. J. Mater. Chem. 2012, 22, 21305–21312. [Google Scholar] [CrossRef]
- Gonzalez-Pizarro, R.; Parrotta, G.; Vera, R.; Sánchez-López, E.; Galindo, R.; Kjeldsen, F.; Badia, J.; Baldoma, L.; Espina, M.; García, M.L. Ocular Penetration of Fluorometholone-Loaded PEG-PLGA Nanoparticles Functionalized with Cell-Penetrating Peptides. Nanomedicine 2019, 14, 3089–3104. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, A.; Vega, E.; Pérez, Y.; Gómara, M.J.; García, M.L.; Haro, I. Conjugation of Cell-Penetrating Peptides with Poly(Lactic-Co-Glycolic Acid)-Polyethylene Glycol Nanoparticles Improves Ocular Drug Delivery. Int. J. Nanomed. 2015, 10, 609–631. [Google Scholar] [CrossRef] [Green Version]
- Galindo, R.; Elena, S.; Espina, M.; Ettcheto, M.; Cano, A.; Haro, I.; Camins, A. Development of Peptide Targeted PLGA-PEGylated Nanoparticles Loading Licochalcone-A for Ocular Inflammation. Pharmaceutics 2022, 14, 285. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Gao, X.; Kang, T.; Jiang, M.; Miao, D.; Gu, G.; Hu, Q. B6 Peptide-Modified PEG-PLA Nanoparticles for Enhanced Brain Delivery of Neuroprotective Peptide. Bioconjug. Chem. 2013, 24, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Ouyang, T.; Peng, T.; Zhang, X.; Xie, B.; Yang, X.; Liang, D.; Zhong, H. Enhanced Oral Absorption of Insulin Using Colon-Specific Nanoparticles Co-Modified with Amphiphilic Chitosan Derivatives and Cell-Penetrating Peptides. Biomater. Sci. 2019, 7, 1493–1506. [Google Scholar] [CrossRef]
- Gonzalez-Pizarro, R.; Carvajal-Vidal, P.; Halbault Bellowa, L.; Calpena, A.C.; Espina, M.; García, M.L. In-Situ Forming Gels Containing Fluorometholone-Loaded Polymeric Nanoparticles for Ocular Inflammatory Conditions. Colloids Surf. B Biointerfaces 2019, 175, 365–374. [Google Scholar] [CrossRef]
- Gilbert, G.H.; Tilashalski, K.R.; Litaker, M.S.; McNeal, S.F.; Boykin, M.J.; Kessler, A.W. Outcomes of Root Canal Treatment in Dental PBRN Practices. Tex. Dent. J. 2013, 130, 351–359. [Google Scholar]
- Kuang, X.; Chen, V.; Xu, X. Novel Approaches to the Control of Oral Microbial Biofilms. Biomed Res. Int. 2018, 2018, 6498932. [Google Scholar] [CrossRef] [Green Version]
- Mahamuni-Badiger, P.P.; Patil, P.M.; Badiger, M.V.; Patel, P.R.; Thorat-Gadgil, B.S.; Pandit, A.; Bohara, R.A. Biofilm Formation to Inhibition: Role of Zinc Oxide-Based Nanoparticles. Mater. Sci. Eng. C 2020, 108, 110319. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Y.; Wu, H.; Høiby, N.; Molin, S.; Song, Z.J. Current Understanding of Multispecies Biofilms. Int. J. Oral Sci. 2011, 3, 74–81. [Google Scholar] [CrossRef]
- Benoit, D.S.W.; Koo, H. Targeted, Triggered Drug Delivery to Tumor and Biofilm Microenvironments. Nanomedicine 2016, 11, 873–879. [Google Scholar] [CrossRef]
- Mao, M.; Zhang, W.; Huang, Z.; Huang, J.; Wang, J.; Li, W.; Gu, S. Graphene Oxide-Copper Nanocomposites Suppress Cariogenic Streptococcus Mutans Biofilm Formation. Int. J. Nanomed. 2021, 16, 7727–7739. [Google Scholar] [CrossRef]
- Parnia, F.; Yazdani, J.; Javaherzadeh, V.; Dizaj, S.M. Overview of Nanoparticle Coating of Dental Implants for Enhanced Osseointegration and Antimicrobial Purposes. J. Pharm. Pharm. Sci. 2017, 20, 148–160. [Google Scholar] [CrossRef]
- Hamouda, I.M. Current Perspectives of Nanoparticles in Medical and Dental Biomaterials. J. Biomed. Res. 2012, 26, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, S.; Sato, M.; Sato, Y.; Ando, N.; Takayama, T.; Fujita, M.; Ishihara, M. Synthesis and Application of Silver Nanoparticles (Ag Nps) for the Prevention of Infection in Healthcare Workers. Int. J. Mol. Sci. 2019, 20, 3620. [Google Scholar] [CrossRef] [Green Version]
- Parolia, A.; Kumar, H.; Ramamurthy, S.; Davamani, F.; Pau, A. Effectiveness of Chitosan-Propolis Nanoparticle against Enterococcus Faecalis Biofilms in the Root Canal. BMC Oral Health 2020, 20, 339. [Google Scholar] [CrossRef]
- Araujo, H.C.; da Silva, A.C.G.; Paião, L.I.; Magario, M.K.W.; Frasnelli, S.C.T.; Oliveira, S.H.P.; Pessan, J.P.; Monteiro, D.R. Antimicrobial, Antibiofilm and Cytotoxic Effects of a Colloidal Nanocarrier Composed by Chitosan-Coated Iron Oxide Nanoparticles Loaded with Chlorhexidine. J. Dent. 2020, 101, 103453. [Google Scholar] [CrossRef]
- Rabea, E.I.; Badawy, M.E.T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as Antimicrobial Agent: Applications and Mode of Action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef]
- Upadya, M.; Shrestha, A.; Kishen, A. Role of Efflux Pump Inhibitors on the Antibiofilm Efficacy of Calcium Hydroxide, Chitosan Nanoparticles, and Light-Activated Disinfection. J. Endod. 2011, 37, 1422–1426. [Google Scholar] [CrossRef] [PubMed]
- Kishen, A.; Shi, Z.; Shrestha, A.; Neoh, K.G. An Investigation on the Antibacterial and Antibiofilm Efficacy of Cationic Nanoparticulates for Root Canal Disinfection. J. Endod. 2008, 34, 1515–1520. [Google Scholar] [CrossRef] [PubMed]
- del Carpio-Perochena, A.; Bramante, C.M.; Duarte, M.A.H.; de Moura, M.R.; Aouada, F.A.; Kishen, A. Chelating and Antibacterial Properties of Chitosan Nanoparticles on Dentin. Restor. Dent. Endod. 2015, 40, 195. [Google Scholar] [CrossRef] [PubMed]
- Barreras, U.S.; Méndez, F.T.; Martínez, R.E.M.; Valencia, C.S.; Rodríguez, P.R.M.; Rodríguez, J.P.L. Chitosan Nanoparticles Enhance the Antibacterial Activity of Chlorhexidine in Collagen Membranes Used for Periapical Guided Tissue Regeneration. Mater. Sci. Eng. C 2016, 58, 1182–1187. [Google Scholar] [CrossRef]
- Li, F.C.; Nicholson, E.; Singh, C.V.; Kishen, A. Microtissue Engineering Root Dentin with Photodynamically Cross-Linked Nanoparticles Improves Fatigue Resistance of Endodontically Treated Teeth. J. Endod. 2020, 46, 668–674. [Google Scholar] [CrossRef]
- Xing, Y.; Xu, Q.; Li, X.; Chen, C.; Ma, L.; Li, S.; Che, Z.; Lin, H. Chitosan-Based Coating with Antimicrobial Agents: Preparation, Property, Mechanism, and Application Effectiveness on Fruits and Vegetables. Int. J. Polym. Sci. 2016, 2016, 4851730. [Google Scholar] [CrossRef] [Green Version]
- Manavitehrani, I.; Fathi, A.; Badr, H.; Daly, S.; Shirazi, A.N.; Dehghani, F. Biomedical Applications of Biodegradable Polyesters. Polymers 2016, 8, 20. [Google Scholar] [CrossRef] [Green Version]
- Coolen, A.L.; Lacroix, C.; Mercier-Gouy, P.; Delaune, E.; Monge, C.; Exposito, J.Y.; Verrier, B. Poly(Lactic Acid) Nanoparticles and Cell-Penetrating Peptide Potentiate MRNA-Based Vaccine Expression in Dendritic Cells Triggering Their Activation. Biomaterials 2019, 195, 23–37. [Google Scholar] [CrossRef]
- Maghsoudi, S.; Shahraki, B.T.; Rabiee, N.; Fatahi, Y.; Dinarvand, R.; Tavakolizadeh, M.; Ahmadi, S.; Rabiee, M.; Bagherzadeh, M.; Pourjavadi, A.; et al. Burgeoning Polymer Nano Blends for Improved Controlled Drug Release: A Review. Int. J. Nanomed. 2020, 15, 4363–4392. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, Y.; Haapasalo, M. Effectiveness of Endodontic Disinfecting Solutions against Young and Old Enterococcus Faecalis Biofilms in Dentin Canals. J. Endod. 2012, 38, 1376–1379. [Google Scholar] [CrossRef]
- Weatherly, L.M.; Gosse, J.A. Triclosan Exposure, Transformation, and Human Health Effects. J. Toxicol. Environ. Health Part B Crit. Rev. 2017, 20, 447–469. [Google Scholar] [CrossRef] [PubMed]
- Piñón-Segundo, E.; Ganem-Quintanar, A.; Alonso-Pérez, V.; Quintanar-Guerrero, D. Preparation and Characterization of Triclosan Nanoparticles for Periodontal Treatment. Int. J. Pharm. 2005, 294, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, B.M.; Mitali, K.; Lu, T.B.; Handral, H.K.; Dubey, N.; Fawzy, A.S. PLGA Nanoparticles as Chlorhexidine-Delivery Carrier to Resin-Dentin Adhesive Interface. Dent. Mater. 2017, 33, 830–846. [Google Scholar] [CrossRef] [PubMed]
- Kishen, A. Advanced Therapeutic Options for Endodontic Biofilms Biofilm as a Therapeutic Target in Root Canal Treatment. Endod. Top. 2012, 22, 99–123. [Google Scholar] [CrossRef]
- Pagonis, T.C.; Chen, J.; Fontana, C.R.; Devalapally, H.; Ruggiero, K.; Song, X.; Foschi, F.; Dunham, J.; Skobe, Z.; Yamazaki, H.; et al. Nanoparticle-Based Endodontic Antimicrobial Photodynamic Therapy. J. Endod. 2010, 36, 322–328. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, A.; Kishen, A. Antibiofilm Efficacy of Photosensitizer-Functionalized Bioactive Nanoparticles on Multispecies Biofilm. J. Endod. 2014, 40, 1604–1610. [Google Scholar] [CrossRef]
- Bernicker, E.; Gaur, P.; Desai, S.; Teh, B.S.; Blackmon, S.H. Chapter 7. Therapy. In Diffuse Malignant Mesothelioma; Springer: New York, NY, USA, 2015; Volume 36, pp. 125–139. ISBN 9781493923748. [Google Scholar]

| Nanoparticle Material | Main Properties | In Vitro Studies | Ex Vivo Studies | References |
|---|---|---|---|---|
| Silica | Most commonly used inorganic materials. Great biocompatibility. Their main applications are diagnostic imaging and drug delivery. | Diagnostic imaging: In Vivo Photoacoustic Imaging of Livers Using Biodegradable Hyaluronic Acid- Conjugated Silica Nanoparticles. | Diagnostic imaging: Photoluminescent and biodegradable porous silicon nanoparticles for biomedical imaging. | [75,76] |
| Drug delivery: Controllable drug release and simultaneously carrier decomposition of SiO2-drug composite nanoparticles. | [57] | |||
| Double loaded self-decomposable SiO2 nanoparticles for sustained drug release. | [58] | |||
| Silica (Cornell dots) | Fluorescent silica nanoparticles for human clinical trials approved by FDA. These can be modified with radioisotopes or optical imaging agents. Moreover, these NPs showed a significantly improved target-background ratio and higher sensitivity for cancer diagnostics. | Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe. | - | [80] |
| Calcium carbonate | Successfully used for gene and drug delivery. | - | Calcium carbonate nanoparticles; Potential in bone and tooth disorders. | [81] |
| Active Compound | Nanoparticle Material | Main Properties | In Vitro Studies | Ex Vivo Studies | References |
|---|---|---|---|---|---|
| - | Gold | The most widely studied in various forms due to their photothermal properties and its capacity to be easily functionalized. Some of them are commercialized (AuroLase®, treatment of head and neck tumours). | Gold nanoshell- localized photothermal ablation of prostate tumours in a clinical pilot device study. | - | [85] |
| - | Iron | Possess superparamagnetic properties at certain sizes, good biocompatibility and great properties for being a contrast agent (Feridex®) or against cancer treatment (NanoTherm®). | Cancer treatment: Plasmonic photothermal therapy (PPTT) using gold nanoparticles. | Contrast agent: Fractionated Feridex and positive contrast: In vivo MR imaging of atherosclerosis. | [86,88] |
| - | Silver | AgNPs stand out especially for their, chemical stability, higher electrical and thermal conductivity of metals, catalytic and antibacterial activity. In the biomedical field they are gaining strength in molecular diagnostics, and as carriers of chemotherapeutics. | Antibacterial properties: Anti-inflammatory effects of silver-polyvinyl pyrrolidone (Ag-PVP) nanoparticles in mouse macrophages infected with live Chlamydia trachomatis. Antibacterial activity of silver nanoparticles (AgNPs) in Staphylococcus aureus and cytotoxicity effect in mammalian cells. substance. | Cancer treatment: Anti-leukaemia activity of PVP-coated silver nanoparticles via generation of reactive oxygen species and release of silver ions | [71,93,97] |
| Antibiotics | Silver | Ag NPs have also been used in combination with antibiotics such as cefazolin (CEF), mupirocin (MUP) or gentamicin (GEN) with good results against Staphylococcus aureus, Pseudomonas aeruginosa and Escherichia coli. | Elucidating pharmacodynamic interaction of silver nanoparticle—Topical deliverable antibiotics. | [95] |
| Active Compound | Nanoparticle Material | Main Properties | In Vitro Studies | Ex Vivo Studies | References |
|---|---|---|---|---|---|
| - | Chitosan | Electrostatic attraction with bacterial cell membranes. Versatile compound in forms and functions. Excellent antibacterial, antiviral and antifungal properties. High biodegradability, non-toxicity. Proven antibiofilm efficacy. Hight root canal penetration. | - | Adherence of E. faecalis to dentin in sectioned single-rooted teeth showing bacterial death and decreased adherence. | [124] |
| Multispecies biofilm infected dentin sections proved the antibiofilm activity and CLSM determined a high penetration. | [138] | ||||
| Bovine dentin sections were infected intra-orally, the treatment result in an inhibition of bacterial recolonization on root dentin. | [125] | ||||
| Chlorhexidine | Chitosan | Antibacterial spectrum that includes most of the microorganisms of the oral cavity. | Collagen membrane with E. faecalis infection, results significant inhibition of bacterial growing. | - | [126] |
| Cross-linked chitosan | Improved resistance to fatigue loads in endodontically treated teeth. | Root canal dentin sections were subjected to nanoindentations before/after treatment, showing a decrease of stress root. | [127] | ||
| Ca(OH)2 | PLGA | Bioabsorbable by simple filtration or metabolism. Prolonged release. Hight root penetration. | - | Single-rooted human teeth were treated with PLGA NPs and observed with confocal microscope, demonstrating higher NPs penetration. | [45] |
| Single-rooted teeth infected with E. faecalis and treated, the result was a decrease in bacterial species and their by-products. | [132] | ||||
| Triclosan | PLGA and PLA | Hight root penetration. Hight encapsulation efficiency. Large surface area. | - | Beagle dogs with induced periodontitis were treated showing a decrease in gingival inflammation. | [134] |
| Chlorhexidine | PLGA | Potent antibacterial efficacy. Slow degradation and gradual chlorhexidine release profile. Increased NPs penetration. | Extracted teeth were connected to experimental setup simulating pulpal hydrostatic pressure, the result was a potent antibacterial efficacy, and gradual degradation pattern. | [135] | |
| Methylene blue | PLGA | Potent antibacterial effects. Novel antimicrobial endodontic treatment. | - | E. faecalis infected root canals were treated and irradiated with red light at 665 nm obtaining a CFU levels significantly lower. | [139] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roig-Soriano, X.; Souto, E.B.; Elmsmari, F.; Garcia, M.L.; Espina, M.; Duran-Sindreu, F.; Sánchez-López, E.; González Sánchez, J.A. Nanoparticles in Endodontics Disinfection: State of the Art. Pharmaceutics 2022, 14, 1519. https://doi.org/10.3390/pharmaceutics14071519
Roig-Soriano X, Souto EB, Elmsmari F, Garcia ML, Espina M, Duran-Sindreu F, Sánchez-López E, González Sánchez JA. Nanoparticles in Endodontics Disinfection: State of the Art. Pharmaceutics. 2022; 14(7):1519. https://doi.org/10.3390/pharmaceutics14071519
Chicago/Turabian StyleRoig-Soriano, Xavier, Eliana B. Souto, Firas Elmsmari, Maria Luisa Garcia, Marta Espina, Fernando Duran-Sindreu, Elena Sánchez-López, and Jose Antonio González Sánchez. 2022. "Nanoparticles in Endodontics Disinfection: State of the Art" Pharmaceutics 14, no. 7: 1519. https://doi.org/10.3390/pharmaceutics14071519