Sustainable Release of Propranolol Hydrochloride Laden with Biconjugated-Ufasomes Chitosan Hydrogel Attenuates Cisplatin-Induced Sciatic Nerve Damage in In Vitro/In Vivo Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of PRO–UFAs
2.3. Characterization and Optimization of PRO–UFAs
2.3.1. Determination of PRO Entrapment Efficiency Percent (EE%)
2.3.2. Particle Size (PS), Zeta Potential (ZP), and Polydispersity Index (PDI) Determination
2.3.3. In Vitro Release Study of PRO–UFAs
2.3.4. Studying the Impact of Formulation Variables Using Full Factorial Design
2.4. Optimization of PRO–UFAs
2.5. Preparation of PRO-CTS-UFAs
2.6. Transmission Electron Microscopy (TEM)
2.7. Physical Stability Study
2.8. Ex Vivo Permeability Study
2.8.1. Skin Preparation
2.8.2. Ex Vivo Permeation Study
2.9. In Vivo Pharmacological Study
2.9.1. Animals
2.9.2. Experimental Design
2.9.3. Methods
Tissue Sampling
2.9.4. ELISA of Tissue Biomarkers
2.9.5. Quantitative Peripheral Myelin 22 Real-Time PCR Tissue Biomarkers Assessment
2.9.6. Histopathological Study
2.9.7. Immunohistochemical Assay
2.9.8. Statistical Analysis
3. Results
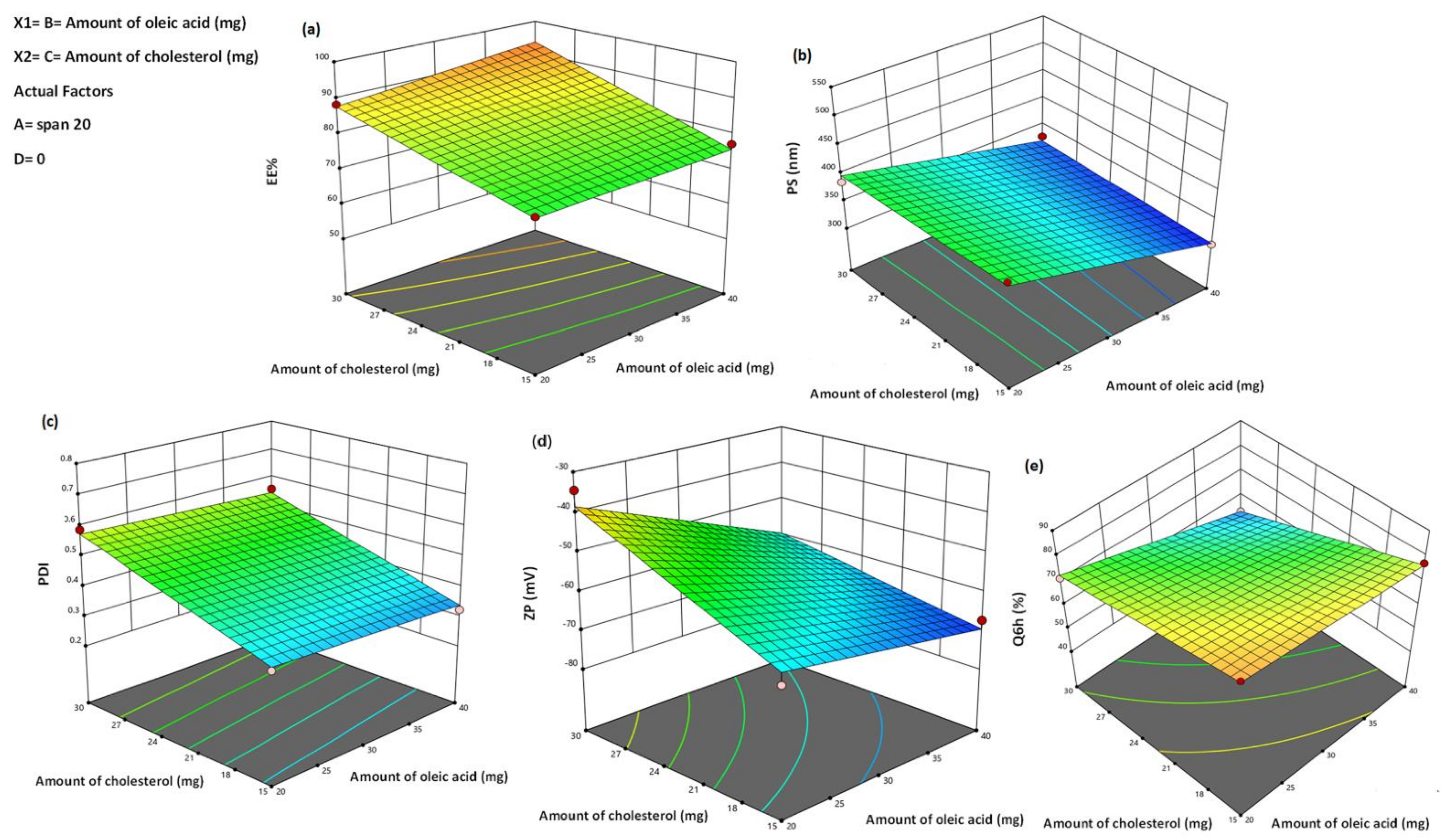
3.1. Analysis of Factorial Design
3.2. PRO–UFAs Characterization
3.2.1. Effect of Formulation Variables on EE%
3.2.2. Effect of Formulation Variables on PS
3.2.3. Effect of Formulation Variables on PDI
3.2.4. Effect Formulation Variables on Zeta Potential (ZP)
3.2.5. Effect of Formulation Variables in In Vitro Drug Release Studies
3.3. Selection of the Optimized Formulation
3.4. Formulation and Characterization of PRO–CTS–UFAs
3.5. Transmission Electron Microscopy (TEM)
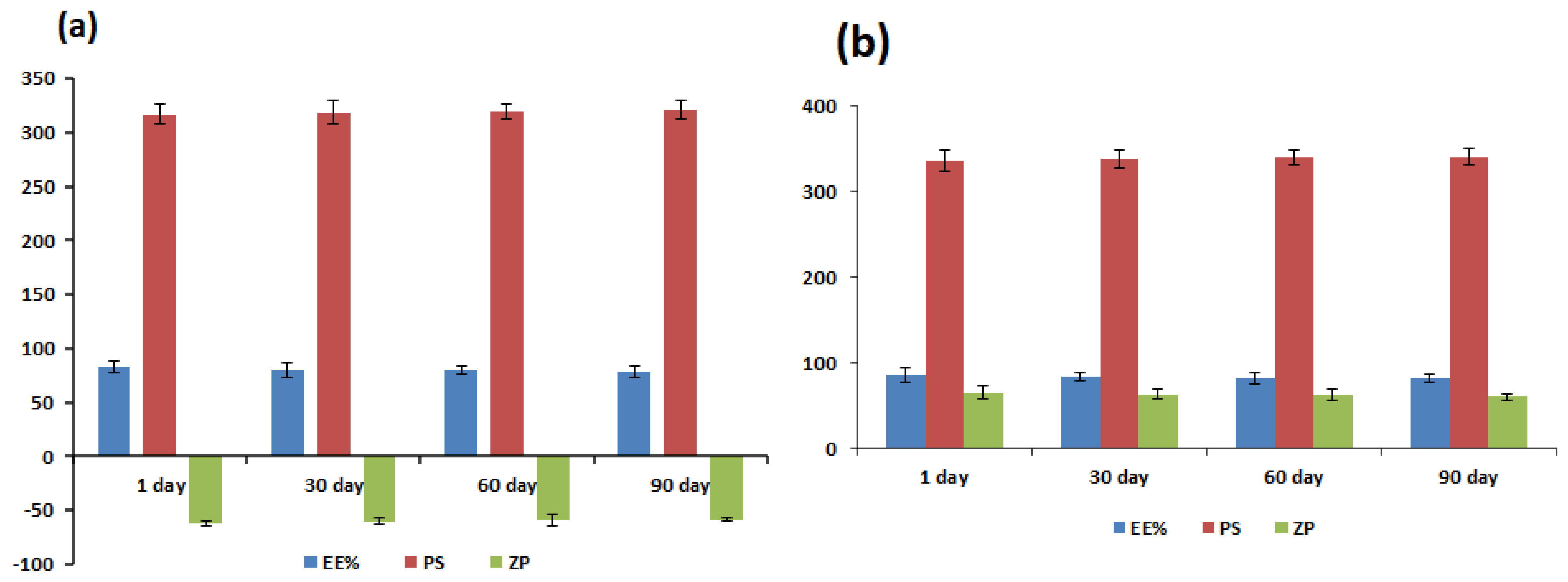
3.6. Physical Stability Study
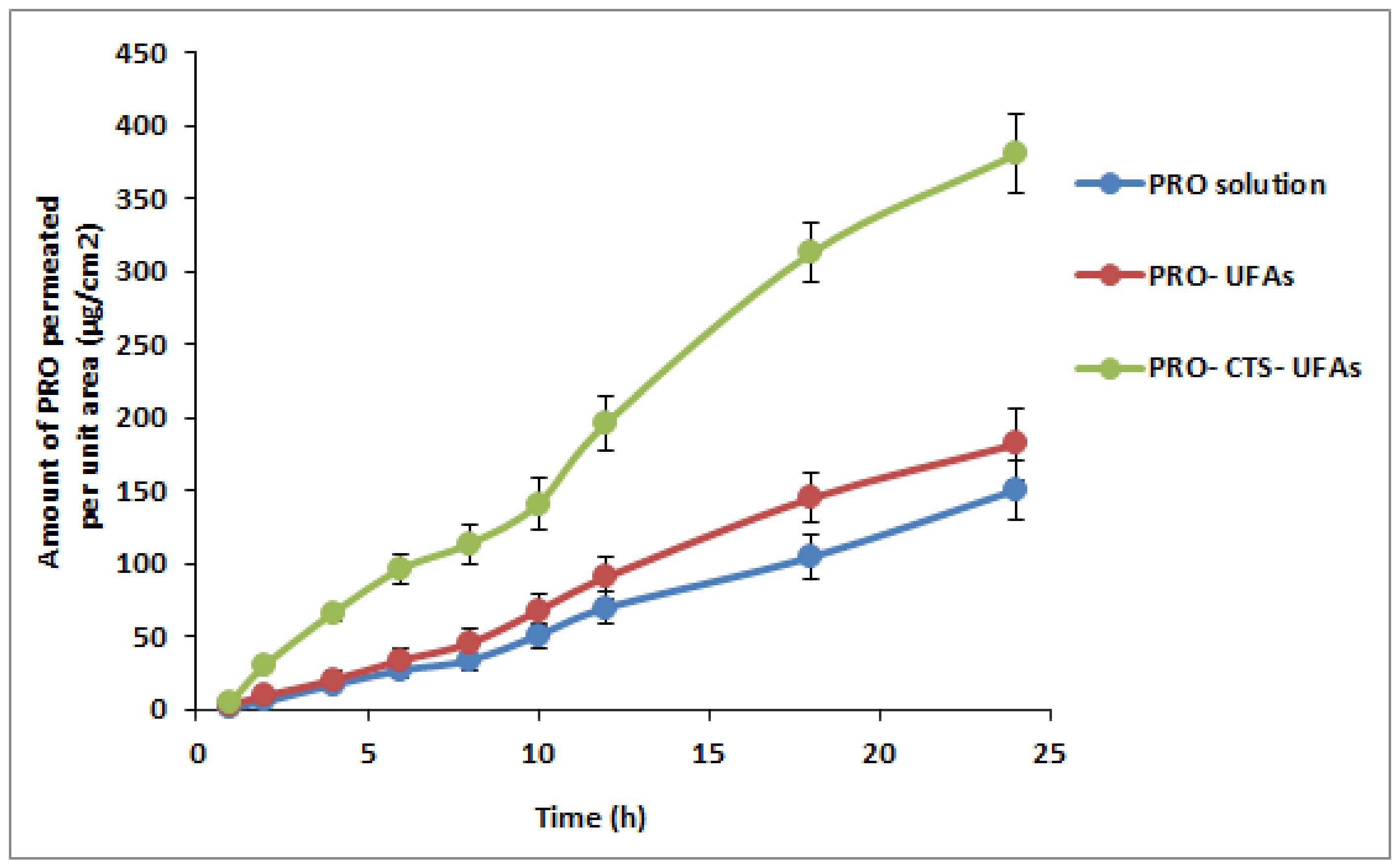
3.7. Ex Vivo Skin Permeation Study
3.8. In Vivo Pharmacological Study
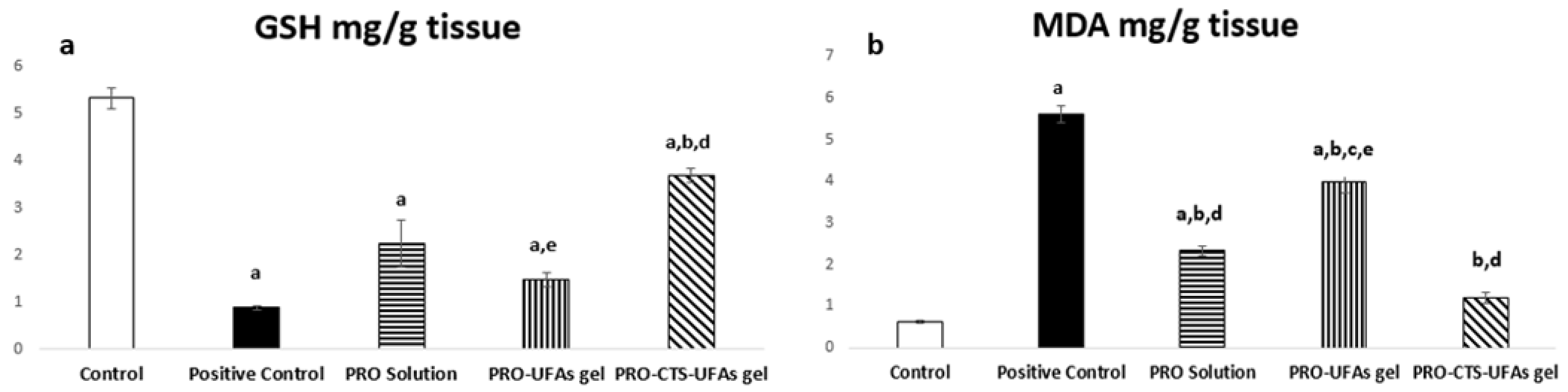
3.8.1. Biochemical Measurement
Catalase Activity
Oxidative Markers
Gene Expression of Peripheral Myelin 22 by the Real-Time PCR
3.8.2. Histopathology
H&E Staining
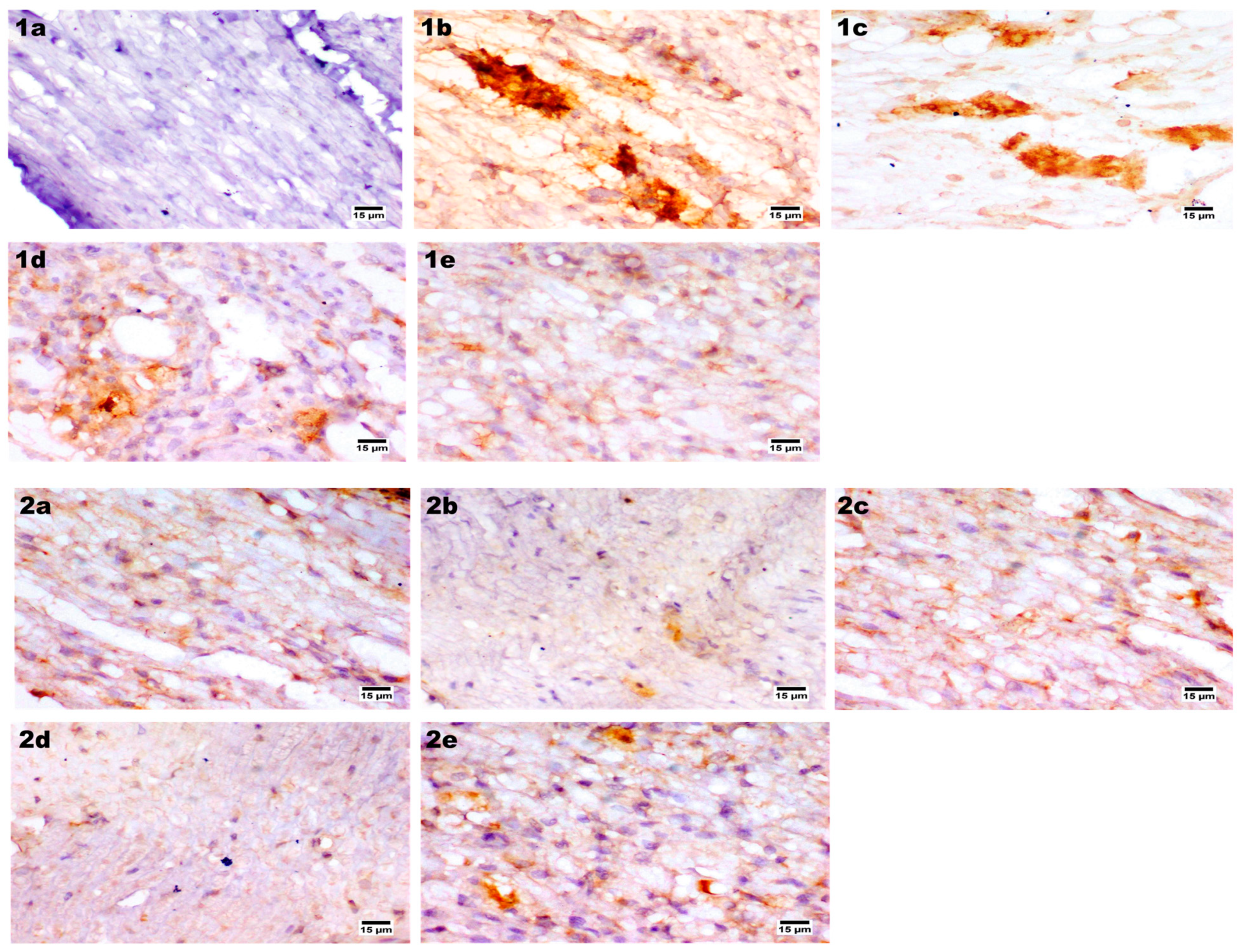
Immunohistochemical Staining
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gershon, M.D.; Nakamura, H. Functional anatomy of the enteric nervous system. In Hirschsprung’s Disease and Allied Disorders; Springer: Cham, Switzerland, 2019; pp. 31–76. [Google Scholar]
- Chen, S.; Ikemoto, T.; Tokunaga, T.; Okikawa, S.; Miyazaki, K.; Yamada, S.; Saito, Y.; Morine, Y.; Shimada, M. Newly Generated 3D Schwann-Like Cell Spheroids from Human Adipose-Derived Stem Cells Using a Modified Protocol. Cell Transplant. 2022, 31, 9636897221093312. [Google Scholar] [CrossRef]
- Samadian, H.; Maleki, H.; Fathollahi, A.; Salehi, M.; Gholizadeh, S.; Derakhshankhah, H.; Allahyari, Z.; Jaymand, M. Naturally occurring biological macromolecules-based hydrogels: Potential biomaterials for peripheral nerve regeneration. Int. J. Biol. Macromol. 2020, 154, 795–817. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, M.; Brown, H.; Quick, T. Clinical Outcome Measures Following Peripheral Nerve Repair: The Future for Assessment of the Processes and Experiences of Nerve Injury. In Peripheral Nerve Tissue Engineering and Regeneration; Springer: Berlin/Heidelberg, Germany, 2022; pp. 491–536. [Google Scholar]
- Joshua, A.M.; Misri, Z. Peripheral Nerve Disorders. In Physiotherapy for Adult Neurological Conditions; Springer: Berlin/Heidelberg, Germany, 2022; pp. 621–729. [Google Scholar]
- Zajączkowska, R.; Kocot-Kępska, M.; Leppert, W.; Wrzosek, A.; Mika, J.; Wordliczek, J. Mechanisms of chemotherapy-induced peripheral neuropathy. Int. J. Mol. Sci. 2019, 20, 1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acklin, S.; Xia, F. The Role of Nucleotide Excision Repair in Cisplatin-Induced Peripheral Neuropathy: Mechanism, Prevention, and Treatment. Int. J. Mol. Sci. 2021, 22, 1975. [Google Scholar] [CrossRef] [PubMed]
- Jindatip, D.; Nopparat, W.; Kobutree, P.; Roumwong, A.; Agthong, S. Pericyte Loss and Detachment in Experimental Cisplatin-Induced Neuropathy. Int. J. Morphol. 2019, 37, 509–514. [Google Scholar] [CrossRef] [Green Version]
- Calls, A.; Torres-Espin, A.; Navarro, X.; Yuste, V.J.; Udina, E.; Bruna, J. Cisplatin-induced peripheral neuropathy is associated with neuronal senescence-like response. Neuro-Oncology 2021, 23, 88–99. [Google Scholar] [CrossRef]
- Szklener, K.; Szklener, S.; Michalski, A.; Żak, K.; Kuryło, W.; Rejdak, K.; Mańdziuk, S. Dietary Supplements in Chemotherapy-Induced Peripheral Neuropathy: A New Hope? Nutrients 2022, 14, 625. [Google Scholar] [CrossRef]
- Dos Santos, N.A.G.; Ferreira, R.S.; Dos Santos, A.C. Overview of cisplatin-induced neurotoxicity and ototoxicity, and the protective agents. Food Chem. Toxicol. 2020, 136, 111079. [Google Scholar] [CrossRef]
- Van den Boogaard, W.M.; Komninos, D.S.; Vermeij, W.P. Chemotherapy Side-Effects: Not All DNA Damage Is Equal. Cancers 2022, 14, 627. [Google Scholar] [CrossRef]
- Berta, T.; Qadri, Y.; Tan, P.H.; Ji, R.R. Targeting dorsal root ganglia and primary sensory neurons for the treatment of chronic pain. Expert Opin. Ther. Targets 2017, 21, 695–703. [Google Scholar] [CrossRef]
- Zhuo, M.; Gorgun, M.F.; Englander, E.W. Neurotoxicity of cytarabine (Ara-C) in dorsal root ganglion neurons originates from impediment of mtDNA synthesis and compromise of mitochondrial function. Free. Radic. Biol. Med. 2018, 121, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Abdelsameea, A.A.; Kabil, S.L. Mitigation of cisplatin-induced peripheral neuropathy by canagliflozin in rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2018, 391, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Avan, A.; Postma, T.J.; Ceresa, C.; Avan, A.; Cavaletti, G.; Giovannetti, E.; Peters, G.J. Platinum-induced neurotoxicity and preventive strategies: Past, present, and future. Oncologist 2015, 20, 411–432. [Google Scholar] [CrossRef] [Green Version]
- Unchiti, K.; Leurcharusmee, P.; Samerchua, A.; Pipanmekaporn, T.; Chattipakorn, N.; Chattipakorn, S.C. The potential role of dexmedetomidine on neuroprotection and its possible mechanisms: Evidence from in vitro and in vivo studies. Eur. J. Neurosci. 2021, 54, 7006–7047. [Google Scholar] [CrossRef] [PubMed]
- Bilir-Yildiz, B.; Sunay, F.B.; Yilmaz, H.F.; Bozkurt-Girit, O. Low-intensity low-frequency pulsed ultrasound ameliorates sciatic nerve dysfunction in a rat model of cisplatin-induced peripheral neuropathy. Sci. Rep. 2022, 12, 8125. [Google Scholar] [CrossRef]
- Carozzi, V.A.; Canta, A.; Chiorazzi, A. Chemotherapy-induced peripheral neuropathy: What do we know about mechanisms? Neurosci. Lett. 2015, 596, 90–107. [Google Scholar] [CrossRef]
- Khadrawy, Y.A.; El-Gizawy, M.M.; Sorour, S.M.; Sawie, H.G.; Hosny, E.N. Effect of curcumin nanoparticles on the cisplatin-induced neurotoxicity in rat. Drug Chem. Toxicol. 2019, 42, 194–202. [Google Scholar] [CrossRef]
- Zaki, S.M.; Mohamed, E.A.; Motawie, A.G.; Fattah, S.A. N-acetylcysteine versus progesterone on the cisplatin-induced peripheral neurotoxicity. Folia Morphol. 2018, 77, 234–245. [Google Scholar] [CrossRef]
- Fan, H.C.; Lee, H.S.; Chang, K.P.; Lee, Y.Y.; Lai, H.C.; Hung, P.L.; Lee, H.F.; Chi, C.S. The impact of anti-epileptic drugs on growth and bone metabolism. Int. J. Mol. Sci. 2016, 17, 1242. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.; Yang, Y.; Sun, P.; Tian, Y.; Gao, F.; Wang, C.; Zong, T.; Li, M.; Zhang, Y.; Yu, T.; et al. βII spectrin (SPTBN1): Biological function and clinical potential in cancer and other diseases. Int. J. Biol. Sci. 2021, 17, 32–49. [Google Scholar] [CrossRef]
- Bosco, F.; Guarnieri, L.; Nucera, S.; Scicchitano, M.; Ruga, S.; Cardamone, A.; Maurotti, S.; Russo, C.; Coppoletta, A.R.; Macrì, R.; et al. Pathophysiology Aspects of Muscle Atrophy and Osteopenia Induced by Chronic Constriction Injury (CCI) of the Sciatic Nerve in Rat. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Stoy, L. Combinational Effects of Body Weight Supported Treadmill Training and Bioengineering Scaffold Releasing Neurotrophins on Forelimb and Hindlimb Bone Biomechanics After Spinal Cord Injury. Master’s Thesis, Widener University, Chester, PA, USA, 2022. [Google Scholar]
- Samadian, H.; Ehterami, A.; Sarrafzadeh, A.; Khastar, H.; Nikbakht, M.; Rezaei, A.; Chegini, L.; Salehi, M. Sophisticated polycaprolactone/gelatin nanofibrous nerve guided conduit containing platelet-rich plasma and citicoline for peripheral nerve regeneration: In vitro and in vivo study. Int. J. Biol. Macromol. 2020, 150, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Muheremu, A.; Ao, Q. Past, present, and future of nerve conduits in the treatment of peripheral nerve injury. BioMed Res. Int. 2015, 2015, 237507. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.S.; Jeng, S.F. Full-thickness skin graft as a one-stage debulking procedure after free flap reconstruction for the lower leg. Plast. Reconstr. Surg. 2006, 118, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Ding, F.; Williams, D.F. Neural tissue engineering options for peripheral nerve regeneration. Biomaterials 2014, 35, 6143–6156. [Google Scholar] [CrossRef]
- Ai, J.; Kiasat-Dolatabadi, A.; Ebrahimi-Barough, S.; Ai, A.; Lotfibakhshaiesh, N.; Norouzi-Javidan, A.; Saberi, H.; Arjmand, B.; Aghayan, H.R. Polymeric scaffolds in neural tissue engineering: A review. Arch. Neurosci. 2014, 1, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Nisbet, D.R.; Crompton, K.E.; Horne, M.K.; Finkelstein, D.I.; Forsythe, J.S. Neural tissue engineering of the CNS using hydrogels. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 87, 251–263. [Google Scholar] [CrossRef]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef]
- Singh, S.K.; Dhyani, A.; Juyal, D. Hydrogel: Preparation, characterization and applications. Pharma Innov. 2017, 6, 25–32. [Google Scholar]
- Yan, J.; Yao, Y.; Yan, S.; Gao, R.; Lu, W.; He, W. Chiral protein supraparticles for tumor suppression and synergistic immunotherapy: An enabling strategy for bioactive supramolecular chirality construction. Nano Lett. 2020, 20, 5844–5852. [Google Scholar] [CrossRef]
- Lai, W.F.; Gui, D.; Wong, M.; Döring, A.; Rogach, A.L.; He, T.; Wong, W.T. A self-indicating cellulose-based gel with tunable performance for bioactive agent delivery. J. Drug Deliv. Sci. Technol. 2021, 63, 102428. [Google Scholar] [CrossRef]
- George, J.; Hsu, C.C.; Nguyen, L.T.B.; Ye, H.; Cui, Z. Neural tissue engineering with structured hydrogels in CNS models and therapies. Biotechnol. Adv. 2020, 42, 107370. [Google Scholar] [CrossRef] [PubMed]
- Madhusudanan, P.; Raju, G.; Shankarappa, S. Hydrogel systems and their role in neural tissue engineering. J. R. Soc. Interface 2020, 17, 20190505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; Lim, S.H.; Mao, H.Q.; Chew, S.Y. Current applications and future perspectives of artificial nerve conduits. Exp. Neurol. 2010, 223, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Angius, D.; Wang, H.; Spinner, R.J. A systematic review of animal models used to study nerve regeneration in tissue-engineered scaffolds. Biomaterials 2012, 33, 8034–8039. [Google Scholar] [CrossRef] [Green Version]
- Sato, T.; Ishii, T.; Okahata, Y. In vitro gene delivery mediated by chitosan. Effect of pH, serum, and molecular mass of chitosan on the transfection efficiency. Biomaterials 2001, 22, 2075–2080. [Google Scholar] [CrossRef]
- Jiang, X.; Dai, H.; Leong, K.W.; Goh, S.H.; Mao, H.Q.; Yang, Y.Y. Chitosan-g-PEG/DNA complexes deliver gene to the rat liver via intrabiliary and intraportal infusions. J. Gene Med. 2006, 8, 477–487. [Google Scholar] [CrossRef]
- Chen, M.H.; Hsu, Y.H.; Lin, C.P.; Chen, Y.J.; Young, T.H. Interactions of acinar cells on biomaterials with various surface properties. J. Biomed. Mater. Res. Part A 2005, 74, 254–262. [Google Scholar] [CrossRef]
- Lin, S.J.; Jee, S.H.; Hsaio, W.C.; Lee, S.J.; Young, T.H. Formation of melanocyte spheroids on the chitosan-coated surface. Biomaterials 2005, 26, 1413–1422. [Google Scholar] [CrossRef]
- Wang, Y.C.; Lin, M.C.; Wang, D.M.; Hsieh, H.J. Fabrication of a novel porous PGA-chitosan hybrid matrix for tissue engineering. Biomaterials 2003, 24, 1047–1057. [Google Scholar] [CrossRef]
- Wang, G.; Wang, X.; Huang, L. Feasibility of chitosan-alginate (Chi-Alg) hydrogel used as scaffold for neural tissue engineering: A pilot study in vitro. Biotechnol. Biotechnol. Equip. 2017, 31, 766–773. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Zhou, X.; He, B.; Dai, T.; Zheng, C.; Yang, C.; Zhu, S.; Zhu, J.; Zhu, Q.; Liu, X. Ginkgo biloba extract (EGb 761) promotes peripheral nerve regeneration and neovascularization after acellular nerve allografts in a rat model. Cell. Mol. Neurobiol. 2015, 35, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Stüssel, P.; Dieckhoff, K.S.; Künzel, S.; Hartmann, V.; Gupta, Y.; Kaiser, G.; Veldkamp, W.; Vidarsson, G.; Visser, R.; Ghorbanalipoor, S.; et al. Propranolol is an effective topical and systemic treatment option for experimental epidermolysis bullosa acquisita. J. Investig. Dermatol. 2020, 140, 2408–2420. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Y.M.; Messiha, B.A.S.; Abo-Saif, A.A. Granisetron and carvedilol can protect experimental rats againstadjuvant-induced arthritis. Immunopharmacol. Immunotoxicol. 2017, 39, 97–104. [Google Scholar] [CrossRef]
- Fujiu, K.; Manabe, I. Nerve–macrophage interactions in cardiovascular disease. Int. Immunol. 2022, 34, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Mallick, R.; Duttaroy, A.K. Modulation of endothelium function by fatty acids. Mol. Cell. Biochem. 2022, 477, 15–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Z.; Zhao, Z.L.; Liu, C.; Zheng, J.W. Exosome-derived miR-196b-5p facilitates intercellular interaction in infantile hemangioma via down-regulating CDKN1B. Ann. Transl. Med. 2021, 9, 394. [Google Scholar] [CrossRef]
- Sun, B.; Dong, C.; Lei, H.; Gong, Y.; Li, M.; Zhang, Y.; Zhang, H.; Sun, L. Propranolol inhibits proliferation and induces apoptosis of hemangioma-derived endothelial cells via Akt pathway by down-regulating Ang-2 expression. Chem. -Biol. Interact. 2020, 316, 108925. [Google Scholar] [CrossRef]
- Li, H. Angiogenesis in the progression from liver fibrosis to cirrhosis and hepatocelluar carcinoma. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 217–233. [Google Scholar] [CrossRef]
- Wang, X.; Xu, J.; Kang, Q. Neuromodulation of bone: Role of different peptides and their interactions. Mol. Med. Rep. 2021, 23, 32. [Google Scholar] [CrossRef]
- Pilipović, I.; Stojić-Vukanić, Z.; Prijić, I.; Jasnić, N.; Leposavić, G. Propranolol diminished severity of rat EAE by enhancing immunoregulatory/protective properties of spinal cord microglia. Neurobiol. Dis. 2020, 134, 104665. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Kanehara, M.; Zhang, Y.; Wang, X.; Ishida, T. β-blocker and other analogous treatments that affect bone mass and sympathetic nerve activity in ovariectomized rats. Am. J. Chin. Med. 2007, 35, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Treyball, A.; Bergeron, A.C.; Brooks, D.J.; Langlais, A.L.; Hashmi, H.; Nagano, K.; Barlow, D.; Neilson, R.J.; Roy, T.A.; Nevola, K.T.; et al. Propranolol promotes bone formation and limits resorption through novel mechanisms during anabolic parathyroid hormone treatment in female C57BL/6J mice. J. Bone Miner. Res. 2022, 37, 954–971. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Hamada, N.; Kim, Y.; Takahashi, Y.; Sasaguri, K.; Ozono, S.; Sato, S. Blockade of sympathetic β-receptors inhibits Porphyromonas gingivalis-induced alveolar bone loss in an experimental rat periodontitis model. Arch. Oral Biol. 2010, 55, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Guyot, M.; Fawaz, F. Design and in vitro evaluation of adhesive matrix for transdermal delivery of propranolol. Int. J. Pharm. 2000, 204, 171–182. [Google Scholar] [CrossRef]
- Padula, C.; Nicoli, S.; Pescina, S.; Santi, P. Thin polymeric films for the topical delivery of propranolol. Colloids Surf. B Biointerfaces 2019, 174, 582–586. [Google Scholar] [CrossRef]
- Calatayud-Pascual, M.A.; Sebastian-Morelló, M.; Balaguer-Fernández, C.; Delgado-Charro, M.B.; López-Castellano, A.; Merino, V. Influence of chemical enhancers and iontophoresis on the in vitro transdermal permeation of propranolol: Evaluation by dermatopharmacokinetics. Pharmaceutics 2018, 10, 265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, L.; Utreja, P. Formulation and characterization of transethosomes for enhanced transdermal delivery of propranolol hydrochloride. Micro Nanosyst. 2020, 12, 38–47. [Google Scholar] [CrossRef]
- Khalil, R.M.; El Arini, S.K.; AbouSamra, M.M.; Zaki, H.S.; El-Gazaerly, O.N.; Elbary, A.A. Development of lecithin/chitosan nanoparticles for promoting topical delivery of propranolol hydrochloride: Design, optimization and in-vivo evaluation. J. Pharm. Sci. 2021, 110, 1337–1348. [Google Scholar] [CrossRef]
- Gebicki, J.M.; Hicks, M. Ufasomes are stable particles surrounded by unsaturated fatty acid membranes. Nature 1973, 243, 232–234. [Google Scholar] [CrossRef]
- Morigaki, K.; Walde, P. Fatty acid vesicles. Curr. Opin. Colloid Interface Sci. 2007, 12, 75–80. [Google Scholar] [CrossRef]
- Fan, Y.; Fang, Y.; Ma, L. The self-crosslinked ufasome of conjugated linoleic acid: Investigation of morphology, bilayer membrane and stability. Colloids Surf. B Biointerfaces 2014, 123, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Zakir, F.; Vaidya, B.; Goyal, A.K.; Malik, B.; Vyas, S.P. Development and characterization of oleic acid vesicles for the topical delivery of fluconazole. Drug Deliv. 2010, 17, 238–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolla, P.K.; Meraz, C.A.; Rodriguez, V.A.; Deaguero, I.; Singh, M.; Yellepeddi, V.K.; Renukuntla, J. Clotrimazole loaded ufosomes for topical delivery: Formulation development and in-vitro studies. Molecules 2019, 24, 3139. [Google Scholar] [CrossRef] [Green Version]
- Al-Mahallawi, A.M.; Khowessah, O.M.; Shoukri, R.A. Enhanced non invasive trans-tympanic delivery of ciprofloxacin through encapsulation into nano-spanlastic vesicles: Fabrication, in-vitro characterization, and comparative ex-vivo permeation studies. Int. J. Pharm. 2017, 522, 157–164. [Google Scholar] [CrossRef]
- Mahmoud, M.O.; Aboud, H.M.; Hassan, A.H.; Ali, A.A.; Johnston, T.P. Transdermal delivery of atorvastatin calcium from novel nanovesicular systems using polyethylene glycol fatty acid esters: Ameliorated effect without liver toxicity in poloxamer 407-induced hyperlipidemic rats. J. Control. Release 2017, 254, 10–22. [Google Scholar] [CrossRef]
- Kamboj, S.; Saini, V.; Bala, S. Formulation and characterization of drug loaded nonionic surfactant vesicles (niosomes) for oral bioavailability enhancement. Sci. World J. 2014, 959741. [Google Scholar] [CrossRef] [Green Version]
- Aboud, H.M.; Hassan, A.H.; Ali, A.A.; Abdel-Razik, A.R.H. Novel in situ gelling vaginal sponges of sildenafil citrate-based cubosomes for uterine targeting. Drug Deliv. 2018, 25, 1328–1339. [Google Scholar] [CrossRef] [Green Version]
- Aboud, H.M.; Hussein, A.K.; Zayan, A.Z.; Makram, T.S.; Sarhan, M.O.; El-Sharawy, D.M. Tailoring of Selenium-Plated Novasomes for Fine-Tuning Pharmacokinetic and Tumor Uptake of Quercetin: In Vitro Optimization and In Vivo Radiobiodistribution Assessment in Ehrlich Tumor-Bearing Mice. Pharmaceutics 2022, 14, 875. [Google Scholar] [CrossRef]
- Barani, M.; Hajinezhad, M.R.; Sargazi, S.; Rahdar, A.; Shahraki, S.; Lohrasbi-Nejad, A.; Baino, F. In vitro and in vivo anticancer effect of pH-responsive paclitaxel-loaded niosomes. J. Mater. Sci. Mater. Med. 2021, 32, 147. [Google Scholar] [CrossRef]
- Pandya, V.M.; Patel, J.K.; Patel, D.J. Formulation and optimization of nanosuspensions for enhancing simvastatin dissolution using central composite design. Dissolution Technol. 2011, 18, 40–45. [Google Scholar] [CrossRef]
- Gonzalez-Rodriguez, M.L.; Barros, L.B.; Palma, J.; González-Rodríguez, P.L.; Rabasco, A.M. Application of statistical experimental design to study the formulation variables influencing the coating process of lidocaine liposomes. Int. J. Pharm. 2007, 337, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Tayel, S.A.; El-Nabarawi, M.A.; Tadros, M.I.; Abd-Elsalam, W.H. Promising ion-sensitive in situ ocular nanoemulsion gels of terbinafine hydrochloride: Design, in vitro characterization and in vivo estimation of the ocular irritation and drug pharmacokinetics in the aqueous humor of rabbits. Int. J. Pharm. 2013, 443, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Al-Mahallawi, A.M.; Abdelbary, A.A.; Aburahma, M.H. Investigating the potential of employing bilosomes as a novel vesicular carrier for transdermal delivery of tenoxicam. Int. J. Pharm. 2015, 485, 329–340. [Google Scholar] [CrossRef]
- Boztaş, N.; Özbilgin, Ş.; Özbilgin, M.; Taylan, E.; Ünlü, M.; Özkardeşler, S.; Akan, M.; Yurtlu, S.; Hancı, V. Effects of Midazolam, Propofol and Thiopental on Gastric Ulcer in Rats Midazolam. Haydarpaşa Numune Med. J. 2021, 61, 24–30. [Google Scholar] [CrossRef]
- Lequin, R.M. Enzyme immunoassay (EIA)/enzyme-linked immunosorbent assay (ELISA). Clin. Chem. 2005, 51, 2415–2418. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Merz, H.; Malisius, R.; Mannweiler, S.; Zhou, R.; Hartmann, W.; Orscheschek, K.; Moubayed, P.; Feller, A.C. ImmunoMax. A maximized immunohistochemical method for the retrieval and enhancement of hidden antigens. Lab. Investig. J. Tech. Methods Pathol. 1995, 73, 149–156. [Google Scholar]
- El-Shoura, E.A.; Sharkawi, S.M.; Messiha, B.A.; Bakr, A.G.; Hemeida, R.A. Perindopril mitigates LPS-induced cardiopulmonary oxidative and inflammatory damage via inhibition of renin angiotensin system, inflammation and oxidative stress. Immunopharmacol. Immunotoxicol. 2019, 41, 630–643. [Google Scholar] [CrossRef]
- Araujo, J.; Gonzalez-Mira, E.; Egea, M.A.; Garcia, M.L.; Souto, E.B. Optimization and physicochemical characterization of a triamcinolone acetonide-loaded NLC for ocular antiangiogenic applications. Int. J. Pharm. 2010, 393, 168–176. [Google Scholar] [CrossRef]
- De Lima, L.S.; Araujo, M.D.M.; Quináia, S.P.; Migliorine, D.W.; Garcia, J.R. Adsorption modeling of Cr, Cd and Cu on activated carbon of different origins by using fractional factorial design. Chem. Eng. J. 2011, 166, 881–889. [Google Scholar] [CrossRef]
- Khallaf, R.A.; Aboud, H.M.; Sayed, O.M. Surface modified niosomes of olanzapine for brain targeting via nasal route; preparation, optimization, and in vivo evaluation. J. Liposome Res. 2020, 30, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, T.; Sternberg, B.; Florence, A.T. Florence, Preparation and properties of vesicles (niosomes) of sorbitan monoesters (Span 20, 40, 60 and 80) and a sorbitan triester (Span 85). Int. J. Pharm. 1994, 105, 1–6. [Google Scholar] [CrossRef]
- El-Ridy, M.S.; Abdelbary, A.; Essam, T.; Abd EL-Salam, R.M.; Aly Kassem, A.A. Niosomes as a potential drug delivery system for increasing the efficacy and safety of nystatin. Drug Dev. Ind. Pharm. 2011, 37, 1491–1508. [Google Scholar] [CrossRef] [PubMed]
- Azeem, A.; Anwer, M.K.; Talegaonkar, S. Niosomes in sustained and targeted drug delivery: Some recent advances. J. Drug Target. 2009, 17, 671–689. [Google Scholar] [CrossRef] [PubMed]
- Abdelbary, G.; El-Gendy, N. Niosome-encapsulated gentamicin for ophthalmic controlled delivery. AAPS PharmSciTech 2008, 9, 740–747. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Han, S.; Qu, Z.; Zhao, J.; Chen, Y.; Chen, Z.; Duan, J.; Pan, Y.; Tang, X. Penetration enhancement of lidocaine hydrochlorid by a novel chitosan coated elastic liposome for transdermal drug delivery. J. Biomed. Nanotechnol. 2011, 7, 704–713. [Google Scholar] [CrossRef]
- Manconi, M.; Sinico, C.; Valenti, D.; Loy, G.; Fadda, A.M. Niosomes as carriers for tretinoin. I. Preparation and properties. Int. J. Pharm. 2002, 234, 237–248. [Google Scholar] [CrossRef]
- Bayindir, Z.S.; Yuksel, N. Characterization of niosomes prepared with various nonionic surfactants for paclitaxel oral delivery. J. Pharm. Sci. 2010, 99, 2049–2060. [Google Scholar] [CrossRef]
- Ribeiro, M.D.M.; Arellano, D.B.; Grosso, C.R.F. The effect of adding oleic acid in the production of stearic acid lipid microparticles with a hydrophilic core by a spray-cooling process. Food Res. Int. 2012, 47, 38–44. [Google Scholar] [CrossRef]
- Gabr, M.M.; Mortada, S.M.; Sallam, M.A. Hexagonal liquid crystalline nanodispersions proven superiority for enhanced oral delivery of rosuvastatin: In vitro characterization and in vivo pharmacokinetic study. J. Pharm. Sci. 2017, 106, 3103–3112. [Google Scholar] [CrossRef]
- El-Nabarawi, M.; Nafady, M.; Elmenshawe, S.; Elkarmalawy, M.; Teaima, M. Liver Targeting of Daclatasvir via Tailoring Sterically Stabilized Bilosomes: Fabrication, Comparative In Vitro/In Vivo Appraisal and Biodistribution Studies. Int. J. Nanomed. 2021, 16, 6413–6426. [Google Scholar] [CrossRef] [PubMed]
- Khalil, R.M.; Abdelbary, A.; Kocova El-Arini, S.; Basha, M.; El-Hashemy, H.A. Evaluation of bilosomes as nanocarriers for transdermal delivery of tizanidine hydrochloride: In vitro and ex vivo optimization. J. Liposome Res. 2019, 29, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Matloub, A.A.; Salama, A.H.; Aglan, H.A.; AbouSamra, M.M.; ElSouda, S.S.M.; Ahmed, H.H. Exploiting bilosomes for delivering bioactive polysaccharide isolated from Enteromorpha intestinalis for hacking hepatocellular carcinoma. Drug Dev. Ind. Pharm. 2018, 44, 523–534. [Google Scholar] [CrossRef] [PubMed]
- El Menshawe, S.F.; Nafady, M.M.; Aboud, H.M.; Kharshoum, R.M.; Elkelawy, A.M.M.H.; Hamad, D.S. Transdermal delivery of fluvastatin sodium via tailored spanlastic nanovesicles: Mitigated Freund’s adjuvant-induced rheumatoid arthritis in rats through suppressing p38 MAPK signaling pathway. Drug Deliv. 2019, 26, 1140–1154. [Google Scholar] [CrossRef] [Green Version]
- Verma, D.D.; Verma, S.; Blume, G.; Fahr, A. Particle size of liposomes influences dermal delivery of substances into skin. Int. J. Pharm. 2003, 258, 141–151. [Google Scholar] [CrossRef]
- Das, S.; Ng, W.K.; Tan, R.B. Tan. Are nanostructured lipid carriers (NLCs) better than solid lipid nanoparticles (SLNs): Development, characterizations and comparative evaluations of clotrimazole-loaded SLNs and NLCs? Eur. J. Pharm. Sci. 2012, 47, 139–151. [Google Scholar] [CrossRef]
- Hathout, R.M.; Mansour, S.; Mortada, N.D.; Guinedi, A.S. Liposomes as an ocular delivery system for acetazolamide: In vitro and in vivo studies. AAPS PharmSciTech 2007, 8, E1–E12. [Google Scholar] [CrossRef]
- Pardakhty, A.; Varshosaz, J.; Rouholamini, A. In vitro study of polyoxyethylene alkyl ether niosomes for delivery of insulin. Int. J. Pharm. 2007, 328, 130–141. [Google Scholar] [CrossRef]
- Balakrishnan, P.; Shanmugam, S.; Lee, W.S.; Lee, W.M.; Kim, J.O.; Oh, D.H.; Kim, D.D.; Kim, J.S.; Yoo, B.K.; Choi, H.G.; et al. Formulation and in vitro assessment of minoxidil niosomes for enhanced skin delivery. Int. J. Pharm. 2009, 377, 1–8. [Google Scholar] [CrossRef]
- Ramana, L.N.; Sethuraman, S.; Ranga, U.; Krishnan, U.M. Development of a liposomal nanodelivery system for nevirapine. J. Biomed. Sci. 2010, 17, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinilla, C.M.B.; Reque, P.M.; Brandelli, A. Effect of oleic acid, cholesterol, and octadecylamine on membrane stability of freeze-dried liposomes encapsulating natural antimicrobials. Food Bioprocess Technol. 2020, 13, 599–610. [Google Scholar] [CrossRef]
- Kelidari, H.R.; Saeedi, M.; Akbari, J.; Morteza-Semnani, K.; Valizadeh, H.; Maniruzzaman, M.; Farmoudeh, A.; Nokhodchi, A. Development and optimisation of spironolactone nanoparticles for enhanced dissolution rates and stability. AAPS PharmSciTech 2017, 18, 1469–1474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsherif, N.I.; Shamma, R.N.; Abdelbary, G. Terbinafine hydrochloride trans-ungual delivery via nanovesicular systems: In vitro characterization and ex vivo evaluation. AAPS PharmSciTech 2017, 18, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.; Dwivedi, P.; Gupta, P.K.; Mishra, P.R. Optimization of novel tocopheryl acetate nanoemulsions for parenteral delivery of curcumin for therapeutic intervention of sepsis. Expert Opin. Drug Deliv. 2014, 11, 1697–1712. [Google Scholar] [CrossRef]
- El-Say, K.M.; Abd-Allah, F.I.; Lila, A.E.; Hassan, A.E.S.A.; Kassem, A.E.A. Diacerein niosomal gel for topical delivery: Development, in vitro and in vivo assessment. J. Liposome Res. 2016, 26, 57–68. [Google Scholar] [CrossRef]
- Zeisig, R.; Shimada, K.; Hirota, S.; Arndt, D. Effect of sterical stabilization on macrophage uptake in vitro and on thickness of the fixed aqueous layer of liposomes made from alkylphosphocholines. Biochim. Biophys. Acta Biomembr. 1996, 1285, 237–245. [Google Scholar] [CrossRef] [Green Version]
- Aithal, B.K.; Kumar, M.S.; Rao, B.N.; Upadhya, R.; Prabhu, V.; Shavi, G.; Arumugam, K.; Sajankila, S.P.; Udupa, N.; Satyamoorthy, K.; et al. Evaluation of pharmacokinetic, biodistribution, pharmacodynamic, and toxicity profile of free juglone and its sterically stabilized liposomes. J. Pharm. Sci. 2011, 100, 3517–3528. [Google Scholar] [CrossRef]
- de Freitas, C.F.; Calori, I.R.; Tessaro, A.L.; Caetano, W.; Hioka, N. Rapid formation of small unilamellar vesicles (suv) through low-frequency sonication: An innovative approach. Colloids Surf. B Biointerfaces 2019, 181, 837–844. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, Y.; Chen, J.; Lai, J.; Sun, J.; Hu, F.; Wu, W. Enhanced bioavailability of the poorly water-soluble drug fenofibrate by using liposomes containing a bile salt. Int. J. Pharm. 2009, 376, 153–160. [Google Scholar] [CrossRef]
- Salem, H.F.; Nafady, M.M.; Kharshoum, R.M.; Abd el-Ghafar, O.A.; Farouk, H.O. Mitigation of rheumatic arthritis in a rat model via transdermal delivery of dapoxetine HCl amalgamated as a nanoplatform: In vitro and in vivo assessment. Int. J. Nanomed. 2020, 15, 1517–1535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abd-Elal, R.M.; Shamma, R.N.; Rashed, H.M.; Bendas, E.R. Trans-nasal zolmitriptan novasomes: In-vitro preparation, optimization and in-vivo evaluation of brain targeting efficiency. Drug Deliv. 2016, 23, 3374–3386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abraham Lingan, M. Formulation and Evaluation of Topical Drug Delivery System Containing Clobetasol Propionate Niosomes. Master’s Thesis, Madurai Medical College, Madurai, India, 2008. [Google Scholar]
- Manca, M.L.; Sinico, C.; Maccioni, A.M.; Diez, O.; Fadda, A.M.; Manconi, M. Composition influence on pulmonary delivery of rifampicin liposomes. Pharmaceutics 2012, 4, 590–606. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jing, Q.; Hu, H.; He, Z.; Wu, T.; Guo, T.; Feng, N. Sodium dodecyl sulfate improved stability and transdermal delivery of salidroside-encapsulated niosomes via effects on zeta potential. Int. J. Pharm. 2020, 580, 119183. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, M.; Sammour, O.A.; Hammad, M.A.; Megrab, N.A. Effect of some formulation parameters on flurbiprofen encapsulation and release rates of niosomes prepared from proniosomes. Int. J. Pharm. 2008, 361, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Sharma, A.; Arora, S. Ufasomes mediated cutaneous delivery of dexamethasone: Formulation and evaluation of anti-inflammatory activity by carrageenin-induced rat paw edema model. J. Pharm. 2013, 2013, 680580. [Google Scholar] [CrossRef]
- Ruckmani, K.; Sankar, V. Formulation and optimization of zidovudine niosomes. AAPS PharmSciTech 2010, 11, 1119–1127. [Google Scholar] [CrossRef] [Green Version]
- Parashar, P.; Rana, P.; Dwivedi, M.; Saraf, S.A. Dextrose modified bilosomes for peroral delivery: Improved therapeutic potential and stability of silymarin in diethylnitrosamine-induced hepatic carcinoma in rats. J. Liposome Res. 2019, 29, 251–263. [Google Scholar] [CrossRef]
- Khelashvili, G.; Johner, N.; Zhao, G.; Harries, D.; Scott, H.L. Molecular origins of bending rigidity in lipids with isolated and conjugated double bonds: The effect of cholesterol. Chem. Phys. Lipids 2014, 178, 18–26. [Google Scholar] [CrossRef]
- Ayee, M.A.; Levitan, I. Paradoxical impact of cholesterol on lipid packing and cell stiffness. Front. Biosci. 2016, 21, 1245–1259. [Google Scholar]
- Wacker, M. Nanocarriers for intravenous injection—The long hard road to the market. Int. J. Pharm. 2013, 457, 50–62. [Google Scholar] [CrossRef] [PubMed]
- El-Nabarawi, M.A.; Ali, A.A.; Aboud, H.M.; Hassan, A.H.; Godah, A.H. Transbuccal delivery of betahistine dihydrochloride from mucoadhesive tablets with a unidirectional drug flow: In vitro, ex vivo and in vivo evaluation. Drug Des. Dev. Ther. 2016, 10, 4031–4045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mady, M.M.; Darwish, M.M.; Khalil, S.; Khalil, W.M. Biophysical studies on chitosan-coated liposomes. Eur. Biophys. J. 2009, 38, 1127–1133. [Google Scholar] [CrossRef]
- Lim, W.M.; Rajinikanth, P.S.; Mallikarjun, C.; Kang, Y.B. Formulation and delivery of itraconazole to the brain using a nanolipid carrier system. Int. J. Nanomed. 2014, 9, 2117–2126. [Google Scholar] [CrossRef] [Green Version]
- Domingos, R.F.; Baalousha, M.A.; Ju-Nam, Y.; Reid, M.M.; Tufenkji, N.; Lead, J.R.; Leppard, G.G.; Wilkinson, K.J. Characterizing manufactured nanoparticles in the environment: Multimethod determination of particle sizes. Environ. Sci. Technol. 2009, 43, 7277–7284. [Google Scholar] [CrossRef]
- Zameer, S.; Ali, J.; Vohora, D.; Najmi, A.K.; Akhtar, M. Development, optimisation and evaluation of chitosan nanoparticles of alendronate against Alzheimer’s disease in intracerebroventricular streptozotocin model for brain delivery. J. Drug Target. 2021, 29, 199–216. [Google Scholar] [CrossRef]
- Salem, H.F.; Kharshoum, R.M.; Sayed, O.M.; Abdel Hakim, L.F. Formulation design and optimization of novel soft glycerosomes for enhanced topical delivery of celecoxib and cupferron by Box–Behnken statistical design. Drug Dev. Ind. Pharm. 2018, 44, 1871–1884. [Google Scholar] [CrossRef]
- El Menshawe, S.F.; Aboud, H.M.; Elkomy, M.H.; Kharshoum, R.M.; Abdeltwab, A.M. A novel nanogel loaded with chitosan decorated bilosomes for transdermal delivery of terbutaline sulfate: Artificial neural network optimization, in vitro characterization and in vivo evaluation. Drug Deliv. Transl. Res. 2020, 10, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.F.; Tang, R.; Wong, W.T. Ionically crosslinked complex gels loaded with oleic acid-containing vesicles for transdermal drug delivery. Pharmaceutics 2020, 12, 725. [Google Scholar] [CrossRef]
- Jia-Li, B.; Yuan-Yuan, Y.; Hong, W.; Hai-Feng, H.; Lin-Lin, W.; Hui-Ping, W. A quantitative description of conductance of human stratum corneum caused by pulse electrical field. In Proceedings of the 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference, Shanghai, China, 17–18 January 2006; IEEE: New York, NY, USA, 2006; pp. 7596–7599. [Google Scholar]
- Rowat, A.C.; Kitson, N.; Thewalt, J.L. Interactions of oleic acid and model stratum corneum membranes as seen by 2H NMR. Int. J. Pharm. 2006, 307, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Harjoh, N.; Wong, T.W.; Caramella, C. Transdermal insulin delivery with microwave and fatty acids as permeation enhancers. Int. J. Pharm. 2020, 584, 119416. [Google Scholar] [CrossRef] [PubMed]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–252. [Google Scholar] [CrossRef]
- Taveira, S.F.; Nomizo, A.; Lopez, R.F. Effect of the iontophoresis of a chitosan gel on doxorubicin skin penetration and cytotoxicity. J. Control. Release 2009, 134, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Wood, E.; Dornish, M. Effect of chitosan on epithelial cell tight junctions. Pharm. Res. 2004, 21, 43–49. [Google Scholar] [CrossRef]
- Zaid Alkilani, A.; McCrudden, M.T.; Donnelly, R.F. Transdermal drug delivery: Innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef] [Green Version]
- Khodaei, F.; Khoshnoud, M.J.; Heidaryfar, S.; Heidari, R.; Karimpour Baseri, M.H.; Azarpira, N.; Rashedinia, M. The effect of ellagic acid on spinal cord and sciatica function in a mice model of multiple sclerosis. J. Biochem. Mol. Toxicol. 2020, 34, e22564. [Google Scholar] [CrossRef]
- Soliman, A.; Wahid, A.; Wahby, M.M.; Bassiouny, A. Study of the possible synergistic protective effects of Melatonin and Pregabalin in Vincristine induced peripheral neuropathy Wistar Albino rats. Life Sci. 2020, 244, 117095. [Google Scholar] [CrossRef]
- Bruno, G.; De Logu, F.; Souza Monteiro de Araujo, D.; Subbiani, A.; Lunardi, F.; Rettori, S.; Nassini, R.; Favre, C.; Calvani, M. β2-and β3-Adrenergic Receptors Contribute to Cancer-Evoked Pain in a Mouse Model of Osteosarcoma via Modulation of Neural Macrophages. Front. Pharmacol. 2021, 12, 697912. [Google Scholar] [CrossRef]
- Xiang, H.; Zhang, Q.; Han, Y.; Yang, L.; Zhang, Y.; Liu, Q.; Zhang, Z.; Zhang, L. Novel brain-targeting 3-n-butylphthalide prodrugs for ischemic stroke treatment. J. Control. Release 2021, 335, 498–514. [Google Scholar] [CrossRef]
- Bharath, G.; Adiga, S.; Reddy, S.K.; Tripathy, A. Comparison of effects of carvedilol and propranolol on learning and memory in rats. Int. J. Adv. Res. 2015, 3, 1164–1168. [Google Scholar]
- Kramer, J.H.; Spurney, C.F.; Iantorno, M.; Tziros, C.; Chmielinska, J.J.; Mak, I.T.; Weglicki, W.B. d-Propranolol protects against oxidative stress and progressive cardiac dysfunction in iron overloaded rats. Can. J. Physiol. Pharmacol. 2012, 90, 1257–1268. [Google Scholar] [CrossRef] [Green Version]
- Ranasinghe, H.N.; Fernando, N.; Handunnetti, S.; Weeratunga, P.N.; Katulanda, P.; Rajapakse, S.; Galappatthy, P.; Constantine, G.R. The impact of propranolol on nitric oxide and total antioxidant capacity in patients with resistant hypertension—Evidence from the APPROPRIATE trial. BMC Res. Notes 2020, 13, 228. [Google Scholar] [CrossRef] [Green Version]
- Hayek, S.S.; Poole, J.C.; Neuman, R.; Morris, A.A.; Khayata, M. Differential. effects of nebivolol and metoprolol on arterial stiffness, circulating progenitor cells, and oxidative stress. J. Am. Soc. Hypertens. 2015, 9, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Ramos, L.P.A.; Justino, A.B.; Tavernelli, N.; Saraiva, A.L.; Franco, R.R.; de Souza, A.V.; Silva, H.C.G.; de Moura, F.B.R.; Botelho, F.V.; Espindola, F.S. Antioxidant compounds from Annona crassiflora fruit peel reduce lipid levels and oxidative damage and maintain the glutathione defense in hepatic tissue of Triton WR-1339-induced hyperlipidemic mice. Biomed. Pharmacother. 2021, 142, 112049. [Google Scholar] [CrossRef] [PubMed]
- Michalovicz, L.T.; Kelly, K.A.; Miller, D.B.; Sullivan, K.; O’Callaghan, J.P. The β-adrenergic receptor blocker and anti-inflammatory drug propranolol mitigates brain cytokine expression in a long-term model of Gulf War Illness. Life Sci. 2021, 285, 119962. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.M.; Ahmad, E.A.; Omran, B.H.; Sakr, A.T.; Ibrahim, I.A.H.; Mahmoud, M.F.; El-Naggar, M.E. Mitigation of dexamethasone-induced nephrotoxicity by modulating the activity of adrenergic receptors: Implication of Wnt/β-arrestin2/β-catenin pathway. Life Sci. 2022, 293, 120304. [Google Scholar] [CrossRef]
- Haas, M.J.; Kurban, W.; Shah, H.; Onstead-Haas, L.; Mooradian, A.D. Beta blockers suppress dextrose-induced endoplasmic reticulum stress, oxidative stress, and apoptosis in human coronary artery endothelial cells. Am. J. Ther. 2016, 23, e1524–e1531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hartung, J.E.; Bortsov, A.V.; Kim, S.; O’Buckley, S.C.; Kozlowski, J.; Nackley, A.G. Sustained stimulation of β2-and β3-adrenergic receptors leads to persistent functional pain and neuroinflammation. Brain Behav. Immun. 2018, 73, 520–532. [Google Scholar] [CrossRef]
- Calvani, M.; Bruno, G.; Dabraio, A.; Subbiani, A.; Bianchini, F.; Fontani, F.; Casazza, G.; Vignoli, M.; De Logu, F.; Frenos, S.; et al. β3-Adrenoreceptor blockade induces stem cells differentiation in melanoma microenvironment. Int. J. Mol. Sci. 2020, 21, 1420. [Google Scholar] [CrossRef] [Green Version]
- Abdel Salam, O.M.E.; Ameen Sleem, A.; Abdel Rahman, R.F.; Morsy, F.A. Effect of Adrenergic Drugs on the Lipopolysaccharide-Induced Oxidative Stress and Liver Damage in the Rat. SOJ Diabet. Endocr. Care 2021, 1, 1–10. [Google Scholar]
- Abdel-Wahab, B.A.; Salem, S.Y.; Mohammed, H.M.; Mohammed, N.A.; Hetta, H.F. The role of vimentin, Connexin-43 proteins, and oxidative stress in the protective effect of propranolol against clozapine-induced myocarditis and apoptosis in rats. Eur. J. Pharmacol. 2021, 890, 173645. [Google Scholar] [CrossRef] [PubMed]
- Harima, M.; Arumugam, S.; Wen, J.; Pitchaimani, V.; Karuppagounder, V.; Afrin, M.R.; Sreedhar, R.; Miyashita, S.; Nomoto, M.; Ueno, K.; et al. Effect of carvedilol against myocardial injury due to ischemia–reperfusion of the brain in rats. Exp. Mol. Pathol. 2015, 98, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Abdou, E.A.I.M.; Elmenshawy, S.H.S.; Hemdan, M.D. Effect of Portulaca Oleracea Extract and Its Interactions with Propranolol in Cirrhotic Portal Hypertensive Rats. Tob. Regul. Sci. 2022, 25, 824–847. [Google Scholar]
- Ahmed, L.A.; Shehata, N.I.; Abdelkader, N.F.; Khattab, M.M. Tempol, a superoxide dismutase mimetic agent, ameliorates cisplatin-induced nephrotoxicity through alleviation of mitochondrial dysfunction in mice. PLoS ONE 2014, 9, e108889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherif, N.A.; El-Banna, A.S.; ElBourini, M.M.; Khalil, N.O. Efficacy of L-carnitine and propranolol in the management of acute theophylline toxicity. Toxicol. Res. 2020, 9, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Jiang, W.; Wang, C.; Xie, J.; Liang, X.; Sun, Y.; Gong, L.; Liu, W.; Qu, L. Jinmaitong, a traditional Chinese compound prescription, ameliorates the streptozocin-induced diabetic peripheral neuropathy rats by increasing sciatic nerve IGF-1 and IGF-1R expression. Front. Pharmacol. 2019, 10, 255. [Google Scholar] [CrossRef]
- Zhai, Q.W.; Ooi, K.J.; Xu, S.Y.; Ong, C.K. Long range electromagnetic field nature of nerve signal propagation in myelinated axons. Chin. Phys. B 2022, 31, 38701. [Google Scholar] [CrossRef]
- Pridmore, M.D.; Glassman, G.E.; Pollins, A.C.; Manzanera Esteve, I.V.; Drolet, B.C.; Weikert, D.R.; Does, M.D.; Perdikis, G.; Thayer, W.P.; Dortch, R.D. Initial findings in traumatic peripheral nerve injury and repair with diffusion tensor imaging. Ann. Clin. Transl. Neurol. 2021, 8, 332–347. [Google Scholar] [CrossRef]
- Araujo, L.P.; Maricato, J.T.; Guereschi, M.G.; Takenaka, M.C.; Nascimento, V.M.; de Melo, F.M.; Quintana, F.J.; Brum, P.C.; Basso, A.S. The sympathetic nervous system mitigates CNS autoimmunity via β2-adrenergic receptor signaling in immune cells. Cell Rep. 2019, 28, 3120–3130.e5. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Deng, Q.W.; Peng, A.N.; Xing, F.L.; Zuo, L.; Li, S.; Gu, Z.T.; Yan, F.L. β-arrestin2 functions as a key regulator in the sympathetic-triggered immunodepression after stroke. J. Neuroinflammation 2018, 15, 102. [Google Scholar] [CrossRef] [Green Version]
- Quatrini, L.; Vivier, E.; Ugolini, S. Neuroendocrine regulation of innate lymphoid cells. Immunol. Rev. 2018, 286, 120–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamisli, S.; Ciftci, O.; Kaya, K.; Cetin, A.; Kamisli, O.; Ozcan, C. Hesperidin protects brain and sciatic nerve tissues against cisplatin-induced oxidative, histological and electromyographical side effects in rats. Toxicol. Ind. Health 2015, 31, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Tiwari, N.; Madar, J.; Mehta, P.; Qiao, L.Y. Beta 2-adrenergic receptor mediates noradrenergic action to induce cyclic adenosine monophosphate response element-binding protein phosphorylation in satellite glial cells of dorsal root ganglia to regulate visceral hypersensitivity. Pain 2022, 163, 180–192. [Google Scholar] [CrossRef]
- Moradi-Arzeloo, M.; Farshid, A.A.; Tamaddonfard, E.; Asri-Rezaei, S. Effects of histidine and vitamin C on isoproterenol-induced acute myocardial infarction in rats. Vet. Res. Forum 2016, 7, 47–54. [Google Scholar]
- Esmaeeli, A.; Keshavarz, Z.; Dehdar, F.; Assadi, M.; Seyedabadi, M. The effects of carvedilol, metoprolol and propranolol on cisplatin-induced kidney injury. Drug Chem. Toxicol. 2022, 45, 1558–1564. [Google Scholar] [CrossRef]
- Xu, W.L.; Aikeremu, D.; Sun, J.G.; Zhang, Y.J.; Xu, J.B.; Zhou, W.Z.; Zhao, X.B.; Wang, H.; Yuan, H. Effect of intensity-modulated radiation therapy on sciatic nerve injury caused by echinococcosis. Neural Regen. Res. 2021, 16, 580–586. [Google Scholar] [CrossRef]
- Dzreyan, V.; Eid, M.; Rodkin, S.; Pitinova, M.; Demyanenko, S. E2F1 Expression and Apoptosis Initiation in Crayfish and Rat Peripheral Neurons and Glial Cells after Axonal Injury. Int. J. Mol. Sci. 2022, 23, 4451. [Google Scholar] [CrossRef] [PubMed]
- Harguindey, S.; Alfarouk, K.; Polo Orozco, J.; Reshkin, S.J.; Devesa, J. Hydrogen Ion Dynamics as the Fundamental Link between Neurodegenerative Diseases and Cancer: Its Application to the Therapeutics of Neurodegenerative Diseases with Special Emphasis on Multiple Sclerosis. Int. J. Mol. Sci. 2022, 23, 2454. [Google Scholar] [CrossRef] [PubMed]
- Grimsley, H.E. Investigating Mechanisms of Acquired Drug Resistance in Triple Negative Breast Cancer. Ph.D. Thesis, University of Kent, Canterbury, UK, 2021. [Google Scholar]
- Worakajit, N.; Thipboonchoo, N.; Chaturongakul, S.; Jutabha, P.; Soontornniyomkij, V.; Tuchinda, P.; Soodvilai, S. Nephroprotective potential of Panduratin A against colistin-induced renal injury via attenuating mitochondrial dysfunction and cell apoptosis. Biomed. Pharmacother. 2022, 148, 112732. [Google Scholar] [CrossRef]
- Tu, Y.; Kim, E.; Gao, Y.; Rankin, G.O.; Li, B.; Chen, Y.C. Theaflavin-3, 3’-digallate induces apoptosis and G2 cell cycle arrest through the Akt/MDM2/p53 pathway in cisplatin-resistant ovarian cancer A2780/CP70 cells. Int. J. Oncol. 2016, 48, 2657–2665. [Google Scholar] [CrossRef] [Green Version]
- Jin, W.; Zhang, Y.; Xue, Y.; Han, X.; Zhang, X.; Ma, Z.; Sun, S.; Chu, X.; Cheng, J.; Guan, S.; et al. Crocin attenuates isoprenaline-induced myocardial fibrosis by targeting TLR4/NF-κB signaling: Connecting oxidative stress, inflammation, and apoptosis. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.Q.; Chen, H.W.; Li, J. Astragaloside IV: An effective drug for the treatment of cardiovascular diseases. Drug Des. Dev. Ther. 2020, 14, 3731–3746. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Demillard, L.J.; Ren, J. Catecholamine-induced cardiotoxicity: A critical element in the pathophysiology of stroke-induced heart injury. Life Sci. 2021, 287, 120106. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Xie, G.; Cui, H.; Yao, Z.; Shao, C.; Yuan, W.; Chen, B. Cyclosporin-A reduced the cytotoxicity of propranolol in HUVECs via p38 MAPK signaling. Medicine 2022, 101, e28329. [Google Scholar] [CrossRef]
- Zhao, S.; Fan, S.; Shi, Y.; Ren, H.; Hong, H.; Gao, X.; Zhang, M.; Qin, Q.; Li, H. Propranolol induced apoptosis and autophagy via the ROS/JNK signaling pathway in Human Ovarian Cancer. J. Cancer 2020, 11, 5900–5910. [Google Scholar] [CrossRef] [PubMed]
- Mak, S.; Liu, Z.; Wu, L.; Guo, B.; Luo, F.; Liu, Z.; Hu, S.; Wang, J.; Cui, G.; Sun, Y.; et al. Pharmacological characterizations of anti-dementia memantine nitrate via neuroprotection and vasodilation in vitro and in vivo. ACS Chem. Neurosci. 2020, 1, 314–327. [Google Scholar] [CrossRef]
- Juranek, I.; Horakova, L.; Rackova, L.; Stefek, M. Antioxidants in treating pathologies involving oxidative damage: An update on medicinal chemistry and biological activity of stobadine and related pyridoindoles. Curr. Med. Chem. 2010, 17, 552–570. [Google Scholar] [CrossRef]
- Zhu, L.; Hao, J.; Cheng, M.; Zhang, C.; Huo, C.; Liu, Y.; Du, W.; Zhang, X. Hyperglycemia-induced Bcl-2/Bax-mediated apoptosis of Schwann cells via mTORC1/S6K1 inhibition in diabetic peripheral neuropathy. Exp. Cell Res. 2018, 367, 186–195. [Google Scholar] [CrossRef]
- Yan, L.; Xie, M.; Lu, H.; Zhang, H.; Shi, M.; Zhang, Y.; Xi, C.; Li, J.; Yang, T. Anti-apoptotic effect of IGF1 on Schwann exposed to hyperglycemia is mediated by neuritin, a novel neurotrophic factor. Mol. Neurobiol. 2018, 55, 495–505. [Google Scholar] [CrossRef]
- Zhu, M.; Gao, S.; Gao, S.; Wang, Y.; Li, N.; Shen, X. Interleukin-17A attenuates photoreceptor cell apoptosis in streptozotocin-induced diabetic mouse model. Bioengineered 2022, 13, 14175–14187. [Google Scholar] [CrossRef]
- Harauz, G.; Boggs, J.M. Myelin management by the 18.5-kDa and 21.5-kDa classic myelin basic protein isoforms. J. Neurochem. 2013, 125, 334–361. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Méndez, J.S.; Herrera-Sánchez, M.P.; Céspedes-Rubio, Á.E.; Rondón-Barragán, I.S. Molecular characterization of myelin basic protein a (mbpa) gene from red-bellied pacu (Piaractus brachypomus). J. Genet. Eng. Biotechnol. 2022, 20, 8. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Xin, W.; Tan, J.R.; Zhu, R.P.; Li, T.; Wang, D.; Kan, S.S.; Xiong, D.K.; Li, H.H.; Zhang, M.M.; et al. Myelin sheath structure and regeneration in peripheral nerve injury repair. Proc. Natl. Acad. Sci. USA 2019, 116, 22347–22352. [Google Scholar] [CrossRef] [Green Version]
- Suo, N.; He, B.; Cui, S.; Yang, Y.; Wang, M.; Yuan, Q.; Xie, X. The orphan G protein-coupled receptor GPR149 is a negative regulator of myelination and remyelination. GLIA 2022. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.K.; Shen, X.Y.; Han, Y.; Guo, Y.S.; Yuan, M.; Bi, X. Enriched Environment Effects on Myelination of the Central Nervous System: Role of Glial Cells. Neural Plast. 2022, 2022, 5766993. [Google Scholar] [CrossRef]
- Wongtawatchai, T.; Agthong, S.; Kaewsema, A.; Chentanez, V. Altered phosphorylation of mitogen-activated protein kinases in dorsal root ganglia and sciatic nerve of rats with cisplatin-induced neuropathy. Asian Biomed. 2012, 6, 397–411. [Google Scholar]
- Abdelrahman, A.; Abd Elhaliem, N.; Elnady, H.; Lotfy, A.; Moghazy, H. A Comparative Study between the Effect of Nerve Growth Factor and All-Trans Retinoic Acid versus their combined use on Taxol Induced Peripheral Neuropathy in Adult Male Albino Rat. Egypt. J. Histol. 2019, 42, 408–424. [Google Scholar]
- Wu, B.Y.; Liu, C.T.; Su, Y.L.; Chen, S.Y.; Chen, Y.H.; Tsai, M.Y. A review of complementary therapies with medicinal plants for chemotherapy-induced peripheral neuropathy. Complement. Ther. Med. 2019, 42, 226–232. [Google Scholar] [CrossRef]
- Taiana, M.M.; Lombardi, R.; Porretta-Serapiglia, C.; Ciusani, E.; Oggioni, N.; Sassone, J.; Bianchi, R.; Lauria, G. Neutralization of schwann cell-secreted VEGF is protective to in vitro and in vivo experimental diabetic neuropathy. PLoS ONE 2014, 9, e108403. [Google Scholar] [CrossRef] [Green Version]
- Vujnović, I.; Pilipović, I.; Jasnić, N.; Petrović, R.; Blagojević, V.; Arsenović-Ranin, N.; Stojić-Vukanić, Z.; Djordjević, J.; Leposavić, G. Noradrenaline through β-adrenoceptor contributes to sexual dimorphism in primary CD4+ T-cell response in DA rat EAE model? Cell. Immunol. 2019, 336, 48–57. [Google Scholar] [CrossRef]
- Wei, W.; Ma, D.; Li, L.; Zhang, L. Progress in the Application of Drugs for the Treatment of Multiple Sclerosis. Front. Pharmacol. 2021, 12, 724718. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha, R.T.; Habib, S.; Sangaraju, S.L.; Yepez, D.; Grandes, X.A. Multiple Sclerosis: Therapeutic Strategies on the Horizon. Cureus 2022, 14, e24895. [Google Scholar] [CrossRef] [PubMed]
- Zakria, M.; Ahmad, N.; Al Kury, L.T.; Alattar, A.; Uddin, Z.; Siraj, S.; Ullah, S.; Alshaman, R.; Khan, M.I.; Shah, F.A. Melatonin rescues the mice brain against cisplatin-induced neurodegeneration, an insight into antioxidant and anti-inflammatory effects. Neurotoxicology 2021, 87, 1–10. [Google Scholar] [CrossRef]
- Domingo, I.K.; Latif, A.; Bhavsar, A.P. Pro-Inflammatory Signalling PRRopels Cisplatin-Induced Toxicity. Int. J. Mol. Sci. 2022, 23, 7227. [Google Scholar] [CrossRef]
- Son, J.Y.; Ju, J.S.; Kim, Y.M.; Ahn, D.K. TNF-α-Mediated RIPK1 Pathway Participates in the Development of Trigeminal Neuropathic Pain in Rats. Int. J. Mol. Sci. 2022, 23, 506. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Ali, G.; Subhan, F.; Naveed, M.; Khan, A.; Khan, J.; Halim, S.A.; Ahmad, N.; Al-Harrasi, A. Attenuation of nociceptive and paclitaxel-induced neuropathic pain by targeting inflammatory, CGRP and substance P signaling using 3-Hydroxyflavone. Neurochem. Int. 2021, 144, 104981. [Google Scholar] [CrossRef]
- Murakami, K.; Kuniyoshi, K.; Iwakura, N.; Matsuura, Y.; Suzuki, T.; Takahashi, K.; Ohtori, S. Vein wrapping for chronic nerve constriction injury in a rat model: Study showing increases in VEGF and HGF production and prevention of pain-associated behaviors and nerve damage. J. Bone Joint Surg. 2014, 96, 859–867. [Google Scholar] [CrossRef]
- Bittner, G.D.; Bushman, J.S.; Ghergherehchi, C.L.; Roballo, K.; Shores, J.T.; Smith, T.A. Typical and atypical properties of peripheral nerve allografts enable novel strategies to repair segmental-loss injuries. J. Neuroinflammation 2022, 19, 60. [Google Scholar] [CrossRef]
- Chen, S.H.; Wu, C.C.; Lin, S.C.; Tseng, W.L.; Huang, T.C.; Yadav, A.; Lu, F.I.; Liu, Y.H.; Lin, S.P.; Hsueh, Y.Y. Investigation of Neuropathology after Nerve Release in Chronic Constriction Injury of Rat Sciatic Nerve. Int. J. Mol. Sci. 2021, 22, 4746. [Google Scholar] [CrossRef]
- Yang, Y.; Song, J.; Liu, N.; Wei, G.; Liu, S.; Zhang, S.; Jiang, N.; Yang, H.; Du, G. Salvianolic acid A relieves cognitive disorder after chronic cerebral ischemia: Involvement of Drd2/Cryab/NF-κB pathway. Pharmacol. Res. 2022, 175, 105989. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, L.; Yin, X.; McCarthy, E.C.; Cheng, M.I.; Hoang, A.T.; Chen, H.C.; Patel, A.Y.; Allard Trout, D.; Xu, E.; et al. Pathogenic TNF-α drives peripheral nerve inflammation in an Aire-deficient model of autoimmunity. Proc. Natl. Acad. Sci. USA 2022, 119, e2114406119. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Fang, L.; Huang, S.; Yang, Z.; Nandi, J.; Thomas, S.; Chen, C.; Camporesi, E. Hyperbaric oxygenation therapy alleviates chronic constrictive injury–induced neuropathic pain and reduces tumor necrosis factor-alpha production. Anesth. Analg. 2011, 113, 626–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, Y.; Han, Q.; Dong, B.; Lin, H.; Jiang, Y.; Zhang, X.; Chen, H.; Yu, Y. PPARα contributes to the therapeutic effect of hydrogen gas against sepsis-associated encephalopathy with the regulation to the CREB-BDNF signaling pathway and hippocampal neuron plasticity-related gene expression. Brain Res. Bull. 2022, 184, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Qiu, H.; Yan, J.; Shen, X.; Wei, X.; Duan, M.; Yang, J. The involvement of TNF-α and TNF-β as proinflammatory cytokines in lymphocyte-mediated adaptive immunity of Nile tilapia by initiating apoptosis. Dev. Comp. Immunol. 2021, 115, 103884. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, H.; Sarmento-Ribeiro, A.B.; Andrade, J.P.; Dourado, M. Apoptosis and (in) Pain—Potential Clinical Implications. Biomedicines 2022, 10, 1255. [Google Scholar] [CrossRef]
- Arab, H.H.; El-Sawalhi, M.M. Carvedilol alleviates adjuvant-induced arthritis and subcutaneous air pouch edema: Modulation of oxidative stress and inflammatory mediators. Toxicol. Appl. Pharmacol. 2013, 268, 241–248. [Google Scholar] [CrossRef]
- Sohn, R.; Roesch, G.; Junker, M.; Meurer, A.; Zaucke, F.; Jenei-Lanzl, Z. Adrenergic signalling in osteoarthritis. Cell. Signal. 2021, 82, 109948. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, S.; Shapouri, R.; Shirkhani, A.; Mahdavi, M. Anti-tumor effects of propranolol: Adjuvant activity on a transplanted murine breast cancer model. Biomed. Pharmacother. 2018, 104, 45–51. [Google Scholar] [CrossRef]
- Pantziarka, P.; Bouche, G.; Sukhatme, V.; Meheus, L.; Rooman, I.; Sukhatme, V.P. Repurposing Drugs in Oncology (ReDO)—Propranolol as an anti-cancer agent. Ecancermedicalscience 2016, 10, 680. [Google Scholar] [CrossRef] [Green Version]
- Faura, J.; Bustamante, A.; Miró-Mur, F.; Montaner, J. Stroke-induced immunosuppression: Implications for the prevention and prediction of post-stroke infections. J. Neuroinflammation 2021, 18, 127. [Google Scholar] [CrossRef]
- Cui, P.; McCullough, L.D.; Hao, J. Brain to periphery in acute ischemic stroke: Mechanisms and clinical significance. Front. Neuroendocrinol. 2021, 63, 100932. [Google Scholar] [CrossRef] [PubMed]
- Saeedi Saravi, S.S.; Saeedi Saravi, S.S.; Arefidoust, A.; Dehpour, A.R. The beneficial effects of HMG-CoA reductase inhibitors in the processes of neurodegeneration. Metab. Brain Dis. 2017, 32, 949–965. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, L.A.; Darwish, H.A.; Abdelsalam, R.M.; Amin, H.A. Role of rho kinase inhibition in the protective effect of fasudil and simvastatin against 3-nitropropionic acid-induced striatal neurodegeneration and mitochondrial dysfunction in rats. Mol. Neurobiol. 2016, 53, 3927–3938. [Google Scholar] [CrossRef] [PubMed]
- Araújo Júnior, R.F.D.; Souza, T.O.; Medeiros, C.A.X.D.; Souza, L.B.D.; Freitas, M.D.L.; de Lucena, H.F. Carvedilol decrease IL-1β and TNF-α, inhibits MMP-2, MMP-9, COX-2, and RANKL expression, and up-regulates OPG in a rat model of periodontitis. PLoS ONE 2013, 8, e66391. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Wang, Y.Y.; Chang, C.Y.; Wu, C.C.; Chen, W.Y.; Kuan, Y.H.; Liao, S.L.; Chen, C.J. Effects of β-adrenergic blockade on metabolic and inflammatory responses in a rat model of ischemic stroke. Cells 2020, 9, 1373. [Google Scholar] [CrossRef] [PubMed]
- Barnard, D.F. The Regulation of Brain Pro-Inflammatory Cytokines: Implications for Stress and Depression. Ph.D. Thesis, Kent State University, Kent, OH, USA, 2020. [Google Scholar]





| Factors | Levels | |
|---|---|---|
| Low (−1) | High (+1) | |
| Independent variables | ||
| A = Span type | Span 20 | Span 60 |
| B = Oleic acid amount (mg) | 20 | 40 |
| C = Cholesterol amount (mg) | 15 | 30 |
| D = Sonication time (min) | 0 | 15 |
| Responses (dependent variables) | Desirability constraints | |
| Y1 = EE% | Maximize | |
| Y2 = PS (nm) | Minimize | |
| Y3 = PDI | ˂0.5 | |
| Y4 = ZP (mV) | Minimize | |
| Y5 = Q6h (%) | Maximize | |
| Ufasomes Formulation | A | B | C | D | Y1 | Y2 | Y3 | Y4 | Y5 |
|---|---|---|---|---|---|---|---|---|---|
| Span Type | Oleic Acid Amount (mg) | Cholesterol Amount (mg) | Sonication Time (min) | EE% | PS (nm) | PDI | ZP (mV) | Q6h % | |
| U1 | span 20 | 40 | 15 | 15 | 64.72 ± 1.12 | 402.62 ± 25.01 | 0.28 ± 0.023 | −69.32 ± 2.25 | 81.31 ± 2.33 |
| U2 | span 60 | 20 | 15 | 0 | 86.84 ± 2.04 | 432.13 ± 15.66 | 0.62 ± 0.012 | −33.52 ± 1.23 | 68.55 ± 1.12 |
| U3 | span 20 | 40 | 15 | 0 | 77.30 ± 1.52 | 302.78 ± 18.52 | 0.32 ± 0.016 | −67.30 ± 1.11 | 77.28 ± 1.65 |
| U4 | span 60 | 40 | 30 | 0 | 96.13 ± 3.22 | 510.44 ± 20.32 | 0.61 ± 0.021 | −33.95 ± 1.06 | 49.61 ± 0.98 |
| U5 | span 20 | 40 | 30 | 0 | 91.22 ± 2.67 | 326.85 ± 17.65 | 0.56 ± 0.015 | −62.60 ± 1.14 | 53.62 ± 2.24 |
| U6 | span 20 | 20 | 30 | 0 | 88.32 ± 1.65 | 385.80 ± 20.31 | 0.59 ± 0.032 | −34.47 ± 2.50 | 71.30 ± 3.23 |
| U7 | span 20 | 20 | 15 | 0 | 74.97 ± 2.66 | 408.29 ± 14.82 | 0.36 ± 0.022 | −64.27 ± 2.14 | 83.21 ± 4.87 |
| U8 | span 20 | 40 | 30 | 15 | 90.44 ± 3.91 | 397.57 ± 22.54 | 0.44 ± 0.032 | −66.24 ± 2.34 | 61.29 ± 2.45 |
| U9 | span 60 | 20 | 15 | 15 | 78.67 ± 2.54 | 351.11 ± 23.70 | 0.52 ± 0.034 | −39.55 ± 3.01 | 74.88 ± 3.21 |
| U10 | span 60 | 20 | 30 | 0 | 90.47 ± 3.43 | 480.26 ± 24.13 | 0.71 ± 0.016 | −31.15 ± 1.13 | 59.57 ± 2.05 |
| U11 | span 20 | 20 | 15 | 15 | 53.64 ± 1.04 | 430.46 ± 26.23 | 0.31 ± 0.034 | −71.12 ± 1.45 | 87.75 ± 3.98 |
| U12 | span 60 | 40 | 15 | 0 | 87.61 ± 2.21 | 470.42 ± 15.74 | 0.59 ± 0.010 | −36.87 ± 1.08 | 65.58 ± 4.11 |
| U13 | span 60 | 40 | 15 | 15 | 79.45 ± 2.34 | 490.21 ± 10.74 | 0.46 ± 0.03 | −40.36 ± 2.23 | 79.63 ± 4.56 |
| U14 | span 60 | 40 | 30 | 15 | 97.52 ± 4.05 | 485.86 ± 18.00 | 0.53 ± 0.016 | −37.41 ± 1.65 | 56.35 ± 1.34 |
| U15 | span 60 | 20 | 30 | 15 | 82.93 ± 2.76 | 356.51 ± 11.65 | 0.57 ± 0.021 | −43.87 ± 1.45 | 70.88 ± 2.12 |
| U16 | span 20 | 20 | 30 | 15 | 79.89 ± 1.94 | 382.23 ± 10.43 | 0.42 ± 0.035 | −43.59 ± 2.06 | 80.44 ± 7.55 |
| Forward Sequence | Reverse Sequence | |
|---|---|---|
| Peripheral myelin 22 | CTCCTCGCAGGCAGAAACTC | TGGCCAGCTCTCCTAAC |
| GAPDH | TGGATTTGGACGCATTGGTC | TTTGCACTGGTACGTGTTGAT |
| Responses | R2 | Adjusted R2 | Predicted R2 | Adequate Precision | Significant Factors |
|---|---|---|---|---|---|
| EE% | 0.97 | 0.92 | 0.74 | 15.59 | A, B, C, D |
| PS (nm) | 0.98 | 0.95 | 0.83 | 18.79 | A, B |
| PDI | 0.98 | 0.94 | 0.80 | 16.76 | A, C, D |
| ZP (mV) | 0.96 | 0.87 | 0.57 | 10.06 | A, B, C |
| Q6h (%) | 0.98 | 0.95 | 0.85 | 20.57 | A, B, C, D |
| Solution | Span Type | Oleic Acid Amount (mg) | Cholesterol Amount (mg) | Sonication Time (min) | EE% | PS (nm) | PDI | ZP (mV) | Q6h % |
|---|---|---|---|---|---|---|---|---|---|
| optimized PRO–UFAs | Span 20 | 40 | 22.52 | 0 | 82.72 ± 2.33 | 317.22 ± 6.43 | 0.441 + 0.02 | −62.06 ± 0.07 | 70.95 ± 8.14 |
| PRO–CTS–UFAs | Span 20 | 40 | 22.52 | 0 | 85.32 ± 2.65 | 336.12 ± 4.9 | 0.445 ± 0.03 | 65.24 ± 0.10 | 64.03 ± 1.9 |
| Formulation | Lag Time (min) | Jss (µg/cm2 h) | Kp (cm/h) | EI |
|---|---|---|---|---|
| PRO–CTS–UFAs | 50.63 ± 2.23 | 16.98 ± 0.12 | 0.0169 ± 0.0007 | 2.45 |
| optimized PRO–UFAs | 66.13 ± 4.34 | 8.12 ± 0.45 | 0.0082 ± 0.0013 | 1.19 |
| PRO solution | 146.78 ± 10.13 | 6.91 ± 0.12 | 0.0069 ± 0.0010 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, Y.M.; Orfali, R.; Hamad, D.S.; Rateb, M.E.; Farouk, H.O. Sustainable Release of Propranolol Hydrochloride Laden with Biconjugated-Ufasomes Chitosan Hydrogel Attenuates Cisplatin-Induced Sciatic Nerve Damage in In Vitro/In Vivo Evaluation. Pharmaceutics 2022, 14, 1536. https://doi.org/10.3390/pharmaceutics14081536
Ahmed YM, Orfali R, Hamad DS, Rateb ME, Farouk HO. Sustainable Release of Propranolol Hydrochloride Laden with Biconjugated-Ufasomes Chitosan Hydrogel Attenuates Cisplatin-Induced Sciatic Nerve Damage in In Vitro/In Vivo Evaluation. Pharmaceutics. 2022; 14(8):1536. https://doi.org/10.3390/pharmaceutics14081536
Chicago/Turabian StyleAhmed, Yasmin M., Raha Orfali, Doaa S. Hamad, Mostafa E. Rateb, and Hanan O. Farouk. 2022. "Sustainable Release of Propranolol Hydrochloride Laden with Biconjugated-Ufasomes Chitosan Hydrogel Attenuates Cisplatin-Induced Sciatic Nerve Damage in In Vitro/In Vivo Evaluation" Pharmaceutics 14, no. 8: 1536. https://doi.org/10.3390/pharmaceutics14081536
APA StyleAhmed, Y. M., Orfali, R., Hamad, D. S., Rateb, M. E., & Farouk, H. O. (2022). Sustainable Release of Propranolol Hydrochloride Laden with Biconjugated-Ufasomes Chitosan Hydrogel Attenuates Cisplatin-Induced Sciatic Nerve Damage in In Vitro/In Vivo Evaluation. Pharmaceutics, 14(8), 1536. https://doi.org/10.3390/pharmaceutics14081536






