Synthesis of Celecoxib-Eutectic Mixture Particles via Supercritical CO2 Process and Celecoxib Immediate Release Tablet Formulation by Quality by Design Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Screening Assisted Solvent for Eutectic Mixture (EM) Preparation
2.2.2. NMR Analysis
2.2.3. DSC Analysis
2.2.4. CEL EM Preparation by Spray Drying
2.2.5. CEL EM Preparation by Supercritical Fluid (SCF) Technique
Supercritical Anti Solvent (SAS) Crystallization
SCF Assisted Mixing Method
2.2.6. Powder X-ray Diffraction (PXRD) Analysis
2.2.7. Fourier Transform Infrared Spectroscopy (FT-IR)
2.2.8. Particle Size Analysis
2.2.9. Scanning Electron Microscopy (SEM)
2.2.10. In Vitro Dissolution Study
2.2.11. CEL Immediate Release (IR) Tablet Formulation Development by a Quality by Design (QbD) Approach
Quality by Design (QbD) Approach
Preparation of CEL IR Tablets
3. Results and Discussion
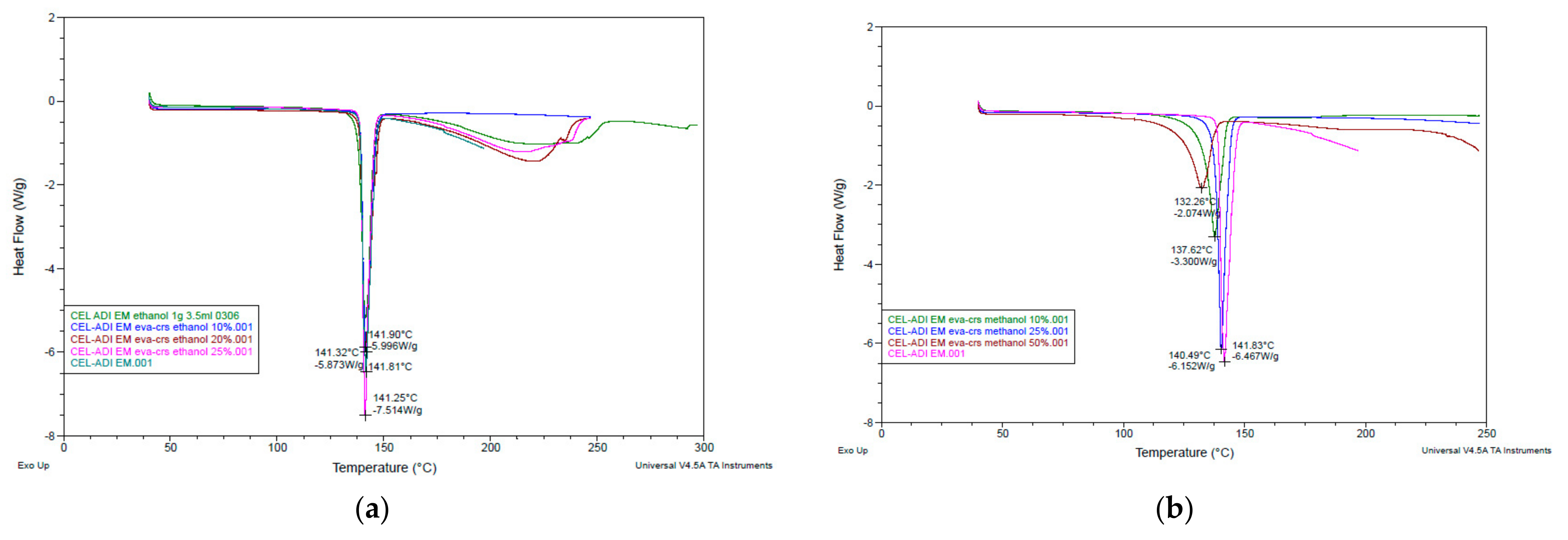
3.1. Assisted Solvent for EM Preparation
3.2. Characterization of CEL-EM
3.2.1. DSC Analysis
3.2.2. FTIR Analysis
3.2.3. NMR Analysis
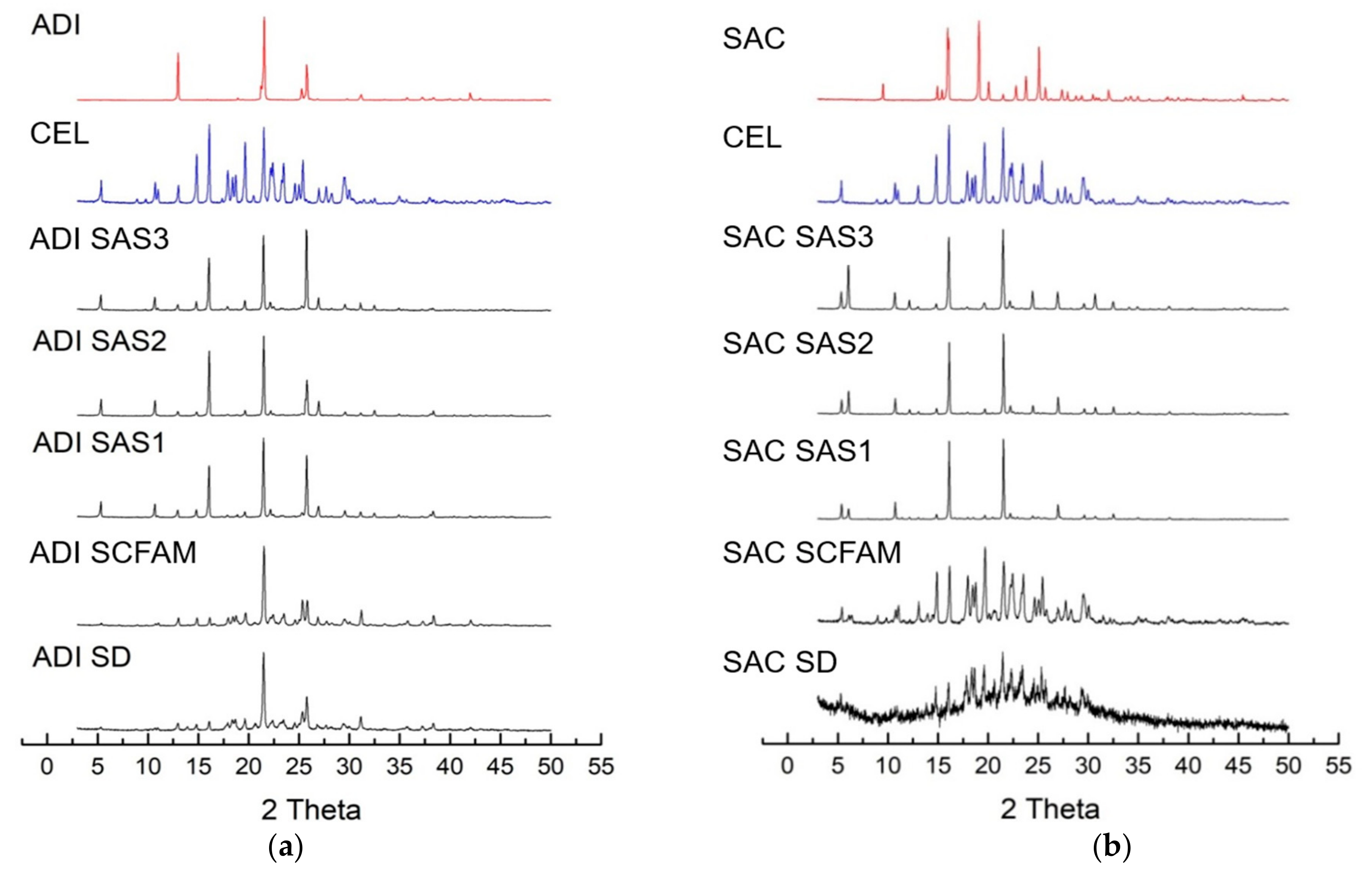
3.2.4. PXRD Analysis
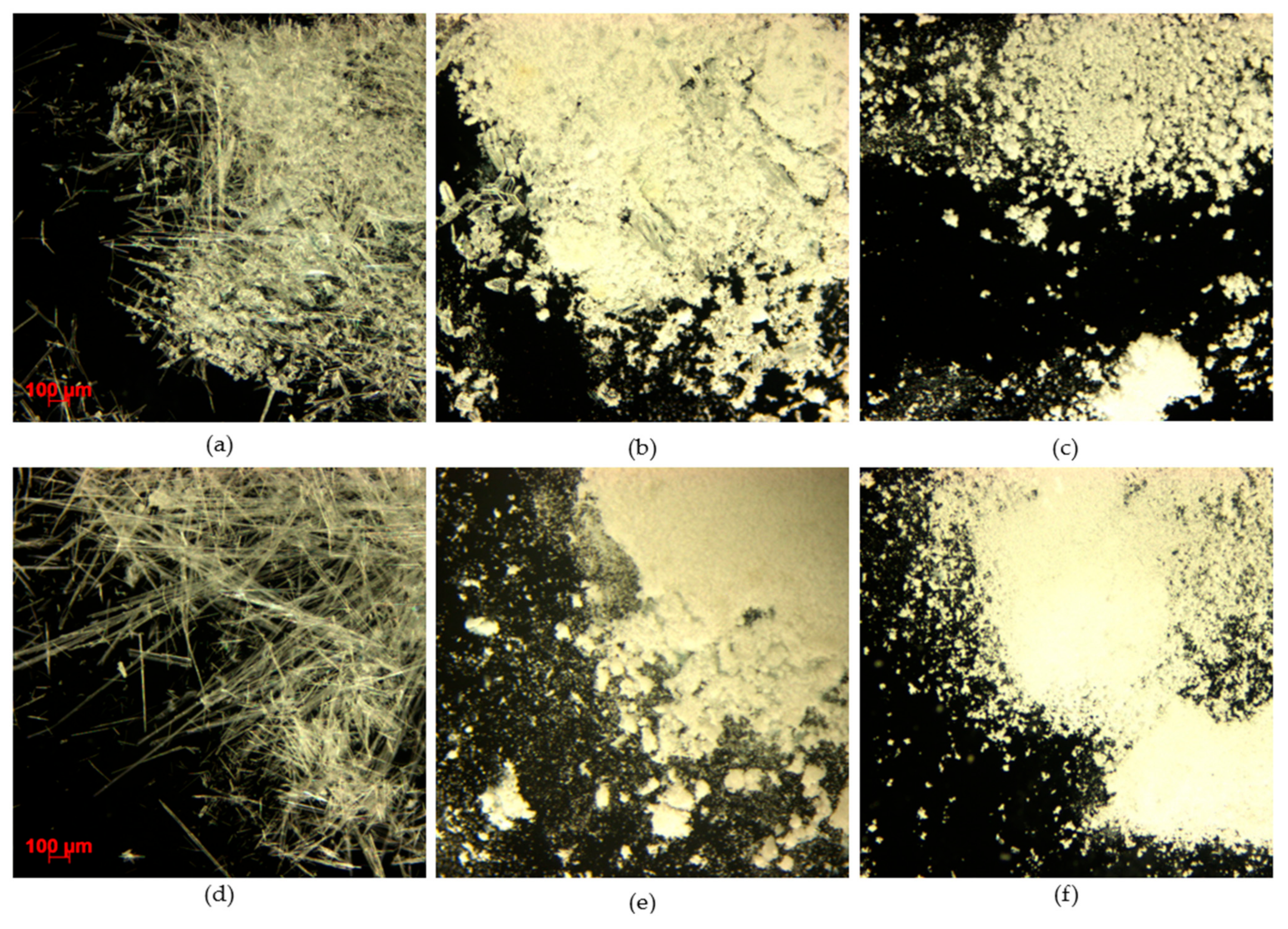
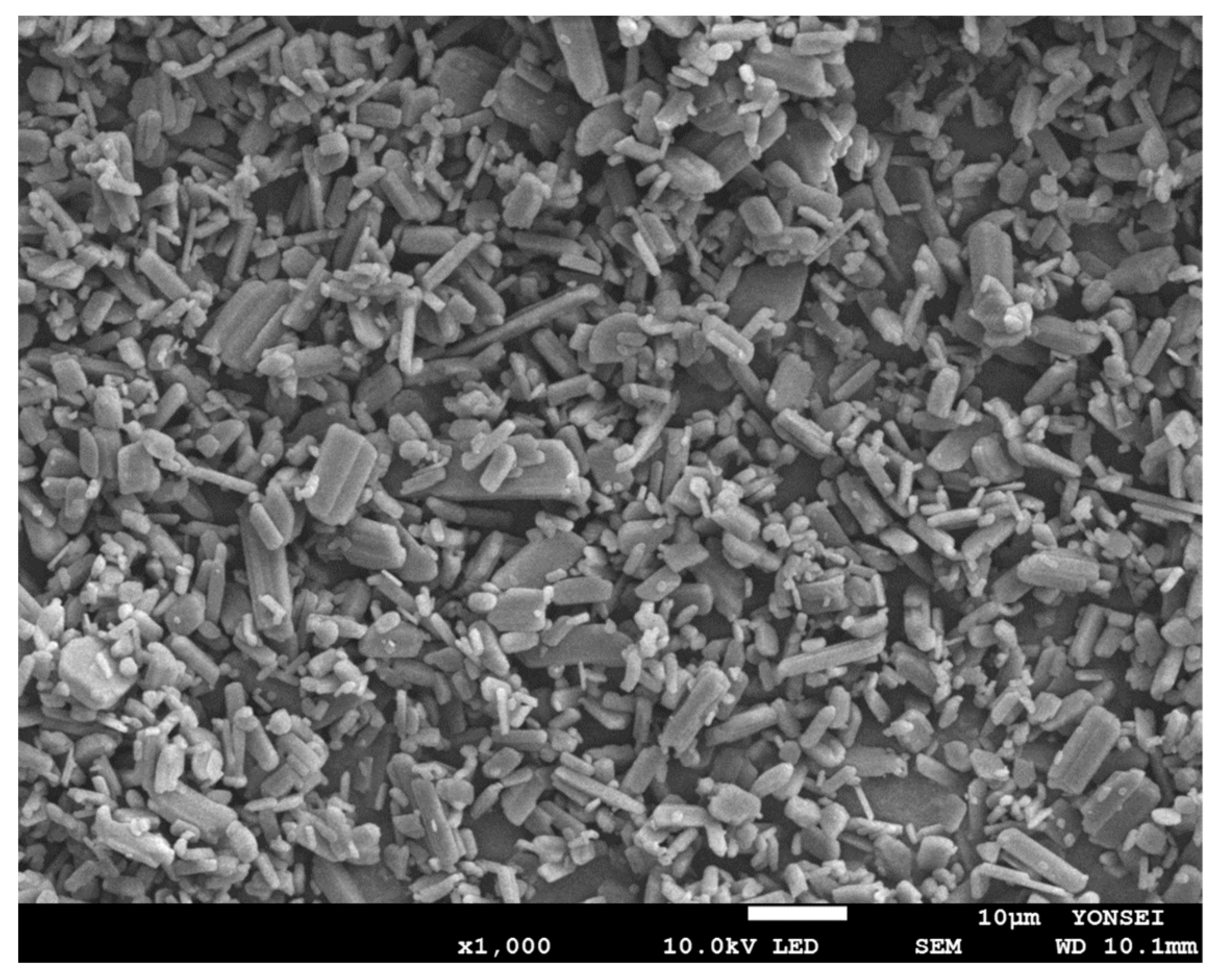
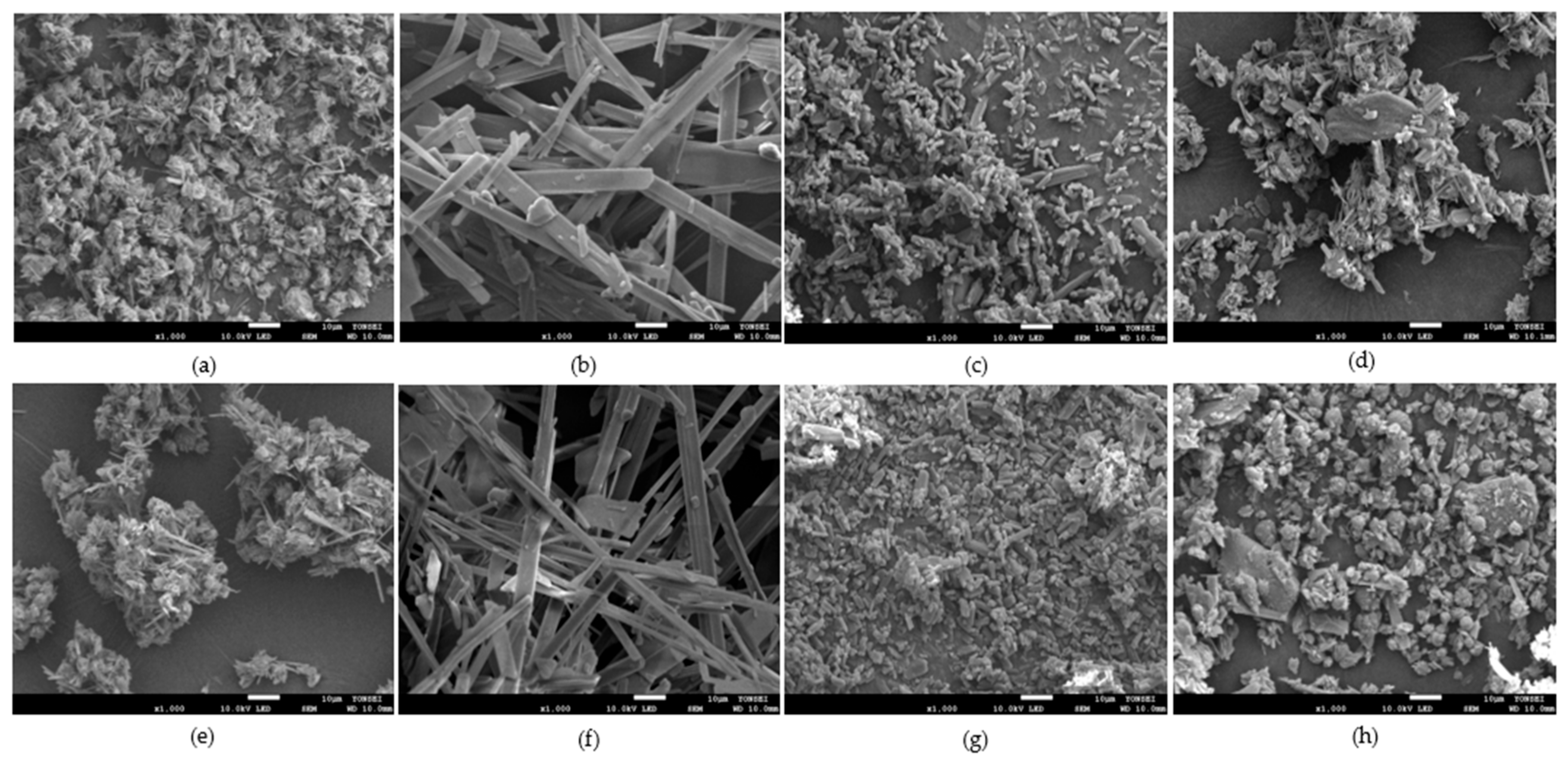
3.2.5. Particle Size and Morphology Analysis
3.2.6. Dissolution Test
3.2.7. Development and Formulation of CEL Immediate Release (IR) Tablet by a Quality by Design (QbD) Approach
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allaj, V.; Guo, C.; Nie, D. Non-steroid anti-inflammatory drugs, prostaglandins, and cancer. Cell Biosci. 2013, 3, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clemett, D.; Goa, K.L. Celecoxib: A review of its use in osteoarthritis, rheumatoid arthritis and acute pain. Drugs 2000, 59, 957–980. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, F.E.; Faich, G.; Goldstein, J.L.; Simon, L.S.; Pincus, T.; Whelton, A.; Makuch, R.; Eisen, G.; Agrawal, N.M.; Stenson, W.F.; et al. Gastrointestinal Toxicity with Celecoxib vs Nonsteroidal Anti-inflammatory Drugs for Osteoarthritis and Rheumatoid Arthritis The CLASS Study: A Randomized Controlled Trial. JAMA 2000, 284, 1247–1255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulson, S.K.; Vaughn, M.B.; Jessen, S.M.; Lawal, Y.; Gresk, C.J.; Yan, B.; Maziasz, T.J.; Cook, C.S.; Karim, A. Pharmacokinetics of celecoxib after oral administration in dogs and humans: Effect of food and site of absorption. J. Pharm. Exp. 2001, 297, 638–645. [Google Scholar]
- Vasconcelos, T.; Sarmento, B.; Costa, P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov. Today Technol. 2007, 12, 1068–1075. [Google Scholar] [CrossRef]
- Hyun, S.-M.; Lee, B.J.; Abuzar, S.M.; Lee, S.; Joo, Y.; Hong, S.-H.; Kang, H.; Kwon, K.-A.; Velaga, S.; Hwang, S.-J. Preparation, characterization, and evaluation of celecoxib eutectic mixtures with adipic acid/saccharin for improvement of wettability and dissolution rate. Int. J. Pharm. 2019, 554, 61–71. [Google Scholar] [CrossRef]
- Stott, P.W.; Williams, A.C.; Barry, B.W. Transdermal delivery from eutectic systems: Enhanced permeation of a model drug, ibuprofen. J. Control. Release 1998, 50, 297–308. [Google Scholar] [CrossRef]
- Leuner, C.; Dressman, J. Improving drug solubility for oral delivery using solid dispersions. Eur. J. Pharm. Biopharm. 2000, 50, 47–60. [Google Scholar] [CrossRef]
- Baumann, J.M.; Adam, M.S.; Wood, J.D. Engineering Advances in Spray Drying for Pharmaceuticals. Annu. Rev. Chem. Biomol. Eng. 2021, 12, 217–240. [Google Scholar] [CrossRef]
- Sun, J.; Wang, F.; Sui, Y.; She, Z.; Zhai, W.; Wang, C.; Deng, Y. Effect of particle size on solubility, dissolution rate, and oral bioavailability: Evaluation using coenzyme Q10 as naked nanocrystals. Int. J. Nanomed. 2012, 7, 5733–5744. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Han, B. Supercritical Carbon Dioxide (CO2) as Green Solvent. In Green Chemistry and Chemical Engineering; Han, B., Wu, T., Eds.; Springer: New York, NY, USA, 2019; pp. 173–197. [Google Scholar]
- Hermans, A.; Abend, A.M.; Kesisoglou, F.; Flanagan, T.; Cohen, M.J.; Diaz, D.A.; Mao, Y.; Zhang, L.; Webster, G.K.; Lin, Y.; et al. Approaches for Establishing Clinically Relevant Dissolution Specifications for Immediate Release Solid Oral Dosage Forms. AAPS J. 2017, 19, 1537–1549. [Google Scholar] [CrossRef]
- Zhang, L.; Mao, S. Application of quality by design in the current drug development. Asian J. Pharm. Sci. 2017, 12, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.X.; Amidon, G.; Khan, M.A.; Hoag, S.W.; Polli, J.; Raju, G.K.; Woodcock, J. Understanding pharmaceutical quality by design. AAPS J. 2014, 16, 771–783. [Google Scholar] [CrossRef] [Green Version]
- Corveleyn, S.; Remon, J.P. Formulation and production of rapidly disintegrating tablets by lyophilisation using hydrochlorothiazide as a model drug. Int. J. Pharm. 1997, 152, 215–225. [Google Scholar] [CrossRef]
- Kushner, J.; Langdon, B.A.; Hicks, I.; Song, D.; Li, F.; Kathiria, L.; Kane, A.; Ranade, G.; Agarwal, K. A quality-by-design study for an immediate-release tablet platform: Examining the relative impact of active pharmaceutical ingredient properties, processing methods, and excipient variability on drug product quality attributes. J. Pharm. Sci. 2014, 103, 527–538. [Google Scholar] [CrossRef]
- Emami, S.; Siahi-Shadbad, M.; Barzegar-Jalali, M.; Adibkia, K. Characterizing eutectic mixtures of gliclazide with succinic acid prepared by electrospray deposition and liquid assisted grinding methods. J. Drug Deliv. Sci. Technol. 2018, 45, 101–109. [Google Scholar] [CrossRef]
- Zhang, S.; Kawakami, K. One-step preparation of chitosan solid nanoparticles by electrospray deposition. Int. J. Pharm. 2010, 397, 211–217. [Google Scholar] [CrossRef]
- Grzybowska, K.; Paluch, M.; Grzybowski, A.; Wojnarowska, Z.; Hawelek, L.; Kolodziejczyk, K.; Ngai, K.L. Molecular dynamics and physical stability of amorphous anti-inflammatory drug: Celecoxib. J. Phys. Chem. B 2010, 114, 12792–12801. [Google Scholar] [CrossRef]
- Thakore, S.D.; Prasad, R.; Dalvi, S.V.; Bansal, A.K. Role of solvent in differential phase behavior of celecoxib during spray drying. Int. J. Pharm. 2020, 585, 119489. [Google Scholar] [CrossRef]
- Li, Q.; Huang, D.; Lu, T.; Seville, J.P.K.; Xing, L.; Leeke, G.A. Supercritical fluid coating of API on excipient enhances drug release. Chem. Eng. J. 2017, 313, 317–327. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-S.; Kim, M.-S.; Park, H.J.; Jin, S.-J.; Lee, S.; Hwang, S.-J. Physicochemical properties and oral bioavailability of amorphous atorvastatin hemi-calcium using spray-drying and SAS process. Int. J. Pharm. 2008, 359, 211–219. [Google Scholar] [CrossRef]
- Park, J.; Park, H.J.; Cho, W.; Cha, K.-H.; Kang, Y.-S.; Hwang, S.-J. Preparation and pharmaceutical characterization of amorphous cefdinir using spray-drying and SAS-process. Int. J. Pharm. 2010, 396, 239–245. [Google Scholar] [CrossRef]
- Debenedetti, P.G.; Tom, J.W.; Kwauk, X.; Yeo, S.D. Rapid expansion of supercritical solutions (ress): Fundamentals and applications. Fluid Phase Equilib. 1993, 82, 311–321. [Google Scholar] [CrossRef]
- Tsai, C.-C.; Lin, H.-M.; Lee, M.-J. Solubility of niflumic acid and celecoxib in supercritical carbon dioxide. J. Supercrit. Fluids 2014, 95, 17–23. [Google Scholar] [CrossRef]
- Isothermal Properties for Carbon Dioxide, NIST Chemistry WebBook, NIST Standard Reference Database No. 69; March 2003 Release; National Institute of Standard and Technology: Gaithersburg, MD, USA, 2003. Available online: http://webbook.nist.gov/chemistry/ (accessed on 28 May 2022).
- Du, M.; Feng, B.; An, H.; Liu, W.; Zhang, L. Effect of addition of weak acids on CO2 desorption from rich amine solvents. Korean J. Chem. Eng. 2012, 29, 362–368. [Google Scholar] [CrossRef]
- Al-Ibraheemi, Z.A.M.; Anuar, M.S.; Taip, F.S.; Amin, M.C.I.; Tahir, S.M.; Mahdi, A.B. Deformation and Mechanical Characteristics of Compacted Binary Mixtures of Plastic (Microcrystalline Cellulose), Elastic (Sodium Starch Glycolate), and Brittle (Lactose Monohydrate) Pharmaceutical Excipients. Part. Sci. Technol. 2013, 31, 561–567. [Google Scholar] [CrossRef]
- Puckhaber, D.; Finke, J.H.; David, S.; Serratoni, M.; Zafar, U.; John, E.; Juhnke, M.; Kwade, A. Prediction of the impact of lubrication on tablet compactibility. Int. J. Pharm. 2022, 617, 121557. [Google Scholar] [CrossRef]
- Rowe, R.C.; Sheskey, P.J.; Owen, S.C. (Eds.) Handbook of Pharmaceutical Excipients, 6th ed.; Pharmaceutical Press: London, UK; American Pharmacists Association: Chicago, IL, USA; Washington, DC, USA, 2009. [Google Scholar]
- Celecoxib Dissolution Method, Dissolution Methods Database. FDA U.S. Food & Drug Administration. Content Current as of: 29 March 2016. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/dissolution-methods-database (accessed on 28 May 2022).




| Process | Material | MAs | PPs | Associated CQAs |
|---|---|---|---|---|
| Mixing | API + co-former (EM) Disintegrants Fillers Surfactant | Particle size Flowability Uniformity Moisture content | Order of addition Hold time Environment (temperature and humidity) | Flowability Uniformity |
| Mixing | API + co-former (EM) Disintegrants Fillers Surfactant + Lubricant | Particle size Flowability Uniformity Moisture content | Order of addition Hold time Environment (temperature and humidity) | Flowability Compressibility Uniformity Disintegration Dissolution |
| Direct compresion | API + co-former (EM) Disintegrants Fillers Surfactant + Lubricant | Particle size Compressibility Uniformity | Compressor type Compression force Rotation speed Environment (temperature and humidity) | Appearance (Size and shape) Weight Thickness Hardness Friability Content uniformity Disintegration Dissolution |
| Quality Attributes (QAs) | Target | Critically |
|---|---|---|
| Dosage form | Solid oral IR tablet containing 100 mg of CEL (API) | |
| Appearance | Suitable size and shape | Critical (related to compressibility, thickness, hardness and patient acceptability) |
| Hardness | 6–10 kp | Critical (able to withstand transport, handling, storage) |
| Friability | ≤1% w/w | Critical (low friability leads to higher hardness of tablets) |
| Moisture content | ≤1% | Not critical, API is not sensitive to hydrolysis |
| Dissolution | Dissolution acceptance criteria: Q ≥ 80% in 15 min (The International Conference on Harmonization (ICH) Q6A guideline) | Critical (IR tablets enabling Tmax in less than 2 h) |
| Disintegration | 2.5 to 10 min [15] | Critical (for IR tablets, related to dissolution, disintegration is before dissolution can occur). ICH allows disintegration time with an upper time limit to be used as the drug release acceptance criteria if Q ≥ 80% is achieved in 15 min at pH 1.2, 4.0, and 6.8. |
| Assay | 95–105% | Critical |
| Content uniformity | Meets USP requirements | Critical |
| QAs | Variables | |||
|---|---|---|---|---|
| API Particle Size | Filler | Disintegrant | Lubricant | |
| Appearance | Low | Low | Low | Low |
| Content uniformity | Medium | Medium | Low | Medium |
| Degradation | Low | Low | Low | Low |
| Disintegration | Medium | Medium | High | High |
| Dissolution | High | Medium | High | High |
| Friability | Low | High | Low | Medium |
| Stability | Low | Medium | Low | Low |
| Ingredient(s) g (%) | Run 1 | Run 2 | Run 3 | Run 4 | Run 5 | Run 6 | Run 7 | Run 8 | Run 9 | Run 10 | Run 11 | Run 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| API | CEL | 100 (25) | 100 (25) | 100 (25) | 100 (25) | 100 (25) | 100 (25) | 100 (25) | 100 (25) | 100 (25) | 100 (25) | 100 (25) | 100 (25) |
| co-former | ADI | 89.75 (22.4) | 89.75 (22.4) | 89.75 (22.4) | 89.75 (22.4) | 89.75 (22.4) | 89.75 (22.4) | 89.75 (22.4) | 89.75 (22.4) | 89.75 (22.4) | 89.75 (22.4) | 89.75 (22.4) | 89.75 (22.4) |
| Filler | lactose monohydrate (75%) & microcrystalline cellulose (25%) | 142.65 (35.7) | 142.65 (35.7) | 142.65 (35.7) | 142.65 (35.7) | 142.65 (35.7) | 142.65 (35.7) | 142.65 (35.7) | 142.65 (35.7) | 142.65 (35.7) | 142.65 (35.7) | 142.65 (35.7) | 142.65 (35.7) |
| Disintegrant | SSG | 32 (8) | - | 64 (16) | - | - | - | - | 21.3 (5.3) | 64 (16) | 32 (8) | 21.3 (5.3) | 32 (8) |
| Disintegrant | crosslinked NaCMC (Ac-Di-Sol®) | - | - | - | - | 64 (16) | 64 (16) | 32 (8) | 21.3 (5.3) | - | - | 21.3 (5.3) | 32 (8) |
| Disintegrant | crosslinked PVP (Kollidon® CL) | 32 (8) | 64 (16) | - | 64 (16) | - | - | 32 (8) | 21.3 (5.3) | - | 32 (8) | 21.3 (5.3) | - |
| Surfactant | SLS | 1.6 (0.4) | 1.6 (0.4) | 1.6 (0.4) | 1.6 (0.4) | 1.6 (0.4) | 1.6 (0.4) | 1.6 (0.4) | 1.6 (0.4) | 1.6 (0.4) | 1.6 (0.4) | 1.6 (0.4) | 1.6 (0.4) |
| Lubricant | Mg-stearate | 2 (0.5) | 2 (0.5) | 2 (0.5) | 2 (0.5) | 2 (0.5) | 2 (0.5) | 2 (0.5) | 2 (0.5) | 2 (0.5) | 2 (0.5) | 2 (0.5) | 2 (0.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, S.-H.; Dinh, L.; Abuzar, S.M.; Lee, E.S.; Hwang, S.-J. Synthesis of Celecoxib-Eutectic Mixture Particles via Supercritical CO2 Process and Celecoxib Immediate Release Tablet Formulation by Quality by Design Approach. Pharmaceutics 2022, 14, 1549. https://doi.org/10.3390/pharmaceutics14081549
Hong S-H, Dinh L, Abuzar SM, Lee ES, Hwang S-J. Synthesis of Celecoxib-Eutectic Mixture Particles via Supercritical CO2 Process and Celecoxib Immediate Release Tablet Formulation by Quality by Design Approach. Pharmaceutics. 2022; 14(8):1549. https://doi.org/10.3390/pharmaceutics14081549
Chicago/Turabian StyleHong, Seung-Hyeon, Linh Dinh, Sharif Md Abuzar, Eun Seok Lee, and Sung-Joo Hwang. 2022. "Synthesis of Celecoxib-Eutectic Mixture Particles via Supercritical CO2 Process and Celecoxib Immediate Release Tablet Formulation by Quality by Design Approach" Pharmaceutics 14, no. 8: 1549. https://doi.org/10.3390/pharmaceutics14081549
APA StyleHong, S.-H., Dinh, L., Abuzar, S. M., Lee, E. S., & Hwang, S.-J. (2022). Synthesis of Celecoxib-Eutectic Mixture Particles via Supercritical CO2 Process and Celecoxib Immediate Release Tablet Formulation by Quality by Design Approach. Pharmaceutics, 14(8), 1549. https://doi.org/10.3390/pharmaceutics14081549








