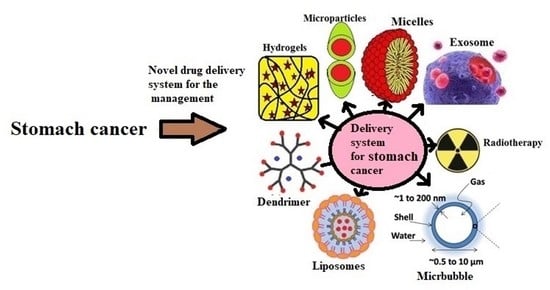
Novel Drug Delivery Systems as an Emerging Platform for Stomach Cancer Therapy
Abstract
1. Introduction
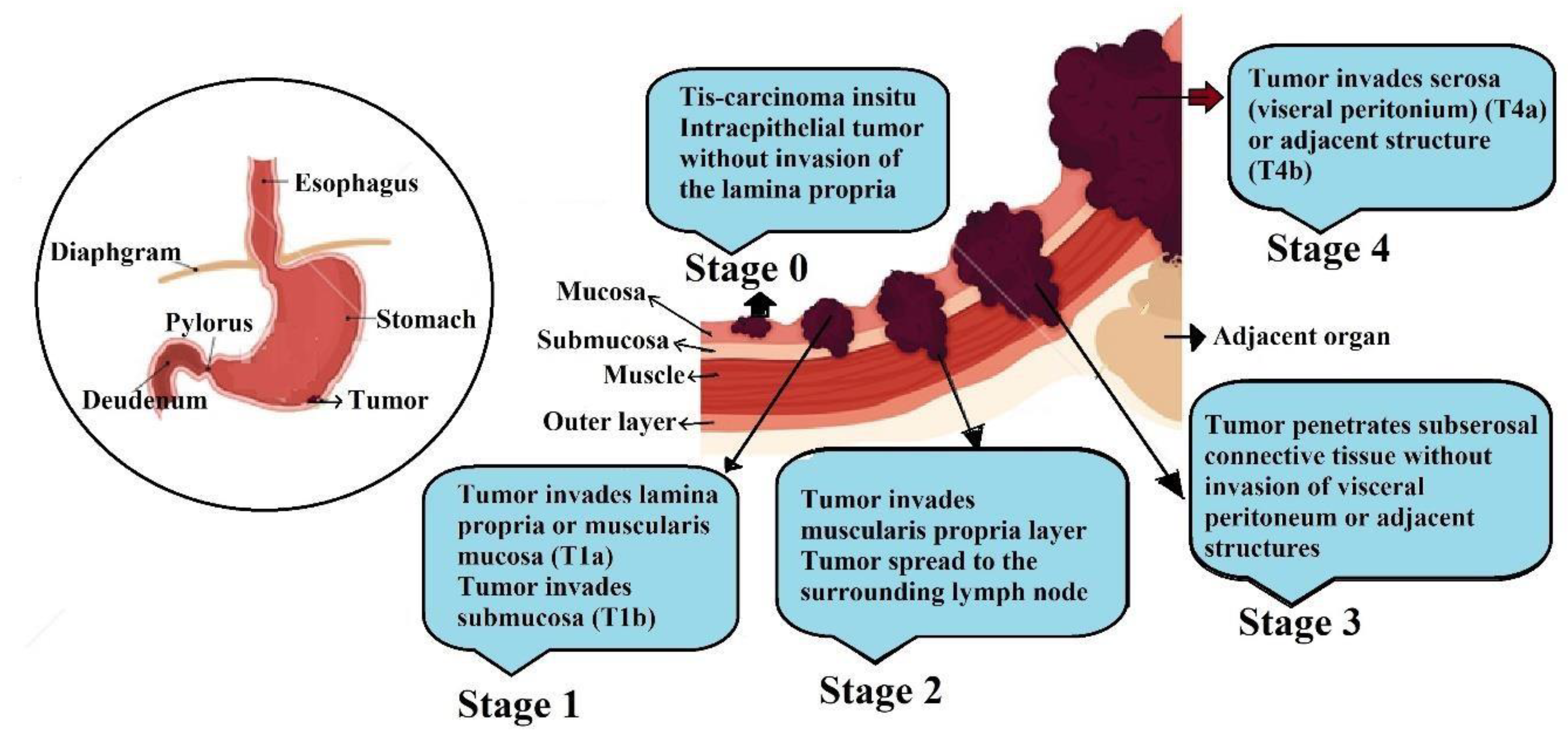
2. Pathophysiology
3. Diagnosis and Therapies
3.1. Diagnosis
3.2. Chemotherapy
3.3. Immunotherapy
3.4. Radiation Therapy
4. Novel Drug Delivery Systems for Gastric Cancer Treatment
4.1. Nanotechnology Based Drug Delivery Systems
4.1.1. Nanoparticles
4.1.2. Polymeric Nanoparticles
4.1.3. Metallic Nanoparticles
4.1.4. Metal-Polymer Composite Nanoparticles
4.1.5. Miscellaneous Nanoparticles
4.2. Dendrimers
4.3. Exosome
4.4. Liposomes
4.5. Polymeric Micelles
5. Other Delivery Systems
5.1. Hydrogels
5.2. Microbubbles
5.3. Microparticles
5.4. Oral Delivery
6. Conclusions and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef]
- Yamaoka, Y. How to eliminate gastric cancer-related death worldwide? Nat. Rev. Clin. Oncol. 2018, 15, 407–408. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Herrero, R. Recent progress in gastric cancer prevention. Best Pract. Res. Clin. Gastroenterol. 2021, 50–51, 101733. [Google Scholar] [CrossRef] [PubMed]
- Kadar, Z.; Jung, I.; Orlowska, J.; Szentirmay, Z.; Sugimura, H.; Turdean, S.; Simona, G. Geographic particularities in incidence and etiopathogenesis of sporadic gastric cancer. Pol. J. Pathol. 2015, 66, 254–259. [Google Scholar] [CrossRef]
- Yamaoka, Y.; Kato, M.; Asaka, M. Geographic differences in gastric cancer incidence can be explained by differences between helicobacter pylori strains. Intern. Med. 2008, 47, 1077–1083. [Google Scholar] [CrossRef]
- Karimi, P.; Islami, F.; Anandasabapathy, S.; Freedman, N.D.; Kamangar, F. Gastric cancer: Descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol. Biomark. Prev. 2014, 23, 700–713. [Google Scholar] [CrossRef]
- Bornschein, J.; Selgrad, M.; Warnecke, M.; Kuester, D.; Wex, T.; Malfertheiner, P. H. pylori infection is a key risk factor for proximal gastric cancer. Dig. Dis. Sci. 2010, 55, 3124–3131. [Google Scholar] [CrossRef]
- Khan, G.; Hashim, M.J. Global burden of deaths from epstein-barr virus attributable malignancies 1990–2010. Infect. Agents Cancer 2014, 9, 38. [Google Scholar] [CrossRef]
- Correa, P. Human gastric carcinogenesis: A multistep and multifactorial process—First american cancer society award lecture on cancer epidemiology and prevention. Cancer Res. 1992, 52, 6735–6740. [Google Scholar] [PubMed]
- Van Cutsem, E.; Sagaert, X.; Topal, B.; Haustermans, K.; Prenen, H. Gastric cancer. Lancet 2016, 388, 2654–2664. [Google Scholar] [CrossRef]
- Nam, S.Y.; Park, B.J.; Ryu, K.H.; Nam, J.H. Effect of Helicobacter pylori eradication on the regression of gastric polyps in national cancer screening program. Korean J. Intern. Med. 2018, 33, 506–511. [Google Scholar] [CrossRef]
- McCullough, M.L.; Robertson, A.S.; Jacobs, E.J.; Chao, A.; Calle, E.E.; Thun, M.J. A prospective study of diet and stomach cancer mortality in United States men and women. Cancer. Epidemiol. Biomark. Prev. 2001, 10, 1201–1205. [Google Scholar]
- Ngoan, L.T.; Mizoue, T.; Fujino, Y.; Tokui, N.; Yoshimura, T. Dietary factors and stomach cancer mortality. Br. J. Cancer 2002, 87, 37–42. [Google Scholar] [CrossRef]
- Strumylaitė, L.; Žičkutė, J.; Dudzevičius, J.; Dregval, L. Salt-preserved foods and risk of gastric cancer. Medicina 2006, 42, 164–170. [Google Scholar] [PubMed]
- Toyoda, T.; Tsukamoto, T.; Yamamoto, M.; Ban, H.; Saito, N.; Takasu, S.; Shi, L.; Saito, A.; Ito, S.; Yamamura, Y.; et al. Gene expression analysis of a Helicobacter pylori-infected and high-salt diet-treated mouse gastric tumor model: Identification of CD177 as a novel prognostic factor in patients with gastric cancer. BMC Gastroenterol. 2013, 13, 122. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Li, K.N.; Bi, J.W.; Wang, B.C. Sodium intake, salt taste and gastric cancer risk according to Helicobacter pylori infection, smoking, histological type and tumor site in china. Asian Pac. J. Cancer Prev. 2012, 13, 2481–2484. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Echizen, K.; Horiuchi, K.; Aoki, Y.; Yamada, Y.; Minamoto, T.; Oshima, H.; Oshima, M. NF-κB-induced NOX1 activation promotes gastric tumorigenesis through the expansion of SOX2-positive epithelial cells. Oncogene 2019, 38, 4250–4263. [Google Scholar] [CrossRef]
- Koh, T.; Wang, T. Tumors of the stomach. Sleisenger & Fordtran’s Gastrointestinal and Liver Disease, 7th ed.; Saunders: Philadelphia, PA, USA, 2002; pp. 829–844. [Google Scholar]
- Cappell, M.S.; Friedel, D. The role of esophagogastroduodenoscopy in the diagnosis and management of upper gastrointestinal disorders. Med. Clin. N. Am. 2002, 86, 1165–1216. [Google Scholar] [CrossRef]
- Low, V.H.; Levine, M.S.; Rubesin, S.E.; Laufer, I.; Herlinger, H. Diagnosis of gastric carcinoma: Sensitivity of double-contrast barium studies. AJR Am. J. Roentgenol. 1994, 162, 329–334. [Google Scholar] [CrossRef]
- Grundmann, R.T.; Hölscher, A.H.; Bembenek, A.; Bollschweiler, E.; Drognitz, O.; Feuerbach, S.; Gastinger, I.; Hermanek, P.; Hopt, U.T.; Hünerbein, M.; et al. Diagnosis of and therapy for gastric cancer--work-flow. Zentralbl. Chir. 2009, 134, 362–374. [Google Scholar] [CrossRef]
- Matsumoto, S.; Ishikawa, S.; Yoshida, Y. Reduction of gastric cancer mortality by endoscopic and radiographic screening in an isolated island: A retrospective cohort study. Aust. J. Rural Health 2013, 21, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Hamashima, C.; Ogoshi, K.; Okamoto, M.; Shabana, M.; Kishimoto, T.; Fukao, A. A community-based, case-control study evaluating mortality reduction from gastric cancer by endoscopic screening in Japan. PLoS ONE 2013, 8, e79088. [Google Scholar] [CrossRef] [PubMed]
- Beeharry, M.K.; Liu, W.T.; Yan, M.; Zhu, Z.G. New blood markers detection technology: A leap in the diagnosis of gastric cancer. World J. Gastroenterol. 2016, 22, 1202–1212. [Google Scholar] [CrossRef]
- Shimada, H.; Noie, T.; Ohashi, M.; Oba, K.; Takahashi, Y. Clinical significance of serum tumor markers for gastric cancer: A systematic review of literature by the task force of the Japanese gastric cancer association. Gastric Cancer 2014, 17, 26–33. [Google Scholar] [CrossRef]
- Asao, T.; Fukuda, T.; Yazawa, S.; Nagamachi, Y. Carcinoembryonic antigen levels in peritoneal washings can predict peritoneal recurrence after curative resection of gastric cancer. Cancer 1991, 68, 44–47. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Xu, J.; Luo, G.H.; Wang, R.C.; Zhu, J.; Zhang, X.Y.; Nilsson-Ehle, P.; Xu, N. Detection of carcinoembryonic antigen mRNA in peritoneal washes from gastric cancer patients and its clinical significance. World J. Gastroenterol. 2006, 12, 1408–1411. [Google Scholar] [CrossRef]
- Feng, F.; Tian, Y.; Xu, G.; Liu, Z.; Liu, S.; Zheng, G.; Guo, M.; Lian, X.; Fan, D.; Zhang, H. Diagnostic and prognostic value of CEA, CA19–9, AFP AND CA125 for early gastric cancer. BMC Cancer 2017, 17, 737. [Google Scholar] [CrossRef]
- Kochi, M.; Fujii, M.; Kanamori, N.; Kaiga, T.; Kawakami, T.; Aizaki, K.; Kasahara, M.; Mochizuki, F.; Kasakura, Y.; Yamagata, M. Evaluation of serum CEA AND CA19-9 levels as prognostic factors in patients with gastric cancer. Gastric Cancer 2000, 3, 177–186. [Google Scholar] [CrossRef]
- Sisik, A.; Kaya, M.; Bas, G.; Basak, F.; Alimoglu, O. CEA and CA 19–9 are still valuable markers for the prognosis of colorectal and gastric cancer patients. Asian Pac. J. Cancer Prev. 2013, 14, 4289–4294. [Google Scholar] [CrossRef]
- Zhou, Y.C.; Zhao, H.J.; Shen, L.Z. Preoperative serum cea and ca19-9 in gastric cancer-a single tertiary hospital study of 1075 cases. Asian Pac. J. Cancer Prev. 2015, 16, 2685–2691. [Google Scholar] [CrossRef]
- Sawayama, H.; Iwatsuki, M.; Kuroda, D.; Toihata, T.; Uchihara, T.; Koga, Y.; Yagi, T.; Kiyozumi, Y.; Eto, T.; Hiyoshi, Y.; et al. The association of the lymph node ratio and serum carbohydrate antigen 19–9 with early recurrence after curative gastrectomy for gastric cancer. Surg. Today 2018, 48, 994–1003. [Google Scholar] [CrossRef]
- Hasbahceci, M.; Malya, F.U.; Kunduz, E.; Guzel, M.; Unver, N.; Akcakaya, A. Use of serum and peritoneal CEA and CA19-9 in prediction of peritoneal dissemination and survival of gastric adenocarcinoma patients: Are they prognostic factors? Ann. R. Coll. Surg. Engl. 2018, 100, 257–266. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, N. Clinical evaluation of CEA, CA19-9, CA72-4 and CA125 in gastric cancer patients with neoadjuvant chemotherapy. World J. Surg. Oncol. 2014, 12, 397. [Google Scholar] [CrossRef]
- Song, Y.X.; Huang, X.Z.; Gao, P.; Sun, J.X.; Chen, X.W.; Yang, Y.C.; Zhang, C.; Liu, H.P.; Wang, H.C.; Wang, Z.N. Clinicopathologic and prognostic value of serum carbohydrate antigen 19–9 in gastric cancer: A meta-analysis. Dis. Markers 2015, 2015, 549843. [Google Scholar] [CrossRef]
- Marrelli, D.; Pinto, E.; De Stefano, A.; Farnetani, M.; Garosi, L.; Roviello, F. Clinical utility of CEA, CA 19–9, and CA 72–4 in the follow-up of patients with resectable gastric cancer. Am. J. Surg. 2001, 181, 16–19. [Google Scholar] [CrossRef]
- Yu, J.; Zheng, W. An alternative method for screening gastric cancer based on serum levels of CEA, CA19-9, and CA72-4. J. Gastrointest. Cancer 2018, 49, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Wang, H.; Wei, K.; Zhang, J.; You, C. Five common tumor biomarkers and CEA for diagnosing early gastric cancer: A protocol for a network meta-analysis of diagnostic test accuracy. Medicine 2018, 97, e0577. [Google Scholar] [CrossRef] [PubMed]
- Machlowska, J.; Baj, J.; Sitarz, M.; Maciejewski, R.; Sitarz, R. Gastric cancer: Epidemiology, risk factors, classification, genomic characteristics and treatment strategies. Int. J. Mol. Sci. 2020, 21, 4012. [Google Scholar] [CrossRef] [PubMed]
- Osmani, R.A.M.; Hani, U.; Bhosale, R.R.; Kulkarni, P.K.; Shanmuganathan, S. Nanosponge carriers—An archetype swing in cancer therapy: A comprehensive review. Curr. Drug Targets. 2017, 18, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Taylor, W.E.; Donohue, J.H.; Gunderson, L.L.; Nelson, H.; Nagorney, D.M.; Devine, R.M.; Haddock, M.G.; Larson, D.R.; Rubin, J.; O’Connell, M.J. The mayo clinic experience with multimodality treatment of locally advanced or recurrent colon cancer. Ann. Surg. Oncol. 2002, 9, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Sato, T.; Yamada, T.; Aoyama, T.; Ogata, T.; Cho, H. Neoadjuvant chemotherapy for gastric cancer, Gan to Kagaku Ryoho. Cancer. Chemother. 2016, 43, 1157–1160. [Google Scholar]
- Chang, A.Y.-C.; Foo, K.F.; Koo, W.H.; Ong, S.; So, J.; Tan, D.; Lim, K.H. Phase II study of neo-adjuvant chemotherapy for locally advanced gastric cancer. BMJ Open Gastroenterol. 2016, 3, e000095. [Google Scholar] [CrossRef]
- Lv, X.; Zhang, L.; Huang, R.; Song, W. A clinical exploration of neoadjuvant chemotherapy with tegafur, gimeracil, and oteracil potassium capsules combined with oxaliplatin for advanced gastric cancer. Int. J. Clin. Exp. Med. 2015, 8, 19030–19036. [Google Scholar] [PubMed]
- Chen, Y.; Guo, Z.Q.; Shi, C.M.; Zhou, Z.F.; Ye, Y.B.; Chen, Q. Efficacy of adjuvant chemotherapy combined with immunotherapy with cytokine-induced killer cells for gastric cancer after d2 gastrectomy. Int. J. Clin. Exp. Med. 2015, 8, 7728–7736. [Google Scholar] [PubMed]
- Neves Filho, E.H.C.; de Sant’Ana, R.O.; Nunes, L.V.S.C.; Pires, A.P.B.; da Cunha, M.D. Histopathological regression of gastric adenocarcinoma after neoadjuvant therapy: A critical review. APMIS 2017, 125, 79–84. [Google Scholar] [CrossRef]
- Funaki, H.; Fujii, Y.; Miura, S.; Fujita, J.; Morioka, E.; Kaida, D.; Ohnishi, T.; Tomita, Y.; Noguchi, M.; Fujita, H.; et al. Treatment outcomes of advanced gastric cancer after neoadjuvant chemotherapy with S-1 and cisplatin. Gan To Kagaku Ryoho 2016, 43, 1421–1423. [Google Scholar]
- Song, Z.; Wu, Y.; Yang, J.; Yang, D.; Fang, X. Progress in the treatment of advanced gastric cancer. Tumour Biol. 2017, 39, 1010428317714626. [Google Scholar] [CrossRef]
- Wilke, H.; Preusser, P.; Fink, U.; Gunzer, U.; Meyer, H.J.; Meyer, J.; Siewert, J.R.; Achterrath, W.; Lenaz, L.; Knipp, H. Preoperative chemotherapy in locally advanced and nonresectable gastric cancer: A phase II study with etoposide, doxorubicin, and cisplatin. J. Clin. Oncol. 1989, 7, 1318–1326. [Google Scholar] [CrossRef]
- Crookes, P.; Leichman, C.G.; Leichman, L.; Tan, M.; Laine, L.; Stain, S.; Baranda, J.; Casagrande, Y.; Groshen, S.; Silberman, H. Systemic chemotherapy for gastric carcinoma followed by postoperative intraperitoneal therapy: A final report. Cancer 1997, 79, 1767–1775. [Google Scholar] [CrossRef]
- Mai, M.; Takahashi, Y.; Fujimoto, T.; Omote, K. Neoadjuvant chemotherapy for far-advanced gastric carcinoma. Gan To Kagaku Ryoho 1994, 21, 431–439. [Google Scholar]
- Gianni, L.; Panzini, I.; Tassinari, D.; Mianulli, A.M.; Desiderio, F.; Ravaioli, A.; Floriani, I.; Dell’Anna, T.; Mari, E.; Torri, V. Meta-analyses of randomized trials of adjuvant chemotherapy in gastric cancer. Ann. Oncol. 2001, 12, 1179–1180. [Google Scholar] [CrossRef]
- Schuhmacher, C.P.; Fink, U.; Becker, K.; Busch, R.; Dittler, H.J.; Mueller, J.; Siewert, J.R. Neoadjuvant therapy for patients with locally advanced gastric carcinoma with etoposide, doxirubicin, and cisplatinum: Closing results after 5 years of follow-up. Cancer 2001, 91, 918–927. [Google Scholar] [CrossRef]
- Mirza, A.; Pritchard, S.; Welch, I. The postoperative component of magic chemotherapy is associated with improved prognosis following surgical resection in gastric and gastrooesophageal junction adenocarcinomas. Int. J. Surg. Oncol. 2013, 2013, 781742. [Google Scholar] [CrossRef]
- Boige, V.; Pignon, J.; Saint-Aubert, B.; Lasser, P.; Conroy, T.; Bouché, O.; Segol, P.; Bedenne, L.; Rougier, P.; Ychou, M. Final results of a randomized trial comparing preoperative 5-fluorouracil (F)/cisplatin (P) to surgery alone in adenocarcinoma of stomach and lower esophagus (ASLE): FNLCC ACCORD07-FFCD 9703 trial. J. Clin. Oncol. 2007, 25, 4510. [Google Scholar] [CrossRef]
- Kim, J.S.; Kang, S.H.; Moon, H.S.; Sung, J.K.; Jeong, H.Y.; Sul, J.Y. Clinical outcome of doublet and triplet neoadjuvant chemotherapy for locally advanced gastric cancer. Korean J. Gastroenterol. 2016, 68, 245–252. [Google Scholar] [CrossRef][Green Version]
- Das, M. Neoadjuvant chemotherapy: Survival benefit in gastric cancer. Lancet Oncol. 2017, 18, e307. [Google Scholar] [CrossRef]
- GASTRIC (Global Advanced/Adjuvant Stomach Tumor Research International Collaboration) Group; Paoletti, X.; Oba, K.; Burzykowski, T.; Michiels, S.; Ohashi, Y.; Pignon, J.P.; Rougier, P.; Sakamoto, J.; Sargent, D.; et al. Benefit of adjuvant chemotherapy for resectable gastric cancer: A meta-analysis. JAMA 2010, 303, 1729–1737. [Google Scholar]
- van den Ende, T.; Ter Veer, E.; Machiels, M.; Mali, R.M.A.; Abe Nijenhuis, F.A.A.; de Waal, L.; Laarman, M.; Gisbertz, S.S.; Hulshof, M.C.C.M.; van Oijen, M.G.H.; et al. The efficacy and safety of (neo) adjuvant therapy for gastric cancer: A network meta-analysis. Cancers 2019, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.D.; Black, D.; Sugarbaker, P.H.; Zhu, J.; Yonemura, Y.; Petrou, G.; Morris, D.L. A systematic review and meta-analysis of the randomized controlled trials on adjuvant intraperitoneal chemotherapy for resectable gastric cancer. Ann. Surg. Oncol. 2007, 14, 2702–2713. [Google Scholar] [CrossRef]
- Ajani, J.A.; Bentrem, D.J.; Besh, S.; D’Amico, T.A.; Das, P.; Denlinger, C.; Fakih, M.G.; Fuchs, C.S.; Gerdes, H.; Glasgow, R.E.; et al. National comprehensive cancer network. J. Natl. Compr. Canc. Netw. 2013, 11, 531–546. [Google Scholar] [CrossRef]
- Okines, A.; Verheij, M.; Allum, W.; Cunningham, D.; Cervantes, A. ESMO guidelines working group. gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2010, 21 (Suppl. S5), v50–v54. [Google Scholar] [CrossRef] [PubMed]
- Pellino, A.; Riello, E.; Nappo, F.; Brignola, S.; Murgioni, S.; Djaballah, S.A.; Lonardi, S.; Zagonel, V.; Rugge, M.; Loupakis, F.; et al. Targeted therapies in metastatic gastric cancer: Current knowledge and future perspectives. World J. Gastroenterol. 2019, 25, 5773–5788. [Google Scholar] [CrossRef]
- Vrána, D.; Matzenauer, M.; Neoral, Č.; Aujeský, R.; Vrba, R.; Melichar, B.; Rušarová, N.; Bartoušková, M.; Jankowski, J. From tumor immunology to immunotherapy in gastric and esophageal cancer. Int. J. Mol. Sci. 2018, 20, 13. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Li, M.; Zhou, R.; Zhang, J.; Sun, H.; Shi, M.; Bin, J.; Li, Y.; Rao, J.; Liao, W. Tumor microenvironment Characterization in gastric cancer identifies prognostic and IM-534 munotherapeutically relevant gene signatures. Cancer Immunol. Resour. 2019, 7, 737–750. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Cao, L.; Guan, L.; Bie, L.; Wang, S.; Xie, B.; Chen, X.; Shen, X.; Cao, F. Immunotherapy for gastric cancer: Dilemmas and prospect. Brief. Funct. Genom. 2019, 18, 107–112. [Google Scholar] [CrossRef]
- Coutzac, C.; Pernot, S.; Chaput, N.; Zaanan, A. Immunotherapy in advanced gastric cancer, is it the future? Crit. Rev. Oncol. Hematol. 2019, 133, 25–32. [Google Scholar] [CrossRef]
- Kelly, R.J. Immunotherapy for Esophageal and Gastric Cancer. In American Society of Clinical Oncology Educational Book; ASCO Publishers: Alexandria, VA, USA, 2017; Volume 37, pp. 292–300. [Google Scholar]
- Gerson, J.N.; Skariah, S.; Denlinger, C.S.; Astsaturov, I. Perspectives of HER2-targeting in gastric and esophageal cancer. Expert Opin. Investig. Drugs 2017, 26, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Johnston, F.M.; Beckman, M. Updates on management of gastric cancer. Curr. Oncol. Rep. 2019, 21, 67. [Google Scholar] [CrossRef]
- De Mello, R.A.; Lordick, F.; Muro, K.; Janjigian, Y.Y. Current and Future Aspects of Immunotherapy for Esophageal and Gastric Malignancies. In American Society of Clinical Oncology Educational Book; ASCO Publishers: Alexandria, VA, USA, 2019; Volume 39, pp. 237–247. [Google Scholar]
- Tey, J.; Soon, Y.Y.; Koh, W.Y.; Leong, C.N.; Choo, B.A.; Ho, F.; Vellayappan, B.; Lim, K.; Tham, I.W. Palliative radiotherapy for gastric cancer: A systematic review and meta-analysis. Oncotarget 2017, 8, 25797–25805. [Google Scholar] [CrossRef]
- Choi, A.H.; Kim, J.; Chao, J. Perioperative chemotherapy for resectable gastric cancer: MAGIC and beyond. World J. Gastroenterol. 2015, 21, 7343–7348. [Google Scholar] [CrossRef]
- Henson, K.E.; Fry, A.; Lyratzopoulos, G.; Peake, M.; Roberts, K.J.; McPhail, S. Sociodemographic variation in the use of chemotherapy and radiotherapy in patients with stage iv lung, oesophageal, stomach and pancreatic cancer: Evidence from population-based data in england during 2013–2014. Br. J. Cancer 2018, 118, 1382–1390. [Google Scholar] [CrossRef]
- Cainap, C.; Vlad, C.; Seicean, A.; Balacescu, O.; Seicean, R.; Constantin, A.M.; Balacescu, L.; Crisan, O.; Marta, M.M.; Cainap, S. Gastric cancer: Adjuvant chemotherapy versus chemoradiation. A clinical point of view. J. BUON 2019, 24, 2209–2219. [Google Scholar]
- Créhange, G.; Huguet, F.; Quero, L.; N’Guyen, T.V.; Mirabel, X.; Lacornerie, T. Radiothérapie des cancers de l’œsophage, du cardia et de l’estomac. Cancer Radiother. 2016, 20, S161–S168. [Google Scholar] [CrossRef]
- Pang, X.; Wei, W.; Leng, W.; Chen, Q.; Xia, H.; Chen, L.; Li, R. Radiotherapy for gastric cancer: A systematic review and meta-analysis. Tumour Biol. 2014, 35, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Yaqoob, A.; Khan, R.; Saddique, A. Review article on applications and classification of gold nanoparticles. Int. J. Respir. 2019, 6, 762–770. [Google Scholar]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric nanoparticles: Production, characterization, toxicology and ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Madkour, L. Nanoparticle and Polymeric Nanoparticle-Based Targeted drug Delivery Systems, Nucleic Acids as Gene Anticancer Drug Delivery Therapy; Academic Press: Cambridge, MA, USA, 2019; pp. 191–240. [Google Scholar]
- Prabha, A.S.; Dorothy, R.; Jancirani, S.; Rajendran, S.; Singh, G.; Kumaran, S.S. Recent Advances in the Study of Toxicity of Polymer-Based Nanomaterials. Nanotoxicity 2020, 143–165. [Google Scholar] [CrossRef]
- Hong, J.; Feng, Z. Synergic fabrication of combination therapy of irinotecan and 5-fluorouracil encapsulated polymeric nanoparticles for the treatment of gastric cancer therapy. Process Biochem. 2021, 106, 191–198. [Google Scholar] [CrossRef]
- Cai, J.; Qian, K.; Zuo, X.; Yue, W.; Bian, Y.; Yang, J.; Wei, J.; Zhao, W.; Qian, H.; Liu, B. PLGA Nanoparticle-based docetaxel/LY294002 drug delivery system enhances antitumor activities against gastric cancer. J. Biomater. Appl. 2019, 33, 1394–1406. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.; Ferreira, D.; Peixoto, A.; Freitas, R.; Relvas-Santos, M.; Palmeira, C.; Martins, G.; Barros, A.; Santos, L.L.; Sarmento, B.; et al. Glycoengineered nanoparticles enhance the delivery of 5-fluoroucil and paclitaxel to gastric cancer cells of high metastatic potential. Int. J. Pharm. 2019, 570, 118646. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Xia, H.; Liang, W.; Huo, X.; Wei, X. Synthesis and characterization of zinc oxide nanoparticles from Morus nigra and its anticancer activity of AGS gastric cancer cells. J. Photochem. Photobiol. B 2020, 202, 111698. [Google Scholar] [CrossRef]
- Sanjeevram, D.; Xu, X.; Wang, R.; Puja, A.M.; Kim, H.; Perumalsamy, H.; Balusamy, S.R.; Kim, Y.-J. Biosynthesis of gold nanoparticles using Nigella sativa and Curtobacterium proimmune K3 and evaluation of their anticancer activity. Mater. Sci. Eng. C 2021, 127, 112214. [Google Scholar]
- Hosseinkhah, M.; Ghasemian, R.; Shokrollahi, F.; Mojdehi, S.R.; Noveiri, M.J.S.; Hedayati, M.; Rezaei, M.; Salehzadeh, A. Cytotoxic potential of nickel oxide nanoparticles functionalized with glutamic acid and conjugated with thiosemicarbazide (NiO@Glu/TSC) against human gastric cancer cells. J. Clust. Sci. 2021, 1–9. [Google Scholar] [CrossRef]
- Yang, C.; Pang, X.; Chen, W.; Wang, X.; Lin, G.; Chu, C.; Zhang, X.; Deng, X.; Chen, X.; Liu, G. Environmentally responsive dual-targeting nanotheranostics for overcoming cancer multidrug resistance. Sci. Bull. 2019, 64, 705–714. [Google Scholar] [CrossRef]
- Wang, Y.; El-Kott, A.F.; El-Kenawy, A.E.; Xue, L. Decorated CuO nanoparticles over chitosan-functionalized magnetic nanoparticles: Investigation of its anti-colon carcinoma and anti-gastric cancer effects. Arab. J. Chem. 2021, 14, 103201. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Z.; Qiu, Y.; Liu, Y.; Ding, M.; Zhang, Y. Anti-miRNA21 and resveratrol-loaded polysaccharide-based mesoporous silica nanoparticle for synergistic activity in gastric carcinoma. J. Drug Target. 2019, 27, 1135–1143. [Google Scholar] [CrossRef]
- Li, D.; Cui, R.; Xu, S.; Liu, Y. Synergism of cisplatin-oleanolic acid co-loaded hybrid nanoparticles on gastric carcinoma cells for enhanced apoptosis and reversed multidrug resistance. Drug Deliv. 2020, 27, 191–199. [Google Scholar]
- Matsuoka, T.; Yashiro, M. The role of PI3K/Akt/mTOR signaling in gastric carcinoma. Cancers 2014, 6, 1441–1463. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Meng, F.; Deng, C.; Zhong, Z. Ligand-directed active tumor-targeting polymeric nanoparticles for cancer chemotherapy. Biomacromolecules 2014, 15, 1955–1969. [Google Scholar] [CrossRef] [PubMed]
- Bayram, B.; Ozgur, A.; Tutar, L.; Tutar, Y. Tumor targeting of polymeric nanoparticles conjugated with peptides, saccharides, and small molecules for anticancer drugs. Curr. Pharm. Des. 2017, 23, 5349–5357. [Google Scholar] [CrossRef] [PubMed]
- Loh, C.Y.; Arya, A.; Naema, A.F.; Wong, W.F.; Sethi, G.; Looi, C.Y. Signal transducer and activator of transcription (STATs) proteins in cancer and inflammation: Functions and therapeutic implication. Front. Oncol. 2019, 9, 48. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Zarrabi, A.; Orouei, S.; Zarrin, V.; Rahmani Moghadam, E.; Zabolian, A.; Mohammadi, S.; Hushmandi, K.; Gharehaghajlou, Y.; Makvandi, P.; et al. STAT3 pathway in gastric cancer: Signaling, therapeutic targeting and future prospects. Biology 2020, 9, 126. [Google Scholar] [CrossRef]
- Yang, H.; Yamazaki, T.; Pietrocola, F.; Zhou, H.; Zitvogel, L.; Ma, Y.; Kroemer, G. STAT3 inhibition enhances the therapeutic efficacy of immunogenic chemotherapy by stimulating type 1 interferon production by cancer cells. Cancer Res. 2015, 75, 3812–3822. [Google Scholar] [CrossRef]
- Sun, C.Y.; Nie, J.; Huang, J.P.; Zheng, G.J.; Feng, B. Targeting STAT3 inhibition to reverse cisplatin resistance. Biomed. Pharmacother. 2019, 117, 109135. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, Z.; Cai, A.; Lin, X.; Jiang, X.; Zhou, B.; Wang, J.; Yao, Q.; Chen, R.; Kou, L. Nanoparticle mediated codelivery of nifuratel and doxorubicin for synergistic anticancer therapy through STAT3 inhibition. Colloids Surf. B Biointerfaces 2020, 193, 111109. [Google Scholar] [CrossRef]
- Sharma, A.; Goyal, A.K.; Rath, G. Recent advances in metal nanoparticles in cancer therapy. J. Drug Target. 2018, 26, 617–632. [Google Scholar] [CrossRef]
- Tinajero-Díaz, E.; Salado-Leza, D.; Gonzalez, C.; Martínez Velázquez, M.; López, Z.; Bravo-Madrigal, J.; Knauth, P.; Flores-Hernández, F.Y.; Herrera-Rodríguez, S.E.; Navarro, R.E.; et al. Green metallic nanoparticles for cancer therapy: Evaluation models and cancer applications. Pharmaceutics 2021, 13, 1719. [Google Scholar] [CrossRef]
- Salapa, J.; Bushman, A.; Lowe, K.; Irudayaraj, J. Nano drug delivery systems in upper gastrointestinal cancer therapy. Nano Converg. 2020, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Mohan, S.K.; Chinnathambi, A.; Alahmadi, T.A.; Manikandan, V.; Rengarajan, T.; Veeraraghavan, V.P. Green synthesized zinc oxide/neodymium nanocomposites from Avaram senna flower extract induces apoptosis in gastric cancer ags cell line through inhibition of the PI3K/AKT/mTOR signaling pathway. J. King Saud Univ. Sci. 2021, 33, 101641. [Google Scholar] [CrossRef]
- Singh, M.; Harris-Birtill, D.C.; Markar, S.R.; Hanna, G.B.; Elson, D.S. Application of gold nanoparticles for gastrointestinal cancer theranostics: A systematic review. Nanomedicine 2015, 11, 2083–2098. [Google Scholar] [CrossRef] [PubMed]
- Jarestan, M.; Khalatbari, K.; Shandiz, S.A.S.; Beigi, S.; Hedayati, M.; Majlesi, A.; Akbari, F.; Salehzadeh, A. Preparation, characterization, and anticancer efficacy of novel cobalt oxide nanoparticles conjugated with thiosemicarbazide. 3 Biotech 2020, 10, 230. [Google Scholar] [CrossRef]
- Ebrahimzadeh, M.A.; Hashemi, Z.; Mohammadyan, M.; Fakhar, M.; Mortazavi-Derazkola, S. In Vitro cytotoxicity against human cancer cell lines (MCF-7 and AGS), antileishmanial and antibacterial activities of green synthesized silver nanoparticles using Scrophularia striata extract. Surf. Interfaces 2021, 23, 100963. [Google Scholar] [CrossRef]
- Shiripoure Ganjineh Ketab, R.; Tafvizi, F.; Khodarahmi, P. Biosynthesis and chemical characterization of silver nanoparticles using Satureja rechingeri jamzad and their apoptotic effects on AGS gastric cancer cells. J. Clust. Sci. 2021, 32, 1389–1399. [Google Scholar] [CrossRef]
- Karuppaiya, P.; Satheeshkumar, E.; Tsay, H.S. Biogenic synthesis of silver nanoparticles using rhizome extract of Dysosma pleiantha and its antiproliferative effect against breast and human gastric cancer cells. Mol. Biol. Rep. 2019, 46, 4725–4734. [Google Scholar] [CrossRef]
- Aslany, S.; Tafvizi, F.; Naseh, V. Characterization and evaluation of cytotoxic and apoptotic effects of green synthesis of silver nanoparticles using Aetemisia ciniformis on human gastric adenocarcinoma. Mater. Today Commun. 2020, 24, 101011. [Google Scholar] [CrossRef]
- Sampath, G.; Shyu, D.J.H.; Rameshkumar, N.; Krishnan, M.; Sivasankar, P.; Kayalvizhi, N. Synthesis and characterization of pyrogallol capped silver nanoparticles and evaluation of their in vitro anti-bacterial, anti-cancer profile against AGS cells. J. Clust. Sci. 2021, 32, 549–557. [Google Scholar] [CrossRef]
- Hashemi, S.F.; Tasharrofi, N.; Saber, M.M. Green synthesis of silver nanoparticles using Teucrium polium leaf extract and assessment of their antitumor effects against mnk45 human gastric cancer cell line. J. Mol. Struct. 2020, 1208, 127889. [Google Scholar] [CrossRef]
- Tamayo, L.; Palza, H.; Bejarano, J.; Zapata, P.A. Polymer composites with metal nanoparticles: Synthesis, properties, and applications. In Polymer Composites with Functionalized Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 249–286. [Google Scholar]
- Liu, Z.; Wang, K.; Wang, T.; Wang, Y.; Ge, Y. Copper nanoparticles supported on polyethylene glycol-modified magnetic Fe3O4 nanoparticles: Its anti-human gastric cancer investigation. Arab. J. Chem. 2022, 15, 103523. [Google Scholar] [CrossRef]
- Lima, J.F.; Carvalho, J.; Pinto-Ribeiro, I.; Almeida, C.; Wengel, J.; Cerqueira, L.; Figueiredo, C.; Oliveira, C.; Azevedo, N.F. Targeting miR-9 in gastric cancer cells using locked nucleic acid oligonucleotides. BMC Mol. Biol. 2018, 19, 6. [Google Scholar] [CrossRef] [PubMed]
- Asefi, Y.; Fahimi, R.; Ghorbian, S. Synergistic Effect of Vitamin C with Superparamagnetic Iron Oxide Nanoparticles for Inhibiting Proliferation of Gastric Cancer Cells. Biointerfaces Res. Appl. Chem. 2021, 12, 3215–3224. [Google Scholar]
- Xu, G.; Shi, C.; Guo, D.; Wang, L.; Ling, Y.; Han, X.; Luo, J. Functional-segregated coumarin-containing telodendrimer nanocarriers for efficient delivery of SN-38 for colon cancer treatment. Acta Biomater. 2015, 21, 85–98. [Google Scholar] [CrossRef]
- Yousefi, M.; Narmani, A.; Jafari, S.M. Dendrimers as efficient nanocarriers for the protection and delivery of bioactive phytochemicals. Adv. Colloid Interface Sci. 2020, 278, 102125. [Google Scholar] [CrossRef]
- Esfand, R.; Tomalia, D.A. Poly(Amidoamine)(PAMAM) Dendrimers: From biomimicry to drug delivery and biomedical applications. Drug Discov. Today 2001, 6, 427–436. [Google Scholar] [CrossRef]
- Stiriba, S.E.; Frey, H.; Haag, R. Dendritic polymers in biomedical applications: From potential to clinical use in diagnostics and therapy. Angew. Chem. Int. Ed. Engl. 2002, 41, 1329–1334. [Google Scholar] [CrossRef]
- Najafi, F.; Pashaei-Sarnaghi, R.; Salami-Kalajahi, M.; Roghani-Mamaqani, H. Application of poly (amidoamine) dendrimer as transfer agent to synthesize poly (amidoamine)-b-poly(methyl acrylate) amphiphilc block copolymers: Self-assembly in aqueous media and drug delivery. J. Drug Deliv. Sci. Technol. 2021, 64, 102626. [Google Scholar] [CrossRef]
- Yoo, H.; Juliano, R.L. Enhanced delivery of antisense oligonucleotides with fluorophore-conjugated pamam dendrimers. Nucleic Acids Res. 2000, 28, 4225–4231. [Google Scholar] [CrossRef]
- Bardelli, A.; Pantel, K. Liquid biopsies, what we do not know (yet). Cancer Cell 2017, 31, 172–179. [Google Scholar] [CrossRef]
- Kalra, H.; Drummen, G.P.; Mathivanan, S. Focus on extracellular vesicles: Introducing the next small big thing. Int. J. Mol. Sci. 2016, 17, 170. [Google Scholar] [CrossRef]
- Fu, M.; Gu, J.; Jiang, P.; Qian, H.; Xu, W.; Zhang, X. Exosomes in gastric cancer: Roles, mechanisms, and applications. Mol. Cancer 2019, 18, 41. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth Liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 2006, 1, 297–315. [Google Scholar]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New developments in liposomal drug delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef]
- Beltrán-Gracia, E.; López-Camacho, A.; Higuera-Ciapara, I.; Velázquez-Fernández, J.B.; Vallejo-Cardona, A.A. Nanomedicine review: Clinical developments in liposomal applications. Cancer Nanotechnol. 2019, 10, 11. [Google Scholar] [CrossRef]
- Zylberberg, C.; Matosevic, S. Pharmaceutical Liposomal Drug Delivery: A review of new delivery systems and a look at the regulatory landscape. Drug Deliv. 2016, 23, 3319–3329. [Google Scholar] [CrossRef]
- Das, M.; Huang, L. Liposomal nanostructures for drug delivery in gastrointestinal cancers. J. Pharmacol. Exp. Ther. 2019, 370, 647–656. [Google Scholar] [CrossRef]
- Tang, W.; Liu, R.; Yan, Y.; Pan, X.; Wang, M.; Han, X.; Ren, H.; Zhang, Z. Expression of estrogen receptors and androgen receptor and their clinical significance in gastric cancer. Oncotarget 2017, 8, 40765–40777. [Google Scholar] [CrossRef]
- Ur Rahman, M.S.U.; Cao, J. Estrogen receptors in gastric cancer: Advances and perspectives. World J. Gastroenterol. 2016, 22, 2475–2482. [Google Scholar] [CrossRef]
- Sun, Y.; Xie, Y.; Tang, H.; Ren, Z.; Luan, X.; Zhang, Y.; Zhu, M.; Lv, Z.; Bao, H.; Li, Y.; et al. In Vitro and in vivo evaluation of a novel estrogen-targeted pegylated oxaliplatin liposome for gastric cancer. Int. J. Nanomed. 2021, 16, 8279–8303. [Google Scholar] [CrossRef]
- Xu, G.; Tang, H.; Chen, J.; Zhu, M.; Xie, Y.; Li, Y.; Hao, Q.; Sun, Y.; Cong, D.; Meng, Q.; et al. Estrone-targeted liposomes for mitoxantrone delivery via estrogen receptor: In Vivo targeting efficacy, antitumor activity, acute toxicity and pharmacokinetics. Eur. J. Pharm. Sci. 2021, 161, 105780. [Google Scholar] [CrossRef] [PubMed]
- Nakonieczna, S.; Grabarska, A.; Kukula-Koch, W. The potential anticancer activity of phytoconstituents against gastric cancer—A review on in vitro, in vivo, and clinical studies. Int. J. Mol. Sci. 2020, 21, 8307. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, X.; Cao, S.; Sun, Y.; He, X.; Jiang, B.; Yu, Y.; Duan, J.; Qiu, F.; Kang, N. Berberine represses human gastric cancer cell growth in vitro and in vivo by inducing cytostatic autophagy via inhibition of MAPK/mTOR/p70S6K and akt signaling pathways. Biomed. Pharmacother. 2020, 128, 110245. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Q.; Liu, Z.; Zheng, X. Preparation, pharmacokinetics and tumour-suppressive activity of berberine liposomes. J. Pharm. Pharmacol. 2017, 69, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Bae, K.; Yoo, H.S.; Cho, S.H. Benefit of adjuvant traditional herbal medicine with chemotherapy for resectable gastric cancer. Integr. Cancer Ther. 2018, 17, 619–627. [Google Scholar] [CrossRef]
- Hong, C.; Wang, D.; Liang, J.; Guo, Y.; Zhu, Y.; Xia, J.; Qin, J.; Zhan, H.; Wang, J. Novel ginsenoside-based multifunctional liposomal delivery system for combination therapy of gastric cancer. Theranostics 2019, 9, 4437–4449. [Google Scholar] [CrossRef]
- Selim, J.H.; Shaheen, S.; Sheu, W.C.; Hsueh, C.T. Targeted and novel therapy in advanced gastric cancer. Exp. Hematol. Oncol. 2019, 8, 25. [Google Scholar] [CrossRef]
- Scott, L.J. Apatinib: A Review in advanced gastric cancer and other advanced cancers. Drugs 2018, 78, 747–758. [Google Scholar] [CrossRef]
- Long, Y.; Wang, Z.; Fan, J.; Yuan, L.; Tong, C.; Zhao, Y.; Liu, B. A hybrid membrane coating nanodrug system against gastric cancer via the VEGFR2/STAT3 Signaling Pathway. J. Mater. Chem. B 2021, 9, 3838–3855. [Google Scholar] [CrossRef]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef]
- Jhaveri, A.M.; Torchilin, V.P. Multifunctional polymeric micelles for delivery of drugs and siRNA. Front. Pharmacol. 2014, 5, 77. [Google Scholar] [CrossRef]
- Sarisozen, C.; Joshi, U.; Mendes, L.P.; Torchilin, V.P. Stimuli-responsive polymeric micelles for extracellular and intracellular drug delivery. In Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 269–304. [Google Scholar]
- Jiang, X.; Ma, M.; Li, M.; Shao, S.; Yuan, H.; Hu, F.; Liu, J.; Huang, X. Preparation and evaluation of novel emodin-loaded stearic acid-g-chitosan oligosaccharide nanomicelles. Nanoscale Res. Lett. 2020, 15, 93. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Wang, J.H.; Gu, L.Y.; Yao, X.M.; Cai, F.Y.; Jing, M.; Li, X.T.; Ju, R.J. Dual variable of drug loaded micelles in both particle and electrical charge on gastric cancer treatment. J. Drug Target. 2020, 28, 1071–1084. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, L.; Zheng, K.; Liu, J.; Tian, P.; Hu, D.; Wang, Q.; Zuo, Q.; Ouyang, X.; Dai, Y.; et al. The design and synthesis of redox-responsive oridonin polymeric prodrug micelle formulation for effective gastric cancer therapy. J. Mater. Chem. B 2021, 9, 3068–3078. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Pang, T.; Yin, X.; Cui, H.; Fang, G.; Xue, X.; Luo, T. Delivery of TSPAN1 siRNA by novel Th17 targeted cationic liposomes for gastric cancer intervention. J. Pharm. Sci. 2020, 109, 2854–2860. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zheng, Z.; Xue, X.; Zheng, L.; Qin, J.; Li, H.; Zhou, Y.; Fang, G. Targeted eradication of gastric cancer stem cells by CD44 targeting USP22 small interfering RNA-loaded nanoliposomes. Future Oncol. 2019, 15, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zheng, Z.; Zheng, L.; Qin, J.; Li, H.; Xue, X.; Gao, J.; Fang, G. SATB1 siRNA-encapsulated immunoliposomes conjugated with CD44 antibodies target and eliminate gastric cancer-initiating cells. Onco Targets Ther. 2018, 11, 6811–6825. [Google Scholar] [CrossRef]
- Shi, J.; Liu, S.; Yu, Y.; He, C.; Tan, L.; Shen, Y.M. RGD peptide-decorated micelles assembled from polymer–paclitaxel conjugates towards gastric cancer therapy. Colloids Surf. B Biointerfaces 2019, 180, 58–67. [Google Scholar] [CrossRef]
- Mori, T.; Hazekawa, M.; Yoshida, M.; Nishinakagawa, T.; Uchida, T.; Ishibashi, D. Enhancing the anticancer efficacy of a ll-37 peptide fragment analog using peptide-linked plga conjugate micelles in tumor cells. Int. J. Pharm. 2021, 606, 120891. [Google Scholar] [CrossRef]
- Qiu, L.; Ge, L.; Long, M.; Mao, J.; Ahmed, K.S.; Shan, X.; Zhang, H.; Qin, L.; Lv, G.; Chen, J. Redox-responsive biocompatible nanocarriers based on novel heparosan polysaccharides for intracellular anticancer drug delivery. Asian J. Pharm. Sci. 2020, 15, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Deng, L.; Chen, Z.; Chen, Z.; Yu, J.; Liu, H.; Li, T.; Lin, T.; Chen, H.; Zhao, M.; et al. Vitamin b12-conjugated sericin micelles for targeting cd320-overexpressed gastric cancer and reversing drug resistance. Nanomedicine 2019, 14, 353–370. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Hydrogels for tissue engineering. Chem. Rev. 2001, 101, 1869–1879. [Google Scholar] [CrossRef]
- Bennett, S.L.; Melanson, D.A.; Torchiana, D.F.; Wiseman, D.M.; Sawhney, A.S. Next-generation hydrogel films as tissue sealants and adhesion barriers. J. Card. Surg. 2003, 18, 494–499. [Google Scholar] [CrossRef]
- Walimbe, T.; Panitch, A.; Sivasankar, P.M. A review of hyaluronic acid and hyaluronic acid-based hydrogels for vocal fold tissue engineering. J. Voice 2017, 31, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, P.; Shantha, K.L.; Rao, K.P. Preparation, swelling characteristics and evaluation of hydrogels for stomach specific drug delivery. Int. J. Pharm. 1997, 154, 89–94. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, S.; Jiang, Y.; Ma, H.; Shi, M.; Wang, Q.; Zhong, W.; Liao, W.; Xing, M.M.Q. Doxorubicin-loaded single wall nanotube thermo-sensitive hydrogel for gastric cancer chemo-photothermal therapy. Adv. Funct. Mater. 2015, 25, 4730–4739. [Google Scholar] [CrossRef]
- Emoto, S.; Yamaguchi, H.; Kamei, T.; Ishigami, H.; Suhara, T.; Suzuki, Y.; Ito, T.; Kitayama, J.; Watanabe, T. Intraperitoneal administration of cisplatin via an in Situ cross-linkable hyaluronic acid-based hydrogel for peritoneal dissemination of gastric cancer. Surg. Today 2014, 44, 919–926. [Google Scholar] [CrossRef]
- Li, S.; Pei, M.; Wan, T.; Yang, H.; Gu, S.; Tao, Y.; Liu, X.; Zhou, Y.; Xu, W.; Xiao, P. Self-healing hyaluronic acid hydrogels based on dynamic schiff base linkages as biomaterials. Carbohydr. Polym. 2020, 250, 116922. [Google Scholar] [CrossRef]
- Suzuki, R.; Klibanov, A.L. Co-administration of Microbubbles and drugs in ultrasound-assisted drug delivery: Comparison with drug-carrying particles. In Therapeutic Ultrasound; Springer: Cham, Switzerland, 2016; pp. 205–220. [Google Scholar]
- Dijkmans, P.A.; Juffermans, L.J.; Musters, R.J.; van Wamel, A.; Ten Cate, F.J.; van Gilst, W.; Visser, C.A.; de Jong, N.; Kamp, O. Microbubbles and ultrasound: From diagnosis to therapy. Eur. J. Echocardiogr. 2004, 5, 245–256. [Google Scholar] [CrossRef]
- Cochran, M.C.; Eisenbrey, J.; Ouma, R.O.; Soulen, M.; Wheatley, M.A. Doxorubicin and paclitaxel loaded microbubbles for ultrasound triggered drug delivery. Int. J. Pharm. 2011, 414, 161–170. [Google Scholar] [CrossRef]
- Lai, B.; Zhu, P.; Li, H.; Hu, L.; Wang, J. Effect of docetaxel-loaded lipid microbubble in combination with ultrasound-triggered microbubble destruction on the growth of a gastric cancer cell line. Oncol. Lett. 2019, 18, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yang, Y.; Yang, Y.; Linghu, E.; Zhan, Q.; Brock, M.V.; Herman, J.G.; Zhang, B.; Guo, M. RASSF10 suppresses colorectal cancer growth by activating P53 signaling and sensitizes colorectal cancer cell to docetaxel. Oncotarget 2015, 6, 4202–4213. [Google Scholar] [CrossRef] [PubMed]
- Pirollo, K.F.; Nemunaitis, J.; Leung, P.K.; Nunan, R.; Adams, J.; Chang, E.H. Safety and efficacy in advanced solid tumors of a targeted nanocomplex carrying the p53 gene used in combination with docetaxel: A phase 1b study. Mol. Ther. 2016, 24, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, J.; Xu, M.; Zhang, L.; Tang, Q.; Chen, J.; Gong, M.; Sun, S.; Ge, H.; Wang, S.; et al. Ultrasound microbubbles mediated sonosensitizer and antibody co-delivery for highly efficient synergistic therapy on HER2-positive gastric cancer. ACS Appl. Mater. Interfaces 2022, 14, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Boisdron-Celle, M.; Menei, P.; Benoit, J.P. Preparation and characterization of 5-fluorouracil-loaded microparticles as biodegradable anticancer drug carriers. J. Pharm. Pharmacol. 1995, 47, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Au, J.L.-S.; Lu, Z.; Wientjes, M.G. Versatility of Particulate Carriers: Development of pharmacodynamically optimized drug-loaded microparticles for treatment of peritoneal cancer. AAPS J. 2015, 17, 1065–1079. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wei, Y.; Yang, H.; Pi, C.; Liu, H.; Ye, Y.; Zhao, L. A 5-fluorouracil-loaded floating gastroretentive hollow microsphere: Development, pharmacokinetic in rabbits, and biodistribution in tumor-bearing mice. Drug Des. Dev. Ther. 2016, 10, 997–1008. [Google Scholar]
- Bar-Zeev, M.; Nativ, L.; Assaraf, Y.G.; Livney, Y.D. Reassembled casein micelles for oral delivery of chemotherapeutic combinations to overcome multidrug resistance in gastric cancer. J. Mol. Clin. Med. 2018, 1, 55–65. [Google Scholar]
- Bhardwaj, P.; Chaurasia, D.; Singh, R.; Swarup, A. Development and characterization of novel site specific hollow floating microspheres bearing 5-Fu for stomach targeting. Sci. World. J. 2014, 2014, 705259. [Google Scholar] [CrossRef]

| Type of Nanoparticles | Drug | Polymers/ Capping/ Reducing Agents | Cell Line/ Animal Model | Application | Ref. |
|---|---|---|---|---|---|
| Polymeric nanoparticles | Irinotecan and 5-fluorouracil | polyethylene glycol and polylactide- coglycolide | NCI-N87 and SGC- 7901 (human gastric cancer cell lines) | To establish synergistic chemotherapy followed by reducing the chemotherapeutic agent related side effects | [85] |
| Polymeric nanoparticles | Docetaxel and LY294002 | Polylactic- coglycolic acid | MKN45 (human gastric cancer cell line)/ tumor-bearing Balb/c nude mice | To enhance the anticancer efficacy of docetaxel by inhibiting the PI3K/AKT pathway using LY294002 | [86] |
| Polymeric nanoparticles | 5-Fluorouracil and paclitaxel | Polylactic- coglycolic acid | NCI-N-87 and AGS (human gastric cancer cell line) | To achieve tumor targeted delivery of chemotherapeutic agents using anti-sLeA monoclonal antibody as a targeting moiety for improved gastric cancer efficacy | [87] |
| Metallic nanoparticles | Zinc oxide nanoparticles | Aqueous leaf extract of Morus nigra | AGS (human gastric cancer cell line) | To achieve anti-gastric cancer effects | [88] |
| Metallic nanoparticles | Gold nanoparticles | Nigella sativa (black cumin) seed extract and membrane vesicles of a Curtobacterium proimmune K3 (probiotic) | AGS (human gastric cancer cell line), RAW264.7 and HaCaT (normal healthy cell line) | To improve the gastric cancer therapy and to overcome the biocompatibility issues associated with chemically synthesized gold nanoparticles | [89] |
| Metallic nanoparticles | Nickel oxide nanoparticles | Glutamic acid and thiosemicarbazide | AGS (human gastric cancer cell line) | A novel therapeutic modality for gastric cancer | [90] |
| Metal- polymer composite nanoparticles | Doxorubicin, XMD8-92 (chemosensitizing agent), and superparamagnetic iron oxide nanoparticles | Poly(ethylene glycol)-blocked- poly(L-leucine) | Gastric cancer-bearing balb/c nude mice (SGC-7901) | To achieve synergistic anti-gastric cancer activity by down- regulating P-gp in gastric cancer cells | [91] |
| Metal-polymer composite nanoparticles | Copper oxide nanoparticles and magnetite nanoparticles | Chitosan | MKN45, AGS, and KATO III (human gastric cancer cell line) | Synergistically suppress the gastric tumors via two metallic nanoparticles | [92] |
| Mesoporous silica nanoparticles | Resveratrol and anti-miR oligonucleotide | Cetyltrimethylammonium bromide and hyaluronic acid | Gastric cancer induced male balb/c nude mice (BGC823) | To enhance the anticancer efficacy of resveratrol by inhibiting the microRNAs-21, which is responsible for cancer cell proliferation | [93] |
| Calcium carbonate nanoparticles | Cisplatin and oleanolic acid | Cancer cell membrane and calcium carbonate | Gastric cancer bearing male balb/c nude mice (MGC-803) | To overcome chemoresistance to cisplatin in gastric cancer | [94] |
| Drug | Polymers/ Lipids | Cell Line/ Animal Model | Application | Ref. |
|---|---|---|---|---|
| Liposomes | ||||
| TSPAN1 siRNA | 1, 2-dioleoyl-3- trimethylammonium-propane, (DOTAP), avanti polar lipids, DSPE-PEG-Mal and cholesterol | Th17 cells/gastric tumor bearing hybrid mice | To decrease in CD4+ T cells polarization to Th17 cells followed by inhibition of gastric tumor formation | [152] |
| ubiquitin- specific proteases-22 (USP22) siRNA | DOTAP, DSPE-mPEG and DSPE- PEG-Mal, and cholesterol | MKN-45 (human gastric cancer cell line)/gastric cancer induced male balb/c nude mice | To improve the therapeutic efficacy of USP22 siRNA against gastric tumor with the help of CD44 antibodies | [153] |
| Special AT-rich sequence binding protein 1 (SATB1) siRNA | DOTAP, DSPE-mPEG, and DSPE-PEG-Mal, and cholesterol | MKN-45 and NCI-N87 (human gastric cancer cell line) | To enhance the therapeutic efficacy of SATB1siRNA against gastric tumor with the help of CD44 antibodies | [154] |
| Mitoxantrone | Phosphatidylcholine, DSPE- mPEG2000, and cholesterol | Tumor induced female balb/c nude mice | To reduce the side effects of mitoxantrone followed by enhancement of gastric cancer therapy via targeted delivery | [137] |
| Berberine | Hydrogenated soy phosphatidylcholine, 2000-(polyethylene glycol) distearoyl phosphatidyl ethanolamine (PEG2000-DSPE), and cholesterol | SGC-7901 (human gastric cancer cell line)/gastric cancer bearing balb/c nude mice (SGC-7901) | To reduce the side effects of berberine followed by enhancement of gastric cancer therapy via targeted delivery | [140] |
| Micelles | ||||
| Paclitaxel | NH2-PEG-OH and 3,3′- Dithiodipropionic acid | SGC-7901 (human gastric cancer cell line)/gastric cancer bearing female balb/c nude mice (SGC-7901) | To achieve gastric tumor targeted controlled delivery of paclitaxel for effective gastric cancer therapy | [155] |
| CKR12 peptide (LL- 37 peptide fragment analog) | Polylactic co-glycolic acid and 3-(2-pyridyldithio) propionyl hydrazide | - | To improve the permeability of CKR12 peptide leading to the improvement of anti-gastric cancer effect | [156] |
| Doxorubicin | Heparosan-cystamine-vitamin E succinate | MGC80-3 (human gastric cancer cell line) | To enhance the anti-gastric cancer effect of doxorubicin with the help of redox- responsive drug delivery | [157] |
| Paclitaxel | Vitamin B12, sericin, synthetic poly(γbenzyl-L-glutamate) | BGC-823 (human gastric cancer cell line) | To improve the gastric cancer therapy by achieving targeted delivery of paclitaxel | [158] |
| Drug + System | Polymer Used | Cell Line | Application | References |
|---|---|---|---|---|
| Hydrogels | ||||
| Doxorubicin-loaded single wall nanotube thermo-sensitive hydrogel for gastric cancer chemo-photothermal therapy | NA | BGC-823 cell line | Efficacy and lesser toxicity | [163] |
| Intraperitoneal administration of cisplatin via an in-situ cross-linkable hyaluronic acid-based hydrogel for peritoneal dissemination of gastric cancer | NA | MKN45P, a human gastric cancer cell line | Sustained drug delivery | [164] |
| Microbubble | ||||
| Docetaxel-loaded lipid microbubble (DLLD) in combination with ultrasound-triggered microbubble destruction (UTMD) on the growth of a gastric cancer cell line | JC-1 | BGC-823 | More efficient in inhibiting cell proliferation and inducing cell apoptosis in the gastric cancer cell line | [169] |
| Ultrasound Microbubbles Mediated Sonosensitizer and Antibody Co-delivery on HER2- Positive Gastric Cancer | NA | HER2-positive gastric cancer NCI-N87 cells | Significant tumor lethal effect in vitro and distinctly inhibited tumor growth in vivo | [172] |
| Microparticles | ||||
| 5-fluorouracil-loaded microparticles as biodegradable anticancer drug carriers | biodegradable poly ((±)-lactide-co-glycolide) (PLAGA) | NA | Sustained drug delivery | [173] |
| Drug-loaded microparticles for treatment of peritoneal cancer | PLGA or poly (lacticco-glycolic acid) copolymer | NA | Less toxic and more effective against several IP metastatic tumors | [174] |
| Oral drug delivery | ||||
| 5-fluorouracil-loaded floating gastroretentive hollow microsphere | polyvinyl pyrrolidone (PVP) and ethyl cellulose (EC) as drug controlled- release polymer blends. | MCF-7 breast cancer cells to induce tumor in mice | 5-FU hollow microspheres exhibited excellent floating and sustained release characteristics. | [175] |
| Re-assembled casein micelles for oral delivery of chemotherapeutic combinations to overcome multidrug resistance in gastric cancer | NA | Human MDR gastric carcinoma cell line | Casein-based oral delivery systems provide a robust natural platform enabling a spectrum of development possibilities for gastric-activated release of synergistic drug combinations Developed oral drug delivery system showed good floating ability and it retained in GIT for a prolonged period of time. | [176] |
| Site Specific Hollow Floating Microspheres Bearing 5-Fu | Eudragit S-100 | NA | system showed good floating ability and it retained in GIT for a prolonged period of time. | [177] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hani, U.; Osmani, R.A.M.; Yasmin, S.; Gowda, B.H.J.; Ather, H.; Ansari, M.Y.; Siddiqua, A.; Ghazwani, M.; Fatease, A.A.; Alamri, A.H.; et al. Novel Drug Delivery Systems as an Emerging Platform for Stomach Cancer Therapy. Pharmaceutics 2022, 14, 1576. https://doi.org/10.3390/pharmaceutics14081576
Hani U, Osmani RAM, Yasmin S, Gowda BHJ, Ather H, Ansari MY, Siddiqua A, Ghazwani M, Fatease AA, Alamri AH, et al. Novel Drug Delivery Systems as an Emerging Platform for Stomach Cancer Therapy. Pharmaceutics. 2022; 14(8):1576. https://doi.org/10.3390/pharmaceutics14081576
Chicago/Turabian StyleHani, Umme, Riyaz Ali M. Osmani, Sabina Yasmin, B. H. Jaswanth Gowda, Hissana Ather, Mohammad Yousuf Ansari, Ayesha Siddiqua, Mohammed Ghazwani, Adel Al Fatease, Ali H. Alamri, and et al. 2022. "Novel Drug Delivery Systems as an Emerging Platform for Stomach Cancer Therapy" Pharmaceutics 14, no. 8: 1576. https://doi.org/10.3390/pharmaceutics14081576
APA StyleHani, U., Osmani, R. A. M., Yasmin, S., Gowda, B. H. J., Ather, H., Ansari, M. Y., Siddiqua, A., Ghazwani, M., Fatease, A. A., Alamri, A. H., Rahamathulla, M., Begum, M. Y., & Wahab, S. (2022). Novel Drug Delivery Systems as an Emerging Platform for Stomach Cancer Therapy. Pharmaceutics, 14(8), 1576. https://doi.org/10.3390/pharmaceutics14081576