Recent Advances in Nanoparticles-Based Platforms Targeting the PD-1/PD-L1 Pathway for Cancer Treatment
Abstract
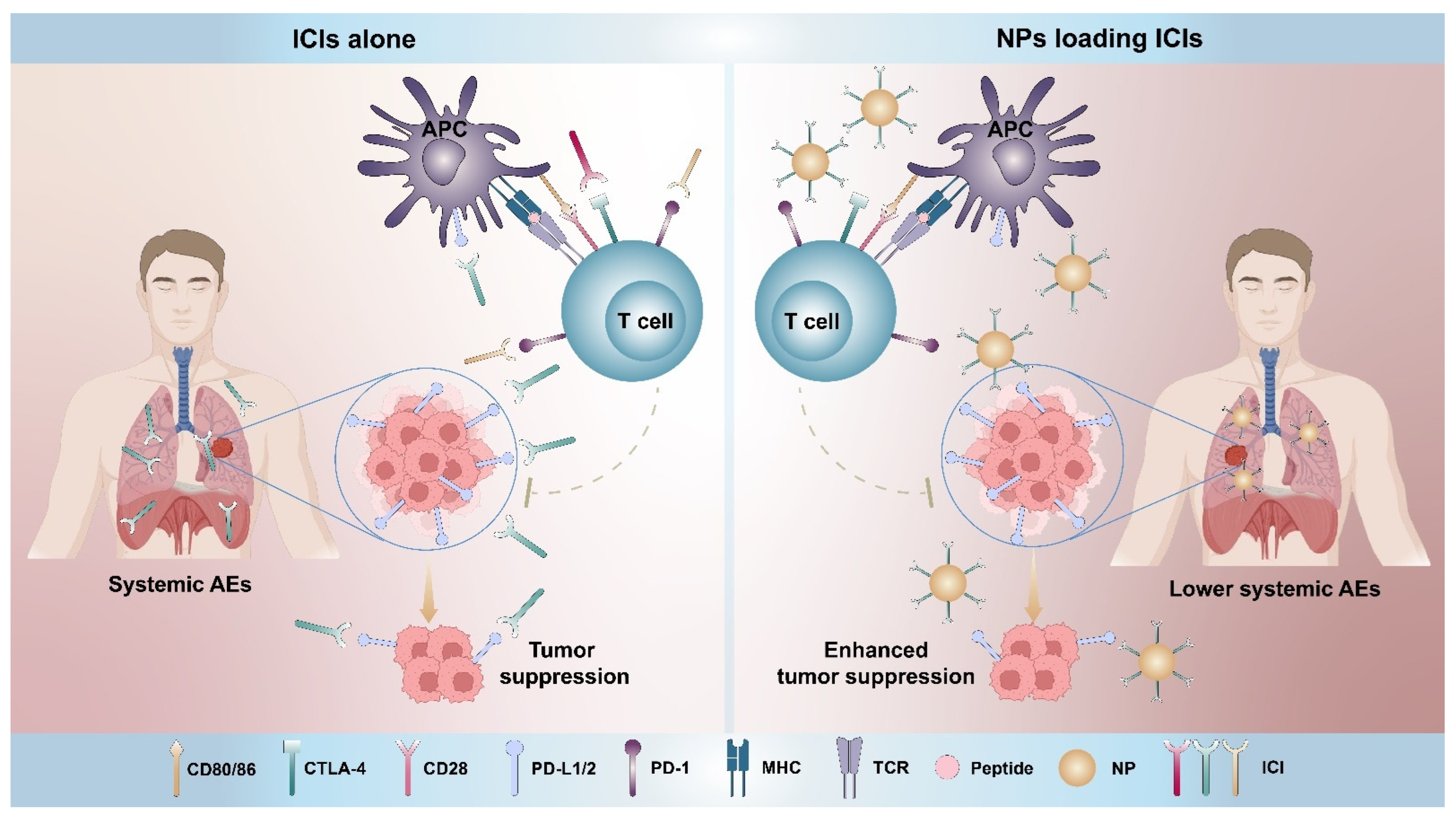
:1. Introduction
2. Nanoparticles Directly Targeting the PD-1/PD-L1 Pathway
2.1. Nanoparticles Delivering Antibodies and Peptides
2.2. Nanoparticles Delivering RNA
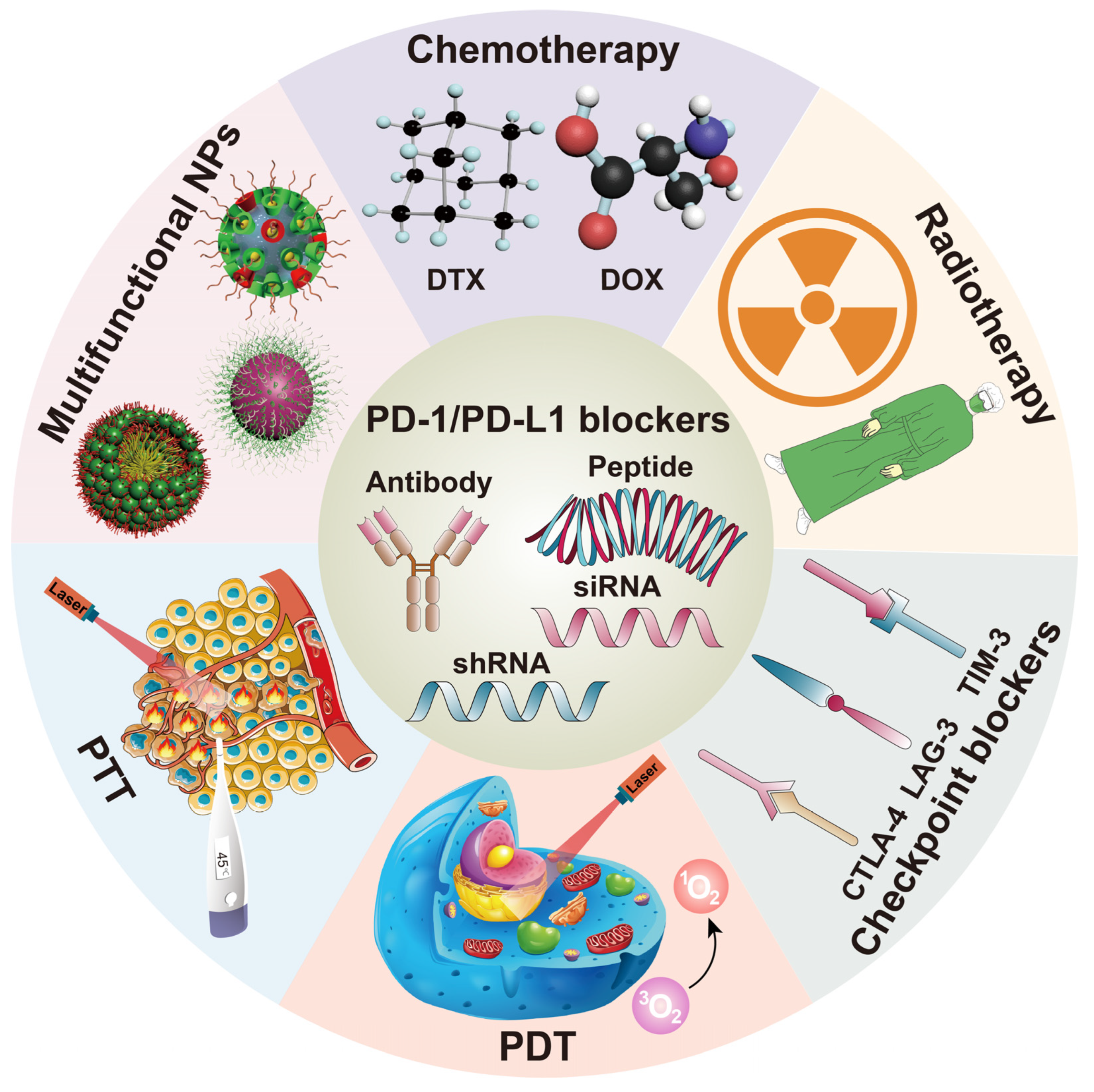
3. Nanoparticles for Combining PD-1/PD-L1 Blocking and Other Therapies
3.1. Nanoparticles Combining PD-1/PD-L1 Blocking and Chemotherapy
3.2. Nanoparticles Combining PD-1/PD-L1 Blocking and Radiotherapy
3.3. Nanoparticles Combining PD-1/PD-L1 Blocking and Other Immune Checkpoint Blockers
3.4. Nanoparticles Combining PD-1/PD-L1 Blocking and Photodynamic Therapy (PDT)
3.5. Nanoparticles Combining PD-1/PD-L1 Blocking and Photothermal Therapy (PTT)
3.6. Multifunctional Nanoparticles Containing PD-1/PD-L1 Blocking
4. Challenges and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qin, S.; Xu, L.; Yi, M.; Yu, S.; Wu, K.; Luo, S. Novel immune checkpoint targets: Moving beyond PD-1 and CTLA-4. Mol. Cancer 2019, 18, 155. [Google Scholar] [CrossRef] [PubMed]
- Kalbasi, A.; Ribas, A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat. Rev. Immunol. 2020, 20, 25–39. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, T.; Zheng, L.; Liu, H.; Song, W.; Liu, D.; Li, Z.; Pan, C.X. Combination strategies to maximize the benefits of cancer immunotherapy. J. Hematol. Oncol. 2021, 14, 156. [Google Scholar] [CrossRef] [PubMed]
- Irvine, D.J.; Dane, E.L. Enhancing cancer immunotherapy with nanomedicine. Nat. Rev. Immunol. 2020, 20, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Lee, J.; Jung, S.; Kim, W.J. Phenylboronic-acid-based nanocomplex as a feasible delivery platform of immune checkpoint inhibitor for potent cancer immunotherapy. J. Control. Release 2021, 330, 1168–1177. [Google Scholar] [CrossRef] [PubMed]
- Bu, J.; Nair, A.; Iida, M.; Jeong, W.J.; Poellmann, M.J.; Mudd, K.; Kubiatowicz, L.J.; Liu, E.W.; Wheeler, D.L.; Hong, S. An Avidity-Based PD-L1 Antagonist Using Nanoparticle-Antibody Conjugates for Enhanced Immunotherapy. Nano Lett. 2020, 20, 4901–4909. [Google Scholar] [CrossRef]
- Guo, H.; Wang, R.; Wang, D.; Wang, S.; Zhou, J.; Chai, Z.; Yao, S.; Li, J.; Lu, L.; Liu, Y.; et al. Deliver anti-PD-L1 into brain by p-hydroxybenzoic acid to enhance immunotherapeutic effect for glioblastoma. J. Control. Release 2020, 320, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Nam, G.H.; Lee, N.K.; Kih, M.; Koh, E.; Kim, Y.K.; Hong, Y.; Kim, S.; Park, S.Y.; Jeong, C.; et al. Nanocage-Therapeutics Prevailing Phagocytosis and Immunogenic Cell Death Awakens Immunity against Cancer. Adv. Mater. 2018, 30, 1705581. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.B.; Sung, H.D.; Nam, G.H.; Kim, W.; Kim, S.; Kang, D.; Lee, E.J.; Kim, I.S. Design of PD-1-decorated nanocages targeting tumor-draining lymph node for promoting T cell activation. J. Control. Release 2021, 333, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Li, W.; Hong, Y.; Zeng, Z.; Zhang, J.; Wu, X.; Zhou, K.; Wu, F. A PD1 targeted nano-delivery system based on epigenetic alterations of T cell responses in the treatment of gastric cancer. Mol. Ther. Oncolytics 2022, 24, 148–159. [Google Scholar] [CrossRef]
- Wang, C.; Sun, W.; Ye, Y.; Hu, Q.; Bomba, H.N.; Gu, Z. In situ activation of platelets with checkpoint inhibitors for post-surgical cancer immunotherapy. Nat. Biomed. Eng. 2017, 1, 11. [Google Scholar] [CrossRef]
- Hu, Q.; Sun, W.; Wang, J.; Ruan, H.; Zhang, X.; Ye, Y.; Shen, S.; Wang, C.; Lu, W.; Cheng, K.; et al. Conjugation of haematopoietic stem cells and platelets decorated with anti-PD-1 antibodies augments anti-leukaemia efficacy. Nat. Biomed. Eng. 2018, 2, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, C.; Wang, J.; Hu, Q.; Langworthy, B.; Ye, Y.; Sun, W.; Lin, J.; Wang, T.; Fine, J.; et al. PD-1 Blockade Cellular Vesicles for Cancer Immunotherapy. Adv. Mater. 2018, 30, e1707112. [Google Scholar] [CrossRef]
- Du, J.; Qin, Y.; Wu, Y.; Zhao, W.; Zhai, W.; Qi, Y.; Wang, C.; Gao, Y. The design of high affinity human PD-1 mutants by using molecular dynamics simulations (MD). Cell Commun. Signal. 2018, 16, 25. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Zhu, X.; Zhou, X.; Wang, X.; Zhai, W.; Li, B.; Du, J.; Li, G.; Sui, X.; Wu, Y.; et al. An orally available PD-1/PD-L1 blocking peptide OPBP-1-loaded trimethyl chitosan hydrogel for cancer immunotherapy. J. Control. Release 2021, 334, 376–388. [Google Scholar] [CrossRef]
- Petrocca, F.; Lieberman, J. Promise and challenge of RNA interference-based therapy for cancer. J. Clin. Oncol. 2011, 29, 747–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitehead, K.A.; Langer, R.; Anderson, D.G. Knocking down barriers: Advances in siRNA delivery. Nat. Rev. Drug Discov. 2009, 8, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Kwak, G.; Kim, D.; Nam, G.H.; Wang, S.Y.; Kim, I.S.; Kim, S.H.; Kwon, I.C.; Yeo, Y. Programmed Cell Death Protein Ligand-1 Silencing with Polyethylenimine-Dermatan Sulfate Complex for Dual Inhibition of Melanoma Growth. ACS Nano 2017, 11, 10135–10146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teo, P.Y.; Yang, C.; Whilding, L.M.; Parente-Pereira, A.C.; Maher, J.; George, A.J.; Hedrick, J.L.; Yang, Y.Y.; Ghaem-Maghami, S. Ovarian cancer immunotherapy using PD-L1 siRNA targeted delivery from folic acid-functionalized polyethylenimine: Strategies to enhance T cell killing. Adv. Healthc. Mater. 2015, 4, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Dowdy, S.F. Overcoming cellular barriers for RNA therapeutics. Nat. Biotechnol. 2017, 35, 222–229. [Google Scholar] [CrossRef]
- Li, C.; Zhou, J.; Wu, Y.; Dong, Y.; Du, L.; Yang, T.; Wang, Y.; Guo, S.; Zhang, M.; Hussain, A.; et al. Core Role of Hydrophobic Core of Polymeric Nanomicelle in Endosomal Escape of siRNA. Nano Lett. 2021, 21, 3680–3689. [Google Scholar] [CrossRef]
- Guan, X.; Lin, L.; Chen, J.; Hu, Y.; Sun, P.; Tian, H.; Maruyama, A.; Chen, X. Efficient PD-L1 gene silence promoted by hyaluronidase for cancer immunotherapy. J. Control. Release 2019, 293, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Emens, L.A.; Middleton, G. The interplay of immunotherapy and chemotherapy: Harnessing potential synergies. Cancer Immunol. Res. 2015, 3, 436–443. [Google Scholar] [CrossRef] [Green Version]
- Leonetti, A.; Wever, B.; Mazzaschi, G.; Assaraf, Y.G.; Rolfo, C.; Quaini, F.; Tiseo, M.; Giovannetti, E. Molecular basis and rationale for combining immune checkpoint inhibitors with chemotherapy in non-small cell lung cancer. Drug Resist. Updat. 2019, 46, 100644. [Google Scholar] [CrossRef]
- Duan, X.; Chan, C.; Han, W.; Guo, N.; Weichselbaum, R.R.; Lin, W. Immunostimulatory nanomedicines synergize with checkpoint blockade immunotherapy to eradicate colorectal tumors. Nat. Commun. 2019, 10, 1899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinoh, H.; Quader, S.; Shibasaki, H.; Liu, X.; Maity, A.; Yamasoba, T.; Cabral, H.; Kataoka, K. Translational Nanomedicine Boosts Anti-PD1 Therapy to Eradicate Orthotopic PTEN-Negative Glioblastoma. ACS Nano 2020, 14, 10127–10140. [Google Scholar] [CrossRef]
- Tournigand, C.; Cervantes, A.; Figer, A.; Lledo, G.; Flesch, M.; Buyse, M.; Mineur, L.; Carola, E.; Etienne, P.L.; Rivera, F.; et al. OPTIMOX1: A randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-Go fashion in advanced colorectal cancer—A GERCOR study. J. Clin. Oncol. 2006, 24, 394–400. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Yu, Z.; Das, M.; Huang, L. Nano Codelivery of Oxaliplatin and Folinic Acid Achieves Synergistic Chemo-Immunotherapy with 5-Fluorouracil for Colorectal Cancer and Liver Metastasis. ACS Nano 2020, 14, 5075–5089. [Google Scholar] [CrossRef]
- Phung, C.D.; Nguyen, H.T.; Choi, J.Y.; Pham, T.T.; Acharya, S.; Timilshina, M.; Chang, J.H.; Kim, J.H.; Jeong, J.H.; Ku, S.K.; et al. Reprogramming the T cell response to cancer by simultaneous, nanoparticle-mediated PD-L1 inhibition and immunogenic cell death. J. Control. Release 2019, 315, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Bugada, L.; Li, R.; Hu, H.; Zhang, L.; Li, C.; Yuan, H.; Rajanayake, K.K.; Truchan, N.A.; Wen, F.; et al. Albumin nanoparticle containing a PI3Kγ inhibitor and paclitaxel in combination with α-PD1 induces tumor remission of breast cancer in mice. Sci. Transl. Med. 2022, 14, eabl3649. [Google Scholar] [CrossRef]
- Li, Q.; Chen, C.; Kong, J.; Li, L.; Li, J.; Huang, Y. Stimuli-responsive nano vehicle enhances cancer immunotherapy by coordinating mitochondria-targeted immunogenic cell death and PD-L1 blockade. Acta Pharm. Sin. B 2022, 12, 2533–2549. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jeon, J.O.; Jun, E.; Jee, J.; Jung, H.K.; Lee, B.H.; Kim, I.S.; Kim, S. Designing Peptide Bunches on Nanocage for Bispecific or Superaffinity Targeting. Biomacromolecules 2016, 17, 1150–1159. [Google Scholar] [CrossRef]
- Jeon, I.S.; Yoo, J.D.; Gurung, S.; Kim, M.; Lee, C.; Park, E.J.; Park, R.W.; Lee, B.; Kim, S. Anticancer nanocage platforms for combined immunotherapy designed to harness immune checkpoints and deliver anticancer drugs. Biomaterials 2021, 270, 120685. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, C.; Zheng, Z.; Chen, S.; Pang, X.; Xiang, X.; Tang, J.; Ren, E.; Chen, Y.; You, M.; et al. Vesicular Antibodies: A Bioactive Multifunctional Combination Platform for Targeted Therapeutic Delivery and Cancer Immunotherapy. Adv. Mater. 2019, 31, e1808294. [Google Scholar] [CrossRef]
- Li, L.; Miao, Q.; Meng, F.; Li, B.; Xue, T.; Fang, T.; Zhang, Z.; Zhang, J.; Ye, X.; Kang, Y.; et al. Genetic engineering cellular vesicles expressing CD64 as checkpoint antibody carrier for cancer immunotherapy. Theranostics 2021, 11, 6033–6043. [Google Scholar] [CrossRef]
- Zhang, P.; Miska, J.; Lee-Chang, C.; Rashidi, A.; Panek, W.K.; An, S.; Zannikou, M.; Lopez-Rosas, A.; Han, Y.; Xiao, T.; et al. Therapeutic targeting of tumor-associated myeloid cells synergizes with radiation therapy for glioblastoma. Proc. Natl. Acad. Sci. USA 2019, 116, 23714–23723. [Google Scholar] [CrossRef] [PubMed]
- Erel-Akbaba, G.; Carvalho, L.A.; Tian, T.; Zinter, M.; Akbaba, H.; Obeid, P.J.; Chiocca, E.A.; Weissleder, R.; Kantarci, A.G.; Tannous, B.A. Radiation-Induced Targeted Nanoparticle-Based Gene Delivery for Brain Tumor Therapy. ACS Nano 2019, 13, 4028–4040. [Google Scholar] [CrossRef] [PubMed]
- Schaue, D.; McBride, W.H. Opportunities and challenges of radiotherapy for treating cancer. Nat. Rev. Clin. Oncol. 2015, 12, 527–540. [Google Scholar] [CrossRef]
- Lan, G.; Ni, K.; Xu, Z.; Veroneau, S.S.; Song, Y.; Lin, W. Nanoscale Metal-Organic Framework Overcomes Hypoxia for Photodynamic Therapy Primed Cancer Immunotherapy. J. Am. Chem. Soc. 2018, 140, 5670–5673. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ye, Y.; Hochu, G.M.; Sadeghifar, H.; Gu, Z. Enhanced Cancer Immunotherapy by Microneedle Patch-Assisted Delivery of Anti-PD1 Antibody. Nano Lett. 2016, 16, 2334–2340. [Google Scholar] [CrossRef]
- Galstyan, A.; Markman, J.L.; Shatalova, E.S.; Chiechi, A.; Korman, A.J.; Patil, R.; Klymyshyn, D.; Tourtellotte, W.G.; Israel, L.L.; Braubach, O.; et al. Blood-brain barrier permeable nano immunoconjugates induce local immune responses for glioma therapy. Nat. Commun. 2019, 10, 3850. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.S.; Choi, D.S.; Ko, M.; Kim, D.; Lee, D.H.; Lee, S.; Lee, A.Y.; Kang, S.G.; Kim, S.H.; Jung, Y.; et al. Extracellular pH modulating injectable gel for enhancing immune checkpoint inhibitor therapy. J. Control. Release 2019, 315, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.Y.; Huang, G.L.; Zhang, C.Y.; Zhuang, B.W.; Liu, B.X.; Su, L.Y.; Ye, J.Y.; Xu, M.; Kuang, M.; Xie, X.Y. Supramolecular Photothermal Nanomedicine Mediated Distant Tumor Inhibition via PD-1 and TIM-3 Blockage. Front. Chem. 2020, 8, 1. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kedzierska, E.; Knap-Czop, K.; Kotlinska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy-mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Huang, P.; Chen, X. Overcoming the Achilles’ heel of photodynamic therapy. Chem. Soc. Rev. 2016, 45, 6488–6519. [Google Scholar] [CrossRef]
- Feng, Z.; Guo, J.; Liu, X.; Song, H.; Zhang, C.; Huang, P.; Dong, A.; Kong, D.; Wang, W. Cascade of reactive oxygen species generation by polyprodrug for combinational photodynamic therapy. Biomaterials 2020, 255, 120210. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Chan, C.; Guo, N.; Han, W.; Weichselbaum, R.R.; Lin, W. Photodynamic Therapy Mediated by Nontoxic Core-Shell Nanoparticles Synergizes with Immune Checkpoint Blockade To Elicit Antitumor Immunity and Antimetastatic Effect on Breast Cancer. J. Am. Chem. Soc. 2016, 138, 16686–16695. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Jeon, Y.H.; Kwon, N.; Park, J.G.; Guo, T.; Kim, H.R.; Huang, J.D.; Lee, D.S.; Yoon, J. In Vivo-assembled phthalocyanine/albumin supramolecular complexes combined with a hypoxia-activated prodrug for enhanced photodynamic immunotherapy of cancer. Biomaterials 2021, 266, 120430. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, Y.; Tang, Q.; Lin, J.; Huang, P. Biomimetic Nanoemulsion for Synergistic Photodynamic-Immunotherapy Against Hypoxic Breast Tumor. Angew. Chem. Int. Ed. Engl. 2021, 60, 10647–10653. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Ying, M.; Spiekermann, K.; Holay, M.; Zhang, Y.; Chen, F.; Gong, H.; Lee, J.H.; Gao, W.; Fang, R.H.; et al. Biomimetic Nanoemulsions for Oxygen Delivery In Vivo. Adv. Mater. 2018, 30, e1804693. [Google Scholar] [CrossRef] [PubMed]
- Wheaton, W.W.; Weinberg, S.E.; Hamanaka, R.B.; Soberanes, S.; Sullivan, L.B.; Anso, E.; Glasauer, A.; Dufour, E.; Mutlu, G.M.; Budigner, G.S.; et al. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. Elife 2014, 3, e02242. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.H.; Yang, W.H.; Xia, W.; Wei, Y.; Chan, L.C.; Lim, S.O.; Li, C.W.; Kim, T.; Chang, S.S.; Lee, H.H.; et al. Metformin Promotes Antitumor Immunity via Endoplasmic-Reticulum-Associated Degradation of PD-L1. Mol. Cell 2018, 71, 606–620.e607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, W.; Qi, L.; Jiang, N.; Zhao, Q.; Chen, L.; Jiang, X.; Li, Y.; Zhou, Z.; Shen, J. Metformin Liposome-Mediated PD-L1 Downregulation for Amplifying the Photodynamic Immunotherapy Efficacy. ACS Appl. Mater. Interfaces 2021, 13, 8026–8041. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, J.; Liu, S.; Li, X.; Miao, L.; Yang, B.; Zhang, C.; He, J.; Ai, S.; Guan, W. Defeating relapsed and refractory malignancies through a nano-enabled mitochondria-mediated respiratory inhibition and damage pathway. Biomaterials 2020, 229, 119580. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, T.; Yu, H.; Feng, B.; Zhou, L.; Zhou, F.; Hou, B.; Zhang, H.; Luo, M.; Li, Y. Engineering nanoparticles to locally activate T cells in the tumor microenvironment. Sci. Immunol. 2019, 4, eaau6584. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xiao, Z.; An, Y.; Han, S.; Wu, W.; Wang, Y.; Guo, Y.; Shuai, X. Nanodrug with dual-sensitivity to tumor microenvironment for immuno-sonodynamic anti-cancer therapy. Biomaterials 2021, 269, 120636. [Google Scholar] [CrossRef]
- Xu, J.; Yu, S.; Wang, X.; Qian, Y.; Wu, W.; Zhang, S.; Zheng, B.; Wei, G.; Gao, S.; Cao, Z.; et al. High Affinity of Chlorin e6 to Immunoglobulin G for Intraoperative Fluorescence Image-Guided Cancer Photodynamic and Checkpoint Blockade Therapy. ACS Nano 2019, 13, 10242–10260. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef]
- Jung, H.S.; Verwilst, P.; Sharma, A.; Shin, J.; Sessler, J.L.; Kim, J.S. Organic molecule-based photothermal agents: An expanding photothermal therapy universe. Chem. Soc. Rev. 2018, 47, 2280–2297. [Google Scholar] [CrossRef]
- Zhang, N.; Song, J.; Liu, Y.; Liu, M.; Zhang, L.; Sheng, D.; Deng, L.; Yi, H.; Wu, M.; Zheng, Y.; et al. Photothermal therapy mediated by phase-transformation nanoparticles facilitates delivery of anti-PD1 antibody and synergizes with antitumor immunotherapy for melanoma. J. Control. Release 2019, 306, 15–28. [Google Scholar] [CrossRef]
- Huang, L.; Li, Y.; Du, Y.; Zhang, Y.; Wang, X.; Ding, Y.; Yang, X.; Meng, F.; Tu, J.; Luo, L.; et al. Mild photothermal therapy potentiates anti-PD-L1 treatment for immunologically cold tumors via an all-in-one and all-in-control strategy. Nat. Commun. 2019, 10, 4871. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, T.; Zhao, M.; Wang, W.; Sun, C.; Liu, L.; Li, Q.; Zhang, F.; Zhao, D.; Li, X. Size and charge dual-transformable mesoporous nanoassemblies for enhanced drug delivery and tumor penetration. Chem. Sci. 2020, 11, 2819–2827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Zhang, C.C.; Wang, X.Y.; Li, L.; Chen, Q.Q.; Liu, W.W.; Cao, Y.; Ran, H.T. Light-Responsive Core-Shell Nanoplatform for Bimodal Imaging-Guided Photothermal Therapy-Primed Cancer Immunotherapy. ACS Appl. Mater. Interfaces 2020, 12, 48420–48431. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Chen, Q.; Guo, Z.; Li, M.; Yang, X.; Wan, G.; Chen, H.; Zhang, Q.; Wang, Y. An Intelligent Biomimetic Nanoplatform for Holistic Treatment of Metastatic Triple-Negative Breast Cancer via Photothermal Ablation and Immune Remodeling. ACS Nano 2020, 14, 15161–15181. [Google Scholar] [CrossRef]
- Fu, L.H.; Qi, C.; Lin, J.; Huang, P. Catalytic chemistry of glucose oxidase in cancer diagnosis and treatment. Chem. Soc. Rev. 2018, 47, 6454–6472. [Google Scholar] [CrossRef]
- Wang, Q.; Niu, D.; Shi, J.; Wang, L. A Three-in-one ZIFs-Derived CuCo(O)/GOx@PCNs Hybrid Cascade Nanozyme for Immunotherapy/Enhanced Starvation/Photothermal Therapy. ACS Appl. Mater. Interfaces 2021, 13, 11683–11695. [Google Scholar] [CrossRef] [PubMed]
- Donskyi, I.S.; Chen, Y.; Nickl, P.; Guday, G.; Qiao, H.; Achazi, K.; Lippitz, A.; Unger, W.E.S.; Böttcher, C.; Chen, W.; et al. Self-degrading graphene sheets for tumor therapy. Nanoscale 2020, 12, 14222–14229. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Wang, D.; Liang, W.; Liu, L.; Zhang, Y.; Chen, X.; Sang, D.K.; Xing, C.; Li, Z.; Dong, B.; et al. Novel concept of the smart NIR-light-controlled drug release of black phosphorus nanostructure for cancer therapy. Proc. Natl. Acad. Sci. USA 2018, 115, 501–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, M.; Liu, Y.; Zhu, X.; Wang, X.; Liu, L.; Sun, H.; Wang, C.; Kong, D.; Ma, G. Nanoscale Reduced Graphene Oxide-Mediated Photothermal Therapy Together with IDO Inhibition and PD-L1 Blockade Synergistically Promote Antitumor Immunity. ACS Appl. Mater. Interfaces 2019, 11, 1876–1885. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, Q.; Chen, Q.; Zhao, X.; Pennycook, S.J.; Chen, H. Highly Efficient 2D NIR-II Photothermal Agent with Fenton Catalytic Activity for Cancer Synergistic Photothermal-Chemodynamic Therapy. Adv. Sci. 2020, 7, 1902576. [Google Scholar] [CrossRef]
- Fang, X.; Wu, X.; Li, Z.; Jiang, L.; Lo, W.S.; Chen, G.; Gu, Y.; Wong, W.T. Biomimetic Anti-PD-1 Peptide-Loaded 2D FePSe3 Nanosheets for Efficient Photothermal and Enhanced Immune Therapy with Multimodal MR/PA/Thermal Imaging. Adv. Sci. 2021, 8, 2003041. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lai, C.; Zeng, G.; Huang, D.; Qin, L.; Zhang, M.; Cheng, M.; Liu, X.; Yi, H.; Zhou, C.; et al. Black Phosphorus, a Rising Star 2D Nanomaterial in the Post-Graphene Era: Synthesis, Properties, Modifications, and Photocatalysis Applications. Small 2019, 15, e1804565. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Liang, X.; Chen, Q.; Miao, Q.; Chen, X.; Zhang, X.; Mei, L. Surgical Tumor-Derived Personalized Photothermal Vaccine Formulation for Cancer Immunotherapy. ACS Nano 2019, 13, 2956–2968. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Ye, X.; Wang, C.; Xing, C.; Miao, Q.; Xie, Z.; Chen, X.; Zhang, X.; Zhang, H.; Mei, L. Photothermal cancer immunotherapy by erythrocyte membrane-coated black phosphorus formulation. J. Control. Release 2019, 296, 150–161. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, L.; Zhou, W.; Wang, J.; Zhang, R.; Wang, Z.; Ran, H.; Li, P.; Li, R. Dual mitigation of immunosuppression combined with photothermal inhibition for highly effective primary tumor and metastases therapy. Biomaterials 2021, 274, 120856. [Google Scholar] [CrossRef]
- Wang, N.; Zhou, Y.; Xu, Y.; Ren, X.; Zhou, S.; Shang, Q.; Jiang, Y.; Luan, Y. Molecular engineering of anti-PD-L1 peptide and photosensitizer for immune checkpoint blockade photodynamic-immunotherapy. Chem. Eng. J. 2020, 400, 125995. [Google Scholar] [CrossRef]
- Cao, Y.; Wei, D.; Yang, L.; Luo, Z.; Yu, P.; Li, H.; Zhang, C.; Liu, X.; Wu, F.; Wu, M.; et al. Nanoplatform Self-Assembly from Small Molecules of Porphyrin Derivatives for NIR-II Fluorescence Imaging Guided Photothermal-Immunotherapy. Adv. Healthc. Mater. 2022, 11, e2102526. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, D.; Zhang, J.; Jiang, Y.; Song, A.; Li, Z.; Luan, Y. A Three-in-One Immunotherapy Nanoweapon via Cascade-Amplifying Cancer-Immunity Cycle against Tumor Metastasis, Relapse, and Postsurgical Regrowth. Nano Lett. 2019, 19, 6647–6657. [Google Scholar] [CrossRef]
- Geng, Z.; Chen, F.; Wang, X.; Wang, L.; Pang, Y.; Liu, J. Combining anti-PD-1 antibodies with Mn2+-drug coordinated multifunctional nanoparticles for enhanced cancer therapy. Biomaterials 2021, 275, 120897. [Google Scholar] [CrossRef]
- Sun, W.; Du, Y.; Liang, X.; Yu, C.; Fang, J.; Lu, W.; Guo, X.; Tian, J.; Jin, Y.; Zheng, J. Synergistic triple-combination therapy with hyaluronic acid-shelled PPy/CPT nanoparticles results in tumor regression and prevents tumor recurrence and metastasis in 4T1 breast cancer. Biomaterials 2019, 217, 119264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Huang, J.; Zeng, Z.; He, S.; Cheng, P.; Li, J.; Pu, K. Catalytical nano-immunocomplexes for remote-controlled sono-metabolic checkpoint trimodal cancer therapy. Nat. Commun. 2022, 13, 3468. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Feng, L.; Jin, P.; Shen, J.; Lu, J.; Song, Y.; Wang, G.; Chen, Q.; Huang, D.; Zhang, Y.; et al. Cavitation assisted endoplasmic reticulum targeted sonodynamic droplets to enhanced anti-PD-L1 immunotherapy in pancreatic cancer. J. Nanobiotechnol. 2022, 20, 283. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Zhao, L.; Cui, X.; Wu, X.; Zhang, Y.; Guan, X.; Ma, J.; Zhang, W. Cooperating minimalist nanovaccine with PD-1 blockade for effective and feasible cancer immunotherapy. J. Adv. Res. 2022, 35, 49–60. [Google Scholar] [CrossRef]
- Xie, X.; Feng, Y.; Zhang, H.; Su, Q.; Song, T.; Yang, G.; Li, N.; Wei, X.; Li, T.; Qin, X.; et al. Remodeling tumor immunosuppressive microenvironment via a novel bioactive nanovaccines potentiates the efficacy of cancer immunotherapy. Bioact. Mater. 2022, 16, 107–119. [Google Scholar] [CrossRef]
- Bockamp, E.; Rosigkeit, S.; Siegl, D.; Schuppan, D. Nano-Enhanced Cancer Immunotherapy: Immunology Encounters Nanotechnology. Cells 2020, 9, 2102. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Barz, M.; De Geest, B.G.; Diken, M.; Hennink, W.E.; Kiessling, F.; Lammers, T.; Shi, Y. Nanomedicine and macroscale materials in immuno-oncology. Chem. Soc. Rev. 2019, 48, 351–381. [Google Scholar] [CrossRef] [PubMed]
| Main Type | Drug Delivery System | Payload | Assisted Ingredient | Responsive Condition | Ref. |
|---|---|---|---|---|---|
| αPD-1/PD-L1 +photodynamic therapy | Fe-TBP | - | αPD-L1 | - | [39] |
| PGCA nanoparticles | pheophorbide A | αPD-1 | pH sensitive | [46] | |
| ZnP nanoparticles | pyrolipid | αPD-L1 | - | [47] | |
| PcN4/albumin complexes | AQ4N | αPD-L1 | - | [48] | |
| cell membrane nanovesicles expressing PD-1 | DVDMS and PFTBA | - | - | [49] | |
| MMP-2-sensitive nanoparticles | indocyanine green and αPD-L1 | - | MMP-2 sensitive | [55] | |
| lipid-based micellar nanoparticles | chlorin e6 and αPD-L1 | - | pH and MMP-2 dual sensitive | [56] | |
| Chloringlobulin nanocomplexes | chlorin e6 and αPD-L1 | - | - | [57] | |
| αPD-1/PD-L1 +photothermal therapy | PLGA-PEG-GRGDS copolymers | αPD-1, iron oxide, and perfluoropentane | - | - | [60] |
| Lipid gels | IR820 and αPD-L1 | - | - | [61] | |
| mSiO2-PFP-PEG nanoparticles | copper sulfide | αPD-1 | - | [63] | |
| DLMSNs | AUNP-12, copper sulfide, and R848 | - | pH sensitive | [64] | |
| The porous nanocarbon | GOx | αPD-1 | - | [66] | |
| rGO nanosheets | IDO inhibitors | αPD-L1 | - | [69] | |
| FePS3-based nanosheets coated with the CT26 cell membrane | APP | - | - | [71] | |
| hydrogel | BPQD-CCNVs | αPD-1 | - | [73] | |
| liposomes | IR780 and SB-505124 | αPD-1 | - | [75] | |
| nanoparticles | IR780 and APP | - | MMP-2 sensitive | [76] | |
| PPor nanoparticles | - | αPD-1 | - | [77] | |
| Multifunctional nanoparticles | nanoparticles | Ce6, 1-mt and αPD-L1 | - | HAase sensitive | [78] |
| nanoparticles | DOX and Ce6 | αPD-1 | - | [79] | |
| nanoparticles | pyrrole and IRDye800CW | αPD-L1 | - | [80] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Fang, C.; Zhang, K.; Su, C. Recent Advances in Nanoparticles-Based Platforms Targeting the PD-1/PD-L1 Pathway for Cancer Treatment. Pharmaceutics 2022, 14, 1581. https://doi.org/10.3390/pharmaceutics14081581
Yu X, Fang C, Zhang K, Su C. Recent Advances in Nanoparticles-Based Platforms Targeting the PD-1/PD-L1 Pathway for Cancer Treatment. Pharmaceutics. 2022; 14(8):1581. https://doi.org/10.3390/pharmaceutics14081581
Chicago/Turabian StyleYu, Xin, Chao Fang, Kun Zhang, and Chunxia Su. 2022. "Recent Advances in Nanoparticles-Based Platforms Targeting the PD-1/PD-L1 Pathway for Cancer Treatment" Pharmaceutics 14, no. 8: 1581. https://doi.org/10.3390/pharmaceutics14081581
APA StyleYu, X., Fang, C., Zhang, K., & Su, C. (2022). Recent Advances in Nanoparticles-Based Platforms Targeting the PD-1/PD-L1 Pathway for Cancer Treatment. Pharmaceutics, 14(8), 1581. https://doi.org/10.3390/pharmaceutics14081581






