Methylphenidate Analogues as a New Class of Potential Disease-Modifying Agents for Parkinson’s Disease: Evidence from Cell Models and Alpha-Synuclein Transgenic Mice
Abstract
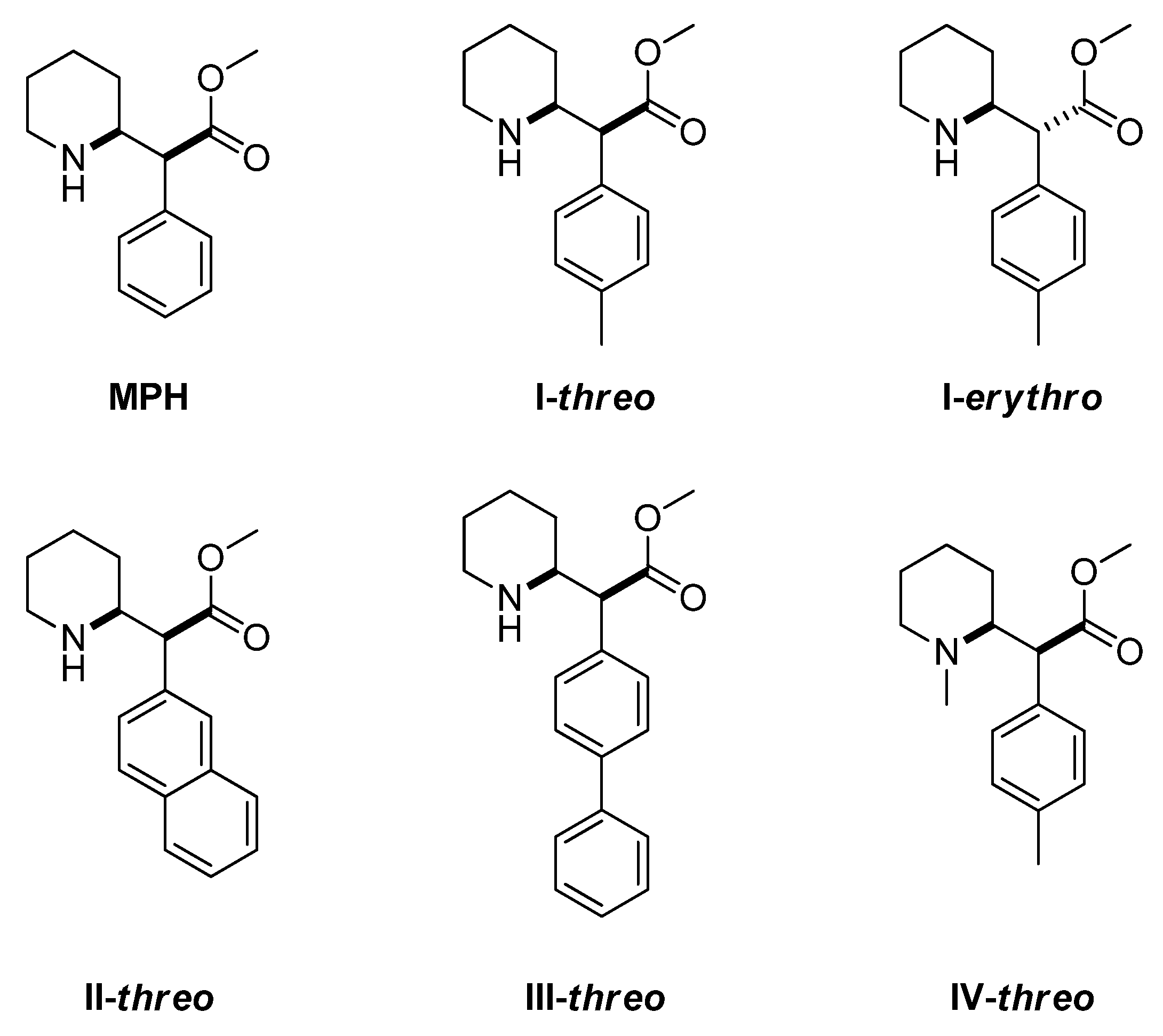
:1. Introduction
2. Materials and Methods
2.1. Chemistry
2.1.1. General
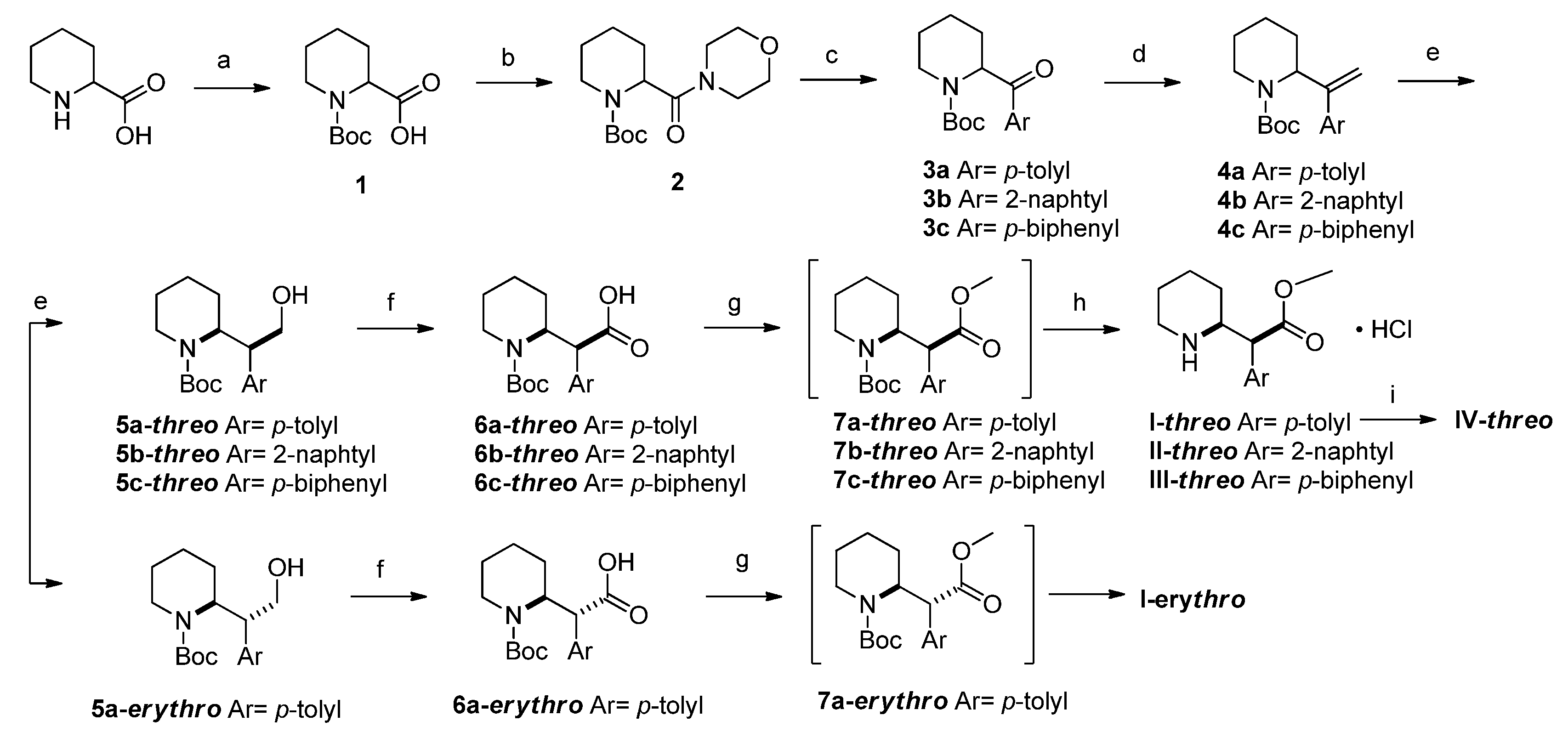
2.1.2. Synthesis
2.2. Animals
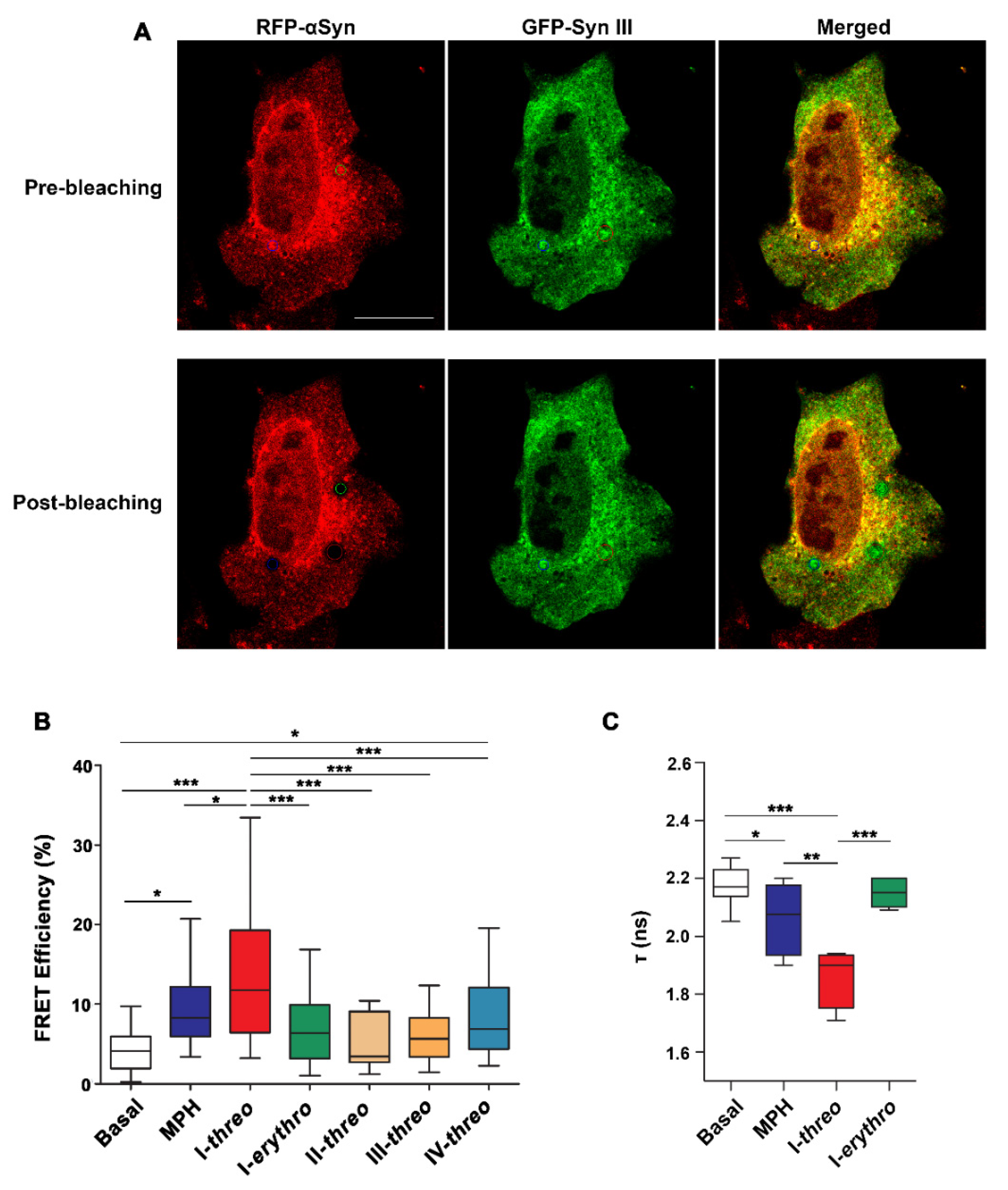
2.3. Acceptor Photobleaching FRET Studies
2.4. Live Fluorescence Lifetime Imaging (FLIM)
2.5. Treatments with MPH-Analogues in the SK-N-SH Cells Overexpressing Human Full Length αSyn
2.6. I-threo and IV-threo Treatments in Primary Mesencephalic Neurons Subjected to Glucose Deprivation (GD)-Induced αSyn Aggregation
2.7. MTT Assay
2.8. Immunocytochemistry
2.9. Open Field Beam Walking Behavioral Tests
2.10. Confocal Microscopy
2.11. Statistical Analysis
2.12. Prediction of Physicochemical Descriptors
2.13. Protein–Protein Docking
2.14. Docking Studies
2.15. MD Simulations
3. Results and Discussion
3.1. FRET Studies Assessing the Ability of MPH Analogues to Influence the Interaction between αSyn and Syn III in SK-N-SH Cells Overexpressing RFP-Tagged αSyn and GFP-Tagged Syn III
3.2. MPH Analogues Did Not Exhibit Toxic Effects at the Concentrations Stimulating the Functional αSyn/Syn III Interaction on Primary Mesencephalic Neurons
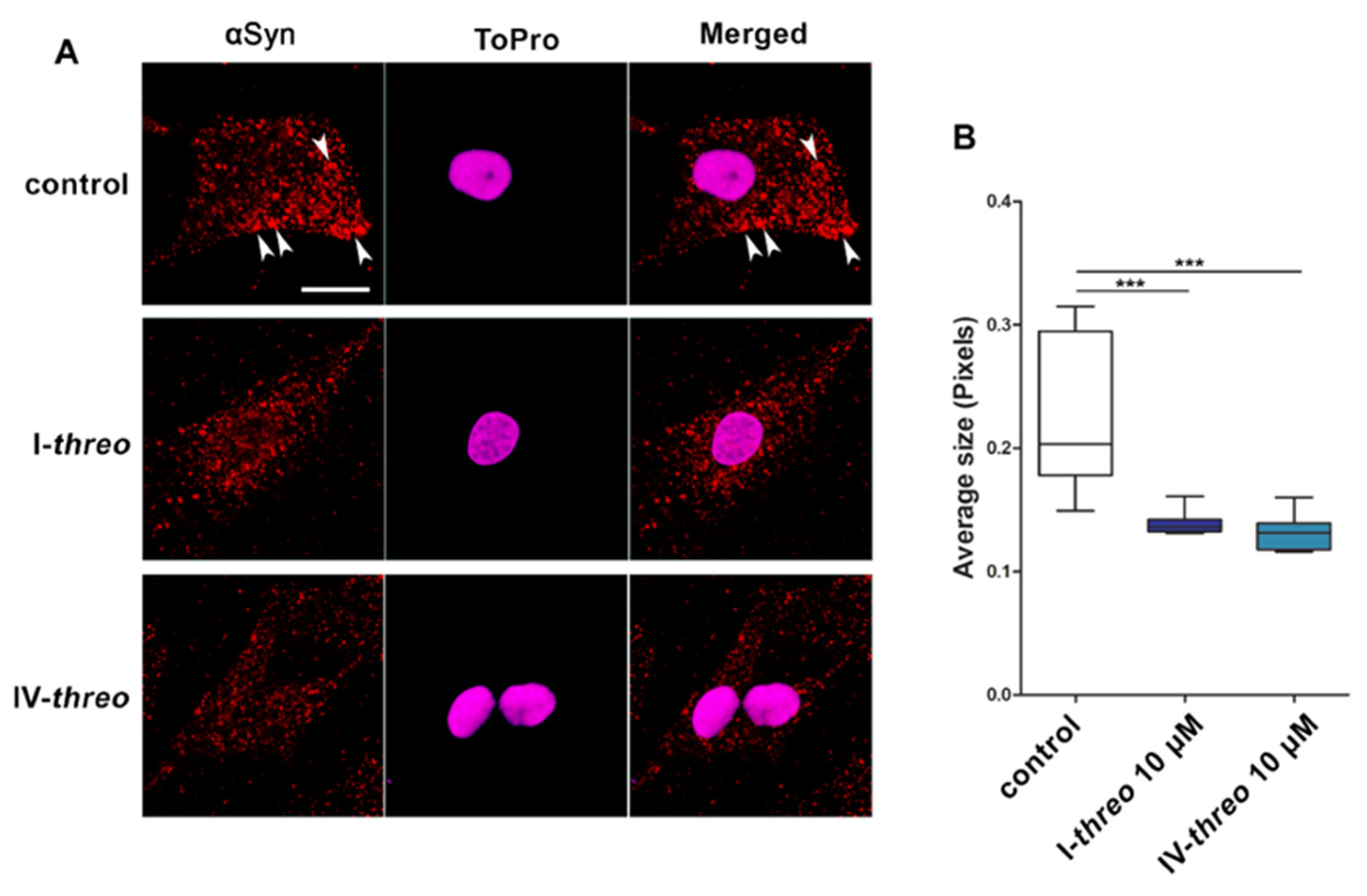
3.3. Compound I-threo Reduced αSyn Aggregation in SK-N-SH Cells Overexpressing αSyn and in Primary Mesencephalic Neurons Exposed to GD
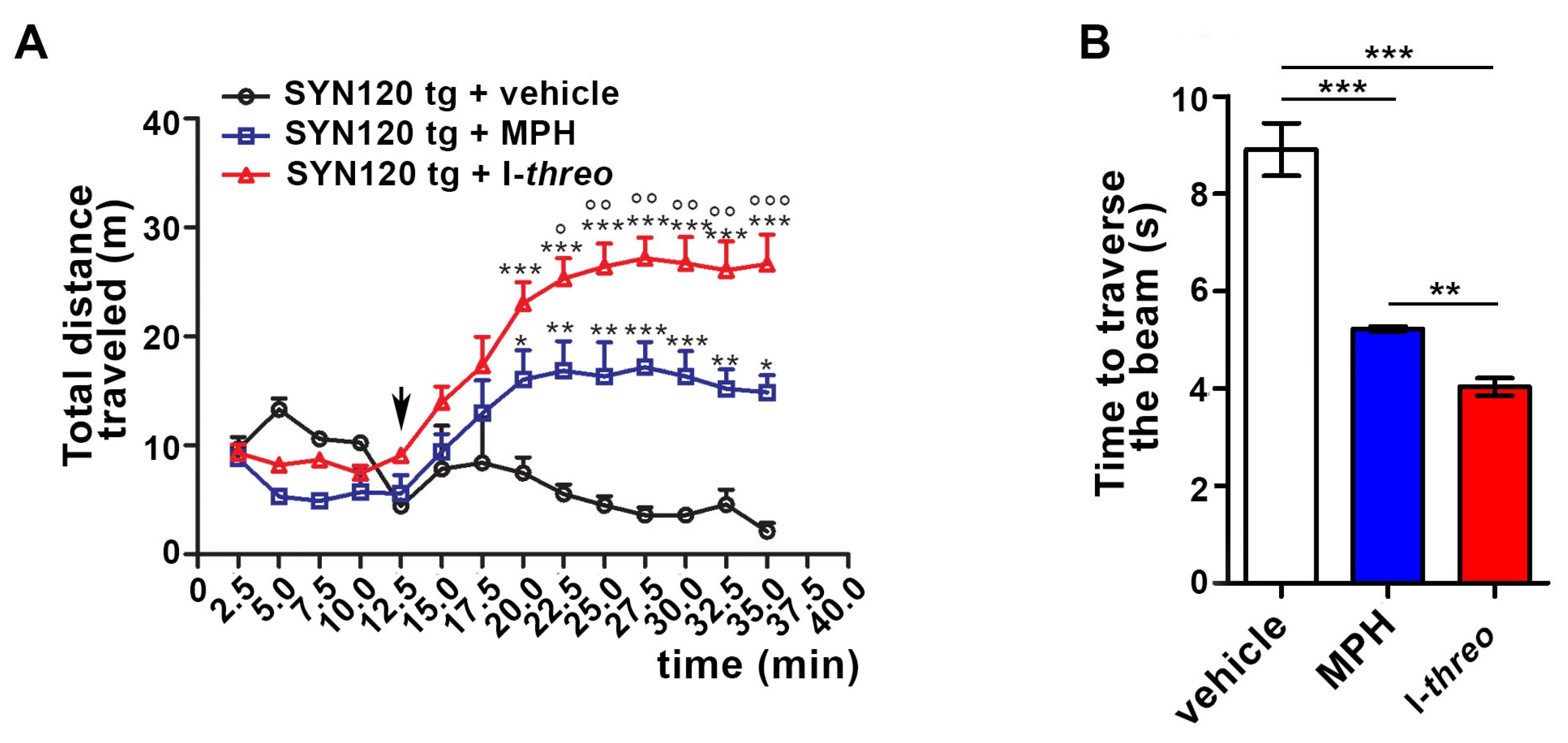
3.4. The Acute i.p. Administration of 10 mg/kg of Compound I-threo Enhanced the Locomotor Activity of 12-Month-Old αSyn tg Mice Significantly More Compared to MPH
3.5. Drug-Likeness
3.6. Generation of the αSyn/Syn III Complex Model
3.7. Docking Studies and Molecular Dynamics Simulations
3.8. Study of the DAT Binding Activity of Compound I-threo
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kile, B.M.; Guillot, T.S.; Venton, B.J.; Wetsel, W.C.; Augustine, G.J.; Wightman, R.M. Synapsins differentially control dopamine and serotonin release. J. Neurosci. 2010, 30, 9762–9770. [Google Scholar] [CrossRef] [PubMed]
- Longhena, F.; Faustini, G.; Spillantini, M.G.; Bellucci, A. Living in Promiscuity: The Multiple Partners of Alpha-Synuclein at the Synapse in Physiology and Pathology. Int. J. Mol. Sci. 2019, 20, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaltieri, M.; Grigoletto, J.; Longhena, F.; Navarria, L.; Favero, G.; Castrezzati, S.; Colivicchi, M.A.; Della Corte, L.; Rezzani, R.; Pizzi, M.; et al. α-synuclein and synapsin III cooperatively regulate synaptic function in dopamine neurons. J. Cell Sci. 2015, 128, 2231–2243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellucci, A.; Mercuri, N.B.; Venneri, A.; Faustini, G.; Longhena, F.; Pizzi, M.; Missale, C.; Spano, P. Review: Parkinson’s disease: From synaptic loss to connectome dysfunction. Neuropathol. Appl. Neurobiol. 2016, 42, 77–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spillantini, M.G.; Goedert, M. Neurodegeneration and the ordered assembly of α-synuclein. Cell Tissue Res. 2018, 373, 137–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goedert, M.; Jakes, R.; Spillantini, M.G. The synucleinopathies: Twenty years on. J. Parkinsons. Dis. 2017, 7, S51–S69. [Google Scholar] [CrossRef] [Green Version]
- Bellucci, A.; Antonini, A.; Pizzi, M.; Spano, P. The End Is the Beginning: Parkinson’s Disease in the Light of Brain Imaging. Front. Aging Neurosci. 2017, 9, 330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunn, B.H.M.; Cragg, S.J.; Bolam, J.P.; Spillantini, M.-G.; Wade-Martins, R. Impaired intracellular trafficking defines early Parkinson’s disease. Trends Neurosci. 2015, 38, 178–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulz-Schaeffer, W.J. The synaptic pathology of alpha-synuclein aggregation in dementia with Lewy bodies, Parkinson’s disease and Parkinson’s disease dementia. Acta Neuropathol. 2010, 120, 131–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kramer, M.L.; Schulz-Schaeffer, W.J. Presynaptic alpha-synuclein aggregates, not Lewy bodies, cause neurodegeneration in dementia with Lewy bodies. J. Neurosci. 2007, 27, 1405–1410. [Google Scholar] [CrossRef] [Green Version]
- Longhena, F.; Faustini, G.; Varanita, T.; Zaltieri, M.; Porrini, V.; Tessari, I.; Poliani, P.L.; Missale, C.; Borroni, B.; Padovani, A.; et al. Synapsin III is a key component of α-synuclein fibrils in Lewy bodies of PD brains. Brain Pathol. 2018, 28, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Faustini, G.; Longhena, F.; Varanita, T.; Bubacco, L.; Pizzi, M.; Missale, C.; Benfenati, F.; Björklund, A.; Spano, P.; Bellucci, A. Synapsin III deficiency hampers α-synuclein aggregation, striatal synaptic damage and nigral cell loss in an AAV-based mouse model of Parkinson’s disease. Acta Neuropathol. 2018, 136, 621–639. [Google Scholar] [CrossRef] [PubMed]
- Faustini, G.; Longhena, F.; Masato, A.; Bassareo, V.; Frau, R.; Klingstedt, T.; Shirani, H.; Brembati, V.; Parrella, E.; Vezzoli, M.; et al. Synapsin III gene silencing redeems alpha-synuclein transgenic mice from Parkinson’s disease-like phenotype. Mol. Ther. 2022, 30, 1465–1483. [Google Scholar] [CrossRef]
- Başay, Ö.; Kabukcu Basay, B.; Alacam, H.; Ozturk, O.; Buber, A.; Gorucu Yilmaz, S.; Kıroğlu, Y.; Erdal, M.E.; Herken, H. The impact of synapsin III gene on the neurometabolite level alterations after single-dose methylphenidate in attention-deficit hyperactivity disorder patients. Neuropsychiatr. Dis. Treat. 2016, 12, 1141–1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chadchankar, H.; Ihalainen, J.; Tanila, H.; Yavich, L. Methylphenidate modifies overflow and presynaptic compartmentalization of dopamine via an α-synuclein-dependent mechanism. J. Pharmacol. Exp. Ther. 2012, 341, 484–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faustini, G.; Longhena, F.; Bruno, A.; Bono, F.; Grigoletto, J.; La Via, L.; Barbon, A.; Casiraghi, A.; Straniero, V.; Valoti, E.; et al. Alpha-synuclein/synapsin III pathological interplay boosts the motor response to methylphenidate. Neurobiol. Dis. 2020, 138, 104789. [Google Scholar] [CrossRef] [PubMed]
- Casiraghi, A.; Longhena, F.; Straniero, V.; Faustini, G.; Newman, A.H.; Bellucci, A.; Valoti, E. Design and Synthesis of Fluorescent Methylphenidate Analogues for a FRET-Based Assay of Synapsin III Binding. ChemMedChem 2020, 15, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Reyes, N.G.D.; Bagnas, M.A.C.; Antonio, A.K.D.; Jamora, R.D.G. High dose methylphenidate in the treatment of freezing of gait in advanced Parkinson’s disease. Basal Ganglia 2018, 11, 8–13. [Google Scholar] [CrossRef]
- Moreau, C.; Delval, A.; Defebvre, L.; Dujardin, K.; Duhamel, A.; Petyt, G.; Vuillaume, I.; Corvol, J.-C.; Brefel-Courbon, C.; Ory-Magne, F.; et al. Methylphenidate for gait hypokinesia and freezing in patients with Parkinson’s disease undergoing subthalamic stimulation: A multicentre, parallel, randomised, placebo-controlled trial. Lancet Neurol. 2012, 11, 589–596. [Google Scholar] [CrossRef]
- Kim, R.; Lee, J.; Kim, Y.; Kim, A.; Jang, M.; Kim, H.-J.; Jeon, B.; Kang, U.J.; Fahn, S. Presynaptic striatal dopaminergic depletion predicts the later development of freezing of gait in de novo Parkinson’s disease: An analysis of the PPMI cohort. Parkinsonism Relat. Disord. 2018, 51, 49–54. [Google Scholar] [CrossRef]
- Bellucci, A.; Casiraghi, A.; Longhena, F.; Straniero, V.; Valoti, E. Structural Analogues of Methylphenidate as Parkinson’s Disease-Modifying. Agents. Patent WO2022/029151 A1, 4 August 2021. [Google Scholar]
- Luethi, D.; Kaeser, P.J.; Brandt, S.D.; Krähenbühl, S.; Hoener, M.C.; Liechti, M.E. Pharmacological profile of methylphenidate-based designer drugs. Neuropharmacology 2018, 134, 133–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellucci, A.; Navarria, L.; Falarti, E.; Zaltieri, M.; Bono, F.; Collo, G.; Spillantini, M.G.; Grazia, M.; Missale, C.; Spano, P. Redistribution of DAT/α-synuclein complexes visualized by “in situ” proximity ligation assay in transgenic mice modelling early Parkinson’s disease. PLoS ONE 2011, 6, e27959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longhena, F.; Faustini, G.; Missale, C.; Pizzi, M.; Bellucci, A. Dopamine Transporter/α-Synuclein Complexes Are Altered in the Post Mortem Caudate Putamen of Parkinson’s Disease: An In Situ Proximity Ligation Assay Study. Int. J. Mol. Sci. 2018, 19, 1611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schweri, M.M.; Deutsch, H.M.; Massey, A.T.; Holtzman, S.G. Biochemical and behavioral characterization of novel methylphenidate analogs. J. Pharmacol. Exp. Ther. 2002, 301, 527–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, H.M.L.; Hopper, D.W.; Hansen, T.; Liu, Q.; Childers, S.R. Synthesis of methylphenidate analogues and their binding affinities at dopamine and serotonin transport sites. Bioorganic Med. Chem. Lett. 2004, 14, 1799–1802. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, H. Synthesis and pharmacology of site specific cocaine abuse treatment agents: A new synthetic methodology for methylphenidate analogs based on the Blaise reaction. Eur. J. Med. Chem. 2001, 36, 303–311. [Google Scholar] [CrossRef]
- Axten, J.M.; Krim, L.; Kung, H.F.; Winkler, J.D. A Stereoselective Synthesis of dl-threo- Methylphenidate: Preparation and Biological Evaluation of Novel Analogues. J. Org. Chem. 1998, 63, 9628–9629. [Google Scholar] [CrossRef]
- Misra, M.; Shi, Q.; Ye, X.; Gruszecka-Kowalik, E.; Bu, W.; Liu, Z.; Schweri, M.M.; Deutsch, H.M.; Venanzi, C.A. Quantitative structure-activity relationship studies of threo-methylphenidate analogs. Bioorganic Med. Chem. 2010, 18, 7221–7238. [Google Scholar] [CrossRef]
- Thai, D.L.; Sapko, M.T.; Reiter, C.T.; Bierer, D.E.; Perel, J.M. Asymmetric synthesis and pharmacology of methylphenidate and its para-substituted derivatives. J. Med. Chem. 1998, 41, 591–601. [Google Scholar] [CrossRef]
- Tofaris, G.K.; Garcia Reitböck, P.; Humby, T.; Lambourne, S.L.; O’Connell, M.; Ghetti, B.; Gossage, H.; Emson, P.C.; Wilkinson, L.S.; Goedert, M.; et al. Pathological changes in dopaminergic nerve cells of the substantia nigra and olfactory bulb in mice transgenic for truncated human alpha-synuclein(1–120): Implications for Lewy body disorders. J. Neurosci. 2006, 26, 3942–3950. [Google Scholar] [CrossRef] [Green Version]
- Winder-Rhodes, S.E.; Garcia-Reitböck, P.; Ban, M.; Evans, J.R.; Jacques, T.S.; Kemppinen, A.; Foltynie, T.; Williams-Gray, C.H.; Chinnery, P.F.; Hudson, G.; et al. Genetic and pathological links between Parkinson’s disease and the lysosomal disorder Sanfilippo syndrome. Mov. Disord. 2012, 27, 312–315. [Google Scholar] [CrossRef]
- Kenworthy, A.K. Imaging protein-protein interactions using fluorescence resonance energy transfer microscopy. Methods 2001, 24, 289–296. [Google Scholar] [CrossRef]
- Vogel, S.S.; van der Meer, B.W.; Blank, P.S. Estimating the distance separating fluorescent protein FRET pairs. Methods 2014, 66, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Bajar, B.T.; Wang, E.S.; Zhang, S.; Lin, M.Z.; Chu, J. A Guide to Fluorescent Protein FRET Pairs. Sensors 2016, 16, 1488. [Google Scholar] [CrossRef] [PubMed]
- Porton, B.; Kao, H.T.; Greengard, P. Characterization of transcripts from the synapsin III gene locus. J. Neurochem. 1999, 73, 2266–2271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarria, L.; Zaltieri, M.; Longhena, F.; Spillantini, M.G.; Missale, C.; Spano, P.; Bellucci, A. Alpha-synuclein modulates NR2B-containing NMDA receptors and decreases their levels after rotenone exposure. Neurochem. Int. 2015, 85–86, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa-Ankerhold, H.C.; Ankerhold, R.; Drummen, G.P.C. Advanced fluorescence microscopy techniques--FRAP, FLIP, FLAP, FRET and FLIM. Molecules 2012, 17, 4047–4132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakowicz, J.R. (Ed.) Principles of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 2006; ISBN 978-0-387-31278-1. [Google Scholar]
- Fares, M.-B.; Maco, B.; Oueslati, A.; Rockenstein, E.; Ninkina, N.; Buchman, V.L.; Masliah, E.; Lashuel, H.A. Induction of de novo α-synuclein fibrillization in a neuronal model for Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2016, 113, E912–E921. [Google Scholar] [CrossRef] [Green Version]
- Daina, A.; Zoete, V. A BOILED-Egg to Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef] [Green Version]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Di, Z.; Zhou, P.; Li, B.; Huang, S.-Y. HDOCK: A web server for protein-protein and protein-DNA/RNA docking based on a hybrid strategy. Nucleic Acids Res. 2017, 45, W365–W373. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Tao, H.; He, J.; Huang, S.-Y. The HDOCK server for integrated protein-protein docking. Nat. Protoc. 2020, 15, 1829–1852. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wen, Z.; Wang, X.; Huang, S.-Y. Addressing recent docking challenges: A hybrid strategy to integrate template-based and free protein-protein docking. Proteins 2017, 85, 497–512. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-Y.; Zou, X. An iterative knowledge-based scoring function for protein-protein recognition. Proteins 2008, 72, 557–579. [Google Scholar] [CrossRef]
- Huang, S.-Y.; Zou, X. A knowledge-based scoring function for protein-RNA interactions derived from a statistical mechanics-based iterative method. Nucleic Acids Res. 2014, 42, e55. [Google Scholar] [CrossRef] [PubMed]
- PDB 2P0A; 10.2210/pdb2p0a/pdb. Available online: https://www.rcsb.org/structure/2p0a (accessed on 17 November 2021).
- Rao, J.N.; Jao, C.C.; Hegde, B.G.; Langen, R.; Ulmer, T.S. A combinatorial NMR and EPR approach for evaluating the structural ensemble of partially folded proteins. J. Am. Chem. Soc. 2010, 132, 8657–8668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez, J.; Doerr, S.; Martínez-Rosell, G.; Rose, A.S.; de Fabritiis, G. DeepSite: Protein-binding site predictor using 3D-convolutional neural networks. Bioinformatics 2017, 33, 3036–3042. [Google Scholar] [CrossRef] [Green Version]
- Galvelis, R.; Doerr, S.; Damas, J.M.; Harvey, M.J.; de Fabritiis, G. A Scalable Molecular Force Field Parameterization Method Based on Density Functional Theory and Quantum-Level Machine Learning. J. Chem. Inf. Model. 2019, 59, 3485–3493. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Rosell, G.; Giorgino, T.; de Fabritiis, G. PlayMolecule ProteinPrepare: A Web Application for Protein Preparation for Molecular Dynamics Simulations. J. Chem. Inf. Model. 2017, 57, 1511–1516. [Google Scholar] [CrossRef]
- Bellucci, A.; Collo, G.; Sarnico, I.; Battistin, L.; Missale, C.; Spano, P. Alpha-synuclein aggregation and cell death triggered by energy deprivation and dopamine overload are counteracted by D2/D3 receptor activation. J. Neurochem. 2008, 106, 560–577. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Reitböck, P.; Anichtchik, O.; Bellucci, A.; Iovino, M.; Ballini, C.; Fineberg, E.; Ghetti, B.; Della Corte, L.; Spano, P.; Tofaris, G.K.; et al. SNARE protein redistribution and synaptic failure in a transgenic mouse model of Parkinson’s disease. Brain 2010, 133, 2032–2044. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, H.M.; Shi, Q.; Gruszecka-Kowalik, E.; Schweri, M.M. Synthesis and pharmacology of potential cocaine antagonists. 2. Structure-activity relationship studies of aromatic ring-substituted methylphenidate analogs. J. Med. Chem. 1996, 39, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, M.; Sharma, M.; Romanos, M.; Lesch, K.-P.; Walitza, S.; Conzelmann, H.A.; Krüger, R.; Renner, T.J. Family-based association study on functional α-synuclein polymorphisms in attention-deficit/hyperactivity disorder. Atten. Defic. Hyperact. Disord. 2019, 11, 107–111. [Google Scholar] [CrossRef]
- Kenar, A.N.İ.; Edgünlü, T.; Herken, H.; Erdal, M.E. Association of synapsin III gene with adult attention deficit hyperactivity disorder. DNA Cell Biol. 2013, 32, 430–434. [Google Scholar] [CrossRef]
- Kong, C.; Jana, N.; Driver, T.G. Rh2(II)-catalyzed selective aminomethylene migration from styryl azides. Org. Lett. 2013, 15, 824–827. [Google Scholar] [CrossRef]
- Jin, J.; Wang, Y.; Wang, F.; Shi, D.; Erhard, K.F.; Wu, Z.; Guida, B.F.; Lawrence, S.K.; Behm, D.J.; Disa, J.; et al. 2-Aminomethyl piperidines as novel urotensin-II receptor antagonists. Bioorg. Med. Chem. Lett. 2008, 18, 2860–2864. [Google Scholar] [CrossRef]
- Kondoh, A.; Ojima, R.; Terada, M. Formal Fluorinative Ring Opening of 2-Benzoylpyrrolidines Utilizing 1,2-Phospha-Brook Rearrangement for Synthesis of 2-Aryl-3-fluoropiperidines. Org. Lett. 2021, 23, 7894–7899. [Google Scholar] [CrossRef]
- Ojo, B. Efficient Synthesis of a New Series of Piperidine Ring–Modified Alcohol and Methyl Ether Analogs of (±)- threo -Methyl Phenyl(piperidin-2-yl)acetate. Synth. Commun. 2012, 42, 2818–2830. [Google Scholar] [CrossRef]
- Guthrie, D.A.; Klein Herenbrink, C.; Lycas, M.D.; Ku, T.; Bonifazi, A.; DeVree, B.T.; Mathiasen, S.; Javitch, J.A.; Grimm, J.B.; Lavis, L.; et al. Novel Fluorescent Ligands Enable Single-Molecule Localization Microscopy of the Dopamine Transporter. ACS Chem. Neurosci. 2020, 11, 3288–3300. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Hernandez, G.A.; Casiraghi, A.; Rudin, D.; Luethi, D.; Ku, T.C.; Guthrie, D.A.; Straniero, V.; Valoti, E.; Schütz, G.J.; Sitte, H.H.; et al. Illuminating the norepinephrine transporter: Fluorescent probes based on nisoxetine and talopram. RSC Med. Chem. 2021, 12, 1174–1186. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casiraghi, A.; Longhena, F.; Faustini, G.; Ribaudo, G.; Suigo, L.; Camacho-Hernandez, G.A.; Bono, F.; Brembati, V.; Newman, A.H.; Gianoncelli, A.; et al. Methylphenidate Analogues as a New Class of Potential Disease-Modifying Agents for Parkinson’s Disease: Evidence from Cell Models and Alpha-Synuclein Transgenic Mice. Pharmaceutics 2022, 14, 1595. https://doi.org/10.3390/pharmaceutics14081595
Casiraghi A, Longhena F, Faustini G, Ribaudo G, Suigo L, Camacho-Hernandez GA, Bono F, Brembati V, Newman AH, Gianoncelli A, et al. Methylphenidate Analogues as a New Class of Potential Disease-Modifying Agents for Parkinson’s Disease: Evidence from Cell Models and Alpha-Synuclein Transgenic Mice. Pharmaceutics. 2022; 14(8):1595. https://doi.org/10.3390/pharmaceutics14081595
Chicago/Turabian StyleCasiraghi, Andrea, Francesca Longhena, Gaia Faustini, Giovanni Ribaudo, Lorenzo Suigo, Gisela Andrea Camacho-Hernandez, Federica Bono, Viviana Brembati, Amy Hauck Newman, Alessandra Gianoncelli, and et al. 2022. "Methylphenidate Analogues as a New Class of Potential Disease-Modifying Agents for Parkinson’s Disease: Evidence from Cell Models and Alpha-Synuclein Transgenic Mice" Pharmaceutics 14, no. 8: 1595. https://doi.org/10.3390/pharmaceutics14081595





