Paracrine Factors of Stressed Peripheral Blood Mononuclear Cells Activate Proangiogenic and Anti-Proteolytic Processes in Whole Blood Cells and Protect the Endothelial Barrier
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
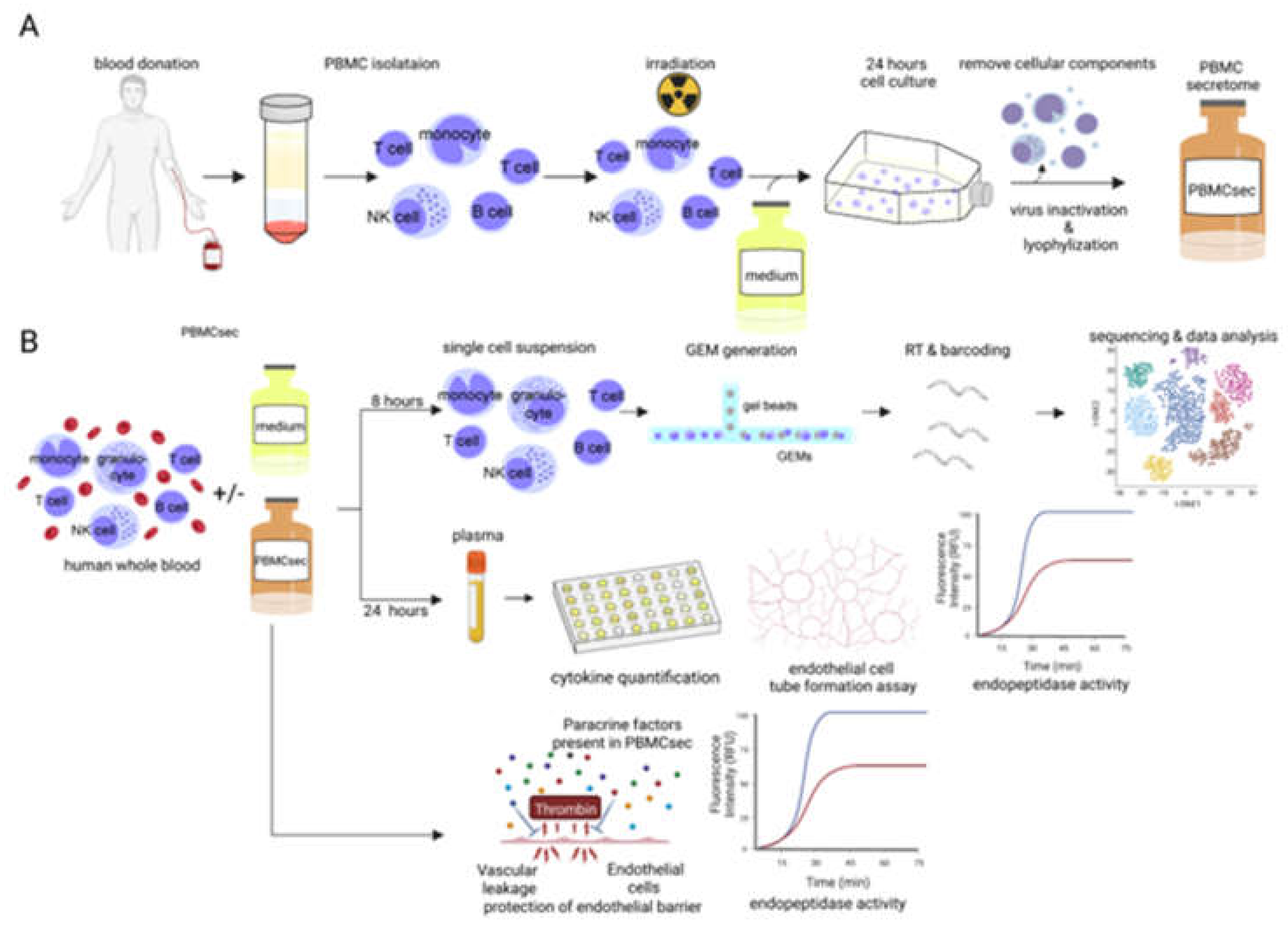
2.2. Generation of PBMCsec
2.3. Preparation of Single-Cell Suspension of Human Whole Blood
2.4. Dermal Microvascular Endothelial Cell Culture
2.5. Tube Formation Assay
2.6. Protein Quantification by Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Protease Activity Assays
2.8. Electrical Cell-Substrate Impedance Sensing (ECIS)
2.9. Gel Bead-In Emulsion (GEMs) and Library Preparation
2.10. Data Analysis
2.11. Statistical Analysis
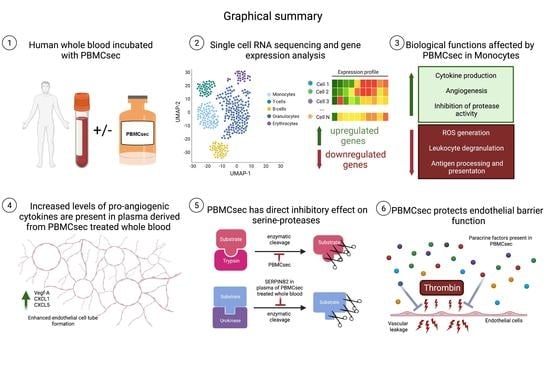
3. Results
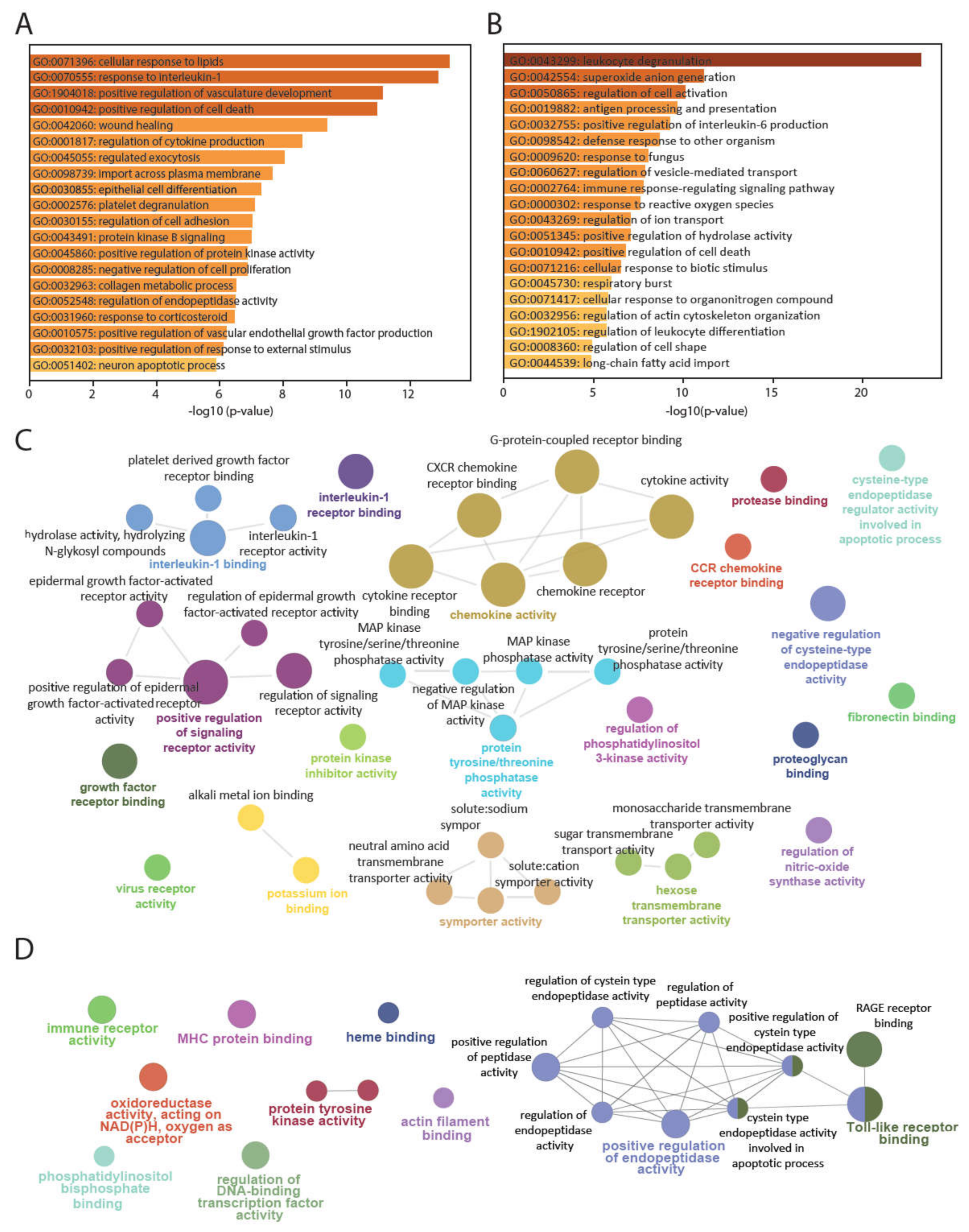
3.1. PBMCsec Modulates the Gene Signature of T Cells, B Cells, Granulocytes, and Monocytes
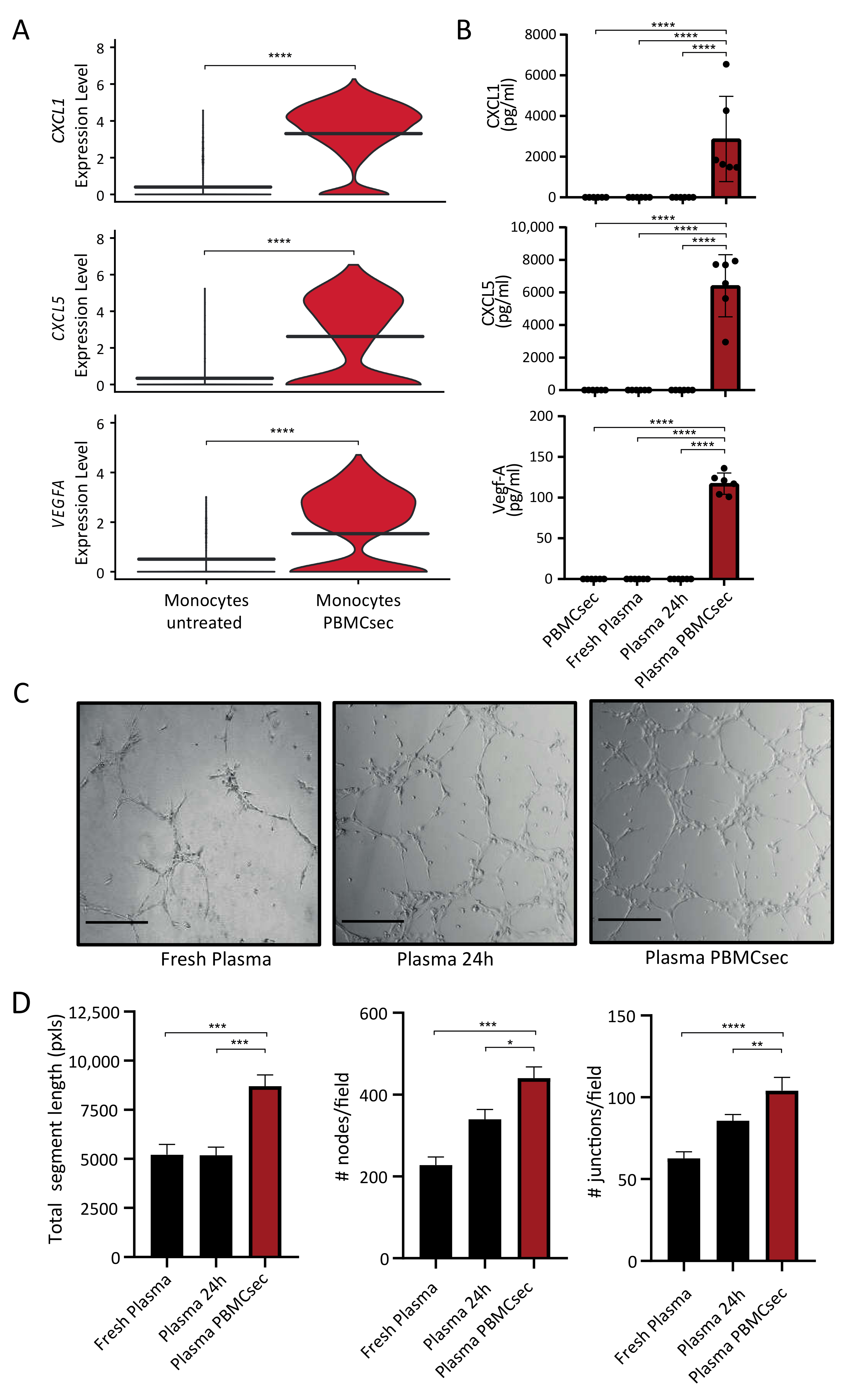
3.2. PBMCsec Induces Tissue-Regenerative Pathways in Monocytes from Human Whole Blood
3.3. Paracrine Factors in the Plasma of PBMCsec-Treated Whole Blood Exert Proangiogenetic Effects In Vitro
3.4. PBMCsec Inhibits Protease Activity In Vitro and Induces the Selective Serine Protease SERPINB2 in Human Whole Blood Ex Vivo
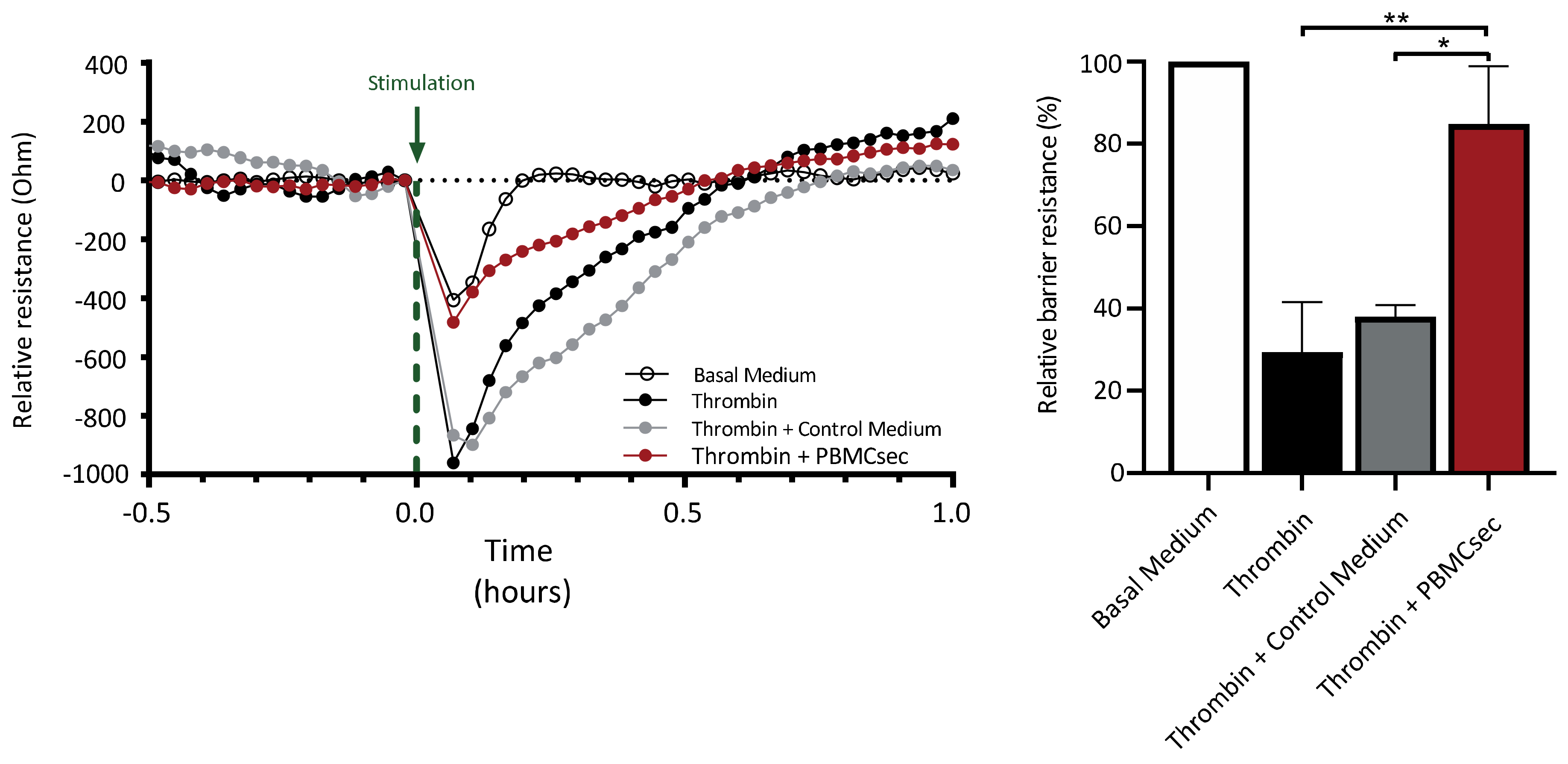
3.5. PBMCsec Ameliorates Thrombin-Induced Decrease in Endothelial Barrier Function
4. Discussion
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, P.; Kandoi, S.; Misra, R.; Vijayalakshmi, S.; Rajagopal, K.; Verma, R.S. The mesenchymal stem cell secretome: A new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev. 2019, 46, 1–9. [Google Scholar]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, B.; Chhatbar, P.Y.; Dong, Y.; Alawieh, A.; Lowe, F.; Hu, X.; Feng, W. Mesenchymal Stem Cell Therapy in Stroke: A Systematic Review of Literature in Pre-Clinical and Clinical Research. Cell Transpl. 2018, 27, 1723–1730. [Google Scholar] [CrossRef]
- Gyöngyösi, M.; Haller, P.M.; Blake, D.J.; Rendon, E.M. Meta-Analysis of Cell Therapy Studies in Heart Failure and Acute Myocardial Infarction. Circ. Res. 2018, 123, 301–308. [Google Scholar] [CrossRef]
- Eggenhofer, E.; Benseler, V.; Kroemer, A.; Popp, F.C.; Geissler, E.; Schlitt, H.J.; Baan, C.C.; Dahlke, M.H.; Hoogduijn, M.J. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front. Immunol. 2012, 3, 297. [Google Scholar] [CrossRef]
- Thum, T.; Bauersachs, J.; Poole-Wilson, P.A.; Volk, H.D.; Anker, S.D. The dying stem cell hypothesis: Immune modulation as a novel mechanism for progenitor cell therapy in cardiac muscle. J. Am. Coll. Cardiol. 2005, 46, 1799–1802. [Google Scholar] [CrossRef]
- Takahashi, M.; Li, T.-S.; Suzuki, R.; Kobayashi, T.; Ito, H.; Ikeda, Y.; Matsuzaki, M.; Hamano, K. Cytokines produced by bone marrow cells can contribute to functional improvement of the infarcted heart by protecting cardiomyocytes from ischemic injury. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H886–H893. [Google Scholar] [CrossRef]
- Gnecchi, M.; He, H.; Noiseux, N.; Liang, O.D.; Zhang, L.; Morello, F.; Mu, H.; Melo, L.G.; Pratt, R.E.; Ingwall, J.S.; et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006, 20, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.-T.; Phuong, T.N.T.; Tien, N.L.B.; Tran, D.K.; Van Thanh, V.; Quang, T.L.; Truong, D.T.; Pham, V.H.; Ngoc, V.T.N.; Chu-Dinh, T.; et al. An Update on the Progress of Isolation, Culture, Storage, and Clinical Application of Human Bone Marrow Mesenchymal Stem/Stromal Cells. Int. J. Mol. Sci. 2020, 21, 708. [Google Scholar] [CrossRef] [PubMed]
- Ankersmit, H.J.; Hoetzenecker, K.; Dietl, W.; Soleiman, A.; Horvat, R.; Wolfsberger, M.; Gerner, C.; Hacker, S.; Mildner, M.; Moser, B.; et al. Irradiated cultured apoptotic peripheral blood mononuclear cells regenerate infarcted myocardium. Eur. J. Clin. Investig. 2009, 39, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Korf-Klingebiel, M.; Kempf, T.; Sauer, T.; Brinkmann, E.; Fischer, P.; Meyer, G.P.; Ganser, A.; Drexler, H.; Wollert, K.C. Bone marrow cells are a rich source of growth factors and cytokines: Implications for cell therapy trials after myocardial infarction. Eur. Heart J. 2008, 29, 2851–2858. [Google Scholar] [CrossRef]
- Wagner, T.; Traxler, D.; Simader, E.; Beer, L.; Narzt, M.-S.; Gruber, F.; Madlener, S.; Laggner, M.; Erb, M.; Vorstandlechner, V.; et al. Different pro-angiogenic potential of γ-irradiated PBMC-derived secretome and its subfractions. Sci. Rep. 2018, 8, 18016. [Google Scholar] [CrossRef]
- Beer, L.; Zimmermann, M.; Mitterbauer, A.; Ellinger, A.; Gruber, F.; Narzt, M.-S.; Zellner, M.; Gyöngyösi, M.; Madlener, S.; Simader, E.; et al. Analysis of the Secretome of Apoptotic Peripheral Blood Mononuclear Cells: Impact of Released Proteins and Exosomes for Tissue Regeneration. Sci. Rep. 2015, 5, 16662. [Google Scholar] [CrossRef]
- Kasiri, M.M.; Beer, L.; Nemec, L.; Gruber, F.; Pietkiewicz, S.; Haider, T.; Simader, E.M.; Traxler-Weidenauer, D.; Schweiger, T.; Janik, S.; et al. Dying blood mononuclear cell secretome exerts antimicrobial activity. Eur. J. Clin. Investig. 2016, 46, 853–863. [Google Scholar] [CrossRef]
- Lichtenauer, M.; Mildner, M.; Hoetzenecker, K.; Zimmermann, M.; Podesser, B.K.; Sipos, W.; Berényi, E.; Dworschak, M.; Tschachler, E.; Gyöngyösi, M.; et al. Secretome of apoptotic peripheral blood cells (APOSEC) confers cytoprotection to cardiomyocytes and inhibits tissue remodelling after acute myocardial infarction: A preclinical study. Basic Res. Cardiol. 2011, 106, 1283–1297. [Google Scholar] [CrossRef]
- Hoetzenecker, K.; Zimmermann, M.; Hoetzenecker, W.; Schweiger, T.; Kollmann, D.; Mildner, M.; Hegedus, B.; Mitterbauer, A.; Hacker, S.; Birner, P.; et al. Mononuclear cell secretome protects from experimental autoimmune myocarditis. Eur. Heart J. 2015, 36, 676–685. [Google Scholar] [CrossRef]
- Mildner, M.; Hacker, S.; Haider, T.; Gschwandtner, M.; Werba, G.; Barresi, C.; Zimmermann, M.; Golabi, B.; Tschachler, E.; Ankersmit, H.J. Secretome of peripheral blood mononuclear cells enhances wound healing. PLoS ONE 2013, 8, e60103. [Google Scholar] [CrossRef]
- Hoetzenecker, K.; Assinger, A.; Lichtenauer, M.; Mildner, M.; Schweiger, T.; Starlinger, P.; Jakab, A.; Berényi, E.; Pavo, N.; Zimmermann, M.; et al. Secretome of apoptotic peripheral blood cells (APOSEC) attenuates microvascular obstruction in a porcine closed chest reperfused acute myocardial infarction model: Role of platelet aggregation and vasodilation. Basic Res. Cardiol. 2012, 107, 292. [Google Scholar] [CrossRef]
- Laggner, M.; Copic, D.; Nemec, L.; Vorstandlechner, V.; Gugerell, A.; Gruber, F.; Peterbauer, A.; Ankersmit, H.J.; Mildner, M. Therapeutic potential of lipids obtained from γ-irradiated PBMCs in dendritic cell-mediated skin inflammation. EBioMedicine 2020, 55, 102774. [Google Scholar] [CrossRef]
- Laggner, M.; Acosta, G.S.; Kitzmüller, C.; Copic, D.; Gruber, F.; Altenburger, L.M.; Vorstandlechner, V.; Gugerell, A.; Direder, M.; Klas, K.; et al. The secretome of irradiated peripheral blood mononuclear cells attenuates activation of mast cells and basophils. eBioMedicine 2022, 81, 104093. [Google Scholar] [CrossRef]
- Beer, L.; Mildner, M.; Gyöngyösi, M.; Ankersmit, H.J. Peripheral blood mononuclear cell secretome for tissue repair. Apoptosis 2016, 21, 1336–1353. [Google Scholar] [CrossRef]
- Hacker, S.; Mittermayr, R.; Traxler, D.; Keibl, C.; Resch, A.; Salminger, S.; Leiss, H.; Hacker, P.; Gabriel, C.; Golabi, B.; et al. The secretome of stressed peripheral blood mononuclear cells increases tissue survival in a rodent epigastric flap model. Bioeng. Transl. Med. 2021, 6, e10186. [Google Scholar] [CrossRef]
- Hacker, S.; Mittermayr, R.; Nickl, S.; Haider, T.; Lebherz-Eichinger, D.; Beer, L.; Mitterbauer, A.; Leiss, H.; Zimmermann, M.; Schweiger, T.; et al. Paracrine Factors from Irradiated Peripheral Blood Mononuclear Cells Improve Skin Regeneration and Angiogenesis in a Porcine Burn Model. Sci. Rep. 2016, 6, 25168. [Google Scholar] [CrossRef]
- Altmann, P.; Mildner, M.; Haider, T.; Traxler, D.; Beer, L.; Ristl, R.; Golabi, B.; Gabriel, C.; Leutmezer, F.; Ankersmit, H.J. Secretomes of apoptotic mononuclear cells ameliorate neurological damage in rats with focal ischemia. F1000Research 2014, 3, 131. [Google Scholar] [CrossRef]
- Haider, T.; Höftberger, R.; Rüger, B.; Mildner, M.; Blumer, R.; Mitterbauer, A.; Buchacher, T.; Sherif, C.; Altmann, P.; Redl, H.; et al. The secretome of apoptotic human peripheral blood mononuclear cells attenuates secondary damage following spinal cord injury in rats. Exp. Neurol. 2015, 267, 230–242. [Google Scholar] [CrossRef]
- Mildner, C.S.; Copic, D.; Zimmermann, M.; Lichtenauer, M.; Direder, M.; Klas, K.; Bormann, D.; Gugerell, A.; Moser, B.; Hoetzenecker, K.; et al. Secretome of Stressed Peripheral Blood Mononuclear Cells Alters Transcriptome Signature in Heart, Liver, and Spleen after an Experimental Acute Myocardial Infarction: An In Silico Analysis. Biology 2022, 11, 116. [Google Scholar] [CrossRef]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Neri, M.; Riezzo, I.; Pascale, N.; Pomara, C.; Turillazzi, E. Ischemia/Reperfusion Injury following Acute Myocardial Infarction: A Critical Issue for Clinicians and Forensic Pathologists. Mediat. Inflamm. 2017, 2017, 7018393. [Google Scholar] [CrossRef]
- Garcia-Dorado, D.; Ruiz-Meana, M.; Inserte, J.; Rodriguez-Sinovas, A.; Piper, H.M. Calcium-mediated cell death during myocardial reperfusion. Cardiovasc. Res. 2012, 94, 168–180. [Google Scholar] [CrossRef]
- Javadov, S.; Karmazyn, M. Mitochondrial permeability transition pore opening as an endpoint to initiate cell death and as a putative target for cardioprotection. Cell Physiol. Biochem. 2007, 20, 1–22. [Google Scholar] [CrossRef]
- He, P.; Talukder, M.A.H.; Gao, F. Oxidative Stress and Microvessel Barrier Dysfunction. Front. Physiol. 2020, 11, 472. [Google Scholar] [CrossRef] [PubMed]
- Toldo, S.; Mauro, A.G.; Cutter, Z.; Abbate, A. Inflammasome, pyroptosis, and cytokines in myocardial ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1553–H1568. [Google Scholar] [CrossRef] [PubMed]
- Sharony, R.; Yu, P.-J.; Park, J.; Galloway, A.C.; Mignatti, P.; Pintucci, G. Protein targets of inflammatory serine proteases and cardiovascular disease. J. Inflamm. 2010, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Carden, D.L.; Granger, D.N. Pathophysiology of ischaemia-reperfusion injury. J. Pathol. 2000, 190, 255–266. [Google Scholar] [CrossRef]
- Laggner, M.; Gugerell, A.; Bachmann, C.; Hofbauer, H.; Vorstandlechner, V.; Seibold, M.; Gouya Lechner, G.; Peterbauer, A.; Madlener, S.; Demyanets, S.; et al. Reproducibility of GMP-compliant production of therapeutic stressed peripheral blood mononuclear cell-derived secretomes, a novel class of biological medicinal products. Stem Cell Res. Ther. 2020, 11, 9. [Google Scholar] [CrossRef]
- DeCicco-Skinner, K.L.; Henry, G.H.; Cataisson, C.; Tabib, T.; Gwilliam, J.C.; Watson, N.J.; Bullwinkle, E.M.; Falkenburg, L.; O’Neill, R.C.; Morin, A.; et al. Endothelial cell tube formation assay for the in vitro study of angiogenesis. J. Vis. Exp. 2014, 91, e51312. [Google Scholar] [CrossRef]
- Carpentier, G.; Berndt, S.; Ferratge, S.; Rasband, W.; Cuendet, M.; Uzan, G.; Albanese, P. Angiogenesis Analyzer for ImageJ—A comparative morphometric analysis of “Endothelial Tube Formation Assay” and “Fibrin Bead Assay”. Sci. Rep. 2020, 10, 11568. [Google Scholar] [CrossRef]
- Holzner, S.; Bromberger, S.; Wenzina, J.; Neumüller, K.; Holper, T.-M.; Petzelbauer, P.; Bauer, W.; Weber, B.; Schossleitner, K. Phosphorylated cingulin localises GEF-H1 at tight junctions to protect vascular barriers in blood endothelial cells. J. Cell Sci. 2021, 134, jcs258557. [Google Scholar] [CrossRef]
- Stuart, T.; Butler, A.; Hoffman, P.; Hafemeister, C.; Papalexi, E.; Mauck, W.M., III; Hao, Y.; Stoeckius, M.; Smibert, P.; Satija, R. Comprehensive Integration of Single-Cell Data. Cell 2019, 177, 1888–1902.e21. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.-H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef]
- Julier, Z.; Park, A.J.; Briquez, P.S.; Martino, M.M. Promoting tissue regeneration by modulating the immune system. Acta Biomater. 2017, 53, 13–28. [Google Scholar] [CrossRef]
- Urso, A.; Prince, A. Anti-Inflammatory Metabolites in the Pathogenesis of Bacterial Infection. Front. Cell Infect. Microbiol. 2022, 12, 925746. [Google Scholar] [CrossRef]
- Kłoczko, J.; Bielawiec, M.; Giedrojć, J.; Radziwon, P.; Galar, M. Human monocytes release plasma serine protease inhibitors in vitro. Haemostasis 1990, 20, 229–232. [Google Scholar] [CrossRef]
- Ritchie, H.; Robbie, L.A.; Kinghorn, S.; Exley, R.; Booth, N.A. Monocyte plasminogen activator inhibitor 2 (PAI-2) inhibits u-PA-mediated fibrin clot lysis and is cross-linked to fibrin. Thromb. Haemost. 1999, 81, 96–103. [Google Scholar]
- Slack, M.A.; Gordon, S.M. Protease Activity in Vascular Disease. Arterioscler. Thromb. Vasc. Biol. 2019, 39, e210–e218. [Google Scholar] [CrossRef]
- Szulcek, R.; Bogaard, H.J.; van Nieuw Amerongen, G.P. Electric cell-substrate impedance sensing for the quantification of endothelial proliferation, barrier function, and motility. J. Vis. Exp. 2014, 3, e51300. [Google Scholar] [CrossRef]
- Park-Windhol, C.; D’Amore, P.A. Disorders of Vascular Permeability. Annu. Rev. Pathol. Mech. Dis. 2016, 11, 251–281. [Google Scholar] [CrossRef]
- Simader, E.; Traxler, D.; Kasiri, M.M.; Hofbauer, H.; Wolzt, M.; Glogner, C.; Storka, A.; Mildner, M.; Gouya, G.; Geusau, A.; et al. Safety and tolerability of topically administered autologous, apoptotic PBMC secretome (APOSEC) in dermal wounds: A randomized Phase 1 trial (MARSYAS I). Sci. Rep. 2017, 7, 6216. [Google Scholar] [CrossRef]
- Gugerell, A.; Gouya-Lechner, G.; Hofbauer, H.; Laggner, M.; Trautinger, F.; Almer, G.; Peterbauer-Scherb, A.; Seibold, M.; Hoetzenecker, W.; Dreschl, C.; et al. Safety and clinical efficacy of the secretome of stressed peripheral blood mononuclear cells in patients with diabetic foot ulcer-study protocol of the randomized, placebo-controlled, double-blind, multicenter, international phase II clinical trial MARSYAS II. Trials 2021, 22, 10. [Google Scholar]
- Beez, C.M.; Haag, M.; Klein, O.; Van Linthout, S.; Sittinger, M.; Seifert, M. Extracellular vesicles from regenerative human cardiac cells act as potent immune modulators by priming monocytes. J. Nanobiotechnol. 2019, 17, 72. [Google Scholar] [CrossRef]
- Guillén, M.I.; Platas, J.; del Caz, M.D.P.; Mirabet, V.; Alcaraz, M.J. Paracrine Anti-inflammatory Effects of Adipose Tissue-Derived Mesenchymal Stem Cells in Human Monocytes. Front. Physiol. 2018, 9, 661. [Google Scholar] [CrossRef]
- Monaco, G.; Lee, B.; Xu, W.; Mustafah, S.; Hwang, Y.Y.; Carré, C.; Burdin, N.; Visan, L.; Ceccarelli, M.; Poidinger, M.; et al. RNA-Seq Signatures Normalized by mRNA Abundance Allow Absolute Deconvolution of Human Immune Cell Types. Cell Rep. 2019, 26, 1627–1640.e7. [Google Scholar] [CrossRef]
- Lee, K.A.; Flores, R.R.; Jang, I.H.; Saathoff, A.; Robbins, P.D. Immune Senescence, Immunosenescence and Aging. Front. Aging 2022, 3, 900028. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, M.; Huang, W.; Chen, W.; Zhao, Y.; Schulte, M.L.; Volberding, P.; Gerbec, Z.; Zimmermann, M.T.; Zeighami, A.; et al. Mitochondrial Metabolic Reprogramming by CD36 Signaling Drives Macrophage Inflammatory Responses. Circ. Res. 2019, 125, 1087–1102. [Google Scholar] [CrossRef]
- Bekkering, S.; Quintin, J.; Joosten, L.A.; van der Meer, J.W.; Netea, M.G.; Riksen, N.P. Oxidized low-density lipoprotein induces long-term proinflammatory cytokine production and foam cell formation via epigenetic reprogramming of monocytes. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1731–1738. [Google Scholar] [CrossRef]
- Vorstandlechner, V.; Laggner, M.; Copic, D.; Klas, K.; Direder, M.; Chen, Y.; Golabi, B.; Haslik, W.; Radtke, C.; Tschachler, E.; et al. The serine proteases dipeptidyl-peptidase 4 and urokinase are key molecules in human and mouse scar formation. Nat. Commun. 2021, 12, 6242. [Google Scholar] [CrossRef]
- Petrera, A.; Gassenhuber, J.; Ruf, S.; Gunasekaran, D.; Esser, J.; Shahinian, J.H.; Hübschle, T.; Rütten, H.; Sadowski, T.; Schilling, O. Cathepsin A inhibition attenuates myocardial infarction-induced heart failure on the functional and proteomic levels. J. Transl. Med. 2016, 14, 153. [Google Scholar] [CrossRef]
- Ogura, Y.; Tajiri, K.; Murakoshi, N.; Xu, D.; Yonebayashi, S.; Li, S.; Okabe, Y.; Feng, D.; Shimoda, Y.; Song, Z.; et al. Neutrophil Elastase Deficiency Ameliorates Myocardial Injury Post Myocardial Infarction in Mice. Int. J. Mol. Sci. 2021, 22, 722. [Google Scholar] [CrossRef]
- Mauro, A.G.; Mezzaroma, E.; Marchetti, C.; Narayan, P.; Del Buono, M.G.; Capuano, M.; Prestamburgo, A.; Catapano, S.; Salloum, F.N.; Abbate, A.; et al. A Preclinical Translational Study of the Cardioprotective Effects of Plasma-Derived Alpha-1 Anti-trypsin in Acute Myocardial Infarction. J. Cardiovasc. Pharmacol. 2017, 69, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Hooshdaran, B.; Kolpakov, M.A.; Guo, X.; Miller, S.A.; Wang, T.; Tilley, D.; Rafiq, K.; Sabri, A. Dual inhibition of cathepsin G and chymase reduces myocyte death and improves cardiac remodeling after myocardial ischemia reperfusion injury. Basic Res. Cardiol. 2017, 112, 62. [Google Scholar] [CrossRef] [PubMed]
- Sen, P.; Helmke, A.; Liao, C.M.; Sörensen-Zender, I.; Rong, S.; Bräsen, J.-H.; Melk, A.; Haller, H.; Von Vietinghoff, S.; Schmitt, R. SerpinB2 Regulates Immune Response in Kidney Injury and Aging. J. Am. Soc. Nephrol. 2020, 31, 983–995. [Google Scholar] [CrossRef] [PubMed]
- BBouchentouf, M.; Paradis, P.; Forner, K.A.; Cuerquis, J.; Boivin, M.N.; Zheng, J.; Boulassel, M.R.; Routy, J.P.; Schiffrin, E.; Galipeau, J. Monocyte derivatives promote angiogenesis and myocyte survival in a model of myocardial infarction. Cell Transpl. 2010, 19, 369–386. [Google Scholar] [CrossRef] [PubMed]
- Simader, E.; Beer, L.; Laggner, M.; Vorstandlechner, V.; Gugerell, A.; Erb, M.; Kalinina, P.; Copic, D.; Moser, D.; Spittler, A.; et al. Tissue-regenerative potential of the secretome of γ-irradiated peripheral blood mononuclear cells is mediated via TNFRSF1B-induced necroptosis. Cell Death Dis. 2019, 10, 729. [Google Scholar] [CrossRef]
- Wuschko, S.; Gugerell, A.; Chabicovsky, M.; Hofbauer, H.; Laggner, M.; Erb, M.; Ostler, T.; Peterbauer, A.; Suessner, S.; Demyanets, S.; et al. Toxicological testing of allogeneic secretome derived from peripheral mononuclear cells (APOSEC): A novel cell-free therapeutic agent in skin disease. Sci. Rep. 2019, 9, 5598. [Google Scholar] [CrossRef]
- Webb, N.J.; Bottomley, M.J.; Watson, C.J.; Brenchley, P.E. Vascular endothelial growth factor (VEGF) is released from platelets during blood clotting: Implications for measurement of circulating VEGF levels in clinical disease. Clin. Sci. 1998, 94, 395–404. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Copic, D.; Direder, M.; Schossleitner, K.; Laggner, M.; Klas, K.; Bormann, D.; Ankersmit, H.J.; Mildner, M. Paracrine Factors of Stressed Peripheral Blood Mononuclear Cells Activate Proangiogenic and Anti-Proteolytic Processes in Whole Blood Cells and Protect the Endothelial Barrier. Pharmaceutics 2022, 14, 1600. https://doi.org/10.3390/pharmaceutics14081600
Copic D, Direder M, Schossleitner K, Laggner M, Klas K, Bormann D, Ankersmit HJ, Mildner M. Paracrine Factors of Stressed Peripheral Blood Mononuclear Cells Activate Proangiogenic and Anti-Proteolytic Processes in Whole Blood Cells and Protect the Endothelial Barrier. Pharmaceutics. 2022; 14(8):1600. https://doi.org/10.3390/pharmaceutics14081600
Chicago/Turabian StyleCopic, Dragan, Martin Direder, Klaudia Schossleitner, Maria Laggner, Katharina Klas, Daniel Bormann, Hendrik Jan Ankersmit, and Michael Mildner. 2022. "Paracrine Factors of Stressed Peripheral Blood Mononuclear Cells Activate Proangiogenic and Anti-Proteolytic Processes in Whole Blood Cells and Protect the Endothelial Barrier" Pharmaceutics 14, no. 8: 1600. https://doi.org/10.3390/pharmaceutics14081600