Ruthenium(II) Polypyridyl Complexes for Antimicrobial Photodynamic Therapy: Prospects for Application in Cystic Fibrosis Lung Airways
Abstract
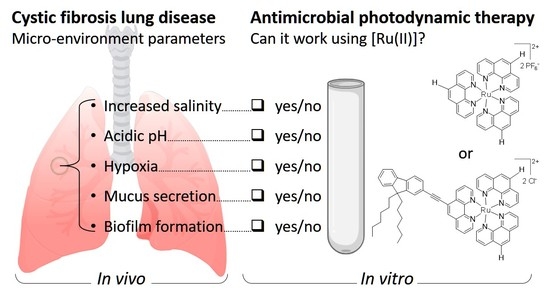
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Photosensitizers
2.1.2. Illumination Set-Up and Light Treatment Procedure
2.1.3. Bacterial Strains
2.1.4. Cells
2.1.5. Specific Media
2.2. Bacteria and Cell Culture Conditions
2.2.1. Bacteria Growing as Planktonic Cells
2.2.2. Bacteria Growing as Sessile Cells in Biofilms
2.2.3. Co-Cultures of Bacteria and Eukaryotic Cells
2.3. Characterizations of [Ru(II)] under Various Experimental Conditions
2.3.1. Fluorescence and UV Visible Spectra
2.3.2. PS interaction with Bacteria
2.3.3. PI Assay
2.3.4. Determination of Singlet Oxygen Production
2.3.5. Determination of Intracellular ROS Production
2.3.6. Hypoxic Condition Assay
2.4. Assessment of Antibacterial PDT Effects
2.4.1. Assessment of PDT Effects on Planktonic Cells
2.4.2. Assessment of PDT Antibiofilm Effects
2.4.3. Assessment of PDT Effects on Bacteria in Co-Culture Experiments
2.5. Statistical Analysis
3. Results
3.1. Characterizations of the Illumination System and PS
3.1.1. Assessment of the Functioning of the Illumination System
3.1.2. Photophysical Characterizations of [Ru(II)]
3.1.3. Singlet Oxygen and ROS Productions
3.1.4. Bacteria/Ru(II) Interaction and PI Assay
3.2. Impact on aPDT of Different Parameters during Light Treatment
3.2.1. Impact of Salinity
3.2.2. Impact of Acidic pH
3.2.3. Impact of Reduced Oxygenation
3.2.4. Impact of Artificial Sputum Medium (ASM)
3.3. PDT towards Bacteria Grown in Different Conditions
3.3.1. PDT towards Polymicrobial Cultures
3.3.2. PDT towards Bacterial Biofilms
3.3.3. PDT towards Bacteria in the Presence of Eukaryotic Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Dai, T.; Huang, Y.-Y.; Hamblin, M.R. Photodynamic Therapy for Localized Infections—State of the Art. Photodiagn. Photodyn. Ther. 2009, 6, 170–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wainwright, M.; Maisch, T.; Nonell, S.; Plaetzer, K.; Almeida, A.; Tegos, G.P.; Hamblin, M.R. Photoantimicrobials—Are We Afraid of the Light? Lancet Infect. Dis. 2017, 17, e49–e55. [Google Scholar] [CrossRef]
- Youf, R.; Müller, M.; Balasini, A.; Thétiot, F.; Müller, M.; Hascoët, A.; Jonas, U.; Schönherr, H.; Lemercier, G.; Montier, T.; et al. Antimicrobial Photodynamic Therapy: Latest Developments with a Focus on Combinatory Strategies. Pharmaceutics 2021, 13, 1995. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Hasan, T. Photodynamic Therapy: A New Antimicrobial Approach to Infectious Disease? Photochem. Photobiol. Sci. 2004, 3, 436–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Fan, T.; Xie, Z.; Zeng, Q.; Xue, P.; Zheng, T.; Chen, Y.; Luo, X.; Zhang, H. Advances in Nanomaterials for Photodynamic Therapy Applications: Status and Challenges. Biomaterials 2020, 237, 119827. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic Therapy—Mechanisms, Photosensitizers and Combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Pereira Rosa, L. Antimicrobial Photodynamic Therapy: A New Therapeutic Option to Combat Infections. J. Med. Microb. Diagn. 2014, 3, 4. [Google Scholar] [CrossRef]
- Hu, X.; Huang, Y.-Y.; Wang, Y.; Wang, X.; Hamblin, M.R. Antimicrobial Photodynamic Therapy to Control Clinically Relevant Biofilm Infections. Front. Microbiol. 2018, 9, 1299. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Laguna, V.; Gilaberte, Y.; Millán-Lou, M.I.; Agut, M.; Nonell, S.; Rezusta, A.; Hamblin, M.R. A Combination of Photodynamic Therapy and Antimicrobial Compounds to Treat Skin and Mucosal Infections: A Systematic Review. Photochem. Photobiol. Sci. 2019, 18, 1020–1029. [Google Scholar] [CrossRef]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef]
- Jakubaszek, M.; Goud, B.; Ferrari, S.; Gasser, G. Mechanisms of Action of Ru(II) Polypyridyl Complexes in Living Cells upon Light Irradiation. Chem. Commun. 2018, 54, 13040–13059. [Google Scholar] [CrossRef] [Green Version]
- Silva, Z.S.; Bussadori, S.K.; Fernandes, K.P.S.; Huang, Y.-Y.; Hamblin, M.R. Animal Models for Photodynamic Therapy (PDT). Biosci. Rep. 2015, 35, e00265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial Photodynamic Therapy—What We Know and What We Don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef] [Green Version]
- Frei, A. Metal Complexes, an Untapped Source of Antibiotic Potential? Antibiotics 2020, 9, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frei, A.; Zuegg, J.; Elliott, A.G.; Baker, M.; Braese, S.; Brown, C.; Chen, F.; Dowson, C.G.; Dujardin, G.; Jung, N.; et al. Metal Complexes as a Promising Source for New Antibiotics. Chem. Sci. 2020, 11, 2627–2639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spesia, M.B.; Durantini, E.N. Evolution of Phthalocyanine Structures as Photodynamic Agents for Bacteria Inactivation. Chem. Rec. 2022, 22, e202100292. [Google Scholar] [CrossRef]
- Mottais, A.; Berchel, M.; Le Gall, T.; Sibiril, Y.; d’Arbonneau, F.; Laurent, V.; Jaffrès, P.-A.; Montier, T. Antibacterial and Transfection Activities of Nebulized Formulations Incorporating Long N-Alkyl Chain Silver N-Heterocyclic Carbene Complexes. Int. J. Pharm. 2019, 567, 118500. [Google Scholar] [CrossRef]
- Rodríguez, I.; Fernández-Vega, L.; Maser-Figueroa, A.N.; Sang, B.; González-Pagán, P.; Tinoco, A.D. Exploring Titanium(IV) Complexes as Potential Antimicrobial Compounds. Antibiotics 2022, 11, 158. [Google Scholar] [CrossRef]
- Frei, A.; Ramu, S.; Lowe, G.J.; Dinh, H.; Semenec, L.; Elliott, A.G.; Zuegg, J.; Deckers, A.; Jung, N.; Bräse, S.; et al. Platinum Cyclooctadiene Complexes with Activity against Gram-positive Bacteria. ChemMedChem 2021, 16, 3165–3171. [Google Scholar] [CrossRef]
- Munteanu, A.-C.; Uivarosi, V. Ruthenium Complexes in the Fight against Pathogenic Microorganisms. An Extensive Review. Pharmaceutics 2021, 13, 874. [Google Scholar] [CrossRef]
- Knežević, N.Ž.; Stojanovic, V.; Chaix, A.; Bouffard, E.; Cheikh, K.E.; Morère, A.; Maynadier, M.; Lemercier, G.; Garcia, M.; Gary-Bobo, M.; et al. Ruthenium(II) Complex-Photosensitized Multifunctionalized Porous Silicon Nanoparticles for Two-Photon near-Infrared Light Responsive Imaging and Photodynamic Cancer Therapy. J. Mater. Chem. B 2016, 4, 1337–1342. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Collins, J.G.; Keene, F.R. Ruthenium Complexes as Antimicrobial Agents. Chem. Soc. Rev. 2015, 44, 2529–2542. [Google Scholar] [CrossRef] [Green Version]
- Le Gall, T.; Lemercier, G.; Chevreux, S.; Tücking, K.-S.; Ravel, J.; Thétiot, F.; Jonas, U.; Schönherr, H.; Montier, T. Ruthenium(II) Polypyridyl Complexes as Photosensitizers for Antibacterial Photodynamic Therapy: A Structure-Activity Study on Clinical Bacterial Strains. ChemMedChem 2018, 13, 2229–2239. [Google Scholar] [CrossRef] [PubMed]
- Lemercier, G.; Four, M.; Chevreux, S. Two-Photon Absorption Properties of 1,10-Phenanthroline-Based Ru(II) Complexes and Related Functionalized Nanoparticles for Potential Application in Two-Photon Excitation Photodynamic Therapy and Optical Power Limiting. Coord. Chem. Rev. 2018, 368, 1–12. [Google Scholar] [CrossRef]
- Karges, J.; Kuang, S.; Maschietto, F.; Blacque, O.; Ciofini, I.; Chao, H.; Gasser, G. Rationally Designed Ruthenium Complexes for 1- and 2-Photon Photodynamic Therapy. Nat. Commun. 2020, 11, 3262. [Google Scholar] [CrossRef]
- Cutting, G.R. Cystic Fibrosis Genetics: From Molecular Understanding to Clinical Application. Nat. Rev. Genet. 2015, 16, 45–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castellani, C.; Assael, B.M. Cystic Fibrosis: A Clinical View. Cell. Mol. Life Sci. 2017, 74, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, R.; Roquefort, P.; Ramel, S.; Laurent, V.; Haute, T.; Le Gall, T.; Aubry, T.; Montier, T. Apparent Yield Stress of Sputum as a Relevant Biomarker in Cystic Fibrosis. Cells 2021, 10, 3107. [Google Scholar] [CrossRef]
- Hurley, M.N.; McKeever, T.M.; Prayle, A.P.; Fogarty, A.W.; Smyth, A.R. Rate of Improvement of CF Life Expectancy Exceeds That of General Population—Observational Death Registration Study. J. Cyst. Fibros. 2014, 13, 410–415. [Google Scholar] [CrossRef] [Green Version]
- den Bossche, S.V.; Broe, E.D.; Coenye, T.; Braeckel, E.V.; Crabbé, A. The Cystic Fibrosis Lung Microenvironment Alters Antibiotic Activity: Causes and Effects. Eur. Respir. Rev. 2021, 30, 210055. [Google Scholar] [CrossRef]
- Stewart, P.S.; Costerton, J.W. Antibiotic Resistance of Bacteria in Biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Chmiel, J.F.; Aksamit, T.R.; Chotirmall, S.H.; Dasenbrook, E.C.; Elborn, J.S.; LiPuma, J.J.; Ranganathan, S.C.; Waters, V.J.; Ratjen, F.A. Antibiotic Management of Lung Infections in Cystic Fibrosis. I. The Microbiome, Methicillin-Resistant Staphylococcus Aureus, Gram-Negative Bacteria, and Multiple Infections. Ann. ATS 2014, 11, 1120–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, D.R.; Namanny, H.; Zobell, J.T. Follow-up Survey of the Utilization of Anti-Pseudomonal Beta-Lactam Antibiotics at U.S. Cystic Fibrosis Centers: Follow-Up Survey of the Utilization of Anti-Pseudomonal. Pediatr. Pulmonol. 2016, 51, 668–669. [Google Scholar] [CrossRef] [PubMed]
- Wagener, J.S.; Rasouliyan, L.; VanDevanter, D.R.; Pasta, D.J.; Regelmann, W.E.; Morgan, W.J.; Konstan, M.W. Oral, Inhaled, and Intravenous Antibiotic Choice for Treating Pulmonary Exacerbations in Cystic Fibrosis. Pediatr. Pulmonol. 2013, 48, 666–673. [Google Scholar] [CrossRef] [Green Version]
- Sherrard, L.J.; Tunney, M.M.; Elborn, J.S. Antimicrobial Resistance in the Respiratory Microbiota of People with Cystic Fibrosis. Lancet 2014, 384, 703–713. [Google Scholar] [CrossRef]
- WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. Available online: http://www.who.int/news-room/detail/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 29 June 2018).
- Moser, C.; Thomsen, T.R.; Høiby, N. Next Generation Microbiology and Cystic Fibrosis Diagnostics: Are We There Yet? Curr. Opin. Pulm. Med. 2018, 24, 599–605. [Google Scholar] [CrossRef]
- Pailhoriès, H.; Herrmann, J.-L.; Velo-Suarez, L.; Lamoureux, C.; Beauruelle, C.; Burgel, P.-R.; Héry-Arnaud, G. Antibiotic Resistance in Chronic Respiratory Diseases: From Susceptibility Testing to the Resistome. Eur. Respir. Rev. 2022, 31, 210259. [Google Scholar] [CrossRef]
- Nair, D.; Memmi, G.; Hernandez, D.; Bard, J.; Beaume, M.; Gill, S.; Francois, P.; Cheung, A.L. Whole-Genome Sequencing of Staphylococcus Aureus Strain RN4220, a Key Laboratory Strain Used in Virulence Research, Identifies Mutations That Affect Not Only Virulence Factors but Also the Fitness of the Strain. J. Bacteriol. 2011, 193, 2332–2335. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, M.; Ohta, T.; Uchiyama, I.; Baba, T.; Yuzawa, H.; Kobayashi, I.; Cui, L.; Oguchi, A.; Aoki, K.; Nagai, Y.; et al. Whole Genome Sequencing of Meticillin-Resistant Staphylococcus Aureus. Lancet 2001, 357, 1225–1240. [Google Scholar] [CrossRef]
- Le Gall, T.; Berchel, M.; Le Hir, S.; Fraix, A.; Salaün, J.Y.; Férec, C.; Lehn, P.; Jaffres, P.-A.; Montier, T. Arsonium-Containing Lipophosphoramides, Poly-Functional Nano-Carriers for Simultaneous Antibacterial Action and Eukaryotic Cell Transfection. Adv. Healthc. Mater. 2013, 2, 1513–1524. [Google Scholar] [CrossRef] [Green Version]
- Goncz, K.K.; Kunzelmann, K.; Xu, Z.; Gruenert, D.C. Targeted Replacement of Normal and Mutant CFTR Sequences in Human Airway Epithelial Cells Using DNA Fragments. Hum. Mol. Genet. 1998, 7, 1913–1919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sriramulu, D.D.; Lünsdorf, H.; Lam, J.S.; Römling, U. 2005 Microcolony Formation: A Novel Biofilm Model of Pseudomonas Aeruginosa for the Cystic Fibrosis Lung. J. Med. Microbiol. 2005, 54, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Agrawal, I.; Singh, P.; Jha, S. Portable, Low-Cost Hypoxia Chamber for Simulating Hypoxic Environments: Development, Characterization and Applications. Med. Devices Sens. 2020, 3, e10064. [Google Scholar] [CrossRef]
- Haney, E.F.; Trimble, M.J.; Hancock, R.E.W. Microtiter Plate Assays to Assess Antibiofilm Activity against Bacteria. Nat. Protoc. 2021, 16, 2615–2632. [Google Scholar] [CrossRef] [PubMed]
- Girardot, C.; Lemercier, G.; Mulatier, J.-C.; Chauvin, J.; Baldeck, P.L.; Andraud, C. Novel Ruthenium(II) and Zinc(II) Complexes for Two-Photon Absorption Related Applications. Dalton Trans. 2007, 3421–3426. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Abrahamse, H. Oxygen-Independent Antimicrobial Photoinactivation: Type III Photochemical Mechanism? Antibiotics 2020, 9, 53. [Google Scholar] [CrossRef] [Green Version]
- Dalecki, A.G.; Crawford, C.L.; Wolschendorf, F. Targeting Biofilm Associated Staphylococcus Aureus Using Resazurin Based Drug-Susceptibility Assay. J. Vis. Exp. 2016, 111, e53925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinemann, F.; Karges, J.; Gasser, G. Critical Overview of the Use of Ru(II) Polypyridyl Complexes as Photosensitizers in One-Photon and Two-Photon Photodynamic Therapy. Acc. Chem. Res. 2017, 50, 2727–2736. [Google Scholar] [CrossRef]
- Le Gall, T.; Berchel, M.; Davies, L.; Mottais, A.; Ghanem, R.; Fautrel, A.; Gill, D.; Hyde, S.; Lehn, P.; Lehn, J.-M.; et al. Aerosol-Mediated Non-Viral Lung Gene Therapy: The Potential of Aminoglycoside-Based Cationic Liposomes. Pharmaceutics 2022, 14, 25. [Google Scholar] [CrossRef]
- Brillault, J.; Tewes, F. Control of the Lung Residence Time of Highly Permeable Molecules after Nebulization: Example of the Fluoroquinolones. Pharmaceutics 2020, 12, E387. [Google Scholar] [CrossRef] [Green Version]
- Kassab, G.; Geralde, M.C.; Inada, N.M.; Achiles, A.E.; Guerra, V.G.; Bagnato, V.S. Nebulization as a Tool for Photosensitizer Delivery to the Respiratory Tract. J. Biophotonics 2019, 12, e201800189. [Google Scholar] [CrossRef] [PubMed]
- Lamy, B.; Tewes, F.; Serrano, D.R.; Lamarche, I.; Gobin, P.; Couet, W.; Healy, A.M.; Marchand, S. New Aerosol Formulation to Control Ciprofloxacin Pulmonary Concentration. J. Control. Release 2018, 271, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Abou Alaiwa, M.H.; Reznikov, L.R.; Gansemer, N.D.; Sheets, K.A.; Horswill, A.R.; Stoltz, D.A.; Zabner, J.; Welsh, M.J. PH Modulates the Activity and Synergism of the Airway Surface Liquid Antimicrobials β-Defensin-3 and LL-37. Proc. Natl. Acad. Sci. USA 2014, 111, 18703–18708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Worlitzsch, D.; Tarran, R.; Ulrich, M.; Schwab, U.; Cekici, A.; Meyer, K.C.; Birrer, P.; Bellon, G.; Berger, J.; Weiss, T.; et al. Effects of Reduced Mucus Oxygen Concentration in Airway Pseudomonas Infections of Cystic Fibrosis Patients. J. Clin. Investig. 2002, 109, 317–325. [Google Scholar] [CrossRef]
- Saint-Criq, V.; Gray, M.A. Role of CFTR in Epithelial Physiology. Cell. Mol. Life Sci. 2017, 74, 93–115. [Google Scholar] [CrossRef] [Green Version]
- Donnelly, R.F.; McCarron, P.A.; Cassidy, C.M.; Elborn, J.S.; Tunney, M.M. Delivery of Photosensitisers and Light through Mucus: Investigations into the Potential Use of Photodynamic Therapy for Treatment of Pseudomonas Aeruginosa Cystic Fibrosis Pulmonary Infection. J. Control. Release 2007, 117, 217–226. [Google Scholar] [CrossRef]
- Pernet, E.; Guillemot, L.; Burgel, P.-R.; Martin, C.; Lambeau, G.; Sermet-Gaudelus, I.; Sands, D.; Leduc, D.; Morand, P.C.; Jeammet, L.; et al. Pseudomonas Aeruginosa Eradicates Staphylococcus Aureus by Manipulating the Host Immunity. Nat. Commun. 2014, 5, 5105. [Google Scholar] [CrossRef] [Green Version]
- Quishida, C.C.C.; Carmello, J.C.; Mima, E.G.d.O.; Bagnato, V.S.; Machado, A.L.; Pavarina, A.C. Susceptibility of Multispecies Biofilm to Photodynamic Therapy Using Photodithazine®. Lasers Med. Sci. 2015, 30, 685–694. [Google Scholar] [CrossRef]
- Lamoureux, C.; Guilloux, C.-A.; Beauruelle, C.; Gouriou, S.; Ramel, S.; Dirou, A.; Le Bihan, J.; Revert, K.; Ropars, T.; Lagrafeuille, R.; et al. An Observational Study of Anaerobic Bacteria in Cystic Fibrosis Lung Using Culture Dependant and Independent Approaches. Sci. Rep. 2021, 11, 6845. [Google Scholar] [CrossRef]
- Bahamondez-Canas, T.F.; Zhang, H.; Tewes, F.; Leal, J.; Smyth, H.D.C. PEGylation of Tobramycin Improves Mucus Penetration and Antimicrobial Activity against Pseudomonas Aeruginosa Biofilms in Vitro. Mol. Pharm. 2018, 15, 1643–1652. [Google Scholar] [CrossRef]
- Boca, S.C.; Four, M.; Bonne, A.; van der Sanden, B.; Astilean, S.; Baldeck, P.L.; Lemercier, G. An Ethylene-Glycol Decorated Ruthenium(II) Complex for Two-Photon Photodynamic Therapy. Chem. Commun. 2009, 31, 4590–4592. [Google Scholar] [CrossRef] [PubMed]
- Bœuf, G.; Roullin, G.V.; Moreau, J.; Van Gulick, L.; Zambrano Pineda, N.; Terryn, C.; Ploton, D.; Andry, M.C.; Chuburu, F.; Dukic, S.; et al. Encapsulated Ruthenium(II) Complexes in Biocompatible Poly(d,l-Lactide-Co-Glycolide) Nanoparticles for Application in Photodynamic Therapy. Chempluschem 2014, 79, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Soliman, N.; Sol, V.; Ouk, T.-S.; Thomas, C.M.; Gasser, G. Encapsulation of a Ru(II) Polypyridyl Complex into Polylactide Nanoparticles for Antimicrobial Photodynamic Therapy. Pharmaceutics 2020, 12, 961. [Google Scholar] [CrossRef] [PubMed]
- Galli, F.; Battistoni, A.; Gambari, R.; Pompella, A.; Bragonzi, A.; Pilolli, F.; Iuliano, L.; Piroddi, M.; Dechecchi, M.C.; Cabrini, G. Oxidative Stress and Antioxidant Therapy in Cystic Fibrosis. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2012, 1822, 690–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kassab, G.; Diaz Tovar, J.S.; Souza, L.M.P.; Costa, R.K.M.; Silva, R.S.; Pimentel, A.S.; Kurachi, C.; Bagnato, V.S. Lung Surfactant Negatively Affects the Photodynamic Inactivation of Bacteria-in Vitro and Molecular Dynamic Simulation Analyses. Proc. Natl. Acad. Sci. USA 2022, 119, e2123564119. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, R.; Laurent, V.; Roquefort, P.; Haute, T.; Ramel, S.; Gall, T.L.; Aubry, T.; Montier, T. Optimizations of In Vitro Mucus and Cell Culture Models to Better Predict In Vivo Gene Transfer in Pathological Lung Respiratory Airways: Cystic Fibrosis as an Example. Pharmaceutics 2020, 13, 47. [Google Scholar] [CrossRef]
- Semaniakou, A.; Croll, R.P.; Chappe, V. Animal Models in the Pathophysiology of Cystic Fibrosis. Front. Pharmacol. 2019, 9, 1475. [Google Scholar] [CrossRef] [Green Version]
- Anthony, E.J.; Bolitho, E.M.; Bridgewater, H.E.; Carter, O.W.L.; Donnelly, J.M.; Imberti, C.; Lant, E.C.; Lermyte, F.; Needham, R.J.; Palau, M.; et al. Metallodrugs Are Unique: Opportunities and Challenges of Discovery and Development. Chem. Sci. 2020, 11, 12888–12917. [Google Scholar] [CrossRef]










| [Ru(II)]1 | [Ru(II)]2 | |||||
|---|---|---|---|---|---|---|
| OFF | ON | OFF | ON | |||
| S. aureus | RN4220 | NaCl | >64 2 | >64 | 64 | 32 |
| Water | >64 | 32 | 64 | 8 | ||
| N315 | NaCl | >64 | >64 | 64 | 64 | |
| Water | >64 | 64 | 64 | 32 | ||
| P. aeruginosa | PA19660 | NaCl | >64 | 64 | >64 | 32 |
| Water | 8 | 8 | 8 | 8 | ||
| PAH | NaCl | >64 | >64 | 8 | 8 | |
| Water | >64 | 64 | Nd | Nd | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youf, R.; Nasir, A.; Müller, M.; Thétiot, F.; Haute, T.; Ghanem, R.; Jonas, U.; Schönherr, H.; Lemercier, G.; Montier, T.; et al. Ruthenium(II) Polypyridyl Complexes for Antimicrobial Photodynamic Therapy: Prospects for Application in Cystic Fibrosis Lung Airways. Pharmaceutics 2022, 14, 1664. https://doi.org/10.3390/pharmaceutics14081664
Youf R, Nasir A, Müller M, Thétiot F, Haute T, Ghanem R, Jonas U, Schönherr H, Lemercier G, Montier T, et al. Ruthenium(II) Polypyridyl Complexes for Antimicrobial Photodynamic Therapy: Prospects for Application in Cystic Fibrosis Lung Airways. Pharmaceutics. 2022; 14(8):1664. https://doi.org/10.3390/pharmaceutics14081664
Chicago/Turabian StyleYouf, Raphaëlle, Adeel Nasir, Mareike Müller, Franck Thétiot, Tanguy Haute, Rosy Ghanem, Ulrich Jonas, Holger Schönherr, Gilles Lemercier, Tristan Montier, and et al. 2022. "Ruthenium(II) Polypyridyl Complexes for Antimicrobial Photodynamic Therapy: Prospects for Application in Cystic Fibrosis Lung Airways" Pharmaceutics 14, no. 8: 1664. https://doi.org/10.3390/pharmaceutics14081664