Supercritical Fluid Technologies for the Incorporation of Synthetic and Natural Active Compounds into Materials for Drug Formulation and Delivery
Abstract
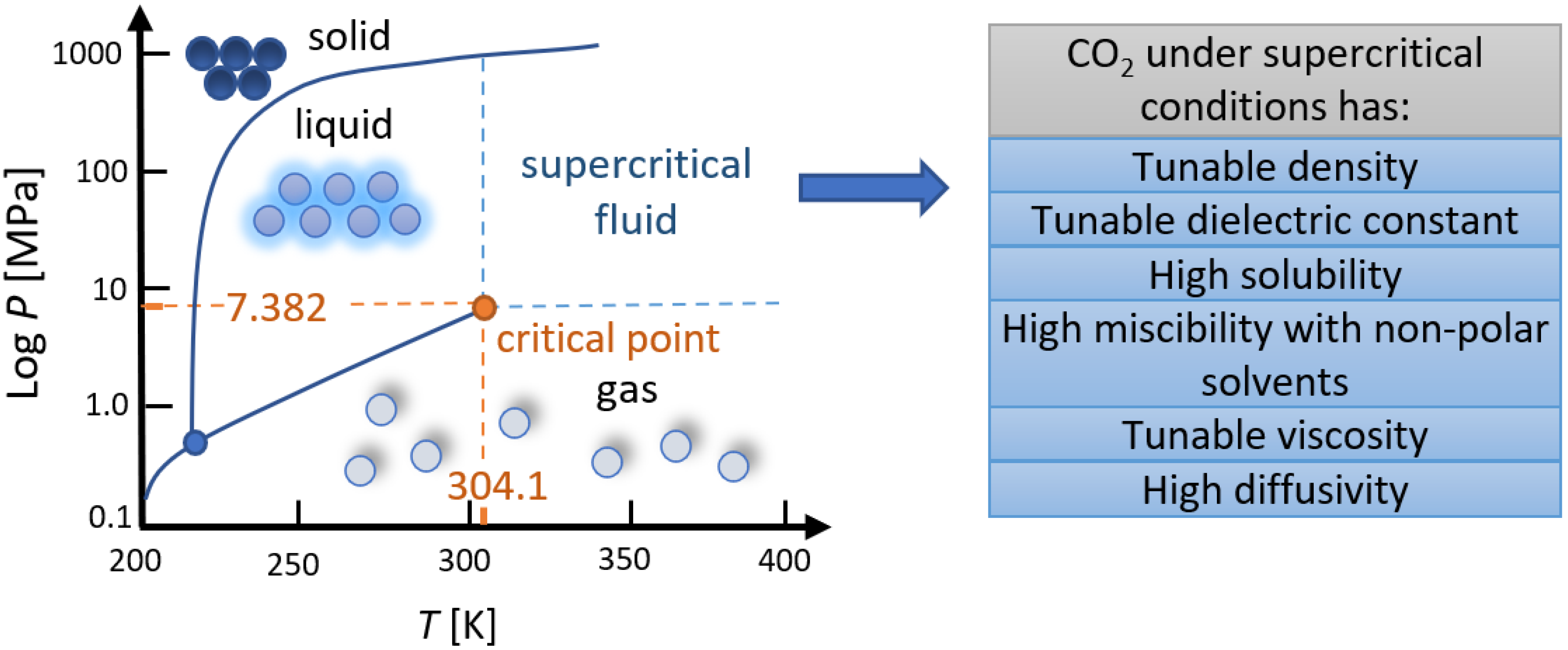
:1. Introduction
2. SCF Technologies for the Incorporation of AIs
2.1. Micronization
2.1.1. RESS
2.1.2. Antisolvent Processes
2.1.3. PGSSTM
2.2. SC Drying for the Preparation of Aerogels
- I.
- a three-dimensional, highly porous structure (with a pore diameter smaller than 100 nm) [75],
- II.
- a very low density (0.0011–0.5 g/cm3) [75],
- III.
- a large specific surface area (70–1600 m2/g) [76],
- IV.
- low thermal conductivity (as low as 0.012 W/mK in air at 1 atm and 300 K) [77],
- V.
- a low dielectric constant,
- VI.
- a low speed of sound, and
- VII.
2.3. SC Foaming
2.4. SC Solvent Impregnation (SSI)
3. Drug Delivery from Formulations Prepared by SCF Technologies
4. Conclusions and Outlooks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sareen, S.; Mathew, G.; Joseph, L. Improvement in solubility of poor water-soluble drugs by solid dispersion. Int. J. Pharm. Investig. 2012, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Kalepu, S.; Nekkanti, V. Insoluble drug delivery strategies: Review of recent advances and business prospects. Acta Pharm. Sin. B 2015, 5, 442–453. [Google Scholar] [CrossRef]
- Atef, M.; Mahdi Ojagh, S. Health benefits and food applications of bioactive compounds from fish byproducts: A review. J. Funct. Foods 2017, 35, 673–681. [Google Scholar] [CrossRef]
- Kravanja, K.A.; Finšgar, M.; Knez, Ž.; Knez Marevci, M. Evaluation of Natural Extracts as Promising Components of Bioactive Coatings for Orthopedic Implants. Front. Mater. 2022, 9, 878176. [Google Scholar] [CrossRef]
- Valor, D.; Montes, A.; García-Casas, I.; Pereyra, C.; Martínez de la Ossa, E.J. Supercritical solvent impregnation of alginate wound dressings with mango leaves extract. J. Supercrit. Fluids 2021, 178, 105357. [Google Scholar] [CrossRef]
- Anggraini, T.; Wilma, S.; Syukri, D.; Azima, F. Total phenolic, anthocyanin, Catechins, DPPH radical scavenging activity, and toxicity of Lepisanthes alata (Blume) Leenh. Int. J. Food Sci. 2019, 2019, 9703176. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Huang, S.; Shi, Y.; Shao, Y.; Qiu, J.; Sedjoah, R.-C.A.-A.; Yan, Z.; Ding, L.; Zou, D.; Xin, Z. Extraction, isolation, characterization and antimicrobial activities of non-extractable polyphenols from pomegranate peel. Food Chem. 2021, 351, 129232. [Google Scholar] [CrossRef]
- Simonetti, G.; Brasili, E.; Pasqua, G. Antifungal activity of phenolic and polyphenolic compounds from different matrices of Vitis vinifera L. against human pathogens. Molecules 2020, 25, 3748. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, C.; Vari, R.; Scazzocchio, B.; De Sanctis, P.; Giovannini, C.; D’Archivio, M.; Masella, R. Anti-inflammatory activity of extra virgin olive oil polyphenols: Which role in the prevention and treatment of immune-mediated inflammatory diseases? Endocr. Metab. Immune Disord.-Drug Targets (Former. Curr. Drug Targets-Immune Endocr. Metab. Disord.) 2018, 18, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Sobeh, M.; Mahmoud, M.F.; Petruk, G.; Rezq, S.; Ashour, M.L.; Youssef, F.S.; El-Shazly, A.M.; Monti, D.M.; Abdel-Naim, A.B.; Wink, M. Syzygium aqueum: A polyphenol-rich leaf extract exhibits antioxidant, hepatoprotective, pain-killing and anti-inflammatory activities in animal models. Front. Pharmacol. 2018, 9, 566. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.; Li, H.; Hou, T.; Bethu, M.; Ren, Z.; Zhang, Z. Phytosynthesis of silver nanoparticles using Perilla frutescens leaf extract: Characterization and evaluation of antibacterial, antioxidant, and anticancer activities. Int. J. Nanomed. 2021, 16, 15. [Google Scholar] [CrossRef]
- Soto-Vaca, A.; Gutierrez, A.; Losso, J.N.; Xu, Z.; Finley, J.W. Evolution of phenolic compounds from color and flavor problems to health benefits. J. Agric. Food Chem. 2012, 60, 6658–6677. [Google Scholar] [CrossRef] [PubMed]
- Serino, A.; Salazar, G. Protective role of polyphenols against vascular inflammation, aging and cardiovascular disease. Nutrients 2018, 11, 53. [Google Scholar] [CrossRef]
- Grgić, J.; Šelo, G.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Role of the Encapsulation in Bioavailability of Phenolic Compounds. Antioxidants 2020, 9, 923. [Google Scholar] [CrossRef]
- Kankala, R.K.; Zhang, Y.S.; Wang, S.B.; Lee, C.H.; Chen, A.Z. Supercritical fluid technology: An emphasis on drug delivery and related biomedical applications. Adv. Healthc. Mater. 2017, 6, 1700433. [Google Scholar] [CrossRef]
- Xin, X.; Liu, Q.Q.; Chen, C.X.; Guan, Y.X.; Yao, S.J. Fabrication of bimodal porous PLGA scaffolds by supercritical CO2 foaming/particle leaching technique. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Khodaverdi, E.; Abbaspour, M.R.; Oroojalian, F.; Omidkhah, N.; Hossein-nezahd, S.; Kamali, H.; Hadizadeh, F. Dexamethasone delivery of porous PEG-PCL-PEG scaffolds with supercritical carbon dioxide gas foaming. J. Drug Deliv. Sci. Technol. 2021, 66, 102547. [Google Scholar] [CrossRef]
- dos Santos, A.E.; Dal Magro, C.; de Britto, L.S.; Aguiar, G.P.S.; de Oliveira, J.V.; Lanza, M. Micronization of luteolin using supercritical carbon dioxide: Characterization of particles and biological activity in vitro. J. Supercrit. Fluids 2022, 181, 105471. [Google Scholar] [CrossRef]
- Ahn, J.B.; Kim, D.-H.; Lee, S.-E.; Pyo, Y.-C.; Park, J.-S. Improvement of the dissolution rate and bioavailability of fenofibrate by the supercritical anti-solvent process. Int. J. Pharm. 2019, 564, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Monteagudo-Olivan, R.; Cocero, M.J.; Coronas, J.; Rodríguez-Rojo, S. Supercritical CO2 encapsulation of bioactive molecules in carboxylate based MOFs. J. CO2 Util. 2019, 30, 38–47. [Google Scholar] [CrossRef]
- Pattnaik, S.; Arun, G.; Swain, K. Supercritical fluid technologies: A green solvent approach for pharmaceutical product development. In Advanced Nanotechnology and Application of Supercritical Fluids; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–14. [Google Scholar]
- Soh, S.H.; Lee, L.Y. Microencapsulation and Nanoencapsulation Using Supercritical Fluid (SCF) Techniques. Pharmaceutics 2019, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, P.; Famili, A.; Nagapudi, K.; Al-Sayah, M.A. Using Supercritical Fluid Technology as a Green Alternative During the Preparation of Drug Delivery Systems. Pharmaceutics 2019, 11, 629. [Google Scholar] [CrossRef]
- Kankala, R.K.; Xu, P.-Y.; Chen, B.-Q.; Wang, S.-B.; Chen, A.-Z. Supercritical fluid (SCF)-assisted fabrication of carrier-free drugs: An eco-friendly welcome to active pharmaceutical ingredients (APIs). Adv. Drug Deliv. Rev. 2021, 176, 113846. [Google Scholar] [CrossRef]
- Champeau, M.; Thomassin, J.M.; Tassaing, T.; Jérôme, C. Drug loading of polymer implants by supercritical CO2 assisted impregnation: A review. J. Control. Release 2015, 209, 248–259. [Google Scholar] [CrossRef]
- Arumugham, T.; Rambabu, K.; Hasan, S.W.; Show, P.L.; Rinklebe, J.; Banat, F. Supercritical carbon dioxide extraction of plant phytochemicals for biological and environmental applications—A review. Chemosphere 2021, 271, 129525. [Google Scholar] [CrossRef]
- Badens, E.; Masmoudi, Y.; Mouahid, A.; Crampon, C. Current situation and perspectives in drug formulation by using supercritical fluid technology. J. Supercrit. Fluids 2018, 134, 274–283. [Google Scholar] [CrossRef]
- Rasenack, N.; Müller, B.W. Micron-Size Drug Particles: Common and Novel Micronization Techniques. Pharm. Dev. Technol. 2004, 9, 1–13. [Google Scholar] [CrossRef]
- Esfandiari, N. Production of micro and nano particles of pharmaceutical by supercritical carbon dioxide. J. Supercrit. Fluids 2015, 100, 129–141. [Google Scholar] [CrossRef]
- Vandana, K.R.; Prasanna Raju, Y.; Harini Chowdary, V.; Sushma, M.; Vijay Kumar, N. An overview on in situ micronization technique—An emerging novel concept in advanced drug delivery. Saudi Pharm. J. 2014, 22, 283–289. [Google Scholar] [CrossRef]
- Munin, A.; Edwards-Lévy, F. Encapsulation of Natural Polyphenolic Compounds; a Review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef]
- Türk, M. Particle synthesis by rapid expansion of supercritical solutions (RESS): Current state, further perspectives and needs. J. Aerosol Sci. 2022, 161, 105950. [Google Scholar] [CrossRef]
- Pocheville, A. Supercritical Antisolvent (SAS) CO-Precipitation of Ethyl Cellulose and Natural Extracts IN SC-CO2. Available online: https://www.researchgate.net/profile/Bruno-Inarra/publication/271499148_SUPERCRITICAL_ANTISOLVENT_SAS_CO-PRECIPITATION_OF_ETHYL_CELLULOSE_AND_NATURAL_EXTRACTS_IN_SC-CO_2/links/54c8f1250cf22d626a3a7526/SUPERCRITICAL-ANTISOLVENT-SAS-CO-PRECIPITATION-OF-ETHYL-CELLULOSE-AND-NATURAL-EXTRACTS-IN-SC-CO-2.pdf (accessed on 30 June 2022).
- Gangapurwala, G.; Vollrath, A.; De San Luis, A.; Schubert, U.S. PLA/PLGA-Based Drug Delivery Systems Produced with Supercritical CO2—A Green Future for Particle Formulation? Pharmaceutics 2020, 12, 1118. [Google Scholar] [CrossRef] [PubMed]
- Matson, D.W.; Petersen, R.C.; Smith, R.D. Production of powders and films by the rapid expansion of supercritical solutions. J. Mater. Sci. 1987, 22, 1919–1928. [Google Scholar] [CrossRef]
- Mundargi, R.; Patil, S.; Kulkarni, P.; Mallikarjuna, N.; Aminabhavi, T. Sequential interpenetrating polymer network hydrogel microspheres of poly (methacrylic acid) and poly (vinyl alcohol) for oral controlled drug delivery to intestine. J. Microencapsul. 2008, 25, 228–240. [Google Scholar] [CrossRef]
- Hsiue, G.H.; Liao, C.M.; Lin, S.Y. Effect of Drug-Polymer Interaction on the Release Characteristics of Methacrylic Acid Copolymer Microcapsules Containing Theophylline. Artif. Organs 1998, 22, 651–656. [Google Scholar] [CrossRef]
- Vergara-Mendoza, M.d.S.; Ortiz-Estrada, C.-H.; González-Martínez, J.; Quezada-Gallo, J.-A. Microencapsulation of Coenzyme Q10 in Poly(ethylene glycol) and Poly(lactic acid) with Supercritical Carbon Dioxide. Ind. Eng. Chem. Res. 2012, 51, 5840–5846. [Google Scholar] [CrossRef]
- Knez, Ž.; Škerget, M.; Hrnčič, M.K.; Čuček, D. Particle formation using sub-and supercritical fluids. In Supercritical Fluid Technology for Energy and Environmental Applications; Elsevier: Amsterdam, The Netherlands, 2014; pp. 31–67. [Google Scholar]
- Sheth, P.; Sandhu, H. Amorphous solid dispersion using supercritical fluid technology. In Amorphous solid dispersions; Springer: Berlin/Heidelberg, Germany, 2014; pp. 579–591. [Google Scholar]
- Reverchon, E. Supercritical antisolvent precipitation of micro-and nano-particles. J. Supercrit. Fluids 1999, 15, 1–21. [Google Scholar] [CrossRef]
- Parhi, R.; Suresh, P. Supercritical fluid technology: A review. J. Adv. Pharm. Sci. Technol. 2013, 1, 13–36. [Google Scholar] [CrossRef]
- Kalani, M.; Yunus, R. Application of supercritical antisolvent method in drug encapsulation: A review. Int. J. Nanomed. 2011, 6, 1429. [Google Scholar] [CrossRef]
- Franco, P.; De Marco, I. Supercritical Antisolvent Process for Pharmaceutical Applications: A Review. Processes 2020, 8, 938. [Google Scholar] [CrossRef]
- Matos, R.L.; Lu, T.; Prosapio, V.; McConville, C.; Leeke, G.; Ingram, A. Coprecipitation of curcumin/PVP with enhanced dissolution properties by the supercritical antisolvent process. J. CO2 Util. 2019, 30, 48–62. [Google Scholar] [CrossRef]
- Gil-Ramírez, A.; Rodriguez-Meizoso, I. Purification of natural products by selective precipitation using supercritical/gas antisolvent techniques (SAS/GAS). Sep. Purif. Rev. 2021, 50, 32–52. [Google Scholar] [CrossRef]
- RC Torres, A.; Santana, L.; T Santos, D.; Meireles, A.A. Perspectives on the application of supercritical antisolvent fractionation process for the purification of plant extracts: Effects of operating parameters and patent survey. Recent Pat. Eng. 2016, 10, 88–97. [Google Scholar] [CrossRef]
- Sánchez-Camargo, A.P.; Mendiola, J.A.; Valdés, A.; Castro-Puyana, M.; García-Cañas, V.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Supercritical antisolvent fractionation of rosemary extracts obtained by pressurized liquid extraction to enhance their antiproliferative activity. J. Supercrit. Fluids 2016, 107, 581–589. [Google Scholar] [CrossRef]
- Villalva, M.; Jaime, L.; Villanueva-Bermejo, D.; Lara, B.; Fornari, T.; Reglero, G.; Santoyo, S. Supercritical anti-solvent fractionation for improving antioxidant and anti-inflammatory activities of an Achillea millefolium L. extract. Food Res. Int. 2019, 115, 128–134. [Google Scholar] [CrossRef]
- Knez, Z.; Weidner, E. Particles formation and particle design using supercritical fluids. Curr. Opin. Solid State Mater. Sci. 2003, 7, 353–361. [Google Scholar] [CrossRef]
- Weidner, E.; Knez, Ž.; Novak, Z. Process for the Production of Particles or Powders. U.S. 6056791A, 7 November 1997. Available online: https://patents.google.com/patent/US6056791A/en (accessed on 30 June 2022).
- Pestieau, A.; Krier, F.; Lebrun, P.; Brouwers, A.; Streel, B.; Evrard, B. Optimization of a PGSS (particles from gas saturated solutions) process for a fenofibrate lipid-based solid dispersion formulation. Int. J. Pharm. 2015, 485, 295–305. [Google Scholar] [CrossRef] [PubMed]
- de Paz, E.; Martín, Á.; Cocero, M.J. Formulation of β-carotene with soybean lecithin by PGSS (Particles from Gas Saturated Solutions)-drying. J. Supercrit. Fluids 2012, 72, 125–133. [Google Scholar] [CrossRef]
- Fraile, M.; Martín, ÿ.; Deodato, D.; Rodriguez-Rojo, S.; Nogueira, I.D.; Simplício, A.L.; Cocero, M.J.; Duarte, C.M.M. Production of new hybrid systems for drug delivery by PGSS (Particles from Gas Saturated Solutions) process. J. Supercrit. Fluids 2013, 81, 226–235. [Google Scholar] [CrossRef]
- Haq, M.; Chun, B.-S. Microencapsulation of omega-3 polyunsaturated fatty acids and astaxanthin-rich salmon oil using particles from gas saturated solutions (PGSS) process. LWT 2018, 92, 523–530. [Google Scholar] [CrossRef]
- Akolade, J.O.; Balogun, M.; Swanepoel, A.; Ibrahim, R.B.; Yusuf, A.A.; Labuschagne, P. Microencapsulation of eucalyptol in polyethylene glycol and polycaprolactone using particles from gas-saturated solutions. RSC Adv. 2019, 9, 34039–34049. [Google Scholar] [CrossRef] [PubMed]
- López-Iglesias, C.; Quílez, C.; Barros, J.; Velasco, D.; Alvarez-Lorenzo, C.; Jorcano, J.L.; Monteiro, F.J.; García-González, C.A. Lidocaine-Loaded Solid Lipid Microparticles (SLMPs) Produced from Gas-Saturated Solutions for Wound Applications. Pharmaceutics 2020, 12, 870. [Google Scholar] [CrossRef] [PubMed]
- Pedro, A.S.; Villa, S.D.; Caliceti, P.; Melo, S.A.B.V.d.; Albuquerque, E.C.; Bertucco, A.; Salmaso, S. Curcumin-loaded solid lipid particles by PGSS technology. J. Supercrit. Fluids 2016, 107, 534–541. [Google Scholar] [CrossRef]
- Kravanja, G.; Knez, Ž.; Kotnik, P.; Ljubec, B.; Knez Hrnčič, M. Formulation of nimodipine, fenofibrate, and o-vanillin with Brij S100 and PEG 4000 using the PGSS™ process. J. Supercrit. Fluids 2018, 135, 245–253. [Google Scholar] [CrossRef]
- Gonçalves, V.; Poejo, J.; Matias, A.; Rodríguez-Rojo, S.; Cocero, M.; Duarte, C. Using different natural origin carriers for development of epigallocatechin gallate (EGCG) solid formulations with improved antioxidant activity by PGSS-drying. RSC Adv. 2016, 6, 67599–67609. [Google Scholar] [CrossRef]
- Varona, S.; Martin, A.; Cocero, M.J. Liposomal incorporation of lavandin essential oil by a thin-film hydration method and by particles from gas-saturated solutions. Ind. Eng. Chem. Res. 2011, 50, 2088–2097. [Google Scholar] [CrossRef]
- Hu, X.; Guo, Y.; Wang, L.; Hua, D.; Hong, Y.; Li, J. Coenzyme Q10 nanoparticles prepared by a supercritical fluid-based method. J. Supercrit. Fluids 2011, 57, 66–72. [Google Scholar] [CrossRef]
- Kistler, S.S. Coherent Expanded Aerogels and Jellies. Nature 1931, 127, 741. [Google Scholar] [CrossRef]
- Zou, F.; Budtova, T. Polysaccharide-based aerogels for thermal insulation and superinsulation: An overview. Carbohydr. Polym. 2021, 266, 118130. [Google Scholar] [CrossRef]
- Smirnova, I.; Gurikov, P. Aerogels in chemical engineering: Strategies toward tailor-made aerogels. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 307–334. [Google Scholar] [CrossRef]
- Pierre, A.C.; Pajonk, G.M. Chemistry of Aerogels and Their Applications. Chem. Rev. 2002, 102, 4243–4266. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, K.; Han, Y.; Wang, D.; Li, X.; Hu, T.; Yi, H.; Ni, Z. A 3D porous graphene aerogel@ GOx based microfluidic biosensor for electrochemical glucose detection. Analyst 2020, 145, 5141–5147. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, Z.; Xia, W.; Zou, R.; Han, R.P. Alkylated phase change composites for thermal energy storage based on surface-modified silica aerogels. J. Mater. Chem. A 2015, 3, 1935–1940. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; Portugal, A. Synthesis of lightweight polymer-reinforced silica aerogels with improved mechanical and thermal insulation properties for space applications. Microporous Mesoporous Mater. 2014, 197, 116–129. [Google Scholar] [CrossRef]
- Horvat, G.; Xhanari, K.; Finšgar, M.; Gradišnik, L.; Maver, U.; Knez, Ž.; Novak, Z. Novel ethanol-induced pectin–xanthan aerogel coatings for orthopedic applications. Carbohydr. Polym. 2017, 166, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Weng, Z.; Liu, X.; Yeung, K.W.K.; Pan, H.; Wu, S. Controlled release and biocompatibility of polymer/titania nanotube array system on titanium implants. Bioact. Mater. 2017, 2, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Follmann, H.D.; Oliveira, O.N.; Lazarin-Bidóia, D.; Nakamura, C.V.; Huang, X.; Asefa, T.; Silva, R. Multifunctional hybrid aerogels: Hyperbranched polymer-trapped mesoporous silica nanoparticles for sustained and prolonged drug release. Nanoscale 2018, 10, 1704–1715. [Google Scholar] [CrossRef]
- Agostinho, D.A.; Paninho, A.I.; Cordeiro, T.; Nunes, A.V.; Fonseca, I.M.; Pereira, C.; Matias, A.; Ventura, M.G. Properties of κ-carrageenan aerogels prepared by using different dissolution media and its application as drug delivery systems. Mater. Chem. Phys. 2020, 253, 123290. [Google Scholar] [CrossRef]
- Kéri, M.; Forgács, A.; Papp, V.; Bányai, I.; Veres, P.; Len, A.; Dudás, Z.; Fábián, I.; Kalmár, J. Gelatin content governs hydration induced structural changes in silica-gelatin hybrid aerogels–Implications in drug delivery. Acta Biomater. 2020, 105, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Barrios, E.; Fox, D.; Li Sip, Y.Y.; Catarata, R.; Calderon, J.E.; Azim, N.; Afrin, S.; Zhang, Z.; Zhai, L. Nanomaterials in advanced, high-performance aerogel composites: A review. Polymers 2019, 11, 726. [Google Scholar] [CrossRef] [PubMed]
- Knez, Ž.; Novak, Z.; Pantić, M. Incorporation of Drugs and Metals into Aerogels Using Supercritical Fluids. In Supercritical and Other High-Pressure Solvent Systems; Royal Society of Chemistry: London, UK, 2018; pp. 374–394. [Google Scholar]
- Hrubesh, L.W.; Pekala, R.W. Thermal properties of organic and inorganic aerogels. J. Mater. Res. 1994, 9, 731–738. [Google Scholar] [CrossRef]
- Zhao, S.; Malfait, W.J.; Guerrero-Alburquerque, N.; Koebel, M.M.; Nyström, G. Biopolymer aerogels and foams: Chemistry, properties, and applications. Angew. Chem. Int. Ed. 2018, 57, 7580–7608. [Google Scholar] [CrossRef]
- Dervin, S.; Pillai, S.C. An introduction to sol-gel processing for aerogels. In Sol-Gel Materials for Energy, Environment and Electronic Applications; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–22. [Google Scholar]
- Landau, M.V. Sol–Gel Process. In Handbook of Heterogeneous Catalysis: Online; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; pp. 119–160. [Google Scholar]
- Şahin, İ.; Özbakır, Y.; İnönü, Z.; Ulker, Z.; Erkey, C. Kinetics of supercritical drying of gels. Gels 2017, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Horvat, G.; Pantić, M.; Knez, Ž.; Novak, Z. Encapsulation and drug release of poorly water soluble nifedipine from bio-carriers. J. Non-Cryst. Solids 2018, 481, 486–493. [Google Scholar] [CrossRef]
- García-González, C.A.; Sosnik, A.; Kalmár, J.; De Marco, I.; Erkey, C.; Concheiro, A.; Alvarez-Lorenzo, C. Aerogels in drug delivery: From design to application. J. Control. Release 2021, 332, 40–63. [Google Scholar] [CrossRef]
- Ulker, Z.; Erkey, C. An advantageous technique to load drugs into aerogels: Gas antisolvent crystallization inside the pores. J. Supercrit. Fluids 2017, 120, 310–319. [Google Scholar] [CrossRef]
- Villegas, M.; Oliveira, A.L.; Bazito, R.C.; Vidinha, P. Development of an integrated one-pot process for the production and impregnation of starch aerogels in supercritical carbon dioxide. J. Supercrit. Fluids 2019, 154, 104592. [Google Scholar] [CrossRef]
- Salgado, M.; Santos, F.; Rodríguez-Rojo, S.; Reis, R.L.; Duarte, A.R.C.; Cocero, M.J. Development of barley and yeast β-glucan aerogels for drug delivery by supercritical fluids. J. CO2 Util. 2017, 22, 262–269. [Google Scholar] [CrossRef]
- Zhang, Y.-G.; Zhu, Y.-J.; Xiong, Z.-C.; Wu, J.; Chen, F. Bioinspired ultralight inorganic aerogel for highly efficient air filtration and oil–water separation. ACS Appl. Mater. Interfaces 2018, 10, 13019–13027. [Google Scholar] [CrossRef]
- Ziegler, C.; Wolf, A.; Liu, W.; Herrmann, A.K.; Gaponik, N.; Eychmüller, A. Modern inorganic aerogels. Angew. Chem. Int. Ed. 2017, 56, 13200–13221. [Google Scholar] [CrossRef] [PubMed]
- Horvat, G.; Pantić, M.; Knez, Ž.; Novak, Z. Preparation and characterization of polysaccharide-silica hybrid aerogels. Sci. Rep. 2019, 9, 16492. [Google Scholar] [CrossRef] [PubMed]
- Zubyk, H.; Mykhailiv, O.; Papathanassiou, A.N.; Sulikowski, B.; Zambrzycka-Szelewa, E.; Bratychak, M.; Plonska-Brzezinska, M.E. A phenol-formaldehyde polymeric network to generate organic aerogels: Synthesis, physicochemical characteristics and potential applications. J. Mater. Chem. A 2018, 6, 845–852. [Google Scholar] [CrossRef]
- Tripathi, A.; Parsons, G.N.; Khan, S.A.; Rojas, O.J. Synthesis of organic aerogels with tailorable morphology and strength by controlled solvent swelling following Hansen solubility. Sci. Rep. 2018, 8, 2106. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Su, Y.; Wang, W.; Fang, Y.; Riffat, S.B.; Jiang, F. The advances of polysaccharide-based aerogels: Preparation and potential application. Carbohydr. Polym. 2019, 226, 115242. [Google Scholar] [CrossRef]
- Qin, L.; Zhao, X.; He, Y.; Wang, H.; Wei, H.; Zhu, Q.; Zhang, T.; Qin, Y.; Du, A. Preparation, characterization, and in vitro evaluation of resveratrol-loaded cellulose aerogel. Materials 2020, 13, 1624. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Moghaddas, J. Mesoporous tablet-shaped potato starch aerogels for loading and release of the poorly water-soluble drug celecoxib. Chin. J. Chem. Eng. 2020, 28, 1778–1787. [Google Scholar] [CrossRef]
- López-Iglesias, C.; Barros, J.; Ardao, I.; Monteiro, F.J.; Alvarez-Lorenzo, C.; Gómez-Amoza, J.L.; García-González, C.A. Vancomycin-loaded chitosan aerogel particles for chronic wound applications. Carbohydr. Polym. 2019, 204, 223–231. [Google Scholar] [CrossRef]
- Trucillo, P.; Cardea, S.; Baldino, L.; Reverchon, E. Production of liposomes loaded alginate aerogels using two supercritical CO2 assisted techniques. J. CO2 Util. 2020, 39, 101161. [Google Scholar] [CrossRef]
- Pantić, M.; Horvat, G.; Knez, Ž.; Novak, Z. Preparation and characterization of chitosan-coated pectin aerogels: Curcumin case study. Molecules 2020, 25, 1187. [Google Scholar] [CrossRef]
- Groult, S.; Buwalda, S.; Budtova, T. Tuning bio-aerogel properties for controlling theophylline delivery. Part 1: Pectin aerogels. Mater. Sci. Eng. C 2021, 126, 112148. [Google Scholar] [CrossRef]
- Mikkonen, K.S.; Parikka, K.; Ghafar, A.; Tenkanen, M. Prospects of polysaccharide aerogels as modern advanced food materials. Trends Food Sci. Technol. 2013, 34, 124–136. [Google Scholar] [CrossRef]
- Pantić, M.; Kravanja, K.A.; Knez, Ž.; Novak, Z. Influence of the Impregnation Technique on the Release of Esomeprazole from Various Bioaerogels. Polymers 2021, 13, 1882. [Google Scholar] [CrossRef]
- Gonçalves, V.S.S.; Gurikov, P.; Poejo, J.; Matias, A.A.; Heinrich, S.; Duarte, C.M.M.; Smirnova, I. Alginate-based hybrid aerogel microparticles for mucosal drug delivery. Eur. J. Pharm. Biopharm. 2016, 107, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Pantić, M.; Knez, Ž.; Novak, Z. Supercritical impregnation as a feasible technique for entrapment of fat-soluble vitamins into alginate aerogels. J. Non-Cryst. Solids 2016, 432, 519–526. [Google Scholar] [CrossRef]
- Di Maio, E.; Kiran, E. Foaming of polymers with supercritical fluids and perspectives on the current knowledge gaps and challenges. J. Supercrit. Fluids 2018, 134, 157–166. [Google Scholar] [CrossRef]
- Duarte, A.R.C.; Mano, J.F.; Reis, R.L. Supercritical fluids in biomedical and tissue engineering applications: A review. Int. Mater. Rev. 2009, 54, 214–222. [Google Scholar] [CrossRef]
- Knez, Ž.; Markočič, E.; Leitgeb, M.; Primožič, M.; Knez Hrnčič, M.; Škerget, M. Industrial applications of supercritical fluids: A review. Energy 2014, 77, 235–243. [Google Scholar] [CrossRef]
- Yang, J.; Wu, M.; Chen, F.; Fei, Z.; Zhong, M. Preparation, characterization, and supercritical carbon dioxide foaming of polystyrene/graphene oxide composites. J. Supercrit. Fluids 2011, 56, 201–207. [Google Scholar] [CrossRef]
- Chen, C.-X.; Peng, H.-H.; Guan, Y.-X.; Yao, S.-J. Morphological study on the pore growth profile of poly (ε-caprolactone) bi-modal porous foams using a modified supercritical CO2 foaming process. J. Supercrit. Fluids 2019, 143, 72–81. [Google Scholar] [CrossRef]
- Corre, Y.-M.; Maazouz, A.; Duchet, J.; Reignier, J. Batch foaming of chain extended PLA with supercritical CO2: Influence of the rheological properties and the process parameters on the cellular structure. J. Supercrit. Fluids 2011, 58, 177–188. [Google Scholar] [CrossRef]
- Li, B.; Zhao, G.; Wang, G.; Zhang, L.; Gong, J. Fabrication of high-expansion microcellular PLA foams based on pre-isothermal cold crystallization and supercritical CO2 foaming. Polym. Degrad. Stab. 2018, 156, 75–88. [Google Scholar] [CrossRef]
- Milovanovic, S.; Markovic, D.; Mrakovic, A.; Kuska, R.; Zizovic, I.; Frerich, S.; Ivanovic, J. Supercritical CO2-assisted production of PLA and PLGA foams for controlled thymol release. Mater. Sci. Eng. C 2019, 99, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Lee, L.Y.; Tong, H.; Wang, C.-H. PLGA/chitosan composites from a combination of spray drying and supercritical fluid foaming techniques: New carriers for DNA delivery. J. Control. Release 2008, 129, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Ong, Y.X.J.; Lee, L.Y.; Davoodi, P.; Wang, C.-H. Production of drug-releasing biodegradable microporous scaffold using a two-step micro-encapsulation/supercritical foaming process. J. Supercrit. Fluids 2018, 133, 263–269. [Google Scholar] [CrossRef]
- Song, C.; Li, S.; Zhang, J.; Xi, Z.; Lu, E.; Zhao, L.; Cen, L. Controllable fabrication of porous PLGA/PCL bilayer membrane for GTR using supercritical carbon dioxide foaming. Appl. Surf. Sci. 2019, 472, 82–92. [Google Scholar] [CrossRef]
- Cabezas, L.I.; Gracia, I.; García, M.T.; de Lucas, A.; Rodríguez, J.F. Production of biodegradable porous scaffolds impregnated with 5-fluorouracil in supercritical CO2. J. Supercrit. Fluids 2013, 80, 1–8. [Google Scholar] [CrossRef]
- Campardelli, R.; Franco, P.; Reverchon, E.; De Marco, I. Polycaprolactone/nimesulide patches obtained by a one-step supercritical foaming + impregnation process. J. Supercrit. Fluids 2019, 146, 47–54. [Google Scholar] [CrossRef]
- Álvarez, I.; Gutiérrez, C.; Rodríguez, J.F.; de Lucas, A.; García, M.T. Production of drug-releasing biodegradable microporous scaffold impregnated with gemcitabine using a CO2 foaming process. J. CO2 Util. 2020, 41, 101227. [Google Scholar] [CrossRef]
- Rojas, A.; Torres, A.; López de Dicastillo, C.; Velásquez, E.; Villegas, C.; Faba, S.; Rivera, P.; Guarda, A.; Romero, J.; Galotto, M.J. Foaming with scCO2 and Impregnation with Cinnamaldehyde of PLA Nanocomposites for Food Packaging. Processes 2022, 10, 376. [Google Scholar] [CrossRef]
- Lee, L.Y.; Ranganath, S.H.; Fu, Y.; Zheng, J.L.; Lee, H.S.; Wang, C.-H.; Smith, K.A. Paclitaxel release from micro-porous PLGA disks. Chem. Eng. Sci. 2009, 64, 4341–4349. [Google Scholar] [CrossRef]
- Kravanja, G.; Primožič, M.; Knez, Ž.; Leitgeb, M. Transglutaminase release and activity from novel poly(ε-caprolactone)-based composites prepared by foaming with supercritical CO2. J. Supercrit. Fluids 2020, 166, 105031. [Google Scholar] [CrossRef]
- Zizovic, I. Potential of supercritical solvent impregnation for development of materials with antibacterial properties. Int. Arch. Med. Microbiol. 2017, 1, 1–6. [Google Scholar]
- Costa, V.P.; Braga, M.E.M.; Guerra, J.P.; Duarte, A.R.C.; Duarte, C.M.M.; Leite, E.O.B.; Gil, M.H.; de Sousa, H.C. Development of therapeutic contact lenses using a supercritical solvent impregnation method. J. Supercrit. Fluids 2010, 52, 306–316. [Google Scholar] [CrossRef]
- Knez, Ž.; Pantić, M.; Cör, D.; Novak, Z.; Hrnčič, M.K. Are supercritical fluids solvents for the future? Chem. Eng. Process.-Process Intensif. 2019, 141, 107532. [Google Scholar] [CrossRef]
- Marizza, P.; Keller, S.S.; Müllertz, A.; Boisen, A. Polymer-filled microcontainers for oral delivery loaded using supercritical impregnation. J. Control. Release 2014, 173, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Elvira, C.; Fanovich, A.; Fernández, M.; Fraile, J.; San Román, J.; Domingo, C. Evaluation of drug delivery characteristics of microspheres of PMMA–PCL–cholesterol obtained by supercritical-CO2 impregnation and by dissolution–evaporation techniques. J. Control. Release 2004, 99, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Shen, S.; Tan, D.C.T.; Ng, W.K.; Liu, X.; Chia, L.S.; Irwan, A.W.; Tan, R.; Nowak, S.A.; Marsh, K. High drug load, stable, manufacturable and bioavailable fenofibrate formulations in mesoporous silica: A comparison of spray drying versus solvent impregnation methods. Drug Deliv. 2016, 23, 316–327. [Google Scholar] [CrossRef]
- Lee, P.I.; Li, J.X. Evolution of oral controlled release dosage forms. In Oral Controlled Release Formulation Design and Drug Delivery; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 21–31. [Google Scholar]
- Park, K. Controlled drug delivery systems: Past forward and future back. J. Control. Release 2014, 190, 3–8. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, S.S.; DeLuca, P.P. Methods to assess in vitro drug release from injectable polymeric particulate systems. Pharm. Res. 2006, 23, 460–474. [Google Scholar] [CrossRef] [PubMed]
- Chalitangkoon, J.; Wongkittisin, M.; Monvisade, P. Silver loaded hydroxyethylacryl chitosan/sodium alginate hydrogel films for controlled drug release wound dressings. Int. J. Biol. Macromol. 2020, 159, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Catarata, R.; Azim, N.; Bhattacharya, S.; Zhai, L. Controlled drug release from polyelectrolyte–drug conjugate nanoparticles. J. Mater. Chem. B 2020, 8, 2887–2894. [Google Scholar] [CrossRef] [PubMed]
- Finšgar, M.; Kovač, J.; Maver, U. The development and characterization of bioactive coatings for local drug delivery in orthopedic applications. Prog. Org. Coat. 2021, 158, 106350. [Google Scholar] [CrossRef]
- Kravanja, K.A.; Finšgar, M. Analytical Techniques for the Characterization of Bioactive Coatings for Orthopaedic Implants. Biomedicines 2021, 9, 1936. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Ma, Y.; Sun, C.; Lu, Z.; Yao, Z.; Wang, J.; Li, D.; Yuan, Y.; Yang, X. ROS-sensitive polymeric nanocarriers with red light-activated size shrinkage for remotely controlled drug release. Chem. Mater. 2018, 30, 517–525. [Google Scholar] [CrossRef]
- Sukhodub, L.F.; Sukhodub, L.B.; Litsis, O.; Prylutskyy, Y. Synthesis and characterization of hydroxyapatite-alginate nanostructured composites for the controlled drug release. Mater. Chem. Phys. 2018, 217, 228–234. [Google Scholar] [CrossRef]
- Baun, D.; Walker, G. Apparatus for determining the rate of drug release from solid dosage forms. J. Pharm. Sci. 1969, 58, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Fotaki, N.; Aivaliotis, A.; Butler, J.; Dressman, J.; Fischbach, M.; Hempenstall, J.; Klein, S.; Reppas, C. A comparative study of different release apparatus in generating in vitro–in vivo correlations for extended release formulations. Eur. J. Pharm. Biopharm. 2009, 73, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Sachett, A.; Gallas-Lopes, M.; Benvenutti, R.; Marcon, M.; Aguiar, G.P.S.; Herrmann, A.P.; Oliveira, J.V.; Siebel, A.M.; Piato, A. Curcumin micronization by supercritical fluid: In vitro and in vivo biological relevance. Ind. Crops Prod. 2022, 177, 114501. [Google Scholar] [CrossRef]
- Matsuyama, K.; Morotomi, K.; Inoue, S.; Nakashima, M.; Nakashima, H.; Okuyama, T.; Kato, T.; Muto, H.; Sugiyama, H. Antibacterial and antifungal properties of Ag nanoparticle-loaded cellulose nanofiber aerogels prepared by supercritical CO2 drying. J. Supercrit. Fluids 2019, 143, 1–7. [Google Scholar] [CrossRef]
- Banchero, M.; Mohamed, S.S.; Leone, F.; Lopez, F.; Ronchetti, S.; Manna, L.; Onida, B. Supercritical solvent impregnation of different drugs in mesoporous nanostructured ZnO. Pharmaceutics 2019, 11, 340. [Google Scholar] [CrossRef] [PubMed]
- Kanikkannan, N. Technologies to improve the solubility, dissolution and bioavailability of poorly soluble drugs. J. Anal. Pharm. Res. 2018, 7, 00198. [Google Scholar] [CrossRef]
- Montes, A.; Wehner, L.; Pereyra, C.; De La Ossa, E.M. Precipitation of submicron particles of rutin using supercritical antisolvent process. J. Supercrit. Fluids 2016, 118, 1–10. [Google Scholar] [CrossRef]
- Veres, P.; López-Periago, A.M.; Lázár, I.; Saurina, J.; Domingo, C. Hybrid aerogel preparations as drug delivery matrices for low water-solubility drugs. Int. J. Pharm. 2015, 496, 360–370. [Google Scholar] [CrossRef]
- Franco, P.; Belvedere, R.; Pessolano, E.; Liparoti, S.; Pantani, R.; Petrella, A.; De Marco, I. PCL/Mesoglycan Devices Obtained by Supercritical Foaming and Impregnation. Pharmaceutics 2019, 11, 631. [Google Scholar] [CrossRef]
- Bolten, D.; Türk, M. Micronisation of carbamazepine through rapid expansion of supercritical solution (RESS). J. Supercrit. Fluids 2012, 62, 32–40. [Google Scholar] [CrossRef]
- Lin, P.-C.; Su, C.-S.; Tang, M.; Chen, Y.-P. Micronization of ethosuximide using the rapid expansion of supercritical solution (RESS) process. J. Supercrit. Fluids 2012, 72, 84–89. [Google Scholar] [CrossRef]
- Hiendrawan, S.; Veriansyah, B.; Tjandrawinata, R.R. Micronization of fenofibrate by rapid expansion of supercritical solution. J. Ind. Eng. Chem. 2014, 20, 54–60. [Google Scholar] [CrossRef]
- Sodeifian, G.; Sajadian, S.A. Solubility measurement and preparation of nanoparticles of an anticancer drug (Letrozole) using rapid expansion of supercritical solutions with solid cosolvent (RESS-SC). J. Supercrit. Fluids 2018, 133, 239–252. [Google Scholar] [CrossRef]
- Chen, B.-Q.; Kankala, R.K.; Wang, S.-B.; Chen, A.-Z. Continuous nanonization of lonidamine by modified-rapid expansion of supercritical solution process. J. Supercrit. Fluids 2018, 133, 486–493. [Google Scholar] [CrossRef]
- Türk, M.; Bolten, D. Formation of submicron poorly water-soluble drugs by rapid expansion of supercritical solution (RESS): Results for Naproxen. J. Supercrit. Fluids 2010, 55, 778–785. [Google Scholar] [CrossRef]
- Huang, Z.; Guo, Y.; Miao, H.; Teng, L. Solubility of progesterone in supercritical carbon dioxide and its micronization through RESS. Powder Technol. 2014, 258, 66–77. [Google Scholar] [CrossRef]
- Franco, P.; De Marco, I. Controlled-release antihistamines using supercritical antisolvent process. J. Supercrit. Fluids 2021, 171, 105201. [Google Scholar] [CrossRef]
- Franco, P.; De Marco, I. Preparation of non-steroidal anti-inflammatory drug/β-cyclodextrin inclusion complexes by supercritical antisolvent process. J. CO2 Util. 2021, 44, 101397. [Google Scholar] [CrossRef]
- Liu, M.; Liu, Y.; Ge, Y.; Zhong, Z.; Wang, Z.; Wu, T.; Zhao, X.; Zu, Y. Solubility, Antioxidation, and Oral Bioavailability Improvement of Mangiferin Microparticles Prepared Using the Supercritical Antisolvent Method. Pharmaceutics 2020, 12, 90. [Google Scholar] [CrossRef] [PubMed]
- Ha, E.-S.; Park, H.; Lee, S.-K.; Sim, W.-Y.; Jeong, J.-S.; Baek, I.-h.; Kim, M.-S. Pure Trans-Resveratrol Nanoparticles Prepared by a Supercritical Antisolvent Process Using Alcohol and Dichloromethane Mixtures: Effect of Particle Size on Dissolution and Bioavailability in Rats. Antioxidants 2020, 9, 342. [Google Scholar] [CrossRef] [PubMed]
- Yesil-Celiktas, O.; Cetin-Uyanikgil, E.O. In vitro release kinetics of polycaprolactone encapsulated plant extract fabricated by supercritical antisolvent process and solvent evaporation method. J. Supercrit. Fluids 2012, 62, 219–225. [Google Scholar] [CrossRef]
- Senčar-Božič, P.; Srčič, S.; Knez, Z.; Kerč, J. Improvement of nifedipine dissolution characteristics using supercritical CO2. Int. J. Pharm. 1997, 148, 123–130. [Google Scholar] [CrossRef]
- Ngo, T.T.; Blair, S.; Kuwahara, K.; Christensen, D.; Barrera, I.; Domingo, M.; Singamneni, S. Drug impregnation for laser sintered poly(methyl methacrylate) biocomposites using supercritical carbon dioxide. J. Supercrit. Fluids 2018, 136, 29–36. [Google Scholar] [CrossRef]
- Obaidat, R.; Alnaief, M.; Jaeger, P. Significant solubility of carbon dioxide in Soluplus® facilitates impregnation of ibuprofen using supercritical fluid technology. Pharm. Dev. Technol. 2018, 23, 697–705. [Google Scholar] [CrossRef]
- Fathi, M.; Sodeifian, G.; Sajadian, S.A. Experimental study of ketoconazole impregnation into polyvinyl pyrrolidone and hydroxyl propyl methyl cellulose using supercritical carbon dioxide: Process optimization. J. Supercrit. Fluids 2022, 188, 105674. [Google Scholar] [CrossRef]





| SC Technique | AI | System | AI Release | Reference |
|---|---|---|---|---|
| RESS | Carbamazepine | AI in SC-CO2 | Submicron carbamazepine has a dissolution rate coefficient that is up to two times higher than that of the original material. | [144] |
| coQ10 | AI with PEG and PLA in SC-CO2 + co-solvent (ethanol/acetone) | PEG: A higher release rate when the concentration of the PEG is higher than that of coQ10 (a smaller particle size is produced) and by ethanol as a co-solvent, maintaining the same PEG/coQ10 ratio.PLA: a higher release rate when the PLA concentration is higher than coQ10.The best dissolution rate occurs at a PLA/coQ10 ratio of 2/1. | [38] | |
| Ethosuximide | AI in SC-CO2 | Enhanced dissolution rate in PBS compared to the unprocessed material. | [145] | |
| Fenofibrate | AI in SC-CO2 | Enhanced dissolution rate in water with 0.05 M SLS: 8.1 times higher dissolution rate coefficient for the micronized AI. | [146] | |
| Letrozole | AI in SC-CO2 + co-solvent (menthol) | Improved dissolution rate: 14.86 times higher dissolution rate coefficient for the micronized drug. | [147] | |
| Lonidamine | AI in SC-CO2 | Improved dissolution rate of the micronized drug in aqueous media. | [148] | |
| Naproxen | AI in SC-CO2 | Improved dissolution rate in SBF: higher dissolution rate coefficients of the micronized drug compared to the unprocessed drug at pH = 2.0 and pH = 7.4. | [149] | |
| Progesterone | AI in SC-CO2 | Enhanced drug dissolution rate after RESS treatment. | [150] | |
| SAS | Cetirizine dihydrochloride and ketotifen | AIs in zein and SC-CO2 | Prolonged (controlled) release of both processed antihistamines. | [151] |
| Curcumin | AI and poly (vinyl pyrrolidone) in an ethanol/acetone mixture with SC-CO2 | Up to 600 times increased solubility of the processed AI compared to unprocessed. | [45] | |
| Fenofibrate | AI in the polymers P407 and TPGS with SC-CO2 | 95.1% ± 2.5% improved drug dissolution rate compared to the unprocessed drug. | [19] | |
| Ketoprofen and nimesulide | AIs in β-cyclodextrin with SC-CO2 | An enhancement of the drug dissolution rate of up to 21 (nimesulide) and 7 (ketoprofen) times. | [152] | |
| Mangiferin | AI with N, N-dimethylformamide (DMF) as the solvent and SC-CO2 as the antisolvent | 4.26, 2.1, and 2.5 times better solubility of the processed AI in water, simulated gastric fluid, and simulated intestinal fluid, respectively. | [153] | |
| Rutin | AI in acetone and DMSO with SC-CO2 | A dissolution rate of micronized AI particles up to 10 times faster than nonprocessed AI. | [141] | |
| Trans-resveratrol | AI in alcohol (methanol or ethanol) and dichloromethane mixtures with SC-CO2 | Improved release rate of the processed drug. | [154] | |
| GAS | Rosemary extract | AI encapsulated in PCL dissolved in dichloromethane, antisolvent SC-CO2 | Burst release in an aqueous medium, first-order kinetic model. | [155] |
| PGSSTM | Epigallocatechin gallate | AI in OSA-starch, soybean lecithin and β-glucan with SC-CO2 | Rapid release for polysaccharide matrices, namely OSA-starch and β-glucan, and somewhat more controlled release for amphiphilic lecithin. | [60] |
| Eucalyptol | PEG and/or PCL with SC-CO2 | Significantly delayed release of AI in PEG and/or PCL compared to the pure AI (an average of 40% released AI from the polymer and 96% released unencapsulated AI in 120 min). | [56] | |
| Fenofibrate | AI in Gelucire® 50/13 with SC-CO2 | Slow, controlled release | [52] | |
| Fenofibrate, nimodipine and o-vanillin | AIs in Brij S100 and PEG 4000 with SC-CO2 | Increased dissolution rate of Brij S100 micronized nimodipine, Brij S100 micronized fenofibrate, and Brij S100/PEG 4000 micronized o-vanillin compared to the unprocessed AIs. | [59] | |
| Ibuprofen | AI in pluronic poloxamers, gelucire and glyceryl monostearate with SC-CO2 | Accelerated release rate of AI in pluronic carriers, prolonged/controlled release in gelucire and glyceryl monostearate. | [54] | |
| Nifedipine | AI in PEG 4000 with SC-CO2 | Increased dissolution rate of micronized AI compared to the unprocessed AIs. | [156] | |
| Omega-3 polyunsaturated fatty acids and astaxanthin-rich salmon oil | AIs in PAG-6000 with SC-CO2 | Rapid release of oil in distilled water: up to 65% within 30 min. | [55] | |
| Aerogels | Ampicillin | AI loaded liposomes entrapped in alginate aerogels | Slow and controlled release of AI from aerogel over 100 h compared to the burst release of pure AI within the first 5 h. | [96] |
| Celecoxib | AI in potato starch aerogel | Faster dissolution rate of AI from aerogel compared to pure AI in simulated gastric and intestinal fluids over a period of 7 h. The release kinetics follow the Korsmeyer–Peppas model. | [94] | |
| Curcumin | AI in pectin- and chitosan-coated pectin aerogels | Enhanced dissolution of AI from aerogels after 2 h in gastric fluid and 22 h in intestinal fluid. The fastest AI release is obtained from pure pectin aerogels. | [97] | |
| Diclofenac sodium, indomethacin | AIs in pectin and xanthan aerogels | Release of the two AIs within 24 h. The release profile of indomethacin showed a higher initial release rate compared with diclofenac and slower release after 5 h of testing. | [70] | |
| Esomeprazole | AI incorporated in alginate, pectin, chitosan, and composite aerogels via diffusion or supercritical impregnation | Slower and more controlled release of AI from aerogels in gastric and intestinal fluids compared to pure AI. The slowest drug release is achieved from pectin and chitosan composite aerogels. | [100] | |
| Ibuprofen, ketoprofen, triflusal | AIs in 14 silica-gelatin aerogels of different composition | Depending on the composition of the aerogels, both immediate and delayed release are possible. | [142] | |
| Ketoprofen, quercetin | AIs loaded in pure alginate and composite pectin, κ-carrageenan, and alginate aerogel microparticles by supercritical impregnation | The release of AIs from the aerogels is slower and more controlled than that of unprocessed AIs within the 60-min test period. | [101] | |
| Nifedipine | Guar, xanthan, pectin, and alginate aerogels prepared by novel ethanol induced gelation | Prolonged release of AI up to 14 days for guar and xanthan aerogels, steady release within 6 h for alginate and pectin aerogels in simulated gastric and intestinal fluids. Drug release from pectin aerogels is controlled by the Hixson-Crowell model, from alginate in PBS by the first-order model, and in HCl media by the Korsmeyer–Peppas model. | [82] | |
| Resveratrol | AI loaded in TEMPO-oxidized cellulose aerogels | After the initial burst release (within the first 15 min), controlled release of AI from aerogels in simulated gastric and intestinal fluids is achieved. After 5 h, 35–50% of the AI is released from the aerogels, compared to 90% of pure AI within the same time period. | [93] | |
| Theophylline | AI loaded in pectin aerogels prepared with different solution pH and calcium concentrations | Drug release from all aerogels shows an initial burst release followed by a more controlled release. The low pH of the pectin starting solutions results in faster release of the AI, while calcium crosslinking decreases the rate of AI release. The main release mechanism is shown to be the Peppas-Sahlin model. | [98] | |
| Tetracycline hydrochloride | κ-carrageenan aerogels prepared with the addition of potassium salts as crosslinking agents | Initial burst release followed by a plateau at approximately 60 min, corresponding to 90% of the released active ingredient in PBS, with a pH of 7.4. | [73] | |
| Vancomycin | AI loaded in chitosan aerogel beads | Burst release within the first hour, followed by a plateau during the remaining test period (2 days). The release profile is fitted to a first-order release model. | [95] | |
| Vitamin D3 | AI loaded in alginate aerogels | Significantly improved dissolution of impregnated AI compared to crystalline AI within 7 h. | [102] | |
| SC foaming | Cinnamaldehyde | AI in PLA foam | An initial burst release, followed by a slowed release over the 300-min test period; Quasi-Fickian diffusion, fitting the Korsmeyer–Peppas mathematical model. | [117] |
| Curcumin, gentamicin | AIs loaded in PLGA foam | Diffusion-controlled release; the drugs were not completely released in the 14-day test period. A slower release is obtained for curcumin. | [112] | |
| Gemcitabine | AI in PLGA foam | An initial burst release with over 80% of the drug released in the first 5 days, followed by prolonged release over the 20-day test period. The drug release is first controlled by a diffusion process, followed by the internal transfer of mass and polymer degradation. | [116] | |
| DNA | DNA loaded in PLGA or composite chitosan/PLGA foams | An initial burst release followed by slow release over a 40-day period for composite foams. | [111] | |
| Mesoglycan | AI loaded in PCL foam | Dissolution tests demonstrated prolonged release of the AI from the PCL foam of up to 70 times longer compared to the pure AI during the 3-day testing period. | [143] | |
| Nimesulide | AI in PCL foam | 3.5 times prolonged release of the AI from the PCL foam compared to the pure AI in the 3-day test period. | [115] | |
| Paclitaxel | AI in PLGA or PLGA-PEG foams | Continuous and nearly linear AI release from the foams, with approximately 50% of release within 8 weeks. | [118] | |
| Thymol | AI loaded in PLA and PLGA foams | Prolonged release of the AI in PBS over the 1.5-month testing period. | [110] | |
| Transglutaminase | AI crosslinked with glutaraldehyde in PCL foam containing chitosan and hydroxyapatite | Prolonged AI release for up to 30 days. | [119] | |
| SSI | Acetylsalicylic acid | AI in barley and yeast β-glucan aerogels | Faster release of AI from barley aerogel and more sustained release from yeast β-glucan aerogel during the 25-h test period. | [86] |
| Cholesterol | AI loaded in PMMA, PMMA/PCL microspheres | Faster dissolution of the AI in PMMA and more sustained release from PMMA/PCL during the 450-h test period. | [124] | |
| Fenofibrate | AI in mesoporous silica | Improved drug dissolution of impregnated AI compared to crystalline AI during the 120-min test period. | [125] | |
| Flurbiprofen | AI in PMMA/β-tricalcium phosphate biocomposites | 50% of the AI released within the first 4 h of measurement in an ethanol solution. | [157] | |
| Ibuprofen | AI in Soluplus® | Improved dissolution of AI loaded by SSI compared to the physical mixture during the 140-h test period. | [158] | |
| Ketoconazole | AI in poly (vinyl pyrrolidone) (PVP) and hydroxy propyl methyl cellulose (HPMC) | Improved dissolution of AI impregnated in polymers by SSI compared to the physical mixture during the 75-min test period. | [159] | |
| Ketoprofen | AI in PVP | Fastest release of AI (87% in the first 30 min) from the impregnated polymer compared to the physical mixture with crystalline or amorphous AI (micro-tablets). Drug dissolution is controlled by polymer degradation. | [123] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kravanja, K.A.; Finšgar, M.; Knez, Ž.; Knez Marevci, M. Supercritical Fluid Technologies for the Incorporation of Synthetic and Natural Active Compounds into Materials for Drug Formulation and Delivery. Pharmaceutics 2022, 14, 1670. https://doi.org/10.3390/pharmaceutics14081670
Kravanja KA, Finšgar M, Knez Ž, Knez Marevci M. Supercritical Fluid Technologies for the Incorporation of Synthetic and Natural Active Compounds into Materials for Drug Formulation and Delivery. Pharmaceutics. 2022; 14(8):1670. https://doi.org/10.3390/pharmaceutics14081670
Chicago/Turabian StyleKravanja, Katja Andrina, Matjaž Finšgar, Željko Knez, and Maša Knez Marevci. 2022. "Supercritical Fluid Technologies for the Incorporation of Synthetic and Natural Active Compounds into Materials for Drug Formulation and Delivery" Pharmaceutics 14, no. 8: 1670. https://doi.org/10.3390/pharmaceutics14081670
APA StyleKravanja, K. A., Finšgar, M., Knez, Ž., & Knez Marevci, M. (2022). Supercritical Fluid Technologies for the Incorporation of Synthetic and Natural Active Compounds into Materials for Drug Formulation and Delivery. Pharmaceutics, 14(8), 1670. https://doi.org/10.3390/pharmaceutics14081670









