Marine Bioactive Compounds Derived from Macroalgae as New Potential Players in Drug Delivery Systems: A Review
Abstract
:1. Introduction
The Diversity of Black Sea Macroalgae Species and Correlation with Their Medical Potential
2. Representative Delivery Systems Developed Based on Bioactive Compounds from Brown, Red, and Green Macroalgae Species
2.1. Phaeophyceae—Brown Algae
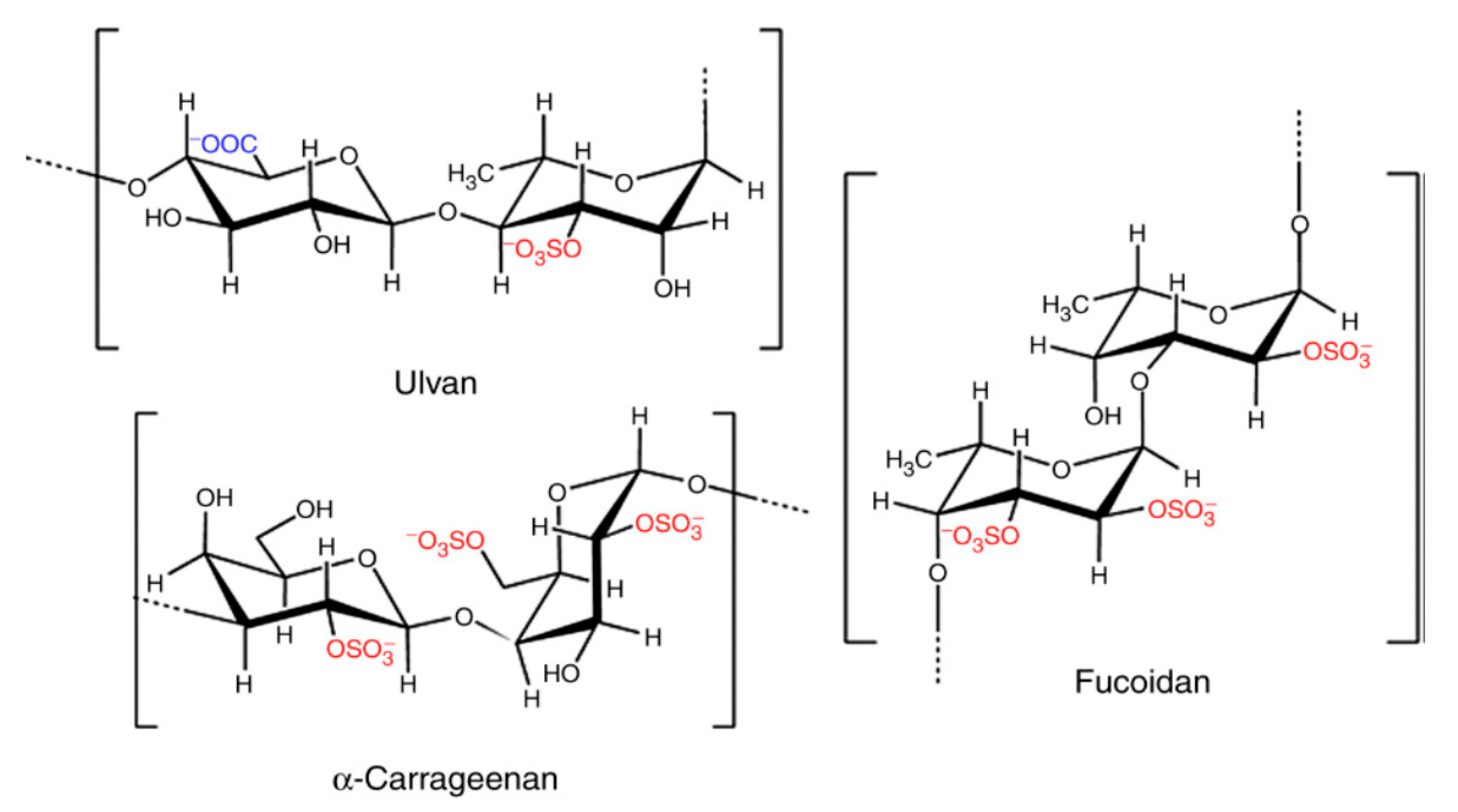
2.1.1. Fucoidan
2.1.2. Drug Delivery Systems with Antiproliferative Potential
2.1.3. Drug Delivery Systems with Antimicrobial Potential
2.1.4. Drug Delivery Systems with Anti-Inflammatory Potential
2.1.5. Drug Delivery Systems with Antidiabetic Potential
| Bioactive Property | Drug Delivery System | Mechanism of Action | Reference |
|---|---|---|---|
| Antitumoral | Oral administration of fucoidan extract | Suppression of tumor in vivo mice model | [36] |
| Oral administration of 100 mg/kg fucoidan extract | Inhibition of tumor growth in vivo mice model | [37] | |
| Agar matrix mix of fucoidan extracts from three algae | Inhibition of SK-MEL-28 human melanoma cells and DLD-1 colon cancer cells | [38] | |
| Purified fucoidan extract | Inhibition of colony formation of DLD-1 cancer cells | [39] | |
| Fucoidan extract | Induce cell apoptosis in B16 murine melanoma cells | [40] | |
| Fucoidan extract | Inhibits DU-145 human prostate cancer cells migration and hiders tumor growth in cancer xenograft | [14] | |
| Fucoidan extract | Induce apoptotic cell death in HCT116 human colorectal carcinoma cells | [41] | |
| Intraperitoneal injection of fucoidan | Inhibits tumor growth and induce apoptosis in 4T1 tumor bearing Balb/c mice | [42] | |
| Oral administration | Hinders metastasis in Lewis tumor-bearing mice | [43] | |
| Fucoidan extract as potential anticancer agent | Inhibits HT-29 human colon adenocarcinoma cells | [44] | |
| Oral administration | Inhibits tumor growth in LLC1-bering mice | [45] | |
| Antioxidant | Purified fucoidan extract | High antioxidant activities due to high sulfate content in new extraction procedure | [46] |
| Fucoidan extract | Presents strong scavenging activity and could be used as natural antioxidant in diseases treatments | [47] | |
| Immune-modulatory effect | Intraperitoneal injection | Up-regulates CD40, CD80, CD86, MHC class I and MHC class II in spleen dendritic cells | [16] |
| Intraperitoneal injection of fucoidan | Enchanced Natural Killer cells activity in spleen of C57BL/6 mice | [48] | |
| Anti-inflammatory | High-molecular-weight product of fucoidan (150 mL/day) | Reducing toxicity in patience going thru chemotherapy | [49] |
| Fucoidan extract—Mei Han Yun product | Stimulates natural immunity | [50] | |
| Dietary supplement | Inhibits atopic dermatitis skin lesions and immune system abnormalities | [51] | |
| Fucoidan extract | Reducing toxicity and inhibition of reactive oxygen species and nitric oxide generation | [52] | |
| Fucoidan extract | Inhibition expression of IL-1B, IL-6, TARC and MDC in TNF-α/IFN-γ induced HaCaT human keratinocyte cell line | [53] | |
| Oral and parenteral administration of fucoidan | Hypocholesterolemic effect and reduce inflammation | [54] | |
| Fucoidan administered as adjuvant | Induce pro-inflammatory cytokine production from spleen in C57BL/6 mice Enhances antigen presentation and antigen specific T cell proliferation in C57BL/6 mice | [55] | |
| Treatment with purified fucoidan extract | Inhibition of nitric oxide production in LPS-exposed zebrafish embryos | [56] | |
| Oral administration of low molecular weight fucoidan | Down regulate expression of IL-6 and up-regulate IL-10 in apoE-knockout mice | [57] | |
| Topical application of lyophilized fucoidan powder | Improve symptoms of atopic dermatitis in AD-induced Nc/Nga mice | [58] |
2.2. Rodophyta—Red Algae
Carrageenans
| Bioactive Property | Drug Delivery System | Use | Reference |
|---|---|---|---|
| Antiviral activity | Extracts for drug formulation, acting as inhibitors for viruses | Holds back Human Rhinoviruses, Herpes Simplex Virus (HSV), Varicella Zoster Virus (VZV) and Human Papillomavirus (HPV) | [73,74,75,76,77,78] |
| Novel core-matrix intravaginal ring | Inhibits HPV and HSV-2 | [79] | |
| Gel formation—Carvir | Bioactive activity against HPV infection | [80] | |
| Iota-carrageenan nasal-spray | Ameliorate cold symptoms and inhibits the multiplication of Human Rhinoviruses (HRV) | [81] | |
| Intranasal iota-carrageenan | Could hold back the Influenza A Virus infection | [82] | |
| κ-carrageenan extract specific targeting | Inhibits H1N1/2009 and other similar viruses | [83] | |
| Intranasal application synergy of carrageenan and zanamivir | Holds back Influenza A Virus strains (pandemic H1N1/09, H3N2, H5N1, H7N7 | [84] | |
| Nasal spray | Inhibits Human Rhinovirus (HRV) 1a, hRV8 and Human Coronavirus OC43 | [85] | |
| κ-carrageenan extract in plaque reduction assay | Bioactive activity against Enterovirus 71 (EV 71) | [86] | |
| λ-carrageenan P32 extract as promising drug | Bioactive activity against Rabies Virus (RABV) | [87] | |
| Polysaccharide carrageenan extract | Bioactive activity against Varicella Zostre Virus (VZV) | [88] | |
| Polysaccharide carrageenan extract | Bioactive activity against Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) | [89] | |
| Antibacterial activity | Polysaccharide carrageenan extract suggested to act as preservatives in processed food | Acts against the growth of different bacterial strains | [90] |
| Local application of iota-carrageenan | Bioactive activity against the ocular infection caused by Chlamydia trachomatis | [91] | |
| k-carrageenan oligosaccharide extract | Is hostile for Saccharomices cerevisiae | [92] | |
| Oxidized κ-carrageenan | Inhibits the growth of Gram-positive and Gram-negative bacteria | [93] | |
| Carrageenan added in sinus rinses | In the presence of Kappa-Carrageenan cells release less IL-6 | [94] | |
| Hydrogel formation | Inhibits the growth of Staphyloccocus aureus and Escherichia coli | [95] | |
| Carboxymethylation of κ-carrageenan for biomaterial applications | Inhibits the growth of Bacillus cereus, Pseudomonas aeruginosa, Staphyloccocus aureus and Escherichia coli | [96] | |
| Antihyperlipidemic activity | Viscous gels | Lowering blood levels of cholesterol | [76] |
| Seaweed powder mix as health supplement Carrageenan microgels | Lowering serum levels of triglycerides, low density lipoprotein cholesterol (LDL-C) and total cholesterol, and raises levels of high-density lipoprotein cholesterol (HDL-C) | [97,98] | |
| Carrageenan extract as supplement | Regulate prostaglandin E2 synthesis and stimulate IL-1 and IL-6 synthesis. Cholesterol reducing properties | [99] | |
| Gel formation for vegetable ingestible jelly | Lowering serum levels of total cholesterol | [100] | |
| Carrageenans as food supplement and prebiotics | Bioactivity in metabolic syndrome | [101,102] | |
| Anticoagulant and antithrombotic activity | Extracts | Most effective anticoagulant tested on rabbits | [103] |
| Carrageenan as excipient | Reduces formation of blood clots | [62,73] | |
| Synthesis of carrageenan derivatives | Acts as an anticoagulant | [104] | |
| Antitumor and immunomodulatory activity | Adjuvants Synthesis of carrageenan oligosaccharide derivatives | Acts as an immunomodulator with anticancer effects | [105,106] |
| Extract of low molecular weight λ-carrageenan | Increases the antitumor effect of 5-Fluorouracil | [107] | |
| λ-carrageenan intratumoral injection | Holds back the growth of tumors in mice with murine melanoma cell line | [108] | |
| Extract as anticancer agent | Kappa-CG and Lambda-CG delays the cell cycle in the G2/M phase, while only the last stalles the cell cycle in the G1 and phase | [109] | |
| Degraded iota-carrageenan | Holds back tumor growth, can induce apoptosis, and stop the G1 phase | [110] | |
| Active principles of extracts | Damages LM2 tumor cells | [111] | |
| Extracts | Inhibits colorectal cancer stem-like cells | [112] | |
| LMW carrageenan degradation products | Modulates the immune system with anticancer effects | [113] | |
| Antioxidant activity | Multilayer coating based on κ-carrageenan and quercetin-loaded lecithin/chitosan nanoparticles | Antioxidant activity in the multilayer coating | [114] |
2.3. Chlorophyta (Green Algae)
2.3.1. Ulvans
2.3.2. Targeted Delivery System
2.3.3. Ulvan-Based Hydrogels as Delivery Systems
2.3.4. Ulvan-Based Polymeric Materials
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ROS | Reactive oxygen species |
| FTIR | Fourier-transform infrared spectroscopy |
| TNF-alpha-induced | tumor necrosis factor |
| HeLa | human cervical cancer cells |
| NDF | neutral detergent fiber |
| LDL | low-density lipoprotein |
| CVDs | cardiovascular diseases |
| SPs | sulphated polysaccharides |
| GAGs | glycosaminoglycans |
| UMA | ulvan methacrylate |
| LCST | lower critical solution temperature |
| PVA | poly(vinyl alcohol) |
| PEO | poly(ethylene oxide) |
| PVC | poly (vinyl chloride) |
| PDLLA | poly-dl-lactic acid |
References
- Bellisle, F.; Blundell, J.E.; Dye, L.; Fantino, M.; Fern, E.; Fletcher, R.J.; Lambert, J.; Roberfroid, M.; Specter, S.; Westenhofer, J. Functional food science and behaviour and psychological functions. Br. J. Nutr. 1998, 80 (Suppl. S1), S173–S193. [Google Scholar] [CrossRef] [PubMed]
- Negreanu-Pirjol, B.S.; Negreanu-Pirjol, T.; Cadar, E.; Paraschiv, G.M. Qualitative Data Regarding the Macrophytic Communities Structure in the Wave Breaking Zone at the Romanian Black Sea Littoral. Eur. J. Med. Nat. Sci. 2019, 2, 31–41. [Google Scholar] [CrossRef]
- Karaçuha, A.; Okudan, E.S. Macroalgae and phanerogams of the Black Sea. In Black Sea Marine Environment: The Turkish Shelf. Turkish Marine Research Foundation; Sezgin, M., Bat, L., Ürkmez, D., Arici, E., Öztürk, B., Eds.; Turkish Marine Research Foundation (TUDAV): Istanbul, Turkey, 2017; pp. 167–177. [Google Scholar]
- Milchakova, N. Marine Plants of the Black Sea: An Illustrated Field Guide; DigitPrint Press: Hazari, India, 2011; 144p. [Google Scholar]
- Marin, O.; Abaza, V.; Roşioru, D.M. The qualitative and quantitative evaluation of macroalgae from the Romanian Black Sea coast-a potential economic resource for future. In Proceedings of the 16th International Multidisciplinary Scientific Geoconference SGEM Conference Proceedings, Albena, Bulgaria, 30 June–6 July 2016; Volume 2, pp. 885–892. [Google Scholar]
- Negreanu-Pirjol, B.; Negreanu-Pirjol, T.; Paraschiv, G.; Bratu, M.; Sirbu, R.; Roncea, F.; Meghea, A. Physical-chemical characterization of some green and red macrophyte algae from Romanian Black Sea littoral. Sci. Study Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2011, 12, 173–184. [Google Scholar]
- Plaza, M.; Cifuentes, A.; Ibáñez, E. In the search of new functional food ingredients from algae. Trends Food Sci. Technol. 2008, 19, 31–39. [Google Scholar] [CrossRef]
- Dilipkumar, P.; Khushboo, R. Bioactive Natural Products for Pharmaceutical Applications. Adv. Struct. Mater. 2021, 140, 587–611. [Google Scholar]
- Kylin, H. Zur Biochemie der Meeresalgen. Hoppe-Seyler’s Zeitschrift für Physiologische Chemie. 1913. Strassbürg; Verlag von, K.J., Ed.; Trübner: New York, NY, USA; Walter de Gruyter Publisher: Berlin, Germany, 2009; Volume 83, pp. 171–197. [Google Scholar]
- Sakai, T.; Ishizuka, K.; Shimanaka, K.; Ikai, K.; Kato, I. Structures of oligosaccharides derived from Cladosiphon okamuranus fucoidan by digestion with marine bacterial enzymes. Mar. Biotechnol. 2003, 5, 536–544. [Google Scholar] [CrossRef]
- Berteau, O.; Mulloy, B. Sulfated fucans, fresh perspectives: Structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology 2003, 13, 29–40. [Google Scholar] [CrossRef]
- Miller, K.D.; Fidler-Benaoudia, M.; Keegan, T.H.; Hipp, H.S.; Jemal, A.; Siegel, R.L. Cancer statistics for adolescents and young adults. CA Cancer J. Clin. 2020, 70, 443–459. [Google Scholar] [CrossRef]
- Qian, C.; Kuang, M.; Wang, Y. Effect of Qianghuo erhuang decoction on T regulatory and T helper 17 cells in treatment of adjuvant-induced arthritis in rats. Sci. Rep. 2017, 7, 17198. [Google Scholar] [CrossRef]
- Rui, X.; Pan, H.F.; Shao, S.L.; Xu, X.M. Anti-tumor and anti-angiogenic effects of Fucoidan on prostate cancer: Possible JAK-STAT3 pathway. BMC Complement. Altern. Med. 2017, 17, 378. [Google Scholar] [CrossRef]
- Zayed, A.; Hahn, T.; Rupp, S.; Kramer, R.; Ulber, R. Fucoidan as a natural anticoagulant, antiviral and anti-cancer drug. Naunyn-Schmiedebergs Arch. Pharmacol. 2018, 391, S7–S8. [Google Scholar] [CrossRef]
- Zhang, W.; Oda, T.; Yu, Q.; Jin, J.O. Fucoidan from Macrocystis pyrifera has powerful immune-modulatory effects compared to three other fucoidans. Mar. Drugs 2015, 13, 1084–1104. [Google Scholar] [CrossRef] [Green Version]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Nifantiev, N.E. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef]
- Atashrazm, F.; Lowenthal, R.M.; Woods, G.M.; Holloway, A.F.; Dickinson, J.L. Fucoidan and cancer: A multifunctional molecule with anti-tumor potential. Mar. Drugs 2015, 13, 2327–2346. [Google Scholar] [CrossRef]
- Cheng, Y.; Sibusiso, L.; Hou, L.; Jiang, H.; Chen, P.; Zhang, X.; Tong, H. Sargassum fusiforme fucoidan modifies the gut microbiota during alleviation of streptozotocin-induced hyperglycemia in mice. Int. J. Biol. Macromol. 2019, 131, 1162–1170. [Google Scholar] [CrossRef]
- Jun, J.Y.; Jung, M.J.; Jeong, I.H.; Yamazaki, K.; Kawai, Y.; Kim, B.M. Antimicrobial and antibiofilm activities of sulfated polysaccharides from marine algae against dental plaque bacteria. Mar. Drugs 2018, 16, 301. [Google Scholar] [CrossRef]
- Zhang, L.; Hao, J.; Zheng, Y.; Su, R.; Liao, Y.; Gong, X.; Liu, L.; Wang, X. Fucoidan protects dopaminergic neurons by enhancing the mitochondrial function in a rotenone-induced rat model of Parkinson’s disease. Aging Dis. 2018, 9, 590. [Google Scholar] [CrossRef]
- Wang, J.; Hu, X.; Xiang, D. Nanoparticle Drug Delivery Systems: An Excellent Carrier for Tumor Peptide Vaccines. Drug Deliv. 2018, 25, 1319–1327. [Google Scholar] [CrossRef]
- Cavalcanti, I.D.L.; Ximenes, R.M.; Pessoa, O.D.L.; Magalhães, N.S.S.; de Britto Lira-Nogueira, M.C. Fucoidan-coated PIBCA nanoparticles containing oncocalyxone A: Activity against metastatic breast cancer cells. J. Drug Deliv. Sci. Technol. 2021, 65, 102698. [Google Scholar] [CrossRef]
- Jafari, M.; Sriram, V.; Xu, Z.; Harris, G.M.; Lee, J.Y. Fucoidan-doxorubicin nanoparticles targeting p-selectin for effective breast cancer therapy. Carbohydr. Polym. 2020, 249, 116837. [Google Scholar] [CrossRef]
- Chiang, C.S.; Huang, B.J.; Chen, J.Y.; Chieng, W.W.; Lim, S.H.; Lee, W.; Jeng, L.B. Fucoidan-Based Nanoparticles with Inherently Therapeutic Efficacy for Cancer Treatment. Pharmaceutics 2021, 13, 1986. [Google Scholar] [CrossRef]
- Oka, S.; Okabe, M.; Tsubura, S.; Mikami, M.; Imai, A. Properties of fucoidans beneficial to oral healthcare. Odontology 2020, 108, 34–42. [Google Scholar] [CrossRef]
- Alghuthaymi, M.A.; El-Sersy, Z.R.; Tayel, A.A.; Alsieni, M.A.; Abd El Maksoud, A.I. Anticandidal potentiality of biosynthesized and decorated nanometals with fucoidan. Green Process. Synth. 2021, 10, 811–823. [Google Scholar] [CrossRef]
- Rao, S.S.; Saptami, K.; Venkatesan, J.; Rekha, P.D. Microwave assisted rapid synthesis of silver nanoparticles using fucoidan: Characterization with assessment of biocompatibility and antimicrobial activity. Int. J. Biol. Macromol. 2020, 163, 745–755. [Google Scholar] [CrossRef]
- Shanthi, N.; Arumugam, P.; Murugan, M.; Sudhakar, M.P.; Arunkumar, K. Extraction of Fucoidan from Turbinaria decurrens and the Synthesis of Fucoidan-Coated AgNPs for Anticoagulant Application. ACS Omega 2021, 6, 30998–31008. [Google Scholar] [CrossRef]
- El-Far, Y.M.; Khodir, A.E.; Emarah, Z.A.; Ebrahim, M.A.; Al-Gayyar, M.M. Fucoidan ameliorates hepatocellular carcinoma induced in rats: Effect on miR143 and inflammation. Nutr. Cancer 2021, 73, 1498–1510. [Google Scholar] [CrossRef]
- Barbosa, A.I.; Coutinho, A.J.; Costa Lima, S.A.; Reis, S. Marine polysaccharides in pharmaceutical applications: Fucoidan and chitosan as key players in the drug delivery match field. Mar. Drugs 2019, 17, 654. [Google Scholar] [CrossRef]
- Barbosa, A.I.; Lima, S.A.C.; Reis, S. Development of methotrexate loaded fucoidan/chitosan nanoparticles with anti-inflammatory potential and enhanced skin permeation. Int. J. Biol. Macromol. 2019, 124, 1115–1122. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, S.; Zhao, H.; Liu, Y.; Xue, M.; Zhang, H.; Qiu, X.; Sun, Z.; Liang, H. Protective effects of fucoidan against ethanol-induced liver injury through maintaining mitochondrial function and mitophagy balance in rats. Food Funct. 2021, 12, 3842–3854. [Google Scholar] [CrossRef]
- Peng, Y.; Ren, D.; Song, Y.; Hu, Y.; Wu, L.; Wang, Q.; He, Y.; Zhou, H.; Liu, S.; Cong, H. Effects of a combined fucoidan and traditional Chinese medicine formula on hyperglycaemia and diabetic nephropathy in a type II diabetes mellitus rat model. Int. J. Biol. Macromol. 2020, 147, 408–419. [Google Scholar] [CrossRef]
- Daub, C.D.; Mabate, B.; Malgas, S.; Pletschke, B.I. Fucoidan from Ecklonia maxima is a powerful inhibitor of the diabetes-related enzyme, α-glucosidase. Int. J. Biol. Macromol. 2020, 151, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Ishihara, T.; Nakamoto, H.; Amaha, T.; Osaki, T.; Tsuka, T.; Imagawa, T.; Minami, S.; Takashima, O.; Ifuku, S.; et al. Effects of Oral Administration of Fucoidan Extracted from Cladosiphon okamuranus on Tumor Growth and Survival Time in a Tumor-Bearing Mouse Model. Mar. Drugs 2012, 10, 2337–2348. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Tomimori, K.; Kimura, R.; Ishikawa, C.; Nowling, T.K.; Mori, N. Anti-tumor activity of fucoidanis mediated by nitric oxide released from macrophages. Int. J. Oncol. 2012, 40, 251–260. [Google Scholar] [CrossRef]
- Ermakova, S.; Sokolova, R.; Kim, S.M.; Um, B.H.; Isakov, V.; Zvyagintseva, T. Fucoidans from brown seaweeds Sargassum hornery, Eclonia cava, Costaria costata: Structural characteristics and anticancer activity. Appl. Biochem. Biotechnol. 2011, 164, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Ermakova, S.; Men’shova, R.; Vishchuk, O.; Kim, S.M.; Um, B.H.; Isakov, V.; Zvyagintseva, T. Water-soluble polysaccharides from the brown alga Eisenia bicyclis: Structural characteristics and antitumor activity. Algal Res. 2013, 2, 51–58. [Google Scholar] [CrossRef]
- Wang, Z.J.; Xu, W.; Liang, J.W.; Wang, C.S.; Kang, Y. Effect of Fucoidan on B16 Murine Melanoma Cell Melanin Formation and Apoptosis. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Park, S.H.; Jeong, J.W.; Yoon, D.; Han, M.H.; Lee, D.S.; Choi, G.; Yim, M.J.; Lee, J.M.; Kim, D.H. Induction of p53-independent apoptosis and G1 cell cycle arrest by fucoidan in HCT116 human colorectal carcinoma cells. Mar. Drugs 2017, 15, 154. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Ge, Y.; Zhang, J.; Wang, Q.; Hou, L.; Liu, Y.; Sun, L.; Li, Q. Anticancer properties and mechanisms of fucoidan on mouse breast cancer in vitro and in vivo. PLoS ONE 2012, 7, e43483. [Google Scholar] [CrossRef]
- Huang, T.-H.; Chiu, Y.-H.; Chan, Y.-L.; Chiu, Y.-H.; Wang, H.; Huang, K.-C.; Li, T.-L.; Hsu, K.-H.; Wu, C.-J. Prophylactic Administration of Fucoidan Represses Cancer Metastasis by Inhibiting Vascular Endothelial Growth Factor (VEGF) and Matrix Metalloproteinases (MMPs) in Lewis Tumor-Bearing Mice. Mar. Drugs 2015, 13, 1882–1900. [Google Scholar] [CrossRef]
- Kim, I.H.; Kwon, M.J.; Nam, T.J. Differences in cell death and cell cycle following fucoidan treatment in high-density HT-29 colon cancer cells. Mol. Med. Rep. 2017, 15, 4116–4122. [Google Scholar] [CrossRef]
- Hsu, H.Y.; Lin, T.Y.; Wu, Y.C.; Tsao, S.M.; Hwang, P.A.; Shih, Y.W.; Hsu, J. Fucoidan inhibition of lung cancer in vivo and in vitro: Role of the Smurf2-dependent ubiquitin proteasome pathway in TGF receptordegradation. Oncotarget 2014, 5, 7870–7885. [Google Scholar] [CrossRef]
- Zhao, D.; Xu, J.; Xu, X. Bioactivity of fucoidan extracted from Laminaria japonica using a novel procedurewith high yield. Food Chem. 2018, 245, 911–918. [Google Scholar] [CrossRef]
- Marudhupandi, T.; Ajith Kumar, T.T.; Lakshmana Senthil, S.; Nanthini Devi, K. In vitro antioxidant properties of fucoidan fractions from Sargassum tenerrimum. Pak. J. Biol. Sci. 2014, 17, 402–407. [Google Scholar] [CrossRef]
- Ale, M.; Maruyama, H.; Tamauchi, H.; Mikkelsen, J.D.; Meyer, A. Fucoidan from Sargassum sp. and Fucus vesiculosus reduces cell viability of lung carcinoma and melanoma cells in vitro and activates natural killercells in mice in vivo. Int. J. Biol. Macromol. 2011, 49, 331–336. [Google Scholar] [CrossRef]
- Ikeguchi, M.; Yamamoto, M.; Arai, Y.; Maeta, Y.; Ashida, K.; Katano, K.; Miki, Y.; Kimura, T. Fucoidan reduces the toxicities of chemotherapy for patients with unresectable advanced or recurrent colorectal cancer. Oncol. Lett. 2011, 2, 319–322. [Google Scholar] [CrossRef]
- Tomori, M.; Nagamine, T.; Miyamoto, T.; Iha, M. Evaluation of the immunomodulatory effects of fucoidan derived from Cladosiphon okamuranus tokida in mice. Mar. Drugs 2019, 17, 547. [Google Scholar] [CrossRef]
- Kim, O.K.; Lee, M.; Kwon, H.O.; Lee, D.; Park, J.; Kim, E.; You, Y.; Lim, Y.T.; Jun, W.; Lee, J. Costaria costataextract suppresses development of atopic dermatitis in chloro-2,4-dinitrobenzene-treated NC/Nga Mice. Skin Pharmacol. Physiol. 2018, 31, 212–219. [Google Scholar] [CrossRef]
- Lee, S.H.; Ko, C.I.; Jee, Y.; Jeong, Y.; Kim, M.; Kim, J.S.; Jeon, Y.J. Anti-inflammatory effect of fucoidanextracted from Ecklonia cava in zebrafish model. Carbohydr. Polym. 2013, 92, 84–89. [Google Scholar] [CrossRef]
- Ryu, M.J.; Chung, H.S. Anti-inflammatory activity of fucoidan with blocking NF-B and STAT1 in humankeratinocytes cells. Nat. Prod. Sci. 2015, 21, 205–209. [Google Scholar]
- Kuznetsova, T.A.; Ivanushko, L.A.; Persiyanova, E.V.; Ermakova, S.P.; Besednova, N.N. Markers of Systemic Inflammation in Experimental Dyslipidemia Induced by P-407: Modulation with Fucoidan from Brown Alga Fucus evanescens. Bull. Exp. Biol. Med. 2019, 166, 766–769. [Google Scholar] [CrossRef]
- Jin, J.O.; Zhang, W.; Du, J.Y.; Wong, K.W.; Oda, T.; Yu, Q. Fucoidan can function as an adjuvant in vivo to enhance dendritic cell maturation and function and promote antigen-specific T cell immune responses. PLoS ONE 2014, 9, e99396. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Jayawardena, T.U.; Kim, S.Y.; Kim, H.S.; Ahn, G.; Kim, J.; Jeon, Y.J. Fucoidan isolated from invasive Sargassum horneri inhibit LPS-induced inflammation via blocking NF-B and MAPK pathways. Algal Res. 2019, 41, 101561. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, J.; Ge, K.; Tian, Q.; Zhao, P.; Guo, Y. Anti-inflammatory effect of low molecular weight fucoidanfrom Saccharina japonica on atherosclerosis in apoE-knockout mice. Int. J. Biol. Macromol. 2018, 118, 365–374. [Google Scholar] [CrossRef]
- Yang, J.H. Topical application of fucoidan improves atopic dermatitis symptoms in NC/Nga mice. Phytother. Res. 2012, 26, 1898–1903. [Google Scholar] [CrossRef]
- Campo, V.L.; Kawano, D.F.; da Silva, D.B.; Carvalho, I. Carrageenans: Biological properties, chemical modifications and structural analysis—A review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Zia, K.M.; Tabasum, S.; Nasif, M.; Sultan, N.; Aslam, N.; Noreen, A.; Zuber, M. A review on synthesis, properties and applications of natural polymer-based carrageenan blends and composites. Int. J. Biol. Macromol. 2017, 96, 282–301. [Google Scholar] [CrossRef]
- Cohen, S.M.; Ito, N. A Critical Review of the Toxicological Effects of Carrageenan and Processed Eucheuma Seaweed on the Gastrointestinal Tract. Crit. Rev. Toxicol. 2002, 32, 413–444. [Google Scholar] [CrossRef]
- Guan, J.; Li, L.; Mao, S. Applications of carrageenan in advanced drug delivery. In Seaweed Polysaccharides: Isolation, Biological and Biomedical Applications; Venkatesan, J., Anil, S., Kim, S.-K., Eds.; Elsevier: Cambridge, UK, 2017; pp. 283–303. [Google Scholar] [CrossRef]
- Pacheco-Quito, E.-M.; Ruiz-Caro, R.; Veiga, M.-D. Carrageenan: Drug Delivery Systems and Other Biomedical Applications. Mar. Drugs 2020, 18, 583. [Google Scholar] [CrossRef]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H. Chemical Structures and Bioactivities of Sulfated Polysaccharides from Marine Algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef]
- Sharifzadeh, G.; Wahit, M.U.; Soheilmoghaddam, M.; Whye, W.T.; Pasbakhsh, P. Kappa-carrageenan/halloysite nanocomposite hydrogels as potential drug delivery systems. J. Taiwan Inst. Chem. Eng. 2016, 67, 426–434. [Google Scholar] [CrossRef]
- Pettinelli, N.; Rodríguez-Llamazares, S.; Farrag, Y.; Bouza, R.; Barral, L.; Feijoo-Bandín, S.; Lago, F. Poly (hydroxybutyrate-co-hydroxyvalerate) microparticles embedded in κ-carrageenan/locust bean gum hydrogel as a dual drug delivery carrier. Int. J. Biol. Macromol. 2020, 146, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, H.M.; Aly, A.A.; Sayed, S.M.; Abou-Okeil, A. К-carrageenan/Na-alginate wound dressing with sustainable drug delivery properties. Polym. Adv. Technol. 2021, 32, 1793–1801. [Google Scholar] [CrossRef]
- Joshi, K.S.; Sonawane, R.O.; Pardeshi, C.V. New κ-Carrageenan-montmorillonite Polyelectrolyte Complex used as a Polymer for the Extended Release Circular Pellets Containing Tapentadol Hydrochloride: Statistical optimization. Mater. Technol. 2020, 37, 367–380. [Google Scholar] [CrossRef]
- Youssouf, L.; Bhaw-Luximon, A.; Diotel, N.; Catan, A.; Giraud, P.; Gimié, F.; Koshel, D.; Casale, S.; Bénard, S.; Meneyrol, V.; et al. Enhanced effects of curcumin encapsulated in polycaprolactone-grafted oligocarrageenan nanomicelles, a novel nanoparticle drug delivery system. Carbohydr. Polym. 2019, 217, 35–45. [Google Scholar] [CrossRef]
- Vinothini, K.; Rajendran, N.K.; Munusamy, M.A.; Alarfaj, A.A.; Rajan, M. Development of biotin molecule targeted cancer cell drug delivery of doxorubicin loaded κ-carrageenan grafted graphene oxide nanocarrier. Mater. Sci. Eng. C 2019, 100, 676–687. [Google Scholar] [CrossRef]
- Rodrigues, S.; Cunha, L.; Rico, J.; da Costa, A.M.R.; Almeida, A.J.; Faleiro, M.L.; Buttini, F.; Grenha, A. Carrageenan from red algae: An application in the development of inhalable tuberculosis therapy targeting the macrophages. Drug Deliv. Transl. Res. 2020, 10, 1675–1687. [Google Scholar] [CrossRef]
- Goel, A.; Meher, M.K.; Gupta, P.; Gulati, K.; Pruthi, V.; Poluri, K.M. Microwave assisted κ-carrageenan capped silver nanocomposites for eradication of bacterial biofilms. Carbohydr. Polym. 2019, 206, 854–862. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L. Carrageenan: A review. Vet. Med. 2013, 58, 187–205. [Google Scholar] [CrossRef]
- Wijesekara, I.; Pangestuti, R.; Kim, S.K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 2011, 84, 14–21. [Google Scholar] [CrossRef]
- Li, L.; Ni, R.; Shao, Y.; Mao, S. Carrageenan and its applications in drug delivery. Carbohydr. Polym. 2014, 103, 1–11. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.-K. Biological Activities of Carrageenan. In Advances in Food and Nutrition Research; Elsevier Inc.: Amsterdam, The Netherlands, 2014; Volume 72, pp. 113–124. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, A.; Lu, Z.; Qin, C.; Hu, J.; Yin, J. Overview on the antiviral activities and mechanisms of marine polysaccharides from seaweeds. Carbohydr. Res. 2017, 453–454, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mahomoodally, M.F.; Lobine, D.; Rengasamy, K.R.; Gowrishankar, S.; Tewari, D.; Zengin, G.; Sivanesan, I. Marine algae: A potential resource of anti-HSV molecules. Processes 2019, 7, 887. [Google Scholar] [CrossRef]
- Ugaonkar, S.R.; Wesenberg, A.; Wilk, J.; Seidor, S.; Mizenina, O.; Kizima, L.; Zydowsky, T.M. A novel intravaginal ring to prevent HIV-1, HSV-2, HPV, and unintended pregnancy. J. Control. Release 2015, 213, 57–68. [Google Scholar] [CrossRef]
- Perino, A.; Consiglio, P.; Maranto, M.; De Franciscis, P.; Marci, R.; Restivo, V.; Manzone, M.; Capra, G.; Cucinella, G.; Calagna, G. Impact of a new carrageenan-based vaginal microbicide in a female population with genital HPV-infection: First experimental results. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 6744–6752. [Google Scholar] [CrossRef]
- Eccles, R.; Winther, B.; Johnston, S.L.; Robinson, P.; Trampisch, M.; Koelsch, S. Efficacy and safety of iota-carrageenan nasal spray versus placebo in early treatment of the common cold in adults: The ICICC trial. Respir. Res. 2015, 16, 121. [Google Scholar] [CrossRef]
- Leibbrandt, A.; Meier, C.; König-Schuster, M.; Weinmüllner, R.; Kalthoff, D.; Pflugfelder, B.; Grassauer, A. Iota-carrageenan is a potent inhibitor of influenza A virus infection. PLoS ONE 2010, 5, e14320. [Google Scholar] [CrossRef]
- Shao, Q.; Guo, Q.; Xu, W.P.; Li, Z.; Zhao, T.T. Specific inhibitory effect of κ-carrageenan polysaccharide on swine pandemic 2009 H1N1 influenza virus. PLoS ONE 2015, 10, e0126577. [Google Scholar] [CrossRef]
- Morokutti-Kurz, M.; König-Schuster, M.; Koller, C.; Graf, C.; Graf, P.; Kirchoff, N.; Reutterer, B.; Seifert, J.-M.; Unger, H.; Grassauer, A.; et al. The intranasal application of Zanamivir and carrageenan is synergistically active against influenza A virus in the murine model. PLoS ONE 2015, 10, e0128794. [Google Scholar] [CrossRef]
- Graf, C.; Bernkop-Schnürch, A.; Egyed, A.; Koller, C.; Prieschl-Grassauer, E.; Morokutti-Kurz, M. Development of a nasal spray containing xylometazoline hydrochloride and iota-carrageenan for the symptomatic relief of nasal congestion caused by rhinitis and sinusitis. Int. J. Gen. Med. 2018, 11, 275–283. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Chan, Y.L.; Tsai, L.W.; Li, T.L.; Wu, C.J. Prevention of human enterovirus 71 infection by kappa carrageenan. Antivir. Res. 2012, 95, 128–134. [Google Scholar] [CrossRef]
- Luo, Z.; Tian, D.; Zhou, M.; Xiao, W.; Zhang, Y.; Li, M.; Zhao, L. λ-carrageenan P32 is a potent inhibitor of rabies virus infection. PLoS ONE 2015, 10, e0140586. [Google Scholar] [CrossRef] [PubMed]
- Abu-Galiyun, E.; Huleihel, M.; Levy-Ontman, O. Antiviral bioactivity of renewable polysaccharides against Varicella Zoster. Cell Cycle 2019, 18, 3540–3549. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Peng, H.; Wang, Q.; Liu, Z.; Dong, X.; Wen, C.; Ai, C.; Zhang, Y.; Wang, Z.; Zhu, B. Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2. Food Funct. 2020, 11, 7415–7420. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Sugita-Konishi, Y.; Shimizu, M. In vitro bacteriostatic effects of dietary polysaccharides. Food Sci. Technol. Res. 2001, 7, 262–264. [Google Scholar] [CrossRef]
- Inic-Kanada, A.; Stein, E.; Stojanovic, M.; Schuerer, N.; Ghasemian, E.; Filipovic, A.; Marinkovic, E.; Kosanovic, D.; Barisani-Asenbauer, T. Effects of iota-carrageenan on ocular Chlamydia trachomatis infection in vitro and in vivo. J. Appl. Phycol. 2018, 30, 2601–2610. [Google Scholar] [CrossRef]
- Wang, F.F.; Yao, Z.; Wu, H.G.; Zhang, S.X.; Zhu, N.N.; Gai, X. Antibacterial activities of kappa-carrageenan oligosaccharides. Appl. Mech. Mater. 2012, 108, 194–199. [Google Scholar]
- Zhu, M.; Ge, L.; Lyu, Y.; Zi, Y.; Li, X.; Li, D.; Mu, C. Preparation, characterization and antibacterial activity of oxidized κ-carrageenan. Carbohydr. Polym. 2017, 174, 1051–1058. [Google Scholar] [CrossRef]
- Bennett, C.; Ramezanpour, M.; Cooksley, C.; Vreugde, S.; Psaltis, A.J. Kappa-carrageenan sinus rinses reduce inflammation and intracellular Staphylococcus aureus infection in airway epithelial cells. Int. Forum Allergy Rhinol. 2019, 9, 918–925. [Google Scholar] [CrossRef]
- Rasool, A.; Ata, S.; Islam, A.; Khan, R.U. Fabrication of novel carrageenan based stimuli responsive injectable hydrogels for controlled release of cephradine. RSC Adv. 2019, 9, 12282–12290. [Google Scholar] [CrossRef]
- Madruga, L.Y.; Sabino, R.M.; Santos, E.C.; Popat, K.C.; Balaban, R.D.C.; Kipper, M.J. Carboxymethyl-kappa-carrageenan: A study of biocompatibility, antioxidant and antibacterial activities. Int. J. Biol. Macromol. 2020, 152, 483–491. [Google Scholar] [CrossRef]
- Amano, H.; Kakinuma, M.; Coury, D.A.; Ohno, H.; Hara, T. Effect of a seaweed mixture on serum lipid level and platelet aggregation in rats. Fish. Sci. 2005, 71, 1160–1166. [Google Scholar] [CrossRef]
- Chen, F.; Deng, Z.; Zhang, Z.; Zhang, R.; Xu, Q.; Fan, G.; Luo, T.; McClements, D.J. Controlling lipid digestion profiles using mixtures of different types of microgel: Alginate beads and carrageenan beads. J. Food Eng. 2018, 238, 156–163. [Google Scholar] [CrossRef]
- Sokolova, E.V.; Kravchenko, A.O.; Sergeeva, N.V.; Davydova, V.N.; Bogdanovich, L.N.; Yermak, I.M. Effect of carrageenans on some lipid metabolism components in vitro. Carbohydr. Polym. 2020, 230, 115629. [Google Scholar] [CrossRef]
- Valado, A.; Pereira, M.; Caseiro, A.; Figueiredo, J.P.; Loureiro, H.; Almeida, C.; Cotas, J.; Pereira, L. Effect of carrageenans on vegetable jelly in humans with hypercholesterolemia. Mar. Drugs 2020, 18, 19. [Google Scholar] [CrossRef]
- Wanyonyi, S.; Du Preez, R.; Brown, L.; Paul, N.A.; Panchal, S.K. Kappaphycus alvarezii as a food supplement prevents diet-induced metabolic syndrome in rats. Nutrients 2017, 9, 1261. [Google Scholar] [CrossRef]
- Du Preez, R.; Paul, N.; Mouatt, P.; Majzoub, M.E.; Thomas, T.; Panchal, S.K.; Brown, L. Carrageenans from the red seaweed Sarconema filiforme attenuate symptoms of diet-induced metabolic syndrome in rats. Mar. Drugs 2020, 18, 97. [Google Scholar] [CrossRef]
- Liang, W.; Mao, X.; Peng, X.; Tang, S. Effects of sulfate group in red seaweed polysaccharides on anticoagulant activity and cytotoxicity. Carbohydr. Polym. 2014, 101, 776–785. [Google Scholar] [CrossRef]
- dos Santos-Fidencio, G.C.; Gonçalves, A.G.; Noseda, M.D.; Duarte, M.E.R.; Ducatti, D.R. Effects of carboxyl group on the anticoagulant activity of oxidized carrageenans. Carbohydr. Polym. 2019, 214, 286–293. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, T.; Yang, Y.; Meng, F.; Zhan, F.; Jiang, Q.; Sun, X. Anti-cancer activity of porphyran and carrageenan from red seaweeds. Molecules 2019, 24, 4286. [Google Scholar] [CrossRef]
- Yuan, H.; Song, J.; Li, X.; Li, N.; Liu, S. Enhanced immunostimulatory and antitumor activity of different derivatives of κ-carrageenan oligosaccharides from Kappaphycus striatum. J. Appl. Phycol. 2011, 23, 59–65. [Google Scholar] [CrossRef]
- Zhou, G.; Xin, H.; Sheng, W.; Sun, Y.; Li, Z.; Xu, Z. In vivo growth-inhibition of S180 tumor by mixture of 5-Fu and low molecular λ-carrageenan from Chondrus ocellatus. Pharmacol. Res. 2005, 51, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Shao, B.; Nie, W.; Wei, X.W.; Li, Y.L.; Wang, B.L.; He, Z.Y.; Liang, X.; Ye, T.H.; Wei, Y.Q. Antitumor and adjuvant activity of λ-carrageenan by stimulating immune response in cancer immunotherapy. Sci. Rep. 2015, 5, 11062. [Google Scholar] [CrossRef] [PubMed]
- Prasedya, E.S.; Miyake, M.; Kobayashi, D.; Hazama, A. Carrageenan delays cell cycle progression in human cancer cells in vitro demonstrated by FUCCI imaging. BMC Complement. Altern. Med. 2016, 16, 270. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Han, Y.X.; Han, X.R. Degraded iota-carrageenan can induce apoptosis in human osteosarcoma cells via the Wnt/β-catenin signaling pathway. Nutr. Cancer 2013, 65, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Calvo, G.H.; Cosenza, V.A.; Sáenz, D.A.; Navarro, D.A.; Stortz, C.A.; Céspedes, M.A.; Di Venosa, G.M. Disaccharides obtained from carrageenans as potential antitumor agents. Sci. Rep. 2019, 9, 6654. [Google Scholar] [CrossRef] [PubMed]
- Cotas, J.; Marques, V.; Afonso, M.B.; Rodrigues, C.M.; Pereira, L. Antitumour potential of Gigartina pistillata carrageenans against colorectal cancer stem cell-enriched tumourspheres. Mar. Drugs 2020, 18, 50. [Google Scholar] [CrossRef] [PubMed]
- Cicinskas, E.; Begun, M.A.; Tiasto, V.A.; Belousov, A.S.; Vikhareva, V.V.; Mikhailova, V.A.; Kalitnik, A.A. In vitro antitumor and immunotropic activity of carrageenans from red algae Chondrus armatus and their low-molecular weight degradation products. J. Biomed. Mater. Res. Part A 2020, 108, 254–266. [Google Scholar] [CrossRef]
- Souza, M.P.; Vaz, A.F.; Costa, T.B.; Cerqueira, M.A.; De Castro, C.M.; Vicente, A.A.; Carneiro-da-Cunha, M.G. Construction of a biocompatible and antioxidant multilayer coating by layer-by-layer assembly of κ-carrageenan and quercetin nanoparticles. Food Bioprocess Technol. 2018, 11, 1050–1060. [Google Scholar] [CrossRef]
- Sirbu, R.; Zaharia, T.; Negreanu-Pirjol, B.; Nicolaev, S.; Bologa, A.; Psegalinschi, I. The Black Sea ecosystem—Important potential source for pharmaceutical industry. J. Environ. Prot. Ecol. 2010, 11, 1336–1348. [Google Scholar]
- Negreanu-Pirjol, B.S.; Negreanu-Pirjol, T.; Mirea, M.; Vasile, M.; Cadar, E. Antioxidant capacity of some marine green macroalgae species fluid extracts. In Proceedings of the International Multidisciplinary Scientific Conferences on Earth & GeoSciences—SGEM Vienna Green, Vienna, Austria, 3–6 December 2018; Issue 6.4, Section 8. Advances in Biotechnology. Volume 51, pp. 63–69. [Google Scholar]
- Lahaye, M.; Robic, A. Structure and functional properties of ulvan, a polysaccharide from green seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar] [CrossRef]
- Popper, Z.A.; Gurvan, M.; Hervé, C.; Domozych, D.S.; Willats, W.G.T.; Tuohy, M.G.; Kloareg, B.; Stengel, B.D. Evolution and Diversity of Plant Cell Walls: From Algae to Flowering Plants. Annu. Rev. Plant Biol. 2011, 62, 567–590. [Google Scholar] [CrossRef]
- Peso-Echarri, P.; González-Bermúdez, C.A.; Ros-Berruezo, G.; Martínez-Graciá, C.; Frontela-Saseta, C. Polysaccharides as Bioactive Components of Functional Food. In Functional Polymers in Food Science: From Technology to Biology, Vol. 2 Food Processing; Cirillo, G., Gianfranco Spizzirri, U., Iemma, F., Eds.; Scrivener Publishing: Beverly, MA, USA, 2015; Chapter 7; pp. 133–158. [Google Scholar]
- Brading, J.W.E.; Georg-Plant, M.M.T.; Hardy, D.M. The polysaccharide from the alga Ulva lactuca—purification, hydrolysis, and methylation of the polysaccharide. J. Chem. Soc. 1954, 3, 319–324. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kurnianto, D. Green Seaweeds-Derived Polysaccharides Ulvan: Occurrence, Medicinal Value and Potential Applications. In Seaweed Polysaccharides. Isolation, Biological and Biomedical Applications; Venkatesan, J., Anil, S., Kim, S.K., Eds.; Elsevier: Oxford, UK, 2017; pp. 205–221. [Google Scholar] [CrossRef]
- McKinnell, J.P.; Percival, E. Acid polysaccharide from green seaweed Ulva lactuca. J. Chem. Soc. 1962, 2082–2083. [Google Scholar] [CrossRef]
- Abdel-Fattah, A.F.; Edrees, M. A study on the polysaccharide, content of Ulva lactuca L. Qual. Plant. Mater. Veg. 1972, 22, 15–22. [Google Scholar] [CrossRef]
- Shao, P.; Qin, M.; Han, L.; Sun, P. Rheology and characteristics of sulfated polysaccharides from chlorophytan seaweeds Ulva fasciata. Carbohyd. Polym. 2014, 113, 365–372. [Google Scholar] [CrossRef]
- Alves, A.; Sousa, R.A.; Reis, R.L. Processing of degradable ulvan 3D porous structures for biomedical applications. J. Biomed. Mater. Res. A 2013, 101, 998–1006. [Google Scholar] [CrossRef]
- Robic, A.; Rondeau-Mouro, C.; Sassi, J.F.; Lerat, Y.; Lahaye, M. Structure and interactions of ulvan in the cell wall of the marine green algae Ulva rotundata (Ulvales Chlorophyceae). Carbohyd. Polym. 2009, 77, 206–216. [Google Scholar] [CrossRef]
- Yaich, H.; Garna, H.; Besbes, S.; Barthélemy, J.P.; Paquot, M.; Blecker, C.; Attia, H. Impact of extraction procedures on the chemical, rheological and textural properties of ulvan from Ulva lactuca of Tunisia coast. Food Hydrocol. 2014, 40, 53–63. [Google Scholar] [CrossRef]
- Jaulneau, V.; Lafitte, C.; Jacquet, C.; Fournier, S.; Salamagne, S.; Briand, X.; Esquerre-Tugaye, M.T.; Dumas, B. Ulvan, a sulfated polysaccharide from green algae, activates plant immunity through the jasmonic acid signaling pathway. J. Biomed. Biotechnol. 2010, 2010, 525291. [Google Scholar] [CrossRef]
- Quemener, B.; Lahaye, M.; Bobin-Dubigeon, C. Sugar determination in ulvans by a chemical-enzymatic method coupled to high performance anion exchange chromatography. J. Appl. Phycol. 1997, 9, 179–188. [Google Scholar] [CrossRef]
- Lahaye, M.; Cimadevilla, E.A.-C.; Kuhlenkamp, R.; Quemener, B.; Lognoné, V.; Dion, P. Chemical composition and 13C NMR spectroscopic characterisation of ulvans from Ulva (Ulvales, Chlorophyta). J. Appl. Phycol. 1999, 11, 1–7. [Google Scholar] [CrossRef]
- Webster, E.A.; Gadd, G.M. Cadmium replaces calcium in the cell wall of Ulva lactuca. BioMetals 1996, 9, 241–244. [Google Scholar] [CrossRef]
- Lahaye, M.; Ray, B. Cell-wall polysaccharides from the marine green alga Ulva rigida (Ulvales, Chlorophyta)—NMR analysis of ulvan oligosaccharides. Carbohyd. Res. 1996, 283, 161–173. [Google Scholar] [CrossRef]
- Paradossi, G.; Cavalieri, F.; Chiessi, E. A conformational study on the algal polysaccharide ulvan. Macromolecules 2002, 35, 6404–6411. [Google Scholar] [CrossRef]
- Siddhanta, A.K.; Goswami, A.M.; Ramavat, B.K.; Mody, K.H.; Mairh, O.P. Water soluble polysaccharides of marine algal species of Ulva (Ulvales, Chlorophyta) of Indian waters. Indian J. Mar. Sci. 2001, 30, 166–172. [Google Scholar]
- Yamamoto, M. Physicochemical studies on sulfated polysaccharides extracted from seaweeds at various temperatures. Agric. Biol. Chem. 1980, 44, 589–593. [Google Scholar] [CrossRef]
- Tziveleka, L.A.; Ioannou, E.; Roussis, V. Ulvan, a bioactive marine sulphated polysaccharide as a key constituent of hybrid biomaterials: A review. Carbohydr. Polym. 2019, 218, 355–370. [Google Scholar] [CrossRef]
- Ciancia, M.; Fernández, P.V.; Leliaert, F. Diversity of Sulfated Polysaccharides From Cell Walls of Coenocytic Green Algae and Their Structural Relationships in View of Green Algal Evolution. Front. Plant Sci. 2020, 11, 554585. [Google Scholar] [CrossRef]
- Yaich, H.; Garna, H.; Besbes, S.; Paquot, M.; Blecker, C.; Attia, H. Chemical composition and functional properties of Ulva lactuca seaweed collected in Tunisia. Food Chem. 2011, 128, 895–901. [Google Scholar] [CrossRef]
- Qi, H.; Zhang, Q.; Zhao, T.; Chen, R.; Zhang, H.; Niu, X.; Li, Z. Antioxidant activity of different sulfate content derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta) in vitro. Int. J. Biol. Macromol. 2005, 37, 195–199. [Google Scholar] [CrossRef]
- Sirbu, R.; Negreanu-Pirjol, T.; Paris, S.; Negreanu-Pirjol, B.S.; Jurja, S.; Tomescu, A. Important bioactive compounds from marine algae—Potential source of pharmaceutical industry. In Proceedings of the International Multidisciplinary Scientific GeoConferences, Albena, Bulgaria, 17–26 June 2014; Volume I, Section Advances in Biotechnology, pp. 381–388. [Google Scholar]
- Sirbu, R.; Zaharia, T.; Maximov, V.; Bechir, A.M.; Maris, M.; Negreanu-Pirjol, B.; Maris, D.A.; Negreanu-Pirjol, T.; Leca, M.; Cadar, E.M.; et al. Clean bio-technologies for obtaining new pharmaceutical formulations based on collagen gels and marine algae extracts for medical applications. J. Environ. Prot. Ecol. 2010, 11, 654–665. [Google Scholar]
- He, J.; Xu, Y.; Chen, H.; Sun, P. Extraction, Structural Characterization, and Potential Antioxidant Activity of the Polysaccharides from Four Seaweeds. Int. J. Mol. Sci. 2016, 17, 1988. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Zhao, T.; Zhang, Q.; Li, Z.; Zhao, Z.; Xing, R. Antioxidant activity of different molecular weight sulfated polysaccharides from Ulva pertusa Kjellm (Chlorophyta). J. Appl. Phycol. 2005, 17, 527–534. [Google Scholar] [CrossRef]
- Redouan, E.; Petit, E.; Beuvain, C.; Courtois, B. Development of new ulvan-like polymer by regioselective oxidation of gellan exopolysaccharide using TEMPO reagent. Carbohydr. Polym. 2010, 80, 485–490. [Google Scholar] [CrossRef]
- Redouan, E.; Petit, E.; Lequart-Pillon, M.; Courtois, B. Evaluation of antioxidant capacity of ulvan-like polymer obtained by regioselective oxidation of gellan exopolysaccharide. Food Chem. 2011, 127, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.S.; Lehmann, V.K.S. Marine pharmacology in 1999: Antitumor and cytotoxic compounds. Anticancer Res. 2001, 21, 2489–2500. [Google Scholar] [PubMed]
- Kaeffer, B.; Benard, C.; Lahaye, M.; Blottiere, H.M.; Cherbut, C. Biological properties of ulvan, a new source of green seaweed sulfated polysaccharides, on cultured normal and cancerous colonic epithelial cells. Planta Med. 1999, 65, 527–531. [Google Scholar] [CrossRef]
- Negreanu-Pirjol, T.; Negreanu-Pirjol, B.; Sirbu, R.; Paraschiv, G.; Meghea, A. Comparative studies regarding the antioxidative activity of some therapeutic marine algae species along Romanian Black Sea Coast. J. Environ. Prot. Ecol. 2012, 13, 1744–1750. [Google Scholar]
- Mao, W.; Zang, X.; Li, Y.; Zhang, H. Sulfated polysaccharides from marine green algae Ulva conglobata and their anticoagulant activity. J. Appl. Phycol. 2006, 18, 9–14. [Google Scholar] [CrossRef]
- Qi, H.; Huang, L.; Liu, X.; Liu, D.; Zhang, Q.; Liu, S. Antihyperlipidemic activity of high sulfate content derivative of polysaccharide extracted from Ulva pertusa (Chlorophyta). Carbohyd. Polym. 2012, 87, 1637–1640. [Google Scholar] [CrossRef]
- Qi, H.; Liu, X.; Zhang, J.; Duan, Y.; Wang, X.; Zhang, Q. Synthesis and antihyperlipidemic activity of acetylated derivative of ulvan from Ulva pertusa. Int. J Biol. Macromol. 2012, 50, 270–272. [Google Scholar] [CrossRef]
- Yu, P.; Li, N.; Liu, X.; Zhou, G.; Zhang, Q.; Li, P. Antihyperlipidemic effects of different molecular weight sulfated polysaccharides from Ulva pertusa (Chlorophyta). Pharmacol. Res. 2003, 48, 543–549. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, Q.; Li, N.; Xu, Z.; Wang, Y.; Li, Z. Polysaccharides from Ulva pertusa (Chlorophyta) and preliminary studies on their antihyperlipidemia activity. J. Appl. Phycol. 2003, 15, 21–27. [Google Scholar] [CrossRef]
- Gomez, L.P.; Alvarez, C.; Zhao, M.; Tiwari, U.; Curtin, J.; Garcia-Vaquero, M. Innovative processing strategies and technologies to obtain hydrocolloids from macroalgae for food applications. Carbohydr. Polym. 2020, 248, 116784. [Google Scholar] [CrossRef]
- Qi, H.; Liu, X.; Ma, J.; Zhang, Q.; Li, Z. In vitro antioxidant activity of acetylated derivatives of polysaccharide extracted from Ulva pertusa (Cholorophta). J. Med. Plants Res. 2010, 4, 2445–2451. [Google Scholar] [CrossRef]
- El-Baky, H.H.A.; Baz, F.K.E.; Baroty, G.S.E. Potential biological properties of sulphated polysaccharides extracted from the macroalgae Ulva lactuca L. Acad. J. Cancer Res. 2009, 2, 1–11. [Google Scholar] [CrossRef]
- Qi, H.; Sun, Y. Antioxidant activity of high sulfate content derivative of ulvan in hyperlipidemic rats. Int. J. Biol. Macromol. 2015, 76, 326–329. [Google Scholar] [CrossRef]
- Ahmed, O.M.; Ahmed, R.R. Anti-proliferative and apoptotic efficacies of ulvan polysaccharides against different types of carcinoma cells in vitro and in vivo. J. Cancer Sci. Ther. 2014, 6, 202–208. [Google Scholar] [CrossRef]
- Veeraperumal, S.; Chinnathambi, A.; Palani, P.; Rengasamy, R. Protective effect of Ulvan from Ulva lactuca against experimentally induced fibrosarcoma in wistar rats. Int. J. Curr. Sci. 2012, 4, 50–56. [Google Scholar]
- Berri, M.; Olivier, M.; Holbert, S.; Dupont, J.; Demais, H.; Le Goff, M.; Collen, P.N. Ulvan from Ulva armoricana (Chlorophyta) activates the PI3K/Akt signalling pathway via TLR4 to induce intestinal cytokine production. Algal Res. 2017, 28, 39–47. [Google Scholar] [CrossRef]
- Tran, T.T.V.; Truong, H.B.; Tran, N.H.V. Structure, conformation in aqueous solution and antimicrobial activity of ulvan extracted from green seaweed Ulva reticulata. Nat. Prod. Res. 2018, 32, 2291–2296. [Google Scholar] [CrossRef]
- Kidgell, J.T.; Magnusson, M.; de Nys, R.; Glasson, C.R.K. Ulvan: A systematic review of extraction, composition and function. Algal Res. 2019, 39, 101422. [Google Scholar] [CrossRef]
- Negreanu-Pirjol, T.; Negreanu-Pirjol, B.; Gorun, E.; Nitu, B.; Nastac, M. Marine algae biomass as a useful resource in green fertilization. In Proceedings of the International Multidisciplinary Scientific GeoConferences—SGEM 2017—Nano, Bio and Green—Technologies for a Sustainable Future, Vienna, Austria, 27–29 November 2017; Volume 17, Section Micro and Nano technologies, Advances in Biotechnology, pp. 797–806. [Google Scholar]
- Margret, R.J.; Kumaresan, S.; Ravikumar, S. A preliminary study on the anti-inflammatory activity of methanol extract of Ulva lactuca in rat. J. Environ. Biol. 2009, 30, 899–902. [Google Scholar]
- Patel, S. Therapeutic importance of sulfated polysaccharides from seaweeds: Updating the recent findings. 3 Biotech 2012, 2, 171–185. [Google Scholar] [CrossRef]
- Hallak, L.K.; Spillmann, D.; Collins, P.L.; Peeples, M.E. Glycosaminoglycan sulfation requirements for respiratory syncytial virus infection. J. Virol. 2000, 74, 10508–10513. [Google Scholar] [CrossRef]
- Leiro, J.M.; Castro, R.; Arranz, J.A.; Lamas, J. Immunomodulating activities of acidic sulphated polysaccharides obtained from the seaweed Ulva rigida C. Agardh. Int. Immunopharmacol. 2007, 7, 879–888. [Google Scholar] [CrossRef]
- Rahimi, F.; Tabarsa, M.; Rezaei, M. Ulvan from green algae Ulva intestinalis: Optimization of ultrasound-assisted extraction and antioxidant activity. J. Appl. Phycol. 2016, 28, 2979–2990. [Google Scholar] [CrossRef]
- Guidara, M.; Yaich, H.; Amor, I.B.; Fakhfakh, J.; Gargouri, J.; Lassoued, S.; Blecker, C.; Richel, A.; Attia, H.; Garna, H. Effect of extraction procedures on the chemical structure, antitumor and anticoagulant properties of ulvan from Ulva lactuca of Tunisia coast. Carbohyd. Polym. 2021, 253, 117283. [Google Scholar] [CrossRef]
- Qiao, L.; Li, Y.; Chi, Y.; Ji, Y.; Gao, Y.; Hwang, H.; Wang, P. Rheological properties, gelling behavior and texture characteristics of polysaccharide from Enteromorpha prolifera. Carbohyd. Polym. 2016, 136, 1307–1314. [Google Scholar] [CrossRef]
- Stiger-Pouvreau, V.; Bourgougnon, N.; Deslandes, E. Carbohydrates from seaweeds. In Seaweed in Health and Disease Prevention; Fleurence, J., Levine, I., Eds.; Academic Press, Elsevier: Cambridge, MA, USA, 2016; pp. 223–274. [Google Scholar] [CrossRef]
- Lee, J.C.; Hou, M.F.; Huang, H.W.; Chang, F.R.; Yeh, C.C.; Tang, J.Y.; Chang, H.W. Marine algal natural products with anti-oxidative, anti-inflammatory, and anti-cancer properties. Cancer Cell Int. 2013, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Kraan, S. Algal Polysaccharides, Novel Applications and Outlook. In Biochemistry, Genetics and Molecular Biology. Carbohydrates—Comprehensive Studies on Glycobiology and Glycotechnology; Chang, C.-F., Ed.; InTech: London, UK, 2012; Chapter 22; pp. 489–532. [Google Scholar] [CrossRef]
- Mestechkina, N.M.; Shcherbukhin, V.D. Sulfated polysaccharides and their anticoagulant activity: A review. Appl. Biochem. Microbiol. 2010, 46, 267–273. [Google Scholar] [CrossRef]
- Morelli, A.; Puppi, D.; Chiellini, F. Polymers from renewable resources: Perspectives in biomedical applications. J. Renew. Mater. 2013, 1, 83–112. [Google Scholar] [CrossRef]
- Morelli, A.; Chiellini, F. Ulvan as a new type of biomaterials from renewable resources: Functionalization and hydrogel preparation. Macromol. Chem. Phy. 2010, 211, 821–832. [Google Scholar] [CrossRef]
- Popa, E.G.; Reis, R.L.; Gomes, M.E. Seaweed polysaccharide-based hydrogels used for the regeneration of articular cartilage. Crit. Rev. Biotechnol. 2015, 35, 410–424. [Google Scholar] [CrossRef]
- Shanmugam, M.; Mody, K.H. Heparinoid-active sulphated polysaccharides from marine algae as potential blood anticoagulant agents. Curr. Sci. 2000, 79, 1672–1683. [Google Scholar] [CrossRef]
- Cunha, L.; Grenha, A. Sulfated seaweed polysaccharides as multifunctional materials in drug delivery applications. Mar. Drugs 2016, 14, 42. [Google Scholar] [CrossRef]
- Wichard, T.; Charrier, B.; Mineur, F.; Bothwell, J.H.; De Clerck, O.; Coates, J.C. The green seaweed Ulva: A model system to study morphogenesis. Front. Plant Sci. 2015, 6, 72. [Google Scholar] [CrossRef]
- Lin, J.; Jiao, G.; Kermanshahi-pour, A. Algal Polysaccharides-Based Hydrogels: Extraction, Synthesis, Characterization, and Applications. Mar. Drugs 2022, 20, 306. [Google Scholar] [CrossRef]
- Lakshmi, D.S.; Sankaranarayanan, S.; Gajaria, T.K.; Li, G.; Kujawski, W.; Kujawa, J.; Navia, R. A Short Review on the Valorization of Green Seaweeds and Ulvan: FEEDSTOCK for Chemicals and Biomaterials. Biomolecules 2020, 10, 991. [Google Scholar] [CrossRef]
- Masarin, F.; Paz Cedeno, F.R.; Solorzano Chavez, E.G.; de Oliveira, L.E.; Gelli, V.C.; Monti, R. Chemical analysis and biorefinery of red algae Kappaphycus alvarezii for efficient production of glucose from residue of carrageenan extraction process. Biotechnol. Biofuels 2016, 9, 122. [Google Scholar] [CrossRef]
- Wahlström, N.; Nylander, F.; Malmhäll-Bah, E.; Sjövold, K.; Edlund, U.; Westman, G.; Albers, E. Composition and structure of cell wall ulvans recovered from Ulva spp. along the Swedish west coast. Carbohyd. Polym. 2020, 233, 115852. [Google Scholar] [CrossRef]
- Robic, A.; Gaillard, C.; Sassi, J.F.; Lerat, Y.; Lahaye, M. Ultrastructure of ulvan: A polysaccharide from green seaweeds. Biopolymers 2009, 91, 652–664. [Google Scholar] [CrossRef]
- Robic, A.; Sassi, J.F.; Lahaye, M. Impact of stabilization treatments of the green seaweed Ulva rotundata (Chlorophyta) on the extraction yield, the physico-chemical and rheological properties of ulvan. Carbohyd. Polym. 2008, 74, 344–352. [Google Scholar] [CrossRef]
- Synytsya, A.; Choi, D.J.; Pohl, R.; Na, Y.S.; Capek, P.; Lattova, E.; Taubner, T.; Choi, J.W.; Lee, C.W.; Park, J.K. Structural features and anti-coagulant activity of the sulfated polysaccharide SPS-CF from a green alga Capsosiphon fulvescens. Mar. Biotechnol. 2015, 17, 718–735. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; Yacoub, M.H. Hydrogel scaffolds for tissue engineering: Progress and challenges. Glob. Cardiol. Sci. Pract. 2013, 3, 316–342. [Google Scholar] [CrossRef] [Green Version]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Oh, J.K.; Drumright, R.; Siegwart, D.J.; Matyjaszewski, K. The development of microgels/nanogels for drug delivery applications. Prog. Polym. Sci. 2008, 33, 448–477. [Google Scholar] [CrossRef]
- Hunt, J.A.; Chen, R.; van Veena, T.; Bryan, N. Hydrogels for tissue engineering and regenerative medicine. J. Mater. Chem. B 2014, 2, 5319–5338. [Google Scholar] [CrossRef]
- Nicodemus, G.D.; Bryant, S.J. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng. Part B Rev. 2008, 14, 149–165. [Google Scholar] [CrossRef]
- Bae, K.H.; Wang, L.S.; Kurisawa, M. Injectable biodegradable hydrogels: Progress and challenges. J. Mater. Chem. B 2013, 1, 5371–5388. [Google Scholar] [CrossRef]
- Metters, A.T.; Lin, C.C. Biodegradable hydrogels: Tailoring properties and functions through chemistry and structure. In Biomaterials; Wong, J.Y., Bronzino, J.D., Eds.; CRC Press, Taylor & Francis Group, LLC: Boca Raton, FL, USA, 2007; Chapter 5. [Google Scholar]
- Pitarresi, G.; Palumbo, F.S.; Giammona, G.; Casadei, M.A.; Moracci, F.M. Biodegradable hydrogels obtained by photocrosslinking of dextran and polyaspartamide derivatives. Biomaterials 2003, 24, 4301–4313. [Google Scholar] [CrossRef]
- Sulastri, E.; Zubair, M.S.; Lesmana, R.; Mohammed, A.F.A.; Wathoni, N. Development and Characterization of Ulvan Polysaccharides-Based Hydrogel Films for Potential Wound Dressing Applications. Drug Des. Devel. Ther. 2021, 15, 4213–4226. [Google Scholar] [CrossRef]
- Dash, M.; Samal, S.K.; Bartoli, C.; Morelli, A.; Smet, P.F.; Dubruel, P.; Chiellini, F. Biofunctionalization of Ulvan scaffolds for bone tissue engineering. ACS Appl. Mater. Interfaces 2014, 6, 3211–3218. [Google Scholar] [CrossRef]
- Toskas, G.; Heinemann, S.; Heinemann, C.; Cherif, C.; Hund, R.D.; Roussis, V.; Hanke, T. Ulvan and ulvan/chitosan polyelectrolyte nanofibrous membranes as a potential substrate material for the cultivation of osteoblasts. Carbohyd. Polym. 2012, 89, 997–1002. [Google Scholar] [CrossRef]
- Morelli, A.; Betti, M.; Puppi, D.; Chiellini, F. Design, preparation and characterization of ulvan based thermosensitive hydrogels. Carbohyd. Polym. 2016, 136, 1108–1117. [Google Scholar] [CrossRef]
- Morelli, A.; Betti, M.; Puppi, D.; Bartoli, C.; Gazzarri, M.; Chiellini, F. Enzymatically crosslinked ulvan hydrogels as injectable systems for cell delivery. Macromol. Chem. Phys. 2016, 217, 581–590. [Google Scholar] [CrossRef]
- Overstreet, D.J.; Dutta, D.; Stabenfeldt, S.E.; Vernon, B.L. Injectable hydrogels. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 881–903. [Google Scholar] [CrossRef]
- Wang, D.A.; Varghese, S.; Sharma, B.; Strehin, I.; Fermanian, S.; Gorham, J.; Fairbrother, D.H.; Cascio, B.; Elisseeff, J.H. Multifunctional chondroitin sulphate for cartilage tissue-biomaterial integration. Nat. Mater. 2007, 6, 385–392. [Google Scholar] [CrossRef]
- Klouda, L.; Mikos, A.G. Thermoresponsive hydrogels in biomedical applications. Eur. J. Pharm. Biopharm. 2008, 68, 34–45. [Google Scholar] [CrossRef]
- Alves, A.; Pinho, E.D.; Neves, N.M.; Sousa, R.A.; Reis, R.L. Processing ulvan into 2D structures: Cross-linked ulvan membranes as new biomaterials for drug delivery applications. Int. J. Pharm. 2012, 426, 76–81. [Google Scholar] [CrossRef]
- Toskas, G.; Hund, R.D.; Laourine, E.; Cherif, C.; Smyrniotopoulos, V.; Roussis, V. Nanofibers based on polysaccharides from the green seaweed Ulva Rigida. Carbohyd. Polym. 2011, 84, 1093–1102. [Google Scholar] [CrossRef]
- Gadenne, V.; Lebrun, L.; Jouenne, T.; Thebault, P. Antiadhesive activity of ulvan polysaccharides covalently immobilized onto titanium surface. Colloids Surf. B 2013, 112, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Gadenne, V.; Lebrun, L.; Jouenne, T.; Thebault, P. Role of molecular properties of ulvans on their ability to elaborate antiadhesive surfaces. J. Biomed. Mater. Res. A 2015, 103, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Bigot, S.; Louarn, G.; Kebir, N.; Burel, F. Click grafting of seaweed polysaccharides onto PVC surfaces using an ionic liquid as solvent and catalyst. Carbohyd. Polym. 2013, 98, 1644–1649. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Duarte, A.R.C.; Mano, J.F.; Sousa, R.A.; Reis, R.L. PDLLA enriched with ulvan particles as a novel 3D porous scaffold targeted for bone engineering. J. Supercrit. Fluids 2012, 65, 32–38. [Google Scholar] [CrossRef]
- Damodaran, V.B.; Murthy, N.S. Bio-inspired strategies for designing antifouling biomaterials. Biomater. Res. 2016, 20, 18. [Google Scholar] [CrossRef] [PubMed]
- Ghisalberti, C. Enhanced Treatment of Joint and Connective Tissue Damage. PCT Patent WO 2010/109256, 30 September 2010. [Google Scholar]
- Larraz, E.; Elvira, C.; Fernandez, M.; Parra, J.; Collia, F.; Lopez-Bravo, A.; Roman, J.S. Self-curing acrylic formulations with applications in intervertebral disk restoration: Drug release and biological behaviour. J. Tissue Eng. Regen. Med. 2007, 1, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Andrès, E.; Molinari, J.; Péterszegi, G.; Mariko, B.; Ruszova, E.; Velebny, V.; Faury, G.; Robert, L. Pharmacological properties of rhamnose-rich polysaccharides, potential interest in age dependent alterations of connectives tissues. Pathol. Biol. 2006, 54, 420–425. [Google Scholar] [CrossRef]
- Faury, G.; Molinari, J.; Rusova, E.; Mariko, B.; Raveaud, S.; Huber, P.; Velebny, V.; Robert, A.M.; Robert, L. Receptors and aging: Structural selectivity of the rhamnose-receptor on fibroblasts as shown by Ca2+—Mobilization and gene-expression profiles. Arch. Gerontol. Geriat. 2011, 53, 106–112. [Google Scholar] [CrossRef]
- Massarelli, I.; Murgia, L.; Bianucci, A.M.; Chiellini, F.; Chiellini, E. Understanding the selectivity mechanism of the human asialoglycoprotein receptor (ASGP-R) toward gal- and man-type ligands for predicting interactions with exogenous sugars. Int. J. Mol. Sci. 2007, 8, 13–28. [Google Scholar] [CrossRef]
- Khotimchenko, Y.S.; Khozhaenko, E.V.; Khotimchenko, M.Y.; Kolenchenko, E.A.; Kovalev, V.V. Carrageenans as a new source of drugs with metal binding properties. Mar. Drugs 2010, 8, 1106–1121. [Google Scholar] [CrossRef]





| Green Algae | Red Algae | Brown Algae |
|---|---|---|
| Bryopsis hypnoides | Apoglossum ruscifolium * | Cladostephus spongiosus * |
| Bryopsis plumosa | Callithamnion corymbosum | Corynophlaea umbellata * |
| Chaetomorpha aerea | Callithamnion granulatum | Cystoseira barbata |
| Chaetomorpha linum | Ceramium arborescens | Cystoseira crinita * |
| Cladophora albida | Ceramium ciliatum | Dictyota dichotoma * |
| Cladophora dalmatica | Ceramium deslongchampsii | Dictyota fasciola * |
| Cladophora laetevirens | Ceramium diaphanum | Dictyota spiralis * |
| Cladophora liniformis | Ceramium virgatum | Ectocarpus siliculosus |
| Cladophora sericea | Chondria capillaris * | Nereia filiformis * |
| Cladophora vagabunda | Chondria dasyphylla * | Padina pavonica |
| Cladophoropsis membranacea * | Coccotylus truncatus * | Scytosiphon lomentaria |
| Codium vermilara * | Corallina elongata * | Spermatochnus paradoxus * |
| Ulva clathrata | Corallina officinalis | Sphacelaria cirrosa * |
| Ulva flexuosa | Dasya baillouviana * | Stilophora tenella * |
| Ulva (Enteromorpha) intestinalis | Dasya hutchinsiae | Zanardinia typus * |
| Ulva linza | Gelidium crinale * | |
| Ulva prolifera | Gelidium spinosum * | |
| Ulva rigida syn. Ulva lactuca | Gracilaria dura * | |
| Gracilaria gracilis | ||
| Grateloupia dichotoma * | ||
| Haliptilon virgatum * | ||
| Jania rubens | ||
| Laurencia coronopus * | ||
| Laurencia obtusa * | ||
| Lomentaria clavellosa | ||
| Nemalion helminthoides * | ||
| Nitophyllum punctatum * | ||
| Osmundea pinnatifida | ||
| Palisada perforata | ||
| Peyssonnelia dubyi | ||
| Phyllophora crispa | ||
| Phymatolithon lenormandii | ||
| Polysiphonia elongata | ||
| Polysiphonia fucoides | ||
| Polysiphonia subulifera | ||
| Porphyra leucosticta |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negreanu-Pirjol, B.-S.; Negreanu-Pirjol, T.; Popoviciu, D.R.; Anton, R.-E.; Prelipcean, A.-M. Marine Bioactive Compounds Derived from Macroalgae as New Potential Players in Drug Delivery Systems: A Review. Pharmaceutics 2022, 14, 1781. https://doi.org/10.3390/pharmaceutics14091781
Negreanu-Pirjol B-S, Negreanu-Pirjol T, Popoviciu DR, Anton R-E, Prelipcean A-M. Marine Bioactive Compounds Derived from Macroalgae as New Potential Players in Drug Delivery Systems: A Review. Pharmaceutics. 2022; 14(9):1781. https://doi.org/10.3390/pharmaceutics14091781
Chicago/Turabian StyleNegreanu-Pirjol, Bogdan-Stefan, Ticuta Negreanu-Pirjol, Dan Razvan Popoviciu, Ruxandra-Elena Anton, and Ana-Maria Prelipcean. 2022. "Marine Bioactive Compounds Derived from Macroalgae as New Potential Players in Drug Delivery Systems: A Review" Pharmaceutics 14, no. 9: 1781. https://doi.org/10.3390/pharmaceutics14091781







