Are There Differences in the Homogeneity of the Parts of Tablets Obtained after Subdivision?—A Preliminary Assessment Using an X-ray Microtomography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tablets Selected for Analysis
2.2. Evaluation of the Whole Tablets
2.3. Evaluation of Parts of the Tablets
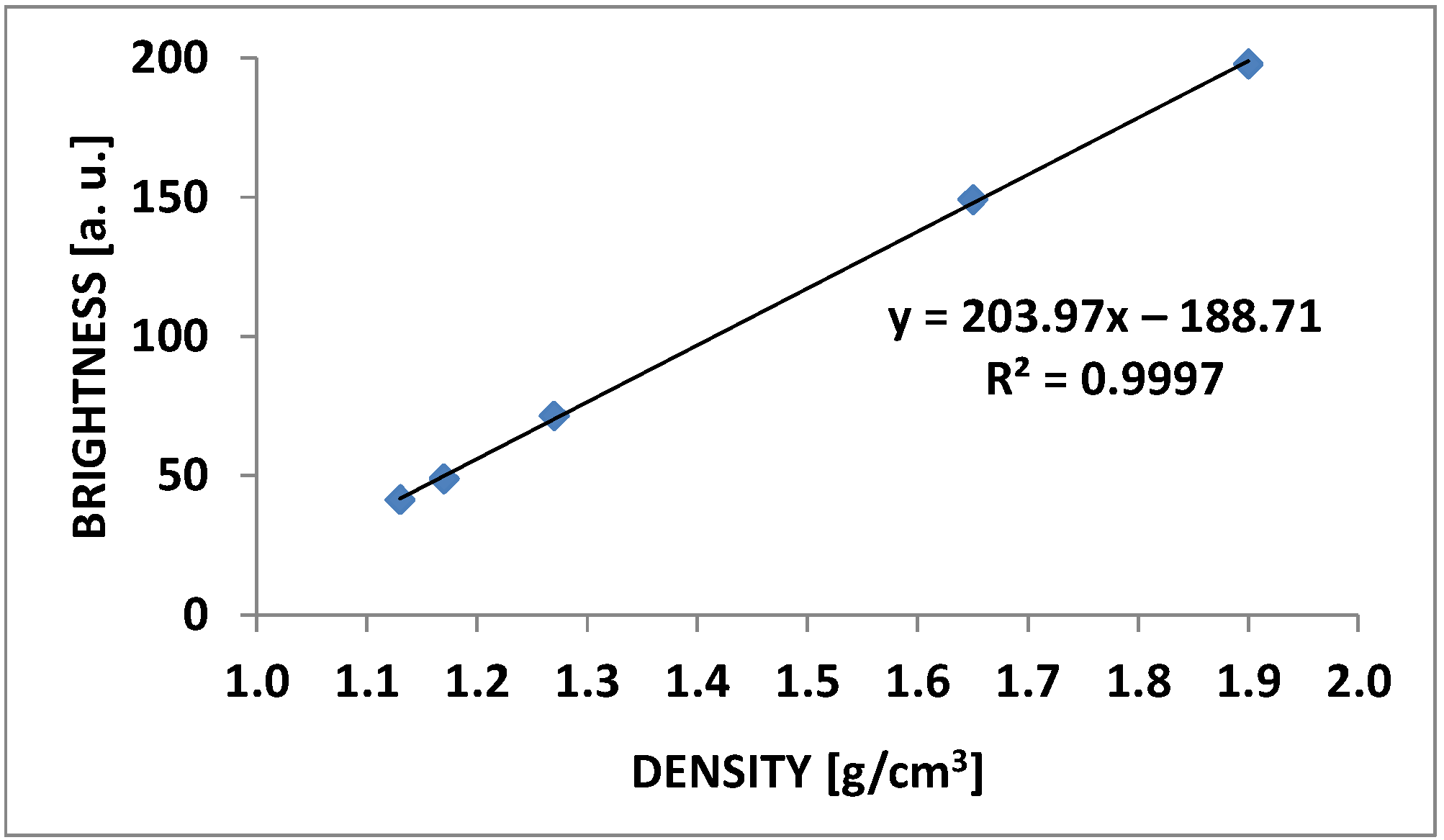
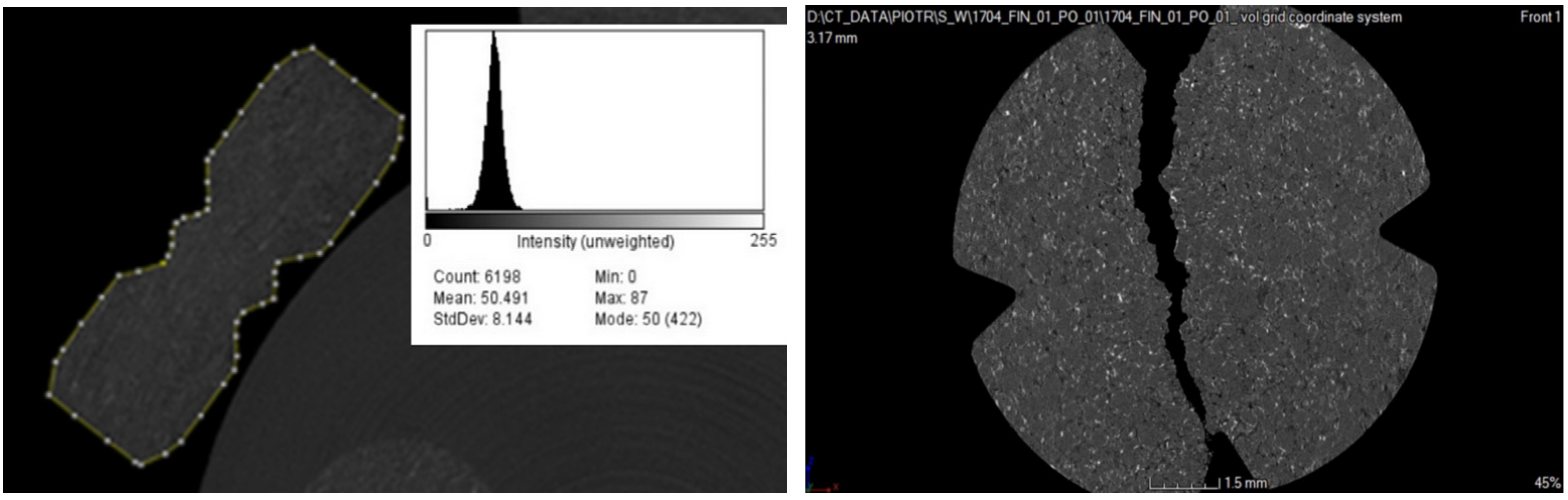
2.4. Microtomographic Analysis
2.5. Statistical Analyses
3. Results
3.1. General Characteristics of the Analyzed Tablets
3.2. Weight Analysis before and after Subdivision
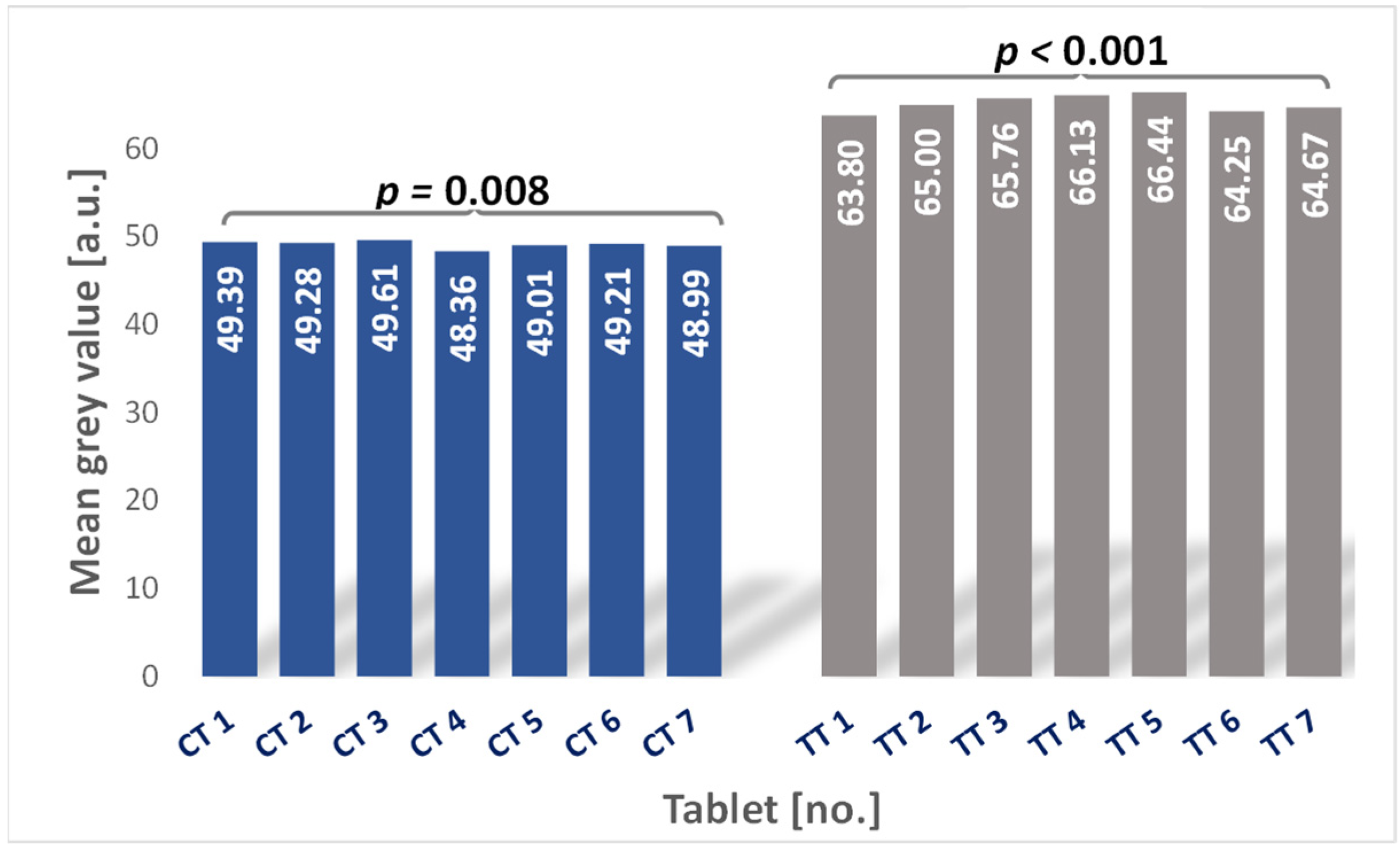
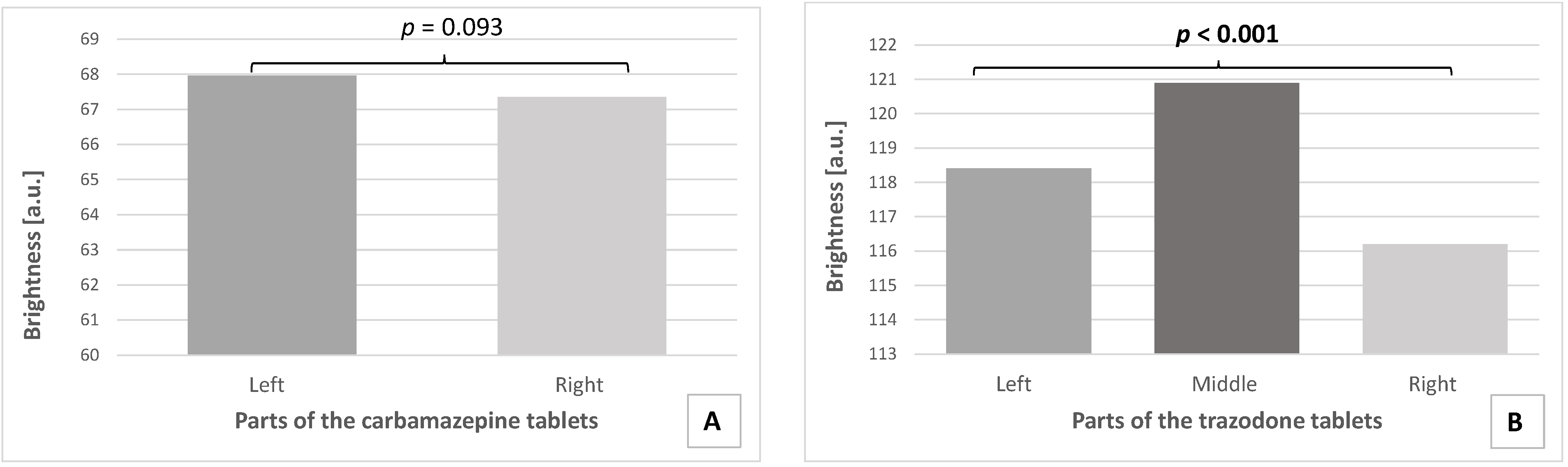
3.3. Microtomographic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jacques, E.R.; Alexandridis, P. Tablet Scoring: Current Practice, Fundamentals, and Knowledge Gaps. Appl. Sci. 2019, 9, 3066. [Google Scholar] [CrossRef]
- Bubić-Pajić, N.; Račić, A.; Gatarić, B. Whole vs. Half-tablets: A case of Diazepam. Scr. Med. 2019, 50, 153–160. [Google Scholar] [CrossRef]
- Ashrafpour, R.; Ayati, N.; Sadeghi, R.; Zare Namdar, S.; Ayati, N.; Ghahremani, S.; Zakavi, S.R. Comparison of Treatment Response Achieved by Tablet Splitting Versus Whole Tablet Administration of Levothyroxine in Patients with Thyroid Cancer. Asia Ocean. J. Nucl. Med. Biol. 2018, 6, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas Starling, F.; Medeiros-Souza, P.; Francisco De Camargos, E.; Ferreira, F.; Rodrigues Silva, A.; Homem-De-Mello, M. Tablet Splitting of Psychotropic Drugs for Patients with Dementia: A Pharmacoepidemiologic Study in a Brazilian Sample. Clin. Ther. 2015, 37, 2332–2338. [Google Scholar] [CrossRef] [PubMed]
- Gracia-Vásquez, S.L.; González-Barranco, P.; Camacho-Mora, I.A.; González-Santiago, O.; Vázquez-Rodríguez, S.A. Medications that should not be crushed. Med. Univ. 2017, 19, 50–63. [Google Scholar] [CrossRef]
- Arnet, I.; Moos, M.; von Hersberger, K.E. Wrongly Prescribed Half Tablets in a Swiss University Hospital. Int. J. Clin. Med. 2012, 03, 637–643. [Google Scholar] [CrossRef]
- Auricchio, M.T.; Yano, H.M.; Santos, A.P.; Bugno, A. Evaluation of the content of atenolol tablets divided with a knife and homemade machine cutter. ACTA Paul. Enferm. 2011, 24, 74–79. [Google Scholar] [CrossRef]
- Habib, W.A.; Alanizi, A.S.; Abdelhamid, M.M.; Alanizi, F.K. Accuracy of tablet splitting: Comparison study between hand splitting and tablet cutter. Saudi Pharm. J. 2014, 22, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Zaid, A.N.; Ghoush, A.A.; Al-Ramahi, R.; Are’r, M. Evaluation of the discrepancy between the European pharmacopoeia test and an adopted United States pharmacopoeia test regarding the weight uniformity of scored tablet halves: Is harmonization required? PDA J. Pharm. Sci. Technol. 2012, 66, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Green, G.; Berg, C.; Polli, J.E.; Dirk, M. Pharmacopeial Standards for the Subdivision Characteristics of Scored Tablets Pharmacopeial Standards for the Subdivision Characteristics of Scored Tablets. Pharmacop. Forum 2015, 35, 1598–1612. [Google Scholar] [CrossRef]
- FDA (Food and Drug Administration). Guidance for Industry-Tablet Scoring: Nomenclature, Labeling, and Data for Evaluation. Center for Drug Evaluation and Research. 2013; docket number: FDA-2011-D-0595. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/tablet-scoringnomenclature-labeling-and-data-evaluation (accessed on 25 August 2022).
- Meisner, M.; Kuśnierz, P.; Sarecka-Hujar, B.; Wilczyński, S. Accuracy and safety of tablet subdivision. Med. Ogólna Nauk. Zdrowiu 2020, 26, 354–359. [Google Scholar] [CrossRef]
- Schomberg, A.K.; Diener, A.; Wünsch, I.; Finke, J.H.; Kwade, A. The use of X-ray microtomography to investigate the microstructure of pharmaceutical tablets: Potentials and comparison to common physical methods. Int. J. Pharm. X 2021, 3, 100090. [Google Scholar] [CrossRef]
- European Directorate for the Quality of Medicine. Tablets Monograph 0478. In European Pharmacopoeia, 6th ed.; Suppl. 6.4. Council of Europe; European Directorate for the Quality of Medicine: Strasbourg, France, 2008. [Google Scholar]
- Starling, F.M.; Camargos, E.F.; Ferreira, F.; Freitas, M.P.D.; Costa, J.R.S.; Medeiros-Souza, P. Unavailability of appropriate doses and need for tablet splitting of psychotropic drugs by geriatric patients. Geriatr. Gerontol. Aging 2019, 13, 126–132. [Google Scholar] [CrossRef]
- Teixeira, M.T.; Sa-Barreto, L.L.; Taveira, S.F.; Gratieri, T.; Gelfuso, G.M.; Marreto, R.N.; Silva, I.C.; Cunha-Filho, M. The Influence of Matrix Technology on the Subdivision of Sustained Release Matrix Tablets. AAPS PharmSciTech 2019, 21, 8. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.T.; Sá-Barreto, L.C.L.; Gratieri, T.; Gelfuso, G.M.; Silva, I.C.R.; Cunha-Filho, M.S.S. Key Technical Aspects Influencing the Accuracy of Tablet Subdivision. AAPS PharmSciTech 2017, 18, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Zidan, A.; Tawakkul, M.; Sayeed, V.A.; Khan, M. Tablet splitting: Product quality assessment of metoprolol succinate extended release tablets. Int. J. Pharm. 2010, 401, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Wilczynski, S.; Koprowski, R.; Duda, P.; Banys, A.; Blonska-Fajfrowska, B. Microtomographic studies of subdivision of modified-release tablets. Int. J. Pharm. 2016, 511, 899–912. [Google Scholar] [CrossRef] [PubMed]
- Wray, P.; Chan, K.L.; Kimber, J.; Kazarian, S.G. Compaction of pharmaceutical tablets with different polymer matrices studied by FTIR imaging and X-ray microtomography. J. Pharm. Sci. 2008, 97, 4269–4277. [Google Scholar] [CrossRef] [PubMed]
- Seong, S.; Shin, J.; Kim, D.; Song, I.; Sun, S.; Kim, I.; Park, S.; Ha, D. The effect of tablet splitting on the mass loss, uniformity, and stability: By hand or splitter? J. Asian Assoc. Sch. Pharm. 2019, 8, 7–14. [Google Scholar]
- Gharaibeh, S.F.; Tahaineh, L. Effect of different splitting techniques on the characteristics of divided tablets of five commonly split drug products in Jordan. Pharm. Pract. 2020, 18, 1776. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Preparation | Active Pharmaceutical Substance; Dose | Manufacturer’s Recommendation on Subdivision | Score Line | Number of Parts Obtained after Subdivision * |
|---|---|---|---|---|
| Trittico CR | trazodone hydrochloride; 75 mg | yes | yes | 3 |
| Finlepsin 200 retard | carbamazepine; 200 mg | yes | yes | 2 |
| Preparation | Shape | Length of the Tablets [mm] M ± SD | Width of the Tablets [mm] M ± SD | The Thickness of the Tablets [mm] M ± SD | Diameter of the Tablets [mm] M ± SD |
|---|---|---|---|---|---|
| Trazodone hydrochloride tablets | oblong | 12.05 ± 0.01 | 4.05 ± 0.04 | 3.03 ± 0.04 | - |
| Carbamazepine tablets | round | - | - | 3.47 ± 0.01 | 10.10 ± 0.01 |
| Analyzed Tablets | Uniformity Test for Subdivided Tablet Acc. To Ph. Eur. | Uniformity Test for Subdivided Tablet Acc. To USP | ||||||
|---|---|---|---|---|---|---|---|---|
| Outside the Range of 85–115% of the Average Mass N | Outside the Range of 75–125% of the Average Mass N | Result of the Test | Number of Tablets | Outside the Range of 85–115% of the Average Mass N | Outside the Range of 75–125% of the Average Mass N | RSD [%] | Result of the Test | |
| Carbamazepine tablets | 0 | 0 | Pass | 10 | 0 | 0 | 5.75 | Pass |
| Trazodone hydrochloride tablets | 1 | 0 | Pass | 10 | 8 | 1 | 13.95 | Fail |
| Before Subdivision | After Subdivision | |||||
|---|---|---|---|---|---|---|
| Whole Tablets N = 30 | Left Parts of the Tablets N = 30 | Middle Parts of the Tablets N = 30 | Right Parts of the Tablets N = 30 | Mass of the Theoretical Parts of the Tablets | p | |
| Trazodone hydrochloride tablets, M ± SD | 0.142 ± 0.001 | 0.043 ± 0.002 | 0.054 ± 0.003 | 0.044 ± 0.002 | 0.047 ± 0.0005 | <0.001 * |
| Carbamazepine tablets, M ± SD | 0.301 ± 0.002 | 0.152 ± 0.008 | - | 0.146 ± 0.007 | 0.150 ± 0.001 | 0.015 ** |
| Mass Loss [g] | Mass Loss [%] | |
|---|---|---|
| Trazodone hydrochloride tablets, M ± SD | 0.0008 ± 0.0005 | 0.544 ± 0.354 |
| Carbamazepine tablets, M ± SD | 0.003 ± 0.003 | 0.932 ± 0.941 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meisner, M.; Kuśnierz, P.; Duda, P.; Wilczyński, S.; Sarecka-Hujar, B. Are There Differences in the Homogeneity of the Parts of Tablets Obtained after Subdivision?—A Preliminary Assessment Using an X-ray Microtomography. Pharmaceutics 2022, 14, 1850. https://doi.org/10.3390/pharmaceutics14091850
Meisner M, Kuśnierz P, Duda P, Wilczyński S, Sarecka-Hujar B. Are There Differences in the Homogeneity of the Parts of Tablets Obtained after Subdivision?—A Preliminary Assessment Using an X-ray Microtomography. Pharmaceutics. 2022; 14(9):1850. https://doi.org/10.3390/pharmaceutics14091850
Chicago/Turabian StyleMeisner, Michał, Piotr Kuśnierz, Piotr Duda, Sławomir Wilczyński, and Beata Sarecka-Hujar. 2022. "Are There Differences in the Homogeneity of the Parts of Tablets Obtained after Subdivision?—A Preliminary Assessment Using an X-ray Microtomography" Pharmaceutics 14, no. 9: 1850. https://doi.org/10.3390/pharmaceutics14091850
APA StyleMeisner, M., Kuśnierz, P., Duda, P., Wilczyński, S., & Sarecka-Hujar, B. (2022). Are There Differences in the Homogeneity of the Parts of Tablets Obtained after Subdivision?—A Preliminary Assessment Using an X-ray Microtomography. Pharmaceutics, 14(9), 1850. https://doi.org/10.3390/pharmaceutics14091850









