Plasmid DNA for Therapeutic Applications in Cancer
Abstract
:1. Introduction
2. Plasmid Design for Cancer Therapy
3. Tumor-Specific Promoters for Gene Therapy
4. Tumor-Specific Antigens for DNA Vaccination
5. DNA Vaccines Encoding Fusion Proteins
5.1. Antigen Fusion to Organelle-Targeting Sequences
5.2. Antigen Fusion to Heat Shock Protein (HSP) 70
5.3. Antigen Fusion to Cytokines
5.4. Antigen Fusion to Other Immune-Stimulating Sequences
6. DNA Cancer Vaccines in Combination with ICB Therapies
7. Antibody Production by DNA Immunization
8. Delivery Methods for Plasmids in Cancer Therapeutics
8.1. Naked DNA Injection
8.2. Electroporation
8.3. Biolistic
8.4. DNA–Liposome Complexes and Lipid Nanoparticles
8.5. Other Nanoparticle Systems
9. Clinical Trials Using DNA Vaccines
| Phase | Type of Cancer | Site of Administration and Delivery Method | Description of Intervention and Key Results | Trial/ Status/ Reference |
|---|---|---|---|---|
| I | Stage III–IV or Recurrent Ovarian Cancer | Intradermal injection | Intervention: pUMVC3-hIGFBP-polyepitope DNA vaccine encoding Insulin-Like Growth Factor Binding Protein-2 (IGFBP-2) mixed with rhuGM-CSF monthly for three months. Key results: Stimulates the production of type 1 T lymphocytes without evidence of regulatory responses | NCT01322802/ Completed/ [193] |
| II | Non-metastasic castration-sensitive prostate cancer (CSPC) | Intradermal injection | Intervention: pTVG-HP DNA vaccine encoding PAP with rhGM-CSF. Key results: No overall increase in 2-year metastasis-free survival (MFS). | NCT01341652/ Completed/ [194] |
| II | Metastatic castration-resistant prostate cancer (CRPC) | Intradermal injection | Intervention: sipuleucel-T with or without pTVG-HP DNA vaccine encoding PAP Key results: The combination of sipuleucel-T with pTVG-HP can increase the diversity of the cellular and humoral immune response. | NCT01706458/ Completed/ [195] |
| II | Metastasic CRPC | Intradermal injection | Intervention: pTVG-HP is a plasmid encoding PAP, with Pembrolizumab, a (PD-1)-blocking antibody No study results are available | NCT04090528/ Recruiting/ [196] |
| I | Head and Neck Cancer | Intramuscular injection and electroporation | Intervention: pNGVL-4a-CRT/E7 (detox) DNA vaccine encoding calreticulin and HPV-16 E7 antigen with cyclophosphamide No study results are available | NCT01493154/ Terminated/ [197] |
| I | Nine types of cancer | Intramuscular injection and electroporation | Intervention: INO-1400 or INO-1401 Plasmid encoding hTERT variants, with or with-out plasmid encoding IL-12 Key results: Survival of patients with pancreatic cancer, tolerance, enhanced CD8+ response | NCT02960594/ Completed/ [164] |
| I | Prostate cancer | Intramuscular injection and electroporation | Intervention: INO-5150 encoding PSA and PSMA with and without INO-9012 encoding IL-12 Key results: Dampening percentage rise in PSA and increased PSA Doubling Time (PSADT) in patients. | NCT02514213/ Completed/ [198] |
| IB | Breast Cancer | Injection and electroporation | Intervention: Mammaglobin-A DNA vaccine No study results are available | NCT02204098/ Recruiting/ [199] |
| I, II | Cervical intraepithelilal neoplasia (CIN) 2/3 | Intramuscular injection | Intervention: VB10.16 vaccine (HPV-16 E7/E6 protein linked to human chemokine MIP-1α) Key results: Tolerance and promising immunogenicity results dependent on specific T lymphocytes | NCT02529930/ Completed/ [200] |
| I, IIA | Cervical Cancer | Intramuscular injection and electroporation | Intervention: INO-3112 DNA vaccine (VGX-3100 encoding for modified HPV-16 and HPV-18, E6 and E7 antigens, and INO-9012 encoding IL-12) No study results are available | NCT02172911/ Completed/ [201] |
| I, IIA | Head and Neck Cancer | Intramuscular injection and electroporation | Intervention: MEDI0457 (DNA immunotherapy targeting HPV16/18 E6/E7 with IL-12 encoding plasmids) in combination with Durvalumab for PD-1/PD-L1 blockade Key results: Durable antigen-specific peripheral and tumor immune responses. | NCT03162224/ Completed/ [202] |
| II | CIN 3 | Intramuscular injection and electroporation | Intervention: GX-188E is a DNA vaccine encoding HPV-16 and HPV-18 E6/E7 fusion proteins Key results: Effective therapeutic vaccine with histopathologic regression and significantly higher fold changes in their IFNγ | NCT02139267/ Completed/ [203] |
| II | Cervical cancer | Intramuscular injection and electroporation | Intervention: GX-188E DNA vaccine plus Pembrolizumab PD-1-blocking antibody Key results: This combination therapy showed preliminary antitumor activity | NCT03444376/ Active, not recruiting/ [204] |
| II | Cervical Cancer | Intramuscular injection | Intervention: VB10.16 vaccine (HPV16 E7/E6 protein linked to human chemokine MIP-1α) in combination with Atezolizumab PD-L1-blocking antibody Key results: No study results are available | NCT04405349/ Active, not recruiting/ [205] |
| II | Merkel Cell Carcinoma | Intratumural injection and electroporation | Intervention: DNA vaccine encoding IL-12 Key results: The vaccine is secure, and produces a systemic immune response, increased peripheral and intratumoral specific T cells | NCT01440816/ Completed/ [190] |
| II | Melanoma | Intratumural injection and electroporation | Intervention: DNA vaccine encoding IL-12 Key results: Circulating PD-1+ CD4+ and CD8+ T cells declined with treatment; specific immune responses to gp100 were also detected and were correlated with an increase in CD8+, CD3+ T cells within the tumor. | NCT01502293/ Completed/ [167] |
10. RNA Vaccines
11. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 2 May 2022).
- Wu, P.; Gao, W.; Su, M.; Nice, E.C.; Zhang, W.; Lin, J.; Xie, N. Adaptive Mechanisms of Tumor Therapy Resistance Driven by Tumor Microenvironment. Front. Cell Dev. Biol. 2021, 9, 641469. [Google Scholar] [CrossRef] [PubMed]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New Approaches and Procedures for Cancer Treatment: Current Perspectives. SAGE Open Med. 2021, 9, 4370. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Chen, X. Drug Resistance and Combating Drug Resistance in Cancer. Cancer Drug Resist. 2019, 2, 141–160. [Google Scholar] [CrossRef] [PubMed]
- Friedmann, T. A Brief History of Gene Therapy. Nat. Genet. 1992, 2, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Van der Bruggen, P.; Traversari, C.; Chomez, P.; Lurquin, C.; De Plaen, E.; Van den Eynde, B.; Knuth, A.; Boon, T. A Gene Encoding an Antigen Recognized by Cytolytic T Lymphocytes on a Human Melanoma. Science 1991, 254, 1643–1647. [Google Scholar] [CrossRef]
- Almeida, A.M.; Eusébio, D.; Queiroz, J.A.; Sousa, F.; Sousa, Â. Minicircle DNA Vaccine Purification and E7 Antigen Expression Assessment. In DNA Vaccines; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2021; Volume 2197, pp. 207–222. [Google Scholar] [CrossRef]
- Kobelt, D.; Aumann, J.; Schmidt, M.; Wittig, B.; Fichtner, I.; Behrens, D.; Lemm, M.; Freundt, G.; Schlag, P.M.; Walther, W. Preclinical Study on Combined Chemo- and Nonviral Gene Therapy for Sensitization of Melanoma Using a Human TNF-Alpha Expressing MIDGE DNA Vector. Mol. Oncol. 2014, 8, 609–619. [Google Scholar] [CrossRef]
- Short, C.; Savelyeva, N. Doggybones, DNA Vaccines and Skin-Penetrating Fluids: Whatever It Takes to Win the Fight against Cancer. Biochemist 2021, 43, 22–25. [Google Scholar] [CrossRef]
- Conforti, A.; Salvatori, E.; Lione, L.; Compagnone, M.; Pinto, E.; Shorrock, C.; Hayward, J.A.; Sun, Y.; Liang, B.M.; Palombo, F.; et al. Linear DNA Amplicons as a Novel Cancer Vaccine Strategy. J. Exp. Clin. Cancer Res. 2022, 41, 195. [Google Scholar] [CrossRef]
- Prazeres, D.M.F.; Monteiro, G.A. Plasmid Biopharmaceuticals. Microbiol. Spectr. 2014, 2, 2.6.02. [Google Scholar] [CrossRef] [Green Version]
- Klein, J.S.; Jiang, S.; Galimidi, R.P.; Keeffe, J.R.; Bjorkman, P.J. Design and Characterization of Structured Protein Linkers with Differing Flexibilities. Protein Eng. Des. Sel. 2014, 27, 325–330. [Google Scholar] [CrossRef]
- Chen, X.; Zaro, J.; Shen, W.-C. Fusion Protein Linkers: Property, Design and Functionality. Adv. Drug Deliv. Rev. 2013, 65, 1357–1369. [Google Scholar] [CrossRef]
- Renaud-Gabardos, E.; Hantelys, F.; Morfoisse, F.; Chaufour, X.; Garmy-Susini, B.; Prats, A.-C. Internal Ribosome Entry Site-Based Vectors for Combined Gene Therapy. World J. Exp. Med. 2015, 5, 11–20. [Google Scholar] [CrossRef]
- Kutzler, M.A.; Weiner, D.B. DNA Vaccines: Ready for Prime Time? Nat. Rev. Genet. 2008, 9, 776–788. [Google Scholar] [CrossRef]
- Kudla, G.; Lipinski, L.; Caffin, F.; Helwak, A.; Zylicz, M. High Guanine and Cytosine Content Increases MRNA Levels in Mammalian Cells. PLoS Biol. 2006, 4, e180. [Google Scholar] [CrossRef]
- Mauro, V.P.; Chappell, S.A. A Critical Analysis of Codon Optimization in Human Therapeutics. Trends Mol. Med. 2014, 20, 604–613. [Google Scholar] [CrossRef]
- Coban, C.; Koyama, S.; Takeshita, F.; Akira, S.; Ishii, K.J. Molecular and Cellular Mechanisms of DNA Vaccines. Hum. Vaccin. 2008, 4, 453–456. [Google Scholar] [CrossRef]
- Hanagata, N. Structure-Dependent Immunostimulatory Effect of CpG Oligodeoxynucleotides and Their Delivery System. Int. J. Nanomed. 2012, 7, 2181–2195. [Google Scholar] [CrossRef]
- Sato, Y.; Roman, M.; Tighe, H.; Lee, D.; Corr, M.; Nguyen, M.D.; Silverman, G.J.; Lotz, M.; Carson, D.A.; Raz, E. Immunostimulatory DNA Sequences Necessary for Effective Intradermal Gene Immunization. Science 1996, 273, 352–354. [Google Scholar] [CrossRef]
- Gómez-Navarro, J.; Arafat, W.; Xiang, J. Gene Therapy for Carcinoma of the Breast: Pro-Apoptotic Gene Therapy. Breast Cancer Res. 2000, 2, 32–44. [Google Scholar] [CrossRef] [Green Version]
- Zarogoulidis, P.; Darwiche, K.; Sakkas, A.; Yarmus, L.; Huang, H.; Li, Q.; Freitag, L.; Zarogoulidis, K.; Malecki, M. Suicide Gene Therapy for Cancer—Current Strategies. J. Genet. Syndr. Gene Ther. 2013, 4, 16849. [Google Scholar] [CrossRef]
- Ardiani, A.; Johnson, A.J.; Ruan, H.; Sanchez-Bonilla, M.; Serve, K.; Black, M.E. Enzymes to Die For: Exploiting Nucleotide Metabolizing Enzymes for Cancer Gene Therapy. Curr. Gene Ther. 2012, 12, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Marqus, S.; Pirogova, E.; Piva, T.J. Evaluation of the Use of Therapeutic Peptides for Cancer Treatment. J. Biomed. Sci. 2017, 24, 21. [Google Scholar] [CrossRef] [PubMed]
- Pahle, J.; Walther, W. Bacterial Toxins for Oncoleaking Suicidal Cancer Gene Therapy. In Current Strategies in Cancer Gene Therapy; Recent Results in Cancer Research; Springer: Cham, Switzerland, 2016; Volume 209, pp. 95–110. [Google Scholar] [CrossRef]
- Ohana, P.; Matouk, I.; Amit, D.; Gilon, M.; Hochberg, A. Chapter 8—Toxin-Based Cancer Gene Therapy: Under the Control of Oncofetal H19 Regulatory Sequences. In Gene Therapy of Cancer, 3rd ed.; Lattime, E.C., Gerson, S.L., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 107–122. ISBN 978-0-12-394295-1. [Google Scholar]
- Gawronski, M.; Kopinski, P.; Jankowski, M.; Goede, A.; Szpechcinski, A.; Chorostowska, J. Inhibition of the Effect of Epidermal Growth Factor (EGF) on Lung Cancer Cells. The Use of Plasmids Encoding Specific SiRNA Molecules. Eur. Respir. J. 2015, 46, PA538. [Google Scholar] [CrossRef]
- Wang, S.-L.; Yao, H.-H.; Qin, Z.-H. Strategies for Short Hairpin RNA Delivery in Cancer Gene Therapy. Expert Opin. Biol. Ther. 2009, 9, 1357–1368. [Google Scholar] [CrossRef]
- Montaño-Samaniego, M.; Bravo-Estupiñan, D.M.; Méndez-Guerrero, O.; Alarcón-Hernández, E.; Ibáñez-Hernández, M. Strategies for Targeting Gene Therapy in Cancer Cells with Tumor-Specific Promoters. Front. Oncol. 2020, 10, 605380. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Zhong, F.; Li, N.; Han, D.; Pan, S. Strategies for Enhancing DNA Vaccine Potency by Targeting Antigen-Presenting Cells. Front. Agric. China 2009, 3, 478. [Google Scholar] [CrossRef]
- Cáceres-Morgado, P.; Lladser, A. Tumor-Specific CD8+ T-Cell Responses Induced by DNA Vaccination. In DNA Vaccines; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2021; Volume 2197, pp. 225–239. [Google Scholar] [CrossRef]
- Johnson, A.O.; Fowler, S.B.; Webster, C.I.; Brown, A.J.; James, D.C. Bioinformatic Design of Dendritic Cell-Specific Synthetic Promoters. ACS Synth. Biol. 2022, 11, 1613–1626. [Google Scholar] [CrossRef]
- Patel, A.; Bah, M.A.; Weiner, D.B. In Vivo Delivery of Nucleic Acid-Encoded Monoclonal Antibodies. BioDrugs 2020, 34, 273–293. [Google Scholar] [CrossRef]
- Cong, Y.S.; Wen, J.; Bacchetti, S. The Human Telomerase Catalytic Subunit HTERT: Organization of the Gene and Characterization of the Promoter. Hum. Mol. Genet. 1999, 8, 137–142. [Google Scholar] [CrossRef]
- Yuan, X.; Larsson, C.; Xu, D. Mechanisms Underlying the Activation of TERT Transcription and Telomerase Activity in Human Cancer: Old Actors and New Players. Oncogene 2019, 38, 6172–6183. [Google Scholar] [CrossRef]
- Fan, Y.; Yao, Y.; Li, L.; Wu, Z.; Xu, F.; Hou, M.; Wu, H.; Shen, Y.; Wan, H.; Zhou, Q. Nm23-H1 Gene Driven by HTERT Promoter Induces Inhibition of Invasive Phenotype and Metastasis of Lung Cancer Xenograft in Mice. Thorac. Cancer 2013, 4, 41–52. [Google Scholar] [CrossRef]
- Lu, Y.; Ma, J.; Lin, J.; Tian, Y.; Ma, Y.; Wang, W.; Li, J.; Zhang, H.; Jiao, P. Cell Membrane Breakage and Triggering T Cell Infiltration Are Involved in Human Telomerase Reverse Transcriptase (HTERT) Promoter-Driven Novel Peptide KK-64 for Liver Cancer Gene Therapy. Bioengineered 2021, 12, 12708–12721. [Google Scholar] [CrossRef]
- Xie, X.; Hsu, J.L.; Choi, M.-G.; Xia, W.; Yamaguchi, H.; Chen, C.-T.; Trinh, B.Q.; Lu, Z.; Ueno, N.T.; Wolf, J.K.; et al. A Novel HTERT Promoter-Driven E1A Therapeutic for Ovarian Cancer. Mol. Cancer Ther. 2009, 8, 2375–2382. [Google Scholar] [CrossRef]
- Li, F.; Aljahdali, I.; Ling, X. Cancer Therapeutics Using Survivin BIRC5 as a Target: What Can We Do after over Two Decades of Study? J. Exp. Clin. Cancer Res. 2019, 38, 368. [Google Scholar] [CrossRef]
- Wang, T.; Chen, Y.; Goodale, D.; Allan, A.L.; Ronald, J.A. A Survivin-Driven, Tumor-Activatable Minicircle System for Prostate Cancer Theranostics. Mol. Ther. Oncolytics 2021, 20, 209–219. [Google Scholar] [CrossRef]
- Alekseenko, I.V.; Pleshkan, V.V.; Sass, A.V.; Filyukova, O.B.; Snezhkov, E.V.; Sverdlov, E.D. A Universal Tumor-Specific Promoter for Cancer Gene Therapy. Dokl. Biochem. Biophys. 2018, 480, 158–161. [Google Scholar] [CrossRef]
- Cordo Russo, R.I.; Chervo, M.F.; Madera, S.; Charreau, E.H.; Elizalde, P.V. Nuclear ErbB-2: A Novel Therapeutic Target in ErbB-2-Positive Breast Cancer? Horm. Cancer 2019, 10, 64–70. [Google Scholar] [CrossRef]
- Mungamuri, S.K.; Murk, W.; Grumolato, L.; Bernstein, E.; Aaronson, S.A. Chromatin Modifications Sequentially Enhance ErbB2 Expression in ErbB2-Positive Breast Cancers. Cell Rep. 2013, 5, 302–313. [Google Scholar] [CrossRef]
- Nami, B.; Ghanaeian, A.; Black, C.; Wang, Z. Epigenetic Silencing of HER2 Expression during Epithelial-Mesenchymal Transition Leads to Trastuzumab Resistance in Breast Cancer. Life 2021, 11, 868. [Google Scholar] [CrossRef]
- Miller, D.; Ingersoll, M.A.; Lin, M.-F. ErbB-2 Signaling in Advanced Prostate Cancer Progression and Potential Therapy. Endocr. Relat. Cancer 2019, 26, R195–R209. [Google Scholar] [CrossRef]
- Vernimmen, D.; Gueders, M.; Pisvin, S.; Delvenne, P.; Winkler, R. Different Mechanisms Are Implicated in ERBB2 Gene Overexpression in Breast and in Other Cancers. Br. J. Cancer 2003, 89, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Hurst, H.C. Update on HER-2 as a Target for Cancer Therapy: The ERBB2 Promoter and Its Exploitation for Cancer Treatment. Breast Cancer Res. 2001, 3, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Pandha, H.S.; Martin, L.A.; Rigg, A.; Hurst, H.C.; Stamp, G.W.; Sikora, K.; Lemoine, N.R. Genetic Prodrug Activation Therapy for Breast Cancer: A Phase I Clinical Trial of ErbB-2-Directed Suicide Gene Expression. J. Clin. Oncol. 1999, 17, 2180–2189. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; O-Wang, J.; Matsubara, H.; Asano, T.; Ochiai, T.; Sakiyama, S.; Tagawa, M. A Minimum C-ErbB-2 Promoter-Mediated Expression of Herpes Simplex Virus Thymidine Kinase Gene Confers Selective Cytotoxicity of Human Breast Cancer Cells to Ganciclovir. Cancer Gene Ther. 2001, 8, 890–896. [Google Scholar] [CrossRef]
- Hokari, S.; Tamura, Y.; Kaneda, A.; Katsura, A.; Morikawa, M.; Murai, F.; Ehata, S.; Tsutsumi, S.; Ishikawa, Y.; Aburatani, H.; et al. Comparative Analysis of TTF-1 Binding DNA Regions in Small-Cell Lung Cancer and Non-Small-Cell Lung Cancer. Mol. Oncol. 2020, 14, 277–293. [Google Scholar] [CrossRef]
- Huang, T.-W.; Lin, K.-F.; Lee, C.-H.; Chang, H.; Lee, S.-C.; Shieh, Y.-S. The Role of Thyroid Transcription Factor-1 and Tumor Differentiation in Resected Lung Adenocarcinoma. Sci. Rep. 2017, 7, 14222. [Google Scholar] [CrossRef]
- Kolla, V.; Gonzales, L.W.; Gonzales, J.; Wang, P.; Angampalli, S.; Feinstein, S.I.; Ballard, P.L. Thyroid Transcription Factor in Differentiating Type II Cells: Regulation, Isoforms, and Target Genes. Am. J. Respir. Cell Mol. Biol. 2007, 36, 213–225. [Google Scholar] [CrossRef] [Green Version]
- Lei, L.; Chen, C.; Zhao, J.; Wang, H.; Guo, M.; Zhou, Y.; Luo, J.; Zhang, J.; Xu, L. Targeted Expression of MiR-7 Operated by TTF-1 Promoter Inhibited the Growth of Human Lung Cancer through the NDUFA4 Pathway. Mol. Ther. Nucleic Acids 2017, 6, 183–197. [Google Scholar] [CrossRef]
- Yeung, F.; Li, X.; Ellett, J.; Trapman, J.; Kao, C.; Chung, L.W. Regions of Prostate-Specific Antigen (PSA) Promoter Confer Androgen-Independent Expression of PSA in Prostate Cancer Cells. J. Biol. Chem. 2000, 275, 40846–40855. [Google Scholar] [CrossRef]
- Pang, S.; Taneja, S.; Dardashti, K.; Cohan, P.; Kaboo, R.; Sokoloff, M.; Tso, C.L.; Dekernion, J.B.; Belldegrun, A.S. Prostate Tissue Specificity of the Prostate-Specific Antigen Promoter Isolated from a Patient with Prostate Cancer. Hum. Gene Ther. 1995, 6, 1417–1426. [Google Scholar] [CrossRef]
- Spitzweg, C.; Zhang, S.; Bergert, E.R.; Castro, M.R.; McIver, B.; Heufelder, A.E.; Tindall, D.J.; Young, C.Y.; Morris, J.C. Prostate-Specific Antigen (PSA) Promoter-Driven Androgen-Inducible Expression of Sodium Iodide Symporter in Prostate Cancer Cell Lines. Cancer Res. 1999, 59, 2136–2141. [Google Scholar]
- Mizutani, K.; Kawakami, K.; Fujita, Y.; Kato, T.; Takai, M.; Kato, D.; Iinuma, K.; Koie, T.; Ito, M. Gene Therapy of Prostate Cancer Using Liposomes Containing Perforin Expression Vector Driven by the Promoter of Prostate-Specific Antigen Gene. Sci. Rep. 2022, 12, 1442. [Google Scholar] [CrossRef]
- Hollingsworth, R.E.; Jansen, K. Turning the Corner on Therapeutic Cancer Vaccines. NPJ Vaccines 2019, 4, 7. [Google Scholar] [CrossRef]
- Karimi, H.; Soleimanjahi, H.; Abdoli, A.; Banijamali, R.S. Combination Therapy Using Human Papillomavirus L1/E6/E7 Genes and Archaeosome: A Nanovaccine Confer Immuneadjuvanting Effects to Fight Cervical Cancer. Sci. Rep. 2020, 10, 5787. [Google Scholar] [CrossRef]
- Akhatova, A.; Chan, C.K.; Azizan, A.; Aimagambetova, G. The Efficacy of Therapeutic DNA Vaccines Expressing the Human Papillomavirus E6 and E7 Oncoproteins for Treatment of Cervical Cancer: Systematic Review. Vaccines 2022, 10, 53. [Google Scholar] [CrossRef]
- De Pooter, D.; Van Gulck, E.; Chen, A.; Evans, C.F.; Neefs, J.-M.; Horton, H.; Boden, D. A Therapeutic Hepatitis B Virus DNA Vaccine Induces Specific Immune Responses in Mice and Non-Human Primates. Vaccines 2021, 9, 969. [Google Scholar] [CrossRef]
- Endmann, A.; Klünder, K.; Kapp, K.; Riede, O.; Oswald, D.; Talman, E.G.; Schroff, M.; Kleuss, C.; Ruiters, M.H.J.; Juhls, C. Cationic Lipid-Formulated DNA Vaccine against Hepatitis B Virus: Immunogenicity of MIDGE-Th1 Vectors Encoding Small and Large Surface Antigen in Comparison to a Licensed Protein Vaccine. PLoS ONE 2014, 9, e101715. [Google Scholar] [CrossRef]
- Münz, C. Redirecting T Cells against Epstein-Barr Virus Infection and Associated Oncogenesis. Cells 2020, 9, 1400. [Google Scholar] [CrossRef]
- Wojtak, K.; Perales-Puchalt, A.; Weiner, D.B. Novel Synthetic DNA Immunogens Targeting Latent Expressed Antigens of Epstein–Barr Virus Elicit Potent Cellular Responses and Inhibit Tumor Growth. Vaccines 2019, 7, 44. [Google Scholar] [CrossRef]
- Fang, X.; Guo, Z.; Liang, J.; Wen, J.; Liu, Y.; Guan, X.; Li, H. Neoantigens and Their Potential Applications in Tumor Immunotherapy. Oncol. Lett. 2022, 23, 88. [Google Scholar] [CrossRef]
- Malekzadeh, P.; Pasetto, A.; Robbins, P.F.; Parkhurst, M.R.; Paria, B.C.; Jia, L.; Gartner, J.J.; Hill, V.; Yu, Z.; Restifo, N.P.; et al. Neoantigen Screening Identifies Broad TP53 Mutant Immunogenicity in Patients with Epithelial Cancers. J. Clin. Investig. 2021, 129, e123791. [Google Scholar] [CrossRef]
- Bear, A.S.; Blanchard, T.; Cesare, J.; Ford, M.J.; Richman, L.P.; Xu, C.; Baroja, M.L.; McCuaig, S.; Costeas, C.; Gabunia, K.; et al. Biochemical and Functional Characterization of Mutant KRAS Epitopes Validates This Oncoprotein for Immunological Targeting. Nat. Commun. 2021, 12, 4365. [Google Scholar] [CrossRef]
- Chandran, S.S.; Ma, J.; Klatt, M.G.; Dündar, F.; Bandlamudi, C.; Razavi, P.; Wen, H.Y.; Weigelt, B.; Zumbo, P.; Fu, S.N.; et al. Immunogenicity and Therapeutic Targeting of a Public Neoantigen Derived from Mutated PIK3CA. Nat. Med. 2022, 28, 946–957. [Google Scholar] [CrossRef]
- Schwartzentruber, J.; Korshunov, A.; Liu, X.-Y.; Jones, D.T.W.; Pfaff, E.; Jacob, K.; Sturm, D.; Fontebasso, A.M.; Quang, D.-A.K.; Tönjes, M.; et al. Driver Mutations in Histone H3.3 and Chromatin Remodelling Genes in Paediatric Glioblastoma. Nature 2012, 482, 226–231. [Google Scholar] [CrossRef]
- Disis, M.L.; Coveler, A.L.; Higgins, D.; Fintak, P.; Waisman, J.R.; Reichow, J.; Slota, M.; Childs, J.; Dang, Y.; Salazar, L.G. A Phase I Trial of the Safety and Immunogenicity of a DNA-Based Vaccine Encoding the HER2/Neu (HER2) Intracellular Domain in Subjects with HER2+ Breast Cancer. J. Clin. Oncol. 2014, 32, 616. [Google Scholar] [CrossRef]
- Nguyen-Hoai, T.; Hohn, O.; Pezzutto, A.; Westermann, J. Gene Gun Her2/Neu DNA Vaccination: Evaluation of Vaccine Efficacy in a Syngeneic Her2/Neu Mouse Tumor Model. In Gene Therapy of Cancer; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2022; Volume 2521, pp. 129–154. [Google Scholar] [CrossRef]
- Lai, M.-D.; Yen, M.-C.; Lin, C.-M.; Tu, C.-F.; Wang, C.-C.; Lin, P.-S.; Yang, H.-J.; Lin, C.-C. The Effects of DNA Formulation and Administration Route on Cancer Therapeutic Efficacy with Xenogenic EGFR DNA Vaccine in a Lung Cancer Animal Model. Genet. Vaccines Ther. 2009, 7, 2. [Google Scholar] [CrossRef]
- Gong, Y.-F.; Zhou, Q.-B.; Liao, Y.-D.; Mai, C.; Chen, T.-J.; Tang, Y.-Q.; Chen, R.-F. Optimized Construction of MUC1-VNTRn DNA Vaccine and Its Anti-Pancreatic Cancer Efficacy. Oncol. Lett. 2017, 13, 2198–2206. [Google Scholar] [CrossRef] [Green Version]
- Lewis, J.D.; Sullivan, L.A.; Byrne, J.A.; de Riese, W.; Bright, R.K. Memory and Cellular Immunity Induced by a DNA Vaccine Encoding Self Antigen TPD52 Administered with Soluble GM-CSF. Cancer Immunol. Immunother. 2009, 58, 1337–1349. [Google Scholar] [CrossRef] [PubMed]
- Sabel, M.S.; Nehs, M.A. Immunologic Approaches to Breast Cancer Treatment. Surg. Oncol. Clin. N. Am. 2005, 14, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Goedegebuure, P.; Gillanders, W.E. Mammaglobin-A Is a Target for Breast Cancer Vaccination. Oncoimmunology 2016, 5, e1069940. [Google Scholar] [CrossRef] [PubMed]
- Cecil, D.L.; Holt, G.E.; Park, K.H.; Gad, E.; Rastetter, L.; Childs, J.; Higgins, D.; Disis, M.L. Elimination of IL-10-Inducing T-Helper Epitopes from an IGFBP-2 Vaccine Ensures Potent Antitumor Activity. Cancer Res. 2014, 74, 2710–2718. [Google Scholar] [CrossRef]
- Ferraro, B.; Cisper, N.J.; Talbott, K.T.; Philipson-Weiner, L.; Lucke, C.E.; Khan, A.S.; Sardesai, N.Y.; Weiner, D.B. Co-Delivery of PSA and PSMA DNA Vaccines with Electroporation Induces Potent Immune Responses. Hum. Vaccin. 2011, 7 (Suppl. 1), 120–127. [Google Scholar] [CrossRef]
- Ren, J.; Zheng, L.; Chen, Q.; Li, H.; Zhang, L.; Zhu, H. Co-Administration of a DNA Vaccine Encoding the Prostate Specific Membrane Antigen and CpG Oligodeoxynucleotides Suppresses Tumor Growth. J. Transl. Med. 2004, 2, 29. [Google Scholar] [CrossRef]
- Spies, E.; Reichardt, W.; Alvarez, G.; Groettrup, M.; Ohlschläger, P. An Artificial PAP Gene Breaks Self-Tolerance and Promotes Tumor Regression in the TRAMP Model for Prostate Carcinoma. Mol. Ther. J. Am. Soc. Gene Ther. 2012, 20, 555–564. [Google Scholar] [CrossRef]
- Johnson, L.E.; Brockstedt, D.; Leong, M.; Lauer, P.; Theisen, E.; Sauer, J.-D.; McNeel, D.G. Heterologous Vaccination Targeting Prostatic Acid Phosphatase (PAP) Using DNA and Listeria Vaccines Elicits Superior Anti-Tumor Immunity Dependent on CD4+ T Cells Elicited by DNA Priming. OncoImmunology 2018, 7, e1456603. [Google Scholar] [CrossRef]
- Roos, A.-K.; Moreno, S.; Leder, C.; Pavlenko, M.; King, A.; Pisa, P. Enhancement of Cellular Immune Response to a Prostate Cancer DNA Vaccine by Intradermal Electroporation. Mol. Ther. J. Am. Soc. Gene Ther. 2006, 13, 320–327. [Google Scholar] [CrossRef]
- Veisi Malekshahi, Z.; Hashemi Goradel, N.; Shakouri Khomartash, M.; Maleksabet, A.; Kadkhodazadeh, M.; Kardar, G.A.; Negahdari, B. CEA Plasmid as Therapeutic DNA Vaccination against Colorectal Cancer. Iran. J. Immunol. 2019, 16, 235–245. [Google Scholar] [CrossRef]
- Yan, J.; Tingey, C.; Lyde, R.; Gorham, T.C.; Choo, D.K.; Muthumani, A.; Myles, D.; Weiner, L.P.; Kraynyak, K.A.; Reuschel, E.L.; et al. Novel and Enhanced Anti-Melanoma DNA Vaccine Targeting the Tyrosinase Protein Inhibits Myeloid-Derived Suppressor Cells and Tumor Growth in a Syngeneic Prophylactic and Therapeutic Murine Model. Cancer Gene Ther. 2014, 21, 507–517. [Google Scholar] [CrossRef]
- Shen, Y.; Qiu, L. Effective Oral Delivery of Gp100 Plasmid Vaccine against Metastatic Melanoma through Multi-Faceted Blending-by-Blending Nanogels. Nanomed. Nanotechnol. Biol. Med. 2019, 22, 102114. [Google Scholar] [CrossRef]
- Qian, J.; Yi, Q. DKK1 as a Novel Target for Myeloma Immunotherapy. Oncoimmunology 2012, 1, 756–758. [Google Scholar] [CrossRef]
- Ye, J.; Chen, G.-S.; Song, H.-P.; Li, Z.-S.; Huang, Y.-Y.; Qu, P.; Sun, Y.-J.; Zhang, X.-M.; Sui, Y.-F. Heat Shock Protein 70/MAGE-1 Tumor Vaccine Can Enhance the Potency of MAGE-1-Specific Cellular Immune Responses in Vivo. Cancer Immunol. Immunother. 2004, 53, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Duperret, E.K.; Liu, S.; Paik, M.; Trautz, A.; Stoltz, R.; Liu, X.; Ze, K.; Perales-Puchalt, A.; Reed, C.; Yan, J.; et al. A Designer Cross-Reactive DNA Immunotherapeutic Vaccine That Targets Multiple MAGE-A Family Members Simultaneously for Cancer Therapy. Clin. Cancer Res. 2018, 24, 6015–6027. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.A.; McNeel, D.G. Vaccines Targeting the Cancer-Testis Antigen SSX-2 Elicit HLA-A2 Epitope-Specific Cytolytic T Cells. J. Immunother. 2011, 34, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Rekoske, B.; Smith, H.A.; McNeel, D.G. Epitope Optimization of a DNA Vaccine Targeting SSX-2 Leads to PD-1 Upregulation on Antigen-Specific CD8 T Cells and PD-L1 Upregulation on Tumor Cells. J. Immunother. Cancer 2013, 1, P233. [Google Scholar] [CrossRef]
- Raza, A.; Merhi, M.; Inchakalody, V.P.; Krishnankutty, R.; Relecom, A.; Uddin, S.; Dermime, S. Unleashing the Immune Response to NY-ESO-1 Cancer Testis Antigen as a Potential Target for Cancer Immunotherapy. J. Transl. Med. 2020, 18, 140. [Google Scholar] [CrossRef]
- Gnjatic, S.; Altorki, N.K.; Tang, D.N.; Tu, S.-M.; Kundra, V.; Old, L.J.; Logothetis, C.J.; Sharma, P. NY-ESO-1 DNA Vaccine Induces T Cell Responses That Are Suppressed by Regulatory T Cells. Clin. Cancer Res. 2009, 15, 2130–2139. [Google Scholar] [CrossRef]
- Zur Hausen, H. Papillomaviruses in the Causation of Human Cancers—A Brief Historical Account. Virology 2009, 384, 260–265. [Google Scholar] [CrossRef] [Green Version]
- Viruses That Can Lead to Cancer. Available online: https://www.cancer.org/healthy/cancer-causes/infectious-agents/infections-that-can-lead-to-cancer/viruses.html (accessed on 21 June 2022).
- Jou, J.; Harrington, K.J.; Zocca, M.-B.; Ehrnrooth, E.; Cohen, E.E.W. The Changing Landscape of Therapeutic Cancer Vaccines-Novel Platforms and Neoantigen Identification. Clin. Cancer Res. 2021, 27, 689–703. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, M.; Qin, Y.; Gao, W.; Tao, L.; Su, W.; Zhong, J. Neoantigen: A New Breakthrough in Tumor Immunotherapy. Front. Immunol. 2021, 12, 672356. [Google Scholar] [CrossRef]
- Bright, R.K.; Bright, J.D.; Byrne, J.A. Overexpressed Oncogenic Tumor-Self Antigens. Hum. Vaccines Immunother. 2014, 10, 3297–3305. [Google Scholar] [CrossRef]
- Vigneron, N. Human Tumor Antigens and Cancer Immunotherapy. BioMed Res. Int. 2015, 2015, 948501. [Google Scholar] [CrossRef]
- Scanlan, M.J.; Gure, A.O.; Jungbluth, A.A.; Old, L.J.; Chen, Y.-T. Cancer/Testis Antigens: An Expanding Family of Targets for Cancer Immunotherapy. Immunol. Rev. 2002, 188, 22–32. [Google Scholar] [CrossRef]
- Yang, X.; Fan, J.; Wu, Y.; Ma, Z.; Huang, J.; Zhang, Y.; Zhou, Z.; Mo, F.; Liu, X.; Yuan, H.; et al. Synthetic Multiepitope Neoantigen DNA Vaccine for Personalized Cancer Immunotherapy. Nanomed. Nanotechnol. Biol. Med. 2021, 37, 102443. [Google Scholar] [CrossRef]
- Peng, S.; Ferrall, L.; Gaillard, S.; Wang, C.; Chi, W.-Y.; Huang, C.-H.; Roden, R.B.S.; Wu, T.-C.; Chang, Y.-N.; Hung, C.-F. Development of DNA Vaccine Targeting E6 and E7 Proteins of Human Papillomavirus 16 (HPV16) and HPV18 for Immunotherapy in Combination with Recombinant Vaccinia Boost and PD-1 Antibody. mBio 2021, 12, e03224-20. [Google Scholar] [CrossRef]
- Walters, J.N.; Ferraro, B.; Duperret, E.K.; Kraynyak, K.A.; Chu, J.; Saint-Fleur, A.; Yan, J.; Levitsky, H.; Khan, A.S.; Sardesai, N.Y.; et al. A Novel DNA Vaccine Platform Enhances Neo-Antigen-like T Cell Responses against WT1 to Break Tolerance and Induce Anti-Tumor Immunity. Mol. Ther. J. Am. Soc. Gene Ther. 2017, 25, 976–988. [Google Scholar] [CrossRef]
- Lowe, D.B.; Shearer, M.H.; Kennedy, R.C. DNA Vaccines: Successes and Limitations in Cancer and Infectious Disease. J. Cell. Biochem. 2006, 98, 235–242. [Google Scholar] [CrossRef]
- Chen, C.H.; Ji, H.; Suh, K.W.; Choti, M.A.; Pardoll, D.M.; Wu, T.C. Gene Gun-Mediated DNA Vaccination Induces Antitumor Immunity against Human Papillomavirus Type 16 E7-Expressing Murine Tumor Metastases in the Liver and Lungs. Gene Ther. 1999, 6, 1972–1981. [Google Scholar] [CrossRef] [Green Version]
- Adhikari, A.S.; Macauley, J.; Johnson, Y.; Connolly, M.; Coleman, T.; Heiland, T. Development and Characterization of an HCMV Multi-Antigen Therapeutic Vaccine for Glioblastoma Using the UNITE Platform. Front. Oncol. 2022, 12, 850546. [Google Scholar] [CrossRef]
- Cheng, W.-F.; Hung, C.-F.; Chai, C.-Y.; Hsu, K.-F.; He, L.; Ling, M.; Wu, T.-C. Tumor-Specific Immunity and Antiangiogenesis Generated by a DNA Vaccine Encoding Calreticulin Linked to a Tumor Antigen. J. Clin. Investig. 2001, 108, 669–678. [Google Scholar] [CrossRef]
- Perez-Trujillo, J.J.; Garza-Morales, R.; Barron-Cantu, J.A.; Figueroa-Parra, G.; Garcia-Garcia, A.; Rodriguez-Rocha, H.; Garcia-Juarez, J.; Muñoz-Maldonado, G.E.; Saucedo-Cardenas, O.; Montes-De-Oca-Luna, R.; et al. DNA Vaccine Encoding Human Papillomavirus Antigens Flanked by a Signal Peptide and a KDEL Sequence Induces a Potent Therapeutic Antitumor Effect. Oncol. Lett. 2017, 13, 1569–1574. [Google Scholar] [CrossRef]
- Pérez-Trujillo, J.J.; Robles-Rodríguez, O.A.; Garza-Morales, R.; García-García, A.; Rodríguez-Rocha, H.; Villanueva-Olivo, A.; Segoviano-Ramírez, J.C.; Esparza-González, S.C.; Saucedo-Cárdenas, O.; Montes-de-Oca-Luna, R.; et al. Antitumor Response by Endoplasmic Reticulum-Targeting DNA Vaccine Is Improved by Adding a KDEL Retention Signal. Nucleic Acid Ther. 2018, 28, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Sher, Y.-P.; Lin, S.-I.; Chai, K.M.; Chen, I.-H.; Liu, S.-J. Endoplasmic Reticulum-Targeting Sequence Enhanced the Cellular Immunity of a Tumor-Associated Antigen L6-Based DNA Vaccine. Am. J. Cancer Res. 2019, 9, 2028–2036. [Google Scholar] [PubMed]
- Oosterhuis, K.; Aleyd, E.; Vrijland, K.; Schumacher, T.N.; Haanen, J.B. Rational Design of DNA Vaccines for the Induction of Human Papillomavirus Type 16 E6- and E7-Specific Cytotoxic T-Cell Responses. Hum. Gene Ther. 2012, 23, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Puente, D.H.; Garza-Morales, R.; Pérez-Trujillo, J.J.; García-García, A.; Villanueva-Olivo, A.; Rodríguez-Rocha, H.; Zavala-Flores, L.M.; Saucedo-Cárdenas, O.; Montes de Oca-Luna, R.; de Loera-Arias, M.J. Targeting E7 Antigen to the Endoplasmic Reticulum Degradation Pathway Promotes a Potent Therapeutic Antitumor Effect. J. Drug Target. 2021, 29, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Puente, D.H.; Garza-Morales, R.; Pérez-Trujillo, J.J.; Bernabé-Acosta, F.; Villanueva-Olivo, A.; García-García, A.; Zavala-Flores, L.M.; Rodríguez-Rocha, H.; Valdés, J.; Saucedo-Cárdenas, O.; et al. Enhanced Antitumor Activity Induced by a DNA Vaccine Encoding E7 Antigen Fused to an ERAD-Targeting Sequence. J. Drug Target. 2022, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, G. Hsp70: A Carrier Molecule with Built-in Adjuvanticity. Experientia 1994, 50, 1061–1066. [Google Scholar] [CrossRef]
- Shevtsov, M.; Multhoff, G. Heat Shock Protein-Peptide and HSP-Based Immunotherapies for the Treatment of Cancer. Front. Immunol. 2016, 7, 171. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.H.; Wang, T.L.; Hung, C.F.; Yang, Y.; Young, R.A.; Pardoll, D.M.; Wu, T.C. Enhancement of DNA Vaccine Potency by Linkage of Antigen Gene to an HSP70 Gene. Cancer Res. 2000, 60, 1035–1042. [Google Scholar]
- Liu, T.-T.; Wu, Y.; Niu, T. Human DKK1 and Human HSP70 Fusion DNA Vaccine Induces an Effective Anti-Tumor Efficacy in Murine Multiple Myeloma. Oncotarget 2018, 9, 178–191. [Google Scholar] [CrossRef]
- Choi, D.-H.; Woo, J.K.; Choi, Y.; Seo, H.-S.; Kim, C.-W. A Novel Chimeric DNA Vaccine: Enhancement of Preventive and Therapeutic Efficacy of DNA Vaccine by Fusion of Mucin 1 to a Heat Shock Protein 70 Gene. Mol. Med. Rep. 2011, 4, 885–890. [Google Scholar] [CrossRef]
- Zong, J.; Wang, C.; Wang, Q.; Peng, Q.; Xu, Y.; Xie, X.; Xu, X. HSP70 and Modified HPV 16 E7 Fusion Gene without the Addition of a Signal Peptide Gene Sequence as a Candidate Therapeutic Tumor Vaccine. Oncol. Rep. 2013, 30, 3020–3026. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Chen, C.-A.; Lee, C.-N.; Chang, M.-C.; Su, Y.-N.; Lin, Y.-C.; Hsieh, C.-Y.; Cheng, W.-F. DNA Vaccine Encoding Heat Shock Protein 60 Co-Linked to HPV16 E6 and E7 Tumor Antigens Generates More Potent Immunotherapeutic Effects than Respective E6 or E7 Tumor Antigens. Gynecol. Oncol. 2007, 107, 404–412. [Google Scholar] [CrossRef]
- Lin, C.-T.; Chang, T.-C.; Chao, A.; Dzeng, E.; Soong, Y.-K.; Hung, C.-F.; Lai, C.-H. Enhancement of DNA Vaccine Potency through Linkage of Antigen Gene to ER Chaperone Molecules, ER-60, Tapasin, and Calnexin. J. Biomed. Sci. 2005, 12, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Kang, Y.; Ascione, R. Cytokine Genes as Molecular Adjuvants for DNA Vaccines. In Gene Vaccines; Thalhamer, J., Weiss, R., Scheiblhofer, S., Eds.; Springer: Vienna, Austria, 2012; pp. 89–107. ISBN 978-3-7091-0439-2. [Google Scholar]
- Nguyen, K.G.; Vrabel, M.R.; Mantooth, S.M.; Hopkins, J.J.; Wagner, E.S.; Gabaldon, T.A.; Zaharoff, D.A. Localized Interleukin-12 for Cancer Immunotherapy. Front. Immunol. 2020, 11, 575597. [Google Scholar] [CrossRef] [PubMed]
- Kamensek, U.; Cemazar, M.; Lampreht Tratar, U.; Ursic, K.; Sersa, G. Antitumor in Situ Vaccination Effect of TNFα and IL-12 Plasmid DNA Electrotransfer in a Murine Melanoma Model. Cancer Immunol. Immunother. 2018, 67, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Nishitani, M.-A.; Sakai, T.; Kanayama, H.-O.; Himeno, K.; Kagawa, S. Cytokine Gene Therapy for Cancer with Naked DNA. Mol. Urol. 2000, 4, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Peng, S.; Yang, A.; Farmer, E.; Wu, T.-C.; Hung, C.-F. Coinjection of IL2 DNA Enhances E7-Specific Antitumor Immunity Elicited by Intravaginal Therapeutic HPV DNA Vaccination with Electroporation. Gene Ther. 2017, 24, 408–415. [Google Scholar] [CrossRef]
- Gordy, J.T.; Luo, K.; Zhang, H.; Biragyn, A.; Markham, R.B. Fusion of the Dendritic Cell-Targeting Chemokine MIP3α to Melanoma Antigen Gp100 in a Therapeutic DNA Vaccine Significantly Enhances Immunogenicity and Survival in a Mouse Melanoma Model. J. Immunother. Cancer 2016, 4, 96. [Google Scholar] [CrossRef] [Green Version]
- Biragyn, A.; Tani, K.; Grimm, M.C.; Weeks, S.; Kwak, L.W. Genetic Fusion of Chemokines to a Self Tumor Antigen Induces Protective, T-Cell Dependent Antitumor Immunity. Nat. Biotechnol. 1999, 17, 253–258. [Google Scholar] [CrossRef]
- Lin, C.-T.; Tsai, Y.-C.; He, L.; Yeh, C.-N.; Chang, T.-C.; Soong, Y.-K.; Monie, A.; Hung, C.-F.; Lai, C.-H. DNA Vaccines Encoding IL-2 Linked to HPV-16 E7 Antigen Generate Enhanced E7-Specific CTL Responses and Antitumor Activity. Immunol. Lett. 2007, 114, 86–93. [Google Scholar] [CrossRef]
- Wang, C.; Zainal, N.S.; Chai, S.J.; Dickie, J.; Gan, C.P.; Zulaziz, N.; Lye, B.K.W.; Sutavani, R.V.; Ottensmeier, C.H.; King, E.V.; et al. DNA Vaccines Targeting Novel Cancer-Associated Antigens Frequently Expressed in Head and Neck Cancer Enhance the Efficacy of Checkpoint Inhibitor. Front. Immunol. 2021, 12, 763086. [Google Scholar] [CrossRef]
- Wu, C.-C.; Wu, F.-C.; Hsu, Y.-T.; Hsiao, Y.-C.; Yang, Y.-C.; Chang, C.A.; Chang, C.-L. Enhanced Anti-Tumor Therapeutic Efficacy of DNA Vaccine by Fusing the E7 Gene to BAFF in Treating Human Papillomavirus-Associated Cancer. Oncotarget 2017, 8, 33024–33036. [Google Scholar] [CrossRef]
- Garza-Morales, R.; Perez-Trujillo, J.J.; Martinez-Jaramillo, E.; Saucedo-Cardenas, O.; Loera-Arias, M.J.; Garcia-Garcia, A.; Rodriguez-Rocha, H.; Yolcu, E.; Shirwan, H.; Gomez-Gutierrez, J.G.; et al. A DNA Vaccine Encoding SA-4-1BBL Fused to HPV-16 E7 Antigen Has Prophylactic and Therapeutic Efficacy in a Cervical Cancer Mouse Model. Cancers 2019, 11, 96. [Google Scholar] [CrossRef]
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune Checkpoint Inhibitors: Recent Progress and Potential Biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef]
- Ledford, H. Melanoma Drug Wins US Approval. Nature 2011, 471, 561. [Google Scholar] [CrossRef]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’Byrne, K.; Kulasinghe, A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Oncol. 2022, 29, 3044–3060. [Google Scholar] [CrossRef]
- Yang, B.; Jeang, J.; Yang, A.; Wu, T.C.; Hung, C.-F. DNA Vaccine for Cancer Immunotherapy. Hum. Vaccines Immunother. 2014, 10, 3153–3164. [Google Scholar] [CrossRef]
- Xue, W.; Metheringham, R.L.; Brentville, V.A.; Gunn, B.; Symonds, P.; Yagita, H.; Ramage, J.M.; Durrant, L.G. SCIB2, an Antibody DNA Vaccine Encoding NY-ESO-1 Epitopes, Induces Potent Antitumor Immunity Which Is Further Enhanced by Checkpoint Blockade. Oncoimmunology 2016, 5, e1169353. [Google Scholar] [CrossRef] [Green Version]
- Kos, S.; Lopes, A.; Preat, V.; Cemazar, M.; Lampreht Tratar, U.; Ucakar, B.; Vanvarenberg, K.; Sersa, G.; Vandermeulen, G. Intradermal DNA Vaccination Combined with Dual CTLA-4 and PD-1 Blockade Provides Robust Tumor Immunity in Murine Melanoma. PLoS ONE 2019, 14, e0217762. [Google Scholar] [CrossRef]
- Lopes, A.; Vanvarenberg, K.; Kos, Š.; Lucas, S.; Colau, D.; Van den Eynde, B.; Préat, V.; Vandermeulen, G. Combination of Immune Checkpoint Blockade with DNA Cancer Vaccine Induces Potent Antitumor Immunity against P815 Mastocytoma. Sci. Rep. 2018, 8, 15732. [Google Scholar] [CrossRef]
- Duperret, E.K.; Wise, M.C.; Trautz, A.; Villarreal, D.O.; Ferraro, B.; Walters, J.; Yan, J.; Khan, A.; Masteller, E.; Humeau, L.; et al. Synergy of Immune Checkpoint Blockade with a Novel Synthetic Consensus DNA Vaccine Targeting TERT. Mol. Ther. J. Am. Soc. Gene Ther. 2018, 26, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Salvatori, E.; Lione, L.; Compagnone, M.; Pinto, E.; Conforti, A.; Ciliberto, G.; Aurisicchio, L.; Palombo, F. Neoantigen Cancer Vaccine Augments Anti-CTLA-4 Efficacy. NPJ Vaccines 2022, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.; Bastiancich, C.; Bausart, M.; Ligot, S.; Lambricht, L.; Vanvarenberg, K.; Ucakar, B.; Gallez, B.; Préat, V.; Vandermeulen, G. New Generation of DNA-Based Immunotherapy Induces a Potent Immune Response and Increases the Survival in Different Tumor Models. J. Immunother. Cancer 2021, 9, e001243. [Google Scholar] [CrossRef] [PubMed]
- Bausart, M.; Vanvarenberg, K.; Ucakar, B.; Lopes, A.; Vandermeulen, G.; Malfanti, A.; Préat, V. Combination of DNA Vaccine and Immune Checkpoint Blockades Improves the Immune Response in an Orthotopic Unresectable Glioblastoma Model. Pharmaceutics 2022, 14, 1025. [Google Scholar] [CrossRef]
- Muthumani, K.; Block, P.; Flingai, S.; Muruganantham, N.; Chaaithanya, I.K.; Tingey, C.; Wise, M.; Reuschel, E.L.; Chung, C.; Muthumani, A.; et al. Rapid and Long-Term Immunity Elicited by DNA-Encoded Antibody Prophylaxis and DNA Vaccination Against Chikungunya Virus. J. Infect. Dis. 2016, 214, 369–378. [Google Scholar] [CrossRef]
- Kim, H.; Danishmalik, S.N.; Hwang, H.; Sin, J.-I.; Oh, J.; Cho, Y.; Lee, H.; Jeong, M.; Kim, S.-H.; Hong, H.J. Gene Therapy Using Plasmid DNA-Encoded Anti-HER2 Antibody for Cancers That Overexpress HER2. Cancer Gene Ther. 2016, 23, 341–347. [Google Scholar] [CrossRef]
- Muthumani, K.; Marnin, L.; Kudchodkar, S.B.; Perales-Puchalt, A.; Choi, H.; Agarwal, S.; Scott, V.L.; Reuschel, E.L.; Zaidi, F.I.; Duperret, E.K.; et al. Novel Prostate Cancer Immunotherapy with a DNA-Encoded Anti-Prostate-Specific Membrane Antigen Monoclonal Antibody. Cancer Immunol. Immunother. 2017, 66, 1577–1588. [Google Scholar] [CrossRef]
- Duperret, E.K.; Trautz, A.; Stoltz, R.; Patel, A.; Wise, M.C.; Perales-Puchalt, A.; Smith, T.; Broderick, K.E.; Masteller, E.; Kim, J.J.; et al. Synthetic DNA-Encoded Monoclonal Antibody Delivery of Anti-CTLA-4 Antibodies Induces Tumor Shrinkage In Vivo. Cancer Res. 2018, 78, 6363–6370. [Google Scholar] [CrossRef] [Green Version]
- Perales-Puchalt, A.; Duperret, E.K.; Yang, X.; Hernandez, P.; Wojtak, K.; Zhu, X.; Jung, S.-H.; Tello-Ruiz, E.; Wise, M.C.; Montaner, L.J.; et al. DNA-Encoded Bispecific T Cell Engagers and Antibodies Present Long-Term Antitumor Activity. JCI Insight 2019, 4, 126086. [Google Scholar] [CrossRef]
- Qin, F.; Xia, F.; Chen, H.; Cui, B.; Feng, Y.; Zhang, P.; Chen, J.; Luo, M. A Guide to Nucleic Acid Vaccines in the Prevention and Treatment of Infectious Diseases and Cancers: From Basic Principles to Current Applications. Front. Cell Dev. Biol. 2021, 9, 633776. [Google Scholar] [CrossRef]
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct Gene Transfer into Mouse Muscle in Vivo. Science 1990, 247, 1465–1468. [Google Scholar] [CrossRef]
- Mincheff, M.; Tchakarov, S.; Zoubak, S.; Loukinov, D.; Botev, C.; Altankova, I.; Georgiev, G.; Petrov, S.; Meryman, H.T. Naked DNA and Adenoviral Immunizations for Immunotherapy of Prostate Cancer: A Phase I/II Clinical Trial. Eur. Urol. 2000, 38, 208–217. [Google Scholar] [CrossRef]
- Maloy, K.J.; Erdmann, I.; Basch, V.; Sierro, S.; Kramps, T.A.; Zinkernagel, R.M.; Oehen, S.; Kündig, T.M. Intralymphatic Immunization Enhances DNA Vaccination. Proc. Natl. Acad. Sci. USA 2001, 98, 3299–3303. [Google Scholar] [CrossRef]
- Wu, X.; Gao, H.; Pasupathy, S.; Tan, P.H.; Ooi, L.L.; Hui, K.M. Systemic Administration of Naked DNA with Targeting Specificity to Mammalian Kidneys. Gene Ther. 2005, 12, 477–486. [Google Scholar] [CrossRef]
- Sokołowska, E.; Błachnio-Zabielska, A.U. A Critical Review of Electroporation as A Plasmid Delivery System in Mouse Skeletal Muscle. Int. J. Mol. Sci. 2019, 20, 2776. [Google Scholar] [CrossRef]
- Neumann, E.; Schaefer-Ridder, M.; Wang, Y.; Hofschneider, P.H. Gene Transfer into Mouse Lyoma Cells by Electroporation in High Electric Fields. EMBO J. 1982, 1, 841–845. [Google Scholar] [CrossRef]
- Lambricht, L.; Lopes, A.; Kos, S.; Sersa, G.; Préat, V.; Vandermeulen, G. Clinical Potential of Electroporation for Gene Therapy and DNA Vaccine Delivery. Expert Opin. Drug Deliv. 2016, 13, 295–310. [Google Scholar] [CrossRef]
- Sales, N.S.; Silva, J.R.; Aps, L.R.M.M.; Silva, M.O.; Porchia, B.F.M.M.; Ferreira, L.C.S.; Diniz, M.O. In Vivo Electroporation Enhances Vaccine-Mediated Therapeutic Control of Human Papilloma Virus-Associated Tumors by the Activation of Multifunctional and Effector Memory CD8+ T Cells. Vaccine 2017, 35, 7240–7249. [Google Scholar] [CrossRef]
- Paolini, F.; Amici, C.; Carosi, M.; Bonomo, C.; Di Bonito, P.; Venuti, A.; Accardi, L. Intrabodies Targeting Human Papillomavirus 16 E6 and E7 Oncoproteins for Therapy of Established HPV-Associated Tumors. J. Exp. Clin. Cancer Res. 2021, 40, 37. [Google Scholar] [CrossRef]
- Jacobs, L.; De Smidt, E.; Geukens, N.; Declerck, P.; Hollevoet, K. DNA-Based Delivery of Checkpoint Inhibitors in Muscle and Tumor Enables Long-Term Responses with Distinct Exposure. Mol. Ther. J. Am. Soc. Gene Ther. 2020, 28, 1068–1077. [Google Scholar] [CrossRef]
- Kim, J.J.; Ayyavoo, V.; Bagarazzi, M.L.; Dang, K.; Chattergoon, M.A.; Wang, B.; Boyer, J.D.; Weiner, D.B. In Vivo Engineering of a Cellular Immune Response by Co-Administration of IL-12 Expression Vector with a DNA Immunogen. Immunol. Lett. 1997, 56, 20–21. [Google Scholar] [CrossRef]
- Kim, J.J.; Yang, J.S.; Dang, K.; Manson, K.H.; Weiner, D.B. Engineering Enhancement of Immune Responses to DNA-Based Vaccines in a Prostate Cancer Model in Rhesus Macaques through the Use of Cytokine Gene Adjuvants. Clin. Cancer Res. 2001, 7, 882s–889s. [Google Scholar] [PubMed]
- Jacobs, L.; Yshii, L.; Junius, S.; Geukens, N.; Liston, A.; Hollevoet, K.; Declerck, P. Intratumoral DNA-Based Delivery of Checkpoint-Inhibiting Antibodies and Interleukin 12 Triggers T Cell Infiltration and Anti-Tumor Response. Cancer Gene Ther. 2022, 29, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Nguyen, B.; Lee, J.Y.; Browning, E.; Zhang, J.; Mukhopadhyay, A.; Gujar, R.; Salazar, J.; Hermiz, R.; Svenson, L.; et al. Intratumoral Electroporation of Plasmid Encoded IL12 and Membrane-Anchored Anti-CD3 Increases Systemic Tumor Immunity. Mol. Cancer Res. 2022, 20, 983–995. [Google Scholar] [CrossRef]
- Komel, T.; Bosnjak, M.; Kranjc Brezar, S.; De Robertis, M.; Mastrodonato, M.; Scillitani, G.; Pesole, G.; Signori, E.; Sersa, G.; Cemazar, M. Gene Electrotransfer of IL-2 and IL-12 Plasmids Effectively Eradicated Murine B16.F10 Melanoma. Bioelectrochemistry 2021, 141, 107843. [Google Scholar] [CrossRef]
- Vonderheide, R.H.; Kraynyak, K.A.; Shields, A.F.; McRee, A.J.; Johnson, J.M.; Sun, W.; Chintakuntlawar, A.V.; Pawlicki, J.; Sylvester, A.J.; McMullan, T.; et al. Phase 1 Study of Safety, Tolerability and Immunogenicity of the Human Telomerase (HTERT)-Encoded DNA Plasmids INO-1400 and INO-1401 with or without IL-12 DNA Plasmid INO-9012 in Adult Patients with Solid Tumors. J. Immunother. Cancer 2021, 9, e003019. [Google Scholar] [CrossRef]
- Telli, M.L.; Nagata, H.; Wapnir, I.; Acharya, C.R.; Zablotsky, K.; Fox, B.A.; Bifulco, C.B.; Jensen, S.M.; Ballesteros-Merino, C.; Le, M.H.; et al. Intratumoral Plasmid IL12 Expands CD8+ T Cells and Induces a CXCR3 Gene Signature in Triple-Negative Breast Tumors That Sensitizes Patients to Anti–PD-1 Therapy. Clin. Cancer Res. 2021, 27, 2481–2493. [Google Scholar] [CrossRef]
- Algazi, A.; Bhatia, S.; Agarwala, S.; Molina, M.; Lewis, K.; Faries, M.; Fong, L.; Levine, L.P.; Franco, M.; Oglesby, A.; et al. Intratumoral Delivery of Tavokinogene Telseplasmid Yields Systemic Immune Responses in Metastatic Melanoma Patients. Ann. Oncol. 2020, 31, 532–540. [Google Scholar] [CrossRef] [Green Version]
- Greaney, S.K.; Algazi, A.P.; Tsai, K.K.; Takamura, K.T.; Chen, L.; Twitty, C.G.; Zhang, L.; Paciorek, A.; Pierce, R.H.; Le, M.H.; et al. Intratumoral Plasmid IL12 Electroporation Therapy in Patients with Advanced Melanoma Induces Systemic and Intratumoral T-Cell Responses. Cancer Immunol. Res. 2020, 8, 246–254. [Google Scholar] [CrossRef]
- Chang, A.E.; Tanigawa, K.; Turner, J.G.; Chang, E.C.; Yu, H. Use of Gene Gun for Genetic Immunotherapy: In Vitro and in Vivo Methods. Methods Mol. Med. 2001, 61, 223–240. [Google Scholar] [CrossRef]
- Kao, C.-Y.; Huang, S.-H.; Lin, C.-M. A Low-Pressure Gene Gun for Genetic Transformation of Maize (Zea mays L.). Plant Biotechnol. Rep. 2008, 2, 267–270. [Google Scholar] [CrossRef]
- O’Brien, J.A.; Lummis, S.C. Nano-Biolistics: A Method of Biolistic Transfection of Cells and Tissues Using a Gene Gun with Novel Nanometer-Sized Projectiles. BMC Biotechnol. 2011, 11, 66. [Google Scholar] [CrossRef]
- Yang, N.-S.; Sun, W.H. Gene Gun and Other Non-Viral Approaches for Cancer Gene Therapy. Nat. Med. 1995, 1, 481–483. [Google Scholar] [CrossRef]
- Chen, C.-A.; Chang, M.-C.; Sun, W.-Z.; Chen, Y.-L.; Chiang, Y.-C.; Hsieh, C.-Y.; Chen, S.M.; Hsiao, P.-N.; Cheng, W.-F. Noncarrier Naked Antigen-Specific DNA Vaccine Generates Potent Antigen-Specific Immunologic Responses and Antitumor Effects. Gene Ther. 2009, 16, 776–787. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, Y.; Zhang, J.; Kuo, J.C.-T.; Zhang, Z.; Xie, H.; Zhu, J.; Liu, T. Modification of Lipid-Based Nanoparticles: An Efficient Delivery System for Nucleic Acid-Based Immunotherapy. Molecules 2022, 27, 1943. [Google Scholar] [CrossRef]
- Yang, J.P.; Huang, L. Direct Gene Transfer to Mouse Melanoma by Intratumor Injection of Free DNA. Gene Ther. 1996, 3, 542–548. [Google Scholar]
- Reimer, D.L.; Kong, S.; Monck, M.; Wyles, J.; Tam, P.; Wasan, E.K.; Bally, M.B. Liposomal Lipid and Plasmid DNA Delivery to B16/BL6 Tumors after Intraperitoneal Administration of Cationic Liposome DNA Aggregates. J. Pharmacol. Exp. Ther. 1999, 289, 807–815. [Google Scholar]
- Reddy, J.A.; Abburi, C.; Hofland, H.; Howard, S.J.; Vlahov, I.; Wils, P.; Leamon, C.P. Folate-Targeted, Cationic Liposome-Mediated Gene Transfer into Disseminated Peritoneal Tumors. Gene Ther. 2002, 9, 1542–1550. [Google Scholar] [CrossRef]
- Ito, I.; Began, G.; Mohiuddin, I.; Saeki, T.; Saito, Y.; Branch, C.D.; Vaporciyan, A.; Stephens, L.C.; Yen, N.; Roth, J.A.; et al. Increased Uptake of Liposomal–Dna Complexes by Lung Metastases Following Intravenous Administration. Mol. Ther. 2003, 7, 409–418. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, L.; Zhu, W.; Guo, R.; Sun, H.; Chen, X.; Deng, N. Barriers and Strategies of Cationic Liposomes for Cancer Gene Therapy. Mol. Ther. Methods Clin. Dev. 2020, 18, 751–764. [Google Scholar] [CrossRef]
- Cong, X.; Tian, H.; Liu, S.; Mao, K.; Chen, H.; Xin, Y.; Liu, F.; Wang, X.; Meng, X.; Zhu, G.; et al. Cationic Liposome/DNA Complexes Mediate Antitumor Immunotherapy by Promoting Immunogenic Tumor Cell Death and Dendritic Cell Activation. ACS Appl. Mater. Interfaces 2020, 12, 28047–28056. [Google Scholar] [CrossRef] [PubMed]
- Kodama, Y.; Nakashima, M.; Nagahara, T.; Oyama, N.; Hashizume, J.; Nakagawa, H.; Harasawa, H.; Muro, T.; Kurosaki, T.; Yamashita, C.; et al. Development of a DNA Vaccine for Melanoma Metastasis by Inhalation Based on an Analysis of Transgene Expression Characteristics of Naked PDNA and a Ternary Complex in Mouse Lung Tissues. Pharmaceutics 2020, 12, 540. [Google Scholar] [CrossRef] [PubMed]
- Moku, G.; Vangala, S.; Gulla, S.K.; Yakati, V. In Vivo Targeting of DNA Vaccines to Dendritic Cells via the Mannose Receptor Induces Long-Lasting Immunity against Melanoma. ChemBioChem Eur. J. Chem. Biol. 2021, 22, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tiruthani, K.; Wang, M.; Zhou, X.; Qiu, N.; Xiong, Y.; Pecot, C.V.; Liu, R.; Huang, L. Tumor-Targeted Gene Therapy with Lipid Nanoparticles Inhibits Tumor-Associated Adipocytes and Remodels the Immunosuppressive Tumor Microenvironment in Triple-Negative Breast Cancer. Nanoscale Horiz. 2021, 6, 319–329. [Google Scholar] [CrossRef]
- Sun, B.; Zhao, X.; Wu, Y.; Cao, P.; Movahedi, F.; Liu, J.; Wang, J.; Xu, Z.P.; Gu, W. Mannose-Functionalized Biodegradable Nanoparticles Efficiently Deliver DNA Vaccine and Promote Anti-Tumor Immunity. ACS Appl. Mater. Interfaces 2021, 13, 14015–14027. [Google Scholar] [CrossRef]
- McCarthy, H.O.; McCaffrey, J.; McCrudden, C.M.; Zholobenko, A.; Ali, A.A.; McBride, J.W.; Massey, A.S.; Pentlavalli, S.; Chen, K.-H.; Cole, G.; et al. Development and Characterization of Self-Assembling Nanoparticles Using a Bio-Inspired Amphipathic Peptide for Gene Delivery. J. Control. Release 2014, 189, 141–149. [Google Scholar] [CrossRef]
- Cole, G.; Ali, A.A.; McErlean, E.; Mulholland, E.J.; Short, A.; McCrudden, C.M.; McCaffrey, J.; Robson, T.; Kett, V.L.; Coulter, J.A.; et al. DNA Vaccination via RALA Nanoparticles in a Microneedle Delivery System Induces a Potent Immune Response against the Endogenous Prostate Cancer Stem Cell Antigen. Acta Biomater. 2019, 96, 480–490. [Google Scholar] [CrossRef]
- Hu, Q.; Wu, M.; Fang, C.; Cheng, C.; Zhao, M.; Fang, W.; Chu, P.K.; Ping, Y.; Tang, G. Engineering Nanoparticle-Coated Bacteria as Oral DNA Vaccines for Cancer Immunotherapy. Nano Lett. 2015, 15, 2732–2739. [Google Scholar] [CrossRef]
- Ledwith, B.J.; Manam, S.; Troilo, P.J.; Barnum, A.B.; Pauley, C.J.; Griffiths, T.G.; Harper, L.B.; Beare, C.M.; Bagdon, W.J.; Nichols, W.W. Plasmid DNA Vaccines: Investigation of Integration into Host Cellular DNA Following Intramuscular Injection in Mice. Intervirology 2000, 43, 258–272. [Google Scholar] [CrossRef]
- Klinman, D.M.; Klaschik, S.; Tross, D.; Shirota, H.; Steinhagen, F. FDA Guidance on Prophylactic DNA Vaccines: Analysis and Recommendations. Vaccine 2010, 28, 2801–2805. [Google Scholar] [CrossRef] [Green Version]
- Sung, Y.K.; Kim, S.W. Recent Advances in the Development of Gene Delivery Systems. Biomater. Res. 2019, 23, 8. [Google Scholar] [CrossRef]
- Bhatia, S.; Longino, N.V.; Miller, N.J.; Kulikauskas, R.; Iyer, J.G.; Ibrani, D.; Blom, A.; Byrd, D.R.; Parvathaneni, U.; Twitty, C.G.; et al. Intratumoral Delivery of Plasmid IL12 Via Electroporation Leads to Regression of Injected and Noninjected Tumors in Merkel Cell Carcinoma. Clin. Cancer Res. 2020, 26, 598–607. [Google Scholar] [CrossRef]
- Wang, Z.; Troilo, P.J.; Wang, X.; Griffiths, T.G.; Pacchione, S.J.; Barnum, A.B.; Harper, L.B.; Pauley, C.J.; Niu, Z.; Denisova, L.; et al. Detection of Integration of Plasmid DNA into Host Genomic DNA Following Intramuscular Injection and Electroporation. Gene Ther. 2004, 11, 711–721. [Google Scholar] [CrossRef]
- Marshall, H.T.; Djamgoz, M.B.A. Immuno-Oncology: Emerging Targets and Combination Therapies. Front. Oncol. 2018, 8, 315. [Google Scholar] [CrossRef]
- Cecil, D.L.; Liao, J.B.; Dang, Y.; Coveler, A.L.; Kask, A.; Yang, Y.; Childs, J.S.; Higgins, D.M.; Disis, M.L. Immunization with a Plasmid DNA Vaccine Encoding the N-Terminus of Insulin-like Growth Factor Binding Protein-2 in Advanced Ovarian Cancer Leads to High-Level Type I Immune Responses. Clin. Cancer Res. 2021, 27, 6405–6412. [Google Scholar] [CrossRef]
- McNeel, D.G.; Eickhoff, J.C.; Johnson, L.E.; Roth, A.R.; Perk, T.G.; Fong, L.; Antonarakis, E.S.; Wargowski, E.; Jeraj, R.; Liu, G. Phase II Trial of a DNA Vaccine Encoding Prostatic Acid Phosphatase (PTVG-HP [MVI-816]) in Patients with Progressive, Nonmetastatic, Castration-Sensitive Prostate Cancer. J. Clin. Oncol. 2019, 37, 3507–3517. [Google Scholar] [CrossRef]
- Wargowski, E.; Johnson, L.E.; Eickhoff, J.C.; Delmastro, L.; Staab, M.J.; Liu, G.; McNeel, D.G. Prime-Boost Vaccination Targeting Prostatic Acid Phosphatase (PAP) in Patients with Metastatic Castration-Resistant Prostate Cancer (MCRPC) Using Sipuleucel-T and a DNA Vaccine. J. Immunother. Cancer 2018, 6, 21. [Google Scholar] [CrossRef]
- University of Wisconsin, Madison. Phase II Trial of pTVG-HP DNA Vaccine with or Without pTVG-AR DNA Vaccine and Pembrolizumab in Patients with Castration-Resistant, Metastatic Prostate Cancer. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04090528 (accessed on 4 August 2022).
- Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins. A Phase I Clinical Trial Assessing the Safety and Feasibility of Administration of PNGVL4a-CRT/E7(Detox) DNA Vaccine Using the Intramuscular TriGridTM Delivery System in Combination with Cyclophosphamide in HPV-16 Associated Head and Neck Cancer Patients. 2018. Available online: https://www.clinicalconnection.com/clinical-trials-from-other-databases/study-details-from-other-databases/317605/44718348/safety-study-of-hpv-dna-vaccine-to-treat-head-and-neck-cancer-patients (accessed on 4 August 2022).
- Shore, N.D.; Morrow, M.P.; McMullan, T.; Kraynyak, K.A.; Sylvester, A.; Bhatt, K.; Cheung, J.; Boyer, J.D.; Liu, L.; Sacchetta, B.; et al. CD8+ T Cells Impact Rising PSA in Biochemically Relapsed Cancer Patients Using Immunotherapy Targeting Tumor-Associated Antigens. Mol. Ther. 2020, 28, 1238–1250. [Google Scholar] [CrossRef]
- Washington University School of Medicine. A Phase 1B Clinical Trial to Evaluate the Safety and Immune Response to a Mammaglobin-A DNA Vaccine in ER+, HER2- Breast Cancer Patients Undergoing Neoadjuvant Endocrine Therapy or Chemotherapy. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT02204098 (accessed on 4 August 2022).
- Hillemanns, P.; Petry, K.U.; Böhmer, G.; Jentschke, M.; Wölber, L.; Skjørestad, I.; Frederiksen, A.; Axelsen, M. P22 An Exploratory Safety and Immunogenicity Study of Human Papillomavirus (HPV16+) Immunotherapy VB10.16 in Women with High Grade Cervical Intraepithelial Neoplasia (HSIL; CIN 2/3). Int. J. Gynecol. Cancer 2019, 29, A64–A65. [Google Scholar] [CrossRef]
- Inovio Pharmaceuticals. Phase I/IIA, Open-Label, Safety, Tolerability, and Immunogenicity Study of INO-3112 Delivered by Electroporation (EP) in Women with Cervical Cancer After Chemoradiation for Newly Diagnosed Disease or Therapy for Recurrent and/or Persistent Disease. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT02172911 (accessed on 4 August 2022).
- Aggarwal, C.; Cohen, R.B.; Morrow, M.P.; Kraynyak, K.A.; Sylvester, A.J.; Knoblock, D.M.; Bauml, J.M.; Weinstein, G.S.; Lin, A.; Boyer, J.; et al. Immunotherapy Targeting HPV16/18 Generates Potent Immune Responses in HPV-Associated Head and Neck Cancer. Clin. Cancer Res. 2019, 25, 110–124. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.J.; Hur, S.Y.; Kim, T.-J.; Hong, S.R.; Lee, J.K.; Cho, C.-H.; Park, K.S.; Woo, J.W.; Sung, Y.C.; Suh, Y.S.; et al. A Phase II, Prospective, Randomized, Multicenter, Open-Label Study of GX-188E, an HPV DNA Vaccine, in Patients with Cervical Intraepithelial Neoplasia 3. Clin. Cancer Res. 2020, 26, 1616–1623. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.W.; Hur, S.-Y.; Woo, J.W.; Kim, Y.-M.; Lim, M.C.; Park, S.Y.; Seo, S.S.; No, J.H.; Kim, B.-G.; Lee, J.-K.; et al. Pembrolizumab plus GX-188E Therapeutic DNA Vaccine in Patients with HPV-16-Positive or HPV-18-Positive Advanced Cervical Cancer: Interim Results of a Single-Arm, Phase 2 Trial. Lancet Oncol. 2020, 21, 1653–1660. [Google Scholar] [CrossRef]
- Nykode Therapeutics ASA. A Multi-Centre, Open-Label Phase 2a Trial of the Combination of VB10.16 and Atezolizumab in Patients with Advanced or Recurrent, Non-Resectable HPV 16-Positive Cervical Cancer. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04405349 (accessed on 7 August 2022).
- Duan, L.-J.; Wang, Q.; Zhang, C.; Yang, D.-X.; Zhang, X.-Y. Potentialities and Challenges of MRNA Vaccine in Cancer Immunotherapy. Front. Immunol. 2022, 13, 923647. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, J.; Xu, Q. Current Developments and Challenges of MRNA Vaccines. Annu. Rev. Biomed. Eng. 2022, 24, 85–109. [Google Scholar] [CrossRef]
- Liu, M.A. A Comparison of Plasmid DNA and MRNA as Vaccine Technologies. Vaccines 2019, 7, 37. [Google Scholar] [CrossRef]
- Ahn, J.Y.; Lee, J.; Suh, Y.S.; Song, Y.G.; Choi, Y.-J.; Lee, K.H.; Seo, S.H.; Song, M.; Oh, J.-W.; Kim, M.; et al. Safety and Immunogenicity of Two Recombinant DNA COVID-19 Vaccines Containing the Coding Regions of the Spike or Spike and Nucleocapsid Proteins: An Interim Analysis of Two Open-Label, Non-Randomised, Phase 1 Trials in Healthy Adults. Lancet Microbe 2022, 3, e173–e183. [Google Scholar] [CrossRef]
- Kraynyak, K.A.; Blackwood, E.; Agnes, J.; Tebas, P.; Giffear, M.; Amante, D.; Reuschel, E.L.; Purwar, M.; Christensen-Quick, A.; Liu, N.; et al. SARS-CoV-2 DNA Vaccine INO-4800 Induces Durable Immune Responses Capable of Being Boosted in a Phase 1 Open-Label Trial. J. Infect. Dis. 2022, 225, 1923–1932. [Google Scholar] [CrossRef]
- Ravi, A.D.; Sadhna, D.; Nagpaal, D.; Chawla, L. Needle Free Injection Technology: A Complete Insight. Int. J. Pharm. Investig. 2015, 5, 192–199. [Google Scholar] [CrossRef]
- Schoppink, J.; Fernandez Rivas, D. Jet Injectors: Perspectives for Small Volume Delivery with Lasers. Adv. Drug Deliv. Rev. 2022, 182, 114109. [Google Scholar] [CrossRef]
- Lukjanov, V.; Koutná, I.; Šimara, P. CAR T-Cell Production Using Nonviral Approaches. J. Immunol. Res. 2021, 2021, 6644685. [Google Scholar] [CrossRef]
- Al Saber, M.; Biswas, P.; Dey, D.; Kaium, M.A.; Islam, M.A.; Tripty, M.I.A.; Rahman, M.H.; Rahaman, T.I.; Biswas, M.Y.; Paul, P.; et al. A Comprehensive Review of Recent Advancements in Cancer Immunotherapy and Generation of CAR T Cell by CRISPR-Cas9. Processes 2022, 10, 16. [Google Scholar] [CrossRef]
- Fritah, H.; Rovelli, R.; Chiang, C.L.-L.; Kandalaft, L.E. The Current Clinical Landscape of Personalized Cancer Vaccines. Cancer Treat. Rev. 2022, 106, 102383. [Google Scholar] [CrossRef]
- Hillemanns, P.; Petry, K.U.; Woelber, L.; Böhmer, G.; Stubsrud, E.; Skjørestad, I.; Schjetne, K.; Fredriksen, A.; Axelsen, M. Abstract CT209: Safety, Efficacy and Immunogenicity of VB10.16, a Therapeutic DNA Vaccine Targeting Human Papillomavirus (HPV) 16 E6 and E7 Proteins for High Grade Cervical Intraepithelial Neoplasia (CIN 2/3): 6-Month Data from an Exploratory Open-Label Phase I/2a Trial. Cancer Res. 2019, 79, CT209. [Google Scholar] [CrossRef]
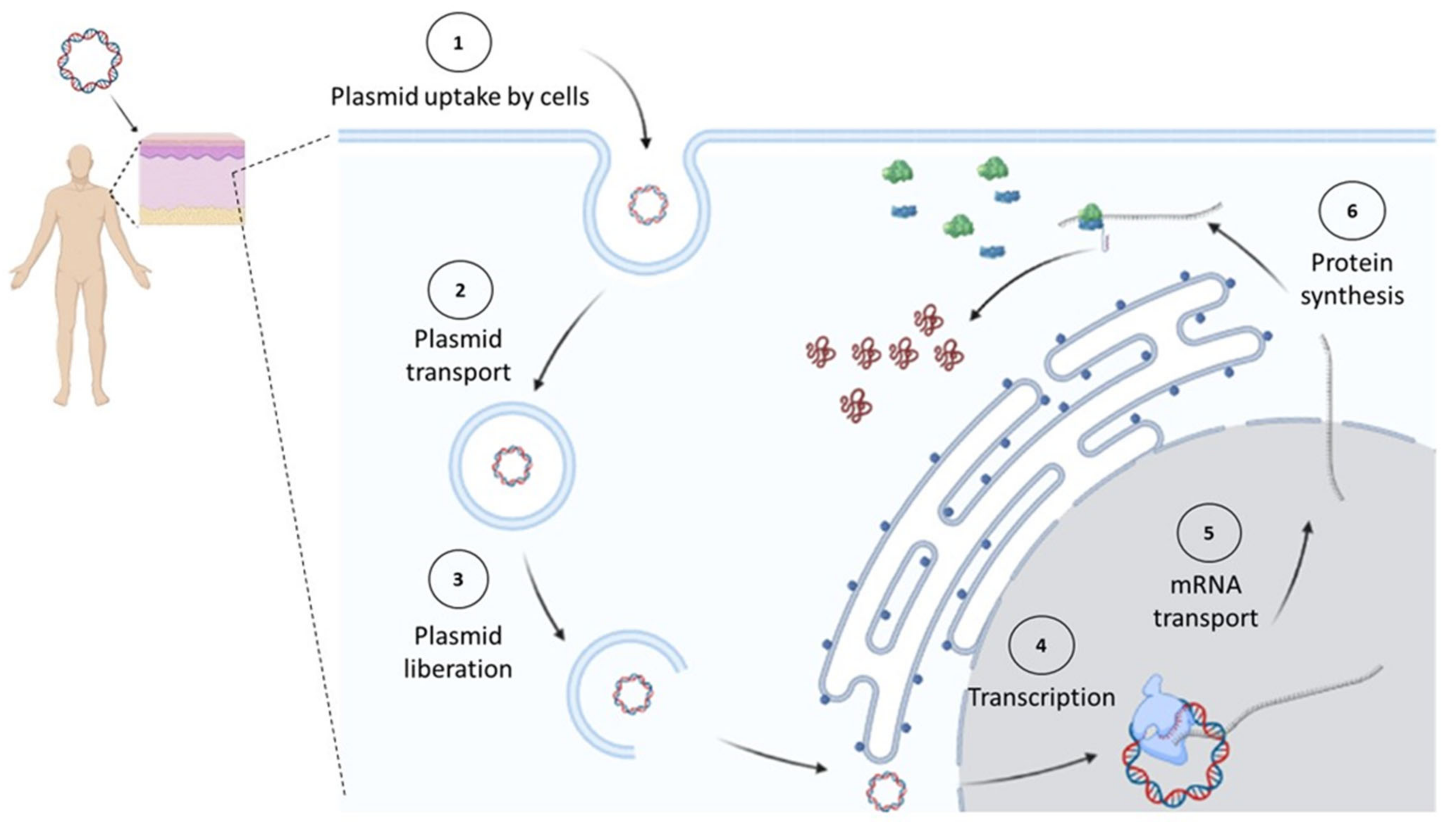
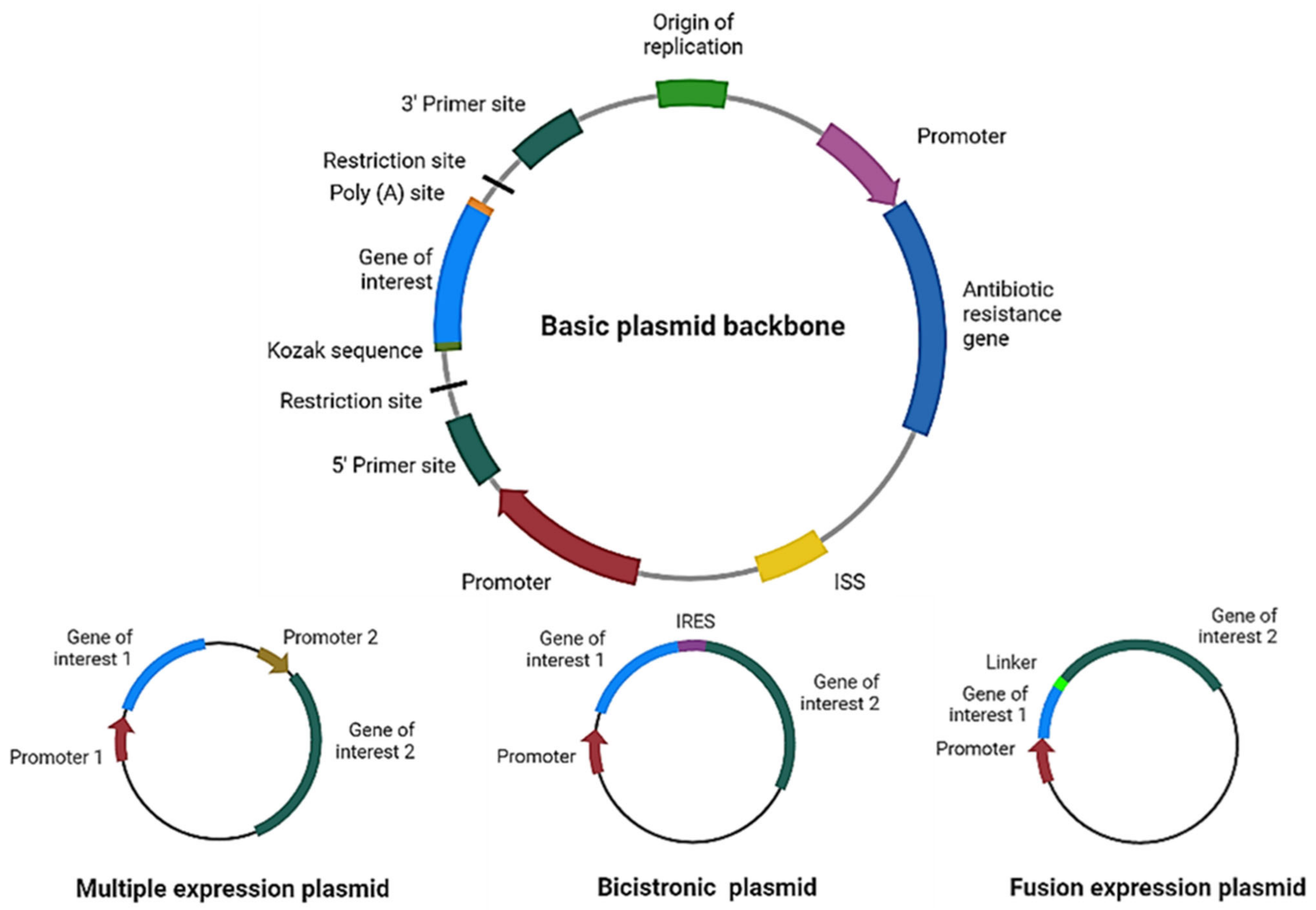


| Categories | Type of Antigen | Examples | References |
|---|---|---|---|
| Tumor-specific antigens | Viral antigens | L1, E6, and E7 from human papillomavirus (HPV) | [59,60] |
| HBsAg from hepatitis B virus (HBV) | [61,62] | ||
| Epstein–Barr nuclear antigens (EBNAs) | [63,64] | ||
| Private neoantigens | Differs from each patient | [65] | |
| Public neoantigens | TP53 | [66] | |
| KRAS | [67] | ||
| PIK3CA | [68] | ||
| Histone H3.3 | [69] | ||
| Tumor-associated antigens | Overexpressed proteins | Receptor tyrosine-protein kinase erbB-2 | [70,71] |
| Epidermal growth factor receptor (EGFR) | [72] | ||
| Mucin 1, cell surface associated (MUC1) | [73] | ||
| Tumor protein D52 (TPD52) | [74] | ||
| Mammaglobin A (Mam-A) | [75,76] | ||
| Insulin-like growth factor (IGF) binding protein 2 (IGFBP-2) | [77] | ||
| Differentiation antigens | Prostate-specific membrane antigen (PSMA) | [78,79] | |
| Prostatic acid phosphatase (PAP) | [80,81] | ||
| Prostatic specific antigen (PSA) | [78,82] | ||
| Carcinoembryonic antigen (CEA) | [83] | ||
| Tyrosinase | [84] | ||
| Glycoprotein 100 (gp100) | [85] | ||
| Dickkopf-1 (DKK1) | [86] | ||
| Cancer testis antigens | MAGE-A | [87,88] | |
| SSX-2 | [89,90] | ||
| NY-ESO-1 | [91,92] |
| DNA | RNA | |
|---|---|---|
| Advantages | Non-infective platforms | Non-infective platforms |
| Easy to design and edit | Easy to design and edit | |
| Economic synthesis | Economic synthesis | |
| Induce specific immune responses | Induce specific immune responses | |
| High stability | Non-genetic integration | |
| Disadvantages | Poor immunogenic | Poor immunogenic |
| Low transfection efficiency | Low transfection efficiency | |
| Unknown side effects | Unwanted inflammatory responses | |
| May require a special administration device | Requires low temperatures for storage | |
| Potential integration into the human genome | Low stability |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Puente, D.H.; Pérez-Trujillo, J.J.; Zavala-Flores, L.M.; García-García, A.; Villanueva-Olivo, A.; Rodríguez-Rocha, H.; Valdés, J.; Saucedo-Cárdenas, O.; Montes de Oca-Luna, R.; Loera-Arias, M.d.J. Plasmid DNA for Therapeutic Applications in Cancer. Pharmaceutics 2022, 14, 1861. https://doi.org/10.3390/pharmaceutics14091861
Martínez-Puente DH, Pérez-Trujillo JJ, Zavala-Flores LM, García-García A, Villanueva-Olivo A, Rodríguez-Rocha H, Valdés J, Saucedo-Cárdenas O, Montes de Oca-Luna R, Loera-Arias MdJ. Plasmid DNA for Therapeutic Applications in Cancer. Pharmaceutics. 2022; 14(9):1861. https://doi.org/10.3390/pharmaceutics14091861
Chicago/Turabian StyleMartínez-Puente, David Hernán, José Juan Pérez-Trujillo, Laura Mireya Zavala-Flores, Aracely García-García, Arnulfo Villanueva-Olivo, Humberto Rodríguez-Rocha, Jesús Valdés, Odila Saucedo-Cárdenas, Roberto Montes de Oca-Luna, and María de Jesús Loera-Arias. 2022. "Plasmid DNA for Therapeutic Applications in Cancer" Pharmaceutics 14, no. 9: 1861. https://doi.org/10.3390/pharmaceutics14091861
APA StyleMartínez-Puente, D. H., Pérez-Trujillo, J. J., Zavala-Flores, L. M., García-García, A., Villanueva-Olivo, A., Rodríguez-Rocha, H., Valdés, J., Saucedo-Cárdenas, O., Montes de Oca-Luna, R., & Loera-Arias, M. d. J. (2022). Plasmid DNA for Therapeutic Applications in Cancer. Pharmaceutics, 14(9), 1861. https://doi.org/10.3390/pharmaceutics14091861







