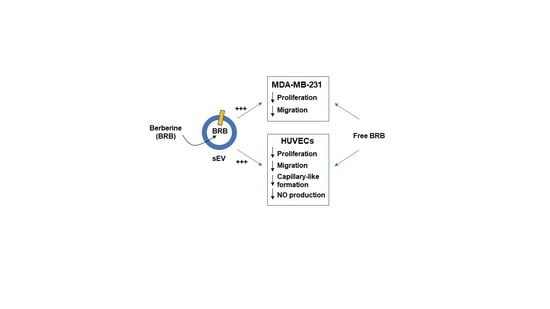
Enhancement of the In Vitro Antitumor Effects of Berberine Chloride When Encapsulated within Small Extracellular Vesicles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. sEV Isolation
2.3. Berberine Chloride (BRB) Loading and Entrapment Efficiency
2.4. Nanoparticle Tracking Analysis (NTA)
2.5. Dynamic Light Scattering
2.6. Western Blotting Analysis
2.7. Proliferation Assay
2.8. Cell Cycle Analysis
2.9. Wound Healing Assay
2.10. In Vitro Capillary Network Formation
2.11. Nitric Oxide (NO) Production Assay
2.12. Statistical Analysis
3. Results
3.1. sEV Characterization, BRB Loading, and Entrapment Efficiency
3.2. Effects of Free BRB and sEV-Loaded BRB on Cell Proliferation
3.3. Effects of Free BRB and sEV-Loaded BRB on Cell Cycle Progression
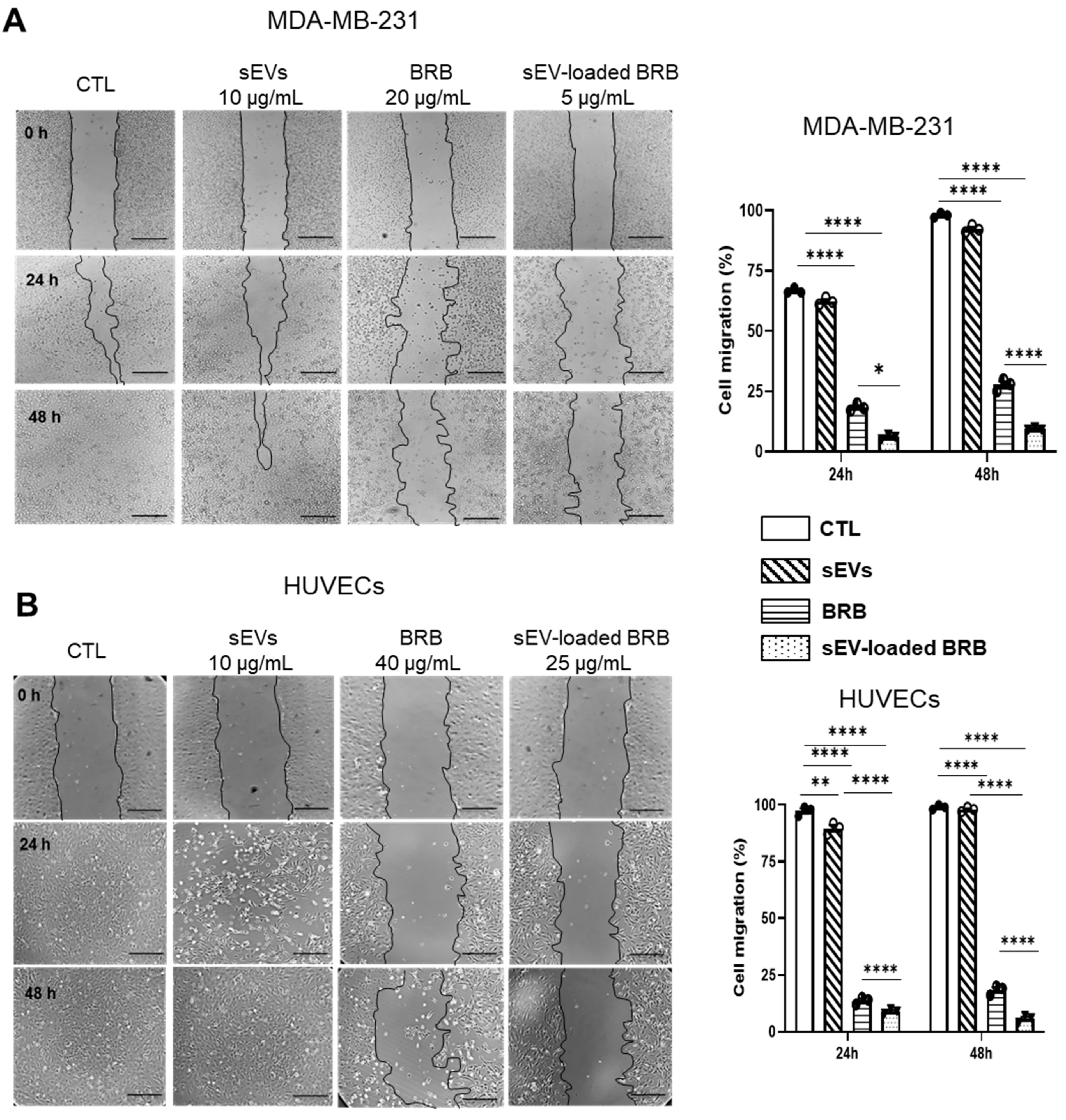
3.4. Effects of Free BRB and sEV-Loaded BRB on Cell Migration
3.5. Effects of Free BRB and sEV-Loaded BRB on Angiogenesis
3.6. Effects of Free BRB and sEV-Loaded BRB on NO Production
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BRB | Berberine hydrochloride |
| %EE | entrapment efficiency |
| FBS | fetal bovine serum |
| HUVECs | human umbilical vein endothelial cells |
| NO | nitric oxide |
| NTA | nanoparticle tracking analysis |
| PMA | Phorbol-12-myristate-13-acetate |
| sEVs | small extracellular vesicles |
| TNBC | triple-negative breast cancer |
References
- DeSantis, C.E.; Ma, J.; Gaudet, M.M.; Newman, L.A.; Miller, K.D.; Goding Sauer, A.; Jemal, A.; Siegel, R.L. Breast cancer statistics. CA Cancer J. Clin. 2019, 69, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef] [PubMed]
- Neag, M.A.; Mocan, A.; Echeverría, J.; Pop, R.M.; Bocsan, C.I.; Crişan, G.; Buzoianu, A.D. Berberine: Botanical occurrence, traditional uses, extraction methods, and relevance in cardiovascular, metabolic, hepatic, and renal disorders. Front. Pharmacol. 2018, 9, 557. [Google Scholar] [CrossRef]
- Kong, W.J.; Xing, X.Y.; Xiao, X.H.; Zhao, Y.L.; Wei, J.H.; Wang, J.B.; Yang, R.C.; Yang, M.H. Effect of berberine on Escherichia coli, Bacillus subtilis, and their mixtures as determined by isothermal microcalorimetry. Appl. Microbiol. Biotechnol. 2012, 96, 503–510. [Google Scholar] [CrossRef]
- Chang, W.; Chen, L.; Hatch, G.M. Berberine as a therapy for type 2 diabetes and its complications: From mechanism of action to clinical studies. Biochem. Cell Biol. 2015, 93, 479–486. [Google Scholar] [CrossRef]
- Fan, X.; Wang, J.; Hou, J.; Lin, C.; Bensoussan, A.; Chang, D.; Liu, J.; Wang, B. Berberine alleviates ox-LDL induced inflammatory factors by up-regulation of autophagy via AMPK/mTOR signaling pathway. J. Transl. Med. 2015, 13, 92. [Google Scholar] [CrossRef]
- Tan, W.; Li, Y.; Chen, M.; Wang, Y. Berberine hydrochloride: Anticancer activity and nanoparticulate delivery system. Int. J. Nanomed. 2011, 6, 1773–1777. [Google Scholar] [CrossRef]
- Kaboli, P.J.; Rahmat, A.; Ismail, P.; Lin, K.H. Targets and mechanisms of berberine, a natural drug with potential to treat cancer with special focus on breast cancer. Eur. J. Pharmacol. 2014, 740, 584–595. [Google Scholar] [CrossRef]
- Seigneuric, R.; Markey, L.; Nuyten, D.S.A.; Dubernet, C.; Evelo, C.T.A.; Finot, E.; Garrido, C. From nanotechnology to nanomedicine: Applications to cancer research. Curr. Mol. Med. 2010, 10, 640–652. [Google Scholar] [CrossRef]
- Soleti, R.; Andriantsitohaina, R.; Martinez, M.C. Impact of polyphenols on extracellular vesicle levels and effects and their properties as tools for drug delivery for nutrition and health. Arch. Biochem. Biophys. 2018, 644, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Taverna, S.; Fontana, S.; Monteleone, F.; Pucci, M.; Saieva, L.; De Caro, V.; Cardinale, V.G.; Giallombardo, M.; Vicario, E.; Rolfo, C.; et al. Curcumin modulates chronic myelogenous leukemia exosomes composition and affects angiogenic phenotype via exosomal miR-21. Oncotarget 2016, 7, 30420–30439. [Google Scholar] [CrossRef] [PubMed]
- Barkallah, M.; Nzoughet-Kouassi, J.; Simard, G.; Thoulouze, L.; Marze, S.; Ropers, M.H.; Andriantsitohaina, R. Enhancement of the anti-angiogenic effects of delphinidin when encapsulated within small extracellular vesicles. Nutrients 2021, 13, 4378. [Google Scholar] [CrossRef]
- Xue, M.; Zhang, L.; Yang, M.X.; Zhang, W.; Li, X.M.; Ou, Z.M.; Li, Z.P.; Liu, S.H.; Li, X.J.; Yang, S.Y. Berberine-loaded solid lipid nanoparticles are concentrated in the liver and ameliorate hepatosteatosis in db/db mice. Int. J. Nanomed. 2015, 10, 5049–5057. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Ouyang, W. Preparation and physicochemical characteristics of berberine hydrochloric nanoemulsion. Chin. Tradit. Herbal. Drugs 2007, 38, 1476–1480. [Google Scholar]
- Chen, J.; Tan, L.; Li, W.; Guoqiang, L. Study on the preparation process of berberine hydrochloride liposomes by orthogonal design. J. Pract. Med. Tech. 2007, 14, 1868–1870. [Google Scholar]
- Quah, B.J.C.; O’Neill, H.C. The immunogenicity of dendritic cell-derived exosomes. Blood Cells Mol. Dis. 2005, 35, 94–110. [Google Scholar] [CrossRef]
- Aqil, F.; Munagala, R.; Jeyaprakash, J.; Agrawal, A.K.; Gupta, R. Exosomes for the enhanced tissue bioavailability and efficacy of curcumin. AAPS J. 2017, 19, 1691–1702. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine 2016, 12, 655–664. [Google Scholar] [CrossRef]
- Milbank, E.; Dragano, N.R.V.; González-García, I.; Garcia, M.R.; Rivas-Limeres, V.; Perdomo, L.; Hilairet, G.; Ruiz-Pino, F.; Mallegol, P.; Morgan, D.A.; et al. Small extracellular vesicle-mediated targeting of hypothalamic AMPKα1 corrects obesity through BAT activation. Nat. Metab. 2021, 3, 1415–1431. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Tan, H.Y.; Li, L.; Yuen, M.F.; Feng, Y. Berberine and coptidis Rhizoma as potential anticancer agents: Recent updates and future perspectives. J. Ethnopharmacol. 2015, 176, 35–48. [Google Scholar] [CrossRef]
- Zhao, Y.; Jing, Z.; Lv, J.; Zhang, Z.; Lin, J.; Cao, X.; Zhao, Z.; Liu, P.; Mao, W. Berberine activates caspase-9/cytochrome c -mediated apoptosis to suppress triple-negative breast cancer cells in vitro and in vivo. Biomed. Pharmacother. 2017, 95, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Favot, L.; Martin, S.; Keravis, T.; Andriantsitohaina, R.; Lugnier, C. Involvement of cyclin-dependent pathway in the inhibitory effect of delphinidin on angiogenesis. Cardiovasc. Res. 2003, 59, 479–487. [Google Scholar] [CrossRef]
- Cianchi, F.; Cuzzocrea, S.; Vinci, M.C.; Messerini, L.; Comin, C.E.; Navarra, G.; Perigli, G.; Centorrino, T.; Marzocco, S.; Lenzi, E.; et al. Heterogeneous expression of cyclooxygenase-2 and inducible nitric oxide synthase within colorectal tumors: Correlation with tumor angiogenesis. Dig. Liver Dis. 2010, 42, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Fukumura, D.; Jain, R.K. Role of nitric oxide in angiogenesis and microcirculation in tumors. Cancer Metastasis Rev. 1998, 17, 77–89. [Google Scholar] [CrossRef]
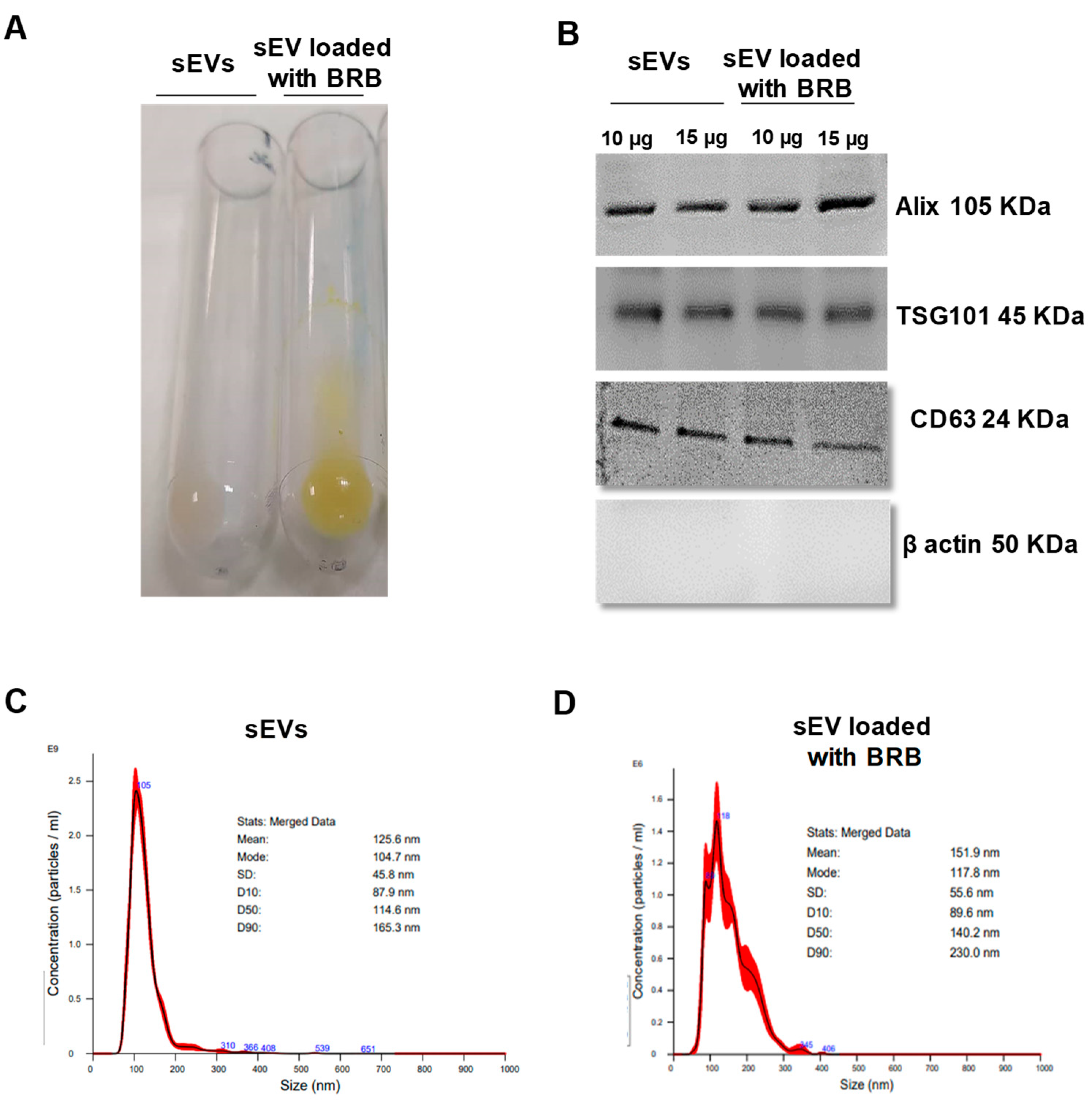


| Entrapment Efficiency (%) | BRB Loading (µg of BRB/µg sEV) | Size (nm) | PDI | Zeta Potential (mV) | |
|---|---|---|---|---|---|
| sEVs | 101.13 ± 16.02 | 0.246 ± 0.01 | −11.3 ± 8.4 | ||
| sEV loaded with BRB | 41.6 ± 1.8 | 3.57 ± 0.05 | 109.6 ± 11.5 | 0.327 ± 0.03 | −5.4 ± 13 **** |
| MDA-MB-231 | HUVECs | |||
|---|---|---|---|---|
| BRB | sEV-Loaded BRB | BRB | sEV-Loaded BRB | |
| 24 h | 20 ± 0.12 | 5 ± 0.06 **** | 40 ± 1.04 | 25 ± 0.76 **** |
| 48 h | 10 ± 0.15 | 2.5 ± 1.3 **** | 25 ± 0.9 | 15 ± 0.3 **** |
| 72 h | 5 ± 0.21 | 1.25 ± 0.01 **** | 15 ± 0.13 | 12 ± 0.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salek, A.; Selmi, M.; Barboura, M.; Martinez, M.C.; Chekir-Ghedira, L.; Andriantsitohaina, R. Enhancement of the In Vitro Antitumor Effects of Berberine Chloride When Encapsulated within Small Extracellular Vesicles. Pharmaceutics 2022, 14, 1913. https://doi.org/10.3390/pharmaceutics14091913
Salek A, Selmi M, Barboura M, Martinez MC, Chekir-Ghedira L, Andriantsitohaina R. Enhancement of the In Vitro Antitumor Effects of Berberine Chloride When Encapsulated within Small Extracellular Vesicles. Pharmaceutics. 2022; 14(9):1913. https://doi.org/10.3390/pharmaceutics14091913
Chicago/Turabian StyleSalek, Abir, Mouna Selmi, Mahassen Barboura, M. Carmen Martinez, Leila Chekir-Ghedira, and Ramaroson Andriantsitohaina. 2022. "Enhancement of the In Vitro Antitumor Effects of Berberine Chloride When Encapsulated within Small Extracellular Vesicles" Pharmaceutics 14, no. 9: 1913. https://doi.org/10.3390/pharmaceutics14091913
APA StyleSalek, A., Selmi, M., Barboura, M., Martinez, M. C., Chekir-Ghedira, L., & Andriantsitohaina, R. (2022). Enhancement of the In Vitro Antitumor Effects of Berberine Chloride When Encapsulated within Small Extracellular Vesicles. Pharmaceutics, 14(9), 1913. https://doi.org/10.3390/pharmaceutics14091913