Ocular Drug Delivery: Advancements and Innovations
Abstract
:1. Introduction
2. Ocular Gene Therapy
2.1. AAV-Based Ocular Gene Therapy
2.1.1. Adeno-Associated Viruses (AAVs)
2.1.2. Age-Related Macular Degeneration (AMD)
2.1.3. Diabetic Retinopathy
2.1.4. Glaucoma
2.1.5. Corneal Diseases
2.2. Organic Nanoparticles (NPs)
2.2.1. Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carriers (NLCs)
2.2.2. Polymer Nanoparticles and Peptide Nanoparticles (PNPs)
2.2.3. Dendrimers
2.2.4. Nanoemulsions
2.2.5. Lipid-Based Nanoparticles
2.2.6. Nanomicelles
2.2.7. Liposomes
2.2.8. Niosomes
3. Carrier Technology for Ocular Therapeutics
3.1. Conventional Carrier Systems
3.1.1. Topical Ophthalmic Solutions
3.1.2. Cyclodextrins
3.1.3. Suspensions
3.2. Innovative Carrier Technology
3.2.1. Punctal Plugs
3.2.2. Drug-Eluting Contact Lenses
4. Advancements in Intraocular Administration Routes and Systemic Drug Delivery
4.1. Anterior Segment
4.1.1. Subconjunctival Administration
4.1.2. Intrastromal Administration
4.1.3. Intracameral Administration
4.2. Posterior Segment
4.2.1. Intravitreal Administration
4.2.2. Subretinal Administration
4.2.3. Suprachoroidal Administration
4.2.4. Systemic Delivery
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Barar, J.; Javadzadeh, A.R.; Omidi, Y. Ocular novel drug delivery: Impacts of membranes and barriers. Expert Opin. Drug Deliv. 2008, 5, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Bill, A. The blood-aqueous barrier. Trans. Ophthalmol. Soc. UK 1986, 105, 149–155. [Google Scholar] [PubMed]
- Runkle, E.A.; Antonetti, D.A. The blood-retinal barrier: Structure and functional significance. Blood-Brain Other Neural Barriers 2011, 2011, 133–148. [Google Scholar]
- Russell, S.; Bennett, J.; Wellman, J.A.; Chung, D.C.; Yu, Z.-F.; Tillman, A.; Wittes, J.; Pappas, J.; Elci, O.; McCague, S. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: A randomised, controlled, open-label, phase 3 trial. Lancet 2017, 390, 849–860. [Google Scholar] [CrossRef]
- Prado, D.A.; Acosta-Acero, M.; Maldonado, R.S. Gene therapy beyond luxturna: A new horizon of the treatment for inherited retinal disease. Curr. Opin. Ophthalmol. 2020, 31, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Musarella, M.A. Gene mapping of ocular diseases. Surv. Ophthalmol. 1992, 36, 285–312. [Google Scholar] [CrossRef]
- Cheng, K.J.; Hsieh, C.M.; Nepali, K.; Liou, J.P. Ocular disease therapeutics: Design and delivery of drugs for diseases of the eye. J. Med. Chem. 2020, 63, 10533–10593. [Google Scholar] [CrossRef]
- Mendell, J.R.; Al-Zaidy, S.A.; Rodino-Klapac, L.R.; Goodspeed, K.; Gray, S.J.; Kay, C.N.; Boye, S.L.; Boye, S.E.; George, L.A.; Salabarria, S. Current clinical applications of in vivo gene therapy with AAVs. Mol. Ther. 2021, 29, 464–488. [Google Scholar] [CrossRef]
- Dunbar, C.E.; High, K.A.; Joung, J.K.; Kohn, D.B.; Ozawa, K.; Sadelain, M. Gene therapy comes of age. Science 2018, 359, eaan4672. [Google Scholar] [CrossRef]
- Carter, B.J. Adeno-associated virus and the development of adeno-associated virus vectors: A historical perspective. Mol. Ther. 2004, 10, 981–989. [Google Scholar] [CrossRef]
- Naso, M.F.; Tomkowicz, B.; Perry, W.L.; Strohl, W.R. Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs 2017, 31, 317–334. [Google Scholar] [CrossRef] [Green Version]
- Philpott, N.J.; Giraud-Wali, C.; Dupuis, C.; Gomos, J.; Hamilton, H.; Berns, K.I.; Falck-Pedersen, E. Efficient integration of recombinant adeno-associated virus DNA vectors requires a p5-rep sequence in cis. J. Virol. 2002, 76, 5411–5421. [Google Scholar] [CrossRef]
- Acland, G.M.; Aguirre, G.D.; Ray, J.; Zhang, Q.; Aleman, T.S.; Cideciyan, A.V.; Pearce-Kelling, S.E.; Anand, V.; Zeng, Y.; Maguire, A.M. Gene therapy restores vision in a canine model of childhood blindness. Nat. Genet. 2001, 28, 92–95. [Google Scholar] [CrossRef]
- Khanani, A.M.; Thomas, M.J.; Aziz, A.A.; Weng, C.Y.; Danzig, C.J.; Yiu, G.; Kiss, S.; Waheed, N.K.; Kaiser, P.K. Review of gene therapies for age-related macular degeneration. Eye 2022, 36, 303–311. [Google Scholar] [CrossRef]
- Wang, J.-H.; Roberts, G.E.; Liu, G.-S. Updates on gene therapy for diabetic retinopathy. Curr. Diabetes Rep. 2020, 20, 22. [Google Scholar] [CrossRef]
- Komáromy, A.M.; Koehl, K.L.; Park, S.A. Looking into the future: Gene and cell therapies for glaucoma. Vet. Ophthalmol. 2021, 24, 16–33. [Google Scholar] [CrossRef]
- Mehta, S. Age-Related Macular Degeneration. Prim. Care Clin. Off. Pract. 2015, 42, 377–391. [Google Scholar] [CrossRef]
- Khandhadia, S.; Cipriani, V.; Yates, J.; Lotery, A.J. Age-related macular degeneration and the complement system. Immunobiology 2012, 217, 127–146. [Google Scholar] [CrossRef]
- Anderson, D.H.; Radeke, M.J.; Gallo, N.B.; Chapin, E.A.; Johnson, P.T.; Curletti, C.R.; Hancox, L.S.; Hu, J.; Ebright, J.N.; Malek, G. The pivotal role of the complement system in aging and age-related macular degeneration: Hypothesis re-visited. Prog. Retin. Eye Res. 2010, 29, 95–112. [Google Scholar] [CrossRef]
- Ferrara, N.; Mass, R.D.; Campa, C.; Kim, R. Targeting VEGF-A to treat cancer and age-related macular degeneration. Annu. Rev. Med. 2007, 58, 491–504. [Google Scholar] [CrossRef]
- Campa, C.; P Harding, S. Anti-VEGF compounds in the treatment of neovascular age related macular degeneration. Curr. Drug Targets 2011, 12, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Senra, H.; Ali, Z.; Balaskas, K.; Aslam, T. Psychological impact of anti-VEGF treatments for wet macular degeneration—A review. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 254, 1873–1880. [Google Scholar] [CrossRef] [PubMed]
- Kansara, V.; Muya, L.; Wan, C.-r.; Ciulla, T.A. Suprachoroidal delivery of viral and nonviral gene therapy for retinal diseases. J. Ocul. Pharmacol. Ther. 2020, 36, 384–392. [Google Scholar] [CrossRef]
- Li, J.Q.; Welchowski, T.; Schmid, M.; Letow, J.; Wolpers, C.; Pascual-Camps, I.; Holz, F.G.; Finger, R.P. Prevalence, incidence and future projection of diabetic eye disease in Europe: A systematic review and meta-analysis. Eur. J. Epidemiol. 2020, 35, 11–23. [Google Scholar] [CrossRef]
- Stewart, M.W. Pathophysiology of diabetic retinopathy. Diabet. Retin. 2010, 2010, 1–30. [Google Scholar]
- Mantravadi, A.V.; Vadhar, N. Glaucoma. Prim. Care Clin. Off. Pract. 2015, 42, 437–449. [Google Scholar] [CrossRef]
- Davanger, M.; Ringvold, A.; Blika, S. The probability of having glaucoma at different IOP levels. Acta Ophthalmol. 1991, 69, 565–568. [Google Scholar] [CrossRef]
- Tham, Y.-C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.-Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Lusthaus, J.; Goldberg, I. Current management of glaucoma. Med. J. Aust. 2019, 210, 180–187. [Google Scholar] [CrossRef]
- Kholdebarin, R.; Campbell, R.J.; Jin, Y.-P.; Buys, Y.M.; Group, C.C.S. Multicenter study of compliance and drop administration in glaucoma. Can. J. Ophthalmol. 2008, 43, 454–461. [Google Scholar] [CrossRef]
- Llobet, A.; Gasull, X.; Gual, A. Understanding trabecular meshwork physiology: A key to the control of intraocular pressure? Physiology 2003, 18, 205–209. [Google Scholar] [CrossRef]
- Borrás, T.; Xue, W.; Choi, V.W.; Bartlett, J.S.; Li, G.; Samulski, R.J.; Chisolm, S.S. Mechanisms of AAV transduction in glaucoma-associated human trabecular meshwork cells. J. Gene Med. A Cross-Discip. J. Res. Sci. Gene Transf. Its Clin. Appl. 2006, 8, 589–602. [Google Scholar] [CrossRef]
- Buie, L.K.; Rasmussen, C.A.; Porterfield, E.C.; Ramgolam, V.S.; Choi, V.W.; Markovic-Plese, S.; Samulski, R.J.; Kaufman, P.L.; Borrás, T. Self-complementary AAV virus (scAAV) safe and long-term gene transfer in the trabecular meshwork of living rats and monkeys. Investig. Ophthalmol. Vis. Sci. 2010, 51, 236–248. [Google Scholar] [CrossRef]
- Rodriguez-Estevez, L.; Asokan, P.; Borrás, T. Transduction optimization of AAV vectors for human gene therapy of glaucoma and their reversed cell entry characteristics. Gene Ther. 2020, 27, 127–142. [Google Scholar] [CrossRef]
- Shen, J.; Wang, Y.; Yao, K. Protection of retinal ganglion cells in glaucoma: Current status and future. Exp. Eye Res. 2021, 205, 108506. [Google Scholar] [CrossRef]
- Abbasi, M.; Gupta, V.K.; Chitranshi, N.; Gupta, V.; Ranjbaran, R.; Rajput, R.; Pushpitha, K.; Devaraj, K.; You, Y.; Salekdeh, G.H. Inner retinal injury in experimental glaucoma is prevented upon AAV mediated Shp2 silencing in a caveolin dependent manner. Theranostics 2021, 11, 6154. [Google Scholar] [CrossRef]
- Liu, D.; Deng, Q.; Lei, X.; Lu, W.; Zhao, Q.; Shen, Y. Overexpression of BMP4 protects retinal ganglion cells in a mouse model of experimental glaucoma. Exp. Eye Res. 2021, 210, 108728. [Google Scholar] [CrossRef]
- Komaromy, A.M.; Harman, C.; Koehl, K.; Anderson, A.; Boye, S.L.; Steibel, J.P.; Teixeira, L.; Toris, C.B.; Moroi, S.E.; Boye, S.E. Four-year follow up of intraocular pressure (IOP) control in a canine model of open-angle glaucoma (OAG) treated with adeno-associated virus (AAV)-mediated modification of gene expression within the aqueous humor outflow pathways (AHOP). Investig. Ophthalmol. Vis. Sci. 2021, 62, 2783. [Google Scholar]
- Visuvanathan, S. The Effects of AAV-Mediated XIAP Gene Therapy in a Mouse Model of Glaucoma. Master’s Thesis, Université d’Ottawa/University of Ottawa, Ottawa, ON, Canada, 2019. [Google Scholar]
- Su, W.; Sun, S.; Tian, B.; Tai, P.W.L.; Luo, Y.; Ko, J.; Zhan, W.; Ke, X.; Zheng, Q.; Li, X.; et al. Efficacious, safe, and stable inhibition of corneal neovascularization by AAV-vectored anti-VEGF therapeutics. Mol. Ther. Methods Clin. Dev. 2021, 22, 107–121. [Google Scholar] [CrossRef]
- Hirsch, M.L.; Conatser, L.M.; Smith, S.M.; Salmon, J.H.; Wu, J.; Buglak, N.E.; Davis, R.; Gilger, B.C. AAV vector-meditated expression of HLA-G reduces injury-induced corneal vascularization, immune cell infiltration, and fibrosis. Sci. Rep. 2017, 7, 17840. [Google Scholar] [CrossRef]
- Mohan, R.R.; Tovey, J.C.; Sharma, A.; Schultz, G.S.; Cowden, J.W.; Tandon, A. Targeted decorin gene therapy delivered with adeno-associated virus effectively retards corneal neovascularization in vivo. PLoS ONE 2011, 6, e26432. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Rodier, J.T.; Sharma, A.; Giuliano, E.A.; Sinha, P.R.; Hesemann, N.P.; Ghosh, A.; Mohan, R.R. Targeted AAV5-Smad7 gene therapy inhibits corneal scarring in vivo. PLoS ONE 2017, 12, e0172928. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.R.; Tandon, A.; Sharma, A.; Cowden, J.W.; Tovey, J.C. Significant inhibition of corneal scarring in vivo with tissue-selective, targeted AAV5 decorin gene therapy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4833–4841. [Google Scholar] [CrossRef] [Green Version]
- Bilsbury, E.; Su, W.; Sun, S.; Doherty, S.; Wood, E.; Ke, X.; Zheng, Q.; Tai, P.W.; Lin, H.; Gao, G. AAV-mediated anti-VEGF therapeutics increase corneal allograft survival-rate in a model of high-risk corneal transplantation. Investig. Ophthalmol. Vis. Sci. 2022, 63, 72-A0045. [Google Scholar]
- Watson, Z.L.; Washington, S.D.; Phelan, D.M.; Lewin, A.S.; Tuli, S.S.; Schultz, G.S.; Neumann, D.M.; Bloom, D.C. In vivo knockdown of the herpes simplex virus 1 latency-associated transcript reduces reactivation from latency. J. Virol. 2018, 92, e00812-18. [Google Scholar] [CrossRef] [PubMed]
- Gilger, B.C.; Salmon, J.H.; Roberts, D.; Song, L.; Bower, J.; Hirsch, M. Intracorneal sequential dosing of AAV-ArsB clears corneal opacity associated with mucopolysaccharidosis VI in a feline model. Investig. Ophthalmol. Vis. Sci. 2021, 62, 801. [Google Scholar]
- Miyadera, K.; Conatser, L.; Llanga, T.A.; Carlin, K.; O’Donnell, P.; Bagel, J.; Song, L.; Kurtzberg, J.; Samulski, R.J.; Gilger, B. Intrastromal gene therapy prevents and reverses advanced corneal clouding in a canine model of mucopolysaccharidosis I. Mol. Ther. 2020, 28, 1455–1463. [Google Scholar] [CrossRef]
- Mathews, P.M.; Lindsley, K.; Aldave, A.J.; Akpek, E.K. Etiology of global corneal blindness and current practices of corneal transplantation: A focused review. Cornea 2018, 37, 1198–1203. [Google Scholar] [CrossRef]
- Di Iorio, E.; Barbaro, V.; Alvisi, G.; Trevisan, M.; Ferrari, S.; Masi, G.; Nespeca, P.; Ghassabian, H.; Ponzin, D.; Palù, G. New frontiers of corneal gene therapy. Hum. Gene Ther. 2019, 30, 923–945. [Google Scholar] [CrossRef]
- Maeno, A.; Naor, J.; Lee, H.M.; Hunter, W.S.; Rootman, D.S. Three decades of corneal transplantation: Indications and patient characteristics. Cornea 2000, 19, 7–11. [Google Scholar] [CrossRef]
- Bastola, P.; Song, L.; Gilger, B.C.; Hirsch, M.L. Adeno-Associated Virus Mediated Gene Therapy for Corneal Diseases. Pharmaceutics 2020, 12, 767. [Google Scholar] [CrossRef]
- Armitage, W.J.; Goodchild, C.; Griffin, M.D.; Gunn, D.J.; Hjortdal, J.; Lohan, P.; Murphy, C.C.; Pleyer, U.; Ritter, T.; Tole, D.M.; et al. High-risk Corneal Transplantation: Recent Developments and Future Possibilities. Transplantation 2019, 103, 2468–2478. [Google Scholar] [CrossRef]
- Dohlman, T.H.; Omoto, M.; Hua, J.; Stevenson, W.; Lee, S.; Chauhan, S.K.; Dana, R. VEGF-trap aflibercept significantly improves long-term graft survival in high-risk corneal transplantation. Transplantation 2015, 99, 678–686. [Google Scholar] [CrossRef]
- Ramamoorth, M.; Narvekar, A. Non viral vectors in gene therapy-an overview. J. Clin. Diagn. Res. 2015, JCDR 9, GE01. [Google Scholar] [CrossRef]
- Zu, H.; Gao, D. Non-viral vectors in gene therapy: Recent development, challenges, and prospects. AAPS J. 2021, 23, 78. [Google Scholar] [CrossRef]
- Agban, Y.; Thakur, S.S.; Mugisho, O.O.; Rupenthal, I.D. Depot formulations to sustain periocular drug delivery to the posterior eye segment. Drug Discov. Today 2019, 24, 1458–1469. [Google Scholar] [CrossRef]
- Huang, H.; Feng, W.; Chen, Y.; Shi, J. Inorganic nanoparticles in clinical trials and translations. Nano Today 2020, 35, 100972. [Google Scholar] [CrossRef]
- Khiev, D.; Mohamed, Z.A.; Vichare, R.; Paulson, R.; Bhatia, S.; Mohapatra, S.; Lobo, G.P.; Valapala, M.; Kerur, N.; Passaglia, C.L.; et al. Emerging Nano-Formulations and Nanomedicines Applications for Ocular Drug Delivery. Nanomaterials 2021, 11, 173. [Google Scholar] [CrossRef]
- Grassiri, B.; Zambito, Y.; Bernkop-Schnürch, A. Strategies to prolong the residence time of drug delivery systems on ocular surface. Adv. Colloid Interface Sci. 2021, 288, 102342. [Google Scholar] [CrossRef]
- Seyfoddin, S.J.; Al-Kassas, R. Solid lipid nanoparticles for ocular drug delivery. Drug Deliv. 2010, 17, 467–489. [Google Scholar] [CrossRef]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Nanostructured lipid matrices for improved microencapsulation of drugs. Int. J. Pharm. 2002, 242, 121–128. [Google Scholar] [CrossRef]
- Sun, D.; Sahu, B.; Gao, S.; Schur, R.M.; Vaidya, A.M.; Maeda, A.; Palczewski, K.; Lu, Z.R. Targeted Multifunctional Lipid ECO Plasmid DNA Nanoparticles as Efficient Non-viral Gene Therapy for Leber’s Congenital Amaurosis. Mol. Ther. Nucleic Acids. 2017, 7, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Kansara, V.S.; Cooper, M.; Sesenoglu-Laird, O.; Muya, L.; Moen, R.; Ciulla, T.A. Suprachoroidally Delivered DNA Nanoparticles Transfect Retina and Retinal Pigment Epithelium/Choroid in Rabbits. Transl. Vis. Sci. Technol. 2020, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, L.; Chonn, A.; Wasserman, J.D.; Duff, C.; Worton, R.G. Condensation of plasmid DNA with polylysine improves liposome-mediated gene transfer into established and primary muscle cells. Gene Ther. 1996, 3, 396–404. [Google Scholar]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Mahapatro, A.; Singh, D.K. Biodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines. J. Nanobiotechnol. 2011, 9, 55. [Google Scholar] [CrossRef] [Green Version]
- Rai, R.; Alwani, S.; Badea, I. Polymeric Nanoparticles in Gene Therapy: New Avenues of Design and Optimization for Delivery Applications. Polymers 2019, 11, 745. [Google Scholar] [CrossRef]
- Cho, Y.K.; Uehara, H.; Young, J.R.; Tyagi, P.; Kompella, U.B.; Zhang, X.; Luo, L.; Singh, N.; Archer, B.; Ambati, B.K. Flt23k nanoparticles offer additive benefit in graft survival and anti-angiogenic effects when combined with triamcinolone. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2328–2336. [Google Scholar] [CrossRef]
- de Oliveira, F.A.; Albuquerque, L.; Delecourt, G.; Bennevault, V.; Guégan, P.; Giacomelli, F.C. Current Designs of Polymeric Platforms Towards the Delivery of Nucleic Acids Inside the Cells with Focus on Polyethylenimine. Curr. Gene Ther. 2021, 21, 431–451. [Google Scholar] [CrossRef]
- Bolhassani, A.; Javanzad, S.; Saleh, T.; Hashemi, M.; Aghasadeghi, M.; Sadat, S. Polymeric nanoparticles potent vectors for vaccine delivery targeting cancer and infectious diseases. Hum. Vaccin. Immunother. 2014, 10, 321–332. [Google Scholar] [CrossRef]
- Conley, S.M.; Naash, M.I. Nanoparticles for retinal gene therapy. Prog. Retin. Eye Res. 2010, 29, 376–397. [Google Scholar] [CrossRef]
- Cai, X.; Conley, S.M.; Nash, Z.; Fliesler, S.J.; Cooper, M.J.; Naash, M.I. Gene delivery to mitotic and postmitotic photoreceptors via compacted DNA nanoparticles results in improved phenotype in a mouse model of retinitis pigmentosa. FASEB J. 2010, 24, 1178–1191. [Google Scholar] [CrossRef]
- Han, Z.; Conley, S.M.; Makkia, R.S.; Cooper, M.J.; Naash, M.I. DNA nanoparticle-mediated ABCA4 delivery rescues Stargardt dystrophy in mice. J. Clin. Investig. 2012, 122, 3221–3226. [Google Scholar] [CrossRef]
- Teixeira, H.F.; Bruxel, F.; Fraga, M.; Schuh, R.S.; Zorzi, G.K.; Matte, U.; Fattal, E. Cationic nanoemulsions as nucleic acids delivery systems. Int. J. Pharm. 2017, 534, 356–367. [Google Scholar] [CrossRef]
- Dhahir, R.K.; Al-Nima, A.M.; Al-Bazzaz, F.Y. Nanoemulsions as Ophthalmic Drug Delivery Systems. Turk. J. Pharm. Sci. 2021, 18, 652–664. [Google Scholar] [CrossRef]
- Garrigue, J.-S.; Lambert, G.; Rabinovich, L.; Daull, P.; Serle, J.B. A Comparative Study of Latanoprost-Cationic Emulsion (Catioprost) and Latanoprost Aqueous Solution (Xalatan) in Preclinical Efficacy and Safety Models. Investig. Ophthalmol. Vis. Sci. 2011, 52, 238. [Google Scholar]
- Omri, S.; Behar-Cohen, F.; de Kozak, Y.; Sennlaub, F.; Verissimo, L.M.; Jonet, L.; Savoldelli, M.; Omri, B.; Crisanti, P. Microglia/Macrophages migrate through retinal epithelium barrier by a transcellular route in diabetic retinopathy: Role of PKC in the goto kakizaki rat model. Am. J. Pathol. 2011, 179, 942–953. [Google Scholar] [CrossRef]
- Gote, V.; Sikder, S.; Sicotte, J.; Pal, D. Ocular drug delivery: Present Innovations and Future Challenges. J. Pharmacol. Investig. Ther. 2019, 370, 602–624. [Google Scholar] [CrossRef]
- Vaishya, R.D.; Khurana, V.; Patel, S.; Mitra, A.K. Controlled ocular drug delivery with nanomicelles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 6, 422–437. [Google Scholar] [CrossRef]
- Lin, S.; Ge, C.; Wang, D.; Xie, Q.; Wu, B.; Wang, J.; Nan, K.; Zheng, Q.; Chen, W. Overcoming the anatomical and physiological barriers in topical eye surface medication using a peptide-decorated polymeric micelle. ACS Appl. Mater. Interfaces 2019, 11, 39603–39612. [Google Scholar] [CrossRef]
- Yoon, K.C.; Ahn, K.Y.; Lee, J.H.; Chun, B.J.; Park, S.W.; Seo, M.S.; Park, Y.G.; Kim, K.K. Lipid-mediated delivery of brain-specific angiogenesis inhibitor 1 gene reduces corneal neovascularization in an in vivo rabbit model. Gene Ther. 2005, 12, 617–624. [Google Scholar] [CrossRef]
- Dos Santos, G.A.; Ferreira-Nunes, R.; Dalmolin, L.F.; dos Santos Re, A.C.; Anjos, J.L.V.; Mendanha, S.A.; Aires, C.P.; Lopez, R.F.; Cunha-Filho, M.; Gelfuso, G.M.; et al. Besifloxacin liposomes with positively charged additives for an improved topical ocular delivery. Sci. Rep. 2020, 10, 19285. [Google Scholar] [CrossRef]
- Verma, A.; Tiwari, A.; Saraf, S.; Panda, P.K.; Jain, A.; Jain, S.K. Emerging potential of niosomes in ocular delivery. Expert Opin. Drug Deliv. 2021, 18, 55–71. [Google Scholar] [CrossRef]
- Biswas, G.R.; Majee, S.B. Niosomes in ocular drug delivery. Eur. J. Pharm. Med. Res 2017, 4, 813–819. [Google Scholar]
- Puras, G.; Mashal, M.; Zarate, J.; Agirre, M.; Ojeda, E.; Grijalvo, S.; Eritja, R.; Diaz-Tahoces, A.; Navarrete, G.M.; Aviles-Tregueros, M.; et al. A novel cationic niosome formulation for gene delivery to the retina. J. Control. Release 2014, 174, 27–36. [Google Scholar] [CrossRef]
- Qin, Y.; Tian, Y.; Liu, Y.; Li, D.; Zhang, H.; Yang, Y.; Qi, J.; Wang, H.; Gan, L. Hyaluronic acid-modified cationic niosomes for ocular gene delivery: Improving transfection efficiency in retinal pigment epithelium. J. Pharm. Pharmacol. 2018, 70, 1139–1151. [Google Scholar] [CrossRef]
- Avti, P.; Patel, S.; Sitharaman, B. Nanobiomaterials Handbook; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Morrison, P.W.; Khutoryanskiy, V.V. Advances in ophthalmic drug delivery. Ther. Deliv. 2014, 5, 1297–1315. [Google Scholar] [CrossRef] [PubMed]
- Shirasaki, Y. Molecular design for enhancement of ocular penetration. J. Pharm. Sci. 2008, 97, 2462–2496. [Google Scholar] [CrossRef] [PubMed]
- Molokhia, S.A.; Thomas, S.C.; Garff, K.J.; Mandell, K.J.; Wirostko, B.M. Anterior eye segment drug delivery systems: Current treatments and future challenges. J. Ocul. Pharmacol. Ther. 2013, 29, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Gupta, H. Updates on drug bioavailability and delivery to posterior segment of eye. J. Pharm. Bioallied Sci. 2013, 5, 173. [Google Scholar] [CrossRef] [PubMed]
- Jumelle, C.; Gholizadeh, S.; Annabi, N.; Dana, R. Advances and limitations of drug delivery systems formulated as eye drops. J. Control. Release 2020, 321, 1–22. [Google Scholar] [CrossRef]
- Achouri, D.; Alhanout, K.; Piccerelle, P.; Andrieu, V. Recent advances in ocular drug delivery. Drug Dev. Ind. Pharm. 2013, 39, 1599–1617. [Google Scholar] [CrossRef]
- Gaudana, R.; Jwala, J.; Boddu, S.H.; Mitra, A.K. Recent perspectives in ocular drug delivery. Pharm. Res. 2009, 26, 1197–1216. [Google Scholar] [CrossRef]
- Vicente-Pascual, M.; Gomez-Aguado, I.; Rodrigues-Castejon, J.; Rodriguez-Gascon, A.; Muntoni, E.; Battaglia, L.; del Pozo-Rodriguez, A.; Solinis Aspiazu, M.A. Topical Administration of SLN-Based Gene Therapy for the Treatment of Corneal Inflammation by De Novo IL-10 Production. Pharmaceutics 2020, 12, 584. [Google Scholar] [CrossRef]
- Liu, C.-H.; Lee, G.-W.; Wu, W.-C.; Wang, C.-C. Encapsulating curcumin in ethylene diamine-β-cyclodextrin nanoparticle improves topical cornea delivery. Colloids Surf. B Biointerfaces 2020, 186, 110726. [Google Scholar] [CrossRef]
- Loftsson, T.; Stefánsson, E. Aqueous eye drops containing drug/cyclodextrin nanoparticles deliver therapeutic drug concentrations to both anterior and posterior segment. Acta Ophthalmol. 2022, 100, 7–25. [Google Scholar] [CrossRef]
- Lorenzo-Veiga, B.; Sigurdsson, H.H.; Loftsson, T.; Alvarez-Lorenzo, C. Cyclodextrin–amphiphilic copolymer supramolecular assemblies for the ocular delivery of natamycin. Nanomaterials 2019, 9, 745. [Google Scholar] [CrossRef]
- Van Ooteghem, M.M. Biopharmaceutics of Ocular Drug Delivery; CRC Press: Boca Raton, FL, USA, 2019; pp. 27–42. [Google Scholar]
- Vooturi, S.; Bourne, D.; Panda, J.J.; Choi, S.; Kim, H.; Yandrapu, S.K.; Kompella, U.B. Effect of particle size and viscosity of suspensions on topical ocular bioavailability of budesonide, a corticosteroid. J. Ocul. Pharmacol. Ther. 2020, 36, 404–409. [Google Scholar] [CrossRef]
- Toropainen, E.; Fraser-Miller, S.J.; Navakovic, D.; Del Amo, E.M.; Vellonen, K.S.; Ruponen, M.; Viitala, T.; Korhonen, O.; Auriola, S.; Hellinen, L.; et al. Biopharmaceutics of topical ophthalmic suspensions: Importance of viscosity and particle size in ocular absorption of indomethacin. Pharmaceutics 2021, 13, 452. [Google Scholar] [CrossRef]
- Varma, D.K. Sustained release drug delivery: Closer than you think. Glaucoma Today 2018, 3, 58–60. [Google Scholar]
- Kompella, U.B.; Hartman, R.R.; Patil, M.A. Extraocular, periocular, and intraocular routes for sustained drug delivery for glaucoma. Prog. Retin. Eye Res. 2021, 82, 100901. [Google Scholar] [CrossRef]
- Sedlavek, J. Possibilities of application of ophthalmic drugs with the aid of gel contact lens. Cesk. Oftalmol. 1965, 21, 509–514. [Google Scholar]
- Xu, J.; Xue, Y.; Hu, G.; Lin, T.; He, H.; Zhang, Y.; Tang, X. A comprehensive review on contact lens for ophthalmic drug delivery. J. Control. Release 2018, 281, 97–118. [Google Scholar] [CrossRef]
- Nayak, K.; Misra, M. A review on recent drug delivery systems for posterior segment of eye. Biomed. Pharmacother. 2018, 107, 1564–1582. [Google Scholar] [CrossRef]
- Rafiei, F.; Tabesh, H.; Farzad, F. Sustained subconjunctival drug delivery systems: Current trends and future perspectives. Int. Ophthalmol. 2020, 40, 2385–2401. [Google Scholar] [CrossRef]
- Chu, H.S.; Hu, F.R.; Yang, C.M.; Yeh, P.T.; Chen, Y.M.; Hou, Y.C.; Chen, W.L. Subconjunctival injection of bevacizumab in the treatment of corneal neovascularization associated with lipid deposition. Cornea 2011, 30, 60–66. [Google Scholar] [CrossRef]
- Zhang, N.; Luo, X.; Zhang, S.; Liu, R.; Liang, L.; Su, W.; Liang, D. Subconjunctival injection of tumor necrosis factor-α pre-stimulated bone marrow-derived mesenchymal stem cells enhances anti-inflammation and anti-fibrosis in ocular alkali burns. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Maleki, A.; Aghaei, H.; Lee, S. Topical interferon alpha 2b in the treatment of refractory pseudophakic cystoid macular edema. Am. J. Ophthalmol. Case Rep. 2018, 10, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Daien, V.; Papinaud, L.; Domerg, C.; Lacombe, S.; Daures, J.P.; Villain, M. Incidence and Characteristics of Cystoid Macular Edema after Cataract Surgery. Ophthalmology 2016, 123, 663–664. [Google Scholar] [CrossRef]
- Saluja, G.; Sharma, N.; Agarwal, R.; Sharma, H.P.; Singhal, D.; Maharana, P.K.; Sinha, R.; Agarwal, T.; Velpandian, T.; Titiyal, J.S.; et al. Comparison of Safety and Efficacy of Intrastromal Injections of Voriconazole, Amphotericin B and Natamycin in Cases of Recalcitrant Fungal Keratitis: A Randomized Controlled Trial. Clin. Ophthalmol. 2020, 15, 2437–2446. [Google Scholar] [CrossRef] [PubMed]
- Konar, P.; Joshi, S.; Mandhare, S.J.; Thakur, R.; Deshpande, M.; Dayal, A. Intrastromal voriconazole: An adjuvant approach for recalcitrant mycotic keratitis. Indian J. Ophthalmol. 2020, 68, 35–38. [Google Scholar] [CrossRef]
- Song, L.; Bower, J.J.; Llanga, T.; Salmon, J.H.; Hirsch, M.L.; Gilger, B.C. Ocular Tolerability and Immune Response to Corneal Intrastromal AAV-IDUA Gene Therapy in New Zealand White Rabbits. Mol. Ther. Methods Clin. Dev. 2020, 18, 24–32. [Google Scholar] [CrossRef]
- Liebmann, J.M.; Barton, K.; Weinreb, R.N.; Eichenbaum, D.A.; Gupta, P.K.; McCabe, C.M.; Wolfe, J.D.; Ahmed, I.; Sheybani, A.; Craven, E.R. Evolving guidelines for intracameral injection. J. Glaucoma 2020, 29, S1–S7. [Google Scholar] [CrossRef]
- Lewis, R.A.; Christie, W.C.; Day, D.G.; Craven, E.R.; Walters, T.; Bejanian, M.; Lee, S.S.; Goodkin, M.L.; Zhang, J.; Whitcup, S.M.; et al. Bimatoprost sustained-release implants for glaucoma therapy: 6-month results from a phase I/II clinical trial. Am. J. Ophthalmol. 2017, 175, 137–147. [Google Scholar] [CrossRef]
- Ramulu, P.Y.; Do, D.V.; Corcoran, K.J.; Corcoran, S.L.; Robin, A.L. Use of retinal procedures in medicare beneficiaries from 1997 to 2007. Arch. Ophthalmol. 2010, 128, 1335–1340. [Google Scholar] [CrossRef]
- Grzybowski, A.; Told, R.; Sacu, S.; Bandello, F.; Moisseiev, E.; Loewenstein, A.; Schmidt-Erfurth, U. 2018 Update on Intravitreal Injections: Euretina Expert Consensus Recommendations. Ophthalmologica 2018, 239, 181–193. [Google Scholar] [CrossRef]
- Sampat, K.M.; Garg, S.J. Complications of intravitreal injections. Curr. Opin. Ophthalmol. 2010, 21, 178–183. [Google Scholar] [CrossRef]
- Ross, M.; Ofri, R. The future of retinal gene therapy: Evolving from subretinal to intravitreal vector delivery. Neural Regen. Res. 2021, 16, 1751–1759. [Google Scholar]
- Cehajic-Kapetanovic, J.; Xue, K.; Edwards, T.L.; Meenink, T.C.; Beelen, M.J.; Naus, G.J.; de Smet, M.D.; MacLaren, R.E. First-in-Human Robot-Assisted Subretinal Drug Delivery Under Local Anesthesia. Am. J. Ophthalmol. 2021, 237, 104–113. [Google Scholar] [CrossRef]
- Allyn, M.M.; Luo, R.H.; Hellwarth, E.B.; Swindle-Reilly, K.E. Considerations for Polymers Used in Ocular Drug Delivery. Front. Med. 2021, 8, 787644. [Google Scholar] [CrossRef]
- Rai, U.D.J.; Young, S.A.; Thrimawithana, T.R.; Abdelkader, H.; Alani, A.W.; Pierscionek, B.; Alany, R.G. The suprachoroidal pathway: A new drug delivery route to the back of the eye. Drug Discov. Today 2015, 20, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Fusi-Rubiano, W.; Saedon, H.; Patel, V.; Yang, Y.C. Oral medications for central serous chorioretinopathy: A literature review. Eye 2020, 34, 809–824. [Google Scholar] [CrossRef] [PubMed]
- Lotery, A.; Sivaprasad, S.; O’Connell, A.; Harris, R.A.; Culliford, L.; Ellis, L.; Cree, A.; Madhusudhan, S.; Behar-Cohen, F.; Chakravarthy, U.; et al. Eplerenone for chronic central serous chorioretinopathy in patients with active, previously untreated disease for more than 4 months (VICI): A randomised, double-blind, placebo-controlled trial. Lancet 2020, 395, 294–303. [Google Scholar] [CrossRef]
- Singh, R.P.; Sears, J.E.; Bedi, R.; Schachat, A.P.; Ehlers, J.P.; Kaiser, P.K. Oral eplerenone for the management of chronic central serous chorioretinopathy. Int. J. Ophthalmol. 2015, 8, 310–314. [Google Scholar] [PubMed]
- Iqbal, F.; Iqbal, K.; Inayat, B.; Arjumand, S.; Ghafoor, Z.; Sattar, W.; Abbas, K. Eplerenone Treatment in Chronic Central Serous Chorioretinopathy. Cureus 2021, 13, e18415. [Google Scholar] [CrossRef] [PubMed]
- Cakir, B.; Fischer, F.; Ehlken, C.; Buhler, A.; Stahl, A.; Schlunck, G.; Bohringer, D.; Agostini, H.; Lange, C. Clinical experience with eplerenone to treat chronic central serous chorioretinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 2151–2157. [Google Scholar] [CrossRef]
- Lotan, I.; Charlson, R.W.; Ryerson, L.Z.; Levy, M.; Kister, I. Effectiveness of subcutaneous tocilizumab in neuromyelitis optica spectrum disorders. Mult. Scler. Relat. Disord. 2019, 39, 101920. [Google Scholar] [CrossRef]
- Silkiss, R.Z.; Paap, M.K.; Roelofs, K.A.; Agi, J.; Weis, E. Treatment of corticosteroid-resistant thyroid eye disease with subcutaneous tocilizumab. Can. J. Ophthalmol. 2021, 56, 66–70. [Google Scholar] [CrossRef]
- Stone, J.H.; Tuckwell, K.; Dimonaco, S.; Klearman, M.; Aringer, M.; Blockmans, D.; Brouwer, E.; Cid, M.C.; Dasgupta, B.; Rech, J.; et al. Trial of Tocilizumab in Giant-Cell Arteritis. N. Engl. J. Med. 2017, 377, 317–328. [Google Scholar] [CrossRef]
- Ramlogan-Steel, C.A.; Murali, A.; Andrzejewski, S.; Dhungel, B.; Steel, J.C.; Layton, C.J. Gene therapy and the adeno-associated virus in the treatment of genetic and acquired ophthalmic diseases in humans: Trials, future directions and safety considerations. Clin. Exp. Ophthalmol. 2019, 47, 521–536. [Google Scholar] [CrossRef]
- Büning, H.; Srivastava, A. Capsid Modifications for Targeting and Improving the Efficacy of AAV Vectors. Mol. Ther. Methods Clin. Dev. 2019, 12, 248–265. [Google Scholar] [CrossRef]
- Silva, M.; Peng, T.; Zhao, X.; Li, S.; Farhan, M.; Zheng, W. Recent trends in drug-delivery systems for the treatment of diabetic retinopathy and associated fibrosis. Adv. Drug Deliv. Rev. 2021, 173, 439–460. [Google Scholar] [CrossRef]
- Wang, Y.; Rajala, A.; Rajala, R.V.S. Lipid Nanoparticles for Ocular Gene Delivery. J. Funct. Biomater. 2015, 6, 379–394. [Google Scholar] [CrossRef] [Green Version]

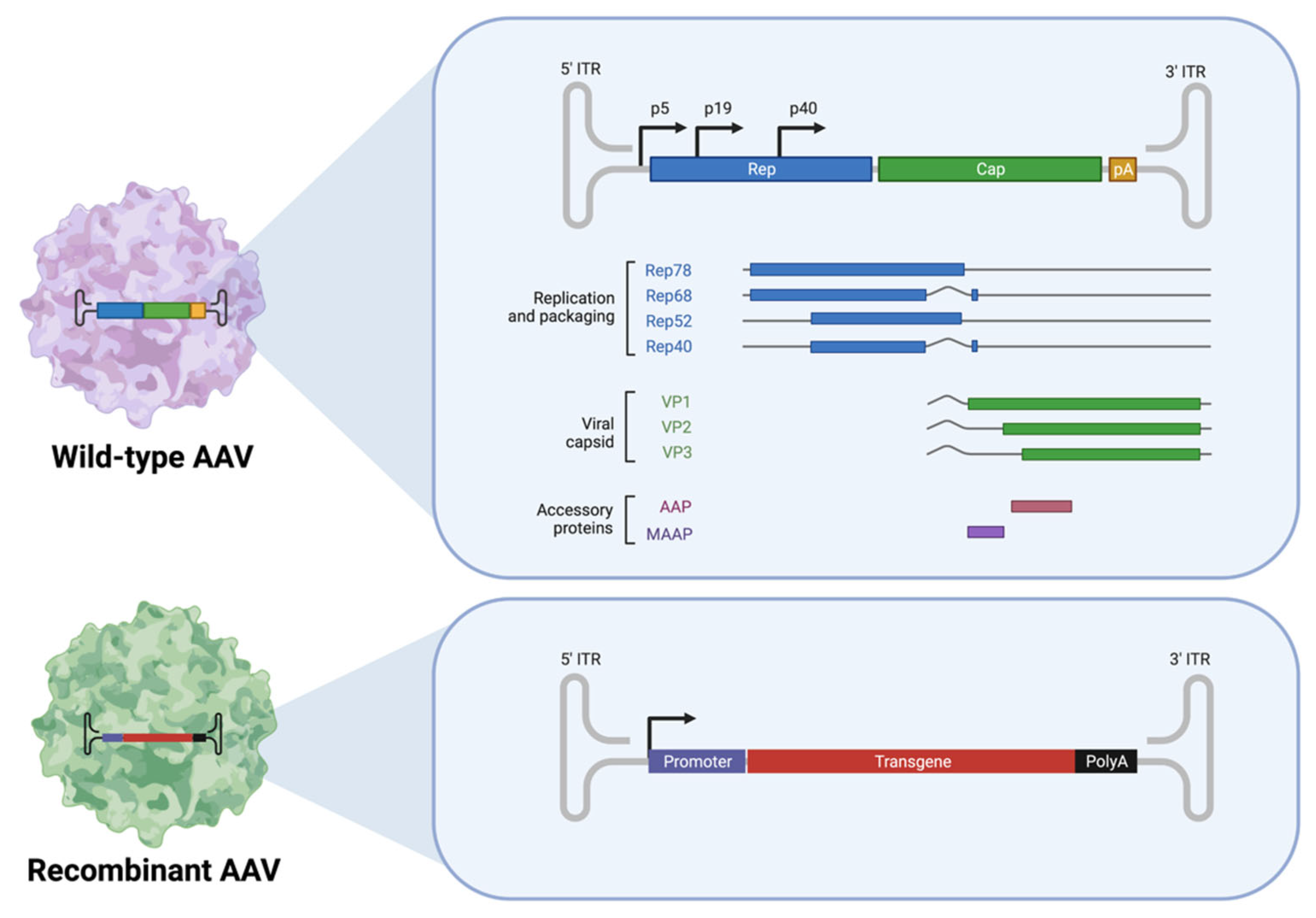
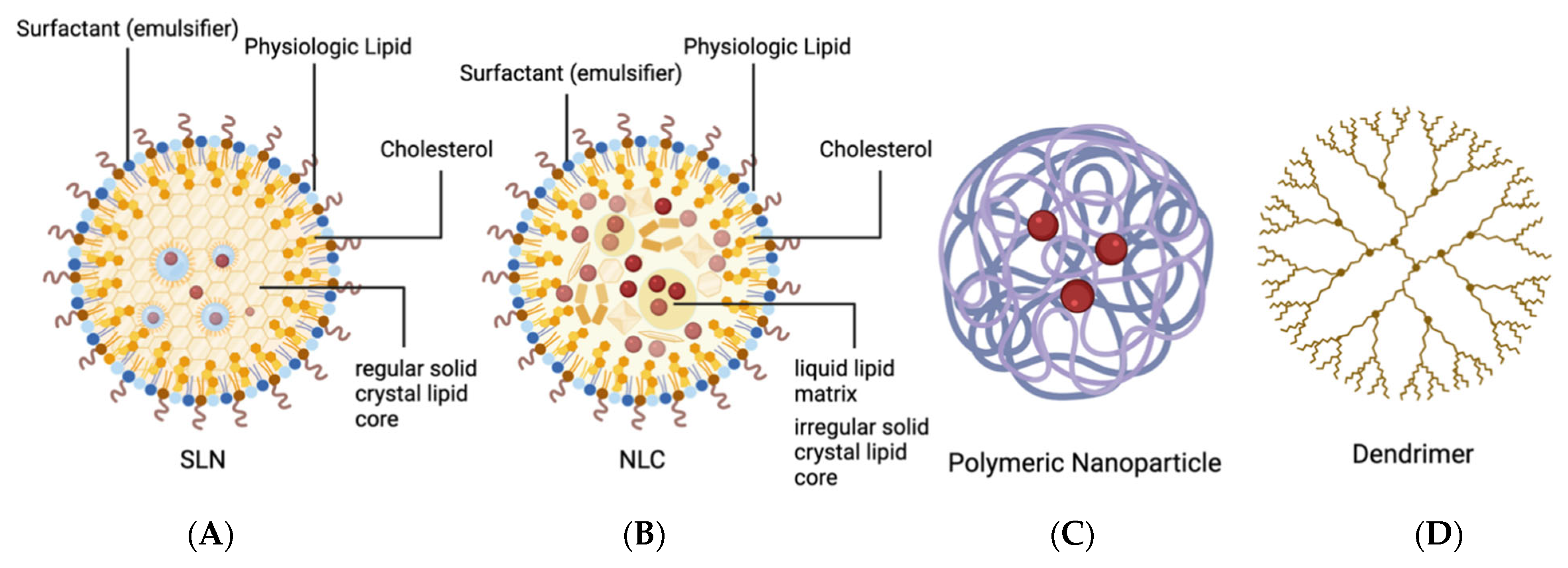

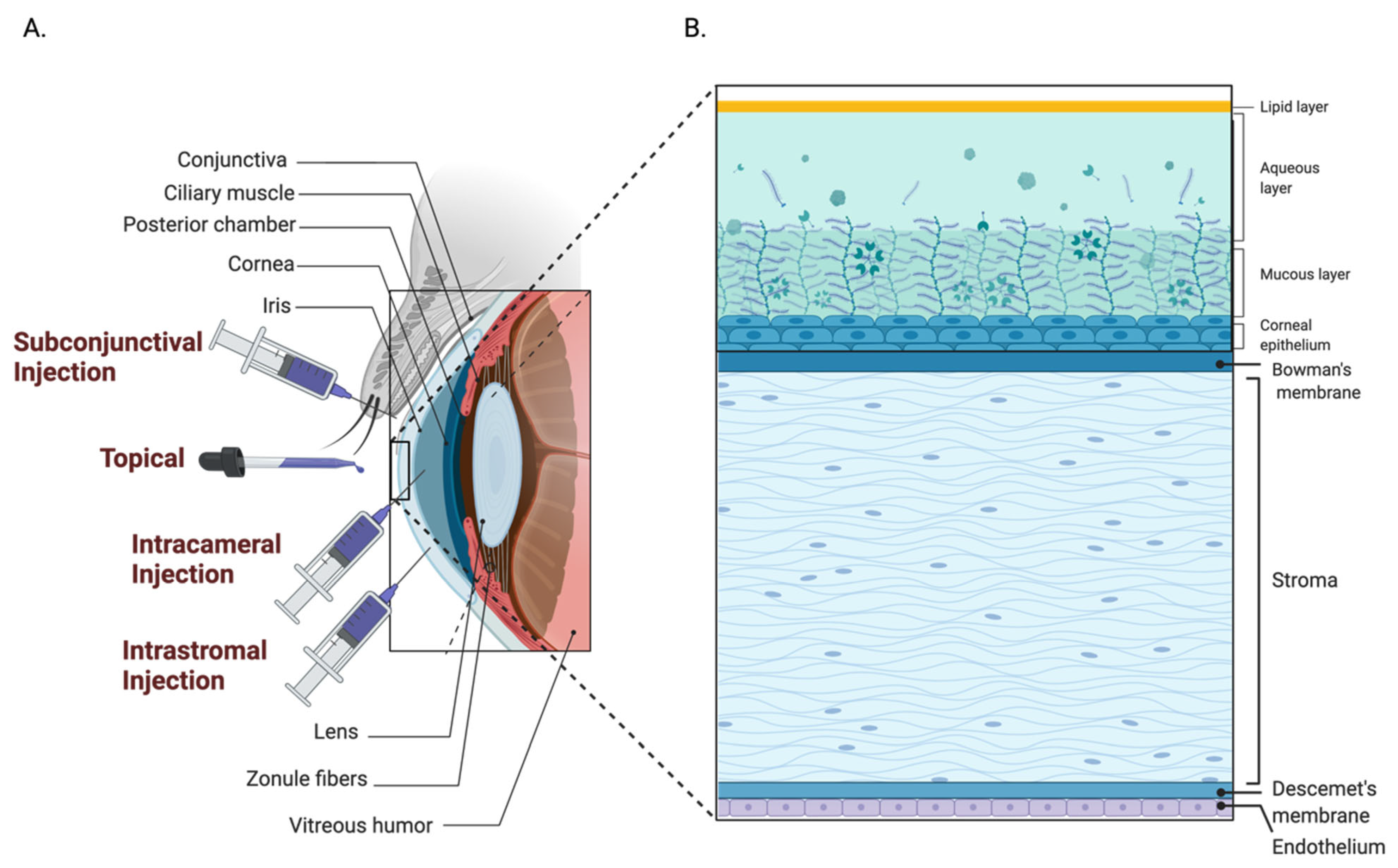
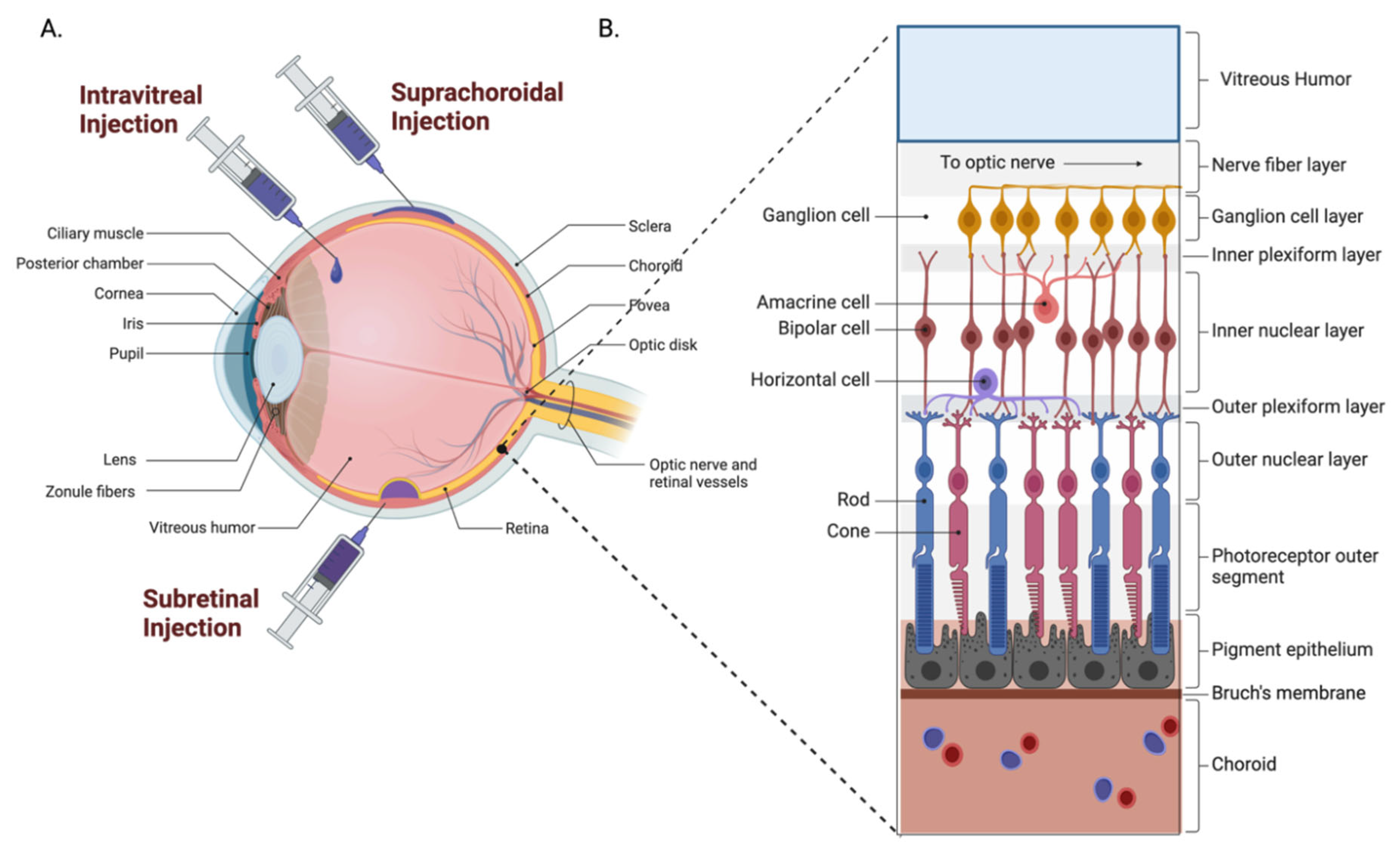
| Conditions | Sponsor | AAV Serotype | Gene Therapy Product | Transgene | Administration Route | Clinical Trial Status | NCT Number(s) |
|---|---|---|---|---|---|---|---|
| Neovascular AMD | Regenxbio Inc. | AAV8 | RGX-314 | mAb fragment, anti-VEGF | Suprachoroidal injection(s) | Phase 1/2a, 2, 2/3, long-term follow-up | NCT03066258, NCT04514653, NCT05210803 |
| Regenxbio Inc. | AAV8 | RGX-314 | mAb fragment, anti-VEGF | One-time intravitreal injection | Phase 2/3 | NCT04704921 | |
| Regenxbio Inc. | AAV8 | RGX-314 | mAb fragment, anti-VEGF | One-time subretinal injection | Phase 2, long-term follow-up | NCT04832724, NCT03999801 | |
| Adverum Biotechnologies, Inc. | AAV7 | ADVM-022 | aflibercept | One-time intravitreal injection | Phase 1, long-term follow-up | NCT03748784, NCT04645212 | |
| Genzyme/Sanofi | AAV2 | AAV2-sFLT01 | sFLT-1 | One-time intravitreal injection | Phase 1 | NCT01024998 | |
| Lions Eye Institute | - | rAAV.sFlt-1 | sFLT-1 | One-time subretinal injection | Phase 1/2 | NCT01494805 | |
| Gyroscope Therapeutics Limited | AAV2 | GT005 | Complement factor I (CFI) gene | One-time subretinal injection | Phase 2 | NCT03846193 | |
| 4D Molecular Therapeutics | R100 capsid | 4D-150 | Anti-VEGF-C miRNA and codon-optimized sequence encoding aflibercept | One-time intravitreal injection | Phase 2 | NCT05197270 | |
| Diabetic macular edema | Adverum Biotechnologies, Inc. | AAV7 | ADVM-022 | aflibercept | One-time intravitreal injection | Phase 2 | NCT04418427 |
| Diabetic retinopathy | Regenxbio Inc. | AAV8 | RGX-314 | mAb fragment anti-VEGF | One or two suprachoroidal injections | Phase 2, long-term follow-up | NCT04567550, NCT05296447 |
| X-linked retinitis pigmentosa | MeiraGT UK II Ltd. | AAV2/5 | AAV2/5-RPGR | RPGR coding sequence | One-time subretinal injection | Phase 1/2, 3 | NCT03252847, NCT04671433 |
| NightstaRx Ltd./Biogen | AAV8 | BIIB112 | RPGR coding sequence | Six-time subretinal injection | Phase 1/2 | NCT03116113 | |
| 4D Molecular Therapeutics | R100 capsid | 4D-125 | Codon-optimized RPGR gene | One-time intravitreal injection | Phase 1/2 | NCT04517149 | |
| Applied Genetic Technologies Corp. | AAV2tYF | AGTC-501 (rAAV2tYF-GRK1-hRPGRco) | G Protein-Coupled Receptor Kinase 1 (GRK1) and RPGR coding sequences | One-time subretinal injection | Phase 1/2, 2/3 | NCT03316560, NCT04850118 | |
| Retinitis pigmentosa | Coave Therapeutics | AAV2/5 | AAV2/5-hPDE6B | PDE6B gene | Subretinal injection | Phase 1/2 | NCT03328130 |
| STZ eye trial | - | rAAV.hPDE6A | PDE6A gene | One-time subretinal injection | Phase 1/2 | NCT04611503 | |
| King Khaled Eye Specialist Hospital | AAV2 | rAAV2-VMD2-hMERTK | VMD2-hMERTK gene vector | Subretinal injection | Phase 1 | NCT01482195 | |
| Nanoscope Therapeutics Inc. | AAV2 | vMCO-1 | Multi-Characteristic Opsin 1 gene expression. cassette | One-time intravitreal injection | Phase 1/2 | NCT04919473 | |
| GenSight Biologics | AAV2 | GS030 (rAAV2.7m8-CAG-ChrimsonR-tdTomato)-Medical Device | Channel rhodopsin ChrimsonR-tdTomato gene with Visual Interface Stimulating Glasses | One-time intravitreal injection | Phase 1/2 | NCT03326336 | |
| Ocugen | AAV5 | OCU400 | Nuclear Hormone Receptor (NR2E3) gene | One-time subretinal injection | Phase 1/2 | NCT03326336 | |
| Nanoscope Therapeutics Inc. | AAV2 | vMCO-101 | Multi-characteristic opsin (MCO) gene expression cassette | One-time intravitreal injection | Phase 2 | NCT04945772 | |
| Choroideremia | University of Oxford | AAV2 | rAAV2.REP1 | Rab-escort Protein 1 (REP1) coding sequence | Subretinal injection | Phase 1/2 | NCT01461213 |
| Spark Therapeutics | AAV2 | AAV2-hCHM (human choroideremia gene, same as REP1) | Rab-escort Protein 1 (REP1) coding sequence | Subretinal injection | Phase 1/2 | NCT02341807 | |
| Byron Lam | AAV2 | AAV2-REP1 | Rab-escort Protein 1 (REP1) coding sequence | Subretinal injection | Phase 2 | NCT02553135 | |
| 4D Molecular Therapeutics | R100 | 4D-R100 | Codon-optimized Rab-escort Protein 1 (REP1) coding sequence | One-time intravitreal injection | Phase 1 | NCT04483440 | |
| STZ eye trial | AAV2 | rAAV2.REP1 | Rab-escort Protein 1 (REP1) coding sequence | One-time subretinal injection | Phase 2 | NCT02671539 | |
| Ian M. MacDonald | AAV2 | rAAV2.REP1 | Rab-escort Protein 1 (REP1) coding sequence | One-time subretinal injection | Phase 1/2 | NCT02077361 | |
| Leber congenital amaurosis | Spark Therapeutics | AAV2 | LUXTURNA, voretigene neparvovec-rzyl (AAV2-hRPE65v2) | RPE65 gene | One-time subretinal injection | Phase 1, 1/2, 5-year follow-up, 3, 15-year follow-up | NCT00516477, NCT01208389, NCT03597399, NCT00999609, NCT03602820 |
| MeiraGTx UK II Ltd. | AAV2 | AAV2/5-OPTIRPE65 | RPE65 gene | One-time subretinal injection | Phase 1/2, long-term follow-up | NCT02781480, NCT02946879 | |
| University College, London | AAV2 | tgAAG76 (rAAV 2/2.hRPE65p.hRPE65) | RPE65 gene | One-time subretinal injection | Phase 1/2 | NCT00643747 | |
| Applied Genetic Technologies Corp | AAV2 | rAAV2-CB-hRPE65 | RPE65 gene | One-time subretinal injection | Phase 1/2 | NCT00749957 | |
| Autosomal recessive Leber congenital amaurosis | Atsena Therapeutics Inc. | AAV5 | SAR-439483 | GUCY2D gene | One-time subretinal injection | Phase 1/2 | NCT03920007 |
| Leber Hereditary Optic Neuropathy | GenSight Biologics | AAV2 | GS010 (rAAV2/2-ND4) | ND4 gene (mitochondrial) | One-time intravitreal injection | Phase 3 | NCT03293524 |
| Byron Lam | Self-complementary AAV2 | scAAV2-P1ND4v2 | ND4 gene (mitochondrial) | One-time intravitreal injection | Phase 1 | NCT02161380 | |
| MeiraGTx UK II Ltd. | AAV2/8 | AAV2/8-hG1.7p.coCNGA3 | CNGA3 gene | One-time subretinal injection | Phase 1/2 | NCT03758404 | |
| Applied Genetic Technologies Corp | AAV2 | AGTC-402 (rAAV2tYF-PR1.7-hCNGA3) | CNGA3 gene | One-time subretinal injection | Phase 1/2 | NCT02935517 | |
| Applied Genetic Technologies Corp | AAV2 | AGTC-401 (rAAV2tYF-PR1.7-hCNGB3) | CNGB3 gene | One-time subretinal injection | Phase 1/2 | NCT02599922 | |
| Variant Late-Infantile Neuronal Ceroid Lipofuscinosis | Amicus Therapeutics | Self-complementary AAV9 | scAAV9.CB.CLN6 | CLN6 Gene | One-time intrathecal injection | Phase 1/2 | NCT02725580 |
| X-linked Juvenile Retinoschisis | National Eye Institute (NEI) | AAV8 | AAV8-scRS/IRBPhRS | RS1 gene | One-time intravitreal injection | Phase 1/2 | NCT02317887 |
| Genetic Technologies Corp | AAV2 | rAAV2tYF-CB-hRS1 | RS1 gene | One-time intravitreal injection | Phase 1/2 | NCT02416622 |
| Condition Treated | Gene Therapy Product | AAV Serotype | Delivery Method | Animal Model | Comments/Mechanism | References |
|---|---|---|---|---|---|---|
| Open-angle-glaucoma | scAAV2.CMV.GFP | AAV2 | Intracameral injection | NHPs | Resulted in fluorescence in TM for 2 years | [34] |
| Open-angle glaucoma | AAV2-Shp2 eGFP-shRNA | AAV2 | Intravitreal injection | Cav-1 deficient mouse model of glaucoma | Prevented inner retinal injury due to ocular hypertension | [36] |
| Open-angle glaucoma | AAV2-BMP4 | AAV2 | Intravitreal injection | Magnetic microbead-induced glaucoma, mouse | Retinal ganglion cell survival was enhanced and the amplitude of the PhNR was restored in ERG | [37] |
| Open-angle glaucoma | AAV2(Y444F)-smCBA-hADAMTS10 | AAV2 | Intracameral injection | Six ADAMTS10-mutant dogs | Treated eyes showed almost complete prevention of extracellular plaque formation | [38] |
| Open-angle glaucoma | AAV2-XIAP | AAV2 | Intravitreal injection | Intracameral injections of microbeads, mouse | XIAP overexpression resulted in significant protection of RGCs | [39] |
| CoNV | AAV8-KH902 | AAV8 | Intrastromal injection | Alkali burn model, mouse | AAV8 showed superior efficacy to AAV2 | [40] |
| CoNV | scAAV8G9-optHLA-G1 + G5 | AAV8 | Intrastromal injection | Burn-induced CoNV, rabbit | HLA-G upregulates Treg cells, preventing foreign body rejection | [41] |
| CoNV | AAV5-decorin | AAV5 | Topical | Corneal micropocket assay model of CoNV, rabbit | Decorin is a TGF-β inhibitor | [42] |
| Corneal Fibrosis | AAV5-Smad7 | AAV5 | Topical | PRK-induced corneal fibrosis, rabbit | Smad7 is a negative regulator of TGF-β | [43] |
| Corneal Fibrosis | AAV5-decorin | AAV5 | Topical | PRK-induced corneal fibrosis, rabbit | Decorin is a TGF-β inhibitor | [44] |
| Corneal Transplant Rejection | AAV8-KH902 | AAV8 | Intrastromal injection | Cornel suture model, rat | CoNV and corneal opacity were decreased, graft survival rate was increased | [45] |
| HSV-mediated keratitis | scAAV2-LAT | AAV2 | Abrasion followed by topical administration | HSV-infected rabbits | Viral reactivation was blocked in 60% or rabbits | [46] |
| Mucopolysaccharidosis VI | AAV8-ArsB | AAV8 | Intrastromal injection and sequential (opposite eye) intrastromal injection | One ArsB homozygous and one heterozygous ArsB feline mutants | Corneal opacity was reversed, and no signs of an inhibitory capsid antibody response observed in opposite eye | [47] |
| Mucopolysaccharidosis I | AAV8G9-opt-IDUA (AAV8 and 9 chimeric capsid-optimized-IDUA) | AAV8 | Intrastromal injection | MPS I canine model, homozygous for the IDUA gene mutation | Treatment was able to prevent and reverse visual impairment | [48] |
| Corneal Dystrophy Type | Gene/Chromosomal Locus | Gene Size | Open-Reading Frame Size | Inheritance Pattern |
|---|---|---|---|---|
| Avellino Type | TGFBI | 34.8 kb | 2.05 kb | AD |
| Congenital Endothelial 1 | 20p11.2–q11.2 locus | unknown | unknown | AD |
| Congenital Stromal | DCN | 42.3 kb | 1.08 kb | AD |
| Epithelial Basement Membrane | TGFBI | 34.8 kb | 2.05 kb | AD |
| Fleck | PIKFYVE | 92.7 kb | 6.29 kb | AD |
| Fuchs Endothelial, Early Onset | COL8A2 | 29.9 kb | 2.11 kb | AD |
| Fuchs Endothelial, Late Onset | ZEB1 | 211.4 kb | 3.37 kb | AD |
| Fuchs Endothelial, Late Onset 2 | TCF4 | 442.6 kb | 2 kb | AD |
| Granular | TGFBI | 34.8 kb | 2.05 kb | AD |
| Lattice Type I | TGFBI | 34.8 kb | 2.05 kb | AD |
| Lattice Type II | GSN | 131.4 kb | 2.35 kb | AD |
| Meesmann | KRT12, KRT3 | 5.92 kb, 6.43 kb | 1.48 kb, 1.88 kb | AD |
| Posterior Amorphous | 12q21.33 deletion | unknown | unknown | AD |
| Posterior Polymorphous 1 | OVOL2 | 102.2 kb | 0.83 kb | AD |
| Posterior Polymorphous 2 | COL8A2 | 29.9 kb | 2.1 kb | AD |
| Posterior Polymorphous 3 | ZEB1 | 211.4 kb | 3.37 kb | AD |
| Posterior Polymorphous 4 | GRHL2 | 188.7 kb | 1.86 kb | AD |
| Recurrent Epithelial Erosions | unknown | unknown | unknown | AD |
| Reis–Bücklers | TGFBI | 34.8 kb | 2.05 kb | AD |
| Schnyder | UBIAD1 | 26.4 kb | 1.01 kb | AD |
| Stocker–Holt | KRT12 | 5.92 kb | 1.48 kb | AD |
| Subepithelial Mucinous | unknown | unknown | unknown | AD |
| Thiel–Behnke | TGFBI | 34.8 kb | 2.05 kb | AD |
| Band-Shaped | unknown | unknown | unknown | Unknown |
| Congenital Endothelial 2 | SLC4A11 | 12.1 kb | 2.63 kb | AR |
| Gelatinous Drop-like | TACSTD2 | 1.82 kb | 0.97 kb | AR |
| Macular | CHST6 | 23.4 kb | 1.19 kb | AR |
| Lisch Epithelial | unknown | unknown | unknown | X-linked, dominant |
| Endothelial X-Linked | Xq25 locus | unknown | unknown | X-linked, unclear |
| Nanoparticle | Drug Carried | Condition Treated | Delivery Method | Sponsor | Clinical Trial Status | NCT Number(s) |
|---|---|---|---|---|---|---|
| Cyclodextrin NP | Dexamethasone | Diabetic macular edema | Topical | King Saud University | Phase 2/3 | NCT01523314 |
| D-4517.2 (Hydroxyl Dendrimer) | VEGFR Tyrosine Kinase Inhibitor | AMD | Subcutaneous injection | Ashvattha Therapeutics, Inc. | Phase 1 | NCT05105607 |
| TLC399 (ProDex) Multi-layered lipid NP | Dexamethasone | Retinal vein occlusion; macular edema | One-time intravitreal injection | Taiwan Liposome Company | Phase 2 | NCT03093701 |
| SeeQ CdSe 655 Alt Nanoparticles (cadmium-selenium) NP | SeeQ Device | Retinitis Pigmentosa | Two intravitreal injections | 2C Tech Corp | Phase 1 | NCT04008771 |
| Albumin-stabilized nanoparticle | Paclitaxel | Intraocular melanoma | Intravenous injections | Ohio State University Comprehensive Cancer Center | Phase 2 | NCT00738361 |
| EggPC liposomes | Latanoprost | Glaucoma | Subconjunctival injection | Singapore Eye Research Institute | Phase 1/2 | NCT01987323 |
| Pluronic® F-127(PF) polymeric NP | Urea | Cataracts | Topical | Assiut University | Phase 2 | NCT03001466 |
| Ethylenediaminetetraacetic acid (EDTA) disodium salt and crocin liposomes | Hyaluronic acid | Meibomian gland dysfunction | Topical | University of Seville | Not applicable | NCT03617315 |
| Nanoemulsion (OCU-310) | Brimonidine Tartrate | Meibomian gland dysfunction | Topical | Ocugen | Phase 3 | NCT03785340 |
| Sunitinib Malate (GB-102) MP | Aflibercept | Neovascular AMD | Intravitreal injection(s) | Graybug Vision | Phase 1 | NCT03249740 |
| (LAMELLEYE) Liposomal NP | Slecithin phospholipids, sphingomyelin and cholesterol, suspended in saline | Dry eye secondary to Sjögren Syndrome | Topical | NHS Greater Glasgow and Clyde | Not applicable | NCT03140111 |
| AXR-159 ophthalmic solution (Micelles) | Integrins α4β1 and α4β7 antagonists | Dry eye | Topical | AxeroVision, Inc. | Phase 2 | NCT03598699 |
| KPI-121 (submicron suspension) | loteprednol etabonate | Ocular infections, irritations, and inflammation | Topical | Kala Pharmaceuticals, Inc. | Phase 3 | NCT02163824 |
| KPI-121 (submicron suspension) | loteprednol etabonate | Dry eye, keratoconjunctivitis sicca | Topical | Kala Pharmaceuticals, Inc. | Phase 3 | NCT02813265 |
| AR-1105 | Dexamethasone | Macular edema due to retinal vein occlusion | Intravitreal implant | Aerie Pharmaceuticals | Phase 2 | NCT03739593 |
| AR-13503 implant | Aflibercept | Neovascular age-related macular degeneration | Intravitreal implant | Aerie Pharmaceuticals | Phase 1 | NCT03835884 |
| REMOGEN® OMEGA (Microemulsion of polyunsaturated fatty acids and hydrating polymers) | Omega-3 fatty acids | Dry eye | Topical | TRB Chemedica AG | Not applicable | NCT02908282 |
| Liposomes | Artificial tears | Dry eye | Spray | Aston University | Not applicable | NCT02420834 |
| ENV 515 | Travoprost | Glaucoma | Intracameral implant | Envisia Therapeutics | Phase 2 | NCT02371746 |
| OCS-01 (Cyclodextrin NP) | Dexamethasone | Corneal inflammation and post-operative pain | Topical | Oculis | Phase 2 | NCT04130802 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, B.; Bilsbury, E.; Doherty, S.; Teebagy, S.; Wood, E.; Su, W.; Gao, G.; Lin, H. Ocular Drug Delivery: Advancements and Innovations. Pharmaceutics 2022, 14, 1931. https://doi.org/10.3390/pharmaceutics14091931
Tian B, Bilsbury E, Doherty S, Teebagy S, Wood E, Su W, Gao G, Lin H. Ocular Drug Delivery: Advancements and Innovations. Pharmaceutics. 2022; 14(9):1931. https://doi.org/10.3390/pharmaceutics14091931
Chicago/Turabian StyleTian, Bo, Evan Bilsbury, Sean Doherty, Sean Teebagy, Emma Wood, Wenqi Su, Guangping Gao, and Haijiang Lin. 2022. "Ocular Drug Delivery: Advancements and Innovations" Pharmaceutics 14, no. 9: 1931. https://doi.org/10.3390/pharmaceutics14091931
APA StyleTian, B., Bilsbury, E., Doherty, S., Teebagy, S., Wood, E., Su, W., Gao, G., & Lin, H. (2022). Ocular Drug Delivery: Advancements and Innovations. Pharmaceutics, 14(9), 1931. https://doi.org/10.3390/pharmaceutics14091931






