Microfluidic Manufacture of Lipid-Based Nanomedicines
Abstract
:1. The Nanomedicine Market and Bottlenecks to Market Entry
2. Lipid-Based Nanomedicines
2.1. Current Methods for Lipid-Based Nanomedicine Manufacture
2.2. Challenges with Lipid-Based Nanomedicine Manufacture and Clinical Translation
3. Microfluidic Manufacture and the Problem of Mixing
3.1. Microfluidic Devices and Principles
3.1.1. Principles of Mass Transfer and Fluid Mixing
3.1.2. Microreactor Design and Mixing
Microreactors with Continuous Flow
Microreactors with Segmented Flow
Micromixer Channel Dimensions and Residence Time Effects
3.1.3. Heat Transfer and Temperature Control
3.2. Materials for Microfluidic Chip Fabrication Applicable for Nanomaterial Production
4. Microfluidic Manufacture of Lipid-Based Nanomedicines: Studies to Date
4.1. Nanomedicines Prepared with T- or Y-Junction (Shaped) Mixers
4.2. Microfluidic Hydrodynamic Flow (MHF) Focusing
4.3. Microfluidic Staggered Herringbone (SHM) (Chaotic) Micromixers
4.4. Bifurcating Mixer
4.5. Baffle Mixers
| Delivery System/Lipids | Drug/API | Chip Design | FRR (aq:org) | TFR (mL·min−1) | In Vitro Findings | In Vivo Findings | Optimized Formula | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean Diameter (nm) | PDI | LE% | ||||||||
| Lipoplex/ DOTAP:EPC:DOPE | pDNA | FF | 10:1 | 140 mm·s−1 | Transfection efficacy ~10 × 107 relative light units (RLU)·mg of protein−1 | NA | ~135 | ~2.3 | NA | [270] |
| Patterned walls FF | Transfection efficacy ~6 × 107 RLU·mg of protein−1 | ~115 | ~2.0 | |||||||
| Lipid nanoparticles/ mPEG-DSPC:POPC | AmB | NASHM | 3:1 | 12 | IC50: 0.085 μg·mL−1, hemolytic at ≥25 μg·mL−1 | NA | ~39 | ~0.115 | 88 | [271] |
| T-junction | 24 | |||||||||
| Liposomes/ SPC:Chol (3:1 w/w) | CBD | T-junction and zig zag or split and combine | 1:5 | 10 | NA | NA | ~110 | ~0.13 | ~73 | [236] |
| Lipoplex/ DOTAP:DOPE:DOPC:DSPE-PEG2000-FolA or DOTAP:DOPE:DOPC:DSPE-PEG2000 | siRNA | HFF | 9:1 | 0.0167 | In vitro studies on wildtype epithelial carcinoma KB cells show endosomal uptake in the perinuclear region | NA | ~40 | NA | ~60 | [242] |
| Targeted Lipoplex/ DODMA:DOTMA or DCChol:EggPC:mPEG-DSPE) modified with Tf | siRNAs (LOR-1284) | HFF | 5:1 | 0.025−2.000 | siRNA complex was stable in serum for 8 h compared to 40% of free siRNA; no significant cytotoxicity in MV4-11 cells; 6.14-fold reduction in IC50 for siRNA complex (105.27 nM) and increased downregulation compared to free siRNA | 20% of IV dose remained in plasma after 24 h (t1/2: 10.2 h, AUC: 5.5 h μg·mL−1) compared to 1% for free siRNA. (t1/2: 2.93 h); 3-fold increase in t1/2 and decreased protein expression by 86%. | ~80 | NA | 91.5 ± 4.5 | [272] |
| Transferrin-conjugated Lipoplex (Tf-LNPs-MF)/ DOTMA:DODMA:EPC:Chol:mPEG-Cho | siRNAs | SHM | 3 inlets: 3:1:1 | NA | Increased permeability in HepG-2 cells by Tf receptor mediated uptake | Tf-LNPs-MF-siRNA in blood was >100 ng·mL−1 and t1/2 was 25.6 h 48 h post IV vs. <10 ng·mL−1 and t1/2 of 15.1 h for free siRNA | 132.6 | 0.129 | N/A | [224] |
| Lipoplex/ DLinKC2-DMA(cationic lipid):Chol:DSPC:PEG2000-C-DMA | siRNAs | SHM | 3:1 | 0.02−4.00 | NA | 50% silencing in hepatocytes at 10 µg·kg−1 in mice | 28−54 | <0.1 | ~100 | [228] |
| Lipoplex/ DSPC:mPEG2000-DMG:Chol:range of cationic lipids | siRNAs | SHM | 1:1:2 (lipids: siRNA: buffer | 1.2 | NA | Gene silencing potency of >90% at 1.0 mg·kg−1 in mice | 90.5 | NA | ∼80 | [258] |
| Cubosomes/ DOTAP:Glycerol monooleate (GMO):GMO-PEG2000 | siRNAs | SHM | 6:1 | 4 | Gene-knockdown efficiency of 73.6% for a ρ = nDOTAP/nNA of 3 vs. Lipofectamine (45.8%) efficiency; Up to ρ = 10, no significant damage to cell membranes. | NA | 77 | 0.06 | >90% | [273] |
| Lipid nanoparticles/ YSK05 (cationic pH-sensitive lipid):Chol:mPEG2000-DMG | siRNA | SHM | 3:1 | 1.5 | Particles with 1%, 1.25%, and 1.5% w/w and <2% mPEG (67.1, 57.3, and 53.8 nm) show high and similar gene silencing efficiencies | FVII gene silencing activity of 50% Chol-rich 1% mPEG-LNPs was higher than 3% mPEG-LNPs | 32−67 | NA | ∼100 | [274] |
| Lipid nanoparticles/ YSK05 (cationic pH-sensitive lipid):Chol:mPEG2000-DMG | siRNA | Baffle mixers | 3:1 | 0.5 | NA | YSK-LNPs showed high FVII gene-silencing activity with no dose dependency | 80 | 0.1 | >90% | [269] |
| Liposomes/ EPC:DMPC:DPPC:DSPC | Metformin (M) and glipizide (G) | NASHM | 5:1 | 5–15 | Sustained release was achieved | NA | 80–90 | 0.11–0.22 | ~20 (M) ~40 (G) | [250] |
| Lipid nanoparticles/ DSPC:D-Lin-MC3-DMA:Chol:PEG-DMG | siRNA | NASHM | 3:1 | 12 | 79% mRNA knockdown produced by 1 μg siRNA | 15 mg·kg−1 (3 doses IV over 24 h) results in a 100% uptake in peripheral blood cells that remain positive until day 10; no liver toxicity or other biochemical alternation; after 10 IV doses over 35 days, luciferase signal decreased 0.75-fold, while it increased in control mice 1.6-fold; 60% knockdown efficiency of BCR-ABL by LNP-anti-BCR-ABL siRNA in sorted leukemia cells from the myelosarcoma mouse tissue | 55.03 | 0.046 | >90 | [275] |
| Liposomes/ DSPC:mPEG2000-DSPE | Dox, ICU | SHM | 10:1 and/or 16:1 | 5 | After 48 h, ~90% of drug was released (first order); less cytotoxic to MCF-7, MDA-MB 231 and BT-474 breast cancer cells vs. free doxorubicin | NA | ~100 | 0.2 | >80 | [276] |
| Liposomes/ DMPC:DPPC:DSPC | Curcumin | NASHM | 5:1 | 17 | Increased 700-fold the aqueous curcumin solubility | When co-administered with cisplatin, it enhances cisplatin’s efficacy in multiple mouse tumor models with decreased nephrotoxicity | ~125 | <0.2 | 87.7 | [277] |
| Nanoemulsions/ Cold-pressed hempseed oil:lecithin:Poloxamer 188 | Hempseed oil | NASHM | 4:1 | 12 | >98% Caco-2 cell viability; increased uptake by 38.2% | NA | 62 | 0.032 | > 99 | [278] |
| Liposomes/ HSPC:DOPC:mPEG2000-DSPE | Dox | NASHM | 9:1 | 10 | Burst release (20–30%) followed by <10% release over 7 days at 37 °C or during 3 weeks storage at 4 °C | Higher tumor accumulation (5–6% dose/g) at days 1 and 4. | ~50 | <0.2 | > 80 | [279] |
| Liposomes/ EPC or DMPC or DPPC or DSPC or PS:Chol | OVA | NASHM | 3:1 | 15 | Longer chain lipids have slower release rates; burst release observed within 12 h followed by a slower release rate | NA | 60–100 | <0.2 | 20–35 | [280] |
| Liposomes/ EPC:Chol | Propofol | NASHM | 3:1 | 2 | Burst release (40%,1 h) reaching 90% within 8 h | NA | ~40 | 0.4 | 85 | [264] |
| Liposomes/DMPC:Chol:DEPE-PEG2000:DSPE-PEG2000—FA or DSPE-PEG2000-Cys-TAT(CYGRKKRRQRRR) 55:40:3:1 molar ratio | Folic acid (FA) | HFF | 16:1 | 28.8 µL/min | FA and TAT liposomes have 37% and 98% increased targeting in SKOV3 cell spheroids compared to TAT liposomes and FA liposomes, respectively | Improved tumor targeting and longer tumor retention (up to 72 h); 140%, 136%, and 62% higher tumor accumulation than pegylated liposomes and FA or TAT targeted liposomes, respectively | ~60 | <0.3 | NA | [223] |
| Lipoplex/DSPC:cholesterol: DOTAB or DDAB or D-Lin-MC3-DMA:DMG-PEG2000, | mRNA or ssDNA, Poly A | NxGen | 5:1–1:1 | 12−200 | NA | NA | <100 | <0.25 | >90 | [266] |
| Liposomes/HSPC:Chol:DSPE-PEG2000 56:38:5 molar ratio and EPC:Chol 45:55 molar ratio | Dox | M110P Microfluidizer® | Pressures of 5–20 Kpsi | 1–3 cycles | NA | NA | 100–110 | <0.2 | 97–98 | [281] |
| Techniques | Mechanism | Separation Marker | Sizes Separated (nm) | Efficiency (%) | Throughput (mL·min−1) | Pros | Cons |
|---|---|---|---|---|---|---|---|
| Field flow fractionation | Asymmetrical flow FFF | Size | 5–250 | 87–88 | 0.4–1.1 | Very high throughput with high separation efficiency | Specific sample/solvent systems and compatible membrane |
| Centrifugal | Centrifugal force | Size, density | 50–200 | - | 0.0075 | High throughput, density gradient, and dilution not required | Discontinuous |
| Optical | Optical force | Size, refractive index, polarizability | 70–1000 | - | 0.010–0.375 | High separation efficiency | Heating and photodamage, low throughput |
| Affinity capture | Surface interactions | Antigenic site, hydrophobicity, charge | 100 | - | 0.010 | High capture efficiency and purity | Expensive, multiple preparation steps |
| Electrophore-sis | Uniform electric field | Size, charge | <50 | 97 | 0.0004 | Very high separation efficiency and resolution | Flow rate change with chemistry (buffers, wall effects) |
| Dielectropho-resis | Nonuniform electric field | Polarizability and size | 30–60 | 85–100 | 0.000009 | High throughput and separation efficiency | Requires high voltage, depends on medium conductivity, very low throughput |
| Magnetopho-resis | Magnetic field | Size, magnetic properties | 5–200 | 90 | 0.300 | Very high throughput, low cost | Long time for magnetic bead antibody labeling |
| Acoustopho-resis | Ultrasonic sound wave | Size, density, compressibility | <200 | >90 | 0.00043–0.00081 | High separation efficiency, controlled cut off separation | Complex fabrication, limited device material to transmit acoustic power efficiently |
| Ion concentration polarization | Electric field | Size, electrophoretic mobility | 100–500 | - | 0.0005 | Low voltage, no need for internal electrode | Low resolution on small size particles, low throughput |
| Electrohydro-dynamic vortices | Traveling waves, ohmic heating | Size, charge | 200 | ~100 | 0.000033 | High separation efficiency | Complex fabrication of microelectrode, low throughput |
| Deterministic lateral displacement | Laminar flow stream | Size, deformability | 190–2000 | ~100 | 0.00001 | Controllable cutoff size, simple and efficient, high separation efficiency (20 nm resolution) | Very low throughput, precise fabrication required, pillar clogging is possible |
| Hydrodyna-mic filtration | Hydrodynamic sieving | Size | 100–1000 | - | 0.001 | Simple, high separation efficiency, medium throughput | Prone to clogging |
| Spiral microfluidics | Dean vortices | Size, shape | 590–7320 | 95 | 0.010 | Very high separation efficiency, simple | Prone to particle-particle interactions and diffusion disruption |
| Inertial microfluidics | Shear and wall lift | Size, shape | 590–1980 | - | - | Very high throughput, separation efficiency, simple | Prone to particle–particle interactions and diffusion disruption |
| Electrostatic sieving | Electric double-layer force | Size, charge | 19–50 | 97 | 0.0006 | Very high separation efficiency, controllable cut-off size | Separation only possible in low ionic strength conditions, low throughput |
| Bacterial chemotaxis | Chemotaxis, diffusion, and bacterial motility | Selective adhesion on bacteria | 320–390 | 81 | 0.000013 | Simple, low cost | Requires antibody conjugation for selective adhesion to bacteria, very low throughput, and relatively medium separation efficiency |
5. Further Application and Processes of Microfluidic Approaches
5.1. Purification Strategies
5.2. Analysis on a Chip and Production with a High Throughput
5.3. Scale-Up Manufacture
6. Future Perspectives and Challenges
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kara, A.; Vassiliadou, A.; Ongoren, B.; Keeble, W.; Hing, R.; Lalatsa, A.; Serrano, D.R. Engineering 3D Printed Microfluidic Chips for the Fabrication of Nanomedicines. Pharmaceutics 2021, 13, 2134. [Google Scholar] [CrossRef] [PubMed]
- Abedinoghli, D.; Charkhpour, M.; Osouli-Bostanabad, K.; Selselehjonban, S.; Emami, S.; Barzegar-Jalali, M.; Adibkia, K. Electrosprayed Nanosystems of Carbamazepine—PVP K30 for Enhancing Its Pharmacologic Effects. Iran. J. Pharm. Res. 2018, 17, 1431–1443. [Google Scholar] [PubMed]
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Specification, P.A. Terminology for Nanomaterials; British Standards Institute: London, UK, 2007. [Google Scholar]
- Bosetti, R.; Jones, S.L. Cost–effectiveness of nanomedicine: Estimating the real size of nano-costs. Nanomedicine 2019, 14, 1367–1370. [Google Scholar] [CrossRef]
- Peptide Therapeutics Market. Reports and Data. 2020, p. 294. Available online: https://www.reportsanddata.com/report-detail/peptide-therapeutics-market (accessed on 5 August 2022).
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Germain, M.; Caputo, F.; Metcalfe, S.; Tosi, G.; Spring, K.; Åslund, A.K.O.; Pottier, A.; Schiffelers, R.; Ceccaldi, A.; Schmid, R. Delivering the power of nanomedicine to patients today. J. Control. Release 2020, 326, 164–171. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef]
- Forssen, E.A. The design and development of DaunoXome® for solid tumor targeting in vivo. Adv. Drug Deliv. Rev. 1997, 24, 133–150. [Google Scholar] [CrossRef]
- Boswell, G.W.; Buell, D.; Bekersky, I. AmBisome (Liposomal Amphotericin B): A Comparative Review. J. Clin. Pharmacol. 1998, 38, 583–592. [Google Scholar] [CrossRef]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef]
- Silverman, J.A.; Deitcher, S.R. Marqibo® (vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother. Pharmacol. 2013, 71, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiang, J.; Chan, R.; Ji, Y.; Lu, J.; Liao, Y.-P.; Okene, M.; Lin, J.; Lin, P.; Chang, C.H.; et al. Improved Efficacy and Reduced Toxicity Using a Custom-Designed Irinotecan-Delivering Silicasome for Orthotopic Colon Cancer. ACS Nano 2019, 13, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Akinc, A.; Maier, M.A.; Manoharan, M.; Fitzgerald, K.; Jayaraman, M.; Barros, S.; Ansell, S.; Du, X.; Hope, M.J.; Madden, T.D.; et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat. Nanotechnol. 2019, 14, 1084–1087. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Han, H.-K. Nanomedicines: Current status and future perspectives in aspect of drug delivery and pharmacokinetics. J. Pharm. Investig. 2018, 48, 43–60. [Google Scholar] [CrossRef]
- Martins, J.P.; das Neves, J.; de la Fuente, M.; Celia, C.; Florindo, H.; Günday-Türeli, N.; Popat, A.; Santos, J.L.; Sousa, F.; Schmid, R.; et al. The solid progress of nanomedicine. Drug Deliv. Transl. Res. 2020, 10, 726–729. [Google Scholar] [CrossRef]
- Thi, T.T.H.; Suys, E.J.A.; Lee, J.S.; Nguyen, D.H.; Park, K.D.; Truong, N.P. Lipid-Based Nanoparticles in the Clinic and Clinical Trials: From Cancer Nanomedicine to COVID-19 Vaccines. Vaccines 2021, 9, 359. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Shin, M.D.; Shukla, S.; Chung, Y.H.; Beiss, V.; Chan, S.K.; Ortega-Rivera, O.A.; Wirth, D.M.; Chen, A.; Sack, M.; Pokorski, J.K.; et al. COVID-19 vaccine development and a potential nanomaterial path forward. Nat. Nanotechnol. 2020, 15, 646–655. [Google Scholar] [CrossRef]
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, Properties, and Regulatory Issues. Front. Chem. 2018, 6, 360. [Google Scholar] [CrossRef]
- Selselehjonban, S.; Garjani, A.; Osouli-Bostanabad, K.; Tanhaei, A.; Emami, S.; Adibkia, K.; Barzegar-Jalali, M. Physicochemical and pharmacological evaluation of carvedilol-eudragit(®) RS100 electrosprayed nanostructures. Iran. J. Basic Med. Sci. 2019, 22, 547–556. [Google Scholar] [CrossRef]
- Kim, B.Y.S.; Rutka, J.T.; Chan, W.C.W. Nanomedicine. N. Engl. J. Med. 2010, 363, 2434–2443. [Google Scholar] [CrossRef] [PubMed]
- Khurana, A.; Allawadhi, P.; Khurana, I.; Allwadhi, S.; Weiskirchen, R.; Banothu, A.K.; Chhabra, D.; Joshi, K.; Bharani, K.K. Role of nanotechnology behind the success of mRNA vaccines for COVID-19. Nano Today 2021, 38, 101142. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Ko, J.; Lee, S.; Yu, J.; Kim, Y.; Jeon, N.L. Microfluidics in nanoparticle drug delivery; From synthesis to pre-clinical screening. Adv. Drug Deliv. Rev. 2018, 128, 29–53. [Google Scholar] [CrossRef] [PubMed]
- Fornaguera, C.; García-Celma, M.J. Personalized Nanomedicine: A Revolution at the Nanoscale. J. Pers. Med. 2017, 7, 12. [Google Scholar] [CrossRef]
- Tapeinos, C.; Battaglini, M.; Ciofani, G. Advances in the design of solid lipid nanoparticles and nanostructured lipid carriers for targeting brain diseases. J. Control. Release 2017, 264, 306–332. [Google Scholar] [CrossRef]
- Fonseca-Santos, B.; Gremião, M.P.D.; Chorilli, M. Nanotechnology-based drug delivery systems for the treatment of Alzheimer’s disease. Int. J. Nanomed. 2015, 10, 4981–5003. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Fenton, O.S.; Olafson, K.N.; Pillai, P.S.; Mitchell, M.J.; Langer, R. Advances in Biomaterials for Drug Delivery. Adv. Mater. 2018, 30, 1705328. [Google Scholar] [CrossRef]
- Sarfraz, M.; Afzal, A.; Yang, T.; Gai, Y.; Raza, S.M.; Khan, M.W.; Cheng, Y.; Ma, X.; Xiang, G. Development of Dual Drug Loaded Nanosized Liposomal Formulation by A Reengineered Ethanolic Injection Method and Its Pre-Clinical Pharmacokinetic Studies. Pharmaceutics 2018, 10, 151. [Google Scholar] [CrossRef]
- Sedighi, M.; Sieber, S.; Rahimi, F.; Shahbazi, M.-A.; Rezayan, A.H.; Huwyler, J.; Witzigmann, D. Rapid optimization of liposome characteristics using a combined microfluidics and design-of-experiment approach. Drug Deliv. Transl. Res. 2019, 9, 404–413. [Google Scholar] [CrossRef]
- Lalatsa, A.; Schätzlein, A.G.; Uchegbu, I.F. Drug delivery across the blood-brain barrier. In Comprehensive Biotechnology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 628–637. [Google Scholar]
- Fernández-García, R.; Lalatsa, A.; Statts, L.; Bolás-Fernández, F.; Ballesteros, M.P.; Serrano, D.R. Transferosomes as nanocarriers for drugs across the skin: Quality by design from lab to industrial scale. Int J. Pharm 2020, 573, 118817. [Google Scholar] [CrossRef]
- Rai, S.; Pandey, V.; Rai, G. Transfersomes as versatile and flexible nano-vesicular carriers in skin cancer therapy: The state of the art. Nano Rev. Exp. 2017, 8, 1325708. [Google Scholar] [CrossRef] [PubMed]
- Opatha, S.A.T.; Titapiwatanakun, V.; Chutoprapat, R. Transfersomes: A Promising Nanoencapsulation Technique for Transdermal Drug Delivery. Pharmaceutics 2020, 12, 855. [Google Scholar] [CrossRef] [PubMed]
- Naik, U.S. The Synthesis and Characterisation of Novel Ultra-Flexible Lipidic Vesicles Using Propanol. Ph.D. Thesis, University of Central Lancashire, Preston, UK, 2013. [Google Scholar]
- Rane, B.R.; Gujarathi, N.A. Transfersomes and Protransfersome: Ultradeformable Vesicular System. In Novel Approaches for Drug Delivery; Keservani, R.K., Sharma, A.K., Kesharwani, R.K., Eds.; IGI Global: Hershey, PA, USA, 2017; pp. 149–169. [Google Scholar]
- Rajan, R.; Jose, S.; Biju Mukund, V.; Vasudevan, D. Transferosomes—A vesicular transdermal delivery system for enhanced drug permeation. J. Adv. Pharm. Technol. Res. 2011, 2, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Jangdey, M.S.; Gupta, A.; Saraf, S.; Saraf, S. Development and optimization of apigenin-loaded transfersomal system for skin cancer delivery: In vitro evaluation. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1452–1462. [Google Scholar] [CrossRef] [PubMed]
- Touitou, E. Compositions for Applying Active Substances to or through the Skin. U.S. Patent No. 5,540,934, 30 July 1996. [Google Scholar]
- Touitou, E.; Dayan, N.; Bergelson, L.; Godin, B.; Eliaz, M. Ethosomes—Novel vesicular carriers for enhanced delivery: Characterization and skin penetration properties. J. Control. Release 2000, 65, 403–418. [Google Scholar] [CrossRef]
- Touitou, E. Composition for Applying Active Substances to or through the Skin. U.S. Patent No. 5,716,638, 10 February 1998. [Google Scholar]
- Saifi, Z.; Rizwanullah, M.; Mir, S.R.; Amin, S. Bilosomes nanocarriers for improved oral bioavailability of acyclovir: A complete characterization through in vitro, ex-vivo and in vivo assessment. J. Drug Deliv. Sci. Technol. 2020, 57, 101634. [Google Scholar] [CrossRef]
- Conacher, M.; Alexander, J.; Brewer, J.M. Oral immunisation with peptide and protein antigens by formulation in lipid vesicles incorporating bile salts (bilosomes). Vaccine 2001, 19, 2965–2974. [Google Scholar] [CrossRef]
- Pavlović, N.; Goločorbin-Kon, S.; Ðanić, M.; Stanimirov, B.; Al-Salami, H.; Stankov, K.; Mikov, M. Bile Acids and Their Derivatives as Potential Modifiers of Drug Release and Pharmacokinetic Profiles. Front. Pharmacol. 2018, 9, 1283. [Google Scholar] [CrossRef]
- Niu, M.; Lu, Y.; Hovgaard, L.; Guan, P.; Tan, Y.; Lian, R.; Qi, J.; Wu, W. Hypoglycemic activity and oral bioavailability of insulin-loaded liposomes containing bile salts in rats: The effect of cholate type, particle size and administered dose. Eur. J. Pharm. Biopharm. 2012, 81, 265–272. [Google Scholar] [CrossRef]
- Aburahma, M.H. Bile salts-containing vesicles: Promising pharmaceutical carriers for oral delivery of poorly water-soluble drugs and peptide/protein-based therapeutics or vaccines. Drug Deliv. 2016, 23, 1847–1867. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Mäder, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery—A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Patel, S.; Ryals, R.C.; Weller, K.K.; Pennesi, M.E.; Sahay, G. Lipid nanoparticles for delivery of messenger RNA to the back of the eye. J. Control. Release 2019, 303, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Vhora, I.; Lalani, R.; Bhatt, P.; Patil, S.; Misra, A. Lipid-nucleic acid nanoparticles of novel ionizable lipids for systemic BMP-9 gene delivery to bone-marrow mesenchymal stem cells for osteoinduction. Int. J. Pharm. 2019, 563, 324–336. [Google Scholar] [CrossRef]
- Duan, Y.; Dhar, A.; Patel, C.; Khimani, M.; Neogi, S.; Sharma, P.; Kumar, N.S.; Vekariya, R.L. A brief review on solid lipid nanoparticles: Part and parcel of contemporary drug delivery systems. RSC Adv. 2020, 10, 26777–26791. [Google Scholar] [CrossRef]
- Laffleur, F.; Keckeis, V. Advances in drug delivery systems: Work in progress still needed? Int. J. Pharm. X 2020, 2, 100050. [Google Scholar] [CrossRef]
- Zarif, L.; Graybill, J.R.; Perlin, D.; Mannino, R.J. Cochleates: New Lipid-Based Drug Delivery System. J. Liposome Res. 2000, 10, 523–538. [Google Scholar] [CrossRef]
- Zarif, L. Elongated supramolecular assemblies in drug delivery. J. Control. Release 2002, 81, 7–23. [Google Scholar] [CrossRef]
- Shende, P.; Khair, R.; Gaud, R.S. Nanostructured cochleates: A multi-layered platform for cellular transportation of therapeutics. Drug Dev. Ind. Pharm. 2019, 45, 869–881. [Google Scholar] [CrossRef]
- Talke, S.; Salunkhe, K.; Chavan, M. A Review on nanocochleates novel approach for drug delivery. World J. Pharm. Pharm. Sci. 2018, 7, 284–294. [Google Scholar]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ho, W.; Zhang, X.; Bertrand, N.; Farokhzad, O. Cancer nanomedicine: From targeted delivery to combination therapy. Trends Mol. Med. 2015, 21, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Yingchoncharoen, P.; Kalinowski, D.S.; Richardson, D.R. Lipid-Based Drug Delivery Systems in Cancer Therapy: What Is Available and What Is Yet to Come. Pharmacol. Rev. 2016, 68, 701–787. [Google Scholar] [CrossRef]
- Ventola, C.L. Progress in Nanomedicine: Approved and Investigational Nanodrugs. Pharm. Ther. 2017, 42, 742–755. [Google Scholar]
- Shah, S.; Dhawan, V.; Holm, R.; Nagarsenker, M.S.; Perrie, Y. Liposomes: Advancements and innovation in the manufacturing process. Adv. Drug Deliv. Rev. 2020, 154–155, 102–122. [Google Scholar] [CrossRef]
- Fan, Y.; Marioli, M.; Zhang, K. Analytical characterization of liposomes and other lipid nanoparticles for drug delivery. J. Pharm. Biomed. Anal. 2021, 192, 113642. [Google Scholar] [CrossRef]
- Lamichhane, N.; Udayakumar, T.S.; D’Souza, W.D.; Simone II, C.B.; Raghavan, S.R.; Polf, J.; Mahmood, J. Liposomes: Clinical Applications and Potential for Image-Guided Drug Delivery. Molecules 2018, 23, 288. [Google Scholar] [CrossRef]
- Spectrum Pharmaceuticals, Inc. Topotecan Liposomes Injection for Small Cell Lung Cancer (SCLC), Ovarian Cancer and Other Advanced Solid Tumors. Available online: https://clinicaltrials.gov/ct2/show/NCT00765973 (accessed on 13 November 2020).
- Swiss Group for Clinical Cancer Research. TLD-1, a Novel Liposomal Doxorubicin, in Patients with Advanced Solid Tumors. Available online: https://clinicaltrials.gov/ct2/show/NCT03387917 (accessed on 7 September 2022).
- Mebiopharm Co., Ltd. Safety Study of MBP-426 (Liposomal Oxaliplatin Suspension for Injection) to Treat Advanced or Metastatic Solid Tumors. Available online: https://clinicaltrials.gov/ct2/show/NCT00355888 (accessed on 2 December 2014).
- Mebiopharm Co., Ltd. Study of MBP-426 in Patients with Second Line Gastric, Gastroesophageal, or Esophageal Adenocarcinoma. Available online: https://www.clinicaltrials.gov/ct2/show/NCT00964080 (accessed on 2 December 2014).
- Munster, P.; Krop, I.E.; LoRusso, P.; Ma, C.; Siegel, B.A.; Shields, A.F.; Molnár, I.; Wickham, T.J.; Reynolds, J.; Campbell, K.; et al. Safety and pharmacokinetics of MM-302, a HER2-targeted antibody–liposomal doxorubicin conjugate, in patients with advanced HER2-positive breast cancer: A phase 1 dose-escalation study. Br. J. Cancer 2018, 119, 1086–1093. [Google Scholar] [CrossRef]
- Celsion. Study of ThermoDox with Standardized Radiofrequency Ablation (RFA) for Treatment of Hepatocellular Carcinoma (HCC) (OPTIMA). Available online: https://clinicaltrials.gov/ct2/show/NCT02112656 (accessed on 24 October 2018).
- Mebiopharm Co., Ltd. Active Targeting Drug Delivery System. Available online: http://www.mebiopharm.com/english/pro.html (accessed on 4 August 2021).
- Yonezawa, S.; Koide, H.; Asai, T. Recent advances in siRNA delivery mediated by lipid-based nanoparticles. Adv. Drug Deliv. Rev. 2020, 154–155, 64–78. [Google Scholar] [CrossRef]
- Ely, A.; Singh, P.; Smith, T.S.; Arbuthnot, P. In vitro transcribed mRNA for expression of designer nucleases: Advantages as a novel therapeutic for the management of chronic HBV infection. Adv. Drug Deliv. Rev. 2021, 168, 134–146. [Google Scholar] [CrossRef]
- McGoron, A.J. Perspectives on the Future of Nanomedicine to Impact Patients: An Analysis of US Federal Funding and Interventional Clinical Trials. Bioconj. Chem. 2020, 31, 436–447. [Google Scholar] [CrossRef] [PubMed]
- ModernaTX, Inc.; AstraZeneca. Dose Escalation Study of mRNA-2752 for Intratumoral Injection to Participants in Advanced Malignancies. Available online: https://clinicaltrials.gov/ct2/show/NCT03739931 (accessed on 18 July 2022).
- ModernaTX, Inc. Dose Escalation and Efficacy Study of mRNA-2416 for Intratumoral Injection Alone and in Combination with Durvalumab for Participants with Advanced Malignancies. Available online: https://clinicaltrials.gov/ct2/show/NCT03323398 (accessed on 11 July 2022).
- National Cancer Institute (NCI). T4N5 Liposomal Lotion in Preventing The Recurrence of Nonmelanoma Skin Cancer in Patients Who Have Undergone a Kidney Transplant. Available online: https://clinicaltrials.gov/ct2/show/NCT00089180 (accessed on 4 December 2015).
- BioNTech, SE. Evaluation of the Safety and Tolerability of i.v. Administration of a Cancer Vaccine in Patients with Advanced Melanoma (Lipo-MERIT). Available online: https://clinicaltrials.gov/ct2/show/NCT02410733 (accessed on 24 August 2022).
- ModernaTX, Inc.; Merck Sharp & Dohme Corp. An Efficacy Study of Adjuvant Treatment with the Personalized Cancer Vaccine mRNA-4157 and Pembrolizumab in Participants with High-Risk Melanoma (KEYNOTE-942). Available online: https://clinicaltrials.gov/ct2/show/NCT03897881 (accessed on 24 August 2022).
- Ebinger, J.E.; Fert-Bober, J.; Printsev, I.; Wu, M.; Sun, N.; Prostko, J.C.; Frias, E.C.; Stewart, J.L.; Van Eyk, J.E.; Braun, J.G.; et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat. Med. 2021, 27, 981–984. [Google Scholar] [CrossRef] [PubMed]
- Weiss, C.; Carriere, M.; Fusco, L.; Capua, I.; Regla-Nava, J.A.; Pasquali, M.; Scott, J.A.; Vitale, F.; Unal, M.A.; Mattevi, C.; et al. Toward Nanotechnology-Enabled Approaches against the COVID-19 Pandemic. ACS Nano 2020, 14, 6383–6406. [Google Scholar] [CrossRef]
- Kulkarni, J.A.; Cullis, P.R.; van der Meel, R. Lipid Nanoparticles Enabling Gene Therapies: From Concepts to Clinical Utility. Nucleic Acid Ther. 2018, 28, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Rudra, A.; Li, J.; Shakur, R.; Bhagchandani, S.; Langer, R. Trends in Therapeutic Conjugates: Bench to Clinic. Bioconj. Chem. 2020, 31, 462–473. [Google Scholar] [CrossRef]
- Van Riel, D.; de Wit, E. Next-generation vaccine platforms for COVID-19. Nat. Mater. 2020, 19, 810–812. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.H.; Liu, X.; Mahalingam, S. Development of vaccines for SARS-CoV-2. F1000Res 2020, 9, 991. [Google Scholar] [CrossRef]
- Ickenstein, L.M.; Garidel, P. Lipid-based nanoparticle formulations for small molecules and RNA drugs. Expert Opin. Drug Deliv. 2019, 16, 1205–1226. [Google Scholar] [CrossRef]
- Hu, B.; Zhong, L.; Weng, Y.; Peng, L.; Huang, Y.; Zhao, Y.; Liang, X.-J. Therapeutic siRNA: State of the art. Signal Transduct. Target. Ther. 2020, 5, 101. [Google Scholar] [CrossRef]
- Anderluzzi, G.; Schmidt, S.T.; Cunliffe, R.; Woods, S.; Roberts, C.W.; Veggi, D.; Ferlenghi, I.; O’Hagan, D.T.; Baudner, B.C.; Perrie, Y. Rational design of adjuvants for subunit vaccines: The format of cationic adjuvants affects the induction of antigen-specific antibody responses. J. Control. Release 2021, 330, 933–944. [Google Scholar] [CrossRef]
- Lee, K.; Kim, S.Y.; Seo, Y.; Kim, M.H.; Chang, J.; Lee, H. Adjuvant incorporated lipid nanoparticles for enhanced mRNA-mediated cancer immunotherapy. Biomater. Sci. 2020, 8, 1101–1105. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Tripathi, S.; Tripathi, P.K. Invasomes: Potential vesicular systems for transdermal delivery of drug molecules. J. Drug Deliv. Sci. Technol. 2021, 61, 102166. [Google Scholar] [CrossRef]
- Nasirizadeh, S.; Malaekeh-Nikouei, B. Solid lipid nanoparticles and nanostructured lipid carriers in oral cancer drug delivery. J. Drug Deliv. Sci. Technol. 2020, 55, 101458. [Google Scholar] [CrossRef]
- Chacko, I.A.; Ghate, V.M.; Dsouza, L.; Lewis, S.A. Lipid vesicles: A versatile drug delivery platform for dermal and transdermal applications. Colloids Surf. B Biointerfaces 2020, 195, 111262. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Michalowski, C.B.; Beloqui, A. Advances in lipid carriers for drug delivery to the gastrointestinal tract. Curr. Opin. Colloid Interface Sci. 2021, 52, 101414. [Google Scholar] [CrossRef]
- Leung, A.K.K.; Tam, Y.Y.C.; Chen, S.; Hafez, I.M.; Cullis, P.R. Microfluidic Mixing: A General Method for Encapsulating Macromolecules in Lipid Nanoparticle Systems. J. Phys. Chem. B 2015, 119, 8698–8706. [Google Scholar] [CrossRef]
- Kulkarni, J.A.; Witzigmann, D.; Leung, J.; Tam, Y.Y.C.; Cullis, P.R. On the role of helper lipids in lipid nanoparticle formulations of siRNA. Nanoscale 2019, 11, 21733–21739. [Google Scholar] [CrossRef]
- Cheng, X.; Lee, R.J. The role of helper lipids in lipid nanoparticles (LNPs) designed for oligonucleotide delivery. Adv. Drug Deliv. Rev. 2016, 99, 129–137. [Google Scholar] [CrossRef]
- Cheng, Q.; Wei, T.; Farbiak, L.; Johnson, L.T.; Dilliard, S.A.; Siegwart, D.J. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing. Nat. Nanotechnol. 2020, 15, 313–320. [Google Scholar] [CrossRef]
- Berraondo, P.; Martini, P.G.V.; Avila, M.A.; Fontanellas, A. Messenger RNA therapy for rare genetic metabolic diseases. Gut 2019, 68, 1323–1330. [Google Scholar] [CrossRef]
- Razavi, H.; Janfaza, S. Ethosome: A nanocarrier for transdermal drug delivery. Arch. Adv. Biosci. 2015, 6, 38–43. [Google Scholar] [CrossRef]
- Abdulbaqi, I.M.; Darwis, Y.; Khan, N.A.K.; Abou Assi, R.; Khan, A.A. Ethosomal nanocarriers: The impact of constituents and formulation techniques on ethosomal properties, in vivo studies, and clinical trials. Int. J. Nanomed. 2016, 11, 2279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipa-Castro, A.; Legrand, F.-X.; Barratt, G. Cochleate drug delivery systems: An approach to their characterization. Int. J. Pharm. 2021, 610, 121225. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. COVID-19 Vaccine Tracker and Landscape. 2021. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 6 September 2022).
- Fang, E.; Liu, X.; Li, M.; Zhang, Z.; Song, L.; Zhu, B.; Wu, X.; Liu, J.; Zhao, D.; Li, Y. Advances in COVID-19 mRNA vaccine development. Signal Transduct. Target. Ther. 2022, 7, 94. [Google Scholar] [CrossRef]
- FDA News Release, Food and Drug Administration. FDA Approves First COVID-19 Vaccine. 2021. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine (accessed on 5 August 2022).
- FDA News Release, Food and Drug Administration. FDA Authorizes Pfizer-BioNTech COVID-19 Vaccine for Emergency Use in Children 5 through 11 Years of Age. 2021. Available online: https://www.fda.gov/news-events/press-announcements/fda-authorizes-pfizer-biontech-covid-19-vaccine-emergency-use-children-5-through-11-years-age (accessed on 5 August 2022).
- Koirala, A.; Joo, Y.J.; Khatami, A.; Chiu, C.; Britton, P.N. Vaccines for COVID-19: The current state of play. Paediatr. Respir. Rev. 2020, 35, 43–49. [Google Scholar] [CrossRef]
- Samaridou, E.; Heyes, J.; Lutwyche, P. Lipid nanoparticles for nucleic acid delivery: Current perspectives. Adv. Drug Deliv. Rev. 2020, 154–155, 37–63. [Google Scholar] [CrossRef]
- Theobald, N. Emerging vaccine delivery systems for COVID-19: Functionalised silica nanoparticles offer a potentially safe and effective alternative delivery system for DNA/RNA vaccines and may be useful in the hunt for a COVID-19 vaccine. Drug Discov. Today 2020, 25, 1556–1558. [Google Scholar] [CrossRef]
- Shih, H.-I.; Wu, C.-J.; Tu, Y.-F.; Chi, C.-Y. Fighting COVID-19: A quick review of diagnoses, therapies, and vaccines. Biomed. J. 2020, 43, 341–354. [Google Scholar] [CrossRef]
- Nakamura, T.; Harashima, H. Dawn of lipid nanoparticles in lymph node targeting: Potential in cancer immunotherapy. Adv. Drug Deliv. Rev. 2020, 167, 78–88. [Google Scholar] [CrossRef]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–252, IN26–IN27. [Google Scholar] [CrossRef]
- Batzri, S.; Korn, E.D. Single bilayer liposomes prepared without sonication. Biochim. Biophys. Acta 1973, 298, 1015–1019. [Google Scholar] [CrossRef]
- Zumbuehl, O.; Weder, H.G. Liposomes of controllable size in the range of 40 to 180 nm by defined dialysis of lipid/detergent mixed micelles. Biochim. Biophys. Acta 1981, 640, 252–262. [Google Scholar] [CrossRef]
- Smith, L.; Serrano, D.R.; Mauger, M.; Bolás-Fernández, F.; Dea-Ayuela, M.A.; Lalatsa, A. Orally Bioavailable and Effective Buparvaquone Lipid-Based Nanomedicines for Visceral Leishmaniasis. Mol. Pharm. 2018, 15, 2570–2583. [Google Scholar] [CrossRef]
- Fernández- García, R.; Statts, L.; de Jesus, J.A.; Dea-Ayuela, M.A.; Bautista, L.; Simão, R.; Bolás-Fernández, F.; Ballesteros, M.P.; Laurenti, M.D.; Passero, L.F.D.; et al. Ultradeformable Lipid Vesicles Localize Amphotericin B in the Dermis for the Treatment of Infectious Skin Diseases. ACS Infect. Dis. 2020, 6, 2647–2660. [Google Scholar] [CrossRef] [PubMed]
- Colombo, S.; Beck-Broichsitter, M.; Bøtker, J.P.; Malmsten, M.; Rantanen, J.; Bohr, A. Transforming nanomedicine manufacturing toward Quality by Design and microfluidics. Adv. Drug Deliv. Rev. 2018, 128, 115–131. [Google Scholar] [CrossRef]
- Worsham, R.D.; Thomas, V.; Farid, S.S. Potential of Continuous Manufacturing for Liposomal Drug Products. Biotechnol. J. 2019, 14, 1700740. [Google Scholar] [CrossRef] [PubMed]
- Maherani, B.; Arab-Tehrany, E.; Mozafari, R.M.; Gaiani, C.; Linder, M. Liposomes: A Review of Manufacturing Techniques and Targeting Strategies. Curr. Nanosci. 2011, 7, 436–452. [Google Scholar] [CrossRef]
- Pandita, D.; Ahuja, A.; Lather, V.; Benjamin, B.; Dutta, T.; Velpandian, T.; Khar, R.K. Development of Lipid-Based Nanoparticles for Enhancing the Oral Bioavailability of Paclitaxel. AAPS PharmSciTech 2011, 12, 712–722. [Google Scholar] [CrossRef]
- Aditya, N.P.; Patankar, S.; Madhusudhan, B.; Murthy, R.S.R.; Souto, E.B. Arthemeter-loaded lipid nanoparticles produced by modified thin-film hydration: Pharmacokinetics, toxicological and in vivo anti-malarial activity. Eur. J. Pharm. Sci. 2010, 40, 448–455. [Google Scholar] [CrossRef]
- Evers, M.J.W.; Kulkarni, J.A.; van der Meel, R.; Cullis, P.R.; Vader, P.; Schiffelers, R.M. State-of-the-Art Design and Rapid-Mixing Production Techniques of Lipid Nanoparticles for Nucleic Acid Delivery. Small Methods 2018, 2, 1700375. [Google Scholar] [CrossRef]
- Pick, U. Liposomes with a large trapping capacity prepared by freezing and thawing of sonicated phospholipid mixtures. Arch. Biochem. Biophys. 1981, 212, 186–194. [Google Scholar] [CrossRef]
- Obeid, M.A.; Tate, R.J.; Mullen, A.B.; Ferro, V.A. Chapter 8—Lipid-based nanoparticles for cancer treatment. In Lipid Nanocarriers for Drug Targeting; Grumezescu, A.M., Ed.; William Andrew Publishing: Oxford, UK, 2018; pp. 313–359. [Google Scholar] [CrossRef]
- Richardson, J.; Caruso, F. Nanomedicine toward 2040. Nano Lett. 2020, 20, 1481–1482. [Google Scholar] [CrossRef] [PubMed]
- Kamb, A. What’s wrong with our cancer models? Nat. Rev. Drug Discov. 2005, 4, 161–165. [Google Scholar] [CrossRef]
- Tyner, K.M.; Zou, P.; Yang, X.; Zhang, H.; Cruz, C.N.; Lee, S.L. Product quality for nanomaterials: Current U.S. experience and perspective. WIREs Nanomed. Nanobiotechnol. 2015, 7, 640–654. [Google Scholar] [CrossRef]
- Burghelea, T.; Segre, E.; Bar-Joseph, I.; Groisman, A.; Steinberg, V. Chaotic flow and efficient mixing in a microchannel with a polymer solution. Phys. Rev. E 2004, 69, 066305. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-Y.; Wang, W.-T.; Liu, C.-C.; Fu, L.-M. Passive mixers in microfluidic systems: A review. Chem. Eng. J. 2016, 288, 146–160. [Google Scholar] [CrossRef]
- Yaralioglu, G.G.; Wygant, I.O.; Marentis, T.C.; Khuri-Yakub, B.T. Ultrasonic Mixing in Microfluidic Channels Using Integrated Transducers. Anal. Chem. 2004, 76, 3694–3698. [Google Scholar] [CrossRef]
- Lee, S.L.; O’Connor, T.F.; Yang, X.; Cruz, C.N.; Chatterjee, S.; Madurawe, R.D.; Moore, C.M.V.; Yu, L.X.; Woodcock, J. Modernizing Pharmaceutical Manufacturing: From Batch to Continuous Production. J. Pharm. Innov. 2015, 10, 191–199. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Tokeshi, M.; Sato, K. Micro/Nano Devices for Chemical Analysis. Micromachines 2016, 7, 164. [Google Scholar] [CrossRef]
- Karnik, R.; Gu, F.; Basto, P.; Cannizzaro, C.; Dean, L.; Kyei-Manu, W.; Langer, R.; Farokhzad, O.C. Microfluidic Platform for Controlled Synthesis of Polymeric Nanoparticles. Nano Lett. 2008, 8, 2906–2912. [Google Scholar] [CrossRef] [PubMed]
- Donno, R.; Gennari, A.; Lallana, E.; De La Rosa, J.M.R.; d’Arcy, R.; Treacher, K.; Hill, K.; Ashford, M.; Tirelli, N. Nanomanufacturing through microfluidic-assisted nanoprecipitation: Advanced analytics and structure-activity relationships. Int. J. Pharm. 2017, 534, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Bramosanti, M.; Chronopoulou, L.; Grillo, F.; Valletta, A.; Palocci, C. Microfluidic-assisted nanoprecipitation of antiviral-loaded polymeric nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2017, 532, 369–376. [Google Scholar] [CrossRef]
- Jaradat, E.; Weaver, E.; Meziane, A.; Lamprou, D.A. Microfluidics Technology for the Design and Formulation of Nanomedicines. Nanomaterials 2021, 11, 3440. [Google Scholar] [CrossRef]
- Beebe, D.J.; Mensing, G.A.; Walker, G.M. Physics and Applications of Microfluidics in Biology. Annu. Rev. Biomed. Eng. 2002, 4, 261–286. [Google Scholar] [CrossRef]
- Nguyen, N.-T.; Wereley, S.T.; Shaegh, S.A.M. Fundamentals and Applications of Microfluidics; Artech House: Norwood, MA, USA, 2019. [Google Scholar]
- Zhang, Z.; Zhao, P.; Xiao, G.; Lin, M.; Cao, X. Focusing-enhanced mixing in microfluidic channels. Biomicrofluidics 2008, 2, 014101. [Google Scholar] [CrossRef]
- Kumar, V.; Paraschivoiu, M.; Nigam, K.D.P. Single-phase fluid flow and mixing in microchannels. Chem. Eng. Sci. 2011, 66, 1329–1373. [Google Scholar] [CrossRef]
- Yang, Z.; Matsumoto, S.; Goto, H.; Matsumoto, M.; Maeda, R. Ultrasonic micromixer for microfluidic systems. Sens. Actuators A Phys. 2001, 93, 266–272. [Google Scholar] [CrossRef]
- Glasgow, I.; Aubry, N. Enhancement of microfluidic mixing using time pulsing. Lab Chip 2003, 3, 114–120. [Google Scholar] [CrossRef]
- Turkyilmazoglu, M. Magnetohydrodynamic Moving Liquid Plug Within a Microchannel: Analytical Solutions. J. Biomech. Eng. 2020, 143, 011012. [Google Scholar] [CrossRef]
- Tsai, J.-H.; Lin, L. Active microfluidic mixer and gas bubble filter driven by thermal bubble micropump. Sens. Actuators A Phys. 2002, 97–98, 665–671. [Google Scholar] [CrossRef]
- Wu, Z.; Nguyen, N.-T. Convective–diffusive transport in parallel lamination micromixers. Microfluid. Nanofluidics 2005, 1, 208–217. [Google Scholar] [CrossRef]
- Knight, J.B.; Vishwanath, A.; Brody, J.P.; Austin, R.H. Hydrodynamic Focusing on a Silicon Chip: Mixing Nanoliters in Microseconds. Phys. Rev. Lett. 1998, 80, 3863–3866. [Google Scholar] [CrossRef]
- Kamholz, A.E.; Yager, P. Molecular diffusive scaling laws in pressure-driven microfluidic channels: Deviation from one-dimensional Einstein approximations. Sens. Actuators B Chem. 2002, 82, 117–121. [Google Scholar] [CrossRef]
- Johnson, T.J.; Ross, D.; Locascio, L.E. Rapid Microfluidic Mixing. Anal. Chem. 2002, 74, 45–51. [Google Scholar] [CrossRef]
- Stroock, A.D.; Dertinger, S.K.W.; Ajdari, A.; Mezić, I.; Stone, H.A.; Whitesides, G.M. Chaotic Mixer for Microchannels. Science 2002, 295, 647–651. [Google Scholar] [CrossRef]
- Günther, A.; Jhunjhunwala, M.; Thalmann, M.; Schmidt, M.A.; Jensen, K.F. Micromixing of Miscible Liquids in Segmented Gas−Liquid Flow. Langmuir 2005, 21, 1547–1555. [Google Scholar] [CrossRef]
- Song, H.; Tice, J.D.; Ismagilov, R.F. A microfluidic system for controlling reaction networks in time. Angew. Chem. 2003, 115, 792–796. [Google Scholar] [CrossRef]
- Capretto, L.; Cheng, W.; Hill, M.; Zhang, X. Micromixing Within Microfluidic Devices. In Microfluidics: Technologies and Applications; Lin, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 27–68. [Google Scholar]
- Osouli-Bostanabad, K.; Masalehdan, T.; Kapsa, R.M.I.; Quigley, A.; Lalatsa, A.; Bruggeman, K.F.; Franks, S.J.; Williams, R.J.; Nisbet, D.R. Traction of 3D and 4D Printing in the Healthcare Industry: From Drug Delivery and Analysis to Regenerative Medicine. ACS Biomater. Sci. Eng. 2022, 8, 2764–2797. [Google Scholar] [CrossRef]
- Chang, C.-H.; Paul, B.K.; Remcho, V.T.; Atre, S.; Hutchison, J.E. Synthesis and post-processing of nanomaterials using microreaction technology. J. Nanopart. Res. 2008, 10, 965–980. [Google Scholar] [CrossRef]
- Bertuit, E.; Neveu, S.; Abou-Hassan, A. High Temperature Continuous Flow Syntheses of Iron Oxide Nanoflowers Using the Polyol Route in a Multi-Parametric Millifluidic Device. Nanomaterials 2022, 12, 119. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, G.; Hui, Y.; Ranaweera, S.; Zhao, C.-X. Microfluidic Nanoparticles for Drug Delivery. Small 2022, 18, 2106580. [Google Scholar] [CrossRef] [PubMed]
- Abou-Hassan, A.; Bazzi, R.; Cabuil, V. Multistep continuous-flow microsynthesis of magnetic and fluorescent γ-Fe2O3@ SiO2 core/shell nanoparticles. Angew. Chem. 2009, 121, 7316–7319. [Google Scholar] [CrossRef]
- Ying, Y.; Chen, G.; Zhao, Y.; Li, S.; Yuan, Q. A high throughput methodology for continuous preparation of monodispersed nanocrystals in microfluidic reactors. Chem. Eng. J. 2008, 135, 209–215. [Google Scholar] [CrossRef]
- Boleininger, J.; Kurz, A.; Reuss, V.; Sönnichsen, C. Microfluidic continuous flow synthesis of rod-shaped gold and silver nanocrystals. Phys. Chem. Chem. Phys. 2006, 8, 3824–3827. [Google Scholar] [CrossRef]
- Ju, J.; Zeng, C.; Zhang, L.; Xu, N. Continuous synthesis of zeolite NaA in a microchannel reactor. Chem. Eng. J. 2006, 116, 115–121. [Google Scholar] [CrossRef]
- She, Q.M.; Liu, J.H.; Aymonier, C.; Zhou, C.H. In situ fabrication of layered double hydroxide film immobilizing gold nanoparticles in capillary microreactor for efficient catalytic carbonylation of glycerol. Mol. Catal. 2021, 513, 111825. [Google Scholar] [CrossRef]
- Takagi, M.; Maki, T.; Miyahara, M.; Mae, K. Production of titania nanoparticles by using a new microreactor assembled with same axle dual pipe. Chem. Eng. J. 2004, 101, 269–276. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, Y.; Yang, B.; Luo, G. Controllable Preparation of Poly(butyl acrylate) by Suspension Polymerization in a Coaxial Capillary Microreactor. Ind. Eng. Chem. Res. 2011, 50, 11853–11862. [Google Scholar] [CrossRef]
- Flögel, O.; Codée, J.D.C.; Seebach, D.; Seeberger, P.H. Microreactor Synthesis of β-Peptides. Angew. Chem. Int. Ed. 2006, 45, 7000–7003. [Google Scholar] [CrossRef]
- Tan, Y.-C.; Hettiarachchi, K.; Siu, M.; Pan, Y.-R.; Lee, A.P. Controlled Microfluidic Encapsulation of Cells, Proteins, and Microbeads in Lipid Vesicles. J. Am. Chem. Soc. 2006, 128, 5656–5658. [Google Scholar] [CrossRef] [PubMed]
- Lorenceau, E.; Utada, A.S.; Link, D.R.; Cristobal, G.; Joanicot, M.; Weitz, D.A. Generation of Polymerosomes from Double-Emulsions. Langmuir 2005, 21, 9183–9186. [Google Scholar] [CrossRef] [PubMed]
- Shum, H.C.; Kim, J.-W.; Weitz, D.A. Microfluidic Fabrication of Monodisperse Biocompatible and Biodegradable Polymersomes with Controlled Permeability. J. Am. Chem. Soc. 2008, 130, 9543–9549. [Google Scholar] [CrossRef]
- Carugo, D.; Bottaro, E.; Owen, J.; Stride, E.; Nastruzzi, C. Liposome production by microfluidics: Potential and limiting factors. Sci. Rep. 2016, 6, 25876. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.S.M.; York, P.; Blagden, N. Preparation of hydrocortisone nanosuspension through a bottom-up nanoprecipitation technique using microfluidic reactors. Int. J. Pharm. 2009, 375, 107–113. [Google Scholar] [CrossRef]
- Edel, J.B.; Fortt, R.; deMello, J.C.; deMello, A.J. Microfluidic routes to the controlled production of nanoparticles. Chem. Commun. 2002, 10, 1136–1137. [Google Scholar] [CrossRef]
- Kastner, E.; Kaur, R.; Lowry, D.; Moghaddam, B.; Wilkinson, A.; Perrie, Y. High-throughput manufacturing of size-tuned liposomes by a new microfluidics method using enhanced statistical tools for characterization. Int. J. Pharm. 2014, 477, 361–368. [Google Scholar] [CrossRef]
- Aranguren, A.; Torres, C.E.; Munoz-Camargo, C.; Osma, J.F.; Cruz, J.C. Synthesis of Nanoscale Liposomes via Low-Cost Microfluidic Systems. Micromachines 2020, 11, 1050. [Google Scholar] [CrossRef]
- Zizzari, A.; Carbone, L.; Cesaria, M.; Bianco, M.; Perrone, E.; Rendina, F.; Arima, V. Continuous flow scalable production of injectable size-monodisperse nanoliposomes in easy-fabrication milli-fluidic reactors. Chem. Eng. Sci. 2021, 235, 116481. [Google Scholar] [CrossRef]
- Shestopalov, I.; Tice, J.D.; Ismagilov, R.F. Multi-step synthesis of nanoparticles performed on millisecond time scale in a microfluidic droplet-based system. Lab Chip 2004, 4, 316–321. [Google Scholar] [CrossRef]
- Chan, E.M.; Alivisatos, A.P.; Mathies, R.A. High-Temperature Microfluidic Synthesis of CdSe Nanocrystals in Nanoliter Droplets. J. Am. Chem. Soc. 2005, 127, 13854–13861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prakash, G.; Shokr, A.; Willemen, N.; Bashir, S.M.; Shin, S.R.; Hassan, S. Microfluidic fabrication of lipid nanoparticles for the delivery of nucleic acids. Adv. Drug Deliv. Rev. 2022, 184, 114197. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Günther, A.; Schmidt, M.A.; Jensen, K.F. Microfluidic Synthesis of Colloidal Silica. Langmuir 2004, 20, 8604–8611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-H.; Shen, S.-C.; Chen, Z.; Yun, J.-X.; Yao, K.-J.; Chen, B.-B.; Chen, J.-Z. Preparation of solid lipid nanoparticles in co-flowing microchannels. Chem. Eng. J. 2008, 144, 324–328. [Google Scholar] [CrossRef]
- Génot, V.; Desportes, S.; Croushore, C.; Lefèvre, J.-P.; Pansu, R.B.; Delaire, J.A.; von Rohr, P.R. Synthesis of organic nanoparticles in a 3D flow focusing microreactor. Chem. Eng. J. 2010, 161, 234–239. [Google Scholar] [CrossRef]
- Jahn, A.; Vreeland, W.N.; Gaitan, M.; Locascio, L.E. Controlled Vesicle Self-Assembly in Microfluidic Channels with Hydrodynamic Focusing. J. Am. Chem. Soc. 2004, 126, 2674–2675. [Google Scholar] [CrossRef]
- Yun, J.; Zhang, S.; Shen, S.; Chen, Z.; Yao, K.; Chen, J. Continuous production of solid lipid nanoparticles by liquid flow-focusing and gas displacing method in microchannels. Chem. Eng. Sci. 2009, 64, 4115–4122. [Google Scholar] [CrossRef]
- Gupta, R.; Fletcher, D.F.; Haynes, B.S. Taylor Flow in Microchannels: A Review of Experimental and Computational Work. J. Comput. Multiph. Flows 2010, 2, 1–31. [Google Scholar] [CrossRef]
- Tice, J.D.; Song, H.; Lyon, A.D.; Ismagilov, R.F. Formation of Droplets and Mixing in Multiphase Microfluidics at Low Values of the Reynolds and the Capillary Numbers. Langmuir 2003, 19, 9127–9133. [Google Scholar] [CrossRef]
- Kreutzer, M.T.; Kapteijn, F.; Moulijn, J.A.; Heiszwolf, J.J. Multiphase monolith reactors: Chemical reaction engineering of segmented flow in microchannels. Chem. Eng. Sci. 2005, 60, 5895–5916. [Google Scholar] [CrossRef]
- Tan, Z.; Lan, W.; Liu, Q.; Wang, K.; Hussain, M.; Ren, M.; Geng, Z.; Zhang, L.; Luo, X.; Zhang, L.; et al. Kinetically Controlled Self-Assembly of Block Copolymers into Segmented Wormlike Micelles in Microfluidic Chips. Langmuir 2019, 35, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Matosevic, S.; Paegel, B.M. Stepwise Synthesis of Giant Unilamellar Vesicles on a Microfluidic Assembly Line. J. Am. Chem. Soc. 2011, 133, 2798–2800. [Google Scholar] [CrossRef]
- Matosevic, S.; Paegel, B.M. Layer-by-layer cell membrane assembly. Nat. Chem. 2013, 5, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Erfle, P.; Riewe, J.; Bunjes, H.; Dietzel, A. Optically monitored segmented flow for controlled ultra-fast mixing and nanoparticle precipitation. Microfluid. Nanofluidics 2017, 21, 179. [Google Scholar] [CrossRef]
- Riewe, J.; Erfle, P.; Melzig, S.; Kwade, A.; Dietzel, A.; Bunjes, H. Antisolvent precipitation of lipid nanoparticles in microfluidic systems—A comparative study. Int. J. Pharm. 2020, 579, 119167. [Google Scholar] [CrossRef]
- Zinoveva, S.; De Silva, R.; Louis, R.D.; Datta, P.; Kumar, C.S.; Goettert, J.; Hormes, J. The wet chemical synthesis of Co nanoparticles in a microreactor system: A time-resolved investigation by X-ray absorption spectroscopy. Nucl. Instrum. Methods Phys. Res. Sect. A: Accel. Spectrometers Detect. Assoc. Equip. 2007, 582, 239–241. [Google Scholar] [CrossRef]
- Erfan, M.; Gnambodoe-Capochichi, M.; Sabry, Y.M.; Khalil, D.; Leprince-Wang, Y.; Bourouina, T. Spatiotemporal dynamics of nanowire growth in a microfluidic reactor. Microsyst. Nanoeng. 2021, 7, 77. [Google Scholar] [CrossRef]
- Li, J.; Šimek, H.; Ilioae, D.; Jung, N.; Braese, S.; Zappe, H.; Dittmeyer, R.; Ladewig, B.P. In Situ Sensors for Flow Reactors—A Review. React. Chem. Eng. 2021, 6, 1497–1507. [Google Scholar] [CrossRef]
- Sounart, T.L.; Safier, P.A.; Voigt, J.A.; Hoyt, J.; Tallant, D.R.; Matzke, C.M.; Michalske, T.A. Spatially-resolved analysis of nanoparticle nucleation and growth in a microfluidic reactor. Lab Chip 2007, 7, 908–915. [Google Scholar] [CrossRef]
- Just, J.; Coughlan, C.; Singh, S.; Ren, H.; Müller, O.; Becker, P.; Unold, T.; Ryan, K.M. Insights into Nucleation and Growth of Colloidal Quaternary Nanocrystals by Multimodal X-ray Analysis. ACS Nano 2021, 15, 6439–6447. [Google Scholar] [CrossRef]
- Herbst, M. Microfluidic and X-ray Techniques for Investigations of Nanoparticle Nucleation and Growth; ProQuest Dissertations Publishing: Bayreuth, Germany, 2021; p. 28485697. [Google Scholar]
- Maeki, M.; Fujishima, Y.; Sato, Y.; Yasui, T.; Kaji, N.; Ishida, A.; Tani, H.; Baba, Y.; Harashima, H.; Tokeshi, M. Understanding the formation mechanism of lipid nanoparticles in microfluidic devices with chaotic micromixers. PLoS ONE 2017, 12, e0187962. [Google Scholar] [CrossRef] [PubMed]
- Wilms, D.; Klos, J.; Frey, H. Microstructured Reactors for Polymer Synthesis: A Renaissance of Continuous Flow Processes for Tailor-Made Macromolecules? Macromol. Chem. Phys. 2008, 209, 343–356. [Google Scholar] [CrossRef]
- Yu, W.; Chen, H.; Wu, H.; Lin, P.; Xu, H.; Xie, Q.; Shi, K.; Xie, G.; Chen, Y. Continuous-flow rapid synthesis of wavelength-tunable luminescent lanthanide metal-organic framework nanorods by a microfluidic reactor. J. Alloys Compd. 2022, 890, 161860. [Google Scholar] [CrossRef]
- Nakamura, H.; Yamaguchi, Y.; Miyazaki, M.; Maeda, H.; Uehara, M.; Mulvaney, P. Preparation of CdSe nanocrystals in a micro-flow-reactor. Chem. Commun. 2002, 1, 2844–2845. [Google Scholar] [CrossRef] [PubMed]
- Zook, J.M.; Vreeland, W.N. Effects of temperature, acyl chain length, and flow-rate ratio on liposome formation and size in a microfluidic hydrodynamic focusing device. Soft Matter 2010, 6, 1352–1360. [Google Scholar] [CrossRef]
- Pradhan, P.; Guan, J.; Lu, D.; Wang, P.G.; Lee, L.J.; Lee, R.J. A Facile Microfluidic Method for Production of Liposomes. Anticancer Res. 2008, 28, 943. [Google Scholar]
- Miranda, I.; Souza, A.; Sousa, P.; Ribeiro, J.; Castanheira, E.M.S.; Lima, R.; Minas, G. Properties and Applications of PDMS for Biomedical Engineering: A Review. J. Funct. Biomater. 2022, 13, 2. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, L.; Tan, M.; Zhao, S.; Liu, H.; Lu, Z.; Li, J.; Liang, Z. Overview of 3D-Printed Silica Glass. Micromachines 2022, 13, 81. [Google Scholar] [CrossRef]
- Damodara, S.; Shahriari, S.; Wu, W.-I.; Rezai, P.; Hsu, H.-H.; Selvaganapathy, R. 1—Materials and methods for microfabrication of microfluidic devices. In Microfluidic Devices for Biomedical Applications, 2nd ed.; Li, X., Zhou, Y., Eds.; Woodhead Publishing: Cambridge, UK, 2021; pp. 1–78. [Google Scholar] [CrossRef]
- Shubhava; Jayarama, A.; Kannarpady, G.K.; Kale, S.; Prabhu, S.; Pinto, R. Chemical etching of glasses in hydrofluoric Acid: A brief review. Mater. Today Proc. 2021, 55, 46–51. [Google Scholar] [CrossRef]
- Bahrani, S.; Ghalamfarsa, F.; Nekoi, S.; Ghaedi, M.; Hashemi, S.A.; Mousavi, S.M. Chapter 17—Microfluidics technology: Past, present, and future prospects for biomarker diagnostics. In The Detection of Biomarkers; Ozkan, S.A., Bakirhan, N.K., Mollarasouli, F., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 457–485. [Google Scholar] [CrossRef]
- Friend, J.; Yeo, L. Fabrication of microfluidic devices using polydimethylsiloxane. Biomicrofluidics 2010, 4, 026502. [Google Scholar] [CrossRef] [Green Version]
- Kim, P.; Kwon, K.W.; Park, M.C.; Lee, S.H.; Kim, S.M.; Suh, K.Y. Soft Lithography for Microfluidics: A Review. Biochip J. 2008, 2, 1–11. [Google Scholar]
- Perez-Toralla, K.; Champ, J.; Mohamadi, M.R.; Braun, O.; Malaquin, L.; Viovy, J.-L.; Descroix, S. New non-covalent strategies for stable surface treatment of thermoplastic chips. Lab Chip 2013, 13, 4409–4418. [Google Scholar] [CrossRef] [PubMed]
- Agha, A.; Waheed, W.; Alamoodi, N.; Mathew, B.; Alnaimat, F.; Abu-Nada, E.; Abderrahmane, A.; Alazzam, A. A Review of Cyclic Olefin Copolymer Applications in Microfluidics and Microdevices. Macromol. Mater. Eng. 2022, 307, 2200053. [Google Scholar] [CrossRef]
- Garcia-Rey, S.; Nielsen, J.B.; Nordin, G.P.; Woolley, A.T.; Basabe-Desmonts, L.; Benito-Lopez, F. High-Resolution 3D Printing Fabrication of a Microfluidic Platform for Blood Plasma Separation. Polymers 2022, 14, 2537. [Google Scholar] [CrossRef] [PubMed]
- Stansbury, J.J.W.; Idacavage, M.J. 3D printing with polymers: Challenges among expanding options and opportunities. Dent. Mater. 2016, 32, 54–64. [Google Scholar] [CrossRef]
- Dizon, J.R.C.; Espera, A.H.; Chen, Q.; Advincula, R.C. Mechanical characterization of 3D-printed polymers. Addit. Manuf. 2018, 20, 44–67. [Google Scholar] [CrossRef]
- Schoerpf, S.; Catel, Y.; Moszner, N.; Gorsche, C.; Liska, R. Enhanced reduction of polymerization-induced shrinkage stress via combination of radical ring opening and addition fragmentation chain transfer. Polym. Chem. 2019, 10, 1357–1366. [Google Scholar] [CrossRef]
- Iedema, P.D.; Schamböck, V.; Boonen, H.; van der Linden, M.N.; Willemse, R. Photocuring of di-acrylate in presence of oxygen. Chem. Eng. Sci. 2019, 207, 130–144. [Google Scholar] [CrossRef]
- Peerzada, M.; Abbasi, S.; Lau, K.T.; Hameed, N. Additive Manufacturing of Epoxy Resins: Materials, Methods, and Latest Trends. Ind. Eng. Chem. Res. 2020, 59, 6375–6390. [Google Scholar] [CrossRef]
- Li, S.; Sun, D.; Li, A.; Cui, Y. Study on curing shrinkage and mechanism of DHOM-modified epoxy-acrylate-based UV-curing 3D printing materials. J. Appl. Polym. Sci. 2021, 138, 49859. [Google Scholar] [CrossRef]
- Mohan, D.; Sajab, M.S.; Bakarudin, S.B.; Roslan, R.; Kaco, H. 3D Printed Polyurethane Reinforced Graphene Nanoplatelets. Mater. Sci. Forum 2021, 1025, 47–52. [Google Scholar] [CrossRef]
- Sun, B.; Jiang, J.; Shi, N.; Xu, W. Application of microfluidics technology in chemical engineering for enhanced safety. Process Saf. Prog. 2016, 35, 365–373. [Google Scholar] [CrossRef]
- Zhang, H.; Anoop, K.; Huang, C.; Sadr, R.; Gupte, R.; Dai, J.; Han, A. A circular gradient-width crossflow microfluidic platform for high-efficiency blood plasma separation. Sens. Actuators B Chem. 2022, 354, 131180. [Google Scholar] [CrossRef]
- Nix, C.; Fillet, M. Chapter 10—Microfluidics in three key aspects of the drug-development process: Biomarker discovery, preclinical studies, and drug delivery systems. In Multidisciplinary Microfluidic and Nanofluidic Lab-on-a-Chip; Li, X., Yang, C., Li, P.C.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 275–295. [Google Scholar] [CrossRef]
- Luo, X.; Su, P.; Zhang, W.; Raston, C.L. Microfluidic Devices in Fabricating Nano or Micromaterials for Biomedical Applications. Adv. Mater. Technol. 2019, 4, 1900488. [Google Scholar] [CrossRef]
- Ran, R.; Wang, H.; Liu, Y.; Hui, Y.; Sun, Q.; Seth, A.; Wibowo, D.; Chen, D.; Zhao, C.-X. Microfluidic self-assembly of a combinatorial library of single- and dual-ligand liposomes for in vitro and in vivo tumor targeting. Eur. J. Pharm. Biopharm. 2018, 130, 1–10. [Google Scholar] [CrossRef]
- Li, Y.; Lee, R.J.; Huang, X.; Li, Y.; Lv, B.; Wang, T.; Qi, Y.; Hao, F.; Lu, J.; Meng, Q.; et al. Single-step microfluidic synthesis of transferrin-conjugated lipid nanoparticles for siRNA delivery. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 371–381. [Google Scholar] [CrossRef]
- Ran, R.; Middelberg, A.P.J.; Zhao, C.-X. Microfluidic synthesis of multifunctional liposomes for tumour targeting. Colloids Surf. B Biointerfaces 2016, 148, 402–410. [Google Scholar] [CrossRef]
- Maeki, M.; Uno, S.; Niwa, A.; Okada, Y.; Tokeshi, M. Microfluidic technologies and devices for lipid nanoparticle-based RNA delivery. J. Control. Release 2022, 344, 80–96. [Google Scholar] [CrossRef]
- Shepherd, S.J.; Issadore, D.; Mitchell, M.J. Microfluidic formulation of nanoparticles for biomedical applications. Biomaterials 2021, 274, 120826. [Google Scholar] [CrossRef]
- Belliveau, N.M.; Huft, J.; Lin, P.J.C.; Chen, S.; Leung, A.K.K.; Leaver, T.J.; Wild, A.W.; Lee, J.B.; Taylor, R.J.; Tam, Y.K.; et al. Microfluidic Synthesis of Highly Potent Limit-size Lipid Nanoparticles for In Vivo Delivery of siRNA. Mol. Ther. Nucleic Acids 2012, 1, e37. [Google Scholar] [CrossRef]
- Zhigaltsev, I.V.; Belliveau, N.; Hafez, I.; Leung, A.K.K.; Huft, J.; Hansen, C.; Cullis, P.R. Bottom-Up Design and Synthesis of Limit Size Lipid Nanoparticle Systems with Aqueous and Triglyceride Cores Using Millisecond Microfluidic Mixing. Langmuir 2012, 28, 3633–3640. [Google Scholar] [CrossRef] [PubMed]
- Maeki, M.; Saito, T.; Sato, Y.; Yasui, T.; Kaji, N.; Ishida, A.; Tani, H.; Baba, Y.; Harashima, H.; Tokeshi, M. A strategy for synthesis of lipid nanoparticles using microfluidic devices with a mixer structure. RSC Adv. 2015, 5, 46181–46185. [Google Scholar] [CrossRef]
- Nguyen, D.P.; Kloosterman, F.; Barbieri, R.; Brown, E.N.; Wilson, M.A.; Klausberger, T.Z. Micromixers—A review. J. Micromech. Microeng. 2005, 15, R1–R16. [Google Scholar] [CrossRef]
- Zimmermann, T.S.; Lee, A.C.; Akinc, A.; Bramlage, B.; Bumcrot, D.; Fedoruk, M.N.; Harborth, J.; Heyes, J.A.; Jeffs, L.B.; John, M.; et al. RNAi-mediated gene silencing in non-human primates. Nature 2006, 441, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, H.; Fontana, F.; Hirvonen, J.T.; Santos, H.A. Current developments and applications of microfluidic technology toward clinical translation of nanomedicines. Adv. Drug Deliv. Rev. 2018, 128, 54–83. [Google Scholar] [CrossRef] [PubMed]
- Schikarski, T.; Trzenschiok, H.; Peukert, W.; Avila, M. Inflow boundary conditions determine T-mixer efficiency. React. Chem. Eng. 2019, 4, 559–568. [Google Scholar] [CrossRef]
- Günther, A.; Khan, S.A.; Thalmann, M.; Trachsel, F.; Jensen, K.F. Transport and reaction in microscale segmented gas–liquid flow. Lab Chip 2004, 4, 278–286. [Google Scholar] [CrossRef]
- Tiboni, M.; Tiboni, M.; Pierro, A.; Del Papa, M.; Sparaventi, S.; Cespi, M.; Casettari, L. Microfluidics for nanomedicines manufacturing: An affordable and low-cost 3D printing approach. Int. J. Pharm. 2021, 599, 120464. [Google Scholar] [CrossRef]
- Camarri, S.; Mariotti, A.; Galletti, C.; Brunazzi, E.; Mauri, R.; Salvetti, M.V. An Overview of Flow Features and Mixing in Micro T and Arrow Mixers. Ind. Eng. Chem. Res. 2020, 59, 3669–3686. [Google Scholar] [CrossRef]
- Jahn, A.; Stavis, S.M.; Hong, J.S.; Vreeland, W.N.; DeVoe, D.L.; Gaitan, M. Microfluidic Mixing and the Formation of Nanoscale Lipid Vesicles. ACS Nano 2010, 4, 2077–2087. [Google Scholar] [CrossRef]
- Jahn, A.; Vreeland, W.N.; DeVoe, D.L.; Locascio, L.E.; Gaitan, M. Microfluidic Directed Formation of Liposomes of Controlled Size. Langmuir 2007, 23, 6289–6293. [Google Scholar] [CrossRef] [PubMed]
- Capretto, L.; Carugo, D.; Mazzitelli, S.; Nastruzzi, C.; Zhang, X. Microfluidic and lab-on-a-chip preparation routes for organic nanoparticles and vesicular systems for nanomedicine applications. Adv. Drug Deliv. Rev. 2013, 65, 1496–1532. [Google Scholar] [CrossRef]
- Webb, C.; Khadke, S.; Tandrup Schmidt, S.; Roces, C.B.; Forbes, N.; Berrie, G.; Perrie, Y. The Impact of Solvent Selection: Strategies to Guide the Manufacturing of Liposomes Using Microfluidics. Pharmaceutics 2019, 11, 653. [Google Scholar] [CrossRef] [PubMed]
- Krzysztoń, R.; Salem, B.; Lee, D.J.; Schwake, G.; Wagner, E.; Rädler, J.O. Microfluidic self-assembly of folate-targeted monomolecular siRNA-lipid nanoparticles. Nanoscale 2017, 9, 7442–7453. [Google Scholar] [CrossRef]
- van Swaay, D.; DeMello, A. Microfluidic methods for forming liposomes. Lab Chip 2013, 13, 752–767. [Google Scholar] [CrossRef]
- Mijajlovic, M.; Wright, D.; Zivkovic, V.; Bi, J.X.; Biggs, M.J. Microfluidic hydrodynamic focusing based synthesis of POPC liposomes for model biological systems. Colloids Surf. B Biointerfaces 2013, 104, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Balbino, T.T.A.; Aoki, N.T.; Gasperini, A.A.M.; Oliveira, C.L.P.; Azzoni, A.R.; Cavalcanti, L.P.; de la Torre, L.G. Continuous flow production of cationic liposomes at high lipid concentration in microfluidic devices for gene delivery applications. Chem. Eng. J. 2013, 226, 423–433. [Google Scholar] [CrossRef]
- Hood, R.R.; Shao, C.; Omiatek, D.M.; Vreeland, W.N.; DeVoe, D.L. Microfluidic Synthesis of PEG- and Folate-Conjugated Liposomes for One-Step Formation of Targeted Stealth Nanocarriers. Pharm. Res. 2013, 30, 1597–1607. [Google Scholar] [CrossRef]
- Church, A.S.; Witting, M.D. Laboratory testing in ethanol, methanol, ethylene glycol, and isopropanol toxicities. J. Emerg. Med. 1997, 15, 687–692. [Google Scholar] [CrossRef]
- Wu, N.; Zhu, Y.; Leech, P.; Sexton, B.; Brown, S.; Easton, C. Effects of Surfactants on the Formation of Microdroplets in the Flow Focusing Microfluidic Device. In Proceedings of the BioMEMS and Nanotechnology III, Canberra, ACT, Australia, 4–7 December 2007; Volume 6799. [Google Scholar]
- ICH. Guideline Q3C (R8) on Impurities: Guideline for Residual Solvents. European Medicines Agency. EMA/CHMP/ICH/82260/2006. 2021. Available online: https://www.ema.europa.eu/en/ich-q3c-r8-residual-solvents (accessed on 4 August 2022).
- Joshi, S.; Hussain, M.T.; Roces, C.B.; Anderluzzi, G.; Kastner, E.; Salmaso, S.; Kirby, D.J.; Perrie, Y. Microfluidics based manufacture of liposomes simultaneously entrapping hydrophilic and lipophilic drugs. Int. J. Pharm. 2016, 514, 160–168. [Google Scholar] [CrossRef] [Green Version]
- Lou, G.; Anderluzzi, G.; Woods, S.; Roberts, C.W.; Perrie, Y. A novel microfluidic-based approach to formulate size-tuneable large unilamellar cationic liposomes: Formulation, cellular uptake and biodistribution investigations. Eur. J. Pharm. Biopharm. 2019, 143, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Hood, R.R.; DeVoe, D.L.; Atencia, J.; Vreeland, W.N.; Omiatek, D.M. A facile route to the synthesis of monodisperse nanoscale liposomes using 3D microfluidic hydrodynamic focusing in a concentric capillary array. Lab Chip 2014, 14, 2403–2409. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Chang, H.I.; Yeh, M.K. Clinical development of liposome-based drugs: Formulation, characterization, and therapeutic efficacy. Int. J. Nanomed. 2012, 7, 49–60. [Google Scholar] [CrossRef]
- Yang, J.-T.; Fang, W.-F.; Tung, K.-Y. Fluids mixing in devices with connected-groove channels. Chem. Eng. Sci. 2008, 63, 1871–1881. [Google Scholar] [CrossRef]
- van Schijndel, T.; Singh, M.K.; Gillies, M.; Kahya, N.; Kharin, A.; den Toonder, J.M.J. Toward Gradient Formation in Microfluidic Devices by using Slanted Ridges. Macromol. Mater. Eng. 2011, 296, 373–379. [Google Scholar] [CrossRef]
- Lin, D.; He, F.; Liao, Y.; Lin, J.; Liu, C.; Song, J.; Cheng, Y. Three-dimensional staggered herringbone mixer fabricated by femtosecond laser direct writing. J. Opt. 2013, 15, 025601. [Google Scholar] [CrossRef]
- Chen, D.; Love, K.T.; Chen, Y.; Eltoukhy, A.A.; Kastrup, C.; Sahay, G.; Jeon, A.; Dong, Y.; Whitehead, K.A.; Anderson, D.G. Rapid Discovery of Potent siRNA-Containing Lipid Nanoparticles Enabled by Controlled Microfluidic Formulation. J. Am. Chem. Soc. 2012, 134, 6948–6951. [Google Scholar] [CrossRef] [PubMed]
- Ianovska, M. Microfluidic Tools for Multidimensional Liquid Chromatography. Ph.D. Thesis, University of Groningen, Groningen, The Netherlands, 2018. [Google Scholar]
- Kauffman, K.J.; Dorkin, J.R.; Yang, J.H.; Heartlein, M.W.; DeRosa, F.; Mir, F.F.; Fenton, O.S.; Anderson, D.G. Optimization of Lipid Nanoparticle Formulations for mRNA Delivery in Vivo with Fractional Factorial and Definitive Screening Designs. Nano Lett. 2015, 15, 7300–7306. [Google Scholar] [CrossRef]
- Gooding, O.W. Process optimization using combinatorial design principles: Parallel synthesis and design of experiment methods. Curr. Opin. Chem. Biol. 2004, 8, 297–304. [Google Scholar] [CrossRef]
- Dahlman, J.E.; Kauffman, K.J.; Xing, Y.; Shaw, T.E.; Mir, F.F.; Dlott, C.C.; Langer, R.; Anderson, D.G.; Wang, E.T. Barcoded nanoparticles for high throughput in vivo discovery of targeted therapeutics. Proc. Natl. Acad. Sci. USA 2017, 114, 2060–2065. [Google Scholar] [CrossRef]
- Guimaraes, P.P.G.; Zhang, R.; Spektor, R.; Tan, M.; Chung, A.; Billingsley, M.M.; El-Mayta, R.; Riley, R.S.; Wang, L.; Wilson, J.M.; et al. Ionizable lipid nanoparticles encapsulating barcoded mRNA for accelerated in vivo delivery screening. J. Control. Release 2019, 316, 404–417. [Google Scholar] [CrossRef]
- Kastner, E.; Verma, V.; Lowry, D.; Perrie, Y. Microfluidic-controlled manufacture of liposomes for the solubilisation of a poorly water soluble drug. Int. J. Pharm. 2015, 485, 122–130. [Google Scholar] [CrossRef]
- Hood, R.R.; DeVoe, D.L. High-Throughput Continuous Flow Production of Nanoscale Liposomes by Microfluidic Vertical Flow Focusing. Small 2015, 11, 5790–5799. [Google Scholar] [CrossRef]
- Roces, C.B.; Lou, G.; Jain, N.; Abraham, S.; Thomas, A.; Halbert, G.W.; Perrie, Y. Manufacturing Considerations for the Development of Lipid Nanoparticles Using Microfluidics. Pharmaceutics 2020, 12, 1095. [Google Scholar] [CrossRef]
- Finn, J.D.; Smith, A.R.; Patel, M.C.; Shaw, L.; Youniss, M.R.; van Heteren, J.; Dirstine, T.; Ciullo, C.; Lescarbeau, R.; Seitzer, J.; et al. A Single Administration of CRISPR/Cas9 Lipid Nanoparticles Achieves Robust and Persistent In Vivo Genome Editing. Cell Rep. 2018, 22, 2227–2235. [Google Scholar] [CrossRef]
- Patel, S.; Ashwanikumar, N.; Robinson, E.; Xia, Y.; Mihai, C.; Griffith, J.P.; Hou, S.; Esposito, A.A.; Ketova, T.; Welsher, K.; et al. Naturally-occurring cholesterol analogues in lipid nanoparticles induce polymorphic shape and enhance intracellular delivery of mRNA. Nat. Commun. 2020, 11, 983. [Google Scholar] [CrossRef]
- Kimura, N.; Maeki, M.; Sato, Y.; Note, Y.; Ishida, A.; Tani, H.; Harashima, H.; Tokeshi, M. Development of the iLiNP Device: Fine Tuning the Lipid Nanoparticle Size within 10 nm for Drug Delivery. ACS Omega 2018, 3, 5044–5051. [Google Scholar] [CrossRef]
- Balbino, T.A.; Azzoni, A.R.; de la Torre, L.G. Microfluidic devices for continuous production of pDNA/cationic liposome complexes for gene delivery and vaccine therapy. Colloids Surf. B Biointerfaces 2013, 111, 203–210. [Google Scholar] [CrossRef]
- Kulkarni, J.A.; Chen, S.; Tam, Y.Y.C. Scalable Production of Lipid Nanoparticles Containing Amphotericin B. Langmuir 2021, 37, 7312–7319. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, B.; Zhu, J.; Huang, X.; Xie, J.; Xu, S.; Yang, X.; Wang, X.; Yung, B.C.; Lee, L.J.; et al. A microfluidic method to synthesize transferrin-lipid nanoparticles loaded with siRNA LOR-1284 for therapy of acute myeloid leukemia. Nanoscale 2014, 6, 9742–9751. [Google Scholar] [CrossRef]
- Kim, H.; Sung, J.; Chang, Y.; Alfeche, A.; Leal, C. Microfluidics Synthesis of Gene Silencing Cubosomes. ACS Nano 2018, 12, 9196–9205. [Google Scholar] [CrossRef]
- Sato, Y.; Note, Y.; Maeki, M.; Kaji, N.; Baba, Y.; Tokeshi, M.; Harashima, H. Elucidation of the physicochemical properties and potency of siRNA-loaded small-sized lipid nanoparticles for siRNA delivery. J. Control. Release 2016, 229, 48–57. [Google Scholar] [CrossRef]
- Jyotsana, N.; Sharma, A.; Chaturvedi, A.; Budida, R.; Scherr, M.; Kuchenbauer, F.; Lindner, R.; Noyan, F.; Sühs, K.-W.; Stangel, M.; et al. Lipid nanoparticle-mediated siRNA delivery for safe targeting of human CML in vivo. Ann. Hematol. 2019, 98, 1905–1918. [Google Scholar] [CrossRef]
- Gkionis, L.; Campbell, R.A.; Aojula, H.; Harris, L.K.; Tirella, A. Manufacturing drug co-loaded liposomal formulations targeting breast cancer: Influence of preparative method on liposomes characteristics and in vitro toxicity. Int. J. Pharm. 2020, 590, 119926. [Google Scholar] [CrossRef]
- Hamano, N.; Böttger, R.; Lee, S.E.; Yang, Y.; Kulkarni, J.A.; Ip, S.; Cullis, P.R.; Li, S.-D. Robust Microfluidic Technology and New Lipid Composition for Fabrication of Curcumin-Loaded Liposomes: Effect on the Anticancer Activity and Safety of Cisplatin. Mol. Pharm. 2019, 16, 3957–3967. [Google Scholar] [CrossRef]
- Fathordoobady, F.; Sannikova, N.; Guo, Y.; Singh, A.; Kitts, D.D.; Pratap-Singh, A. Comparing microfluidics and ultrasonication as formulation methods for developing hempseed oil nanoemulsions for oral delivery applications. Sci. Rep. 2021, 11, 72. [Google Scholar] [CrossRef]
- Dong, Y.-D.; Tchung, E.; Nowell, C.; Kaga, S.; Leong, N.; Mehta, D.; Kaminskas, L.M.; Boyd, B.J. Microfluidic preparation of drug-loaded PEGylated liposomes, and the impact of liposome size on tumour retention and penetration. J. Liposome Res. 2019, 29, 1–9. [Google Scholar] [CrossRef]
- Forbes, N.; Hussain, M.T.; Briuglia, M.L.; Edwards, D.P.; Horst, J.H.t.; Szita, N.; Perrie, Y. Rapid and scale-independent microfluidic manufacture of liposomes entrapping protein incorporating in-line purification and at-line size monitoring. Int. J. Pharm. 2019, 556, 68–81. [Google Scholar] [CrossRef]
- Khadke, S.; Roces, C.B.; Donaghey, R.; Giacobbo, V.; Su, Y.; Perrie, Y. Scalable solvent-free production of liposomes. J. Pharm. Pharmacol. 2020, 72, 1328–1340. [Google Scholar] [CrossRef]
- Salafi, T.; Zeming, K.K.; Zhang, Y. Advancements in microfluidics for nanoparticle separation. Lab Chip 2017, 17, 11–33. [Google Scholar] [CrossRef]
- Petreus, T.; Cadogan, E.; Hughes, G.; Smith, A.; Pilla Reddy, V.; Lau, A.; O’Connor, M.J.; Critchlow, S.; Ashford, M.; Oplustil O’Connor, L. Tumour-on-chip microfluidic platform for assessment of drug pharmacokinetics and treatment response. Commun. Biol. 2021, 4, 1001. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Bhatia, V. Magnetic nanoparticles in microfluidics-based diagnostics: An appraisal. Nanomedicine 2021, 16, 1329–1342. [Google Scholar] [CrossRef]
- Lapierre, F.; Piret, G.; Drobecq, H.; Melnyk, O.; Coffinier, Y.; Thomy, V.; Boukherroub, R. High sensitive matrix-free mass spectrometry analysis of peptides using silicon nanowires-based digital microfluidic device. Lab Chip 2011, 11, 1620–1628. [Google Scholar] [CrossRef] [PubMed]
- Ran, R.; Wang, H.-F.; Hou, F.; Liu, Y.; Hui, Y.; Petrovsky, N.; Zhang, F.; Zhao, C.-X. A Microfluidic Tumor-on-a-Chip for Assessing Multifunctional Liposomes’ Tumor Targeting and Anticancer Efficacy. Adv. Healthc. Mater. 2019, 8, 1900015. [Google Scholar] [CrossRef] [PubMed]
- Zizzari, A.; Bianco, M.; Carbone, L.; Perrone, E.; Amato, F.; Maruccio, G.; Rendina, F.; Arima, V. Continuous-Flow Production of Injectable Liposomes via a Microfluidic Approach. Materials 2017, 10, 1411. [Google Scholar] [CrossRef]
- Yanar, F.; Mosayyebi, A.; Nastruzzi, C.; Carugo, D.; Zhang, X. Continuous-Flow Production of Liposomes with a Millireactor under Varying Fluidic Conditions. Pharmaceutics 2020, 12, 1001. [Google Scholar] [CrossRef]
- Amador, C.; Gavriilidis, A.; Angeli, P. Flow distribution in different microreactor scale-out geometries and the effect of manufacturing tolerances and channel blockage. Chem. Eng. J. 2004, 101, 379–390. [Google Scholar] [CrossRef]
- Shah, V.M.; Nguyen, D.X.; Patel, P.; Cote, B.; Al-Fatease, A.; Pham, Y.; Huynh, M.G.; Woo, Y.; Alani, A.W.G. Liposomes produced by microfluidics and extrusion: A comparison for scale-up purposes. Nanomed. Nanotechnol. Biol. Med. 2019, 18, 146–156. [Google Scholar] [CrossRef]
- Roces, C.B.; Port, E.C.; Daskalakis, N.N.; Watts, J.A.; Aylott, J.W.; Halbert, G.W.; Perrie, Y. Rapid scale-up and production of active-loaded PEGylated liposomes. Int. J. Pharm. 2020, 586, 119566. [Google Scholar] [CrossRef]
- Lamb, Y.N. BNT162b2 mRNA COVID-19 Vaccine: First Approval. Drugs 2021, 81, 495–501. [Google Scholar] [CrossRef]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J.A. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.A.; Parikh, N.; Blower, R.J.; Agrawal, N. SPIN: Rapid synthesis, purification, and concentration of small drug-loaded liposomes. J. Liposome Res. 2018, 28, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles─From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef] [PubMed]
- Stone, N.R.H.; Bicanic, T.; Salim, R.; Hope, W. Liposomal Amphotericin B (AmBisome®): A Review of the Pharmacokinetics, Pharmacodynamics, Clinical Experience and Future Directions. Drugs 2016, 76, 485–500. [Google Scholar] [CrossRef]
- O’Brien, M.E.R.; Wigler, N.; Inbar, M.; Rosso, R.; Grischke, E.; Santoro, A.; Catane, R.; Kieback, D.G.; Tomczak, P.; Ackland, S.P.; et al. Reduced cardiotoxicity and comparable efficacy in a phase IIItrial of pegylated liposomal doxorubicin HCl(CAELYX™/Doxil®) versus conventional doxorubicin forfirst-line treatment of metastatic breast cancer. Ann. Oncol. 2004, 15, 440–449. [Google Scholar] [CrossRef]
- Zhang, X.; Goel, V.; Robbie, G.J. Pharmacokinetics of Patisiran, the First Approved RNA Interference Therapy in Patients With Hereditary Transthyretin-Mediated Amyloidosis. J. Clin. Pharmacol. 2020, 60, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Matsuura-Sawada, Y.; Maeki, M.; Nishioka, T.; Niwa, A.; Yamauchi, J.; Mizoguchi, M.; Wada, K.; Tokeshi, M. Microfluidic Device-Enabled Mass Production of Lipid-Based Nanoparticles for Applications in Nanomedicine and Cosmetics. ACS Appl. Nano Mater. 2022, 5, 7867–7876. [Google Scholar] [CrossRef]
- Bresseleers, J.; Bagheri, M.; Lebleu, C.; Lecommandoux, S.; Sandre, O.; Pijpers, I.A.B.; Mason, A.F.; Meeuwissen, S.; Nostrum, C.F.v.; Hennink, W.E.; et al. Tuning Size and Morphology of mPEG-b-p(HPMA-Bz) Copolymer Self-Assemblies Using Microfluidics. Polymers 2020, 12, 2572. [Google Scholar] [CrossRef]
- Giraldo, K.A.; Bermudez, J.S.; Torres, C.E.; Reyes, L.H.; Osma, J.F.; Cruz, J.C. Microfluidics for Multiphase Mixing and Liposomal Encapsulation of Nanobioconjugates: Passive vs. Acoustic Systems. Fluids 2021, 6, 309. [Google Scholar] [CrossRef]
- Rasouli, M.R.; Tabrizian, M. An ultra-rapid acoustic micromixer for synthesis of organic nanoparticles. Lab Chip 2019, 19, 3316–3325. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, S.J.; Warzecha, C.C.; Yadavali, S.; El-Mayta, R.; Alameh, M.G.; Wang, L.; Weissman, D.; Wilson, J.M.; Issadore, D.; Mitchell, M.J. Scalable mRNA and siRNA Lipid Nanoparticle Production Using a Parallelized Microfluidic Device. Nano Lett. 2021, 21, 5671–5680. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, D.; Gutkin, A.; Kedmi, R.; Ramishetti, S.; Veiga, N.; Jacobi, A.M.; Schubert, M.S.; Friedmann-Morvinski, D.; Cohen, Z.R.; Behlke, M.A.; et al. CRISPR-Cas9 genome editing using targeted lipid nanoparticles for cancer therapy. Sci Adv. 2020, 6, eabc9450. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.S.; Kashyap, M.V.; Billingsley, M.M.; White, B.; Alameh, M.G.; Bose, S.K.; Zoltick, P.W.; Li, H.; Zhang, R.; Cheng, A.Y.; et al. Ionizable lipid nanoparticles for in utero mRNA delivery. Sci Adv. 2021, 7, eaba1028. [Google Scholar] [CrossRef]
- Sealy, A. How Pfizer Makes Its Millions of COVID-19 Vaccine Doses. Available online: https://edition.cnn.com/2021/03/31/health/pfizer-vaccine-manufacturing/index.html (accessed on 2 April 2021).

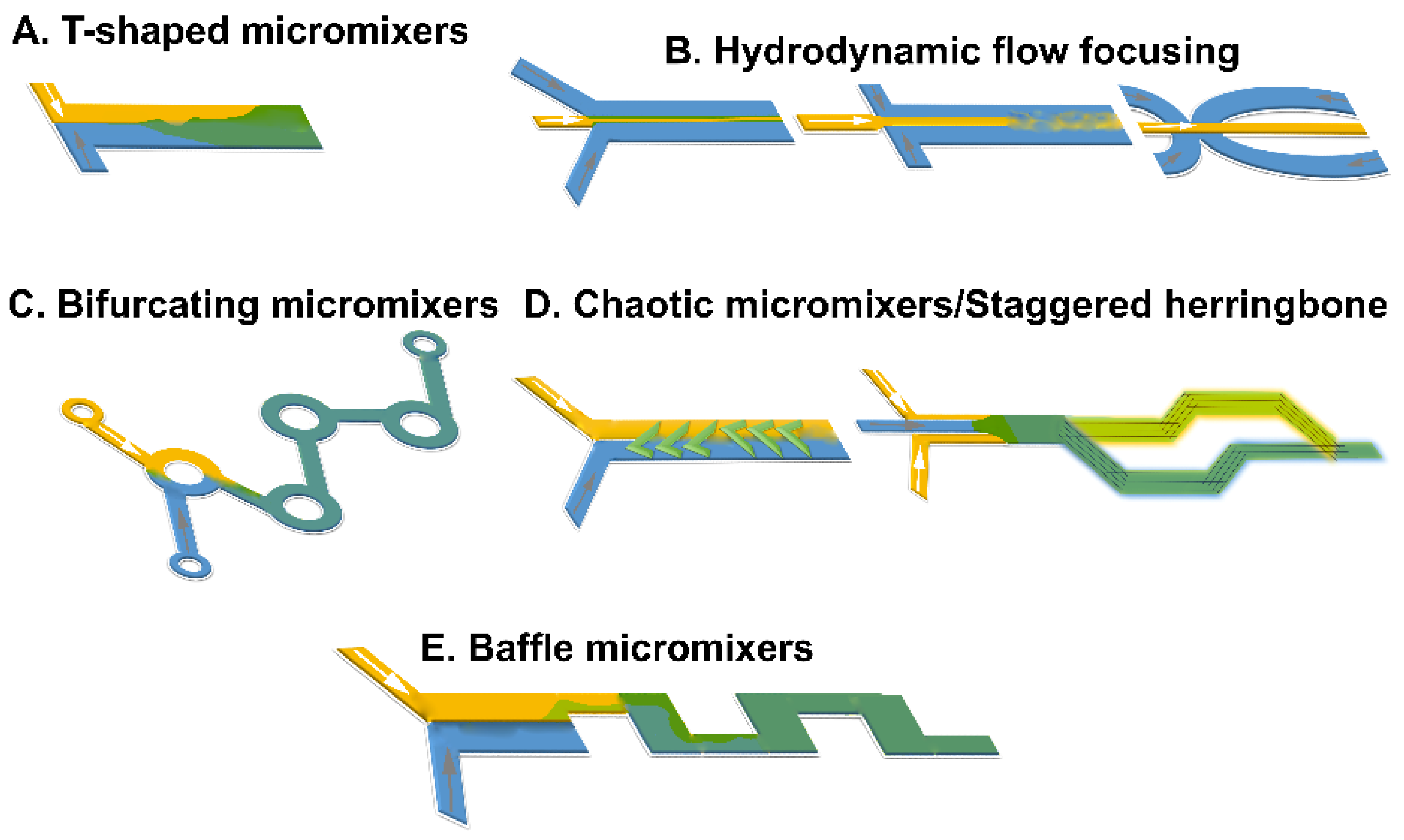
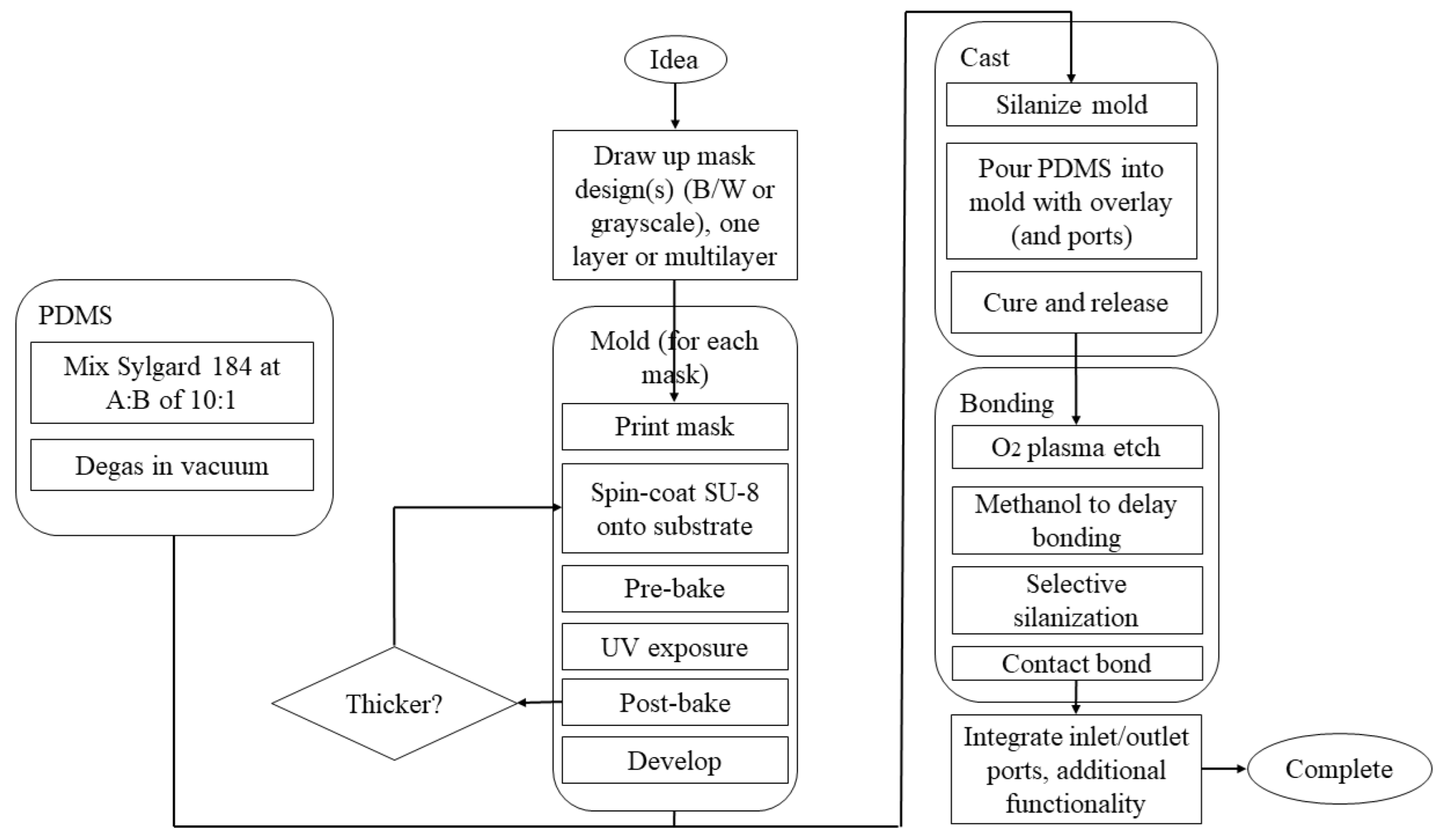

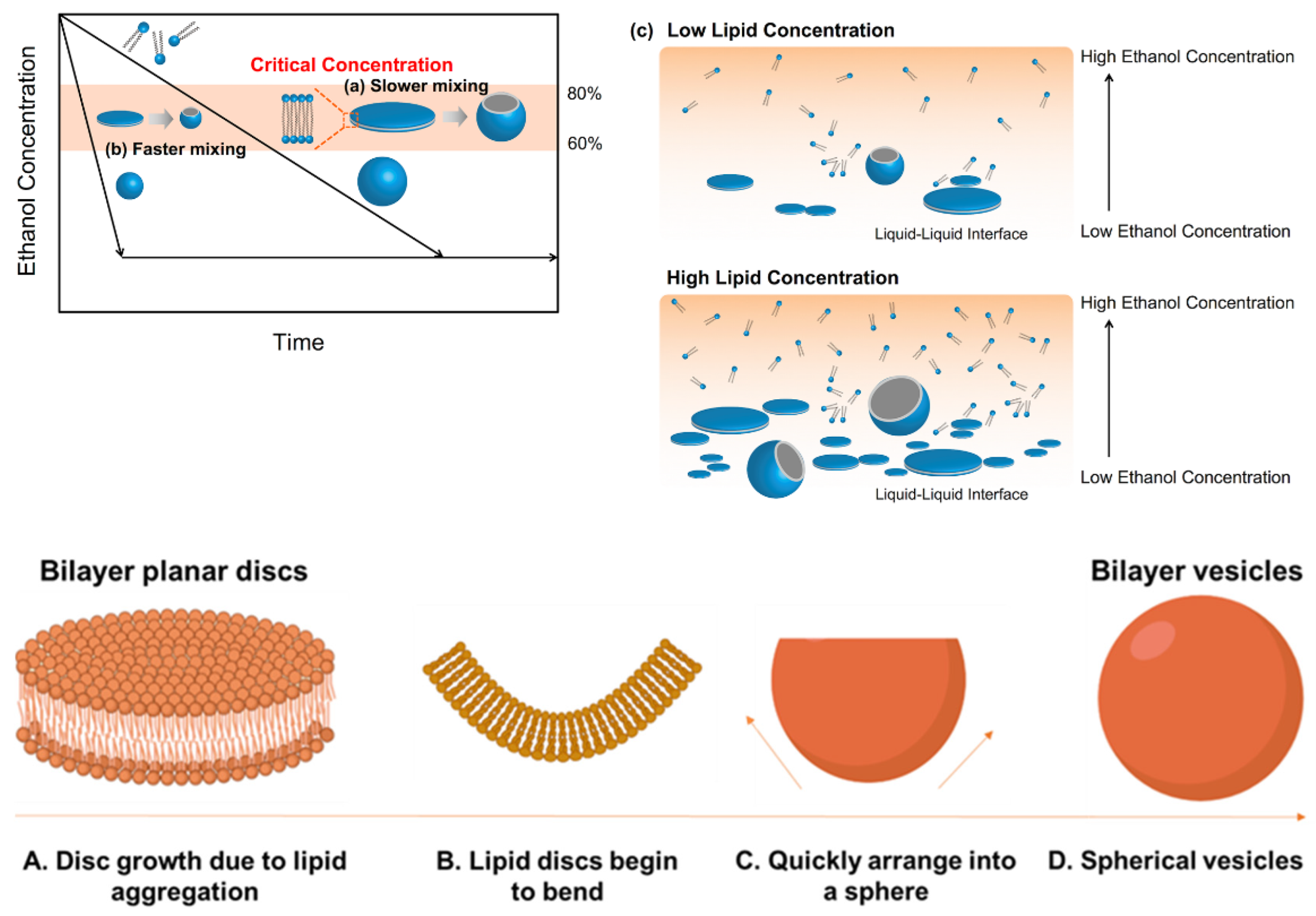
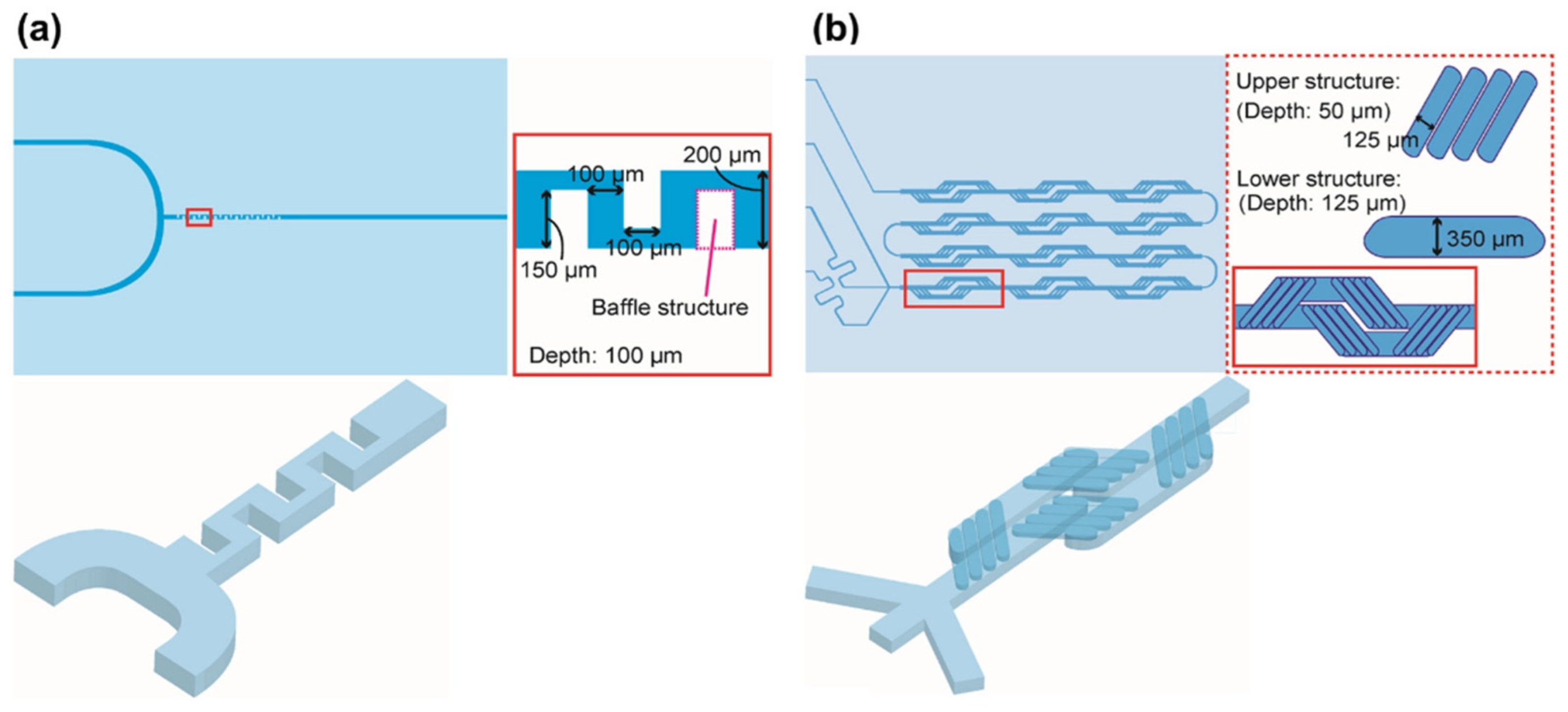
| System | Components | Diameter (nm)/Shape | Manufacturing | Pros | Cons | Refs. |
|---|---|---|---|---|---|---|
| Transferosomes | Edge activators, phospholipids | Less than 300/spherical bilayer | Vortexing, sonication, rotary film, or reverse-phase evaporations | Good stability, higher penetration | Susceptible to oxidative degradation | [36,90] |
| Liposomes | Cholesterol, phospholipids, essential oils | 10–1000/spherical bilayers | Solvent dispersion, mechanical dispersion, detergent removal | Controlled release, drug protection, solubility improvement for hydrophobic drugs, high biodistribution and bioavailability | Rigid structure, limited penetration across the stratum corneum | [28,29,32,33,62,90] |
| Nanostructured lipid carriers (NLCs) | Liquid and solid lipids, surfactants | 50–1000/spherical single layer | Sonication, micro emulsification, high-pressure homogenization | High cell uptake, appropriate protection of therapeutics in acidic pH, biodegradable and biocompatible, simplicity of drug entrapment, long shelf-life, more sustainable drug dissolution, high payload, reduced loss of drug during storage | Solid/liquid lipid ratio optimization difficulties | [52,53,91,92,93] |
| Solid lipid nanoparticles (SLNs) | Surfactants, solid lipids | 50–1000/spherical single layer | Sonication, micro emulsification, high-pressure homogenization | High cell uptake, appropriate protection of therapeutics in acidic pH, biodegradable and biocompatible ingredients, simplicity of drug entrapment, long shelf-life | Gelling tendency | [50,51,52,91,92,93,94,95,96,97,98] |
| Ethosomes | Phospholipids, edge activator, high concentration of a low-molecular-weight alcohol (ethanol ≤45% w/w), water | <200 or 300/spherical with diverse lamellarities | Solvent dispersion, ethanol injection–sonication, thin-film hydration, reverse-phase evaporation, transmembrane pH gradient, extrusion, and sonication | Increased efficacy and therapeutic index, reduce toxicity of API, improved permeation, entrapment of lipophilic, hydrophilic, and amphiphilic agents, simplicity of manufacturing, noninvasive, better solubility and stability, selective passive targeting | Low production yield, only for potent drugs, skin dermatitis or irritation may occur, drug leakage during transfer from organic to water media | [99,100] |
| Cochleates | Phospholipid–cation precipitates (formed by a continuous, solid, lipid bilayer sheet rolled up in a spiral) | 100–1000/cylindrical shape | Trapping method (a bridging agent is mixed with an aqueous lipid suspension), hydrogel method, dialysis, emulsification–lyophilization, microfluidic method, solvent injection | Nonimmunogenic, noninflammatory, and nontoxic, a stable structure due to their tightly packed nature, less susceptible to oxidation of both the encapsulated drug and the phospholipids, sustained release is achievable, enhanced shelf-life, improvements in oral bioavailability of drugs | Aggregation on storage, high production cost | [56,101] |
| Method | Mechanism | Suitability for Continuous Manufacturing |
|---|---|---|
| Bangham | Rehydration of thin lipid film | Not practical—needs continuous dehydration/rehydration steps |
| Sonication | Sonication of aqueous lipid suspensions | Requires small-scale batch operation to ensure sonication efficiency |
| Reverse-phase evaporation | Aqueous phase added to organic phase and evaporated to form liposomes | Very complex to regulate continuous solvent evaporation, sterile boundary hard to establish |
| Detergent depletion | Liposomes forms through detergent–lipid interaction | Slow process with difficult-to-establish sterile boundary, detergent use generally disadvantageous |
| Microfluidic channel | Intersection of lipid and API solutions in micromixers | Continuous but small/medium scale that can be upscaled in parallel |
| High-pressure homogenization | Liposome formation through high-pressure mixing | Very high pressures required, difficulty in sterilizing equipment |
| Heating | Heating of lipid aqueous/glycerol solution to form liposomes | Hydration step and high temperatures make continuous production impractical |
| Supercritical fluid methods | Use of supercritical fluids as solvent for lipids instead | High pressures required for feed vessels make resupply/continuous operation impractical |
| Dense gas | Use of dense gas as solvent for lipids | High pressures required for feed vessels make resupply/continuous operation impractical |
| Dual asymmetric centrifugation | Mechanical turbulence and cavitation | Only for batch sizes ~1 g or less |
| Ethanol/ether injection | Precipitation of liposome from organic phase into aqueous | Simple process with inherently continuous liposome formation step |
| Crossflow | In-line precipitation of liposome from organic phase into aqueous | Simple process with inherently continuous liposome formation step |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osouli-Bostanabad, K.; Puliga, S.; Serrano, D.R.; Bucchi, A.; Halbert, G.; Lalatsa, A. Microfluidic Manufacture of Lipid-Based Nanomedicines. Pharmaceutics 2022, 14, 1940. https://doi.org/10.3390/pharmaceutics14091940
Osouli-Bostanabad K, Puliga S, Serrano DR, Bucchi A, Halbert G, Lalatsa A. Microfluidic Manufacture of Lipid-Based Nanomedicines. Pharmaceutics. 2022; 14(9):1940. https://doi.org/10.3390/pharmaceutics14091940
Chicago/Turabian StyleOsouli-Bostanabad, Karim, Sara Puliga, Dolores R. Serrano, Andrea Bucchi, Gavin Halbert, and Aikaterini Lalatsa. 2022. "Microfluidic Manufacture of Lipid-Based Nanomedicines" Pharmaceutics 14, no. 9: 1940. https://doi.org/10.3390/pharmaceutics14091940
APA StyleOsouli-Bostanabad, K., Puliga, S., Serrano, D. R., Bucchi, A., Halbert, G., & Lalatsa, A. (2022). Microfluidic Manufacture of Lipid-Based Nanomedicines. Pharmaceutics, 14(9), 1940. https://doi.org/10.3390/pharmaceutics14091940








