A Biomimetic, Silaffin R5-Based Antigen Delivery Platform
Abstract
:1. Introduction
2. Experimental Section
3. Results
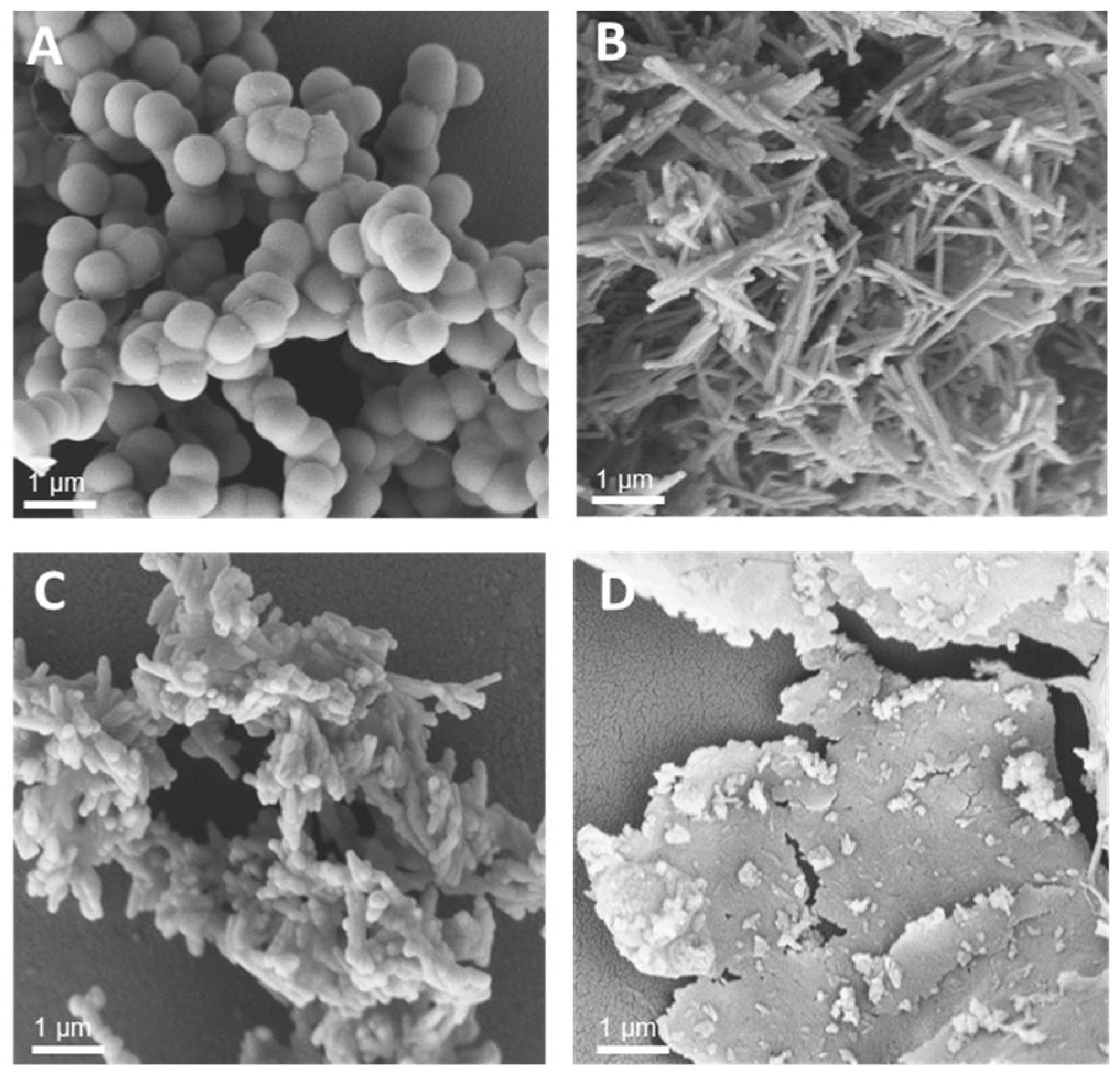
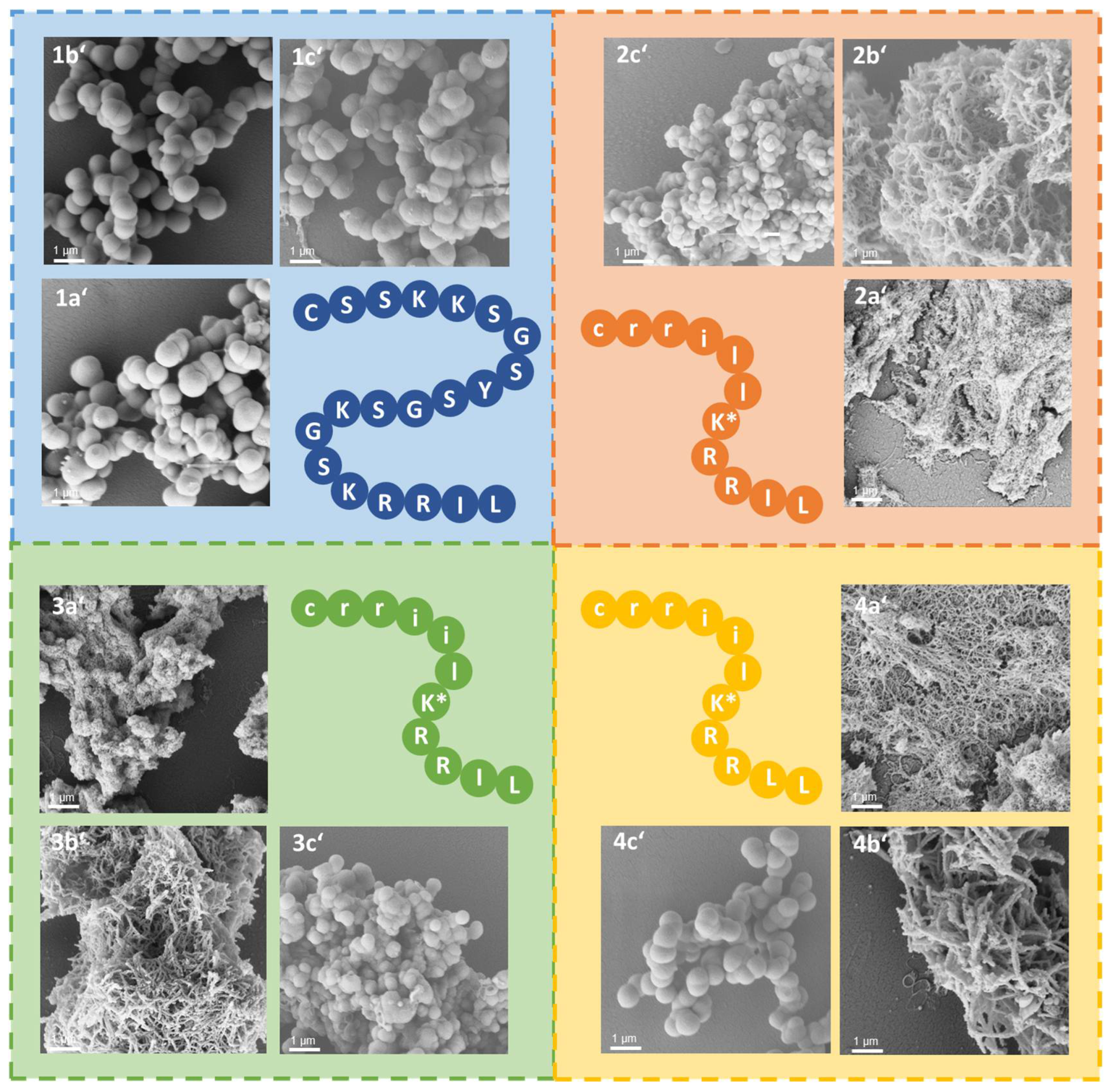
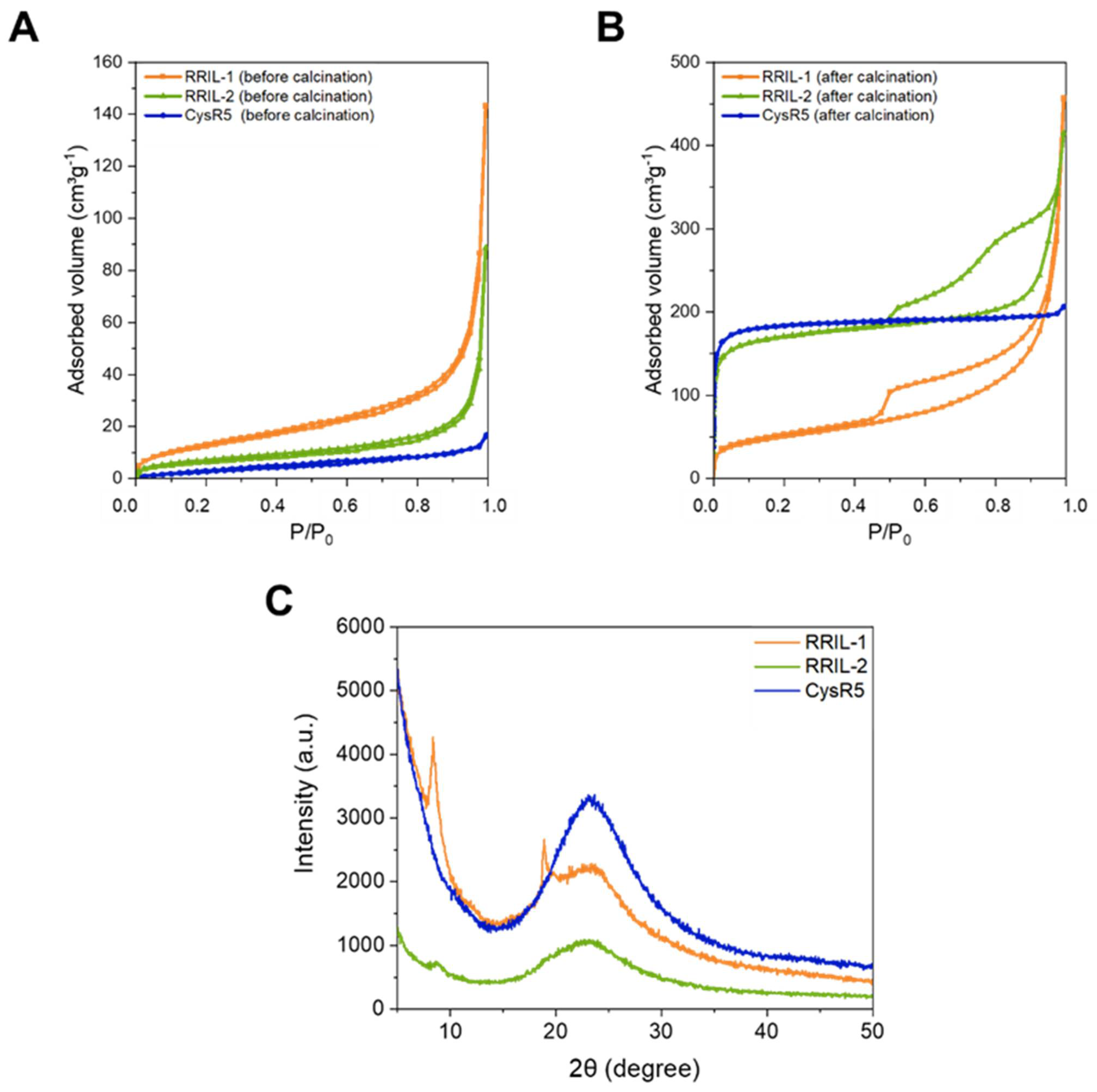
3.1. Synthesis of Silica-Precipitating Peptides and Morphological Evaluation of Silica Particles Resulting from Biomimetic Precipitation
3.2. Silaffin-Derived Silica Particles Induce NET Release
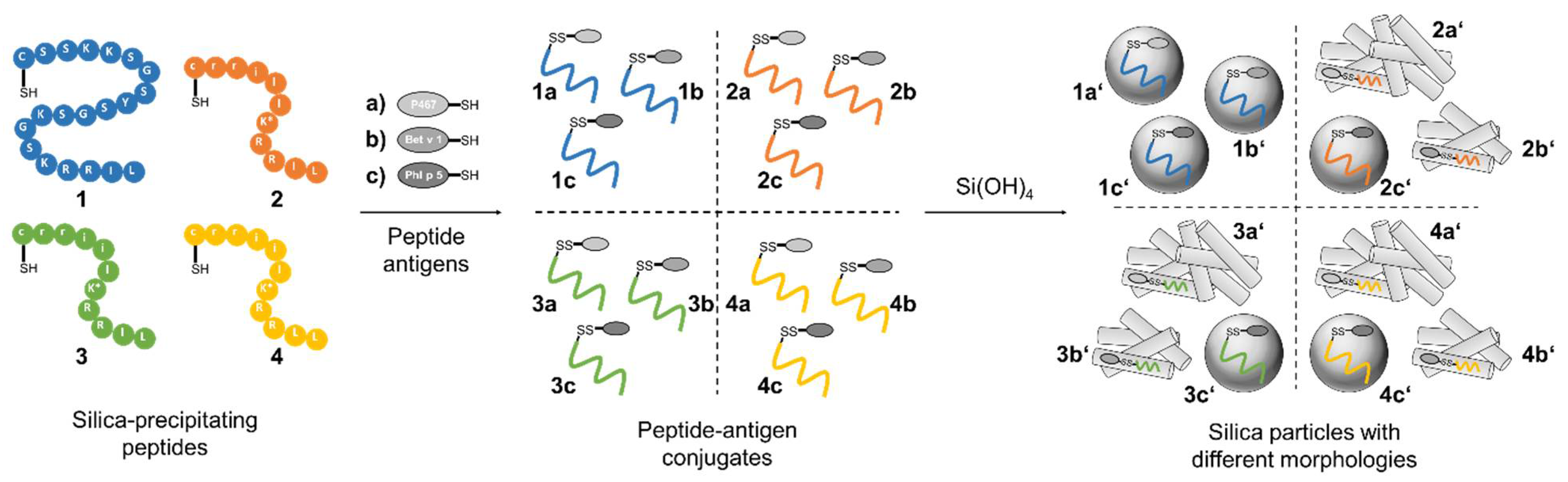
3.3. Silica Particles Loaded with Peptide–Antigen Conjugates
3.4. Biophysical Characterization of Silica-Precipitating Peptides
3.5. Activation of Murine Bone Marrow-Derived Dendritic Cells by Silica Particles Generated from Peptide–Antigen Conjugates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fenner, F. Smallpox Eradication: The Vindication of Jenner’s Prophesy. In History of Vaccine Development; Plotkin, S.A., Ed.; Springer: New York, NY, USA, 2011; pp. 27–32. [Google Scholar] [CrossRef]
- Plotkin, S.A. Vaccines: The Fourth Century. Clin. Vaccine Immunol. 2009, 16, 1709–1719. [Google Scholar] [CrossRef] [Green Version]
- Moyle, P.M.; Toth, I. Self-Adjuvanting Lipopeptide Vaccines. Curr. Med. Chem. 2008, 15, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Possible Applications for Replicating HIV-1 Vectors. HIV Ther. 2010, 4, 361–369. [CrossRef] [PubMed] [Green Version]
- Kaufmann, S.H.; Juliana McElrath, M.; Lewis, D.J.; Del Giudice, G. Challenges and Responses in Human Vaccine Development. Curr. Opin. Immunol. 2014, 28, 18–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pulendran, B.; Ahmed, R. Immunological Mechanisms of Vaccination. Nat. Immunol. 2011, 12, 509–517. [Google Scholar] [CrossRef]
- Boland, G.; Beran, J.; Lievens, M.; Sasadeusz, J.; Dentico, P.; Nothdurft, H.; Zuckerman, J.N.; Genton, B.; Steffen, R.; Loutan, L.; et al. Safety and Immunogenicity Profile of an Experimental Hepatitis B Vaccine Adjuvanted with AS04. Vaccine 2004, 23, 316–320. [Google Scholar] [CrossRef]
- Giannini, S.L.; Hanon, E.; Moris, P.; Van Mechelen, M.; Morel, S.; Dessy, F.; Fourneau, M.A.; Colau, B.; Suzich, J.; Losonksy, G.; et al. Enhanced Humoral and Memory B Cellular Immunity Using HPV16/18 L1 VLP Vaccine Formulated with the MPL/Aluminium Salt Combination (AS04) Compared to Aluminium Salt Only. Vaccine 2006, 24, 5937–5949. [Google Scholar] [CrossRef]
- Vesikari, T.; Groth, N.; Karvonen, A.; Borkowski, A.; Pellegrini, M. MF59®-Adjuvanted Influenza Vaccine (FLUAD®) in Children: Safety and Immunogenicity Following a Second Year Seasonal Vaccination. Vaccine 2009, 27, 6291–6295. [Google Scholar] [CrossRef]
- Ott, G.; Barchfeld, G.L.; Nest, G.V. Enhancement of Humoral Response against Human Influenza Vaccine with the Simple Submicron Oil/Water Emulsion Adjuvant MF59. Vaccine 1995, 13, 1557–1562. [Google Scholar] [CrossRef]
- Durando, P.; Fenoglio, D.; Boschini, A.; Ansaldi, F.; Icardi, G.; Sticchi, L.; Renzoni, A.; Fabbri, P.; Ferrera, A.; Parodi, A.; et al. Safety and Immunogenicity of Two Influenza Virus Subunit Vaccines, with or without MF59 Adjuvant, Administered to Human Immunodeficiency Virus Type 1-Seropositive and -Seronegative Adults. Clin. Vaccine Immunol. 2008, 15, 253–259. [Google Scholar] [CrossRef] [Green Version]
- Chu, D.W.-S.; Hwang, S.-J.; Lim, F.S.; Oh, H.M.L.; Thongcharoen, P.; Yang, P.-C.; Bock, H.L.; Dramé, M.; Gillard, P.; Hutagalung, Y.; et al. Immunogenicity and Tolerability of an AS03A-Adjuvanted Prepandemic Influenza Vaccine: A Phase III Study in a Large Population of Asian Adults. Vaccine 2009, 27, 7428–7435. [Google Scholar] [CrossRef]
- Schwarz, T.F.; Horacek, T.; Knuf, M.; Damman, H.-G.; Roman, F.; Dramé, M.; Gillard, P.; Jilg, W. Single Dose Vaccination with AS03-Adjuvanted H5N1 Vaccines in a Randomized Trial Induces Strong and Broad Immune Responsiveness to Booster Vaccination in Adults. Vaccine 2009, 27, 6284–6290. [Google Scholar] [CrossRef]
- World Health Organization. Biotechnology and World Health : Risks and Benefits of Vaccines and Other Medical Products Produced by Genetic Engineering : Proceedings of a WHO Meeting; World Health Organization: Geneva, Switzerland, 1997. [Google Scholar]
- Clements, C.J.; Griffiths, E. The Global Impact of Vaccines Containing Aluminium Adjuvants. Vaccine 2002, 20, S24–S33. [Google Scholar] [CrossRef]
- Glenny, A.T.; Pope, C.G.; Waddington, H.; Wallace, U. Immunological Notes. XVII–XXIV. J. Pathol. Bacteriol. 1926, 29, 31–40. [Google Scholar] [CrossRef]
- HogenEsch, H.; O’Hagan, D.T.; Fox, C.B. Optimizing the Utilization of Aluminum Adjuvants in Vaccines: You Might Just Get What You Want. Npj Vaccines 2018, 3, 51. [Google Scholar] [CrossRef] [Green Version]
- Eisenbarth, S.C.; Colegio, O.R.; O’Connor, W.; Sutterwala, F.S.; Flavell, R.A. Crucial Role for the Nalp3 Inflammasome in the Immunostimulatory Properties of Aluminium Adjuvants. Nature 2008, 453, 1122–1126. [Google Scholar] [CrossRef] [Green Version]
- Marrack, P.; McKee, A.S.; Munks, M.W. Towards an Understanding of the Adjuvant Action of Aluminium. Nat. Rev. Immunol. 2009, 9, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Reed, S.G.; Bertholet, S.; Coler, R.N.; Friede, M. New Horizons in Adjuvants for Vaccine Development. Trends Immunol. 2009, 30, 23–32. [Google Scholar] [CrossRef]
- McKee, A.S.; MacLeod, M.K.; Kappler, J.W.; Marrack, P. Immune Mechanisms of Protection: Can Adjuvants Rise to the Challenge? BMC Biol. 2010, 8, 37. [Google Scholar] [CrossRef]
- Aguilar, J.C.; Rodríguez, E.G. Vaccine Adjuvants Revisited. Vaccine 2007, 25, 3752–3762. [Google Scholar] [CrossRef]
- Foged, C.; Hansen, J.; Agger, E.M. License to Kill: Formulation Requirements for Optimal Priming of CD8+ CTL Responses with Particulate Vaccine Delivery Systems. Eur. J. Pharm. Sci. 2012, 45, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.L.; Cha, B.G.; Choi, Y.; Im, J.; Kim, J. Injectable Dual-Scale Mesoporous Silica Cancer Vaccine Enabling Efficient Delivery of Antigen/Adjuvant-Loaded Nanoparticles to Dendritic Cells Recruited in Local Macroporous Scaffold. Biomaterials 2020, 239, 119859. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.; Roche, K.C.; Tian, S.; Eblan, M.J.; McKinnon, K.P.; Caster, J.M.; Chai, S.; Herring, L.E.; Zhang, L.; Zhang, T.; et al. Antigen-Capturing Nanoparticles Improve the Abscopal Effect and Cancer Immunotherapy. Nat. Nanotechnol. 2017, 12, 877–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuai, R.; Ochyl, L.J.; Bahjat, K.S.; Schwendeman, A.; Moon, J.J. Designer Vaccine Nanodiscs for Personalized Cancer Immunotherapy. Nat. Mater. 2017, 16, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Peppas, N.A. Hydrogels and Scaffolds for Immunomodulation. Adv. Mater. 2014, 26, 6530–6541. [Google Scholar] [CrossRef]
- Treuel, L.; Jiang, X.; Nienhaus, G.U. New Views on Cellular Uptake and Trafficking of Manufactured Nanoparticles. J. R. Soc. Interface 2013, 10, 20120939. [Google Scholar] [CrossRef]
- Wang, D.; Xu, Z.; Chen, Z.; Liu, X.; Hou, C.; Zhang, X.; Zhang, H. Fabrication of Single-Hole Glutathione-Responsive Degradable Hollow Silica Nanoparticles for Drug Delivery. ACS Appl. Mater. Interfaces 2014, 6, 12600–12608. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, S.; Ma, J.; Fan, L.; Yin, C.; Lin, G.; Li, Q. Double Loaded Self-Decomposable SiO2 Nanoparticles for Sustained Drug Release. Nanoscale 2015, 7, 16389–16398. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Chu, Z.; Yin, C.; Zhang, C.; Lin, G.; Li, Q. Controllable Drug Release and Simultaneously Carrier Decomposition of SiO2-Drug Composite Nanoparticles. J. Am. Chem. Soc. 2013, 135, 5709–5716. [Google Scholar] [CrossRef]
- Li, A.W.; Sobral, M.C.; Badrinath, S.; Choi, Y.; Graveline, A.; Stafford, A.G.; Weaver, J.C.; Dellacherie, M.O.; Shih, T.-Y.; Ali, O.A.; et al. A Facile Approach to Enhance Antigen Response for Personalized Cancer Vaccination. Nat. Mater. 2018, 17, 528–534. [Google Scholar] [CrossRef]
- Kim, J.; Li, W.A.; Choi, Y.; Lewin, S.A.; Verbeke, C.S.; Dranoff, G.; Mooney, D.J. Injectable, Spontaneously Assembling, Inorganic Scaffolds Modulate Immune Cells in Vivo and Increase Vaccine Efficacy. Nat. Biotechnol. 2015, 33, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Dellacherie, M.O.; Li, A.W.; Lu, B.Y.; Mooney, D.J. Covalent Conjugation of Peptide Antigen to Mesoporous Silica Rods to Enhance Cellular Responses. Bioconjug. Chem. 2018, 29, 733–741. [Google Scholar] [CrossRef]
- Mody, K.T.; Popat, A.; Mahony, D.; Cavallaro, A.S.; Yu, C.; Mitter, N. Mesoporous Silica Nanoparticles as Antigen Carriers and Adjuvants for Vaccine Delivery. Nanoscale 2013, 5, 5167–5179. [Google Scholar] [CrossRef]
- Li, Z.; Barnes, J.C.; Bosoy, A.; Stoddart, J.F.; Zink, J.I. Mesoporous Silica Nanoparticles in Biomedical Applications. Chem. Soc. Rev. 2012, 41, 2590–2605. [Google Scholar] [CrossRef]
- CFR-Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=172.480 (accessed on 26 March 2020).
- Popplewell, J.F.; King, S.J.; Day, J.P.; Ackrill, P.; Fifield, L.K.; Cresswell, R.G.; di Tada, M.L.; Liu, K. Kinetics of Uptake and Elimination of Silicic Acid by a Human Subject: A Novel Application of 32Si and Accelerator Mass Spectrometry. J. Inorg. Biochem. 1998, 69, 177–180. [Google Scholar] [CrossRef]
- Croissant, J.G.; Fatieiev, Y.; Khashab, N.M. Degradability and Clearance of Silicon, Organosilica, Silsesquioxane, Silica Mixed Oxide, and Mesoporous Silica Nanoparticles. Adv. Mater. 2017, 29, 1604634. [Google Scholar] [CrossRef]
- Park, J.-H.; Gu, L.; von Maltzahn, G.; Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Biodegradable Luminescent Porous Silicon Nanoparticles for in Vivo Applications. Nat. Mater. 2009, 8, 331–336. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Dong, X.; Liang, J.; Shi, J. Preparation of Mesoporous Calcium Doped Silica Spheres with Narrow Size Dispersion and Their Drug Loading and Degradation Behavior. Microporous Mesoporous Mater. 2007, 102, 151–158. [Google Scholar] [CrossRef]
- Rogalla, S.; Flisikowski, K.; Gorpas, D.; Mayer, A.T.; Flisikowska, T.; Mandella, M.J.; Ma, X.; Casey, K.M.; Felt, S.A.; Saur, D.; et al. Biodegradable Fluorescent Nanoparticles for Endoscopic Detection of Colorectal Carcinogenesis. Adv. Funct. Mater. 2019, 29, 1904992. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, J.E.; Lee, J.H.; Jeong, J.H.; Kim, J. A Biodegradation Study of SBA-15 Microparticles in Simulated Body Fluid and In Vivo. Langmuir 2015, 31, 6457–6462. [Google Scholar] [CrossRef]
- Strobl, J.; Kozak, F.; Kamalov, M.; Reichinger, D.; Kurzbach, D.; Becker, C.F.W. Understanding Self-Assembly of Silica Precipitating Peptides to Control Silica. Adv. Mater. 2022, 2207586. [Google Scholar] [CrossRef] [PubMed]
- Calzetti, F.; Tamassia, N.; Arruda-Silva, F.; Gasperini, S.; Cassatella, M.A. The Importance of Being “Pure” Neutrophils. J. Allergy Clin. Immunol. 2017, 139, 352–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drinić, M.; Wagner, A.; Sarate, P.; Zwicker, C.; Korb, E.; Loupal, G.; Peschke, R.; Joachim, A.; Wiedermann, U.; Schabussova, I. Toxoplasma Gondii Tachyzoite-Extract Acts as a Potent Immunomodulator against Allergic Sensitization and Airway Inflammation. Sci. Rep. 2017, 7, 15211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kröger, N.; Deutzmann, R.; Sumper, M. Polycationic Peptides from Diatom Biosilica That Direct Silica Nanosphere Formation. Science 1999, 286, 1129–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kröger, N.; Deutzmann, R.; Sumper, M. Silica-Precipitating Peptides from Diatoms The Chemical Structure of Silaffin-1A from Cylindrotheca Fusiformis. J. Biol. Chem. 2001, 276, 26066–26070. [Google Scholar] [CrossRef] [Green Version]
- Kamalov, M.; Capel, P.D.; Rentenberger, C.; Müllner, A.R.M.; Peterlik, H.; Becker, C.F.W. Silaffin-Inspired Peptide Assemblies Template Silica Particles with Variable Morphologies. ChemNanoMat 2018, 4, 1209–1213. [Google Scholar] [CrossRef]
- Lechner, C.C.; Becker, C.F.W. Silaffins in Silica Biomineralization and Biomimetic Silica Precipitation. Mar. Drugs 2015, 13, 5297–5333. [Google Scholar] [CrossRef] [Green Version]
- Lechner, C.C.; Becker, C.F.W. Modified Silaffin R5 Peptides Enable Encapsulation and Release of Cargo Molecules from Biomimetic Silica Particles. Bioorg. Med. Chem. 2013, 21, 3533–3541. [Google Scholar] [CrossRef]
- Lechner, C.C.; Becker, C.F. Immobilising Proteins on Silica with Site-Specifically Attached Modified Silaffin Peptides. Biomater. Sci. 2015, 3, 288–297. [Google Scholar] [CrossRef]
- Lechner, C.C.; Becker, C.F.W. A Sequence-Function Analysis of the Silica Precipitating Silaffin R5 Peptide. J. Pept. Sci. 2014, 20, 152–158. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Stephen, J.; Scales, H.E.; Benson, R.A.; Erben, D.; Garside, P.; Brewer, J.M. Neutrophil Swarming and Extracellular Trap Formation Play a Significant Role in Alum Adjuvant Activity. Npj Vaccines 2017, 2, 1. [Google Scholar] [CrossRef]
- Munks, M.W.; McKee, A.S.; MacLeod, M.K.; Powell, R.L.; Degen, J.L.; Reisdorph, N.A.; Kappler, J.W.; Marrack, P. Aluminum Adjuvants Elicit Fibrin-Dependent Extracellular Traps In Vivo. Blood 2010, 116, 5191–5199. [Google Scholar] [CrossRef]
- Reithofer, M.; Karacs, J.; Strobl, J.; Kitzmüller, C.; Polak, D.; Seif, K.; Kamalov, M.; Becker, C.F.W.; Greiner, G.; Schmetterer, K.; et al. Alum Triggers Infiltration of Human Neutrophils Ex Vivo and Causes Lysosomal Destabilization and Mitochondrial Membrane Potential-Dependent NET-Formation. FASEB J. 2020, 34, 14024–14041. [Google Scholar] [CrossRef]
- Douda, D.N.; Khan, M.A.; Grasemann, H.; Palaniyar, N. SK3 Channel and Mitochondrial ROS Mediate NADPH Oxidase-Independent NETosis Induced by Calcium Influx. Proc. Natl. Acad. Sci. USA 2015, 112, 2817–2822. [Google Scholar] [CrossRef] [Green Version]
- Gajhede, M.; Osmark, P.; Poulsen, F.M.; Ipsen, H.; Larsen, J.N.; van Neerven, R.J.J.; Schou, C.; Løwenstein, H.; Spangfort, M.D. X-ray and NMR Structure of Bet v 1, the Origin of Birch Pollen Allergy. Nat. Struct. Biol. 1996, 3, 1040–1045. [Google Scholar] [CrossRef]
- Ebner, C.; Hirschwehr, R.; Bauer, L.; Breiteneder, H.; Valenta, R.; Ebner, H.; Kraft, D.; Scheiner, O. Identification of Allergens in Fruits and Vegetables: IgE Cross-Reactivities with the Important Birch Pollen Allergens Bet v 1 and Bet v 2 (Birch Profilin). J. Allergy Clin. Immunol. 1995, 95, 962–969. [Google Scholar] [CrossRef]
- Hufnagl, K.; Winkler, B.; Focke, M.; Valenta, R.; Scheiner, O.; Renz, H.; Wiedermann, U. Intranasal Tolerance Induction with Polypeptides Derived from 3 Noncross-Reactive Major Aeroallergens Prevents Allergic Polysensitization in Mice. J. Allergy Clin. Immunol. 2005, 116, 370–376. [Google Scholar] [CrossRef]
- Vrtala, S.; Sperr, W.R.; Reimitzer, I.; van Ree, R.; Laffer, S.; Müller, W.D.; Valent, P.; Lechner, K.; Rumpold, H.; Kraft, D. CDNA Cloning of a Major Allergen from Timothy Grass (Phleum Pratense) Pollen; Characterization of the Recombinant Phl PV Allergen. J. Immunol. 1993, 151, 4773–4781. [Google Scholar]
- Wagner, S.; Jasinska, J.; Breiteneder, H.; Kundi, M.; Pehamberger, H.; Scheiner, O.; Zielinski, C.C.; Wiedermann, U. Delayed Tumor Onset and Reduced Tumor Growth Progression after Immunization with a Her-2/Neu Multi-Peptide Vaccine and IL-12 in c-Neu Transgenic Mice. Breast Cancer Res. Treat. 2007, 106, 29–38. [Google Scholar] [CrossRef]
- Wiedermann, U.; Davis, A.B.; Zielinski, C.C. Vaccination for the Prevention and Treatment of Breast Cancer with Special Focus on Her-2/Neu Peptide Vaccines. Breast Cancer Res. Treat. 2013, 138, 1–12. [Google Scholar] [CrossRef]
- Tobias, J.; Garner-Spitzer, E.; Drinić, M.; Wiedermann, U. Vaccination against Her-2/Neu, with Focus on Peptide-Based Vaccines. ESMO Open 2022, 7, 100361. [Google Scholar] [CrossRef] [PubMed]
- Sulpizi, M.; Gaigeot, M.-P.; Sprik, M. The Silica–Water Interface: How the Silanols Determine the Surface Acidity and Modulate the Water Properties. J. Chem. Theory Comput. 2012, 8, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Favero, G.D.; Bialas, F.; Grabher, S.; Wittig, A.; Bräuer, B.; Gerthsen, D.; Echalier, C.; Kamalov, M.; Marko, D.; Becker, C.F.W. Silica Particles with a Quercetin–R5 Peptide Conjugate Are Taken up into HT-29 Cells and Translocate into the Nucleus. Chem. Commun. 2019, 55, 9649–9652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sikora, A.; Bartczak, D.; Geißler, D.; Kestens, V.; Roebben, G.; Ramaye, Y.; Varga, Z.; Palmai, M.; Shard, A.G.; Goenaga-Infante, H.; et al. A Systematic Comparison of Different Techniques to Determine the Zeta Potential of Silica Nanoparticles in Biological Medium. Anal. Methods 2015, 7, 9835–9843. [Google Scholar] [CrossRef] [Green Version]
- Al-Garawi, Z.S.; Kostakis, G.E.; Serpell, L.C. Chemically and Thermally Stable Silica Nanowires with a β-Sheet Peptide Core for Bionanotechnology. J. Nanobiotechnology 2016, 14, 79. [Google Scholar] [CrossRef] [Green Version]
- von Baeckmann, C.; Guillet-Nicolas, R.; Renfer, D.; Kählig, H.; Kleitz, F. A Toolbox for the Synthesis of Multifunctionalized Mesoporous Silica Nanoparticles for Biomedical Applications. ACS Omega 2018, 3, 17496–17510. [Google Scholar] [CrossRef]
- Möller, K.; Bein, T. Talented Mesoporous Silica Nanoparticles. Chem. Mater. 2017, 29, 371–388. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Cychosz, K.A.; Guillet-Nicolas, R.; García-Martínez, J.; Thommes, M. Recent Advances in the Textural Characterization of Hierarchically Structured Nanoporous Materials. Chem. Soc. Rev. 2017, 46, 389–414. [Google Scholar] [CrossRef]
- Schlumberger, C.; Thommes, M. Characterization of Hierarchically Ordered Porous Materials by Physisorption and Mercury Porosimetry—A Tutorial Review. Adv. Mater. Interfaces 2021, 8, 2002181. [Google Scholar] [CrossRef]
- Rasmussen, C.J.; Vishnyakov, A.; Thommes, M.; Smarsly, B.M.; Kleitz, F.; Neimark, A.V. Cavitation in Metastable Liquid Nitrogen Confined to Nanoscale Pores. Langmuir 2010, 26, 10147–10157. [Google Scholar] [CrossRef]
- Thommes, M. Physical Adsorption Characterization of Nanoporous Materials. Chem. Ing. Tech. 2010, 82, 1059–1073. [Google Scholar] [CrossRef]
- Landers, J.; Gor, G.Y.; Neimark, A.V. Density Functional Theory Methods for Characterization of Porous Materials. Colloids Surf. Physicochem. Eng. Asp. 2013, 437, 3–32. [Google Scholar] [CrossRef]
- Thommes, M.; Cychosz, K.A. Physical Adsorption Characterization of Nanoporous Materials: Progress and Challenges. Adsorption 2014, 20, 233–250. [Google Scholar] [CrossRef]
- Juère, E.; Kleitz, F. On the Nanopore Confinement of Therapeutic Drugs into Mesoporous Silica Materials and Its Implications. Microporous Mesoporous Mater. 2018, 270, 109–119. [Google Scholar] [CrossRef]
- Velazquez-Salinas, L.; Verdugo-Rodriguez, A.; Rodriguez, L.L.; Borca, M.V. The Role of Interleukin 6 During Viral Infections. Front. Microbiol. 2019, 10, 1057. [Google Scholar] [CrossRef] [Green Version]
- Angelone, D.F.; Wessels, M.R.; Coughlin, M.; Suter, E.E.; Valentini, P.; Kalish, L.A.; Levy, O. Innate Immunity of the Human Newborn Is Polarized Toward a High Ratio of IL-6/TNF-α Production In Vitro and In Vivo. Pediatr. Res. 2006, 60, 205–209. [Google Scholar] [CrossRef] [Green Version]
- Neumann, A.; Berends, E.T.M.; Nerlich, A.; Molhoek, E.M.; Gallo, R.L.; Meerloo, T.; Nizet, V.; Naim, H.Y.; von Köckritz-Blickwede, M. The Antimicrobial Peptide LL-37 Facilitates the Formation of Neutrophil Extracellular Traps. Biochem. J. 2014, 464, 3–11. [Google Scholar] [CrossRef]
- Hosoda, H.; Nakamura, K.; Hu, Z.; Tamura, H.; Reich, J.; Kuwahara-Arai, K.; Iba, T.; Tabe, Y.; Nagaoaka, I. Antimicrobial Cathelicidin Peptide LL-37 Induces NET Formation and Suppresses the Inflammatory Response in a Mouse Septic Model. Mol. Med. Rep. 2017, 16, 5618–5626. [Google Scholar] [CrossRef]
- Gorgojo, J.; Scharrig, E.; Gómez, R.M.; Harvill, E.T.; Rodríguez, M.E. Bordetella Parapertussis Circumvents Neutrophil Extracellular Bactericidal Mechanisms. PLoS ONE 2017, 12, e0169936. [Google Scholar] [CrossRef] [Green Version]
- Oren, Z.; Lerman, J.C.; Gudmundsson, G.H.; Agerberth, B.; Shai, Y. Structure and Organization of the Human Antimicrobial Peptide LL-37 in Phospholipid Membranes: Relevance to the Molecular Basis for Its Non-Cell-Selective Activity. Biochem. J. 1999, 341 Pt 3, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Desai, J.; Foresto-Neto, O.; Honarpisheh, M.; Steiger, S.; Nakazawa, D.; Popper, B.; Buhl, E.M.; Boor, P.; Mulay, S.R.; Anders, H.-J. Particles of Different Sizes and Shapes Induce Neutrophil Necroptosis Followed by the Release of Neutrophil Extracellular Trap-like Chromatin. Sci. Rep. 2017, 7, 15003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, K.; Lim, J.-S. Induction of Functional Changes of Dendritic Cells by Silica Nanoparticles. Immune Netw. 2012, 12, 104–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallhov, H.; Gabrielsson, S.; Strømme, M.; Scheynius, A.; Garcia-Bennett, A.E. Mesoporous Silica Particles Induce Size Dependent Effects on Human Dendritic Cells. Nano Lett. 2007, 7, 3576–3582. [Google Scholar] [CrossRef]
- Mamaeva, V.; Sahlgren, C.; Lindén, M. Mesoporous Silica Nanoparticles in Medicine—Recent Advances. Adv. Drug Deliv. Rev. 2013, 65, 689–702. [Google Scholar] [CrossRef]
- Heidegger, S.; Gößl, D.; Schmidt, A.; Niedermayer, S.; Argyo, C.; Endres, S.; Bein, T.; Bourquin, C. Immune Response to Functionalized Mesoporous Silica Nanoparticles for Targeted Drug Delivery. Nanoscale 2015, 8, 938–948. [Google Scholar] [CrossRef] [Green Version]
- Meraz, I.M.; Melendez, B.; Gu, J.; Wong, S.T.C.; Liu, X.; Andersson, H.A.; Serda, R.E. Activation of the Inflammasome and Enhanced Migration of Microparticle-Stimulated Dendritic Cells to the Draining Lymph Node. Mol. Pharm. 2012, 9, 2049–2062. [Google Scholar] [CrossRef] [Green Version]
- Skuland, T.; Låg, M.; Gutleb, A.C.; Brinchmann, B.C.; Serchi, T.; Øvrevik, J.; Holme, J.A.; Refsnes, M. Pro-Inflammatory Effects of Crystalline- and Nano-Sized Non-Crystalline Silica Particles in a 3D Alveolar Model. Part. Fibre Toxicol. 2020, 17, 13. [Google Scholar] [CrossRef] [Green Version]
- Napierska, D.; Thomassen, L.C.J.; Vanaudenaerde, B.; Luyts, K.; Lison, D.; Martens, J.A.; Nemery, B.; Hoet, P.H.M. Cytokine Production by Co-Cultures Exposed to Monodisperse Amorphous Silica Nanoparticles: The Role of Size and Surface Area. Toxicol. Lett. 2012, 211, 98–104. [Google Scholar] [CrossRef]
- Pétrilli, V.; Dostert, C.; Muruve, D.A.; Tschopp, J. The Inflammasome: A Danger Sensing Complex Triggering Innate Immunity. Curr. Opin. Immunol. 2007, 19, 615–622. [Google Scholar] [CrossRef]
- Innate Instruction of Adaptive Immunity Revisited: The Inflammasome. EMBO Mol. Med. 2009, 1, 92–98. [CrossRef]
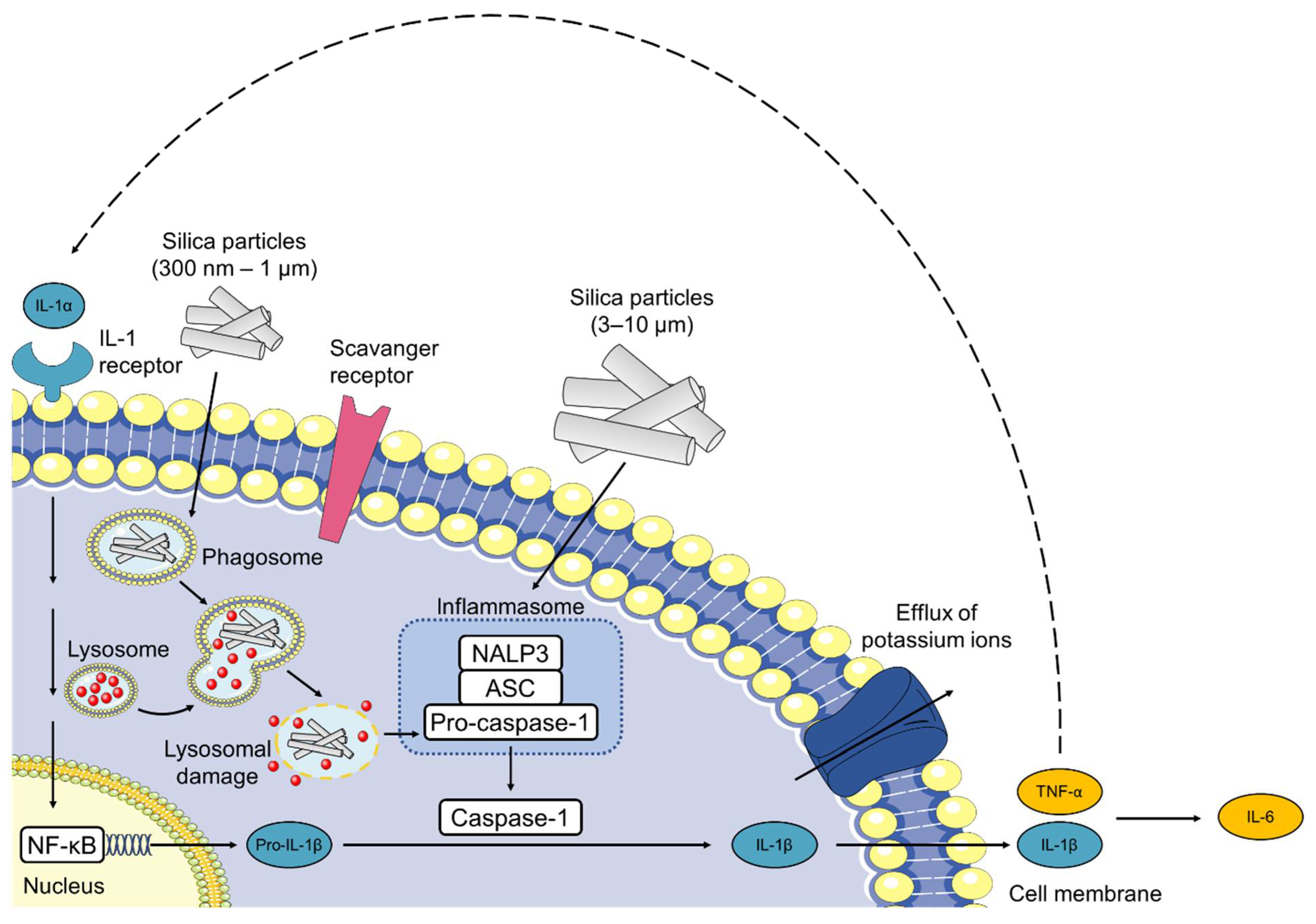
- Hornung, V.; Bauernfeind, F.; Halle, A.; Samstad, E.O.; Kono, H.; Rock, K.L.; Fitzgerald, K.A.; Latz, E. Silica Crystals and Aluminum Salts Activate the NALP3 Inflammasome through Phagosomal Destabilization. Nat. Immunol. 2008, 9, 847–856. [Google Scholar] [CrossRef]
- Sharp, F.A.; Ruane, D.; Claass, B.; Creagh, E.; Harris, J.; Malyala, P.; Singh, M.; O’Hagan, D.T.; Pétrilli, V.; Tschopp, J.; et al. Uptake of Particulate Vaccine Adjuvants by Dendritic Cells Activates the NALP3 Inflammasome. Proc. Natl. Acad. Sci. USA 2009, 106, 870–875. [Google Scholar] [CrossRef] [Green Version]
- Sandberg, W.J.; Låg, M.; Holme, J.A.; Friede, B.; Gualtieri, M.; Kruszewski, M.; Schwarze, P.E.; Skuland, T.; Refsnes, M. Comparison of Non-Crystalline Silica Nanoparticles in IL-1β Release from Macrophages. Part. Fibre Toxicol. 2012, 9, 32. [Google Scholar] [CrossRef] [Green Version]
- Winter, M.; Beer, H.-D.; Hornung, V.; Krämer, U.; Schins, R.P.F.; Förster, I. Activation of the Inflammasome by Amorphous Silica and TiO2 Nanoparticles in Murine Dendritic Cells. Nanotoxicology 2011, 5, 326–340. [Google Scholar] [CrossRef]
- Kojima, S.; Negishi, Y.; Tsukimoto, M.; Takenouchi, T.; Kitani, H.; Takeda, K. Purinergic Signaling via P2X7 Receptor Mediates IL-1β Production in Kupffer Cells Exposed to Silica Nanoparticle. Toxicology 2014, 321, 13–20. [Google Scholar] [CrossRef]
- Kusaka, T.; Nakayama, M.; Nakamura, K.; Ishimiya, M.; Furusawa, E.; Ogasawara, K. Effect of Silica Particle Size on Macrophage Inflammatory Responses. PLoS ONE 2014, 9, e92634. [Google Scholar] [CrossRef]
- Yazdi, A.S.; Guarda, G.; Riteau, N.; Drexler, S.K.; Tardivel, A.; Couillin, I.; Tschopp, J. Nanoparticles Activate the NLR Pyrin Domain Containing 3 (Nlrp3) Inflammasome and Cause Pulmonary Inflammation through Release of IL-1α and IL-1β. Proc. Natl. Acad. Sci. USA 2010, 107, 19449–19454. [Google Scholar] [CrossRef] [Green Version]
- Fubini, B.; Hubbard, A. Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) Generation by Silica in Inflammation and Fibrosis. Free Radic. Biol. Med. 2003, 34, 1507–1516. [Google Scholar] [CrossRef]
- Joshi, G.N.; Knecht, D.A. Silica Phagocytosis Causes Apoptosis and Necrosis by Different Temporal and Molecular Pathways in Alveolar Macrophages. Apoptosis 2013, 18, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-D.; Cheng, M.; Shang, P.-P.; Yang, Y.-Q. Role of IL-6 in Dendritic Cell Functions. J. Leukoc. Biol. 2022, 111, 695–709. [Google Scholar] [CrossRef] [PubMed]
- Herseth, J.I.; Refsnes, M.; Låg, M.; Schwarze, P.E. Role of IL-1β and COX2 in Silica-Induced IL-6 Release and Loss of Pneumocytes in Co-Cultures. Toxicol. In Vitro 2009, 23, 1342–1353. [Google Scholar] [CrossRef] [PubMed]
- Franchi, L.; Eigenbrod, T.; Núñez, G. Cutting Edge: TNF-α Mediates Sensitization to ATP and Silica via the NLRP3 Inflammasome in the Absence of Microbial Stimulation. J. Immunol. 2009, 183, 792–796. [Google Scholar] [CrossRef] [Green Version]




| Peptide | Compound | Sequence | Incorporation Efficiency/% |
|---|---|---|---|
| CysR5 | 1 | H-CSSKKSGSYSGSKGSKRRIL-OH | 84.4 ± 4.3 |
| RRIL-1 | 2 | 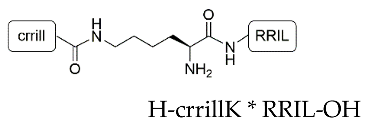 | 84.0 ± 5.4 |
| RRIL-2 | 3 |  | 89.0 ± 0.6 |
| RRLL | 4 |  | 95.3 ± 2.8 |
| Peptide Conjugate | Compound | Incorporation Efficiency/% |
|---|---|---|
| CysR5-Bet v 1 | 1b | 42.7 ± 7.9 |
| CysR5-Phl p 5 | 1c | 32.4 ± 10.7 |
| RRIL-2-Bet v 1 | 3b | 89.9 ± 1.6 |
| RRIL-2-Phl p 5 | 3c | 85.3 ± 1.6 |
| Sample | Compound | SBETa/m2 g−1 | TPV b/cm³ g−1 | Wads c/nm |
|---|---|---|---|---|
| Before calcination | ||||
| CysR5 | 1′ | 13 | 0.01 | n.d. |
| RRIL-1 | 2′ | 48 | 0.03 | n.d. |
| RRIL-2 | 3′ | 22 | 0.01 | n.d. |
| After calcination | ||||
| CysR5 | 1″ | 727 | 0.3 | 1.1 |
| RRIL-1 | 2″ | 180 | 0.1 | 6.5 |
| RRIL-2 | 3″ | 650 | 0.3 | 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reichinger, D.; Reithofer, M.; Hohagen, M.; Drinic, M.; Tobias, J.; Wiedermann, U.; Kleitz, F.; Jahn-Schmid, B.; Becker, C.F.W. A Biomimetic, Silaffin R5-Based Antigen Delivery Platform. Pharmaceutics 2023, 15, 121. https://doi.org/10.3390/pharmaceutics15010121
Reichinger D, Reithofer M, Hohagen M, Drinic M, Tobias J, Wiedermann U, Kleitz F, Jahn-Schmid B, Becker CFW. A Biomimetic, Silaffin R5-Based Antigen Delivery Platform. Pharmaceutics. 2023; 15(1):121. https://doi.org/10.3390/pharmaceutics15010121
Chicago/Turabian StyleReichinger, Daniela, Manuel Reithofer, Mariam Hohagen, Mirjana Drinic, Joshua Tobias, Ursula Wiedermann, Freddy Kleitz, Beatrice Jahn-Schmid, and Christian F. W. Becker. 2023. "A Biomimetic, Silaffin R5-Based Antigen Delivery Platform" Pharmaceutics 15, no. 1: 121. https://doi.org/10.3390/pharmaceutics15010121
APA StyleReichinger, D., Reithofer, M., Hohagen, M., Drinic, M., Tobias, J., Wiedermann, U., Kleitz, F., Jahn-Schmid, B., & Becker, C. F. W. (2023). A Biomimetic, Silaffin R5-Based Antigen Delivery Platform. Pharmaceutics, 15(1), 121. https://doi.org/10.3390/pharmaceutics15010121






