Abstract
Paclitaxel (PTX), etoposide (ETP), and rapamycin (RAPA) have different mechanisms, allowing multiple pathways to be targeted simultaneously, effectively treating various cancers. However, these drugs have a low hydrosolubility, limiting clinical applications. Therefore, we used pH-sensitive polymeric micelles to effectively control the drug release in cancer cells and to improve the water solubility of PTX, ETP, and RAPA. The synergistic effect of PTX, ETP, and RAPA was evaluated in gastric cancer, and the combination index values were evaluated. Thin-film hydration was used to prepare PTX/ETP/RAPA-loaded mPEG-pH-PCL micelles, and various physicochemical properties of these micelles were evaluated. In vitro cytotoxicity, pH-sensitivity, drug release profiles, in vivo pharmacokinetics, and biodistribution studies of PTX/ETP/RAPA-loaded mPEG-pH-PCL micelles were evaluated. In the pH-sensitivity evaluation, the size of the micelles increased more rapidly at a pH of 5.5 than at a pH of 7.4. The release rate of each drug increased with decreasing pH values in PTX/ETP/RAPA-loaded mPEG-pH-PCL micelles. In vitro and in vivo studies demonstrated that PTX/ETP/RAPA-loaded mPEG-pH-PCL micelles exhibit different drug release behaviors depending on the pH of the tumor and normal tissues and increased bioavailability and circulation time in the blood than solutions. Therefore, we propose that PTX/ETP/RAPA- loaded mPEG-pH-PCL micelles are advantageous for gastric cancer treatment in drug delivery systems.
1. Introduction
Gastric cancer (GC), a solid malignancy that occurs within the gastric mucosa, is the third major reason for cancer-related mortality [1,2]. GC is often detected after it has already advanced or metastasized as it exhibits no symptoms in the early stages [3]. Surgery and chemotherapy have been used as the main treatment methods for GC; however, they do not sufficiently meet expectations due to inadequate accumulation between tumors, distant metastasis, drug resistance, and serious side effects [4,5,6,7]. Therefore, new therapeutic strategies for GC with improved efficacy and low toxicity need to be studied.
Drug combination therapy using several different mechanisms has emerged as a way to improve the limitations of multidrug resistance and side effects associated with high doses of single dosage forms [8,9]. Such combination therapy has been regarded as a promising therapeutic strategy as it reduces anticancer drug resistance, overcomes tumor heterogeneity, and provides synergistic anticancer effects [10,11].
Paclitaxel (PTX) is a representative taxane-based anticancer drug that has been widely used against various cancers, including ovarian cancer, lung cancer, GC, and breast cancer. PTX inhibits the proliferation of cancer cells by interfering with the separation of microtubules, which are used as a mechanism for division and self-replication during cell division [12,13]. Etoposide (ETP), a drug that can form a four-dimensional complex structure with DNA and topoisomerase II, causes damage by preventing DNA strand recombination. ETP is used to treat glioblastoma, lung cancer, sarcoma, non-lymphocytic leukemia, and lymphoma; however, it may result in myelosuppression as a side effect [14,15]. Rapamycin (RAPA) is well-known as a drug that affects cell signaling pathways that determine cell cycle progression and cell growth by selectively inhibiting the mammalian target of rapamycin (mTOR) [16,17]. Previous studies have shown that the two-drug combination of PTX, ETP, and RAPA has a synergistic effect in various carcinomas. Wang et al. demonstrated that the formulation co-loaded with PTX and ETP enhances cytotoxicity in MG63 and Saos-2 osteosarcoma cell lines more than when each of these drugs is used alone [18]. Shafer et al. demonstrated that RAPA enhances the effects of PTX by increasing tubulin polymerization and acetylation, inhibiting cell proliferation, and inducing apoptosis in endometrial cancer cells [19]. Itamochi et al. showed that chemotherapy in combination with RAPA and ETP produced a synergistic cytotoxic effect in ovarian cancer cells and extend the survival of mice with ovarian cancer xenografts [20]. In addition, it has been reported that combinations of drugs with similar mechanisms to PTX, ETP, and RAPA are effective in GC. Yildiz et al. demonstrated the moderate effectiveness and safety of the combination of oral ETP and docetaxel as a second-line treatment for advanced GC after failure of platinum-based therapy [21]. Fukamachi et al. found that the Wnt-mTOR pathway is strongly involved in the growth regulation of diffuse GC initiating cells, and based on this, they demonstrated that mTOR inhibitors and checkpoint inhibitors could be useful in treating a subset of diffuse GCs [22]. Zhang et al. demonstrated that BEZ235, a dual PI3K/mTOR inhibitor, enhances the effects of nab-paclitaxel in GC through regulating the PI3K/mTOR pathway and inhibiting cell proliferation [23]. Based on these results, we predict that the combination of PTX, ETP, and RAPA will be effective in GC cell lines. However, all of these drugs are poorly soluble in water, limiting their clinical application.
Polymeric micelles have been widely used as a method to overcome the low water solubility of hydrophobic drugs [24,25]. Polymeric micelles are known to have advantages such as excellent biocompatibility, extended blood circulation time after intravenous injection, and effective tumor targeting [26,27]. However, drug release after in vivo administration is generally uncontrolled, limiting the rate of drug accumulation in tumors [28,29]. To overcome these problems, pH-sensitive polymeric micelles for controlled drug release have emerged as an effective strategy [30,31,32]. Such pH-sensitive polymeric micelles can trigger drug release by using the pH differences between tumor tissues and normal tissues [33,34,35]. Previous studies have reported that the pH of tumors is slightly acidic (pH < 7.0), whereas that of normal tissues remains constant at a pH of 7.2–7.4 [36,37,38]. As a result, pH-sensitive polymeric micelles maintain a stable state in normal tissues, then reach the tumor site through the enhanced permeation and retention (EPR) effect and change to an unstable state in response to the low pH of the tumor and release the drugs [39,40,41].
Methoxy poly(ethylene glycol)-b-poly(ε-caprolactone; mPEG-b-PCL) is an amphiphilic block copolymer composed of a hydrophilic PEG outer shell and a hydrophobic PCL inner core [42]. Cao et al. reported that novel pH-sensitive mPEG-pH-PCL micelles were successfully synthesized by introducing weak acid cleavable citraconic acid amide linkages into the copolymer. Moreover, a previous study has shown that the hydroxyl end group of PEG is transferred to an amino group, and citraconic anhydride is used to link the PCL and PEG blocks [43]. Therefore, the drug-encapsulated pH-sensitive mPEG-PCL micelles release the drug by breaking the citraconic amide bond through the weak acidity of cancer cells.
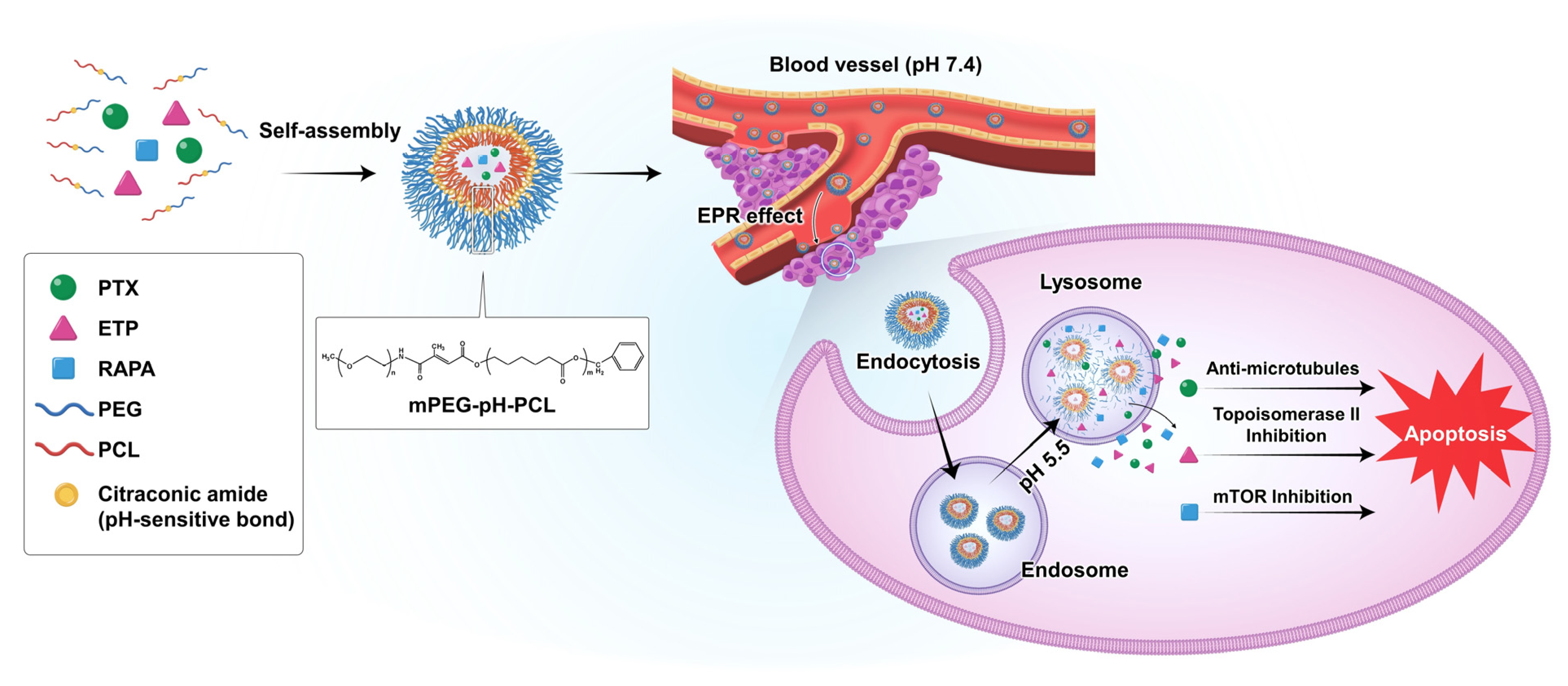
In this study, we used pH-sensitive mPEG-pH-PCL copolymer micelles as carriers for the controlled drug release and delivery of anti-cancer drugs (Figure 1). In addition, we assessed the combination index (CI) of PTX, ETP, and RAPA and investigated the in vitro cytotoxicity, in vitro pH-sensitivity assessment, in vitro release profiles, and pharmacokinetic profiles of micelles.
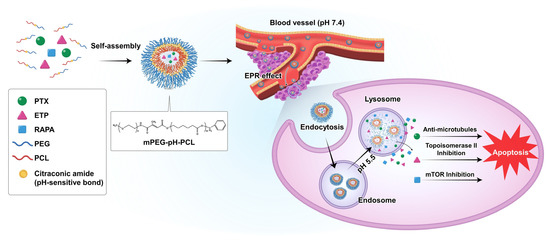
Figure 1.
Schematic diagram of the drug release of paclitaxel (PTX), etoposide (ETP), and rapamycin (RAPA)-loaded mPEG-pH-PCL micelles in response to pH changes.
2. Materials and Methods
2.1. Materials and Reagents
Methoxy poly(ethylene glycol)-b-poly(ε-caprolactone) copolymer with citraconic amide as a pH-sensitive bond (mPEG-pH-PCL) was purchased from Creative PEGWorks (Chapel Hill, NC, USA). Cremophor EL®, dimethyl sulfoxide (DMSO), thiazolyl blue tetrazolium bromide (MTT), and crystal violet were purchased from Sigma-Aldrich Corp. (St. Louis, MO, USA). Distilled water (DW), ethanol (EtOH), acetonitrile (ACN), and methanol (MeOH) were purchased from Fisher Scientific (Waltham, MA, USA). PTX, RAPA, and sorafenib were purchased from LC Laboratories® (Woburn, MA, USA). ETP was purchased from Tokyo Chemical Industry Co., Ltd. (Chuo-ku, Tokyo, Japan). Sodium chloride (NaCl), potassium phosphate monobasic (KH2PO4), potassium chloride (KCl), sodium phosphate dibasic anhydrous (Na2HPO4), and 0.1 N hydrochloric acid (HCI) standard solution were purchased from Duksan (Seoul, Korea) and were used for the manufacturing of phosphate-buffered saline (PBS). All other reagents and solvents were analytical grade or high-performance liquid chromatography (HPLC) grade.
2.2. Cell Line and Cell Culture
AGS-Luc2 cells, used as a human GC luciferase-expressing cell line, were obtained from the American Type Culture Collection (Manassas, VA, USA). Dulbecco’s phosphate-buffered saline (DPBS), Roswell Park Memorial Institute medium (RPMI 1640), fetal bovine serum (FBS), trypsin, and penicillin–streptomycin solution were purchased from Corning Inc. (Corning, NY, USA). AGS-Luc2 cells were cultured in RPMI 1640 medium using a CO2 incubator (Panasonic, Osaka, Japan) maintained at 37 °C and a 5% CO2 atmosphere. The media were supplemented with 1% (w/v) penicillin–streptomycin solution and 10% (v/v) FBS.
2.3. CI Analysis
CI analysis was conducted according to the Chou–Talalay method to assess the synergistic, antagonistic, or additive effects between the drugs [44]. The CI values were calculated using the following formula:
where (Dx)1, (Dx)2, and (Dx)3 represent the half-maximal inhibitory concentration (IC50) values of a single drug, and (D)1, (D)2, and (D)3 represent the IC50 values of each drug when used in combination. CI < 1 indicates synergism, CI = 1 indicates additivity, and CI > 1 indicates antagonism.
2.4. Preparation of PTX/ETP/RAPA-Loaded Polymeric Micelles
The thin-film hydration method was used to prepare PTX/ETP/RAPA-loaded mPEG-pH-PCL micelles [45]. PTX (6 mg), ETP (6 mg), RAPA (3 mg), and mPEG-pH-PCL (150 mg) were dissolved in 1 mL of ACN. The organic solvent was evaporated under reduced pressure in a 60 °C water bath for 10 min using a rotary vacuum evaporator (EYELA®, Bohemia, NY, USA). Then, the thin film was hydrated with 1 mL of DW at 60 °C for 30 min. The PTX/ETP/RAPA-loaded micelle solution was centrifuged at 13,000 rpm for 5 min at 4 °C (Hanil Science Inc., Gimpo, Korea), and a 0.2 μm sterile filter was used to filter the supernatant (Corning, NY, USA).
2.5. Physicochemical Characterization of Micelles
A dynamic light-scattering (DLS) instrument was used to assess the particle size, zeta potential, and poly-dispersity index (PDI) of the PTX/ETP/RAPA-loaded mPEG-pH-PCL micelles (Litesizer 500, Anton Paar, Graz, Austria). The micelle solution was diluted 10-fold with DW before DLS measurement and with ACN before HPLC analysis. The drug loading (DL, %) and encapsulation efficiency (EE, %) of PTX, ETP, and RAPA loaded into micelles were calculated according to the following formulas [46,47]:
2.6. Transmission Electron Microscopy (TEM) Observation
The morphology of PTX/ETP/RAPA-loaded mPEG-pH-PCL micelles was observed using TEM (JEOL Ltd., Tokyo, Japan). The micelle solution diluted with DW was dropped onto a 200-mesh copper grid, dried in a drying oven for 12 h at 60 °C, and measured at a voltage of 200 kV.
2.7. In Vitro pH-Sensitivity Assessment of Micelles
PTX/ETP/RAPA-loaded mPEG-pH-PCL micelles were diluted 10-fold with PBS (pH 7.4 and 5.5) to measure the particle size, zeta potential, and PDI. Each sample was stored at room temperature and measured at 0, 2, 4, 6, and 8 h. Particle size and PDI were measured to investigate the stability of the micelles in the tumor microenvironment (pH 5.5) and physiological conditions (pH 7.4).
2.8. In Vitro Cytotoxicity Assay
The cytotoxicity to AGS-Luc2 cells was evaluated by MTT assay [48]. AGS-Luc2 cells were seeded at a density of 5 × 103 cells/well in 96-well plates. After 24 h of incubation, the medium was aspirated, and the cells were treated with free PTX, free ETP, free RAPA, free PTX/ETP/RAPA, PTX-loaded mPEG-pH-PCL micelles, ETP-loaded mPEG-pH-PCL micelles, RAPA-loaded mPEG-pH-PCL micelles, or PTX/ETP/RAPA-loaded mPEG-pH-PCL micelles. The drug-encapsulated mPEG-pH-PCL micelles were diluted 10-fold with RPMI medium. The free drugs were dissolved in 100 μL of DMSO and then diluted 1000-fold with RPMI medium to be used as an initial concentration. After incubation for 48 h, the medium was aspirated, and 100 μL of 0.5 mg/mL MTT solution was added. After 4 h of MTT treatment, the MTT solution was aspirated, and 100 μL of DMSO was added, followed by shaking at 200 rpm for 10 min using an orbital shaker (N-BIOTEK, NB-101S, Bucheon, Korea). The absorbance was measured using a microplate reader (Molecular Devices, Spectra Max ID3, San Jose, CA, USA) at a wavelength of 540 nm. All cell-related data processing was performed in GraphPad Prism v. 5 (GraphPad Software, La Jolla, CA, USA).
2.9. In Vitro Clonogenic Assay
Clonogenic assays were performed to assess the ability of single cells to form colonies and long-term cell survival [49,50]. AGS-Luc2 cells were seeded at a density of 200 cells/well in 6-well plates. After 24 h of incubation, the cells were treated with PTX-loaded mPEG-pH-PCL micelles, ETP-loaded mPEG-pH-PCL micelles, RAPA-loaded mPEG-pH-PCL micelles, or PTX/ETP/RAPA-loaded mPEG-pH-PCL micelles. The medium was removed after two weeks of incubation, and the colonies were stained with 1 mL of 0.5% w/v crystal violet. The dye was removed with fresh water after 30 min, and the number of colonies was counted. The following equation was used to calculate the colony formation [51]:
2.10. In Vitro Drug Release Assay
The drug release of micelles encapsulated with PTX/ETP/RAPA was investigated using the dialysis method [52,53]. Briefly, PTX/ETP/RAPA-loaded mPEG-pH-PCL micelles were put in a dialysis membrane (MWCO = 20 kDa) and stirred in 2 L of PBS (pH 7.4 and 5.5) at 37 °C and 200 rpm. The pH of PBS (pH 7.4 and 5.5) was measured with a pH meter (Mettler Toledo, Columbus, OH, USA), and the pH values were titrated by 0.1 N HCI. At predetermined sampling times (0, 2, 4, 6, 8, 24, 48, 72, 168, 240, and 336 h), 20 µL of a sample was taken and diluted 10-fold with ACN. The concentrations of PTX, ETP, and RAPA were determined by HPLC. At 8, 24, 72, 168, and 240 h, fresh medium was substituted for the PBS (pH 7.4 and 5.5) release medium. Data were evaluated using the Peppas model of SigmaPlot v. 10.0 (Systat Software, Inc., San Jose, CA, USA).
2.11. In Vivo Pharmacokinetic Study
Sprague–Dawley rats (male, 7–8 weeks old) were purchased from Samtako Bio Korea (Osan, Korea) for pharmacokinetic and biodistribution studies. The animal experiments in this study were approved by the Institutional Animal Care and Use Committee (IACUC) of Chungbuk National University (No. CBNUA-1711-22-01, 27 April 2022). All rats used in the experiment were housed in a constant temperature–humidity and well-ventilated environment and were provided with sufficient food and water. The rats were administered either PTX/ETP/RAPA-loaded mPEG-pH-PCL micelles or PTX/ETP/RAPA solution through the tail vein. The PTX/ETP/RAPA solution was dissolved in EtOH:Cremophor EL® (50:50, v/v) and was used as a control. The respective doses of PTX, ETP, and RAPA were 10 mg/kg, 10 mg/kg, and 5 mg/kg. At 5, 15, 30, 60, 120, 240, and 480 min after administration, blood (<500 μL) was collected from the retro-orbital plexus and centrifuged at 5000 rpm for 5 min to obtain plasma samples. Plasma samples were stored in a −70 °C deep freezer and were analyzed according to the pretreatment procedure described in the Section 2.13.2. The pharmacokinetic parameters of PTX, ETP, and RAPA were analyzed using a one-compartment model and were calculated using SigmaPlot v. 10.0 (Systat Software, Inc., San Jose, CA, USA).
2.12. Biodistribution Study
A biodistribution study was conducted to assess the tissue distribution of the drug at 8 h after intravenous injection of PTX/ETP/RAPA-loaded mPEG-pH-PCL micelles and PTX/ETP/RAPA solution. The rats were euthanized with CO2 gas, and liver, spleen, kidney, heart, lung, and muscle tissues were collected. Tissue samples were cleaned with PBS and stored in a −70 °C deep freezer until analysis.
2.13. HPLC Analysis
2.13.1. Assay Conditions
The HPLC system (Waters, Milford, MA, USA), consisting of a 2996 photodiode array detector and a 2695 separation module, was used to detect the concentration of PTX, ETP, RAPA, and sorafenib (internal standard, IS). For HPLC analysis, a Fortis C18 HPLC column (5 µm, 4.6 × 250 mm) (Fortis® Technologies Ltd., Cheshire, UK) was used, and the column temperature was maintained at 30 °C. The sample injection volume was 10 µL, and the isocratic mode was used to elute PTX, ETP, RAPA, and IS. The ACN:DW ratio of the mobile phase was 70:30 (v/v), and the flow rate was 1.0 mL/min. The retention times of PTX, ETP, RAPA, and IS were 5.8, 2.8, 21.7, and 8.9 min, and the wavelengths were detected at 227, 284, 277, and 264 nm, respectively. Each drug concentration was determined by substituting its peak area into a standard curve.
2.13.2. Preparation of Biological Samples
Frozen plasma samples were thawed at 25 ℃ room temperature, and 50 μL of IS and 400 μL of MeOH were added to 200 μL of plasma sample. The mixture was vortexed and centrifuged for 5 min at 13,000 rpm. The supernatant was passed through a 0.2 μm sterile filter, and the concentrations of PTX, ETP, RAPA, and IS were measured by HPLC. The amounts of PTX, ETP, and RAPA in each tissue were measured using the homogenization method [54]. Briefly, tissue samples were homogenized in an Ultra-Turrax T25 homogenizer (IKA Works Inc., Staufen, Germany) using a Teflon pestle. Then, 200 μL of tissue sample was pretreated in the same manner as above, and the concentration of each drug was analyzed by HPLC.
2.14. Statistical Analysis
All data are presented as the mean ± standard deviation (SD). Drug release experiment results are expressed as the mean ± standard error (SE). All experiments were performed more than three times. An unpaired t-test was conducted for the statistical analysis with GraphPad Prism v. 5.0 (GraphPad Software, La Jolla, CA, USA); a p-value < 0.05 was regarded as statistically significant.
3. Results
3.1. Evaluation of the Synergistic Effect of PTX, ETP, and RAPA
Table 1 shows the IC50 values of PTX, ETP, and RAPA in two ratios and the CI values of each ratio. PTX and ETP exhibited similar IC50 values in both ratios, while RAPA showed a lower IC50 value in the 2:2:1 ratio, showing a significant difference (p < 0.05). As a result of CI value analysis, the 2:2:1 and 1:1:1 ratios both showed a synergistic effect with a CI value of 0.06.

Table 1.
Half-maximal inhibitory concentration (IC50) and combination index (CI) values at two ratios of paclitaxel (PTX), etoposide (ETP), and rapamycin (RAPA) (n = 18).
3.2. Physicochemical Characterization of PTX/ETP/RAPA-Loaded mPEG-pH-PCL Micelles
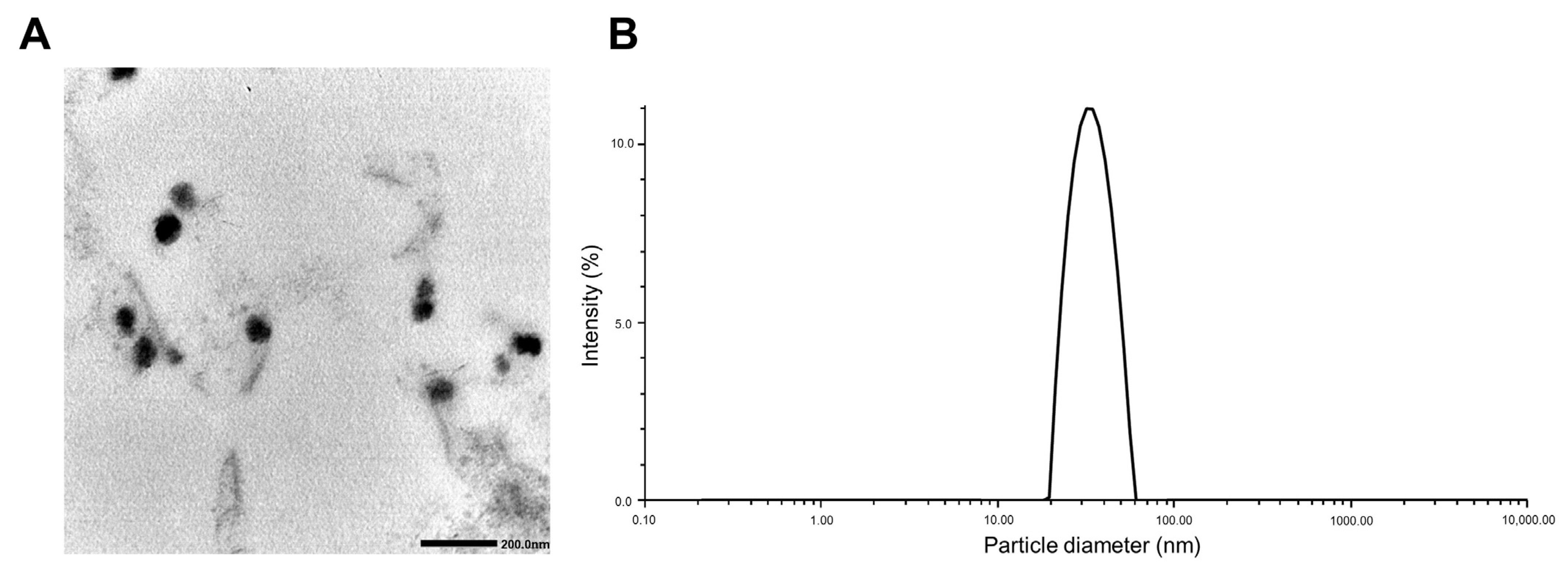
Table 2 shows the EE (%), DL (%), particle size, zeta potential, and PDI of PTX/ETP/RAPA-loaded mPEG-pH-PCL micelles at a 2:2:1 ratio. The EE (%) of PTX, ETP, and RAPA encapsulated in mPEG-pH-PCL micelles were 64.8%, 67.3%, and 70.3%, and the DL (%) was 2.49%, 2.59%, and 1.38%, respectively. In addition, the particle size was 35.0 ± 0.24 nm, the PDI value was 0.03 ± 0.70, and the zeta potential was −0.22 ± 0.03 mV. The morphology and dispersion of the PTX/ETP/RAPA-loaded mPEG-pH-PCL micelles were characterized by TEM and DLS. The TEM image indicates that the morphology of the micelles is uniformly spherical (Figure 2A), and the particle size distribution indicates that the micelle size is <100 nm (Figure 2B).

Table 2.
Physicochemical characterization of paclitaxel (PTX), etoposide (ETP), and rapamycin (RAPA)-loaded mPEG-pH-PCL micelles (n = 3).
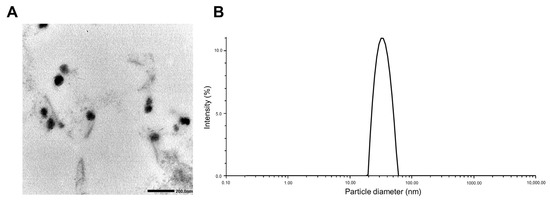
Figure 2.
(A) Transmission electron microscopy (TEM) image of PTX/ETP/RAPA-loaded mPEG-pH-PCL micelles. (B) Representative particle size distributions of PTX/ETP/RAPA-loaded mPEG-pH-PCL micelles (n = 3).
3.3. In Vitro pH-Sensitivity Assessment of PTX/ETP/RAPA-Loaded mPEG-pH-PCL Micelles
Table 3 shows the particle size, zeta potential, and PDI of PTX/ETP/RAPA-loaded mPEG-pH-PCL micelles at a pH of 7.4 and a pH of 5.5. At a pH of 7.4, the size of the micelles increased significantly at 8 h compared to 0 h (Table 3A). On the other hand, at a pH of 5.5, the size of the micelles increased significantly at 6 h compared to 0 h, which is 2 h faster than at a pH of 7.4 (Table 3B) (p < 0.05).

Table 3.
Particle size, zeta potential, and poly-dispersity index (PDI) of paclitaxel (PTX), etoposide (ETP), and rapamycin (RAPA)-loaded mPEG-pH-PCL micelles at (A) a pH of 7.4 and (B) a pH of 5.5 (n = 3).
3.4. In Vitro Cytotoxicity Assay
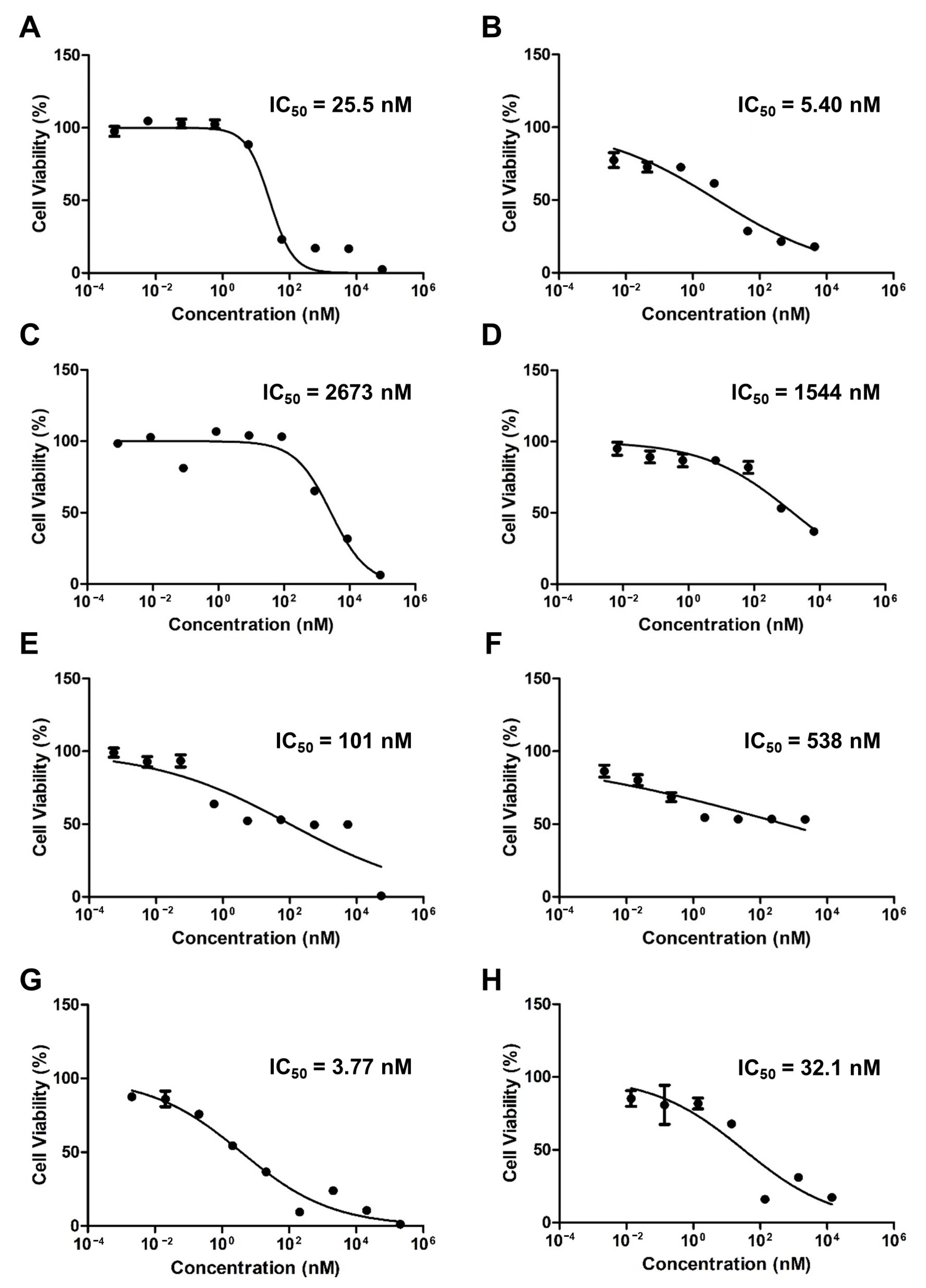
Figure 3 shows the cytotoxicity results for the free drugs and micelles when PTX, ETP, and RAPA were used alone or in combination. The IC50 value of free PTX was 25.5 nM and the PTX-loaded mPEG-pH-PCL micelle was 5.40 nM (Figure 3A,B). The IC50 value of free ETP was 2673 nM and the ETP-loaded mPEG-pH-PCL micelle was 1544 nM (Figure 3C,D). The IC50 value of free RAPA was 101 nM and the RAPA-loaded mPEG-pH-PCL micelle was 538 nM (Figure 3E,F). Finally, the IC50 value of free PTX/ETP/RAPA was 3.77 nM and the PTX/ETP/RAPA-loaded mPEG-pH-PCL micelle was 32.1 nM (Figure 3G,H).
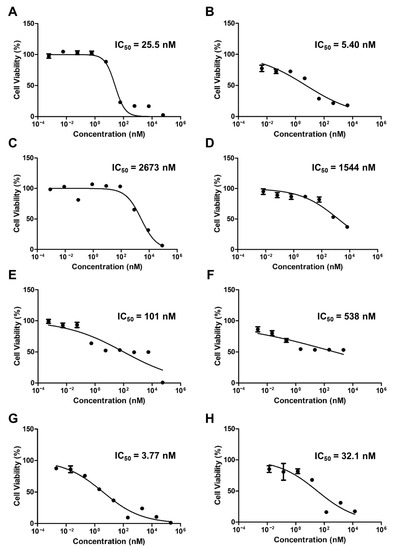
Figure 3.
In vitro cytotoxicity analysis of AGS-Luc2 cells after 48 h of treatment with (A) free PTX, (B) PTX-loaded mPEG-pH-PCL micelles, (C) free ETP, (D) ETP-loaded mPEG-pH-PCL micelles, (E) free RAPA, (F) RAPA-loaded mPEG-pH-PCL micelles, (G) free PTX/ETP/RAPA, and (H) PTX/ETP/RAPA-loaded mPEG-pH-PCL micelles (n = 18).
3.5. In Vitro Clonogenic Assay
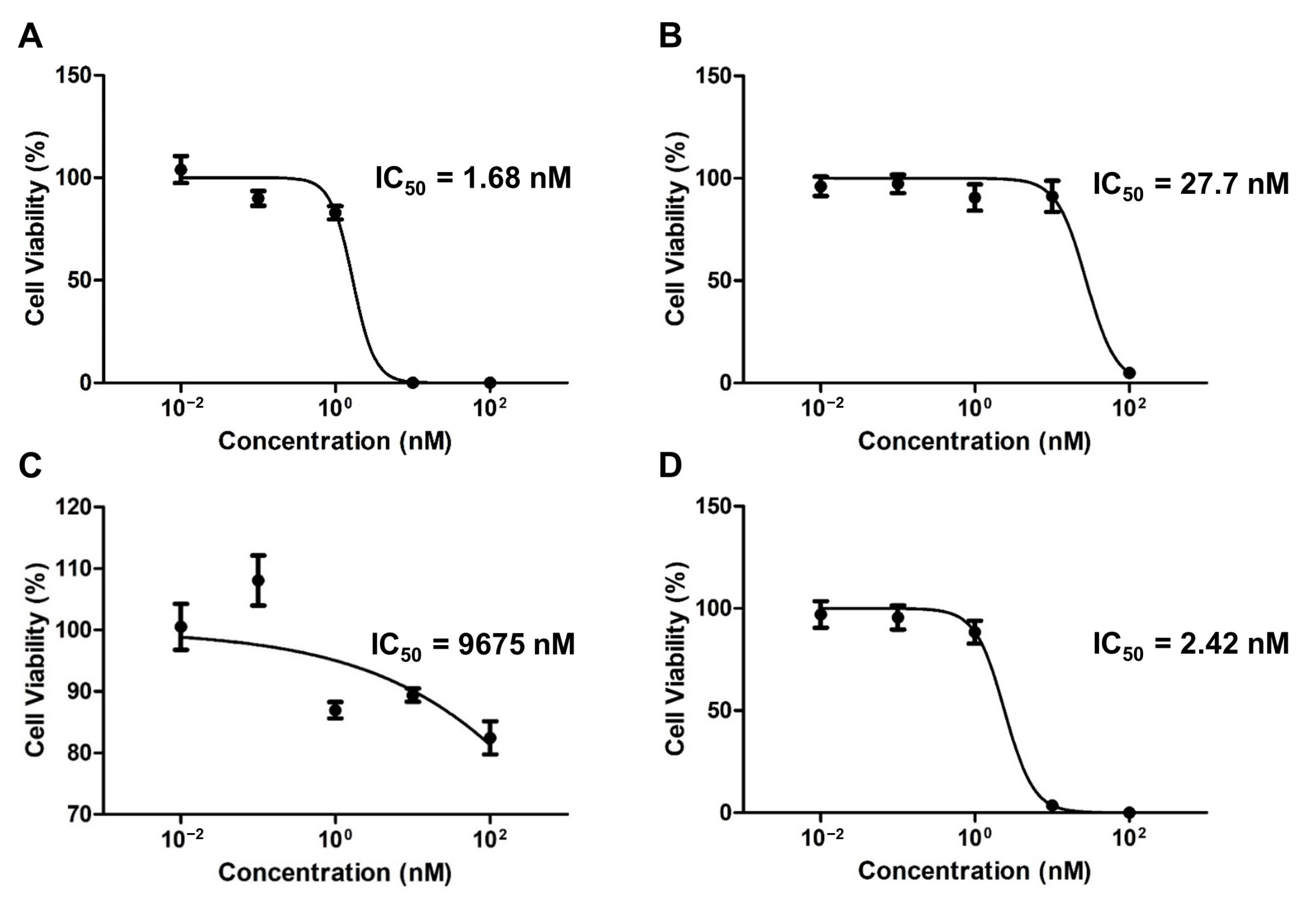
The colony inhibition rate of the drug-encapsulated micelles was evaluated using a clonogenic assay over two weeks (Figure 4). The respective IC50 values of the PTX-, ETP-, RAPA-, and PTX/ETP/RAPA-loaded mPEG-pH-PCL micelles were 1.68 nM (Figure 4A), 27.7 nM (Figure 4B), 9675 nM (Figure 4C), and 2.42 nM (Figure 4D). The CI value was 0.54, indicating synergism.
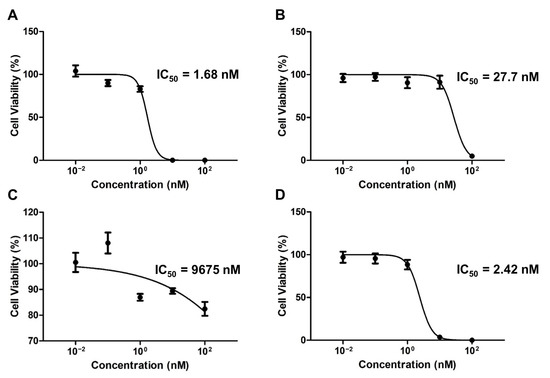
Figure 4.
In vitro clonogenic analysis of AGS-Luc2 cells after two weeks of treatment with (A) PTX-loaded mPEG-pH-PCL micelles, (B) ETP-loaded mPEG-pH-PCL micelles, (C) RAPA-loaded mPEG-pH-PCL micelles, and (D) PTX/ETP/RAPA-loaded mPEG-pH-PCL micelles (n = 3).
3.6. In Vitro Drug Release Assay
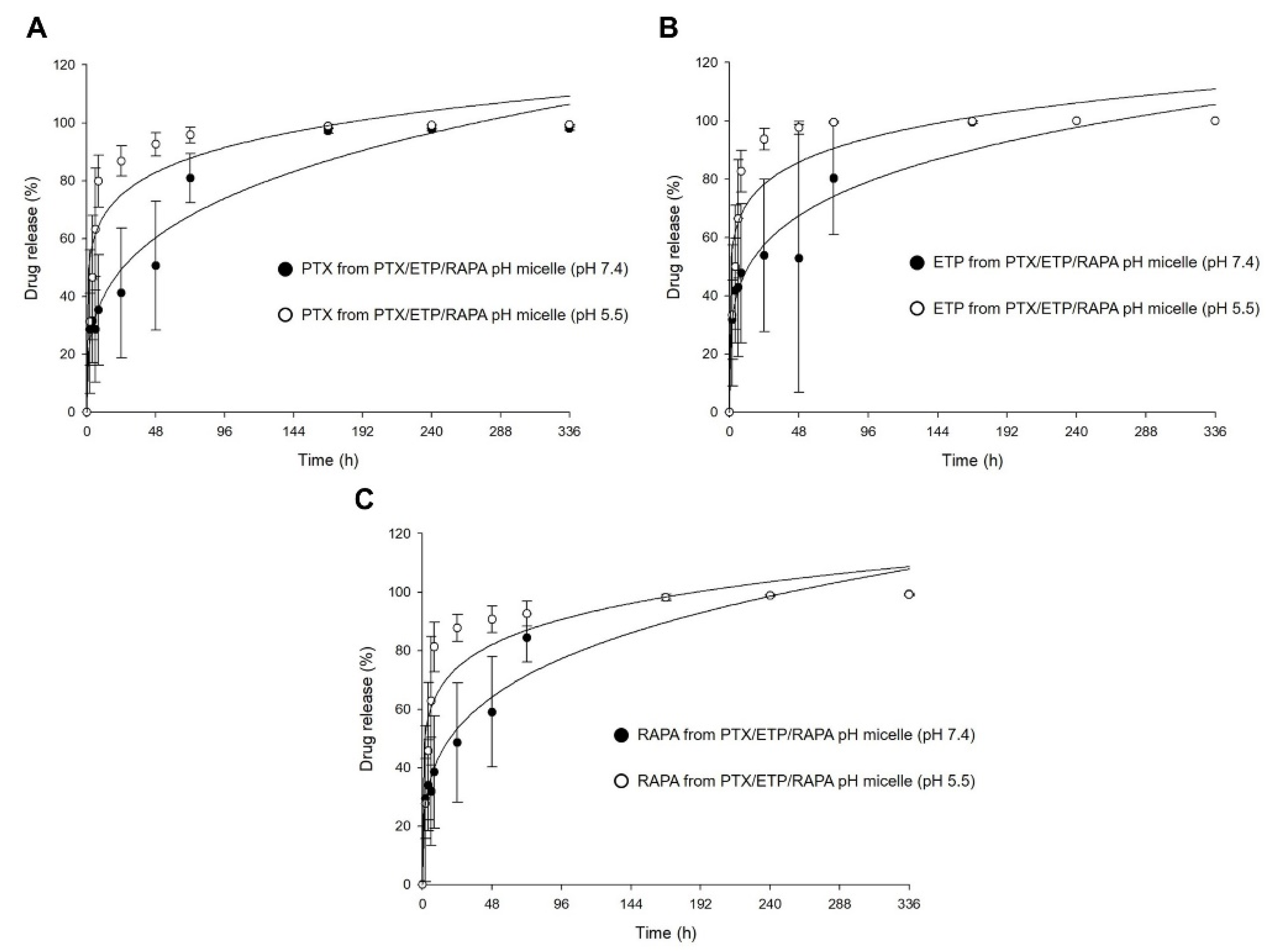
Figure 5 shows the in vitro release profiles of PTX, ETP, and RAPA in PTX/ETP/RAPA-loaded mPEG-pH-PCL micelles. In Figure 5A, the 48 h release rate of PTX was 50% at a pH of 7.4 and 92% at a pH of 5.5. The 48 h release rate of ETP was 52% at a pH of 7.4 and 97% at a pH of 5.5 (Figure 5B). The 48 h release rate of RAPA was 59% at a pH of 7.4 and 90% at a pH of 5.5 (Figure 5C). At a pH of 5.5, PTX showed a rapid release rate >90% after 48 h, ETP after 24 h, and RAPA after 48 h. In contrast, at a pH of 7.4, all three drugs showed a release rate >90% after 168 h. Consequently, the drug release for the PTX/ETP/RAPA-loaded mPEG-pH-PCL micelles at a pH of 5.5 was much faster than at a pH of 7.4.
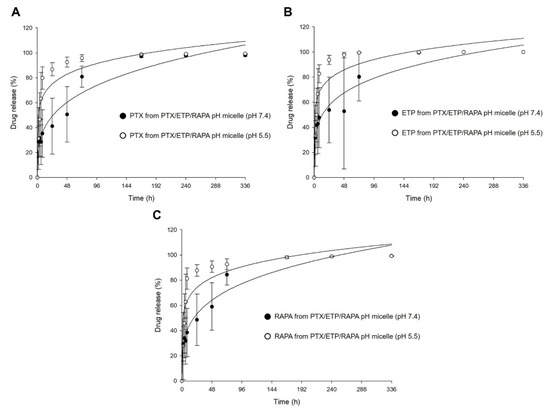
Figure 5.
In vitro release profiles at different pH values of (A) PTX, (B) ETP, and (C) RAPA release in PTX/ETP/RAPA-loaded mPEG-pH-PCL micelles (n = 3–4).
3.7. In Vivo Pharmacokinetic Study
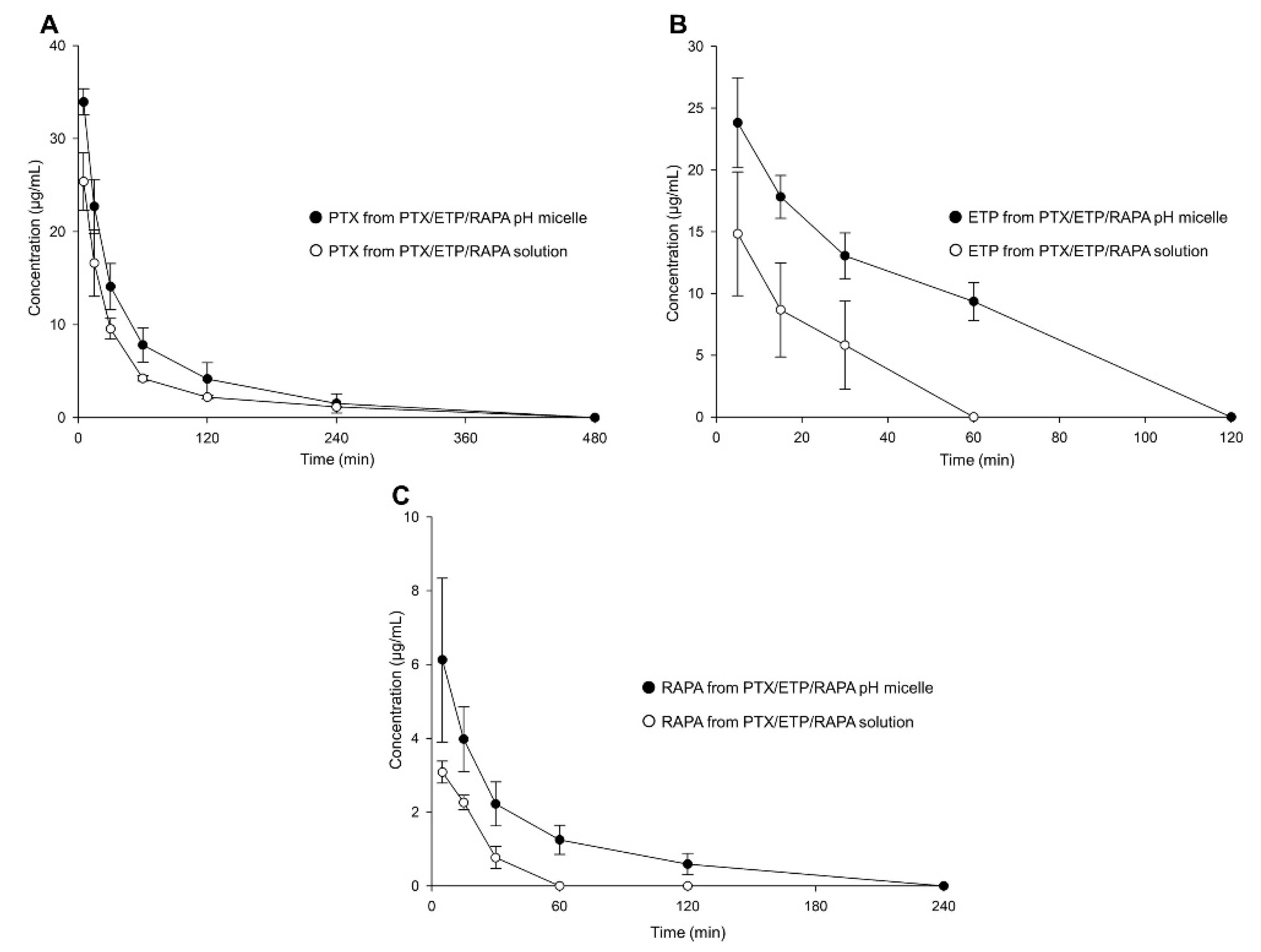
Figure 6 and Table 4 show the plasma concentration–time profiles and pharmacokinetic parameters of the PTX/ETP/RAPA-loaded mPEG-pH-PCL micelles and the PTX/ETP/RAPA solution. In Figure 6A, the plasma concentrations of PTX were detected at up to 240 min, both in the micelles and in the solution. Those of ETP were detected at up to 60 min in the micelles and at up to 30 min in the solution (Figure 6B). Finally, the plasma concentrations of RAPA were detected at up to 120 min in the micelles and at up to 30 min in the solution (Figure 6C). All three drugs were not found at concentrations below the limit of detection (LOD). In Table 4, the PTX area-under-the-curve (AUC) value of the PTX/ETP/RAPA-loaded mPEG-pH-PCL micelles was 1.6-fold higher in the micelles than in the solution. The AUC value of ETP was 3.0-fold higher in the micelles than in the solution (p < 0.05), and that of RAPA was 2.4-fold higher in the micelles than in the solution (p < 0.05). Overall, the micelle formulation showed higher AUC values and lower total clearance (CLt) and volume of distribution (Vd) values than the solution.
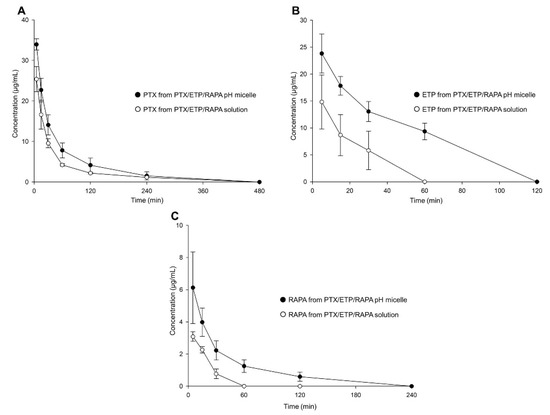
Figure 6.
Plasma concentration–time profiles of PTX, ETP, and RAPA after intravenous injection. (A) PTX, (B) ETP, and (C) RAPA concentration in PTX/ETP/RAPA-loaded mPEG-pH-PCL micelles and PTX/ETP/RAPA solution (n = 3).

Table 4.
Pharmacokinetic parameters of paclitaxel (PTX), etoposide (ETP), and rapamycin (RAPA) in PTX/ETP/RAPA-loaded mPEG-pH-PCL micelles and PTX/ETP/RAPA solution after intravenous injection (n = 3).
3.8. Biodistribution Study
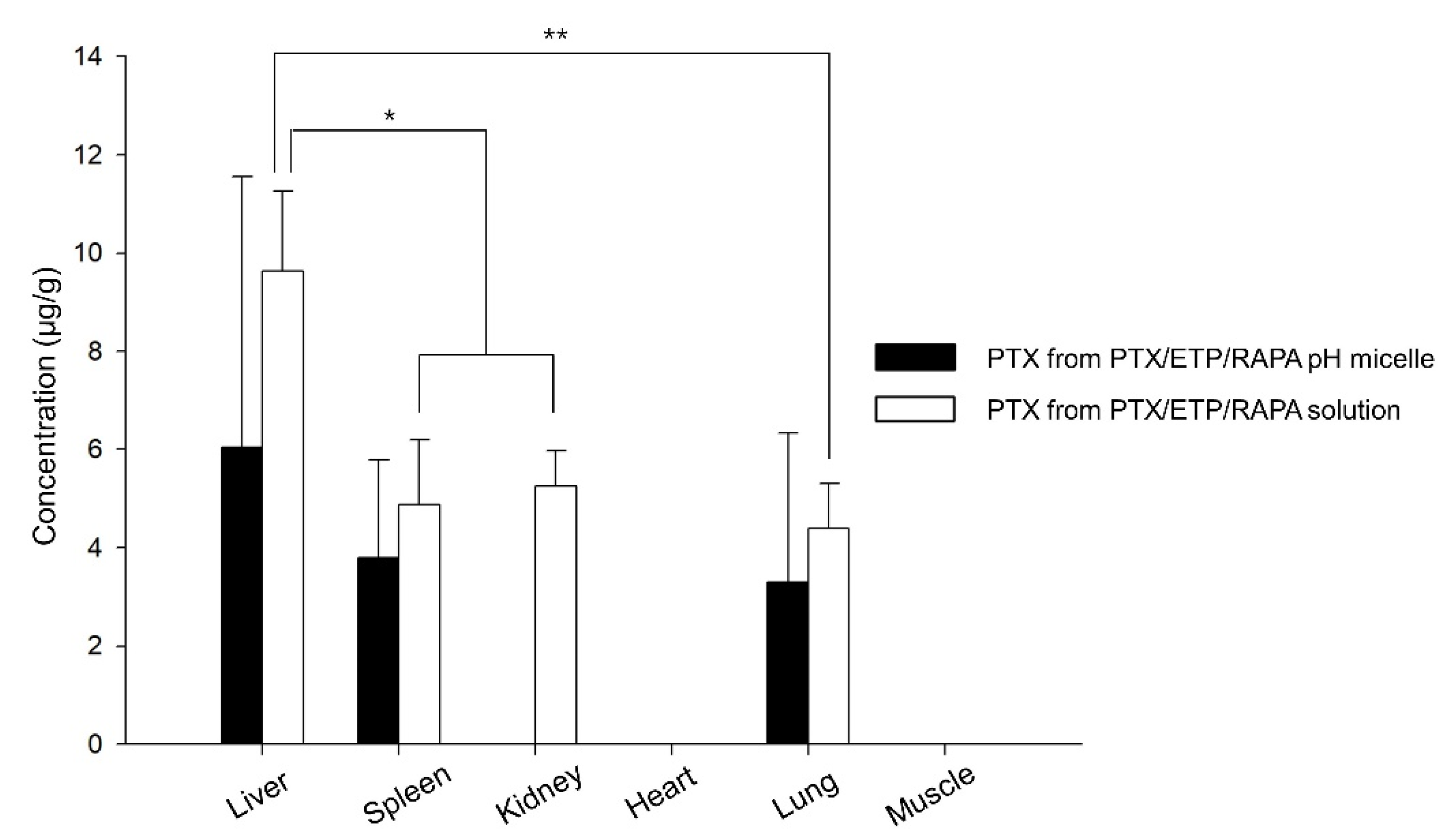
Figure 7 shows the drug distribution of the PTX/ETP/RAPA-loaded mPEG-pH-PCL micelles and the PTX/ETP/RAPA solution in major organs 8 h after intravenous injection. PTX was detected in four organs, whereas ETP and RAPA were not detected in the liver, spleen, kidneys, heart, lungs, and muscles. The solution was detected in the liver, spleen, kidneys, and lungs, but micelles were not detected in the kidneys due to values below the LOD. The highest amount of PTX was detected in the liver for both the micelles and solutions, and the solution was detected at a 1.6-fold higher concentration than that of the micelles. Overall, a higher concentration of PTX was detected in the solution than in the micelles.
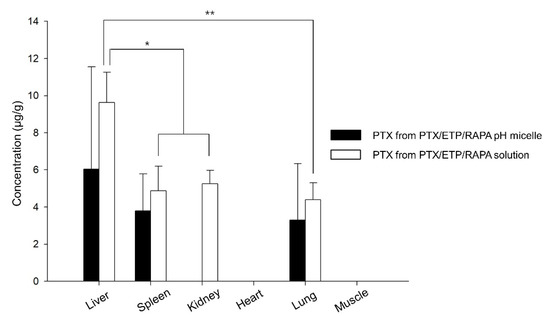
Figure 7.
Biodistribution of PTX in PTX/ETP/RAPA-loaded mPEG-pH-PCL micelles and PTX/ETP/RAPA solution in each tissue at 8 h after intravenous injection (* p < 0.05, ** p < 0.01) (n = 3).
4. Discussion
PTX, ETP, and RAPA have been reported to be effective against various cancers, and due to the different mechanisms of each drug, they can simultaneously target multiple pathways of cancer cells when combined [55,56]. However, all three drugs are limited in their clinical application due to their low solubility in water [57,58,59]. Therefore, pH-sensitive polymeric micelles were used to improve the water solubility of PTX, ETP, and RAPA, and effectively control the drug release from cancer cells. These pH-sensitive polymeric micelles have attracted much attention as an effective strategy for the targeted delivery of antitumor drugs. Based on previous studies, pH-sensitive polymeric micelles are prepared using the mPEG-pH-PCL polymer [43]. As a result, we expected to observe synergism of the three drugs in GC and the controlled drug release of the micelles.
To evaluate the synergistic effect of PTX, ETP, and RAPA in GC, CI values were evaluated at the weight ratios of 2:2:1 and 1:1:1. Results showed that both ratios had a CI value of 0.06, indicating synergism. Intravenous doses of PTX, ETP, and RAPA have been reported to be 10–20 mg/kg, 10–30 mg/kg, and 2–10 mg/kg, respectively [60,61,62,63,64,65,66,67]. Consequently, we chose a final ratio of 2:2:1, considering that RAPA has a relatively small intravenous dose and that RAPA exhibited a lower IC50 value at the 2:2:1 ratio. The evaluation of the physicochemical properties by manufacturing micelles at a ratio of 2:2:1 revealed that the EE (%) of all three drugs was >60% and that the spherical micelles had a particle size of <100 nm. Such small particle sizes (< 100 nm) enhanced the vascular permeability at the target site, favoring preferential accumulation at the tumor site [68]. Moreover, the size of the micelle increased at a faster rate at a pH of 5.5 than at a pH of 7.4. These results indicate that citraconic amide bonds are cleaved and micelles are dissociated under weakly acidic conditions, resulting in an enlarged hydrodynamic diameter.
The IC50 values of free PTX/ETP/RAPA were lower than those of the PTX/ETP/RAPA-loaded mPEG-pH-PCL micelles according to in vitro cytotoxicity analyses. These results may be related to the in vitro drug release profiles. In the PTX/ETP/RAPA-loaded mPEG-pH-PCL micelles, the 48 h release rates of PTX, ETP, and RAPA were 50%, 52%, and 59%, respectively. Therefore, considering the slow release rate of the drug-encapsulated in micelles at 48 h and the fact that endocytosis of micelles may require time, micelles are considered to have a lower cytotoxicity than free drugs [69,70]. As a result of evaluating the long-term colony suppression rate for two weeks in the in vitro clonogenic assay, the CI value of the PTX/ETP/RAPA-loaded mPEG-pH-PCL micelles was shown to be 0.54, indicating synergism. The in vitro drug release profiles showed that the release rate of each drug increased as the pH value decreased for the PTX/ETP/RAPA-loaded mPEG-pH-PCL micelles. These results indicate that the release of the drug is accelerated by breaking the citraconic amide bond at a slightly acidic pH of 5.5. Therefore, in vitro release results suggest that PTX/ETP/RAPA-loaded mPEG-pH-PCL micelles are pH-dependent and exhibit different drug release behaviors depending on the pH of the tumors and normal tissues, which will be effective in treating targeted cancer.
In vivo pharmacokinetic studies have shown that PTX/ETP/RAPA-loaded mPEG-pH-PCL micelles are detected at higher concentrations in the blood for a longer time than solutions. The AUC values of PTX, ETP, and RAPA in the micelles were 1.6-, 3.0-, and 2.4-fold higher than in their respective solutions. In addition, the clearance values were 1.5-, 3.8-, and 2.3-fold lower in the micelles than in their solutions, respectively. These results suggest that micelle formulations can promote long-lasting drug release in the body through improved bioavailability and increased circulation time in the blood than solutions [71]. In the biodistribution study, PTX was detected most in the liver, both in the micelles and in the solution, which is interpreted as drug accumulation due to absorption of the reticuloendothelial system (RES) [72]. Moreover, PTX accumulation in the micelles was lower than in the solution, indicating that the micelle formulation could reduce hepatotoxicity [73]. Regarding the PK parameter table, the solution accumulates more in the organs because the solution had higher CLt and Vd values than the micelles [74].
5. Conclusions
In conclusion, we evaluated the synergistic effects of PTX, ETP, and RAPA and evaluated the physicochemical properties of PTX/ETP/RAPA-loaded mPEG-pH-PCL micelles at a 2:2:1 ratio. In vitro studies showed that the size of micelles increases more rapidly at a pH of 5.5 than at a pH of 7.4, and the release rate of each drug increases with decreasing pH values in PTX/ETP/RAPA-loaded mPEG-pH-PCL micelles. In vivo studies demonstrated that micelle formulations exhibit an improved bioavailability and an increased circulation time in the blood than solutions. Therefore, we suggest that PTX/ETP/RAPA-loaded mPEG-pH-PCL micelles are an advantageous drug delivery strategy for GC treatment.
Author Contributions
Conceptualization, M.J.J. and D.H.S.; methodology, M.J.J.; software, M.J.J.; validation, M.J.J. and D.H.S.; formal analysis, M.J.J.; investigation, M.J.J.; resources, D.H.S.; data curation, M.J.J.; interpretation, M.J.J. and D.H.S.; writing—original draft preparation, M.J.J.; writing—review and editing, M.J.J., H.J.S., M.S.Y., S.Y.K., C.E.J. and D.H.S.; visualization, M.J.J. and H.J.S.; supervision, C.-W.P., J.-S.K. and D.H.S.; project administration, M.J.J. and D.H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, grant number NRF-2022R1C1C1007107. This research was supported by a National Research Foundation (NRF) of Korea Grant funded by the Korean government (MSIP) (No. MRC2017R1A5A2015541). This research was supported by "Regional Innovation Strategy (RIS)" through the National Research Foundation of Korea (NRF) funded by the Ministry of Education(MOE) (2021RIS-001).
Institutional Review Board Statement
The experimental protocols and animal experiments were performed with the approval of the Institutional Animal Care and Use Committee (IACUC) of Chungbuk National University (No. CBNUA-1711-22-01; approval date: 27 April 2022). The treatment and welfare of laboratory animals were performed in accordance with the guidelines of the Ministry of Food and Drug Safety (MFDS) in the Republic of Korea.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this article are contained in the manuscript.
Acknowledgments
The authors would like to thank Yu Been Shin and Jae Min Lee for their technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Padmanabhan, N.; Ushijima, T.; Tan, P. How to stomach an epigenetic insult: The gastric cancer epigenome. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Thrift, A.P.; El-Serag, H.B. Burden of Gastric Cancer. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2020, 18, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Correa, P. Gastric cancer: Overview. Gastroenterol. Clin. N. Am. 2013, 42, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Wang, D.; Liang, J.; Guo, Y.; Zhu, Y.; Xia, J.; Qin, J.; Zhan, H.; Wang, J. Novel ginsenoside-based multifunctional liposomal delivery system for combination therapy of gastric cancer. Theranostics 2019, 9, 4437–4449. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Wang, J.; Ding, N.; Chen, W.; Chen, H.; Xue, M.; Chen, F.; Ni, J.; Wang, Z.; Lin, Z.; et al. Prodrug polymeric micelles integrating cancer-associated fibroblasts deactivation and synergistic chemotherapy for gastric cancer. J. Nanobiotechnol. 2021, 19, 381. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhu, L.; Zheng, K.; Liu, J.; Tian, P.; Hu, D.; Wang, Q.; Zuo, Q.; Ouyang, X.; Dai, Y.; et al. The design and synthesis of redox-responsive oridonin polymeric prodrug micelle formulation for effective gastric cancer therapy. J. Mater. Chem. B 2021, 9, 3068–3078. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Wang, J.H.; Gu, L.Y.; Yao, X.M.; Cai, F.Y.; Jing, M.; Li, X.T.; Ju, R.J. Dual variable of drug loaded micelles in both particle and electrical charge on gastric cancer treatment. J. Drug Target. 2020, 28, 1071–1084. [Google Scholar] [CrossRef] [PubMed]
- Debele, T.A.; Lee, K.-Y.; Hsu, N.-Y.; Chiang, Y.-T.; Yu, L.-Y.; Shen, Y.-A.; Lo, C.-L. A pH sensitive polymeric micelle for co-delivery of doxorubicin and α-TOS for colon cancer therapy. J. Mater. Chem. B 2017, 5, 5870–5880. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, Y.; Zhang, C.Y.; Fang, T. Co-delivery of paclitaxel and doxorubicin by pH-responsive prodrug micelles for cancer therapy. Int. J. Nanomed. 2020, 15, 3319. [Google Scholar] [CrossRef]
- Cho, H.; Lai, T.C.; Tomoda, K.; Kwon, G.S. Polymeric micelles for multi-drug delivery in cancer. AAPS Pharmscitech 2015, 16, 10–20. [Google Scholar] [CrossRef]
- Shin, H.J.; Jo, M.J.; Jin, I.S.; Park, C.-W.; Kim, J.-S.; Shin, D.H. Optimization and Pharmacokinetic Evaluation of Synergistic Fenbendazole and Rapamycin Co-Encapsulated in Methoxy Poly (Ethylene Glycol)-b-Poly (Caprolactone) Polymeric Micelles. Int. J. Nanomed. 2021, 16, 4873. [Google Scholar] [CrossRef]
- Kato, K.; Chin, K.; Yoshikawa, T.; Yamaguchi, K.; Tsuji, Y.; Esaki, T.; Sakai, K.; Kimura, M.; Hamaguchi, T.; Shimada, Y. Phase II study of NK105, a paclitaxel-incorporating micellar nanoparticle, for previously treated advanced or recurrent gastric cancer. Investig. New Drugs 2012, 30, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, S. Taxol (paclitaxel): Mechanisms of action. Ann. Oncol. 1994, 5, S3–S6. [Google Scholar] [PubMed]
- Nitiss, J.L. Targeting DNA topoisomerase II in cancer chemotherapy. Nat. Rev. Cancer 2009, 9, 338–350. [Google Scholar] [CrossRef]
- Baldwin, E.; Osheroff, N. Etoposide, topoisomerase II and cancer. Curr. Med. Chem. Anticancer Agents 2005, 5, 363–372. [Google Scholar] [CrossRef]
- Dumont, F.J.; Su, Q. Mechanism of action of the immunosuppressant rapamycin. Life Sci. 1996, 58, 373–395. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, S.N. Rapamune (RAPA, rapamycin, sirolimus): Mechanism of action immunosuppressive effect results from blockade of signal transduction and inhibition of cell cycle progression. Clin. Biochem. 1998, 31, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yu, X.-C.; Xu, S.-F.; Xu, M. Paclitaxel and etoposide co-loaded polymeric nanoparticles for the effective combination therapy against human osteosarcoma. J. Nanobiotechnol. 2015, 13, 22. [Google Scholar] [CrossRef]
- Shafer, A.; Zhou, C.; Gehrig, P.A.; Boggess, J.F.; Bae-Jump, V.L. Rapamycin potentiates the effects of paclitaxel in endometrial cancer cells through inhibition of cell proliferation and induction of apoptosis. Int. J. Cancer 2010, 126, 1144–1154. [Google Scholar] [CrossRef]
- Itamochi, H.; Oishi, T.; Shimada, M.; Sato, S.; Uegaki, K.; Naniwa, J.; Sato, S.; Nonaka, M.; Terakawa, N.; Kigawa, J. Inhibiting the mTOR pathway synergistically enhances cytotoxicity in ovarian cancer cells induced by etoposide through upregulation of c-Jun. Clin. Cancer Res. 2011, 17, 4742–4750. [Google Scholar] [CrossRef]
- Yildiz, R.; Kalender, M.E.; Dane, F.; Sevinc, A.; Gumus, M.; Camci, C.; Alici, S.; Kaya, A.O.; Yaman, E.; Ozturk, B.; et al. Docetaxel combined with oral etoposide as second-line treatment for advanced gastric carcinoma after failure of platinum- and fluoropyrimidine-based regimens. J. Oncol. Pharm. Pract. 2009, 16, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Fukamachi, H.; Kim, S.K.; Koh, J.; Lee, H.S.; Sasaki, Y.; Yamashita, K.; Nishikawaji, T.; Shimada, S.; Akiyama, Y.; Byeon, S.J.; et al. A subset of diffuse-type gastric cancer is susceptible to mTOR inhibitors and checkpoint inhibitors. J. Exp. Clin. Cancer Res. 2019, 38, 127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Awasthi, N.; Schwarz, M.A.; Schwarz, R.E. The dual PI3K/mTOR inhibitor NVP-BEZ235 enhances nab-paclitaxel antitumor response in experimental gastric cancer. Int. J. Oncol. 2013, 43, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.H.; Tam, Y.T.; Kwon, G.S. Polymeric micelle nanocarriers in cancer research. Front. Chem. Sci. Eng. 2016, 10, 348–359. [Google Scholar] [CrossRef]
- Jo, M.J.; Jo, Y.H.; Lee, Y.J.; Park, C.-W.; Kim, J.-S.; Hong, J.T.; Chung, Y.B.; Lee, M.K.; Shin, D.H. Physicochemical, pharmacokinetic, and toxicity evaluation of methoxy poly(ethylene glycol)-b-poly(d, l-Lactide) polymeric micelles encapsulating alpinumisoflavone extracted from unripe Cudrania tricuspidata fruit. Pharmaceutics 2019, 11, 366. [Google Scholar] [CrossRef]
- Torchilin, V.P. Structure and design of polymeric surfactant-based drug delivery systems. J. Control. Release Off. J. Control. Release Soc. 2001, 73, 137–172. [Google Scholar] [CrossRef]
- Kwon, G.S.; Okano, T. Polymeric micelles as new drug carriers. Adv. Drug Deliv. Rev. 1996, 21, 107–116. [Google Scholar] [CrossRef]
- Yang, H.Y.; Jang, M.-S.; Gao, G.H.; Lee, J.H.; Lee, D.S. Construction of redox/pH dual stimuli-responsive PEGylated polymeric micelles for intracellular doxorubicin delivery in liver cancer. Polym. Chem. 2016, 7, 1813–1825. [Google Scholar] [CrossRef]
- Zhou, H.; Qi, Z.; Xue, X.; Wang, C. Novel pH-sensitive urushiol-loaded polymeric micelles for enhanced anticancer activity. Int. J. Nanomed. 2020, 15, 3851. [Google Scholar] [CrossRef]
- Bae, Y.; Fukushima, S.; Harada, A.; Kataoka, K. Design of environment-sensitive supramolecular assemblies for intracellular drug delivery: Polymeric micelles that are responsive to intracellular pH change. Angew. Chem. (Int. Ed. Engl.) 2003, 42, 4640–4643. [Google Scholar] [CrossRef]
- Bui, Q.N.; Li, Y.; Jang, M.-S.; Huynh, D.P.; Lee, J.H.; Lee, D.S. Redox-and pH-sensitive polymeric micelles based on poly(β-amino ester)-grafted disulfide methylene oxide poly(ethylene glycol) for anticancer drug delivery. Macromolecules 2015, 48, 4046–4054. [Google Scholar] [CrossRef]
- Huang, X.; Liao, W.; Zhang, G.; Kang, S.; Zhang, C.Y. pH-sensitive micelles self-assembled from polymer brush (PAE-g-cholesterol)-b-PEG-b-(PAE-g-cholesterol) for anticancer drug delivery and controlled release. Int J. Nanomed. 2017, 12, 2215–2226. [Google Scholar] [CrossRef] [PubMed]
- Felber, A.E.; Dufresne, M.-H.; Leroux, J.-C. pH-sensitive vesicles, polymeric micelles, and nanospheres prepared with polycarboxylates. Adv. Drug Deliv. Rev. 2012, 64, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Han, S.S.; Li, Z.Y.; Zhu, J.Y.; Han, K.; Zeng, Z.Y.; Hong, W.; Li, W.X.; Jia, H.Z.; Liu, Y.; Zhuo, R.X. Dual-pH sensitive charge-reversal polypeptide micelles for tumor-triggered targeting uptake and nuclear drug delivery. Small 2015, 11, 2543–2554. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhang, Q.; Wang, J.; Chen, M.; Li, S.; Lin, Z.; Li, J. Tumor-targeted aggregation of pH-sensitive nanocarriers for enhanced retention and rapid intracellular drug release. Polym. Chem. 2014, 5, 5668–5679. [Google Scholar] [CrossRef]
- Vaupel, P.; Kallinowski, F.; Okunieff, P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: A review. Cancer Res. 1989, 49, 6449–6465. [Google Scholar]
- Schmaljohann, D. Thermo-and pH-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef]
- Wike-Hooley, J.; Haveman, J.; Reinhold, H. The relevance of tumour pH to the treatment of malignant disease. Radiother. Oncol. 1984, 2, 343–366. [Google Scholar] [CrossRef]
- Wang, Z.; Deng, X.; Ding, J.; Zhou, W.; Zheng, X.; Tang, G. Mechanisms of drug release in pH-sensitive micelles for tumour targeted drug delivery system: A review. Int. J. Pharm. 2018, 535, 253–260. [Google Scholar] [CrossRef]
- Gao, G.H.; Li, Y.; Lee, D.S. Environmental pH-sensitive polymeric micelles for cancer diagnosis and targeted therapy. J. Control. Release Off. J. Control. Release Soc. 2013, 169, 180–184. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Yang, J.; Zhou, C.; Sun, J. pH-sensitive polymeric micelles triggered drug release for extracellular and intracellular drug targeting delivery. Asian J. Pharm. Sci. 2013, 8, 159–167. [Google Scholar] [CrossRef]
- Shao, J.; Zheng, D.; Jiang, Z.; Xu, H.; Hu, Y.; Li, X.; Lu, X. Curcumin delivery by methoxy polyethylene glycol–poly(caprolactone) nanoparticles inhibits the growth of C6 glioma cells. Acta Biochim. Biophys. Sin. 2011, 43, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Su, T.; Zhang, L.; Liu, R.; Wang, G.; He, B.; Gu, Z. Polymeric micelles with citraconic amide as pH-sensitive bond in backbone for anticancer drug delivery. Int. J. Pharm. 2014, 471, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef]
- Zhang, H. Thin-Film Hydration Followed by Extrusion Method for Liposome Preparation. Methods Mol. Biol. (Clifton N.J.) 2017, 1522, 17–22. [Google Scholar] [CrossRef]
- Xu, H.; Hou, Z.; Zhang, H.; Kong, H.; Li, X.; Wang, H.; Xie, W. An efficient Trojan delivery of tetrandrine by poly(N-vinylpyrrolidone)-block-poly(ε-caprolactone) (PVP-b-PCL) nanoparticles shows enhanced apoptotic induction of lung cancer cells and inhibition of its migration and invasion. Int J. Nanomed. 2014, 9, 231–242. [Google Scholar] [CrossRef]
- Sun, C.; Liang, Y.; Hao, N.; Xu, L.; Cheng, F.; Su, T.; Cao, J.; Gao, W.; Pu, Y.; He, B. A ROS-responsive polymeric micelle with a π-conjugated thioketal moiety for enhanced drug loading and efficient drug delivery. Org. Biomol. Chem. 2017, 15, 9176–9185. [Google Scholar] [CrossRef]
- Twentyman, P.R.; Luscombe, M. A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity. Br. J. Cancer 1987, 56, 279–285. [Google Scholar] [CrossRef]
- Mikhail, A.S.; Eetezadi, S.; Allen, C. Multicellular tumor spheroids for evaluation of cytotoxicity and tumor growth inhibitory effects of nanomedicines in vitro: A comparison of docetaxel-loaded block copolymer micelles and Taxotere®. PLoS ONE 2013, 8, e62630. [Google Scholar] [CrossRef]
- Franken, N.A.; Rodermond, H.M.; Stap, J.; Haveman, J.; Van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef]
- Berger, D.P.; Henss, H.; Winterhalter, B.R.; Fiebig, H.H. The clonogenic assay with human tumor xenografts: Evaluation, predictive value and application for drug screening. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 1990, 1, 333–341. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, S. A review of in vitro drug release test methods for nano-sized dosage forms. Adv. Pharm. 2014, 2014, 304757. [Google Scholar] [CrossRef]
- Modi, S.; Anderson, B.D. Determination of drug release kinetics from nanoparticles: Overcoming pitfalls of the dynamic dialysis method. Mol. Pharm. 2013, 10, 3076–3089. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.H.; Park, S.H.; Jeong, S.W.; Park, C.-W.; Han, K.; Chung, Y.B. Hepatic uptake of epirubicin by isolated rat hepatocytes and its biliary excretion after intravenous infusion in rats. Arch. Pharm. Res. 2014, 37, 1599–1606. [Google Scholar] [CrossRef]
- Aw, M.S.; Kurian, M.; Losic, D. Polymeric micelles for multidrug delivery and combination therapy. Chem. Eur. J. 2013, 19, 12586–12601. [Google Scholar] [CrossRef]
- Jo, M.J.; Jin, I.S.; Park, C.-W.; Hwang, B.Y.; Chung, Y.B.; Kim, J.-S.; Shin, D.H. Revolutionizing technologies of nanomicelles for combinatorial anticancer drug delivery. Arch. Pharm. Res. 2020, 43, 100–109. [Google Scholar] [CrossRef]
- Huh, K.M.; Lee, S.C.; Cho, Y.W.; Lee, J.; Jeong, J.H.; Park, K. Hydrotropic polymer micelle system for delivery of paclitaxel. J. Control. Release Off. J. Control. Release Soc. 2005, 101, 59–68. [Google Scholar] [CrossRef]
- Jiang, H.; Pei, L.; Liu, N.; Li, J.; Li, Z.; Zhang, S. Etoposide-loaded nanostructured lipid carriers for gastric cancer therapy. Drug Deliv. 2016, 23, 1379–1382. [Google Scholar] [CrossRef]
- Yáñez, J.A.; Forrest, M.L.; Ohgami, Y.; Kwon, G.S.; Davies, N.M. Pharmacometrics and delivery of novel nanoformulated PEG-b-poly (ε-caprolactone) micelles of rapamycin. Cancer Chemother. Pharmacol. 2008, 61, 133–144. [Google Scholar] [CrossRef]
- Gao, L.; Liu, G.; Kang, J.; Niu, M.; Wang, Z.; Wang, H.; Ma, J.; Wang, X. Paclitaxel nanosuspensions coated with P-gp inhibitory surfactants: I. Acute toxicity and pharmacokinetics studies. Colloids Surf. B Biointerfaces 2013, 111, 277–281. [Google Scholar] [CrossRef]
- Kim, S.C.; Kim, D.W.; Shim, Y.H.; Bang, J.S.; Oh, H.S.; Kim, S.W.; Seo, M.H. In vivo evaluation of polymeric micellar paclitaxel formulation: Toxicity and efficacy. J. Control. Release Off. J. Control. Release Soc. 2001, 72, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Al-Ali, A.A.A.; Quach, J.R.C.; Bundgaard, C.; Steffansen, B.; Holm, R.; Nielsen, C.U. Polysorbate 20 alters the oral bioavailability of etoposide in wild type and mdr1a deficient Sprague-Dawley rats. Int. J. Pharm. 2018, 543, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, P.; Harisudhan, T.; Choudhury, H.; Mullangi, R.; Srinivas, N.R. Pharmacokinetics of etoposide in rats with uranyl nitrate (UN)-induced acute renal failure (ARF): Optimization of the duration of UN dosing. Eur. J. Drug Metab. Pharm. 2007, 32, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Zhang, L.; Niu, Y.; Fan, D.; Wu, X.; Tang, X.; Cai, C. A stable and practical etoposide-containing intravenous long-/medium-chain triglycerides-based lipid emulsion formulation: Pharmacokinetics, biodistribution, toxicity, and antitumor efficacy. Expert Opin. Drug Deliv. 2013, 10, 559–571. [Google Scholar] [CrossRef]
- Wang, F.; Yang, K.; Wang, Z.; Ma, Y.; Gutkind, J.S.; Hida, N.; Niu, G.; Tian, J. Combined image guided monitoring the pharmacokinetics of rapamycin loaded human serum albumin nanoparticles with a split luciferase reporter. Nanoscale 2016, 8, 3991–4000. [Google Scholar] [CrossRef]
- Zhao, R.; Zhu, M.; Zhou, S.; Feng, W.; Chen, H. Rapamycin-loaded mPEG-PLGA nanoparticles ameliorate hepatic steatosis and liver injury in non-alcoholic fatty liver disease. Front. Chem. 2020, 8, 407. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Q.; Zhu, W.-T.; Lin, C.-Y.; Yuan, Z.-W.; Li, Z.-H.; Yan, P.-K. Delivery of rapamycin by liposomes synergistically enhances the chemotherapy effect of 5-fluorouracil on colorectal cancer. Int. J. Nanomed. 2021, 16, 269. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 131–135. [Google Scholar] [CrossRef]
- Liao, J.; Song, Y.; Liu, C.; Li, D.; Zheng, H.; Lu, B. Dual-drug delivery based charge-conversional polymeric micelles for enhanced cellular uptake and combination therapy. Polym. Chem. 2019, 10, 5879–5893. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, Y.; Nyström, A.M. Endocytic uptake and intracellular trafficking of bis-MPA-based hyperbranched copolymer micelles in breast cancer cells. Biomacromolecules 2012, 13, 3814–3822. [Google Scholar] [CrossRef]
- Manjili, H.K.; Malvandi, H.; Mousavi, M.S.; Attari, E.; Danafar, H. In vitro and in vivo delivery of artemisinin loaded PCL–PEG–PCL micelles and its pharmacokinetic study. Artif. Cells Nanomed. Biotechnol. 2018, 46, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Qu, G.; Sun, Y.; Wu, X.; Yao, Z.; Guo, Q.; Ding, Q.; Yuan, S.; Shen, Z.; Ping, Q. Pharmacokinetics, biodistribution, efficacy and safety of N-octyl-O-sulfate chitosan micelles loaded with paclitaxel. Biomaterials 2008, 29, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Li, X.; Sang, Z.; Mei, L.; Yang, T.; Li, Z.; Zhou, L.; Zheng, Y.; He, G.; Guo, G. Improving the pharmacokinetics and tissue distribution of pyrinezolid by self-assembled polymeric micelles. Colloids Surf. B 2017, 156, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.J.; Chen, Y.C.; Lin, C.C.; Chen, C.F.; Chen, J.W. Characterization of pegylated copolymeric micelles and in vivo pharmacokinetics and biodistribution studies. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 77, 188–194. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).