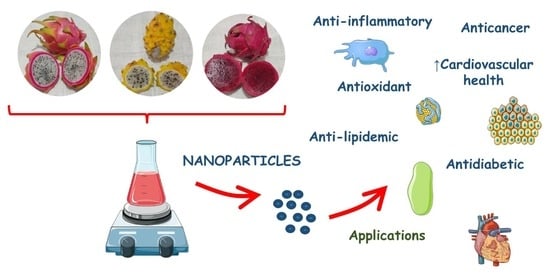
Anti-Inflammatory, Antioxidant, and Other Health Effects of Dragon Fruit and Potential Delivery Systems for Its Bioactive Compounds
Abstract
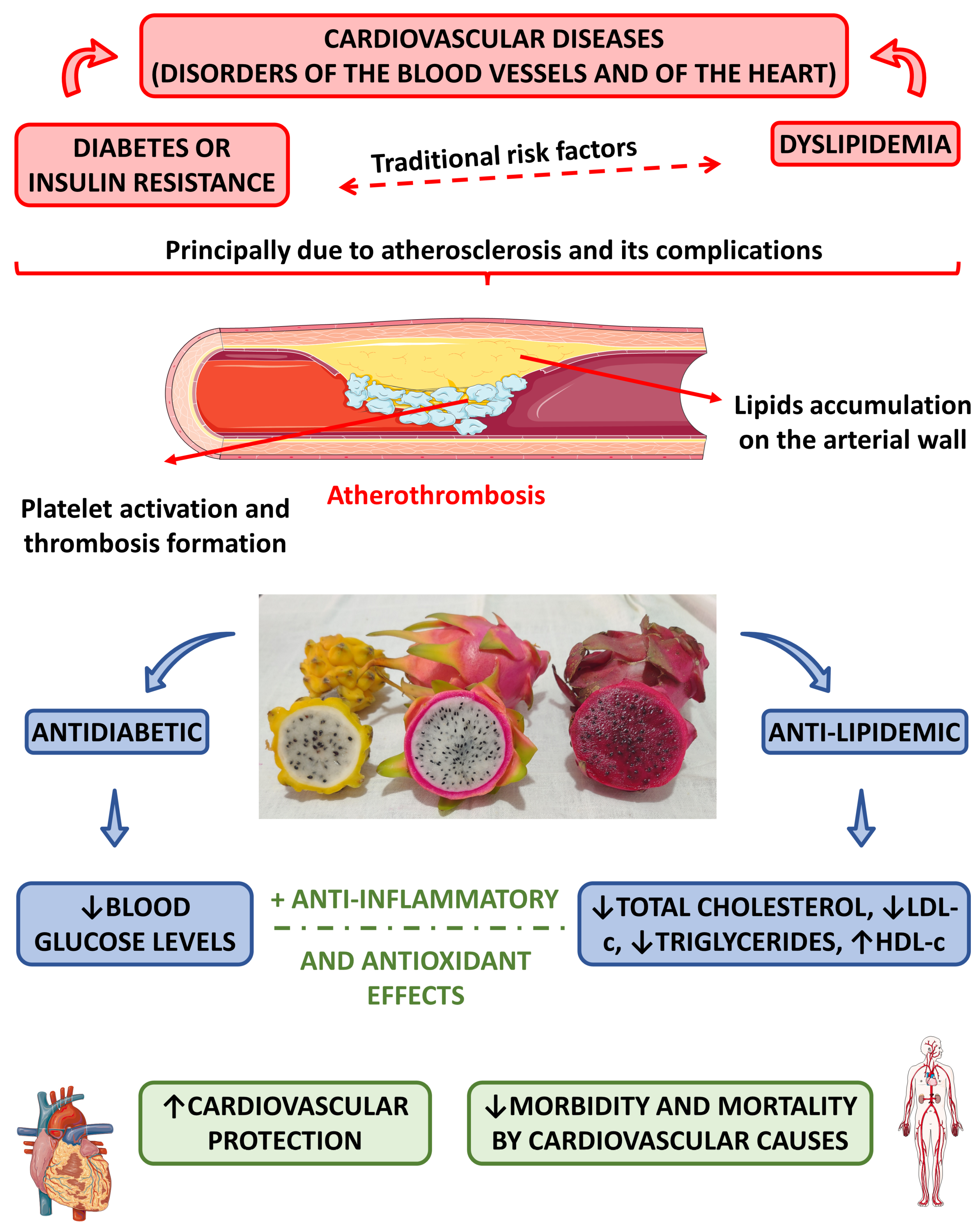
:1. Introduction
2. Materials and Methods
2.1. Focused Question
2.2. Language
2.3. Databases
2.4. Study Selection
2.5. Data Extraction
2.6. Quality Assessment
2.7. Evaluation of Economic Importance and Technological Applications
3. Results
4. Discussion
4.1. Bioactive Compounds of Hylocereus Species
| References | Bioactive Compounds | Pulp, Peel, or Seed | Molecular Structures | Health Effects | Concentration (Dry Weight) |
|---|---|---|---|---|---|
| [45,46] | Anthocyanins (cyanidin 3-glucoside, delphinidin 3-glucoside, and pelargonidin 3-glucoside) | Peel and pulp |  | Anti-inflammatory, antioxidant | 0.82 ± 0.17 a 12.67 ± 0.63 mg/100 g (pulp) and 44.3865 ± 1.3125 mg/100 g (peel) |
| [6,47,48,49] | Ascorbic acid | Peel and pulp |  | Antioxidant, anti-cancer, anti-diabetic, anti-lipidemic | 2.94 ± 1.1 a 5.64 ± 0.7 mg/100 g (pulp) and 175 ± 15.7 µmol Trolox equivalents antioxidant capacity (TEAC)/g (peel) |
| [49,50] | Betacyanin | Peel and pulp |  | Anti-microbial, anti-viral, pigments, hypolipidemic | 10.3 ± 0.22 a 82.79 ± 0.55 (pulp) and 13.8 ± 0.85 a 18.67 ± 0.50 mg/100 g (peel) |
| [51,52] | Lycopene | Pulp |  | Antioxidant, anti-diabetic, anti-cancer | 3.2 ± 0.6 µg/100 g (pulp) |
| [6,52,53] | β-carotene | Pulp |  | Antioxidant, anti-diabetic, anti-cardiovascular diseases | 209.1 ± 0.1 µg/100 g (pulp) |
| [10,54] | Tocopherol | Seed |  | Antioxidant, anti-cancer, anti-diabetes, anti-cardiovascular diseases | 150 µg/100 g (seed) |
| [6,55] | p-Coumaric acid | Pulp and seed |  | Antioxidant, anti-diabetic, anti-lipidemic | 0.365 a 1.52 ± 1,4 mg/100 g (seed) and 0.16 mg 100 g (pulp) |
| [10,49,56,57] | Gallic acid | Peel, pulp, and seed |  | Antioxidant, anti-diabetic, anti-obesity | 24.0 ± 2.7 a 53.2 ± 6.2 mg GAE/100 g (pulp), 39.9 mg GAE/100 g (peel)and 375.1 mg GAE/100 g (seed) |
| [6,58] | Syringic acid | Peel, pulp, and seed |  | Antioxidant, anti-diabetes, anti-cardiovascular diseases, anti-cancer | Variando de 0.095 a 65.10 ± 0.04 µg/100 g (peel and pulp) Seed (NA) |
| [5,49,59] | Vanillic acid | Peel and pulp |  | Antioxidant, anti-inflammatory, anti-diabetic, anti-proliferative, and anti-atherogenic activities | 0.64 mg/100 g (peel and pulp) |
| [6] | Phthalic acid | Peel |  | Antioxidant | (NA) |
| [39] | Benzoic acids derivatives: salicylic acid (a), 3- hydroxyl benzoic acid (b), 4-hydroxy benzoic acid (c). | Peel and Pulp |  | Antioxidant | Variando de 0.48 ± 0.0 a 11.97 ± 0.8 µg/100 g (pulp and peel) |
| [6,49,60] | Caffeic acid | Peel and seed |  | Antioxidant | 0.08 mg/100 g (pulp and peel) |
| [45,61] | Quercetin | Peel and pulp |  | Antioxidant, anti-inflammatory, anti-diabetic | 3.43 mg/100 g (pulp) Peel (NA) |
| [44,48,62,63,64] | Fatty acids: oleic acid (a), linoleic acid (b), linolenic acid (c), and palmitic acid (d) | Pulp and seeds |  | Nutraceutical activity | 129.11 (a), 252.65 (b), 5.33 (c), 62.74 (d) mg/100 g (pulp) and 60 (a), 21 (c), 13 (d) g/100 g (seeds) |
4.2. Healthy Benefits of Hylocereus Species
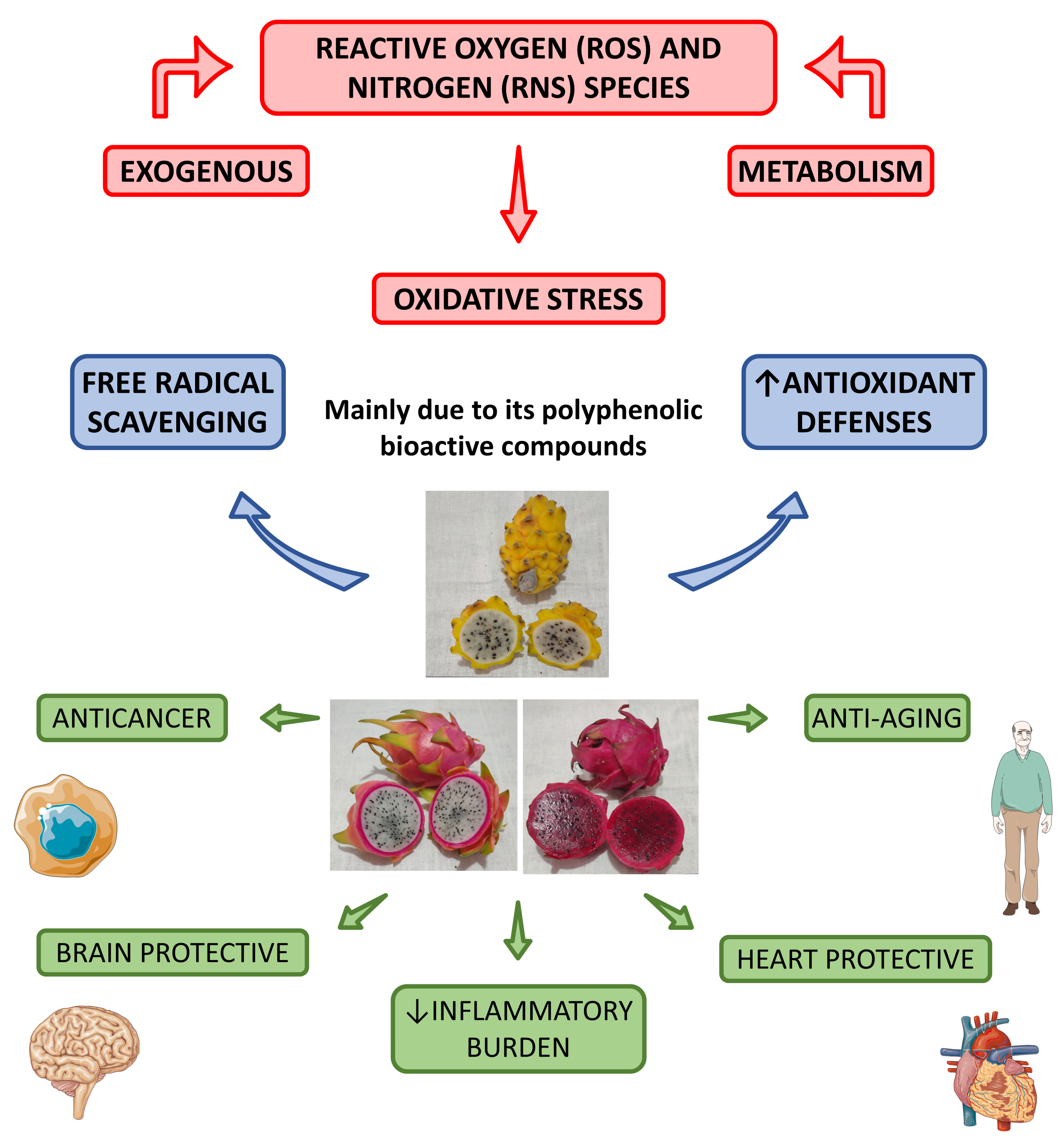
4.2.1. Antioxidant Effects by Species
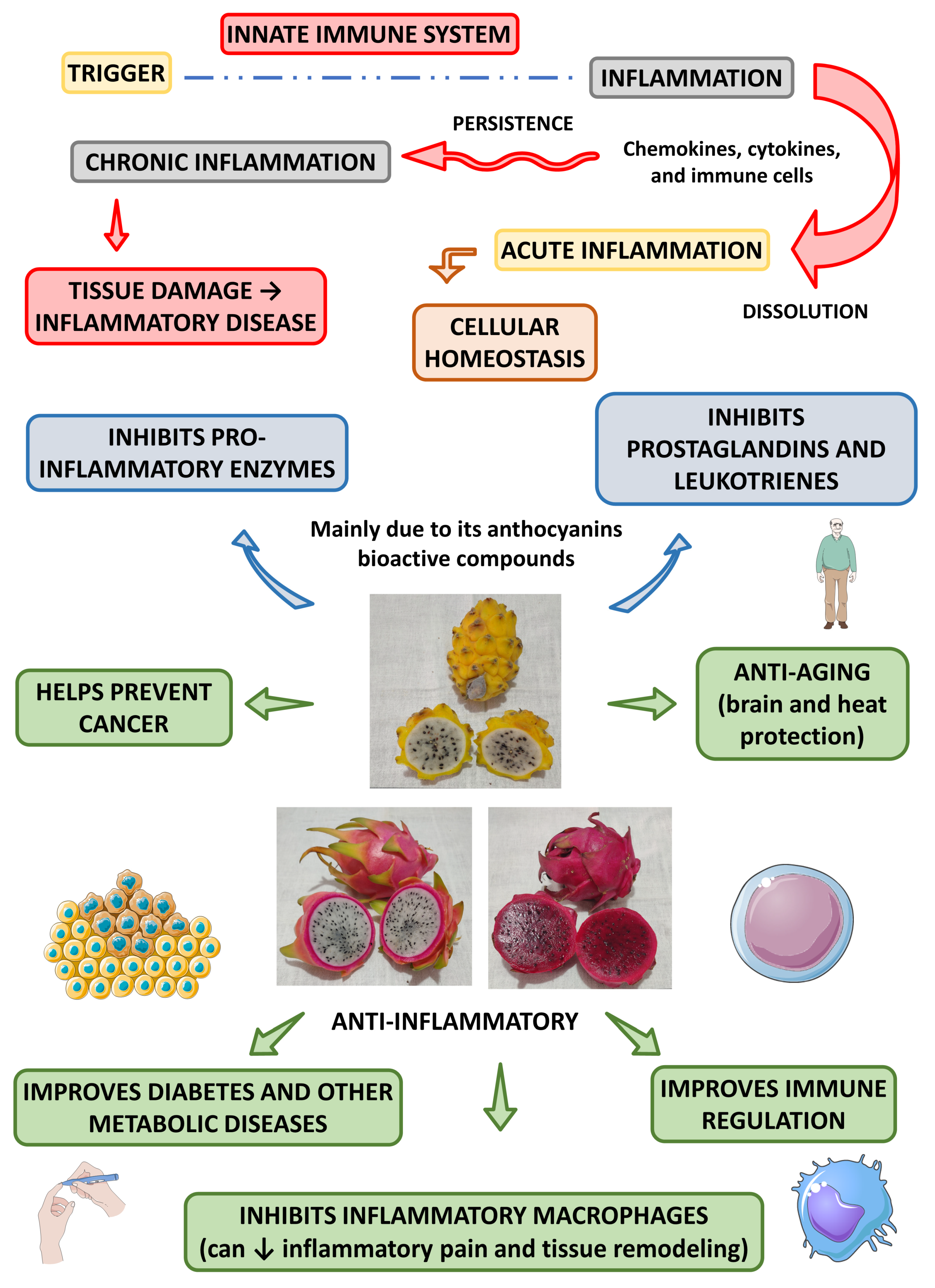
4.2.2. Anti-Inflammatory Effects
4.2.3. Prebiotic Effects
4.2.4. Antimicrobial Effects
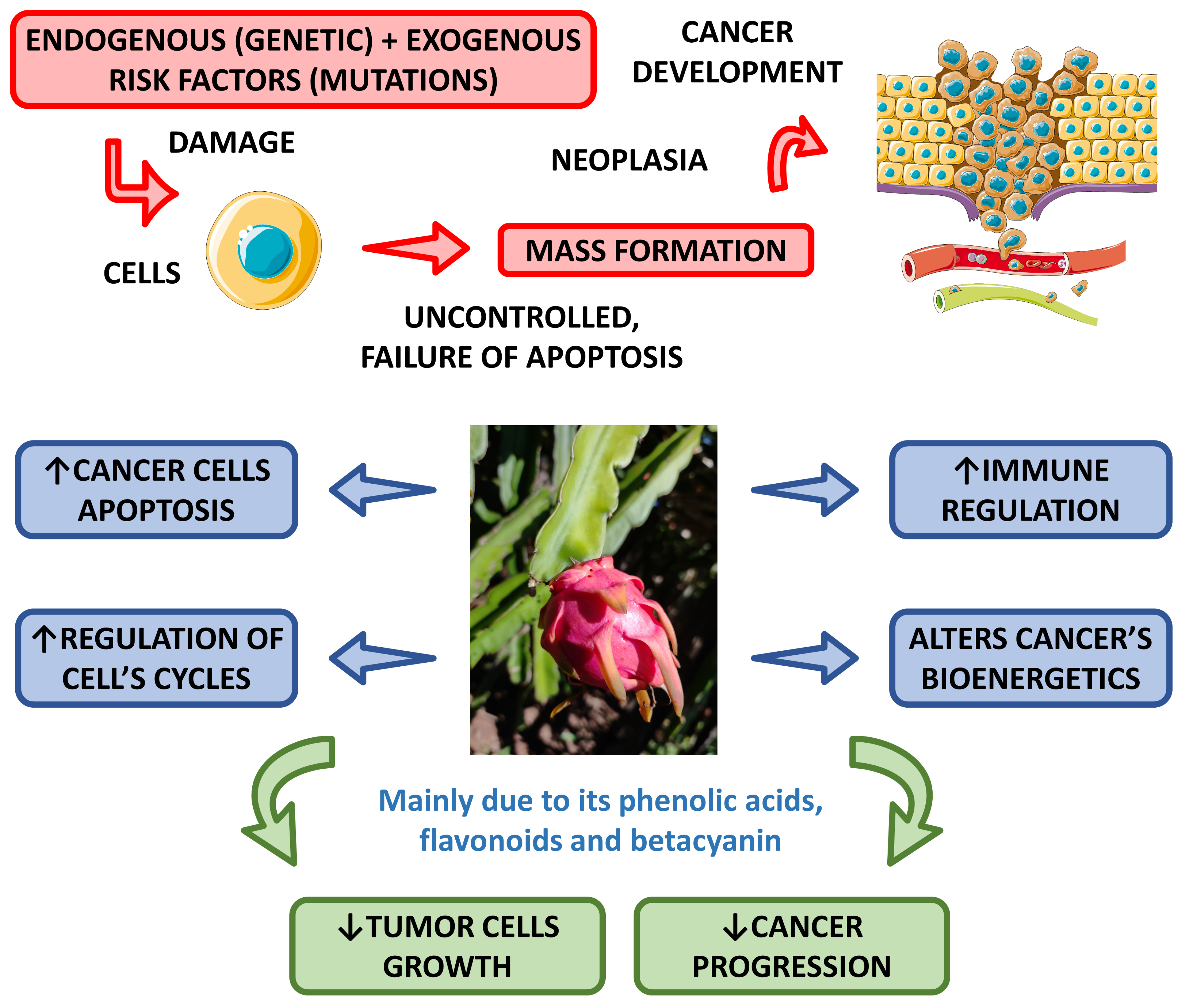
4.2.5. Anti-Cancer Effects
4.2.6. Anti-Diabetic Effects
4.2.7. Anti-Lipidemic Effects
4.2.8. The Effects of Dragon Fruit on Humans: Evaluation of Clinical Trials
4.3. Technological Applications and Economic Importance
4.4. Delivery Systems for Hylocereus Compounds
4.5. Waste Generated by Dragon Fruit Chain
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grosso, G.; Godos, J.; Currenti, W.; Micek, A.; Falzone, L.; Libra, M.; Giampieri, F.; Forbes-Hernández, T.Y.; Quiles, J.L.; Battino, M.; et al. The Effect of Dietary Polyphenols on Vascular Health and Hypertension: Current Evidence and Mechanisms of Action. Nutrients 2022, 14, 545. [Google Scholar] [CrossRef]
- Cosme, F.; Pinto, T.; Aires, A.; Morais, M.C.; Bacelar, E.; Anjos, R.; Ferreira-Cardoso, J.; Oliveira, I.; Vilela, A.; Gonçalves, B. Red Fruits Composition and Their Health Benefits-A Review. Foods 2022, 11, 644. [Google Scholar] [CrossRef]
- Bin-Jumah, M.N.; Nadeem, M.S.; Gilani, S.J.; Mubeen, B.; Ullah, I.; Alzarea, S.I.; Ghoneim, M.M.; Alshehri, S.; Al-Abbasi, F.A.; Kazmi, I. Lycopene: A Natural Arsenal in the War against Oxidative Stress and Cardiovascular Diseases. Antioxidants 2022, 11, 232. [Google Scholar] [CrossRef]
- Imaizumi, V.M.; Laurindo, L.F.; Manzan, B.; Guiguer, E.L.; Oshiiwa, M.; Otoboni, A.M.M.B.; Araujo, A.C.; Tofano, R.J.; Barbalho, S.M. Garlic: A systematic review of the effects on cardiovascular diseases. Crit. Rev. Food Sci. Nutr. 2022, 1–23. [Google Scholar] [CrossRef]
- Huang, Y.; Brennan, M.A.; Kasapis, S.; Richardson, S.J.; Brennan, C.S. Maturation Process, Nutritional Profile, Bioactivities and Utilisation in Food Products of Red Pitaya Fruits: A Review. Foods 2021, 10, 2862. [Google Scholar] [CrossRef]
- Joshi, M.; Prabhakar, B. Phytoconstituents and pharmaco-therapeutic benefits of pitaya: A wonder fruit. J. Food Biochem. 2020, 44, e13260. [Google Scholar] [CrossRef]
- Cheok, A.; George, T.W.; Rodriguez-Mateos, A.; Caton, P.W. The effects of betalain-rich cacti (dragon fruit and cactus pear) on endothelial and vascular function: A systematic review of animal and human studies. Food Funct. 2020, 11, 6807–6817. [Google Scholar] [CrossRef]
- Poolsup, N.; Suksomboon, N.; Paw, N.J. Effect of dragon fruit on glycemic control in prediabetes and type 2 diabetes: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0184577. [Google Scholar] [CrossRef] [Green Version]
- Dartsch, P.C.; Kler, A.; Kriesl, E. Antioxidative and antiinflammatory potential of different functional drink concepts in vitro. Phytother. Res. 2009, 23, 165–171. [Google Scholar] [CrossRef]
- Lim, H.K.; Tan, C.P.; Karim, R.; Ariffin, A.A.; Bakar, J. Chemical composition and DSC thermal properties of two species of Hylocereus cacti seed oil: Hylocereus undatus and Hylocereus polyrhizus. Food Chem. 2010, 119, 1326–1331. [Google Scholar] [CrossRef]
- Wong, Y.-M.; Siow, L.-F. Effects of heat, pH, antioxidant, agitation and light on betacyanin stability using red-fleshed dragon fruit (Hylocereus polyrhizus) juice and concentrate as models. J. Food Sci. Technol. 2015, 52, 3086–3092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, Y.; Wu, X.; Wang, Y.; Ye, W.-C.; Zhang, Q.-W. Studies on the flavonoids from the flowers of Hylocereus undatus. Zhong Yao Cai = Zhongyaocai = J. Chin. Med. Mater. 2011, 34, 712–716. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [Green Version]
- Akhiruddin, M.A.-S. Nutritional Composition, Antioxidant Properties of Hylocereus Polyrhizus Powder and Their Effects on Plasma Glucose Level and Lipid Profiles in Diabetic Rats and Pre-diabetic Subjects. Ph.D. Thesis, Universiti Putra Malaysia, Selangor, Malaysia, 2013. [Google Scholar]
- Abd Hadi, N.; Mohamad, M.; Rohin, M.A.K.; Yusof, R.M.J.B.S. Effects of Red pitaya fruit (Hylocereus polyrhizus) consumption on blood glucose level and lipid profile in type 2 diabetic subjects. Borneo Sci. 2016, 31. [Google Scholar]
- Shafie, S.R. Nutritional Composition and Antioxidant Properties of Spray Pitaya Powder (Hylocereus Polyrhizus [Weber] Briton & Rose) and Its Supplementation Effects on Selected Biomarkers in Normocholestrolemic Subjects. Ph.D. Thesis, Universiti Putra Malaysia, Selangor, Malaysia, 2012. [Google Scholar]
- Fadlilah, S.; Sucipto, A. Dragon Fruit (Hylocereuspolyrhizus) Effectively Reduces Fasting Blood Sugar Levels and Blood Pressure on Excessive Nutritional Status. Pak. J. Med. Health Sci. 2020, 14, 1405–1412. [Google Scholar]
- Cheok, A.; Xu, Y.; Zhang, Z.; Caton, P.W.; Rodriguez-Mateos, A. Betalain-rich dragon fruit (pitaya) consumption improves vascular function in adult men and women: A double-blind, randomized controlled crossover trial. Am. J. Clin. Nutr. 2021, 115, 1418–1431. [Google Scholar] [CrossRef]
- Lira, S.M.; Dionísio, A.P.; Holanda, M.O.; Marques, C.G.; Silva, G.S.D.; Correa, L.C.; Santos, G.B.M.; de Abreu, F.A.P.; Magalhães, F.E.A.; Rebouças, E.L.; et al. Metabolic profile of pitaya (Hylocereus polyrhizus (F.A.C. Weber) Britton & Rose) by UPLC-QTOF-MS(E) and assessment of its toxicity and anxiolytic-like effect in adult zebrafish. Food Res. Int. 2020, 127, 108701. [Google Scholar] [CrossRef]
- Anand Swarup, K.R.; Sattar, M.A.; Abdullah, N.A.; Abdulla, M.H.; Salman, I.M.; Rathore, H.A.; Johns, E.J. Effect of dragon fruit extract on oxidative stress and aortic stiffness in streptozotocin-induced diabetes in rats. Pharmacogn. Res. 2010, 2, 31–35. [Google Scholar] [CrossRef] [Green Version]
- Holanda, M.O. Potencial Terapêutico da Polpa Com Semente da Pitaia Vermelha [Hylocereus Polyrhizus (Weber) Britton & Rose] em Modelo Experimental de Dislipidemia Induzida Por Dieta Hiperlipídica; Embrapa: Fortaleza, Brazil, 2019. [Google Scholar]
- SILVA, F.d.S. Efeito Hipoglicemiante do Extrato Concentrado de Pitaia Vermelha (Hylocereus spp.) em Modelo de Zebrafish (Danio rerio). 2021. Available online: http://www.alice.cnptia.embrapa.br/alice/handle/doc/1143420 (accessed on 20 November 2022).
- Yeh, W.J.; Tsai, C.C.; Ko, J.; Yang, H.Y. Hylocereus polyrhizus Peel Extract Retards Alcoholic Liver Disease Progression by Modulating Oxidative Stress and Inflammatory Responses in C57BL/6 Mice. Nutrients 2020, 12, 3884. [Google Scholar] [CrossRef]
- Ramli, N.S.; Brown, L.; Ismail, P.; Rahmat, A. Effects of red pitaya juice supplementation on cardiovascular and hepatic changes in high-carbohydrate, high-fat diet-induced metabolic syndrome rats. BMC Complement. Altern. Med. 2014, 14, 189. [Google Scholar] [CrossRef] [Green Version]
- Ramli, N.S.; Ismail, P.; Rahmat, A. Red pitaya juice supplementation ameliorates energy balance homeostasis by modulating obesity-related genes in high-carbohydrate, high-fat diet-induced metabolic syndrome rats. BMC Complement. Altern. Med. 2016, 16, 243. [Google Scholar] [CrossRef] [Green Version]
- Song, H.; Chu, Q.; Xu, D.; Xu, Y.; Zheng, X. Purified Betacyanins from Hylocereus undatus Peel Ameliorate Obesity and Insulin Resistance in High-Fat-Diet-Fed Mice. J. Agric. Food Chem. 2016, 64, 236–244. [Google Scholar] [CrossRef]
- Song, H.; Chu, Q.; Yan, F.; Yang, Y.; Han, W.; Zheng, X. Red pitaya betacyanins protects from diet-induced obesity, liver steatosis and insulin resistance in association with modulation of gut microbiota in mice. J. Gastroenterol. Hepatol. 2016, 31, 1462–1469. [Google Scholar] [CrossRef]
- Song, H.; Zheng, Z.; Wu, J.; Lai, J.; Chu, Q.; Zheng, X. White Pitaya (Hylocereus undatus) Juice Attenuates Insulin Resistance and Hepatic Steatosis in Diet-Induced Obese Mice. PLoS ONE 2016, 11, e0149670. [Google Scholar] [CrossRef] [Green Version]
- Macias-Ceja, D.C.; Cosín-Roger, J.; Ortiz-Masiá, D.; Salvador, P.; Hernández, C.; Calatayud, S.; Esplugues, J.V.; Barrachina, M.D. The flesh ethanolic extract of Hylocereus polyrhizus exerts anti-inflammatory effects and prevents murine colitis. Clin. Nutr. 2016, 35, 1333–1339. [Google Scholar] [CrossRef]
- Perez, G.R.; Vargas, S.R.; Ortiz, H.Y. Wound healing properties of Hylocereus undatus on diabetic rats. Phytother. Res. 2005, 19, 665–668. [Google Scholar] [CrossRef]
- Priatni, S.; Pradita, A. Stability study of betacyanin extract from red dragon fruit (Hylocereus polyrhizus) peels. Procedia Chem. 2015, 16, 438–444. [Google Scholar] [CrossRef] [Green Version]
- Ramli, N.S.; Ismail, P.; Rahmat, A. Influence of conventional and ultrasonic-assisted extraction on phenolic contents, betacyanin contents, and antioxidant capacity of red dragon fruit (Hylocereus polyrhizus). Sci. World J. 2014, 2014, 964731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charoensiri, R.; Kongkachuichai, R.; Suknicom, S.; Sungpuag, P. Beta-carotene, lycopene, and alpha-tocopherol contents of selected Thai fruits. Food Chem. 2009, 113, 202–207. [Google Scholar] [CrossRef]
- Luo, H.; Cai, Y.; Peng, Z.; Liu, T.; Yang, S. Chemical composition and in vitro evaluation of the cytotoxic and antioxidant activities of supercritical carbon dioxide extracts of pitaya (dragon fruit) peel. Chem. Cent J. 2014, 8, 1. [Google Scholar] [CrossRef] [Green Version]
- Padmavathy, K.; Kanakarajan, S.; Karthika, S.; Selvaraj, R.; Kamalanathan, A. Phytochemical Profiling And Anticancer Activity Of Dragon Fruit Hylocereus Undatus Extracts Against Human Hepatocellular Carcinoma Cancer (Hepg-2) Cells. Int. J. Pharm. Sci. Res. 2021, 12, 2770–2778. [Google Scholar] [CrossRef]
- Al-Mekhlafi, N.A.; Mediani, A.; Ismail, N.H.; Abas, F.; Dymerski, T.; Lubinska-Szczygeł, M.; Vearasilp, S.; Gorinstein, S. Metabolomic and antioxidant properties of different varieties and origins of Dragon fruit. Microchem. J. 2021, 160, 105687. [Google Scholar] [CrossRef]
- Arivalagan, M.; Karunakaran, G.; Roy, T.K.; Dinsha, M.; Sindhu, B.C.; Shilpashree, V.M.; Satisha, G.C.; Shivashankara, K.S. Biochemical and nutritional characterization of dragon fruit (Hylocereus species). Food Chem. 2021, 353, 129426. [Google Scholar] [CrossRef]
- Jayameena, P.; Sivakumari, K.; Ashok, K.; Rajesh, S. Rutin: A potential anticancer drug against human colon cancer (hct116) cells. Int. J. Biol. Pharm. Allied Sci. 2018, 7, 1731–1745. [Google Scholar] [CrossRef]
- Chia, S.L.; Chong, G.H. Effect of Drum Drying on Physico-chemical Characteristics of Dragon Fruit Peel (Hylocereus polyrhizus). Int. J. Food Eng. 2015, 11, 285–293. [Google Scholar] [CrossRef]
- Seth, S.; Kaba, L.; Ahirwar, S.; Jain, P.; Pagare, M.; Sinha, R.; Barde, L.; Rathore, P. Characterization of Dragon Fruit (Hylocereus undatus) Antioxidative Components to Explore Their Utility as a Natural Food Additive. Egypt. J. Food Sci. 2021, 49, 297–304. [Google Scholar] [CrossRef]
- Choo, K.Y.; Ong, Y.Y.; Lim, R.L.H.; Tan, C.P.; Ho, C.W. Study on bioaccessibility of betacyanins from red dragon fruit (Hylocereus polyrhizus). Food Sci. Biotechnol. 2019, 28, 1163–1169. [Google Scholar] [CrossRef]
- Ariffin, A.A.; Bakar, J.; Tan, C.P.; Rahman, R.A.; Karim, R.; Loi, C.C. Essential fatty acids of pitaya (dragon fruit) seed oil. Food Chem. 2009, 114, 561–564. [Google Scholar] [CrossRef]
- Saenjum, C.; Pattananandecha, T.; Nakagawa, K. Antioxidative and anti-inflammatory phytochemicals and related stable paramagnetic species in different parts of dragon fruit. Molecules 2021, 26, 3565. [Google Scholar] [CrossRef]
- Vargas, M.d.L.V.; Cortez, J.A.T.; Duch, E.S.; Lizama, A.P.; Méndez, C.H.H. Extraction and stability of anthocyanins present in the skin of the dragon fruit (Hylocereus undatus). Food Nutr. Sci. 2013, 4, 1221. [Google Scholar]
- Namkhah, Z.; Ashtary-Larky, D.; Naeini, F.; Clark, C.C.T.; Asbaghi, O. Does vitamin C supplementation exert profitable effects on serum lipid profile in patients with type 2 diabetes? A systematic review and dose-response meta-analysis. Pharmacol. Res. 2021, 169, 105665. [Google Scholar] [CrossRef]
- Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [CrossRef]
- Pires, I.V.; Sakurai, Y.C.N.; Ferreira, N.R.; Moreira, S.G.C.; da Cruz Rodrigues, A.M.; da Silva, L.H.M. Elaboration and Characterization of Natural Deep Eutectic Solvents (NADESs): Application in the Extraction of Phenolic Compounds from pitaya. Molecules 2022, 27, 8310. [Google Scholar] [CrossRef]
- Kumorkiewicz-Jamro, A.; Świergosz, T.; Sutor, K.; Spórna-Kucab, A.; Wybraniec, S. Multi-colored shades of betalains: Recent advances in betacyanin chemistry. Nat. Prod. Rep. 2021, 38, 2315–2346. [Google Scholar] [CrossRef]
- Leh, H.E.; Lee, L.K. Lycopene: A Potent Antioxidant for the Amelioration of Type II Diabetes Mellitus. Molecules 2022, 27, 2335. [Google Scholar] [CrossRef]
- Moo-Huchin, V.M.; Gonzlez-Aguilar, G.A.; Moo-Huchin, M.; Ortiz-V, E. Carotenoid Composition and Antioxidant Activity of Extracts from Tropical Fruits. 2017. Available online: http://cmuir.cmu.ac.th/jspui/handle/6653943832/63885 (accessed on 15 November 2022).
- Jiang, Y.W.; Sun, Z.H.; Tong, W.W.; Yang, K.; Guo, K.Q.; Liu, G.; Pan, A. Dietary Intake and Circulating Concentrations of Carotenoids and Risk of Type 2 Diabetes: A Dose-Response Meta-Analysis of Prospective Observational Studies. Adv. Nutr. 2021, 12, 1723–1733. [Google Scholar] [CrossRef]
- Ungurianu, A.; Zanfirescu, A.; Nițulescu, G.; Margină, D. Vitamin E beyond Its Antioxidant Label. Antioxidants 2021, 10, 634. [Google Scholar] [CrossRef]
- Attar, H.; Gündeşli, M.A.; Urün, I.; Kafkas, S.; Kafkas, N.E.; Ercisli, S.; Ge, C.; Mlcek, J.; Adamkova, A. Nutritional Analysis of Red-Purple and White-Fleshed Pitaya (Hylocereus) Species. Molecules 2022, 27, 808. [Google Scholar] [CrossRef]
- Xu, Y.; Tang, G.; Zhang, C.; Wang, N.; Feng, Y. Gallic Acid and Diabetes Mellitus: Its Association with Oxidative Stress. Molecules 2021, 26, 7115. [Google Scholar] [CrossRef]
- Jiang, H.; Tang, W.; Song, Y.; Jin, W.; Du, Q. Induction of Apoptosis by Metabolites of Rhei Radix et Rhizoma (Da Huang): A Review of the Potential Mechanism in Hepatocellular Carcinoma. Front. Pharmacol. 2022, 13, 806175. [Google Scholar] [CrossRef]
- Srinivasulu, C.; Ramgopal, M.; Ramanjaneyulu, G.; Anuradha, C.M.; Suresh Kumar, C. Syringic acid (SA)—A Review of Its Occurrence, Biosynthesis, Pharmacological and Industrial Importance. Biomed. Pharmacother. 2018, 108, 547–557. [Google Scholar] [CrossRef]
- Xu, J.; Wang, W.; Zhao, Y. Phenolic Compounds in Whole Grain Sorghum and Their Health Benefits. Foods 2021, 10, 1921. [Google Scholar] [CrossRef]
- Nunes, E.N.; de Sousa, A.S.B.; de Lucena, C.M.; Silva, S.D.M.; de Lucena, R.F.P.; Alves, C.A.B.; Alves, R.E. Pitaia (Hylocereus sp.): Uma revisão para o Brasil. Gaia Sci. 2014, 8, 90–98. [Google Scholar]
- Castro-Enríquez, D.D.; Montaño-Leyva, B.; Del Toro-Sánchez, C.L.; Juárez-Onofre, J.E.; Carvajal-Millán, E.; López-Ahumada, G.A.; Barreras-Urbina, C.G.; Tapia-Hernández, J.A.; Rodríguez-Félix, F. Effect of Ultrafiltration of Pitaya Extract (Stenocereus thurberi) on Its Phytochemical Content, Antioxidant Capacity, and UPLC-DAD-MS Profile. Molecules 2020, 25, 281. [Google Scholar] [CrossRef]
- Jernimo, M.C.; Orsine, J.V.C.; Borges, K.K.; Novaes, M.R.C.G. Chemical and Physical-Chemical Properties, Antioxidant Activity and Fatty Acids Profile of Red Pitaya [Hylocereus Undatus (Haw.) Britton & Rose] Grown In Brazil. J. Drug Metab. Toxicol. 2015, 6, 1–6. [Google Scholar]
- Luu, T.-T.; Le, T.-L.; Huynh, N.; Quintela-Alonso, P. Dragon fruit: A review of health benefits and nutrients and its sustainable development under climate changes in Vietnam. Czech J. Food Sci. 2021, 39, 71–94. [Google Scholar] [CrossRef]
- Jeronimo, M.C. Caracterização Química, Físico-Química, Atividade Antioxidante e Avaliação dos Efeitos Citotóxicos da Pitaia-Vermelha [Hylocereus undatus (Haw.) Britton & Rose] Cultivada no Brasil. 2016. Available online: https://repositorio.unb.br/handle/10482/22448 (accessed on 6 November 2022).
- Hernández, Y.D.O.; Salazar, J.A.C. Pitahaya (Hylocereus spp.): A short review. Comun. Sci. 2012, 3, 220–237. [Google Scholar]
- Jeronimo, M.C.; Orsine, J.V.C.; Novaes, M.R.C.G. Nutritional pharmacological and toxicological characteristics of pitaya (Hylocereus undatus): A review of the literature. Afr. J. Pharm. Pharmacol. 2017, 11, 300–304. [Google Scholar]
- Safira, A.; Savitri, S.L.; Putri, A.R.B.; Hamonangan, J.M.; Safinda, B.; Solikhah, T.I.; Khairullah, A.R.; Puspitarani, G.A. Review on the pharmacological and health aspects of Hylocereus or Pitaya: An update. J. Drug Deliv. Ther. 2021, 11, 297–303. [Google Scholar] [CrossRef]
- Kumar, S.B.; Issac, R.; Prabha, M.L. Functional and health-promoting bioactivities of dragon fruit. Drug Invent. Today 2018, 10, 3307–3310. [Google Scholar]
- Hitendraprasad, P.P.; Hegde, K.; Shabaraya, A.R. Hylocereus undatus (Dragon Fruit): A Brief Review. Int. J. Pharm. Sci. Rev. Res. 2020, 60, 55–57. [Google Scholar]
- Harahap, N.S.; Amelia, R. Red dragon fruit (Hylocereus polyrhizus) extract decreases lactic acid level and creatine kinase activity in rats receiving heavy physical exercise. Open Access Maced. J. Med. Sci. 2019, 7, 2232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rusip, G.; Ilyas, S.; Lister, I.N.E.; Ginting, C.N.; Mukti, I. The effect of ingestion of red dragon fruit extract on levels of malondialdehyde and superoxide dismutase after strenuous exercise in rats (Rattus norvegicus). F1000Research 2021, 10, 1061. [Google Scholar] [CrossRef]
- Putri, M.D.; Wiboworini, B.; Dirgahayu, P. Red dragon fruit juice in reducing ros levels and insulin resistance In rats with type 2 diabetes mellitus model. J. Nutr. 2021, 10, 6–14. [Google Scholar] [CrossRef]
- Eldeen, I.M.S.; Foong, S.Y.; Ismail, N.; Wong, K.C. Regulation of pro-inflammatory enzymes by the dragon fruits from Hylocereus undatus (Haworth) and squalene-its major volatile constituents. Pharmacogn. Mag. 2020, 16, 81. [Google Scholar] [CrossRef]
- Amin, M.; Nur, M.; Uddin, R.; Meghla, N.S.; Uddin, M.J. In Vitro Anti-Oxidant, Anti-Inflammatory, Anti-Bacterial, and Cytotoxic Effects of Different Extracted Colorants from Two Species of Dragon Fruit (Hylocereus Spp.); Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Al-Radadi, N.S. Biogenic proficient synthesis of (Au-NPs) via aqueous extract of Red Dragon Pulp and seed oil: Characterization, antioxidant, cytotoxic properties, anti-diabetic anti-inflammatory, anti-Alzheimer and their anti-proliferative potential against cancer cell lines. Saudi J. Biol. Sci. 2022, 29, 2836–2855. [Google Scholar] [CrossRef]
- Wichienchot, S.; Jatupornpipat, M.; Rastall, R. Oligosaccharides of pitaya (dragon fruit) flesh and their prebiotic properties. Food Chem. 2010, 120, 850–857. [Google Scholar] [CrossRef]
- Dasaesamoh, R.; Youravong, W.; Wichienchot, S. Digestibility, fecal fermentation and anti-cancer of dragon fruit oligosaccharides. Int. Food Res. J. 2016, 23, 2581–2587. [Google Scholar]
- Temak, Y.; Cholke, P.; Mule, A.; Shingade, A.; Narote, S.; Kagde, A.; Lagad, R.; Sake, V. In vivo and in-vitro evaluation of antimicrobial activity of peel extracts of red dragon fruit (Hylocereus polyrhizus). Res. J. Pharmacogn. Phytochem. 2019, 11, 23–26. [Google Scholar] [CrossRef]
- Sushmitha, H.S.; Roy, C.L.; Gogoi, D.; Velagala, R.D.; Nagarathna, A.; Balasubramanian, S.; Rajadurai, M. Phytochemical and Pharmacological Studies on Hylocereus undatus Seeds: An In Vitro Approach. World J. Pharmacol. Res. 2018, 7, 986–1006. [Google Scholar]
- Tenore, G.C.; Novellino, E.; Basile, A. Nutraceutical potential and antioxidant benefits of red pitaya (Hylocereus polyrhizus) extracts. J. Funct. Foods 2012, 4, 129–136. [Google Scholar] [CrossRef]
- Divakaran, D.; Lakkakula, J.R.; Thakur, M.; Kumawat, M.K.; Srivastava, R. Dragon fruit extract capped gold nanoparticles: Synthesis and their differential cytotoxicity effect on breast cancer cells. Mater. Lett. 2019, 236, 498–502. [Google Scholar] [CrossRef]
- Wu, L.C.; Hsu, H.W.; Chen, Y.C.; Chiu, C.C.; Lin, Y.I.; Ho, J.A.A. Antioxidant and antiproliferative activities of red pitaya. Food Chem. 2006, 95, 319–327. [Google Scholar] [CrossRef]
- Khoo, H.E.; He, X.; Tang, Y.; Li, Z.; Li, C.; Zeng, Y.; Tang, J.; Sun, J. Betacyanins and Anthocyanins in Pulp and Peel of Red Pitaya (Hylocereus polyrhizus cv. Jindu), Inhibition of Oxidative Stress, Lipid Reducing, and Cytotoxic Effects. Front. Nutr. 2022, 9, 1390. [Google Scholar] [CrossRef]
- Guimarães, D.A.B.; De Castro, D.; de Oliveira, F.L.; Nogueira, E.M.; da Silva, M.A.M.; Teodoro, A.J. Pitaya Extracts Induce Growth Inhibition and Proapoptotic Effects on Human Cell Lines of Breast Cancer via Downregulation of Estrogen Receptor Gene Expression. Oxid Med. Cell Longev. 2017, 2017, 7865073. [Google Scholar] [CrossRef] [Green Version]
- Holanda, M.O.; Lira, S.M.; da Silva, J.Y.G.; Marques, C.G.; Coelho, L.C.; Lima, C.L.S.; Costa, J.T.G.; da Silva, G.S.; Santos, G.B.M.; Zocolo, G.J.; et al. Intake of pitaya (Hylocereus polyrhizus (FAC Weber) Britton & Rose) beneficially affects the cholesterolemic profile of dyslipidemic C57BL/6 mice. Food Biosci. 2021, 42, 101181. [Google Scholar]
- Marietta, S.; Budhiarta, A.; Weta, I.W. The supplementation effect of Red Dragon fruit’s skin extract on the fasting blood glucose and lipid profiles in male Wistar rats with diabetes mellitus and dyslipidemia. Neurol. Spinale Medico Chir. 2020, 3, 87–92. [Google Scholar] [CrossRef]
- Setiawan, N.; Shintawati, R.; Priyandoko, D. The role of red dragon fruit peel (Hylocereus polyrhizus) to improvement blood lipid levels of hyperlipidaemia male mice. J. Phys. Conf. Ser. 2018, 1013, 012167. [Google Scholar]
- Ravichandran, G.; Lakshmanan, D.K.; Murugesan, S.; Elangovan, A.; Rajasekaran, N.S.; Thilagar, S. Attenuation of protein glycation by functional polyphenolics of dragon fruit (Hylocereus polyrhizus); an in vitro and in silico evaluation. Food Res. Int. 2021, 140, 110081. [Google Scholar] [CrossRef]
- Rosa Martha, P.G.; Susana Gabriela, E.A. Ursane derivatives isolated from leaves of Hylocereus undatus inhibit glycation at multiple stages. Chin. J. Nat. Med. 2018, 16, 856–865. [Google Scholar] [CrossRef]
- Sewidan, N.; Abu Khalaf, R.; Mohammad, H.; Hammad, W. In-Vitro Studies on Selected Jordanian Plants as Dipeptidyl Peptidase-IV Inhibitors for Management of Diabetes Mellitus. Iran. J. Pharm. Res. 2020, 19, 95–102. [Google Scholar] [CrossRef]
- Hendra, R.; Khodijah, R.; Putri, R.; Amalia, R.; Haryani, Y.; Teruna, H.Y.; Abdulah, R. Cytotoxicity and Antiplasmodial Properties of Different Hylocereus polyrhizus Peel Extracts. Med. Sci. Monit. Basic Res. 2021, 27, e931118. [Google Scholar] [CrossRef]
- Shen, L.; Xiong, X.; Zhang, D.; Zekrumah, M.; Hu, Y.; Gu, X.; Wang, C.; Zou, X. Optimization of betacyanins from agricultural by-products using pressurized hot water extraction for antioxidant and in vitro oleic acid-induced steatohepatitis inhibitory activity. J. Food Biochem. 2019, 43, e13044. [Google Scholar] [CrossRef]
- Chang, Y.J.; Pong, L.Y.; Hassan, S.S.; Choo, W.S. Antiviral activity of betacyanins from red pitahaya (Hylocereus polyrhizus) and red spinach (Amaranthus dubius) against dengue virus type 2 (GenBank accession no. MH488959). Access Microbiol. 2020, 2, acmi000073. [Google Scholar] [CrossRef]
- Wahdaningsih, S.; Wahyuono, S.; Riyanto, S.; Murwanti, R. Terpenoid-lupeol of red dragon fruit (Hylocereus polyrhizus) and its immunomodulatory activity. Pak. J. Pharm. Sci. 2020, 33, 505–510. [Google Scholar]
- Hartono, M.R.; Suardita, K.; Yuliati, A. Proliferation and osteogenic differentiation of bone marrow-derived mesenchymal stem cell after exposure to red flesh dragon fruit extract. Dent. Res. J. 2020, 17, 107–113. [Google Scholar]
- Deshmukh, R.K.; Gaikwad, K.K. Natural antimicrobial and antioxidant compounds for active food packaging applications. Biomass Convers. Biorefinery 2022, 1–22. [Google Scholar] [CrossRef]
- Ai, Y.; Wang, G.; Fang, F.; Zhang, F.; Liao, H. Development of real-time intelligent films from red pitaya peel and its application in monitoring the freshness of pork. J. Sci. Food Agric. 2022, 102, 5512–5522. [Google Scholar] [CrossRef]
- Kim, H.; Choi, H.K.; Moon, J.Y.; Kim, Y.S.; Mosaddik, A.; Cho, S.K. Comparative antioxidant and antiproliferative activities of red and white pitayas and their correlation with flavonoid and polyphenol content. J. Food Sci. 2011, 76, C38–C45. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, J.; He, Y.; Shi, M.; Han, X.; Li, W.; Zhang, X.; Wen, X. Metabolic Profiling of Pitaya (Hylocereus polyrhizus) during Fruit Development and Maturation. Molecules 2019, 24, 1114. [Google Scholar] [CrossRef] [Green Version]
- Pires, E.; Krauze, C. Análise econômica da produção de Pitaya na agricultura familiar do sul de Santa Catarina. Metodol. E Aprendizado 2020, 2, 181–189. [Google Scholar] [CrossRef]
- Tien, N.N.T.; Le, N.L.; Khoi, T.T.; Richel, A. Influence of location, weather condition, maturity, and plant disease on chemical profiles of dragon fruit (Hylocereus spp.) branches grown in Vietnam. Biomass Convers. Biorefinery 2022, 2022, 1–13. [Google Scholar] [CrossRef]
- Holanda, M.O. Prospecção e Caracterização Química da Polpa de Pitaia Vermelha [Hylocereus Polyrhizus (Weber) Britton & Rose] e Sua Ação Frente à Dislipidemia; Universidade Estadual do Ceará: Santa Catarina, Brazil, 2021. [Google Scholar]
- Pérez-Orozco, A.F.; Sosa, V. Comparative estimations of betalains and sugars in fruits of five species of Selenicereus (Cactaceae). Acta Botánica Mex. 2022, 129. [Google Scholar] [CrossRef]
- Calva-Estrada, S.; Jiménez-Fernández, M.; Lugo-Cervantes, E. Betalains and their applications in food: The current state of processing, stability and future opportunities in the industry. Food Chem. Mol. Sci. 2022, 4, 100089. [Google Scholar] [CrossRef]
- de Lima, A.C.V.; Dionisio, A.P.; de Abreu, F.A.P.; da Silva, G.S.; Junior, R.D.L.; Magalhães, H.C.R.; Garruti, D.D.S.; Araújo, I.M.D.S.; Artur, A.G.; Taniguchi, C.A.K.; et al. Microfiltered red–purple pitaya colorant: UPLC-ESI-QTOF-MSE-based metabolic profile and its potential application as a natural food ingredient. Food Chem. 2020, 330, 127222. [Google Scholar] [CrossRef]
- Bellucci, E.R.B.; Munekata, P.E.; Pateiro, M.; Lorenzo, J.M.; Barretto, A.C.D.S. Red pitaya extract as natural antioxidant in pork patties with total replacement of animal fat. Meat Sci. 2021, 171, 108284. [Google Scholar] [CrossRef]
- Filho, J.G.D.O.; Bertolo, M.R.V.; Rodrigues, M.V.; Silva, G.D.C.; de Mendonça, G.M.N.; Junior, S.B.; Ferreira, M.D.; Egea, M.B. Recent advances in the development of smart, active, and bioactive biodegradable biopolymer-based films containing betalains. Food Chem. 2022, 390, 133149. [Google Scholar] [CrossRef]
- Utpott, M. Desenvolvimento de Farinha de Pitaya de Polpa Vermelha (Hylocereus polyrhizus) e Microcápsulas de Betalaínas como Ingredientes Alimentares. 2019. Available online: http://hdl.handle.net/10183/196659 (accessed on 6 November 2022).
- Liu, W.; Shen, Y.; Li, N.; Mei, J.; Xie, J. Application of Gelatin Incorporated with Red Pitaya Peel Methanol Extract as Edible Coating for Quality Enhancement of Crayfish (Procambarus clarkii) during Refrigerated Storage. J. Food Qual. 2019, 2019, 1715946. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Liu, Y.; Zhang, X.; Liu, J. Development of active and intelligent packaging by incorporating betalains from red pitaya (Hylocereus polyrhizus) peel into starch/polyvinyl alcohol films. Food Hydrocoll. 2020, 100, 105410. [Google Scholar] [CrossRef]
- Amorim, M.G.R. Utilização da Farinha da Casca de Pitaia Vermelha em Pães: Uma Alternativa de Reaproveitamento Agroindustrial. 2021. Available online: http://www.repositorio.ufc.br/handle/riufc/61759 (accessed on 6 November 2022).
- Tamagno, W.A.; Santini, W.; Santos, A.; Alves, C.; Bilibio, D.; Sutorillo, N.T.; Zamberlan, D.C.; Kaizer, R.R.; Gil Barcellos, L.J. Pitaya fruit extract ameliorates the healthspan on copper-induced toxicity of Caenorhabditis elegans. J. Food Biochem. 2022, 46, e14050. [Google Scholar] [CrossRef] [PubMed]
- Hübner, D.S. Produção de Cerveja Estilo Catharina Sour Com polpa de Pitaia (Hylocereus polyrhizus) e Gengibre (Zingiber officinale Roscoe). 2019. Available online: https://repositorio.ufsc.br/handle/123456789/203986 (accessed on 18 November 2022).
- Vijayakumar, R.; Abd Gani, S.S.; Zaidan, U.H.; Halmi, M.I.E.; Karunakaran, T.; Hamdan, M.R. Exploring the potential use of Hylocereus polyrhizus peels as a source of cosmeceutical sunscreen agent for its antioxidant and photoprotective properties. Evid. Based Complement. Altern. Med. 2020, 2020, 7520736. [Google Scholar] [CrossRef] [PubMed]
- Piragine, E.; Citi, V.; Lawson, K.; Calderone, V.; Martelli, A. Regulation of blood pressure by natural sulfur compounds: Focus on their mechanisms of action. Biochem. Pharmacol. 2022, 206, 115302. [Google Scholar] [CrossRef] [PubMed]
- Hajibabaie, F.; Abedpoor, N.; Safavi, K.; Taghian, F. Natural remedies medicine derived from flaxseed (secoisolariciresinol diglucoside, lignans, and α-linolenic acid) improve network targeting efficiency of diabetic heart conditions based on computational chemistry techniques and pharmacophore modeling. J. Food Biochem. 2022, 2022, e14480. [Google Scholar] [CrossRef] [PubMed]
- Saqib, S.; Ullah, F.; Naeem, M.; Younas, M.; Ayaz, A.; Ali, S.; Zaman, W. Mentha: Nutritional and Health Attributes to Treat Various Ailments Including Cardiovascular Diseases. Molecules 2022, 27, 6728. [Google Scholar] [CrossRef] [PubMed]
- Sahiner, M.; Yilmaz, A.S.; Gungor, B.; Ayoubi, Y.; Sahiner, N. Therapeutic and Nutraceutical Effects of Polyphenolics from Natural Sources. Molecules 2022, 27, 6225. [Google Scholar] [CrossRef] [PubMed]
- Metibemu, D.S.; Ogungbe, I.V. Carotenoids in Drug Discovery and Medicine: Pathways and Molecular Targets Implicated in Human Diseases. Molecules 2022, 27, 6005. [Google Scholar] [CrossRef]
- Tiwari, P.; Mishra, K.P. Role of Plant-Derived Flavonoids in Cancer Treatment. Nutr. Cancer 2022, 1–20. [Google Scholar] [CrossRef]
- Beylerli, O.; Beilerli, A.; Shumadalova, A.; Wang, X.; Yang, M.; Sun, H.; Teng, L. Therapeutic effect of natural polyphenols against glioblastoma. Front. Cell Dev. Biol. 2022, 10, 1036809. [Google Scholar] [CrossRef]
- Kenari, R.E.; Razavi, R. Encapsulation of bougainvillea (Bougainvillea spectabilis) flower extract in Urtica dioica L. seed gum: Characterization, antioxidant/antimicrobial properties, and in vitro digestion. Food Sci. Nutr. 2022, 10, 3436–3443. [Google Scholar] [CrossRef]
- Palrasu, M.; Wright, L.; Patel, M.; Leech, L.; Branch, S.; Harrelson, S.; Khan, S. Perspectives on Challenges in Cannabis Drug Delivery Systems: Where Are We? Med. Cannabis Cannabinoids 2022, 5, 102–119. [Google Scholar] [CrossRef] [PubMed]
- Muschiolik, G.; Dickinson, E. Double emulsions relevant to food systems: Preparation, stability, and applications. Compr. Rev. Food Sci. Food Saf. 2017, 16, 532–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amjadi, S.; Almasi, H.; Hamishehkar, H.; Alizadeh Khaledabad, M.; Lim, L.T. Coating of betanin and carvone Co-loaded nanoliposomes with synthesized cationic inulin: A strategy for enhancing the stability and bioavailability. Food Chem. 2022, 373, 131403. [Google Scholar] [CrossRef] [PubMed]
- Kanner, J.; Harel, S.; Granit, R. Betalains a new class of dietary cationized antioxidants. J. Agric. Food Chem. 2001, 49, 5178–5185. [Google Scholar] [CrossRef] [PubMed]
- Tesoriere, L.; Gentile, C.; Angileri, F.; Attanzio, A.; Tutone, M.; Allegra, M.; Livrea, M.A. Trans-epithelial transport of the betalain pigments indicaxanthin and betanin across Caco-2 cell monolayers and influence of food matrix. Eur. J. Nutr. 2013, 52, 1077–1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawicki, T.; Topolska, J.; Bączek, N.; Szawara-Nowak, D.; Juśkiewicz, J.; Wiczkowski, W. Characterization of the profile and concentration of betacyanin in the gastric content, blood and urine of rats after an intragastric administration of fermented red beet juice. Food Chem. 2020, 313, 126169. [Google Scholar] [CrossRef]
- Krantz, C.; Monier, M.; Wahlström, B. Absorption, excretion, metabolism and cardiovascular effects of beetroot extract in the rat. Food Cosmet. Toxicol. 1980, 18, 363–366. [Google Scholar] [CrossRef]
- Harimurti, N.; Nasikin, M.; Mulia, K. Water-in-Oil-in-Water Nanoemulsions Containing Temulawak (Curcuma xanthorriza Roxb) and Red Dragon Fruit (Hylocereus polyrhizus) Extracts. Molecules 2021, 26, 196. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Schieber, A.; Carle, R. Betacyanins in fruits from red-purple pitaya, Hylocereus polyrhizus (Weber) Britton & Rose. Food Chem. 2002, 77, 101–106. [Google Scholar]
- Amjadi, S.; Ghorbani, M.; Hamishehkar, H.; Roufegarinejad, L. Improvement in the stability of betanin by liposomal nanocarriers: Its application in gummy candy as a food model. Food Chem. 2018, 256, 156–162. [Google Scholar] [CrossRef]
- Gengatharan, A.; Dykes, G.A.; Choo, W.S. Stability of betacyanin from red pitahaya (Hylocereus polyrhizus) and its potential application as a natural colourant in milk. Int. J. Food Sci. Technol. 2016, 51, 427–434. [Google Scholar] [CrossRef]
- Kaimainen, M.; Marze, S.; Järvenpää, E.; Anton, M.; Huopalahti, R. Encapsulation of betalain into w/o/w double emulsion and release during in vitro intestinal lipid digestion. LWT 2015, 60, 899–904. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Chung, I.M.; Samynathan, R.; Chandar, S.R.H.; Venkidasamy, B.; Sarkar, T.; Rebezov, M.; Gorelik, O.; Shariati, M.A.; Simal-Gandara, J. A comprehensive review of beetroot (Beta vulgaris L.) bioactive components in the food and pharmaceutical industries. Crit. Rev. Food Sci. Nutr. 2022, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Campos, L.; Valle-Guadarrama, S.; Martínez-Bustos, F.; Salinas-Moreno, Y.; Lobato-Calleros, C.; Calvo-López, A.D. Encapsulation and pigmenting potential of betalains of pitaya (Stenocereus pruinosus) fruit. J. Food Sci. Technol. 2018, 55, 2436–2445. [Google Scholar] [CrossRef]
- Pavoković, D.; Krsnik-Rasol, M. Complex biochemistry and biotechnological production of betalains. Food Technol. Biotechnol. 2011, 49, 145–155. [Google Scholar]
- Rizvi, M.; Tiwari, N.; Mishra, A.; Gupta, R. Kinetic and Computational Study of Degradation of Two Azo Dyes, Metanil Yellow and Orange II, by Iron Oxide Nanoparticles Synthesized Using Hylocereus undatus. ACS Omega 2022, 7, 31667–31681. [Google Scholar] [CrossRef]
- Siew Lian, C.; Chong, G.H. Effect of rotation speed and steam pressure on physico-chemical properties of drum dried pitaya (Hylocereus polyrhizus) peel. Int. Food Res. J. 2014, 22, 372–376. [Google Scholar]
- Bakar, J.; Shu, C.E.; Kharidah, M.; Dzulkily, M.A.; Noranizan, A. Physico-chemical characteristics of red pitaya (Hylocereus polyrhizus) peel. Int. Food Res. J. 2011, 18, 279–286. [Google Scholar]
- Zaid, R.M.; Mishra, P.; Tabassum, S.; Wahid, Z.A.; Sakinah, A.M.M. High methoxyl pectin extracts from Hylocereus polyrhizus’s peels: Extraction kinetics and thermodynamic studies. Int. J. Biol. Macromol. 2019, 141, 1147–1157. [Google Scholar] [CrossRef]
- Muhammad, K.; Zahari, N.I.M.; Gannasin, S.P.; Adzahan, N.M.; Bakar, J. High methoxyl pectin from dragon fruit (Hylocereus polyrhizus) peel. Food Hydrocoll. 2014, 42, 289–297. [Google Scholar] [CrossRef]
- Lim, Y.Y.; Lim, T.T.; Tee, J.J. Antioxidant properties of several tropical fruits: A comparative study. Food Chem. 2007, 103, 1003–1008. [Google Scholar] [CrossRef]



| Properties | Plant Part or Active Compounds | Species | Extraction | Experimental Model | Dose | Route of Administration | Methods of Analysis | Observations | References |
|---|---|---|---|---|---|---|---|---|---|
| Anxiolytic-like effects | Pulp and peel rich in maltotriose, quercetin-3-O-hexoside, and betalains bioactive compounds. | H. polyrhizus. | Ethanolic and aqueous extracts. | Zebrafish from both sexes. | 0.1 mg/mL or 0.5 mg/mL or 1.0 mg/mL, 20 μL. | Dissolved in the fish’s water. | Anxiolytic activities and toxicity assays. | The extracts showed no toxicity in the fish model and exerted significant anxiolytic effects as the fish reduced their permanence in the clear zone of the experimentation area compared to controls. | [21] |
| Metabolic effects | Betalain-rich pitaya pulp. | H. undatus. | Aqueous extract. | Streptozotocin-induced diabetes in male Sprague-Dawley rats. | 250 or 500 mg per kg body weight. | Intragastric gavage. | Pulse wave velocities, surgical procedures, and oxidative stress analyses. | The treated rats had reduced blood pressure, pulse wave velocities, and pulse pressures. | [22] |
| Betalain-rich pitaya juice. | H. polyrhizus. | Aqueous juice. | High-carbohydrate, high-fat diet-induced metabolic syndrome male Wistar rats. | 5% of red pitaya juice during the diet. | Oral by feeding. | Biochemical and physical tests, as well as histopathological assessments. | There was a significant reduction in the diastolic stiffness of the treated rats. Additionally, the treated rats presented reductions in the alkaline phosphatase and alanine transaminase serum concentrations. | [26] | |
| Pitaya’s juice. | H. polyrhizus. | Aqueous juice. | High-carbohydrate, high-fat diet-induced metabolic syndrome male Wistar rats. | 5% of red pitaya juice during the diet. | Oral by feeding. | Biochemical and physical tests, as well as genetic assessments. | During the treatment, the omental and epididymal fat of the rats increased. The pitaya treatment reversed the rats’ metabolic changes by up-regulating the obesity-related Pomc and Insr genes in the liver tissues. | [27] | |
| Pitaya’s peel purified betacyanins. | H. undatus. | Purified betacyanins. | High-fat diet-fed male C57BL/6J mice. | Different concentrations of different purified betacyanins. | Oral by feeding. | Biochemical and histopathological analyses. | The purified betacyanin ameliorated the AT’s hypertrophy, liver steatosis, body glucose intolerance, and the body’s IR. In the liver, the purified betacyanins also augmented the genetic expression of lipid metabolism genes, such as the Acox1, Cpt1b, Cpt1a, Insig1, PPARγ, AdipoR2, and Insig2 and of FGF-21 genes. The purified betacyanins alleviated the liver’s FGF21 resistance, decreased the liver’s fatty acid biosynthesis, and elevated the liver’s fatty acid oxidation. | [28] | |
| Betacyanin-rich pitaya fruit. | H. polyrhizus. | Red pitaya’s fruit betacyanins. | Diet-induced obesity, liver steatosis, and insulin resistance C57BL/6J mice. | 200 mg/kg. | Intragastric gavage. | Biochemical, sequencing, and histological analyses. | The fruit protected the mice from obesity and its related metabolic disorders. There were improvements in the inflammatory statuses of the treated rats, as well as their gut microbiota (there was a decrease in the ratio of Firmicutes and Bacteroidetes and an increase in the relative amount of Akkermansia). | [31] | |
| Pitaya juice rich in polyphenols and flavonoids bioactive compounds. | H. undatus. | Aqueous juice. | Steatosis diet-induced obese C57BL/6J mice. | - | Oral by drinking. | Biochemical and histopathological assessments. | There were improvements in the FGF-21 resistance and lipid metabolisms of the treated rats. Additionally, the juice protected the rats against hepatic steatosis and IR. | [32] | |
| Concentrated Pitaya Pulp with seeds | H. polyrhizus. | Pulp with seed | Hyperlipidemic female C57BL/6 mice | 100, 200, and 400 mg/kg/day | Oral by feeding | Biochemical assessments. | There was an increase in the levels of HDL-c and a significant reduction in the levels of Total cholesterol, LDL, triglycerides, glycemia, AST, and ALT. | [23] | |
| Betacyanin-rich pitaya peel extract. | H. polyrhizus. | Methanolic extract. | Alcoholic-progressive liver disease ethanolic-diet C57BL/6 mice. | 500 and 1000 mg/kg of body weight. | Intragastric gavage. | Biochemical and histopathological assessments. | The treated group presented diminished liver injury and improved liver lipid metabolism via decreases in the SREBP-1 and increases in AMPK and PPAR-α protein expressions. The extract also inhibited the Nrf2 and CYP2E1 expression, reduced endotoxin levels, and decreased TLR4, MyD88, TNF-α, and IL-1β expression in the treated rats’ liver. | [25] | |
| Concentrated Pitaya Pulp with seeds | H. polyrhizus. | Pulp with seed | Hyperglycemic Zebrafish | 5–100% of pitaya pulp | Oral by feeding | Biochemical assessments. | There was a significant decrease in glycemia in all concentrations compared to placebo and with the use of metformin. | [24] | |
| Gastrointestinal | Pitaya’s fruit extract. | H. polyrhizus. | Ethanolic extract. | Balb/c mice induced colitis by trinitrobenzene sulphonic. | 1 g/kg. | Intraperitoneally. | Biochemical and histopathological analysis. | The extract exerted anti-inflammatory (decreases in the Ikb-a degradation and nuclear NF-kb protein levels) effects and prevented colitis development (reduced histological damage score) in the treated mice. | [29] |
| Wound-healing | Pitaya’s leaves, rind, fruit pulp, and flower extracts. | H. undatus. | Aqueous extract. | Streptozotocin diabetic Wistar rats | 200 µL/wound at concentrations of 0.05%, 0.1%, 0.2%, 0.4% and 0.5% twice daily. | Topically. | Wound healing assays and DNA and protein estimation. | The use of the extract facilitated wound healing by enhancing tensile strength, hydroxyproline, DNA, total proteins, collagen content, and epithelization. | [30] |
| Properties | Study | Sequence Generation | Baseline Characteristics | Allocation Concealment | Random Housing | Binding (Intervention) | Random Outcome Assessment | Blinding (Outcome) | Incomplete Outcome Data | Selective Outcome Reporting | Other Sources of Bias |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anxiolytic-like effects | [21] | Yes | Yes | Yes | Yes | Unclear | Yes | Unclear | Yes | Yes | Yes |
| Metabolic effects | [22] | Yes | Yes | Yes | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes |
| [25] | Yes | Yes | Yes | Unclear | Unclear | Unclear | Yes | Yes | Yes | Yes | |
| [26] | Yes | Yes | Yes | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes | |
| [27] | Yes | Yes | Yes | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes | |
| [28] | Yes | Yes | Yes | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes | |
| [31] | Yes | Yes | Yes | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes | |
| [32] | Yes | Yes | Yes | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes | |
| [23] | Yes | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes | |
| [23] | Yes | Yes | Yes | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes | |
| [24] | Yes | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes | |
| Gastrointestinal | [29] | Yes | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes |
| Wound-healing | [30] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | No | Yes | No |
| Properties | Plant Part Used or Compounds | Species | Models | Type of Extract | Tested Concentrations | Methods | Observations | References |
|---|---|---|---|---|---|---|---|---|
| Anti-glycation | Freeze-dried powder rich in phenolic bioactive compounds. | H. polyrhizus. | Bovine serum albumin. | Ethanolic, hydro-ethanolic, methanolic, hydro-methanolic, acetone, aqueous, and petroleum ether extracts. | Concentrations range from 20 µg/mL to 100 µg/mL. | Glycation and aggregation of protein assays, carbohydrate cleaving enzyme inhibitory activity assessments, antioxidant studies, and determination of fructosamine inhibition. | Methanolic and acetone extracts were the most significant anti-glycation and antioxidant agents. The potential polyphenolic compounds associated with these effects were mainly 4-prenylresveratrol, vicenin, and luteolin. | [88] |
| Leaves rich in triterpenes. | H. undatus. | Bovine serum albumin. | Chloroform extract. | 0.5–2.0 mg·mL−1, 3 mmol·L−1, and 5 mmol·L−1. | Protein glycation and aggregation assessments. | The studied triterpenes could inhibit protein glycation at multiple stages, decreasing protein oxidation and protecting against diabetic-related complications. | [89] | |
| Anti-diabetic | Bulb and peel. | H. undatus. | Dipeptidyl peptidase-IV enzyme. | Ethanolic and hydro- ethanolic extracts of pitaya’s fruit and ethanolic extract of pitaya’s peel. | 10 mg/100 µL. | In vitro dipeptidyl peptidase-IV enzyme inhibition. | The hydro-ethanolic extract of pitaya’s fruit exerted 26.8 ± 0.55% of DPP-IV inhibition, the ethanolic extract of pitaya’s peel exerted 62.3 ± 0.63%, and the ethanolic extract of pitaya’s fruit exerted 84.2 ± 0.72%. | [90] |
| Anti-plasmodium | Peel. | H. polyrhizus. | Plasmodium falciparum 3D7 and W2 strains, HeLa cells, and SK-OV-3 cells. | Pigmented, n-hexane, dichloromethane, and ethyl acetate extracts. | - | Anti-plasmodium and cytotoxicity assays. | The dichloromethane extract demonstrated the most prominent anti-plasmodium activity at concentrations of 2.13 ± 0.42 µg/mL. The extracts showed cytotoxicity against the cancer cells at final concentrations of more than 1000 µg/mL. | [91] |
| Cytotoxicity | Pulp and peel rich in maltotriose, quercetin-3-O-hexoside, and betalains bioactive compounds. | H. polyrhizus. | Vero cell lines. | Ethanolic and aqueous extracts. | - | Cytotoxicity assays. | The toxicity against the cells ranged from 2.18 to 2.36 mg/mL for the peel and pulp. | [21] |
| Peel. | H. polyrhizus and H. undatus. | PC3, Bcap-37, and MGC-803 cell lines. | Supercritical carbon dioxide extracts. | Maximum concentrations of 0.7 mg/mL. | Cytotoxicity assays. | All extracts demonstrated significant cytotoxicity against the cancer cell lines at concentrations ranging from 0.61 to 0.73 mg/mL. | [36] | |
| Hepatoprotective | Peel rich in betacyanins. | H. undatus. | HepG2 cells. | Pressurized in the hot water extract. | Concentrations range from 20 μg/mL to 100 μg/mL. | Measurements of ROS and lipid accumulation, as well as biochemical tests. | The betacyanin-rich extracts could effectively exert antioxidant and hepatoprotective effects in the treated cells by controlling their lipid metabolisms and diminishing their TG contents. These compounds inhibited the liberation of the liver enzymes AST and ALT and probably regulated the mRNA expressions of the fatty acid synthase and carnitine palmitoyl transferase 1. | [92] |
| Anti-viral | Pulp rich in betacyanins. | H. polyrhizus. | Denv-2 strains and Vero cells. | Methanolic extracts. | Maximum concentrations at 4.346 mg·mL−1. | Cytotoxicity and anti-viral assays. | The extract was considered non-toxic for the cells at concentrations below 2.50 mg·mL−1. The most prominent anti-viral potential (95.0% virus inhibition) against the denv-2 was achieved at concentrations of 126.70 μg mL−1. | [93] |
| Immunomodulatory | Peel rich in the terpenoid lupeol. | H. polyrhizus. | Mice macrophages. | The compound was dry isolated. | 100, 50, 25, 12.5 and 6.25 µg/mL. | Phagocytosis assessments and other macrophagic tests. | The terpenoid lupeol isolated from the mice was effectively associated with the increase of macrophage phagocytosis of latex beads, demonstrating potent immunomodulatory effects. | [94] |
| Bone effects | Fruit extract. | H. polyrhizus. | Bone marrow-derived mesenchymal stem cells. | Aqueous. | 50, 100, 200, 300, and 400 µg/mL. | Cells osteogenic and proliferation assessments. | The fruit extract could promote the osteogenic differentiation and proliferation of the treated cells. | [95] |
| Reference | Local | Model and Patients | Intervention | Outcomes | Adverse Effects |
|---|---|---|---|---|---|
| [20] | United Kingdom | Randomized, double-blind, placebo-controlled, cross-over trial with 19 healthy subjects (8 ♂, 11 ♀; 18–40 y) | Participants consumed 24 g of whole dragon fruit powder (33 mg of betalains) or a placebo daily/for 14 days. Blood pressure, Flow-mediated dilation (FMD), and arterial stiffness were evaluated (0, 1, 2, 3, and 4 h and 14 days after daily consumption). | Pitaya consumption significantly improved acute FMD at 2, 3, and 4 h post-consumption compared with placebo. This was also observed until 14 d. Pulse wave velocity was acutely significantly decreased at 3 h, and the augmentation index improved after 14 days compared with the placebo. No significant differences were observed for BP. | Not reported by patients. |
| [19] | Indonesia | Quantitative research type quasi-experiment (pre-test and post-test nonequivalent control group) with 32 students (4 ♂, 28 ♀; 21–26 y) who presented excess nutritional status. | Participants consumed 180 g of red pitaya/7 days. | The consumption of dragon fruit effectively reduced glycemia and blood pressure in subjects with excess nutritional status. | Not reported by the authors. |
| [17] | Malaysia | Randomized trial with 28 subjects (14 ♂, 14 ♀, 21 with type 2 diabetes; 20–55 y) | Participants were divided into 4 groups: 400 g of red pitaya/d, 600 g of red pitaya/d, negative control (diabetic subjects with normal diet), and the last group (healthy subjects with normal diet)/7 w. The study included 4 weeks of treatment and 2 weeks of wash-out. | After 4w: Group 1 showed a significant increase in HDL-c and a significant reduction of glycemia, LDL-c, and triglyceride. There was a significant augment of total cholesterol level on 7th w. No significant modifications were seen in group 2. No significant modifications were seen in weight and body fat in any group. | Not reported |
| [16] | Malaysia | Single-blinded cross-over trial with 36 Pre-diabetic participants | Pre-diabetic participants received 60, 80 or 100 g of spray pitaya (red) powder daily/4 week. | A significant decrease was seen in glycemia, total cholesterol, LDL-c, and triglycerides. A significant increase in HDL-c and total antioxidant status was observed. | Not reported |
| [18] | Malaysia | Single-blinded cross-over trial with 60 normocholesterolemic subjects | Participants were divided to receive 3, 4 or 5 sachets of spray pitaya (red) powder (20 g each) daily/4 weeks. | Significant reduction in glycemia, total cholesterol, LDL-c, and triglycerides. A significant increase in HDL-c and total antioxidant status. | Not reported by the participants. |
| Study | Question Focus | Appropriate Randomization | Allocation Blinding | Double-Blind | Losses (<20%) | Prognostics or Demographic Characteristics | Outcomes | Intention to Treat Analysis | Sample Calculation | Adequate Follow-Up |
|---|---|---|---|---|---|---|---|---|---|---|
| [20] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | NR | NR | Yes |
| [19] | Yes | No | No | No | NR | Yes | Yes | NR | NR | No |
| [17] | Yes | No | NR | NR | NR | Yes | Yes | No | No | Yes |
| [16] | Yes | No | Yes | No | NR | No | Yes | No | No | Yes |
| [18] | Yes | No | Yes | No | NR | No | Yes | No | No | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishikito, D.F.; Borges, A.C.A.; Laurindo, L.F.; Otoboni, A.M.M.B.; Direito, R.; Goulart, R.d.A.; Nicolau, C.C.T.; Fiorini, A.M.R.; Sinatora, R.V.; Barbalho, S.M. Anti-Inflammatory, Antioxidant, and Other Health Effects of Dragon Fruit and Potential Delivery Systems for Its Bioactive Compounds. Pharmaceutics 2023, 15, 159. https://doi.org/10.3390/pharmaceutics15010159
Nishikito DF, Borges ACA, Laurindo LF, Otoboni AMMB, Direito R, Goulart RdA, Nicolau CCT, Fiorini AMR, Sinatora RV, Barbalho SM. Anti-Inflammatory, Antioxidant, and Other Health Effects of Dragon Fruit and Potential Delivery Systems for Its Bioactive Compounds. Pharmaceutics. 2023; 15(1):159. https://doi.org/10.3390/pharmaceutics15010159
Chicago/Turabian StyleNishikito, Daniela Franceschi, Ana Claudia Abdalla Borges, Lucas Fornari Laurindo, Alda M. M. Bueno Otoboni, Rosa Direito, Ricardo de Alvares Goulart, Claudia C. T. Nicolau, Adriana M. R. Fiorini, Renata Vargas Sinatora, and Sandra M. Barbalho. 2023. "Anti-Inflammatory, Antioxidant, and Other Health Effects of Dragon Fruit and Potential Delivery Systems for Its Bioactive Compounds" Pharmaceutics 15, no. 1: 159. https://doi.org/10.3390/pharmaceutics15010159
APA StyleNishikito, D. F., Borges, A. C. A., Laurindo, L. F., Otoboni, A. M. M. B., Direito, R., Goulart, R. d. A., Nicolau, C. C. T., Fiorini, A. M. R., Sinatora, R. V., & Barbalho, S. M. (2023). Anti-Inflammatory, Antioxidant, and Other Health Effects of Dragon Fruit and Potential Delivery Systems for Its Bioactive Compounds. Pharmaceutics, 15(1), 159. https://doi.org/10.3390/pharmaceutics15010159