IgG Fc Affinity Ligands and Their Applications in Antibody-Involved Drug Delivery: A Brief Review
Abstract
:1. Introduction
2. IgG Fc Affinity Ligands
3. Applications of Affinity Ligands in Antibody Delivery
| Application | Sequence | Affinity Ligand |
|---|---|---|
| Local Delivery of Antibodies | pG_EAK/EAK [46] | Protein G |
| C12-Z33 [47] | Z33 | |
| EAK16-II-EAKH6 [50] | Protein A/G | |
| EAK-EAKH6 [51] | Protein A/G | |
| EAK16-II [20] | Z15 | |
| Intracellular Delivery of Antibodies | CPD-prtA [52] | Protein A |
| CPD-TRIM21 [52] | TRIM21 | |
| CPP-pAd [53] | Protein Ad | |
| TAT-B2C [54] | B domain | |
| FcBP-Tat [55] | FCⅢ |
4. Applications of Affinity Ligands in Non-Covalent Antibody–Drug Conjugates
5. Applications of Affinity Ligands in Active Targeting Drug Delivery Systems
| Carriers | Linker | Application |
|---|---|---|
| Bionanocapsules (BNCs) [75,77,80,81,82] | C2 domain of protein G | As promising drug delivery system nanocarriers for targeting delivery to macrophages. |
| Z domain | ZZ-BNCs displaying α-DC or α-hEGFR antibodies on the surface of BNCs and enhancing the therapeutic efficacy to target cell. | |
| Virus-like particle (VLP) of JC polyomavirus (JCPyV) [83] | Z domain | VLP-Z can be armed with cell-specific antibody and be used to deliver therapeutic genes to target cells. |
| Long-lived red blood cells (RBCs) [79] | Protein A | The RBC-PEG-SpA-antibody complexes can circulate in vivo and efficiently scavenge target antigen molecules. |
| Gold nanoparticles (AuNPs) [84] | Z domain | AuNP-antibody conjugates (immuno-AuNPs) are of particular value in biomedical applications such as immunoassays for the detection of target antigens. |
| Adenovirus (Ad) [85,86,87] | C domain of Protein A | The complex can efficiently deliver transgenes to target cells by using the cell entry pathway determined by its ligand component. |
| Z33 | The gene therapy using Ad-FZ33 with anti-EpCAM or anti-EGFR or anti-CEA antibodies is a potentially effective and safe therapeutic strategy for various cancers. | |
| Liposomes [74] | PAR28 | PAR28 can be exploited to easily modify the liposomes with various antibodies and selectively enhance drug delivery into specific cells. |
| Apoferritins [78] | HWRGWV | Encapsulating DOX in prostate cancer-targeted APO lowers the influence of DOX on off-target organs. |
6. Challenges and Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sivaccumar, J.; Sandomenico, A.; Vitagliano, L.; Ruvo, M. Monoclonal Antibodies: A Prospective and Retrospective View. Curr. Med. Chem. 2021, 28, 435–471. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Gauthami, S.; Bayry, J.; Kaveri, S.V.; Hegde, N.R. Antibody Therapy: From Diphtheria to Cancer, COVID-19, and Beyond. Monoclon. Antibodies Immunodiagn. Immunother. 2021, 40, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Goulet, D.R.; Atkins, W.M. Considerations for the Design of Antibody-Based Therapeutics. J. Pharm. Sci. 2020, 109, 74–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, T.H.; Jung, S.T. Boosting therapeutic potency of antibodies by taming Fc domain functions. Exp. Mol. Med. 2019, 51, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaughan, C.L. The present state of the art in expression, production and characterization of monoclonal antibodies. Mol. Divers. 2016, 20, 255–270. [Google Scholar] [CrossRef]
- Gupta, N.; Ansari, A.; Dhoke, G.V.; Chilamari, M.; Sivaccumar, J.; Kumari, S.; Chatterjee, S.; Goyal, R.; Dutta, P.K.; Samarla, M.; et al. Computationally designed antibody-drug conjugates self-assembled via affinity ligands. Nat. Biomed. Eng. 2019, 3, 917–929. [Google Scholar] [CrossRef]
- Kabir, S. Immunoglobulin purification by affinity chromatography using protein A mimetic ligands prepared by combinatorial chemical synthesis. Immunol. Investig. 2002, 31, 263–278. [Google Scholar] [CrossRef]
- Roque, A.C.; Silva, C.S.; Taipa, M.A. Affinity-based methodologies and ligands for antibody purification: Advances and perspectives. J. Chromatogr. A 2007, 1160, 44–55. [Google Scholar] [CrossRef]
- Moks, T.; Abrahmsen, L.; Nilsson, B.; Hellman, U.; Sjoquist, J.; Uhlen, M. Staphylococcal protein A consists of five IgG-binding domains. Eur. J. Biochem. 1986, 156, 637–643. [Google Scholar] [CrossRef]
- Gong, Y.; Zhang, L.; Li, J.; Feng, S.; Deng, H. Development of the Double Cyclic Peptide Ligand for Antibody Purification and Protein Detection. Bioconjug. Chem. 2016, 27, 1569–1573. [Google Scholar] [CrossRef]
- Saha, K.; Bender, F.; Gizeli, E. Comparative study of IgG binding to proteins G and A: Nonequilibrium kinetic and binding constant determination with the acoustic waveguide device. Anal. Chem. 2003, 75, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Kang, H.J.; Lee, J.M.; Jung, S.O.; Yun, W.S.; Chung, S.J.; Chung, B.H. Controlled antibody immobilization onto immunoanalytical platforms by synthetic peptide. Anal. Biochem. 2008, 374, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Sockolosky, J.T.; Kivimae, S.; Szoka, F.C. Fusion of a short peptide that binds immunoglobulin G to a recombinant protein substantially increases its plasma half-life in mice. PLoS ONE 2014, 9, e102566. [Google Scholar] [CrossRef]
- Muguruma, K.; Yakushiji, F.; Kawamata, R.; Akiyama, D.; Arima, R.; Shirasaka, T.; Kikkawa, Y.; Taguchi, A.; Takayama, K.; Fukuhara, T.; et al. Novel Hybrid Compound of a Plinabulin Prodrug with an IgG Binding Peptide for Generating a Tumor Selective Noncovalent-Type Antibody-Drug Conjugate. Bioconjugate Chem. 2016, 27, 1606–1613. [Google Scholar] [CrossRef]
- Choe, W.; Durgannavar, T.A.; Chung, S.J. Fc-Binding Ligands of Immunoglobulin G: An Overview of High Affinity Proteins and Peptides. Materials 2016, 9, 994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruljec, N.; Bratkovic, T. Alternative Affinity Ligands for Immunoglobulins. Bioconjug. Chem. 2017, 28, 2009–2030. [Google Scholar] [CrossRef]
- Deisenhofer, J. Crystallographic refinement and atomic models of a human Fc fragment and its complex with fragment B of protein A from Staphylococcus aureus at 2.9- and 2.8-A resolution. Biochemistry 1981, 20, 2361–2370. [Google Scholar] [CrossRef]
- Akerstrom, B.; Bjorck, L. A physicochemical study of protein G, a molecule with unique immunoglobulin G-binding properties. J. Biol. Chem. 1986, 261, 10240–10247. [Google Scholar] [CrossRef]
- Braisted, A.C.; Wells, J.A. Minimizing a binding domain from protein A. Proc. Natl. Acad. Sci. USA 1996, 93, 5688–5692. [Google Scholar] [CrossRef] [Green Version]
- Pham, N.B.; Liu, W.; Schueller, N.R.; Gawalt, E.S.; Fan, Y.; Meng, W.S. Toward reducing biomaterial antigenic potential: A miniaturized Fc-binding domain for local deposition of antibodies. Biomater. Sci. 2019, 7, 760–772. [Google Scholar] [CrossRef]
- Yang, H.; Gurgel, P.V.; Williams, D.K., Jr.; Bobay, B.G.; Cavanagh, J.; Muddiman, D.C.; Carbonell, R.G. Binding site on human immunoglobulin G for the affinity ligand HWRGWV. J. Mol. Recognit. 2010, 23, 271–282. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Gurgel, P.V.; Carbonell, R.G. Purification of human immunoglobulin G via Fc-specific small peptide ligand affinity chromatography. J. Chromatogr. A 2009, 1216, 910–918. [Google Scholar] [CrossRef]
- Menegatti, S.; Bobay, B.G.; Ward, K.L.; Islam, T.; Kish, W.S.; Naik, A.D.; Carbonell, R.G. Design of protease-resistant peptide ligands for the purification of antibodies from human plasma. J. Chromatogr. A 2016, 1445, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Reese, H.R.; Xiao, X.; Shanahan, C.C.; Chu, W.; Van Den Driessche, G.A.; Fourches, D.; Carbonell, R.G.; Hall, C.K.; Menegatti, S. Novel peptide ligands for antibody purification provide superior clearance of host cell protein impurities. J. Chromatogr. A 2020, 1625, 461237. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.-W.; Liu, F.-F.; Shi, Q.-H.; Dong, X.-Y.; Sun, Y. Biomimetic design of affinity peptide ligands for human IgG based on protein A-IgG complex. Biochem. Eng. J. 2014, 88, 1–11. [Google Scholar] [CrossRef]
- Zhao, W.W.; Shi, Q.H.; Sun, Y. FYWHCLDE-based affinity chromatography of IgG: Effect of ligand density and purifications of human IgG and monoclonal antibody. J. Chromatogr. A 2014, 1355, 107–114. [Google Scholar] [CrossRef]
- Zhao, W.W.; Liu, F.F.; Shi, Q.H.; Sun, Y. Octapeptide-based affinity chromatography of human immunoglobulin G: Comparisons of three different ligands. J. Chromatogr. A 2014, 1359, 100–111. [Google Scholar] [CrossRef]
- Sugita, T.; Katayama, M.; Okochi, M.; Kato, R.; Ichihara, T.; Honda, H. Screening of peptide ligands that bind to the Fc region of IgG using peptide array and its application to affinity purification of antibody. Biochem. Eng. J. 2013, 79, 33–40. [Google Scholar] [CrossRef]
- Kruljec, N.; Molek, P.; Hodnik, V.; Anderluh, G.; Bratkovic, T. Development and Characterization of Peptide Ligands of Immunoglobulin G Fc Region. Bioconjug. Chem. 2018, 29, 2763–2775. [Google Scholar] [CrossRef]
- Wei, Y.; Xu, J.; Zhang, L.; Fu, Y.; Xu, X. Development of novel small peptide ligands for antibody purification. RSC Adv. 2015, 5, 67093–67101. [Google Scholar] [CrossRef]
- Tsai, C.W.; Jheng, S.L.; Chen, W.Y.; Ruaan, R.C. Strategy of Fc-recognizable Peptide ligand design for oriented immobilization of antibody. Anal. Chem. 2014, 86, 2931–2938. [Google Scholar] [CrossRef]
- DeLano, W.L.; Ultsch, M.H.; de Vos, A.M.; Wells, J.A. Convergent solutions to binding at a protein-protein interface. Science 2000, 287, 1279–1283. [Google Scholar] [CrossRef] [Green Version]
- Dias, R.L.; Fasan, R.; Moehle, K.; Renard, A.; Obrecht, D.; Robinson, J.A. Protein ligand design: From phage display to synthetic protein epitope mimetics in human antibody Fc-binding peptidomimetics. J. Am. Chem. Soc. 2006, 128, 2726–2732. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Dowd, V.; Stewart, D.J.; Burton, S.J.; Lowe, C.R. Design, synthesis, and application of a protein A mimetic. Nat. Biotechnol. 1998, 16, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Teng, S.F.; Sproule, K.; Husain, A.; Lowe, C.R. Affinity chromatography on immobilized “biomimetic” ligands: Synthesis, immobilization and chromatographic assessment of an immunoglobulin G-binding ligand. J. Chromatogr. B Biomed. Sci. Appl. 2000, 740, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Li, M.Y.; Wang, H.Q.; Lin, D.Q.; Qu, J.P. Antibody-ligand interactions for hydrophobic charge-induction chromatography: A surface plasmon resonance study. Langmuir 2015, 31, 3422–3430. [Google Scholar] [CrossRef]
- Hansel, T.T.; Kropshofer, H.; Singer, T.; Mitchell, J.A.; George, A.J. The safety and side effects of monoclonal antibodies. Nat. Rev. Drug Discov. 2010, 9, 325–338. [Google Scholar] [CrossRef]
- Herrmann, J. Adverse cardiac effects of cancer therapies: Cardiotoxicity and arrhythmia. Nat. Rev. Cardiol. 2020, 17, 474–502. [Google Scholar] [CrossRef]
- Schneider, A.F.L.; Kithil, M.; Cardoso, M.C.; Lehmann, M.; Hackenberger, C.P.R. Cellular uptake of large biomolecules enabled by cell-surface-reactive cell-penetrating peptide additives. Nat. Chem. 2021, 13, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Jing, W.; Chen, Y.; Wang, G.; Abdalla, M.; Gao, L.; Han, M.; Shi, C.; Li, A.; Sun, P.; et al. Intracavity generation of glioma stem cell-specific CAR macrophages primes locoregional immunity for postoperative glioblastoma therapy. Sci. Transl. Med. 2022, 14, eabn1128. [Google Scholar] [CrossRef]
- Xu, J.; Li, Z.; Fan, Q.; Lv, J.; Li, Y.; Cheng, Y. Dynamic Polymer Amphiphiles for Efficient Intracellular and In Vivo Protein Delivery. Adv. Mater. 2021, 33, e2104355. [Google Scholar] [CrossRef]
- Liao, J.; Huang, H. Review on Magnetic Natural Polymer Constructed Hydrogels as Vehicles for Drug Delivery. Biomacromolecules 2020, 21, 2574–2594. [Google Scholar] [CrossRef]
- Kaplan, J.A.; Barthelemy, P.; Grinstaff, M.W. Self-assembled nanofiber hydrogels for mechanoresponsive therapeutic anti-TNFalpha antibody delivery. Chem. Commun. 2016, 52, 5860–5863. [Google Scholar] [CrossRef]
- Jeong, B.; Bae, Y.H.; Kim, S.W. Drug release from biodegradable injectable thermosensitive hydrogel of PEG-PLGA-PEG triblock copolymers. J. Control. Release 2000, 63, 155–163. [Google Scholar] [CrossRef]
- Huynh, V.; Wylie, R.G. Competitive Affinity Release for Long-Term Delivery of Antibodies from Hydrogels. Angew. Chem. Int. Ed. Engl. 2018, 57, 3406–3410. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wong-Noonan, S.; Pham, N.B.; Pradhan, I.; Spigelmyer, A.; Funk, R.; Nedzesky, J.; Cohen, H.; Gawalt, E.S.; Fan, Y.; et al. A genetically engineered Fc-binding amphiphilic polypeptide for congregating antibodies in vivo. Acta Biomater. 2019, 88, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lock, L.L.; Wang, Y.; Ou, S.H.; Stern, D.; Schon, A.; Freire, E.; Xu, X.; Ghose, S.; Li, Z.J.; et al. Bioinspired supramolecular engineering of self-assembling immunofibers for high affinity binding of immunoglobulin G. Biomaterials 2018, 178, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Metters, A.T. Bifunctional monolithic affinity hydrogels for dual-protein delivery. Biomacromolecules 2008, 9, 789–795. [Google Scholar] [CrossRef]
- Lin, C.C.; Metters, A.T. Enhanced protein delivery from photopolymerized hydrogels using a pseudospecific metal chelating ligand. Pharm. Res. 2006, 23, 614–622. [Google Scholar] [CrossRef]
- Wen, Y.; Kolonich, H.R.; Kruszewski, K.M.; Giannoukakis, N.; Gawalt, E.S.; Meng, W.S. Retaining antibodies in tumors with a self-assembling injectable system. Mol. Pharm. 2013, 10, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wen, Y.; George, A.M.; Steinbach, A.M.; Phillips, B.E.; Giannoukakis, N.; Gawalt, E.S.; Meng, W.S. A peptide-based material platform for displaying antibodies to engage T cells. Biomaterials 2011, 32, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Liew, S.S.; Zhang, C.W.; Du, W.; Lang, W.; Yao, C.C.Y.; Li, L.; Ge, J.; Yao, S.Q. Cell-Permeant Bioadaptors for Cytosolic Delivery of Native Antibodies: A “Mix-and-Go” Approach. ACS Cent. Sci. 2020, 6, 2362–2376. [Google Scholar] [CrossRef]
- Itakura, S.; Hama, S.; Ikeda, H.; Mitsuhashi, N.; Majima, E.; Kogure, K. Effective capture of proteins inside living cells by antibodies indirectly linked to a novel cell-penetrating polymer-modified protein A derivative. FEBS J. 2015, 282, 142–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mie, M.; Takahashi, F.; Funabashi, H.; Yanagida, Y.; Aizawa, M.; Kobatake, E. Intracellular delivery of antibodies using TAT fusion protein A. Biochem. Biophys. Res. Commun. 2003, 310, 730–734. [Google Scholar] [CrossRef]
- Kang, H.J.; Choe, W.; Kim, B.M.; Chung, S.J. IgG Fc-binding peptide (FcBP)-tat conjugate as a smart antibody carrier into live cells. Macromol. Res. 2015, 23, 876–881. [Google Scholar] [CrossRef]
- Fu, A.; Tang, R.; Hardie, J.; Farkas, M.E.; Rotello, V.M. Promises and pitfalls of intracellular delivery of proteins. Bioconjug. Chem. 2014, 25, 1602–1608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narang, A.S.; Chang, R.K.; Hussain, M.A. Pharmaceutical development and regulatory considerations for nanoparticles and nanoparticulate drug delivery systems. J. Pharm. Sci. 2013, 102, 3867–3882. [Google Scholar] [CrossRef]
- Sousa, F.; Castro, P.; Fonte, P.; Kennedy, P.J.; Neves-Petersen, M.T.; Sarmento, B. Nanoparticles for the delivery of therapeutic antibodies: Dogma or promising strategy? Expert Opin. Drug Deliv. 2017, 14, 1163–1176. [Google Scholar] [CrossRef]
- Freund, G.; Sibler, A.P.; Desplancq, D.; Oulad-Abdelghani, M.; Vigneron, M.; Gannon, J.; Van Regenmortel, M.H.; Weiss, E. Targeting endogenous nuclear antigens by electrotransfer of monoclonal antibodies in living cells. mAbs 2013, 5, 518–522. [Google Scholar] [CrossRef] [Green Version]
- Chong, S.E.; Oh, J.H.; Min, K.; Park, S.; Choi, S.; Ahn, J.H.; Chun, D.; Lee, H.H.; Yu, J.; Lee, Y. Intracellular delivery of immunoglobulin G at nanomolar concentrations with domain Z-fused multimeric alpha-helical cell penetrating peptides. J. Control. Release 2021, 330, 161–172. [Google Scholar] [CrossRef]
- Lim, S.I.; Lukianov, C.I.; Champion, J.A. Self-assembled protein nanocarrier for intracellular delivery of antibody. J. Control. Release 2017, 249, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, R.M.; Mele, S.; Cheung, A.; Larcombe-Young, D.; Bucaite, G.; Sachouli, E.; Zlatareva, I.; Morad, H.O.J.; Marlow, R.; McDonnell, J.M.; et al. Rapid conjugation of antibodies to toxins to select candidates for the development of anticancer Antibody-Drug Conjugates (ADCs). Sci. Rep. 2020, 10, 8869. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, Y.; Mendelsohn, B.A. An overview of process development for antibody-drug conjugates produced by chemical conjugation technology. Expert Opin. Biol. Ther 2021, 21, 963–975. [Google Scholar] [CrossRef]
- Rudnick, S.I.; Lou, J.; Shaller, C.C.; Tang, Y.; Klein-Szanto, A.J.; Weiner, L.M.; Marks, J.D.; Adams, G.P. Influence of affinity and antigen internalization on the uptake and penetration of Anti-HER2 antibodies in solid tumors. Cancer Res. 2011, 71, 2250–2259. [Google Scholar] [CrossRef] [Green Version]
- Zimmerman, E.S.; Heibeck, T.H.; Gill, A.; Li, X.; Murray, C.J.; Madlansacay, M.R.; Tran, C.; Uter, N.T.; Yin, G.; Rivers, P.J.; et al. Production of site-specific antibody-drug conjugates using optimized non-natural amino acids in a cell-free expression system. Bioconjug. Chem. 2014, 25, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Nervig, C.S.; Owen, S.C. Affinity-bound antibody-drug conjugates. Nat. Biomed. Eng. 2019, 3, 850–851. [Google Scholar] [CrossRef]
- Maso, K.; Montagner, I.M.; Grigoletto, A.; Schiavon, O.; Rosato, A.; Pasut, G. A non-covalent antibody complex for the delivery of anti-cancer drugs. Eur. J. Pharm. Biopharm. 2019, 142, 49–60. [Google Scholar] [CrossRef]
- Nakase, I.; Aoki, A.; Sakai, Y.; Hirase, S.; Ishimura, M.; Takatani-Nakase, T.; Hattori, Y.; Kirihata, M. Antibody-Based Receptor Targeting Using an Fc-Binding Peptide-Dodecaborate Conjugate and Macropinocytosis Induction for Boron Neutron Capture Therapy. ACS Omega 2020, 5, 22731–22738. [Google Scholar] [CrossRef]
- Torchilin, V.P. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat. Rev. Drug Discov. 2014, 13, 813–827. [Google Scholar] [CrossRef] [Green Version]
- Trapani, G.; Denora, N.; Trapani, A.; Laquintana, V. Recent advances in ligand targeted therapy. J. Drug Target. 2012, 20, 1–22. [Google Scholar] [CrossRef]
- Sirskyj, D.; Weltzin, R.; Golshani, A.; Anderson, D.; Bozic, J.; Diaz-Mitoma, F.; Azizi, A. Detection of influenza A and B neutralizing antibodies in vaccinated ferrets and macaques using specific biotin-streptavidin conjugated antibodies. J. Virol. Methods 2010, 163, 459–464. [Google Scholar] [CrossRef]
- Dostalova, S.; Cerna, T.; Hynek, D.; Koudelkova, Z.; Vaculovic, T.; Kopel, P.; Hrabeta, J.; Heger, Z.; Vaculovicova, M.; Eckschlager, T.; et al. Site-Directed Conjugation of Antibodies to Apoferritin Nanocarrier for Targeted Drug Delivery to Prostate Cancer Cells. ACS Appl. Mater. Interfaces 2016, 8, 14430–14441. [Google Scholar] [CrossRef]
- Eloy, J.O.; Petrilli, R.; Trevizan, L.N.F.; Chorilli, M. Immunoliposomes: A review on functionalization strategies and targets for drug delivery. Colloids Surf. B Biointerfaces 2017, 159, 454–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hama, S.; Sakai, M.; Itakura, S.; Majima, E.; Kogure, K. Rapid modification of antibodies on the surface of liposomes composed of high-affinity protein A-conjugated phospholipid for selective drug delivery. Biochem. Biophys. Rep. 2021, 27, 101067. [Google Scholar] [CrossRef] [PubMed]
- Iijima, M.; Araki, K.; Liu, Q.; Somiya, M.; Kuroda, S. Oriented immobilization to nanoparticles enhanced the therapeutic efficacy of antibody drugs. Acta Biomater. 2019, 86, 373–380. [Google Scholar] [CrossRef]
- Yamada, T.; Iwasaki, Y.; Tada, H.; Iwabuki, H.; Chuah, M.K.; VandenDriessche, T.; Fukuda, H.; Kondo, A.; Ueda, M.; Seno, M.; et al. Nanoparticles for the delivery of genes and drugs to human hepatocytes. Nat. Biotechnol. 2003, 21, 885–890. [Google Scholar] [CrossRef]
- Tsutsui, Y.; Tomizawa, K.; Nagita, M.; Michiue, H.; Nishiki, T.; Ohmori, I.; Seno, M.; Matsui, H. Development of bionanocapsules targeting brain tumors. J. Control. Release 2007, 122, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Dostalova, S.; Polanska, H.; Svobodova, M.; Balvan, J.; Krystofova, O.; Haddad, Y.; Krizkova, S.; Masarik, M.; Eckschlager, T.; Stiborova, M.; et al. Prostate-Specific Membrane Antigen-Targeted Site-Directed Antibody-Conjugated Apoferritin Nanovehicle Favorably Influences In Vivo Side Effects of Doxorubicin. Sci. Rep. 2018, 8, 8867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, W.; Smith, P.N.; Koepsel, R.R.; Andersen, J.D.; Baker, S.L.; Zhang, L.; Carmali, S.; Myerson, J.W.; Muzykantov, V.; Russell, A.J. Erythrocytes as carriers of immunoglobulin-based therapeutics. Acta Biomater. 2020, 101, 422–435. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tatematsu, K.; Somiya, M.; Iijima, M.; Kuroda, S. Development of a macrophage-targeting and phagocytosis-inducing bio-nanocapsule-based nanocarrier for drug delivery. Acta Biomater. 2018, 73, 412–423. [Google Scholar] [CrossRef]
- Matsuo, H.; Yoshimoto, N.; Iijima, M.; Niimi, T.; Jung, J.; Jeong, S.Y.; Choi, E.K.; Sewaki, T.; Arakawa, T.; Kuroda, S. Engineered hepatitis B virus surface antigen L protein particles for in vivo active targeting of splenic dendritic cells. Int. J. Nanomed. 2012, 7, 3341–3350. [Google Scholar] [CrossRef] [Green Version]
- Kurata, N.; Shishido, T.; Muraoka, M.; Tanaka, T.; Ogino, C.; Fukuda, H.; Kondo, A. Specific protein delivery to target cells by antibody-displaying bionanocapsules. J. Biochem. 2008, 144, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.N.; Zeng, J.Y.; Su, H.; Qu, Q.M. Recombinant VLP-Z of JC Polyomavirus: A Novel Vector for Targeting Gene Delivery. Intervirology 2015, 58, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Shimojo, K.; Niide, T.; Taguchi, T.; Naganawa, H.; Kamiya, N.; Goto, M. Facile, rapid and efficient biofabrication of gold nanoparticles decorated with functional proteins. Analyst 2012, 137, 2300–2303. [Google Scholar] [CrossRef]
- Korokhov, N.; Mikheeva, G.; Krendelshchikov, A.; Belousova, N.; Simonenko, V.; Krendelshchikova, V.; Pereboev, A.; Kotov, A.; Kotova, O.; Triozzi, P.L.; et al. Targeting of adenovirus via genetic modification of the viral capsid combined with a protein bridge. J. Virol. 2003, 77, 12931–12940. [Google Scholar] [CrossRef] [Green Version]
- Kawashima, R.; Abei, M.; Fukuda, K.; Nakamura, K.; Murata, T.; Wakayama, M.; Seo, E.; Hasegawa, N.; Mizuguchi, H.; Obata, Y.; et al. EpCAM- and EGFR-targeted selective gene therapy for biliary cancers using Z33-fiber-modified adenovirus. Int. J. Cancer 2011, 129, 1244–1253. [Google Scholar] [CrossRef]
- Tanaka, T.; Huang, J.; Hirai, S.; Kuroki, M.; Kuroki, M.; Watanabe, N.; Tomihara, K.; Kato, K.; Hamada, H. Carcinoembryonic antigen-targeted selective gene therapy for gastric cancer through FZ33 fiber-modified adenovirus vectors. Clin. Cancer Res. 2006, 12, 3803–3813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neupane, S.; Pan, Y.; Takalkar, S.; Bentz, K.; Farmakes, J.; Xu, Y.; Chen, B.; Liu, G.; Qian, S.Y.; Yang, Z. Probing the Aggregation Mechanism of Gold Nanoparticles Triggered by a Globular Protein. J. Phys. Chem. C 2017, 121, 1377–1386. [Google Scholar] [CrossRef]
- Lyski, R.D.; Bou, L.B.; Lau, U.Y.; Meyer, D.W.; Cochran, J.H.; Okeley, N.M.; Emmerton, K.K.; Zapata, F.; Simmons, J.K.; Trueblood, E.S.; et al. Development of Novel Antibody-Camptothecin Conjugates. Mol. Cancer Ther 2021, 20, 329–339. [Google Scholar] [CrossRef]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
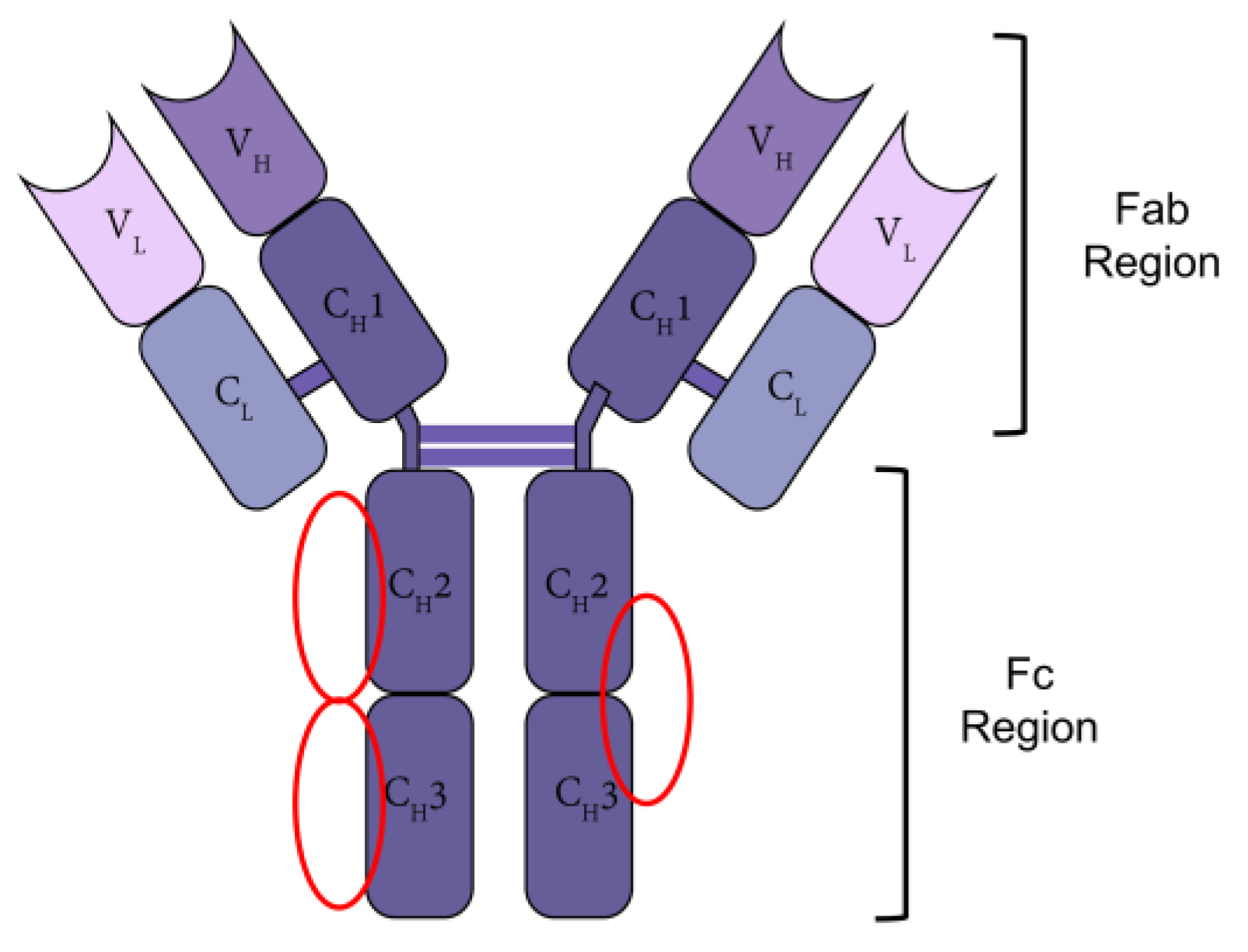




| Category | Name | Source | Kd | Binding Site |
|---|---|---|---|---|
| Natural Proteins | Protein A [17] | Staphylococcus aureus | 7.14 × 10−7 M | Fc region (CH2–CH3 interface) |
| Fab region (VH domain) | ||||
| Protein G [18] | Streptococcal groups C and G | 1.49 × 10−8 M | Fc region (CH2 and CH3 interface) | |
| Fab region (CH1) | ||||
| Engineered Variants of Natural Proteins | Z domain [19] | B domain of the protein A | 1 × 10−9 M | Fc region |
| Z38 [20] | B domain of the protein A | 2 × 10−8 M | Fc region (CH2 and CH3 interface) | |
| Z33 [19] | Z domain of the protein A | 4.3 × 10−9 M | Fc region | |
| Short Peptides | HWRGWV [21] | synthetic solid-phase random hexamer peptide library | 3.8 × 10−6 M | CH3 domain |
| HYFKFD [22] | 1.1 × 10−5 M | |||
| HFRRHL [23] | 2.6 × 10−5 M | |||
| HWCitGWV [23] | 1.08 × 10−4 M | |||
| WQRHGI [24] | peptide search algorithm | 3.5 × 10−7 M | CH2 domain | |
| FYWHCLDE [25] | biomimetic design strategy | 2.4–3.6 × 10−6 M | CH2–CH3 interface | |
| FYCHWALE [26] | 6.1 × 10−6 M | |||
| FYCHTIDE [27] | 5.7 × 10−6 M | |||
| NKFRGKYK [28] | spot-synthesized peptide array | 1.16 × 10−7 M | Fc region | |
| NARKFYKG [28] | 1.54 × 10−7 M | |||
| GSYWYQVWF [29] | phage-display library of random dodecapeptides | 0.6 × 10−6 M | CH2–CH3 interface | |
| DWHW [30] | molecular simulation | 1.1 × 10−5 M | Fc region | |
| CEWW [30] | 1.2 × 10−5 M | |||
| HEYW [30] | 1.7 × 10−5 M | |||
| RRGW [31] | computer design strategy | 0.5 × 10−9 M | Hydrophobic spots on the surface of lower Fc region | |
| Fc-III * [32] | phage-display cyclic peptide library | 1.85 × 10−7 M | CH2–CH3 interface | |
| FcBP-2 [33] | peptidomimetics of Fc-III | 1.8 × 10−9 M | ||
| Fc-Ⅲ-4C [10] | rational design of Fc-III | 2.45 × 10−9 M | ||
| Synthetic Low-Molecular-Weight Ligands | ApA [34] | nonpeptidyl mimic for Protein A | 1 × 10−4 M | Fc region (CH2–CH3 interface) |
| Ligand 22/8 [35] | IgG-binding ligand library | 0.714 × 10−5 M | Fc region (CH2–CH3 interface) | |
| 4-mercaptoethyl-pyridine(MEP) [36] | Hydrophobic charge-induction chromatography (HCIC) | 1.16 × 10−7 M | Fc region and Fab region conserved binding sites |
| Antibody | Linker | Drug | Application |
|---|---|---|---|
| Trastuzumab [14] | Z33 | Plinabulin | Tumor-selective non-covalent ADC can be formed in situ and has potential as an antitumor agent. |
| Trastuzumab [67] | Protein G | Tubulysin A | Obtain more homogeneous non-covalent ADC with significant cytotoxic effect. |
| Cetuximab [68] | Z33 | Dodecaborate | Achieve the intracellular delivery of boron compounds in BNCT. |
| Cetuximab Trastuzumab [6] | MEP | Gemcitabine | ADCs for the targeted delivery of cytotoxic payloads. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; He, B.; Zhang, H.; Wang, X.; Zhang, Q.; Dai, W. IgG Fc Affinity Ligands and Their Applications in Antibody-Involved Drug Delivery: A Brief Review. Pharmaceutics 2023, 15, 187. https://doi.org/10.3390/pharmaceutics15010187
Yang C, He B, Zhang H, Wang X, Zhang Q, Dai W. IgG Fc Affinity Ligands and Their Applications in Antibody-Involved Drug Delivery: A Brief Review. Pharmaceutics. 2023; 15(1):187. https://doi.org/10.3390/pharmaceutics15010187
Chicago/Turabian StyleYang, Chang, Bing He, Hua Zhang, Xueqing Wang, Qiang Zhang, and Wenbing Dai. 2023. "IgG Fc Affinity Ligands and Their Applications in Antibody-Involved Drug Delivery: A Brief Review" Pharmaceutics 15, no. 1: 187. https://doi.org/10.3390/pharmaceutics15010187





