Curcumin-Based Nanomedicines in the Treatment of Inflammatory and Immunomodulated Diseases: An Evidence-Based Comprehensive Review
Abstract
1. Introduction
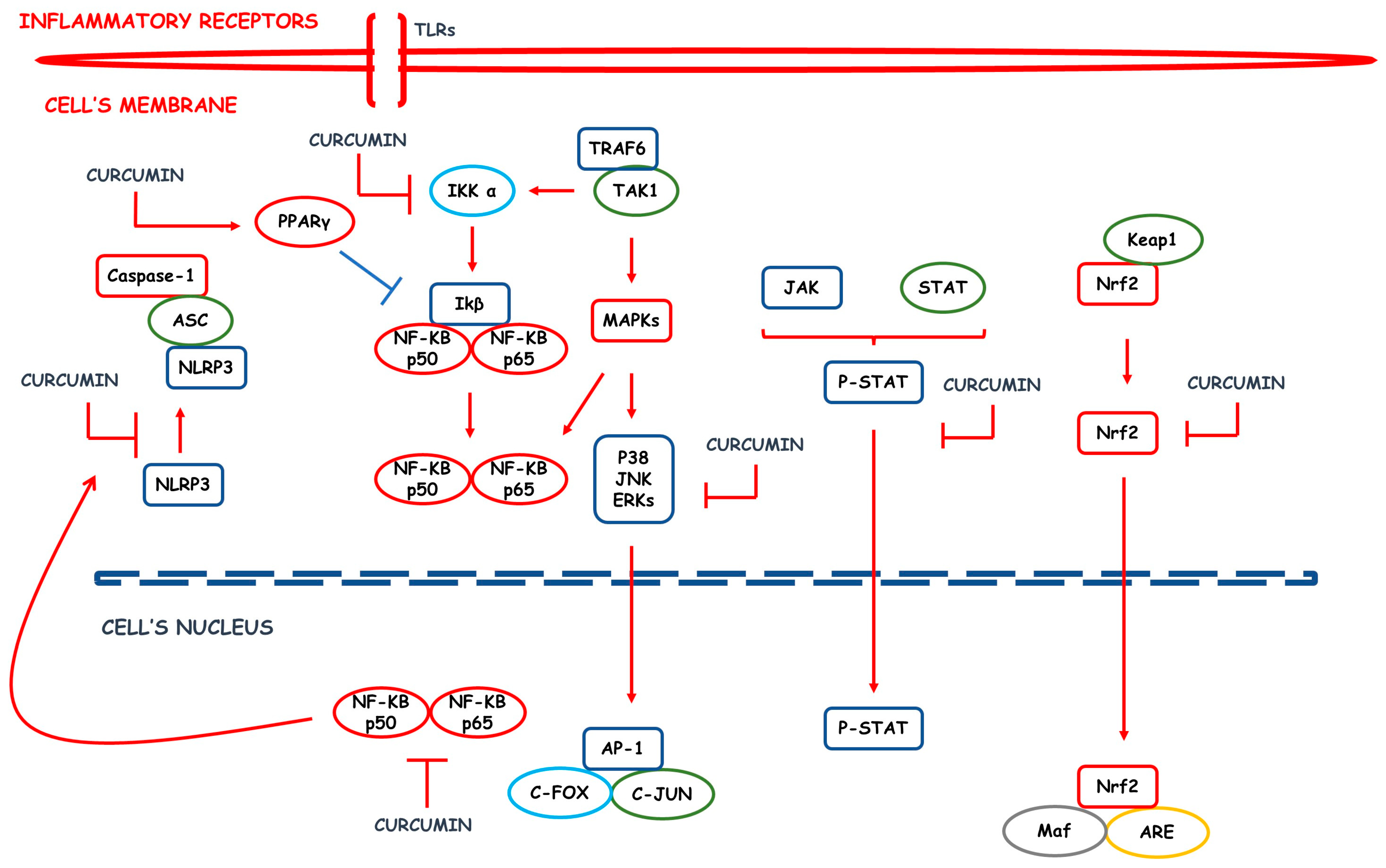
2. Inflammatory Process: An Overview
3. CUR, a Phenolic Compound Derived from Curcuma longa
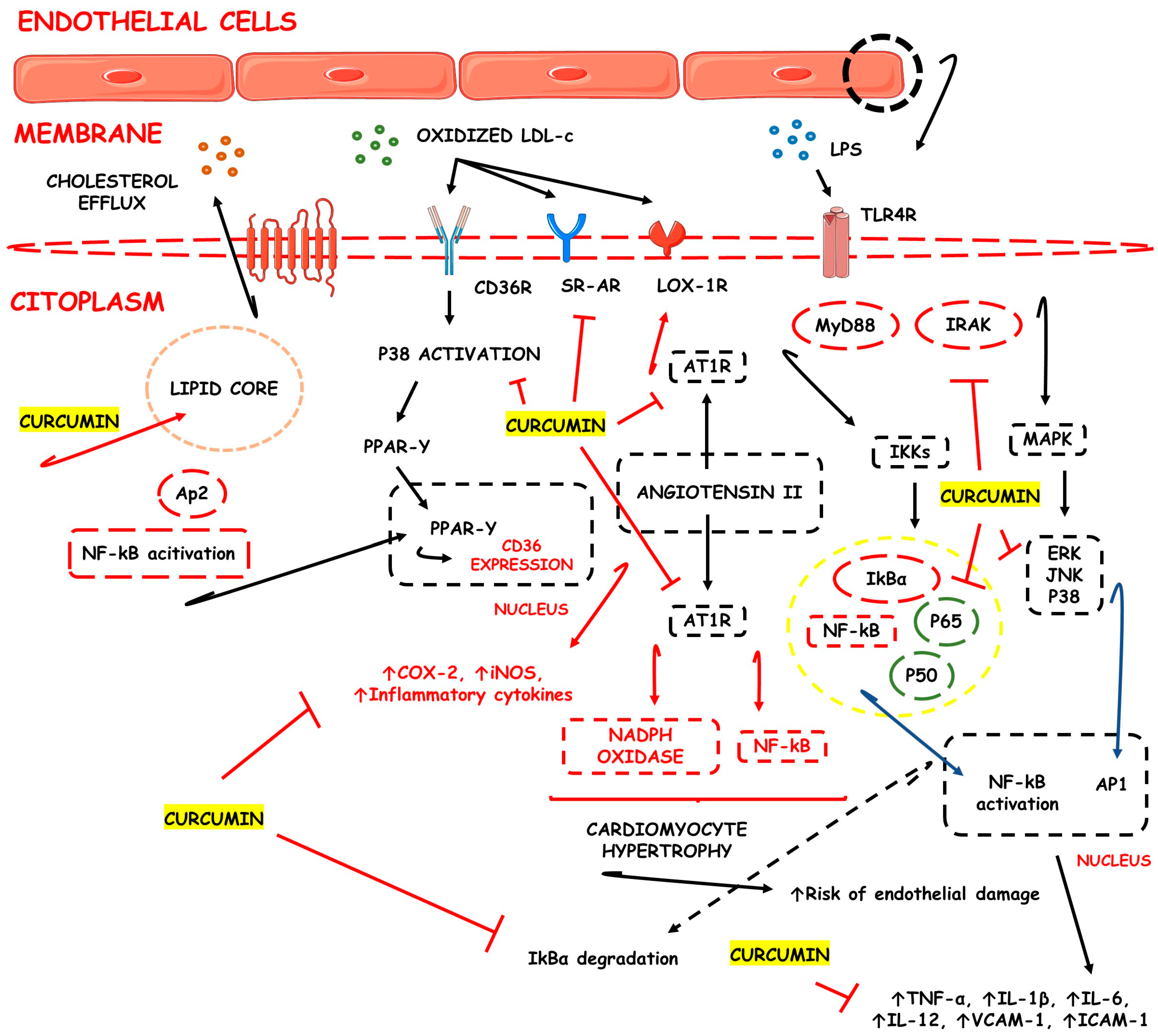
3.1. CUR-Based Nanomedicines in Atherosclerosis
3.2. CUR-Based Nanomedicines in Rheumatoid Arthritis
3.3. CUR-Based Nanomedicines in Osteoarthritis
3.4. CUR-Based Nanomedicines and Neurodegenerative Diseases
3.4.1. Alzheimer’s Disease
3.4.2. Parkinson’s Disease
3.4.3. Multiple Sclerosis
3.4.4. Huntington’s Disease
3.5. CUR-Based Nanomedicines in Epilepsy
3.6. CUR-Based Nanomedicines in Inflammatory Bowel Diseases (IBD)
3.7. CUR-Based Nanomedicines in Psoriasis
3.8. CUR-Based Nanomedicines in Liver Fibrosis
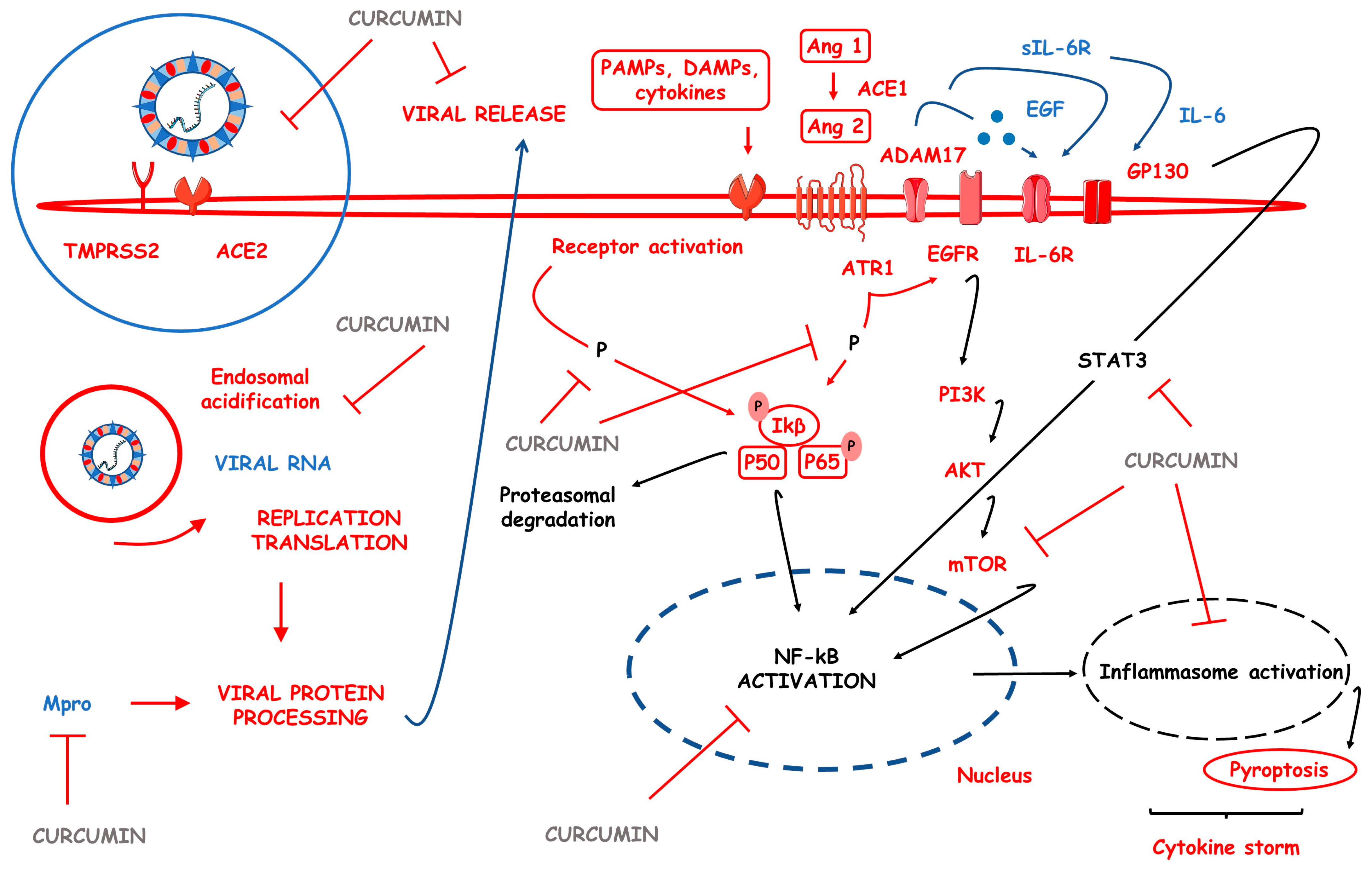
3.9. CUR-Based Nanomedicines in COVID-19
4. Limitations and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quispe, C.; Cruz-Martins, N.; Manca, M.L.; Manconi, M.; Sytar, O.; Hudz, N.; Shanaida, M.; Kumar, M.; Taheri, Y.; Martorell, M.; et al. Nano-Derived Therapeutic Formulations with Curcumin in Inflammation-Related Diseases. Oxid. Med. Cell Longev. 2021, 2021, 3149223. [Google Scholar] [CrossRef]
- Lee, K.H.; Ahn, B.S.; Cha, D.; Jang, W.W.; Choi, E.; Park, S.; Park, J.H.; Oh, J.; Jung, D.E.; Park, H.; et al. Understanding the immunopathogenesis of autoimmune diseases by animal studies using gene modulation: A comprehensive review. Autoimmun. Rev. 2020, 19, 102469. [Google Scholar] [CrossRef] [PubMed]
- Marton, L.T.; Barbalho, S.M.; Sloan, K.P.; Sloan, L.A.; Goulart, R.A.; Araújo, A.C.; Bechara, M.D. Curcumin, autoimmune and inflammatory diseases: Going beyond conventional therapy—A systematic review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2140–2157. [Google Scholar] [CrossRef] [PubMed]
- Akaberi, M.; Sahebkar, A.; Emami, S.A. Turmeric and Curcumin: From Traditional to Modern Medicine. Adv. Exp. Med. Biol. 2021, 1291, 15–39. [Google Scholar] [CrossRef]
- Hafez Ghoran, S.; Calcaterra, A.; Abbasi, M.; Taktaz, F.; Nieselt, K.; Babaei, E. Curcumin-Based Nanoformulations: A Promising Adjuvant towards Cancer Treatment. Molecules 2022, 27, 5236. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Dong, Z.; Chen, M.; Feng, L. Phenolic molecules constructed nanomedicine for innovative cancer treatment. Coord. Chem. Rev. 2021, 439, 213912. [Google Scholar] [CrossRef]
- Yavarpour-Bali, H.; Ghasemi-Kasman, M.; Pirzadeh, M. Curcumin-loaded nanoparticles: A novel therapeutic strategy in treatment of central nervous system disorders. Int. J. Nanomed. 2019, 14, 4449–4460. [Google Scholar] [CrossRef]
- Lakshmanan, V.K.; Jindal, S.; Packirisamy, G.; Ojha, S.; Lian, S.; Kaushik, A.; Alzarooni, A.; Metwally, Y.A.F.; Thyagarajan, S.P.; Do Jung, Y.; et al. Nanomedicine-based cancer immunotherapy: Recent trends and future perspectives. Cancer Gene Ther. 2021, 28, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Moballegh Nasery, M.; Abadi, B.; Poormoghadam, D.; Zarrabi, A.; Keyhanvar, P.; Khanbabaei, H.; Ashrafizadeh, M.; Mohammadinejad, R.; Tavakol, S.; Sethi, G. Curcumin Delivery Mediated by Bio-Based Nanoparticles: A Review. Molecules 2020, 25, 689. [Google Scholar] [CrossRef]
- Salehi, B.; Del Prado-Audelo, M.L.; Cortés, H.; Leyva-Gómez, G.; Stojanović-Radić, Z.; Singh, Y.D.; Patra, J.K.; Das, G.; Martins, N.; Martorell, M.; et al. Therapeutic Applications of Curcumin Nanomedicine Formulations in Cardiovascular Diseases. J. Clin. Med. 2020, 9, 746. [Google Scholar] [CrossRef]
- Kumar, V.; Abbas, A.K.; Aster, J.C. Robbins & Cotran Pathologic Basis of Disease; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Hammer, G.D.; McPhee, S.J. Pathophysiology of Disease: An Introduction to Clinical Medicine 8E; McGraw-Hill Education: New York, NY, USA, 2018. [Google Scholar]
- Barbalho, S.M.; Bosso, H.; Salzedas-Pescinini, L.M.; de Alvares Goulart, R. Green tea: A possibility in the therapeutic approach of inflammatory bowel diseases?: Green tea and inflammatory bowel diseases. Complement. Ther. Med. 2019, 43, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K. Chronic Inflammation as an Immunological Abnormality and Effectiveness of Exercise. Biomolecules 2019, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Zindel, J.; Kubes, P. DAMPs, PAMPs, and LAMPs in Immunity and Sterile Inflammation. Annu. Rev. Pathol. 2020, 15, 493–518. [Google Scholar] [CrossRef]
- Krysko, D.V.; Garg, A.D.; Kaczmarek, A.; Krysko, O.; Agostinis, P.; Vandenabeele, P. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 2012, 12, 860–875. [Google Scholar] [CrossRef]
- Qi, X.; Yu, Y.; Sun, R.; Huang, J.; Liu, L.; Yang, Y.; Rui, T.; Sun, B. Identification and characterization of neutrophil heterogeneity in sepsis. Crit. Care 2021, 25, 50. [Google Scholar] [CrossRef] [PubMed]
- Ratter-Rieck, J.M.; Maalmi, H.; Trenkamp, S.; Zaharia, O.P.; Rathmann, W.; Schloot, N.C.; Straßburger, K.; Szendroedi, J.; Herder, C.; Roden, M. Leukocyte Counts and T-Cell Frequencies Differ Between Novel Subgroups of Diabetes and Are Associated With Metabolic Parameters and Biomarkers of Inflammation. Diabetes 2021, 70, 2652–2662. [Google Scholar] [CrossRef]
- Liu, G.; Zhou, X.; Zhang, L.; Zou, Y.; Xue, J.; Xia, R.; Abuduxiku, N.; Xuejing, G.; Liu, R.; Chen, Z.; et al. Cell-free immunomodulatory biomaterials mediated in situ periodontal multi-tissue regeneration and their immunopathophysiological processes. Mater. Today Bio 2022, 16, 100432. [Google Scholar] [CrossRef]
- Roe, K. An inflammation classification system using cytokine parameters. Scand. J. Immunol. 2021, 93, e12970. [Google Scholar] [CrossRef]
- Feehan, K.T.; Gilroy, D.W. Is Resolution the End of Inflammation? Trends Mol. Med. 2019, 25, 198–214. [Google Scholar] [CrossRef]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef]
- Soleimani, V.; Sahebkar, A.; Hosseinzadeh, H. Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances: Review. Phytother. Res. PTR 2018, 32, 985–995. [Google Scholar] [CrossRef]
- Dei Cas, M.; Ghidoni, R. Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Hu, C.; Xu, R. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des. Dev. Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef] [PubMed]
- Witika, B.A.; Makoni, P.A.; Matafwali, S.K.; Mweetwa, L.L.; Shandele, G.C.; Walker, R.B. Enhancement of Biological and Pharmacological Properties of an Encapsulated Polyphenol: Curcumin. Molecules 2021, 26, 4244. [Google Scholar] [CrossRef]
- Razavi, B.M.; Ghasemzadeh Rahbardar, M.; Hosseinzadeh, H. A review of therapeutic potentials of turmeric (Curcuma longa) and its active constituent, curcumin, on inflammatory disorders, pain, and their related patents. Phytother. Res. PTR 2021, 35, 6489–6513. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, B.M.; Kim, H.S. Potential protective roles of curcumin against cadmium-induced toxicity and oxidative stress. J. Toxicol. Environ. Health Part B Crit. Rev. 2021, 24, 95–118. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.-S.; Chen, T.-H.; Weng, L.; Huang, L.; Lai, D.; Weng, C.-F. Pharmacological properties and underlying mechanisms of curcumin and prospects in medicinal potential. Biomed. Pharmacother. 2021, 141, 111888. [Google Scholar] [CrossRef] [PubMed]
- Urošević, M.; Nikolić, L.; Gajić, I.; Nikolić, V.; Dinić, A.; Miljković, V. Curcumin: Biological Activities and Modern Pharmaceutical Forms. Antibiotics 2022, 11, 135. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Bai, D.; Wei, Z.; Zhang, Y.; Huang, Y.; Deng, H.; Huang, X. Curcumin attenuates oxidative stress in RAW264.7 cells by increasing the activity of antioxidant enzymes and activating the Nrf2-Keap1 pathway. PLoS ONE 2019, 14, e0216711. [Google Scholar] [CrossRef]
- Salehi, B.; Stojanović-Radić, Z.; Matejić, J.; Sharifi-Rad, M.; Anil Kumar, N.V.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of curcumin: A review of clinical trials. Eur. J. Med. Chem. 2019, 163, 527–545. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Senthil, N.; Min, T. Nanocurcumin: A Promising Candidate for Therapeutic Applications. Front. Pharmacol. 2020, 11, 487. [Google Scholar] [CrossRef]
- Del Prado-Audelo, M.L.; Caballero-Florán, I.H.; Meza-Toledo, J.A.; Mendoza-Muñoz, N.; González-Torres, M.; Florán, B.; Cortés, H.; Leyva-Gómez, G. Formulations of Curcumin Nanoparticles for Brain Diseases. Biomolecules 2019, 9, 56. [Google Scholar] [CrossRef]
- Chopra, H.; Dey, P.S.; Das, D.; Bhattacharya, T.; Shah, M.; Mubin, S.; Maishu, S.P.; Akter, R.; Rahman, M.H.; Karthika, C.; et al. Curcumin Nanoparticles as Promising Therapeutic Agents for Drug Targets. Molecules 2021, 26, 4998. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, J.; Jiang, J.; Zhang, C.; Zhao, M.; Chen, Z.; Wang, N.; Hu, D.; Liu, X.; Peng, H.; et al. Sonodynamic therapy in atherosclerosis by curcumin nanosuspensions: Preparation design, efficacy evaluation, and mechanisms analysis. Eur. J. Pharm. Biopharm. 2020, 146, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Meng, N.; Gong, Y.; Zhang, J.; Mu, X.; Song, Z.; Feng, R.; Zhang, H. A novel curcumin-loaded nanoparticle restricts atherosclerosis development and promotes plaques stability in apolipoprotein E deficient mice. J. Biomater. Appl. 2019, 33, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xiao, H.; Lin, C.; Sun, W.; Wu, T.; Wang, J.; Chen, B.; Chen, X.; Cheng, D. Synergistic effects of liposomes encapsulating atorvastatin calcium and curcumin and targeting dysfunctional endothelial cells in reducing atherosclerosis. Int. J. Nanomed. 2019, 14, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Sun, Y.; Liu, Z.; Zhang, M.; Li, C.; Cai, H. The effect of curcumin and its nanoformulation on adjuvant-induced arthritis in rats. Drug Des. Dev. Ther. 2015, 9, 4931–4942. [Google Scholar] [CrossRef]
- Arora, R.; Kuhad, A.; Kaur, I.P.; Chopra, K. Curcumin loaded solid lipid nanoparticles ameliorate adjuvant-induced arthritis in rats. Eur. J. Pain 2015, 19, 940–952. [Google Scholar] [CrossRef]
- Yan, F.; Li, H.; Zhong, Z.; Zhou, M.; Lin, Y.; Tang, C.; Li, C. Co-Delivery of Prednisolone and Curcumin in Human Serum Albumin Nanoparticles for Effective Treatment of Rheumatoid Arthritis. Int. J. Nanomed. 2019, 14, 9113–9125. [Google Scholar] [CrossRef]
- Javadi, M.; Khadem Haghighian, H.; Goodarzy, S.; Abbasi, M.; Nassiri-Asl, M. Effect of curcumin nanomicelle on the clinical symptoms of patients with rheumatoid arthritis: A randomized, double-blind, controlled trial. Int. J. Rheum. Dis. 2019, 22, 1857–1862. [Google Scholar] [CrossRef]
- Zhang, Z.; Leong, D.J.; Xu, L.; He, Z.; Wang, A.; Navati, M.; Kim, S.J.; Hirsh, D.M.; Hardin, J.A.; Cobelli, N.J.; et al. Curcumin slows osteoarthritis progression and relieves osteoarthritis-associated pain symptoms in a post-traumatic osteoarthritis mouse model. Arthritis Res. Ther. 2016, 18, 128. [Google Scholar] [CrossRef]
- Yeh, C.C.; Su, Y.H.; Lin, Y.J.; Chen, P.J.; Shi, C.S.; Chen, C.N.; Chang, H.I. Evaluation of the protective effects of curcuminoid (curcumin and bisdemethoxycurcumin)-loaded liposomes against bone turnover in a cell-based model of osteoarthritis. Drug Des. Dev. Ther. 2015, 9, 2285–2300. [Google Scholar] [CrossRef]
- Niazvand, F.; Khorsandi, L.; Abbaspour, M.; Orazizadeh, M.; Varaa, N.; Maghzi, M.; Ahmadi, K. Curcumin-loaded poly lactic-co-glycolic acid nanoparticles effects on mono-iodoacetate -induced osteoarthritis in rats. Vet. Res. Forum 2017, 8, 155–161. [Google Scholar]
- Li, S.; Stöckl, S.; Lukas, C.; Herrmann, M.; Brochhausen, C.; König, M.A.; Johnstone, B.; Grässel, S. Curcumin-primed human BMSC-derived extracellular vesicles reverse IL-1β-induced catabolic responses of OA chondrocytes by upregulating miR-126-3p. Stem Cell Res. Ther. 2021, 12, 252. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Jung, E.; Hyeon, H.; Seon, S.; Lee, D. Acid-activatable polymeric curcumin nanoparticles as therapeutic agents for osteoarthritis. Nanomedicine 2020, 23, 102104. [Google Scholar] [CrossRef] [PubMed]
- Crivelli, B.; Bari, E.; Perteghella, S.; Catenacci, L.; Sorrenti, M.; Mocchi, M.; Faragò, S.; Tripodo, G.; Prina-Mello, A.; Torre, M.L. Silk fibroin nanoparticles for celecoxib and curcumin delivery: ROS-scavenging and anti-inflammatory activities in an in vitro model of osteoarthritis. Eur. J. Pharm. Biopharm. 2019, 137, 37–45. [Google Scholar] [CrossRef]
- Taylor, M.; Moore, S.; Mourtas, S.; Niarakis, A.; Re, F.; Zona, C.; La Ferla, B.; Nicotra, F.; Masserini, M.; Antimisiaris, S.G.; et al. Effect of curcumin-associated and lipid ligand-functionalized nanoliposomes on aggregation of the Alzheimer’s Aβ peptide. Nanomedicine 2011, 7, 541–550. [Google Scholar] [CrossRef]
- Mathew, A.; Fukuda, T.; Nagaoka, Y.; Hasumura, T.; Morimoto, H.; Yoshida, Y.; Maekawa, T.; Venugopal, K.; Kumar, D.S. Curcumin loaded-PLGA nanoparticles conjugated with Tet-1 peptide for potential use in Alzheimer’s disease. PLoS ONE 2012, 7, e32616. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Agarwal, S.; Seth, B.; Yadav, A.; Nair, S.; Bhatnagar, P.; Karmakar, M.; Kumari, M.; Chauhan, L.K.; Patel, D.K.; et al. Curcumin-loaded nanoparticles potently induce adult neurogenesis and reverse cognitive deficits in Alzheimer’s disease model via canonical Wnt/β-catenin pathway. ACS Nano 2014, 8, 76–103. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Lin, C.C. Rescuing apoptotic neurons in Alzheimer’s disease using wheat germ agglutinin-conjugated and cardiolipin-conjugated liposomes with encapsulated nerve growth factor and curcumin. Int. J. Nanomed. 2015, 10, 2653–2672. [Google Scholar] [CrossRef]
- Fan, S.; Zheng, Y.; Liu, X.; Fang, W.; Chen, X.; Liao, W.; Jing, X.; Lei, M.; Tao, E.; Ma, Q.; et al. Curcumin-loaded PLGA-PEG nanoparticles conjugated with B6 peptide for potential use in Alzheimer’s disease. Drug Deliv. 2018, 25, 1091–1102. [Google Scholar] [CrossRef]
- SoukhakLari, R.; Moezi, L.; Pirsalami, F.; Moosavi, M. The Effect of BSA-Based Curcumin Nanoparticles on Memory and Hippocampal MMP-2, MMP-9, and MAPKs in Adult Mice. J. Mol. Neurosci. 2018, 65, 319–326. [Google Scholar] [CrossRef]
- Huo, X.; Zhang, Y.; Jin, X.; Li, Y.; Zhang, L. A novel synthesis of selenium nanoparticles encapsulated PLGA nanospheres with curcumin molecules for the inhibition of amyloid β aggregation in Alzheimer’s disease. J. Photochem. Photobiol. B 2019, 190, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, S.; Wong, L.R.; Xie, H.; Ho, P.C. In Vitro and In Vivo Comparison of Curcumin-Encapsulated Chitosan-Coated Poly(lactic-co-glycolic acid) Nanoparticles and Curcumin/Hydroxypropyl-β-Cyclodextrin Inclusion Complexes Administered Intranasally as Therapeutic Strategies for Alzheimer’s Disease. Mol. Pharm. 2020, 17, 4256–4269. [Google Scholar] [CrossRef]
- Lin, Y.W.; Fang, C.H.; Yang, C.Y.; Liang, Y.J.; Lin, F.H. Investigating a Curcumin-Loaded PLGA-PEG-PLGA Thermo-Sensitive Hydrogel for the Prevention of Alzheimer’s Disease. Antioxidants 2022, 11, 727. [Google Scholar] [CrossRef] [PubMed]
- Campisi, A.; Sposito, G.; Pellitteri, R.; Santonocito, D.; Bisicchia, J.; Raciti, G.; Russo, C.; Nardiello, P.; Pignatello, R.; Casamenti, F.; et al. Effect of Unloaded and Curcumin-Loaded Solid Lipid Nanoparticles on Tissue Transglutaminase Isoforms Expression Levels in an Experimental Model of Alzheimer’s Disease. Antioxidants 2022, 11, 1863. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Xiong, Y.; Fang, W.; Yu, Q.; Mai, Y.; Cao, Z.; Wang, K.; Lei, M.; Xu, J.; Liu, Y.; et al. Highly sensitive Curcumin-conjugated nanotheranostic platform for detecting amyloid-beta plaques by magnetic resonance imaging and reversing cognitive deficits of Alzheimer’s disease via NLRP3-inhibition. J. Nanobiotechnology 2022, 20, 322. [Google Scholar] [CrossRef]
- Patil, R.; Gangalum, P.R.; Wagner, S.; Portilla-Arias, J.; Ding, H.; Rekechenetskiy, A.; Konda, B.; Inoue, S.; Black, K.L.; Ljubimova, J.Y.; et al. Curcumin Targeted, Polymalic Acid-Based MRI Contrast Agent for the Detection of Aβ Plaques in Alzheimer’s Disease. Macromol. Biosci. 2015, 15, 1212–1217. [Google Scholar] [CrossRef]
- Giacomeli, R.; Izoton, J.C.; Dos Santos, R.B.; Boeira, S.P.; Jesse, C.R.; Haas, S.E. Neuroprotective effects of curcumin lipid-core nanocapsules in a model Alzheimer’s disease induced by β-amyloid 1-42 peptide in aged female mice. Brain Res. 2019, 1721, 146325. [Google Scholar] [CrossRef]
- Bollimpelli, V.S.; Kumar, P.; Kumari, S.; Kondapi, A.K. Neuroprotective effect of curcumin-loaded lactoferrin nano particles against rotenone induced neurotoxicity. Neurochem. Int. 2016, 95, 37–45. [Google Scholar] [CrossRef]
- Zhang, N.; Yan, F.; Liang, X.; Wu, M.; Shen, Y.; Chen, M.; Xu, Y.; Zou, G.; Jiang, P.; Tang, C.; et al. Localized delivery of curcumin into brain with polysorbate 80-modified cerasomes by ultrasound-targeted microbubble destruction for improved Parkinson’s disease therapy. Theranostics 2018, 8, 2264–2277. [Google Scholar] [CrossRef] [PubMed]
- Sookhaklari, R.; Geramizadeh, B.; Abkar, M.; Moosavi, M. The neuroprotective effect of BSA-based nanocurcumin against 6-OHDA-induced cell death in SH-SY5Y cells. Avicenna J. Phytomed. 2019, 9, 92–100. [Google Scholar] [PubMed]
- Liu, L.; Li, Y.; Peng, H.; Liu, R.; Ji, W.; Shi, Z.; Shen, J.; Ma, G.; Zhang, X. Targeted exosome coating gene-chem nanocomplex as “nanoscavenger” for clearing α-synuclein and immune activation of Parkinson’s disease. Sci. Adv. 2020, 6, eaba3967. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Chen, Y.; Liu, Z.; Cai, F.; Niu, W.; Song, L.; Liang, H.; Su, Z.; Yu, B.; Yan, F. Brain Delivery of Curcumin Through Low-Intensity Ultrasound-Induced Blood-Brain Barrier Opening via Lipid-PLGA Nanobubbles. Int. J. Nanomed. 2021, 16, 7433–7447. [Google Scholar] [CrossRef] [PubMed]
- de Guzman, A.C.V.; Razzak, M.A.; Cho, J.H.; Kim, J.Y.; Choi, S.S. Curcumin-Loaded Human Serum Albumin Nanoparticles Prevent Parkinson’s Disease-like Symptoms in C. elegans. Nanomaterials 2022, 12, 758. [Google Scholar] [CrossRef]
- Motavaf, M.; Sadeghizadeh, M.; Babashah, S.; Zare, L.; Javan, M. Dendrosomal nanocurcumin promotes remyelination through induction of oligodendrogenesis in experimental demyelination animal model. J. Tissue Eng. Regen. Med. 2020, 14, 1449–1464. [Google Scholar] [CrossRef]
- Motavaf, M.; Sadeghizadeh, M.; Babashah, S.; Zare, L.; Javan, M. Protective Effects of a Nano-Formulation of Curcumin against Cuprizone-Induced Demyelination in the Mouse Corpus Callosum. Iran. J. Pharm. Res. 2020, 19, 310–320. [Google Scholar] [CrossRef]
- Lu, L.; Qi, S.; Chen, Y.; Luo, H.; Huang, S.; Yu, X.; Luo, Q.; Zhang, Z. Targeted immunomodulation of inflammatory monocytes across the blood-brain barrier by curcumin-loaded nanoparticles delays the progression of experimental autoimmune encephalomyelitis. Biomaterials 2020, 245, 119987. [Google Scholar] [CrossRef]
- Dolati, S.; Babaloo, Z.; Ayromlou, H.; Ahmadi, M.; Rikhtegar, R.; Rostamzadeh, D.; Roshangar, L.; Nouri, M.; Mehdizadeh, A.; Younesi, V.; et al. Nanocurcumin improves regulatory T-cell frequency and function in patients with multiple sclerosis. J. Neuroimmunol. 2019, 327, 15–21. [Google Scholar] [CrossRef]
- Dolati, S.; Ahmadi, M.; Aghebti-Maleki, L.; Nikmaram, A.; Marofi, F.; Rikhtegar, R.; Ayromlou, H.; Yousefi, M. Nanocurcumin is a potential novel therapy for multiple sclerosis by influencing inflammatory mediators. Pharmacol. Rep. 2018, 70, 1158–1167. [Google Scholar] [CrossRef]
- Sandhir, R.; Yadav, A.; Mehrotra, A.; Sunkaria, A.; Singh, A.; Sharma, S. Curcumin nanoparticles attenuate neurochemical and neurobehavioral deficits in experimental model of Huntington’s disease. Neuromolecular. Med. 2014, 16, 106–118. [Google Scholar] [CrossRef]
- Huang, Y.; Canup, B.S.B.; Gou, S.; Chen, N.; Dai, F.; Xiao, B.; Li, C. Oral nanotherapeutics with enhanced mucus penetration and ROS-responsive drug release capacities for delivery of curcumin to colitis tissues. J. Mater. Chem. B 2021, 9, 1604–1615. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yan, X.; Zhang, Y.; Yang, M.; Ma, Y.; Zhang, Y.; Xu, Q.; Tu, K.; Zhang, M. Oral administration of turmeric-derived exosome-like nanovesicles with anti-inflammatory and pro-resolving bioactions for murine colitis therapy. J. Nanobiotechnology 2022, 20, 206. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jin, M.; Wu, Y.; Jung, S.; Li, D.; He, N.; Lee, M.S. An efficient enzyme-triggered controlled release system for colon-targeted oral delivery to combat dextran sodium sulfate (DSS)-induced colitis in mice. Drug Deliv. 2021, 28, 1120–1131. [Google Scholar] [CrossRef]
- Wang, C.; Han, Z.; Wu, Y.; Lu, X.; Tang, X.; Xiao, J.; Li, N. Enhancing stability and anti-inflammatory properties of curcumin in ulcerative colitis therapy using liposomes mediated colon-specific drug delivery system. Food Chem. Toxicol. 2021, 151, 112123. [Google Scholar] [CrossRef]
- Oshi, M.A.; Lee, J.; Naeem, M.; Hasan, N.; Kim, J.; Kim, H.J.; Lee, E.H.; Jung, Y.; Yoo, J.W. Curcumin Nanocrystal/pH-Responsive Polyelectrolyte Multilayer Core-Shell Nanoparticles for Inflammation-Targeted Alleviation of Ulcerative Colitis. Biomacromolecules 2020, 21, 3571–3581. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Y.; Ma, L.; Zu, M.; Song, H.; Xiao, B. Oral administration of chondroitin sulfate-functionalized nanoparticles for colonic macrophage-targeted drug delivery. Carbohydr. Polym. 2019, 223, 115126. [Google Scholar] [CrossRef]
- Hlaing, S.P.; Cao, J.; Lee, J.; Kim, J.; Saparbayeva, A.; Kwak, D.; Kim, H.; Hwang, S.; Yun, H.; Moon, H.R.; et al. Hyaluronic Acid-Conjugated PLGA Nanoparticles Alleviate Ulcerative Colitis via CD44-Mediated Dual Targeting to Inflamed Colitis Tissue and Macrophages. Pharmaceutics 2022, 14, 2118. [Google Scholar] [CrossRef]
- Chen, Q.; Gou, S.; Ma, P.; Song, H.; Zhou, X.; Huang, Y.; Kwon Han, M.; Wan, Y.; Kang, Y.; Xiao, B. Oral administration of colitis tissue-accumulating porous nanoparticles for ulcerative colitis therapy. Int. J. Pharm. 2019, 557, 135–144. [Google Scholar] [CrossRef]
- Arafat, E.A.; Marzouk, R.E.; Mostafa, S.A.; Hamed, W.H.E. Identification of the Molecular Basis of Nanocurcumin-Induced Telocyte Preservation within the Colon of Ulcerative Colitis Rat Model. Mediat. Inflamm. 2021, 2021, 7534601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xia, Q.; Li, Y.; He, Z.; Li, Z.; Guo, T.; Wu, Z.; Feng, N. CD44 Assists the Topical Anti-Psoriatic Efficacy of Curcumin-Loaded Hyaluronan-Modified Ethosomes: A New Strategy for Clustering Drug in Inflammatory Skin. Theranostics 2019, 9, 48–64. [Google Scholar] [CrossRef]
- Rapalli, V.K.; Kaul, V.; Waghule, T.; Gorantla, S.; Sharma, S.; Roy, A.; Dubey, S.K.; Singhvi, G. Curcumin loaded nanostructured lipid carriers for enhanced skin retained topical delivery: Optimization, scale-up, in-vitro characterization and assessment of ex-vivo skin deposition. Eur. J. Pharm. Sci. 2020, 152, 105438. [Google Scholar] [CrossRef]
- Gomez, C.; Muangnoi, C.; Sorasitthiyanukarn, F.N.; Wongpiyabovorn, J.; Rojsitthisak, P.; Rojsitthisak, P. Synergistic Effects of Photo-Irradiation and Curcumin-Chitosan/Alginate Nanoparticles on Tumor Necrosis Factor-Alpha-Induced Psoriasis-Like Proliferation of Keratinocytes. Molecules 2019, 24, 1388. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Ahmad, M.Z.; Nourein, I.H.; Ahmad, J. Co-Delivery of Imiquimod and Curcumin by Nanoemugel for Improved Topical Delivery and Reduced Psoriasis-Like Skin Lesions. Biomolecules 2020, 10, 968. [Google Scholar] [CrossRef] [PubMed]
- Bilia, A.R.; Bergonzi, M.C.; Isacchi, B.; Antiga, E.; Caproni, M. Curcumin nanoparticles potentiate therapeutic effectiveness of acitrein in moderate-to-severe psoriasis patients and control serum cholesterol levels. J. Pharm. Pharmacol. 2018, 70, 919–928. [Google Scholar] [CrossRef]
- Wang, J.; Pan, W.; Wang, Y.; Lei, W.; Feng, B.; Du, C.; Wang, X.J. Enhanced efficacy of curcumin with phosphatidylserine-decorated nanoparticles in the treatment of hepatic fibrosis. Drug Deliv. 2018, 25, 1–11. [Google Scholar] [CrossRef]
- Elzoheiry, A.; Ayad, E.; Omar, N.; Elbakry, K.; Hyder, A. Anti-liver fibrosis activity of curcumin/chitosan-coated green silver nanoparticles. Sci. Rep. 2022, 12, 18403. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.N.; Hsu, S.L.; Liao, M.Y.; Liu, Y.T.; Lai, C.H.; Chen, J.F.; Nguyen, M.T.; Su, Y.H.; Chen, S.T.; Wu, L.C. Ameliorative Effect of Curcumin-Encapsulated Hyaluronic Acid-PLA Nanoparticles on Thioacetamide-Induced Murine Hepatic Fibrosis. Int. J. Environ. Res. Public Health 2016, 14, 11. [Google Scholar] [CrossRef]
- Atia, M.M.; Abdel-Tawab, H.S.; Mostafa, A.M.; Mobarak, S.A. Nanocurcumin and curcumin prevent N, N’-methylenebisacrylamide-induced liver damage and promotion of hepatic cancer cell growth. Sci. Rep. 2022, 12, 8319. [Google Scholar] [CrossRef] [PubMed]
- Adlia, A.; Tomagola, I.; Damayanti, S.; Mulya, A.; Rachmawati, H. Antifibrotic Activity and In Ovo Toxicity Study of Liver-Targeted Curcumin-Gold Nanoparticle. Sci. Pharm. 2018, 86, 41. [Google Scholar] [CrossRef]
- Huang, R.; Zhu, Y.; Lin, L.; Song, S.; Cheng, L.; Zhu, R. Solid Lipid Nanoparticles Enhanced the Neuroprotective Role of Curcumin against Epilepsy through Activation of Bcl-2 Family and P38 MAPK Pathways. ACS Chem. Neurosci. 2020, 11, 1985–1995. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, S.R.; Hashemian, M.; Khalili-Fomeshi, M.; Ashrafpour, M.; Moghadamnia, A.A.; Ghasemi-Kasman, M. Upregulation of klotho and erythropoietin contributes to the neuroprotection induced by curcumin-loaded nanoparticles in experimental model of chronic epilepsy. Brain Res. Bull. 2018, 142, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Hashemian, M.; Anissian, D.; Ghasemi-Kasman, M.; Akbari, A.; Khalili-Fomeshi, M.; Ghasemi, S.; Ahmadi, F.; Moghadamnia, A.A.; Ebrahimpour, A. Curcumin-loaded chitosan-alginate-STPP nanoparticles ameliorate memory deficits and reduce glial activation in pentylenetetrazol-induced kindling model of epilepsy. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 79, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, H.; Abdolmohammadi-Vahid, S.; Danshina, S.; Ziya Gencer, M.; Ammari, A.; Sadeghi, A.; Roshangar, L.; Aslani, S.; Esmaeilzadeh, A.; Ghaebi, M.; et al. Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients. Int. Immunopharmacol. 2020, 89, 107088. [Google Scholar] [CrossRef]
- Saber-Moghaddam, N.; Salari, S.; Hejazi, S.; Amini, M.; Taherzadeh, Z.; Eslami, S.; Rezayat, S.M.; Jaafari, M.R.; Elyasi, S. Oral nano-curcumin formulation efficacy in management of mild to moderate hospitalized coronavirus disease-19 patients: An open label nonrandomized clinical trial. Phytother. Res. 2021, 35, 2616–2623. [Google Scholar] [CrossRef]
- Hassaniazad, M.; Eftekhar, E.; Inchehsablagh, B.R.; Kamali, H.; Tousi, A.; Jaafari, M.R.; Rafat, M.; Fathalipour, M.; Nikoofal-Sahlabadi, S.; Gouklani, H.; et al. A triple-blind, placebo-controlled, randomized clinical trial to evaluate the effect of curcumin-containing nanomicelles on cellular immune responses subtypes and clinical outcome in COVID-19 patients. Phytother. Res. 2021, 35, 6417–6427. [Google Scholar] [CrossRef]
- Hanafy, N.A.N.; El-Kemary, M.A. Silymarin/curcumin loaded albumin nanoparticles coated by chitosan as muco-inhalable delivery system observing anti-inflammatory and anti COVID-19 characterizations in oleic acid triggered lung injury and in vitro COVID-19 experiment. Int. J. Biol. Macromol. 2022, 198, 101–110. [Google Scholar] [CrossRef]
- Asadirad, A.; Nashibi, R.; Khodadadi, A.; Ghadiri, A.A.; Sadeghi, M.; Aminian, A.; Dehnavi, S. Antiinflammatory potential of nano-curcumin as an alternative therapeutic agent for the treatment of mild-to-moderate hospitalized COVID-19 patients in a placebo-controlled clinical trial. Phytother. Res. 2022, 36, 1023–1031. [Google Scholar] [CrossRef]
- Ahmadi, R.; Salari, S.; Sharifi, M.D.; Reihani, H.; Rostamiani, M.B.; Behmadi, M.; Taherzadeh, Z.; Eslami, S.; Rezayat, S.M.; Jaafari, M.R.; et al. Oral nano-curcumin formulation efficacy in the management of mild to moderate outpatient COVID-19: A randomized triple-blind placebo-controlled clinical trial. Food Sci. Nutr. 2021, 9, 4068–4075. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, V.M.; Laurindo, L.F.; Manzan, B.; Guiguer, E.L.; Oshiiwa, M.; Otoboni, A.; Araujo, A.C.; Tofano, R.J.; Barbalho, S.M. Garlic: A systematic review of the effects on cardiovascular diseases. Crit. Rev. Food Sci. Nutr. 2022, 1–23. [Google Scholar] [CrossRef]
- Fan, J.; Watanabe, T. Atherosclerosis: Known and unknown. Pathol. Int. 2022, 72, 151–160. [Google Scholar] [CrossRef]
- Sima, P.; Vannucci, L.; Vetvicka, V. Atherosclerosis as autoimmune disease. Ann. Transl. Med. 2018, 6, 116. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, E.; Atzeni, F.; Sarzi-Puttini, P.; Turiel, M.; Lopez, L.R.; Nurmohamed, M.T. Is atherosclerosis an autoimmune disease? BMC Med. 2014, 12, 47. [Google Scholar] [CrossRef]
- Lin, Y.J.; Anzaghe, M.; Schülke, S. Update on the Pathomechanism, Diagnosis, and Treatment Options for Rheumatoid Arthritis. Cells 2020, 9, 880. [Google Scholar] [CrossRef] [PubMed]
- Kondo, N.; Kuroda, T.; Kobayashi, D. Cytokine Networks in the Pathogenesis of Rheumatoid Arthritis. Int. J. Mol. Sci. 2021, 22, 10922. [Google Scholar] [CrossRef]
- Testa, D.; Calvacchi, S.; Petrelli, F.; Giannini, D.; Bilia, S.; Alunno, A.; Puxeddu, I. One year in review 2021: Pathogenesis of rheumatoid arthritis. Clin. Exp. Rheumatol. 2021, 39, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Firestein, G.S.; McInnes, I.B. Immunopathogenesis of Rheumatoid Arthritis. Immunity 2017, 46, 183–196. [Google Scholar] [CrossRef]
- Deane, K.D.; Holers, V.M. Rheumatoid Arthritis Pathogenesis, Prediction, and Prevention: An Emerging Paradigm Shift. Arthritis Rheumatol. 2021, 73, 181–193. [Google Scholar] [CrossRef]
- Hebert, S.K. Ortopedia e Traumatologia: Princípios e Prática, 5th ed.; Deitos, D., Ed.; Artmed Editora Ltda: Porto Alegre, Brazil, 2017; Volume 1. [Google Scholar]
- Sanchez-Lopez, E.; Coras, R.; Torres, A.; Lane, N.E.; Guma, M. Synovial inflammation in osteoarthritis progression. Nat. Rev. Rheumatol. 2022, 18, 258–275. [Google Scholar] [CrossRef]
- Moonen, S.; Koper, M.J.; Van Schoor, E.; Schaeverbeke, J.M.; Vandenberghe, R.; von Arnim, C.A.F.; Tousseyn, T.; De Strooper, B.; Thal, D.R. Pyroptosis in Alzheimer’s disease: Cell type-specific activation in microglia, astrocytes and neurons. Acta Neuropathol. 2022. [Google Scholar] [CrossRef]
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef]
- Alkanli, S.S.; Alkanli, N.; Ay, A.; Albeniz, I. CRISPR/Cas9 Mediated Therapeutic Approach in Huntington’s Disease. Mol. Neurobiol. 2022. [Google Scholar] [CrossRef]
- Soria Lopez, J.A.; González, H.M.; Léger, G.C. Alzheimer’s disease. Handb. Clin. Neurol. 2019, 167, 231–255. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R.J.M. Comprehensive review on Alzheimer’s disease: Causes and treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zheng, Y. β Amyloid Hypothesis in Alzheimer’s Disease: Pathogenesis, Prevention, and Management. Zhongguo yi xue ke xue yuan xue bao Acta Acad. Med. Sin. 2019, 41, 702–708. [Google Scholar] [CrossRef]
- Ozben, T.; Ozben, S. Neuro-inflammation and anti-inflammatory treatment options for Alzheimer’s disease. Clin. Biochem. 2019, 72, 87–89. [Google Scholar] [CrossRef]
- Forloni, G.; Balducci, C. Alzheimer’s Disease, Oligomers, and Inflammation. J. Alzheimers Dis. 2018, 62, 1261–1276. [Google Scholar] [CrossRef]
- Radhakrishnan, D.M.; Goyal, V. Parkinson’s disease: A review. Neurol. India 2018, 66, S26–S35. [Google Scholar] [CrossRef]
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the diagnosis of Parkinson’s disease. Lancet. Neurol. 2021, 20, 385–397. [Google Scholar] [CrossRef]
- Chia, S.J.; Tan, E.K.; Chao, Y.X. Historical Perspective: Models of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 2464. [Google Scholar] [CrossRef] [PubMed]
- Pajares, M.; Rojo, I.A.; Manda, G.; Boscá, L.; Cuadrado, A. Inflammation in Parkinson’s Disease: Mechanisms and Therapeutic Implications. Cells 2020, 9, 1687. [Google Scholar] [CrossRef]
- Marogianni, C.; Sokratous, M.; Dardiotis, E.; Hadjigeorgiou, G.M.; Bogdanos, D.; Xiromerisiou, G. Neurodegeneration and Inflammation-An Interesting Interplay in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 8421. [Google Scholar] [CrossRef]
- Theodosis-Nobelos, P.; Rekka, E.A. The Multiple Sclerosis Modulatory Potential of Natural Multi-Targeting Antioxidants. Molecules 2022, 27, 8402. [Google Scholar] [CrossRef] [PubMed]
- Nataf, S.; Guillen, M.; Pays, L. Irrespective of Plaque Activity, Multiple Sclerosis Brain Periplaques Exhibit Alterations of Myelin Genes and a TGF-Beta Signature. Int. J. Mol. Sci. 2022, 23, 14993. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Rajamma, U. Huntington’s disease: The coming of age. J. Genet. 2018, 97, 649–664. [Google Scholar] [CrossRef]
- Kim, A.; Lalonde, K.; Truesdell, A.; Gomes Welter, P.; Brocardo, P.S.; Rosenstock, T.R.; Gil-Mohapel, J. New Avenues for the Treatment of Huntington’s Disease. Int. J. Mol. Sci. 2021, 22, 8363. [Google Scholar] [CrossRef] [PubMed]
- Stacchiotti, A.; Corsetti, G. Natural Compounds and Autophagy: Allies Against Neurodegeneration. Front. Cell Dev. Biol. 2020, 8, 555409. [Google Scholar] [CrossRef]
- Mahjoob, M.; Stochaj, U. Curcumin nanoformulations to combat aging-related diseases. Ageing Res. Rev. 2021, 69, 101364. [Google Scholar] [CrossRef]
- Abrahams, S.; Haylett, W.L.; Johnson, G.; Carr, J.A.; Bardien, S. Antioxidant effects of curcumin in models of neurodegeneration, aging, oxidative and nitrosative stress: A review. Neuroscience 2019, 406, 1–21. [Google Scholar] [CrossRef]
- Valadão, P.A.C.; Santos, K.B.S.; Ferreira, E.V.T.H.; Macedo, E.C.T.; Teixeira, A.L.; Guatimosim, C.; de Miranda, A.S. Inflammation in Huntington’s disease: A few new twists on an old tale. J. Neuroimmunol. 2020, 348, 577380. [Google Scholar] [CrossRef]
- Tabrizi, S.J.; Estevez-Fraga, C.; van Roon-Mom, W.M.C.; Flower, M.D.; Scahill, R.I.; Wild, E.J.; Muñoz-Sanjuan, I.; Sampaio, C.; Rosser, A.E.; Leavitt, B.R. Potential disease-modifying therapies for Huntington’s disease: Lessons learned and future opportunities. Lancet Neurol. 2022, 21, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Illarioshkin, S.N.; Klyushnikov, S.A.; Vigont, V.A.; Seliverstov, Y.A.; Kaznacheyeva, E.V. Molecular Pathogenesis in Huntington’s Disease. Biochemistry 2018, 83, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef] [PubMed]
- Falco-Walter, J. Epilepsy-Definition, Classification, Pathophysiology, and Epidemiology. Semin. Neurol. 2020, 40, 617–623. [Google Scholar] [CrossRef]
- Beghi, E.; Giussani, G.; Sander, J.W. The natural history and prognosis of epilepsy. Epileptic. Disord. 2015, 17, 243–253. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, D. Ameliorative effect of curcumin on altered expression of CACNA1A and GABRD in the pathogenesis of FeCl(3)-induced epilepsy. Mol. Biol. Rep. 2020, 47, 5699–5710. [Google Scholar] [CrossRef]
- Erfani, M.; Ashrafzadeh, F.; Rahimi, H.R.; Ebrahimi, S.A.; Kalali, K.; Beiraghi Toosi, M.; Faraji Rad, E. Effect of Curcumin on Pediatric Intractable Epilepsy. Iran. J. Child Neurol. 2022, 16, 35–45. [Google Scholar] [CrossRef]
- Yuen, A.W.C.; Keezer, M.R.; Sander, J.W. Epilepsy is a neurological and a systemic disorder. Epilepsy Behav. 2018, 78, 57–61. [Google Scholar] [CrossRef]
- Vezzani, A.; French, J.; Bartfai, T.; Baram, T.Z. The role of inflammation in epilepsy. Nat. Rev. Neurol. 2011, 7, 31–40. [Google Scholar] [CrossRef]
- Vezzani, A.; Fujinami, R.S.; White, H.S.; Preux, P.M.; Blümcke, I.; Sander, J.W.; Löscher, W. Infections, inflammation and epilepsy. Acta Neuropathol. 2016, 131, 211–234. [Google Scholar] [CrossRef]
- Terrone, G.; Balosso, S.; Pauletti, A.; Ravizza, T.; Vezzani, A. Inflammation and reactive oxygen species as disease modifiers in epilepsy. Neuropharmacology 2020, 167, 107742. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Musto, A.E. The role of inflammation in the development of epilepsy. J. Neuroinflammation 2018, 15, 144. [Google Scholar] [CrossRef]
- Brandt, L.J.F.; Friedman, M.; Lawrance, S. Tratado Gastrointestinal e Doenças do Fígado; Elsevier Editora Ltd.a.: Rio de Janeiro, Brazil, 2009; Volumes 1 and 2. [Google Scholar]
- Goulart, R.A.; Barbalho, S.M. Can vitamin D induce remission in patients with inflammatory bowel disease? Ann. Gastroenterol. 2022, 35, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef]
- Rendon, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.E.M.; Armstrong, A.W.; Gudjonsson, J.E.; Barker, J. Psoriasis. Lancet 2021, 397, 1301–1315. [Google Scholar] [CrossRef]
- Blauvelt, A.; Chiricozzi, A. The Immunologic Role of IL-17 in Psoriasis and Psoriatic Arthritis Pathogenesis. Clin. Rev. Allergy Immunol. 2018, 55, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Petit, R.G.; Cano, A.; Ortiz, A.; Espina, M.; Prat, J.; Muñoz, M.; Severino, P.; Souto, E.B.; García, M.L.; Pujol, M.; et al. Psoriasis: From Pathogenesis to Pharmacological and Nano-Technological-Based Therapeutics. Int. J. Mol. Sci. 2021, 22, 4983. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Wu, G.R.; Zhou, Q.; Yue, H.; Rao, L.Z.; Yuan, T.; Mo, B.; Wang, F.X.; Chen, L.M.; et al. MBD2 serves as a viable target against pulmonary fibrosis by inhibiting macrophage M2 program. Sci. Adv. 2021, 7, eabb6075. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, B.; Mao, J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J. Infect. 2020, 80, 607–613. [Google Scholar] [CrossRef]
- Hu, B.; Huang, S.; Yin, L. The cytokine storm and COVID-19. J. Med. Virol. 2021, 93, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Soy, M.; Keser, G.; Atagündüz, P.; Tabak, F.; Atagündüz, I.; Kayhan, S. Cytokine storm in COVID-19: Pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020, 39, 2085–2094. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.H.; Zhu, Y.; Mao, J.; Du, R.C. T cell immunobiology and cytokine storm of COVID-19. Scand. J. Immunol. 2021, 93, e12989. [Google Scholar] [CrossRef]
- Españo, E.; Kim, J.; Lee, K.; Kim, J.K. Phytochemicals for the treatment of COVID-19. J. Microbiol. 2021, 59, 959–977. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ (Clin. Res. Ed.) 2021, 372, n71. [Google Scholar] [CrossRef]
- Stohs, S.J.; Chen, O.; Ray, S.D.; Ji, J.; Bucci, L.R.; Preuss, H.G. Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research and Application: A Review. Molecules 2020, 25, 1397. [Google Scholar] [CrossRef]
- Nayak, A.P.; Mills, T.; Norton, I. Lipid Based Nanosystems for Curcumin: Past, Present and Future. Curr. Pharm. Des. 2016, 22, 4247–4256. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, D.; Liu, Y.; Guo, X.; Chen, S.; Zeng, L.; Ma, J.; Zhang, X.; Tian, Z.; Yang, Z. Immunogenic-cell-killing and immunosuppression-inhibiting nanomedicine. Bioact. Mater. 2021, 6, 1513–1527. [Google Scholar] [CrossRef]
- Bilia, A.R.; Piazzini, V.; Guccione, C.; Risaliti, L.; Asprea, M.; Capecchi, G.; Bergonzi, M.C. Improving on Nature: The Role of Nanomedicine in the Development of Clinical Natural Drugs. Planta Med. 2017, 83, 366–381. [Google Scholar] [CrossRef]
| Diseases | Cur-Based Nanomedicines | Effects | References |
|---|---|---|---|
| Atherosclerosis | Polyvinylpyrrolidone and sodium dodecyl sulfate nanosuspensions, nanoparticles, and liposomes | ↓TC, ↓LDL-c, ↓M1 macrophage polarization, and ↑M2 macrophage polarization, ↓progression of atheroma plaques, ↑inflammatory macrophages apoptosis, ↑atheroma plaques stability, ↓intraplaque micro vessels concentration, ↓MMP-2, ↓MMP-9, ↓pro-inflammatory cytokines production and release, ↓adhesion molecule expression, ↓monocyte migration into the intima layer of large to medium-sized arteries and ↓endothelial dysfunction | [36,37,38] |
| Rheumatoid arthritis | Nanoemulsions, solid lipid nanoparticles, nanoparticulate systems, and nanomicelles | ↓NF-kB activation and signaling, ↓IL-1β, ↓IL-6, ↑IL-10, ↓TNF-α, ↑pain threshold, ↑joint mobility and stiffness, ↓inflammatory leukocytes recruitment, ↓ROS, ↓anti-CCP levels, ↓pannus formation, ↓bone destruction | [39,40,41,42] |
| Osteoarthritis | Nanoparticles, liposomes, CUR-loaded poly lactic-co-glycolic acid nanoparticles, extracellular vesicles and Poly(β -amino ester) amphiphilic polymers | ↓IL-1β, ↓TNF-α, ↑expression of chondroprotective genes, ↓macrophages inflammatory differentiation in cartilages, ↓COX-2, ↓MMP-3, ↑cartilage glycosaminoglycan synthesis, ↓inflammatory cells migration to arthritis sites, ↓cartilage catabolic processes and ↓ROS | [43,44,45,46,47,48] |
| Alzheimer’s disease | Nanoliposomes, CUR-loaded PLGA nanoparticles, PLGA nanoparticles encapsulated in CUR, CUR liposomes conjugated with WGA and CL, CUR-loaded PLGA-PEG nanoparticles conjugated with B6 peptide, BSA-based CUR nanoparticles, selenium nanoparticles encapsulated PLGA nanospheres with CUR, solid lipid nanoparticles, highly-sensitive CUR-conjugated nanotheranostic platform and CUR lipid-core nanocapsules | ↓Aβ aggregation, ↓amyloid fibril formation, ↑Aβ aggregates breakdown, ↑neurogenesis, ↑neuronal differentiation, ↑proliferation of endogenous neural stem cells, ↑β-catenin nuclear translocation, ↑GSK-3β phosphorylation, ↑expression of pro-neurogenic genes, ↑neuronal cells viability, ↑spatial learning, ↑memory capacity, ↓Tau phosphorylation, ↑microglial modulation, ↓brain inflammation (↓mRNA expression of TNF-α, IL-1β and IL-6, IFN-γ and NF-κB), ↓brain OS, ↓TG2 | [49,50,51,52,53,54,55,56,57,58,59,60,61] |
| Parkinson’s disease | CUR-loaded lactoferrin n noparticles, CUR-loaded modified CPC nanoparticles, BSA-based nanoCUR, peptide-modified exosome chemical complex CURa/phenylboronic acid-poly(2 (dimethylamino) ethyl acr late) nanoparticle, and PLGA-lipid nanobubbles | ↓α-synuclein expression, ↓brain OS, ↓TH, ↓Lewy body formation, ↓behavioral disturbances, ↓dopamine depletion, ↓neuronal cells death, ↑neuronal repair, ↑IL-10, ↓IL-2, ↓IL-17, and ↑dopamine transport to synaptic neurons | [62,63,64,65,66,67] |
| Multiple sclerosis | Dendrosomal CUR nanoparticles, CUR-HPPS, and simple nano CUR | ↑Oligodendrogenesis, ↑remyelination, ↑neuronal myelin content, ↓astrocytes and microglia cells accumulation and actions, ↓microglial proliferation, ↓disease’s morbidity, ↓NF-kB activation and signaling, ↓adhesion and migration-related proteins, ↓peripheral Treg cell frequency and function, ↓TGF-β, IL-10 and FoxP3 expression levels, ↓inflammatory miR-145, miR-132, and miR-16 expression levels, ↓STAT1 activation and signaling, ↑STAT5 mRNA expression levels, ↓IL-1β, IL-6, CCL2, CCL5, IFN-γ, and TNF-α mRNA expression levels | [68,69,70,71,72] |
| Huntington’s disease | Solid lipid CUR nanoparticles | ↓Striatum’s Complex II activity, ↑mitochondrial complexes activity, ↑cytochrome levels, ↓brain OS, ↑GSH, ↑SOD, ↓mitochondrial brain swelling, ↓brain lipid peroxidation, ↓protein carbonyls formation, ↓ROS production, ↑neuromotor coordination, and ↑Nrf2 activation and signaling | [73] |
| Inflammatory bowel diseases | PLGA-based CUR nanoparticles, hydrodynamic size CUR nanoparticles, chitosan capsule and unsaturated alginate resulting CUR nanoparticles, liposomes, nanocrystals, chondroitin sulfate CUR nanoparticles and porous CUR-loaded PLGA with PF127 nanoparticles | ↓TNF-α, ↓IL-1β, ↓IL-6, ↓ROS, ↑HO-1, ↑IL-10, ↓NF-kB activation and signaling, ↓weight loss, ↓reduction in colon length, ↓increase in spleen size, ↓intestinal bleeding, ↓diarrhea, ↓levels of infiltrated neutrophils and macrophages and ↑maintenance of the original intestinal tissue architecture | [74,75,76,77,78,79,80,81,82] |
| Psoriasis | CUR-loaded HA-ES, CUR-loaded NLC, CUR-loaded Cur-CS/Alg nanoparticles, CUR nanuemulgel-based delivery system, and simple CUR nanoparticles | ↓Inflammatory symptoms, ↓PASI, ↓TNF-α, ↓IL-17, ↓IL- 22, ↓IL-1β, ↓CCR6, ↓proliferation of psoriatic cells and ↓occurrence of psoriatic lesions | [83,84,85,86,87] |
| Liver fibrosis | mNLCs containing CUR, CUR encapsulated in AuNPs, CUR HPNPs, AgNPs, and simple nanoCUR | ↓Hepatocytes, centrilobular vein and sinusoid capillaries collagen deposition and fibrosis, ↑HGF, ↓AST, ↓ALT, ↑albumin hepatic production, ↓hepatic fibrosis-related genes expression, ↑apoptosis of pro-inflammatory and pro-fibrotic cells and ↓COL1A1 mRNA expression | [88,89,90,91,92] |
| Epilepsy | CUR solid lipid NPs, CUR-loaded NPs, and CUR-loaded chitosan-alginate STPP NPs | ↑Bcl-2 family progenitors activation, ↑P38 MAPK pathways activation, ↑behavioral performance, ↓neuronal apoptosis, ↓neuronal OS, ↑klotho levels, ↑EPO levels, ↓TNF-α mRNA levels, ↓microglia inflammatory activation and ↓memory deficits | [93,94,95] |
| COVID-19 | Sinacurcumin soft gel containing 40 mg of curcuminoids as nanomicelles and NanoCUR capsules | ↓IFN-γ, ↓TNF-α, ↓IL-6, ↓IL-17, ↓IL-4, ↓IL-1β, ↓TGF-β, ↓COVID-19 clinical aspects, ↑recuperation velocity from fever and chills, myalgia, tachypnea, cough and smell and taste disturbances, ↑SaO2, ↓duration of supplemented O2 and ↓duration of hospitalization, ↓TBX21 genetic expression and ↑FoxP3 genetic expression, ↑lymphocyte count | [96,97,98,99,100,101] |
| Reference | Study | Population | Intervention | Duration | Outcomes |
| [100] | Placebo-controlled clinical trial (Iran) | 60 COVID-19 patients randomly divided equally into nanoCUR (56 ± 14.02 y, 24♂ and 6♀) and placebo (50.2 ± 12.01 y, 24♂ and 6♀) groups | 240 mg/day of nanoCUR orally | 7 days | ↓IFN-γ mRNA, ↓TNF-α mRNA, ↓IL-6 mRNA, ↓IL-1β and ↓COVID-19 clinical aspects |
| [97] | Open-label, nonrandomized clinical trial (Iran) | 41 mild to moderate COVID-19 patients allocated into nanoCUR (n = 21, 53.48 ± 12.21 y, 5♂ and 16♀) and placebo (n = 20, 58.45 ± 17.71 y, 9♂ and 11♀) groups | SinaCUR soft gel containing 40 mg of CURoids as nanomicelles orally | Two capsules twice a day/2 weeks | ↑Recuperation velocity from fever and chills, myalgia, tachypnea and cough, ↑SaO2, ↓duration of supplemented O2, and ↓duration of hospitalization |
| [98] | Triple-blind, placebo-controlled, randomized clinical trial (Iran) | 40 mild to severe COVID-19 patients allocated equally into nanoCUR (48.7 ± 10.8 y, 10♂ and 10♀) and placebo (48.3 ± 11 y, 12♂ and 8♀) groups | NanoCUR capsules containing 40 mg of CUR as nanomicelles orally | One capsule four times a day for 2 weeks | ↓IFN-γ, ↓IL-17, ↓IL-4, ↓TGF-β, ↓TBX21 genetic expression and ↑FoxP3 genetic expression |
| [101] | Triple-blind, placebo-controlled, randomized clinical trial (Iran) | 60 mild to moderate COVID-19 patients equally allocated into nanoCUR (41.33 ± 12.04 y, 20♂ and 10♀) and placebo (44.97 ± 11 y, 15♂ and 15♀) groups | SinaCUR soft gel containing 40 mg of CURoids as nanomicelles orally | Two capsules twice a day/2 weeks | ↑Recuperation velocity from chills, cough and smell and taste disturbances and ↑lymphocyte count |
| [96] | Double-blind, placebo-controlled, randomized clinical trial (Iran) | 40 COVID-19 patients equally divided into nanoCUR (53.3 ± 8.4 y, 15♂ and 5♀) and placebo (51.4 ± 7.9 y, 16♂ and 4♀) groups | SinaCUR soft gel containing 40 mg of CURoids as nanomicelles orally | Two capsules twice a day/2 weeks | ↓IL-6, ↓IL-18, ↓IL-1β and ↓TNF-α |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laurindo, L.F.; de Carvalho, G.M.; de Oliveira Zanuso, B.; Figueira, M.E.; Direito, R.; de Alvares Goulart, R.; Buglio, D.S.; Barbalho, S.M. Curcumin-Based Nanomedicines in the Treatment of Inflammatory and Immunomodulated Diseases: An Evidence-Based Comprehensive Review. Pharmaceutics 2023, 15, 229. https://doi.org/10.3390/pharmaceutics15010229
Laurindo LF, de Carvalho GM, de Oliveira Zanuso B, Figueira ME, Direito R, de Alvares Goulart R, Buglio DS, Barbalho SM. Curcumin-Based Nanomedicines in the Treatment of Inflammatory and Immunomodulated Diseases: An Evidence-Based Comprehensive Review. Pharmaceutics. 2023; 15(1):229. https://doi.org/10.3390/pharmaceutics15010229
Chicago/Turabian StyleLaurindo, Lucas Fornari, Gabriel Magno de Carvalho, Bárbara de Oliveira Zanuso, Maria Eduardo Figueira, Rosa Direito, Ricardo de Alvares Goulart, Daiene Santos Buglio, and Sandra Maria Barbalho. 2023. "Curcumin-Based Nanomedicines in the Treatment of Inflammatory and Immunomodulated Diseases: An Evidence-Based Comprehensive Review" Pharmaceutics 15, no. 1: 229. https://doi.org/10.3390/pharmaceutics15010229
APA StyleLaurindo, L. F., de Carvalho, G. M., de Oliveira Zanuso, B., Figueira, M. E., Direito, R., de Alvares Goulart, R., Buglio, D. S., & Barbalho, S. M. (2023). Curcumin-Based Nanomedicines in the Treatment of Inflammatory and Immunomodulated Diseases: An Evidence-Based Comprehensive Review. Pharmaceutics, 15(1), 229. https://doi.org/10.3390/pharmaceutics15010229